Abstract
Background:
One of the most important limitations of osteochondral autograft transplant is the adverse effect on donor sites in the knee. Ultrapurified alginate (UPAL) gel is a novel biomaterial that enhances hyaline-like cartilage repair for articular defects. To avoid the need for knee cartilage autografting when treating osteochondritis dissecans (OCD) of the capitellum, we developed a surgical procedure involving a bone marrow stimulation technique (BMST) augmented by implantation of UPAL gel.
Hypothesis:
BMST augmented by UPAL gel implantation improves the cartilage repair capacity and provides satisfactory clinical outcomes in OCD of the capitellum.
Study Design:
Case series; Level of evidence, 4.
Methods:
A total of 5 athletes with advanced capitellar OCD in the dominant elbow underwent BMST augmented by implantation of UPAL gel. The osteochondral defects were filled with UPAL gel after BMST. At a mean follow-up of 97 weeks, all patients were evaluated clinically and radiographically.
Results:
At final follow-up, all 5 patients had returned to competitive-level sports, and 4 patients were free from elbow pain. The mean Timmerman-Andrews score significantly improved from 100 to 194 points. Radiographically, all patients exhibited graft incorporation and a normal contour of the subchondral cortex. Magnetic resonance imaging showed that the preoperative heterogeneity of the lesion had disappeared, and the signal intensity had returned to normal. Arthroscopic examinations consistently exhibited improvement in the International Cartilage Regeneration and Joint Preservation Society (ICRS) grade of lesions from 3 or 4 to 1 or 2 in 4 patients at 85 weeks postoperatively. Histologic analysis of biopsy specimens revealed an average total ICRS Visual Assessment Scale II histologic score of 1060.
Conclusion:
The acellular cartilage repair technique using UPAL gel for advanced capitellar OCD provided satisfactory clinical and radiographic results. The present results suggest that this novel technique is a useful, minimally invasive approach for treating cartilaginous lesions in athletes.
Keywords: capitellar osteochondritis dissecans, elbow, biomaterial, ultrapurified alginate gel
Osteochondritis dissecans (OCD) of the humeral capitellum is a common injury among throwing athletes. 7,18,27 In terms of treatment options, young patients with early-stage capitellar OCD are good candidates for nonoperative treatment because of their potential for healing. 25,26 In contrast, surgical treatment is usually required for patients with advanced-stage capitellar OCD. #
Studies have advocated several surgical procedures for patients with large or unstable OCD lesions, 20,25,29,35,37 among which autologous osteochondral grafting, also known as mosaicplasty, is considered a good option. 16,17,21,24,36,41 Studies have demonstrated that mosaicplasty provides satisfactory clinical outcomes and reliable return to play for young athletes with large or unstable OCD lesions. 16,17,24 One of the most important limitations of mosaicplasty is the possible adverse effects on donor knees. Previous experimental studies have shown that the donor tunnels are covered with initial repair tissue by 6 weeks after the graft harvest and that fibrocartilage coverage develops within 8 to 12 weeks. 5,8 Using magnetic resonance imaging (MRI) evaluation, Iwasaki et al 15 suggested that the donor site was resurfaced with fibrous tissue after mosaicplasty for capitellar OCD. Reddy et al 31 reported that osteochondral harvest from normal knee joints for talar osteochondral lesions led to deterioration of knee function. To overcome this limitation, we sought to establish a novel cartilage repair strategy that does not require autograft harvesting.
Igarashi et al 13 developed an in situ–shaping ultrapurified alginate (UPAL) gel exhibiting quite low toxicity. Previous experimental studies in rabbit and canine models have demonstrated that administration of UPAL gel without cell implantation enhanced hyaline-like cartilage repair of osteochondral defects. 1,2 Considering these results, we hypothesized that a bone marrow stimulation technique (BMST) augmented by UPAL gel administration would improve the capacity for resurfacing osteochondral defects by enhancing cartilage repair. The objective of this study was to prospectively assess the clinical outcomes of UPAL gel implantation alone in patients with advanced capitellar OCD.
Methods
This study was conducted according to the Declaration of Helsinki, International Committee on Harmonisation Good Clinical Practice Guidelines, and applicable local laws and regulations. The protocol was approved by the regulatory authorities in Japan and by the ethics committees of each study site.
Patients
Between January 2016 and April 2017, a total of 5 patients (5 elbows) underwent BMST augmented by UPAL gel administration for advanced capitellar OCD. The inclusion criteria were symptomatic advanced capitellar OCD in athletes identified on anteroposterior plain radiographs with the elbow at 45° of flexion. Indications for this surgery included failure of nonoperative treatment of >6 months in duration or evidence of unstable lesions on plain radiographs and MRI. Patients with osteoarthritis of the elbow, an open growth plate of the capitellum, age <13 or >65 years, Timmerman-Andrews score 39 >140, or lesions <1.0 cm2 were excluded.
Data were collected regarding clinical outcomes and imaging. Images were evaluated by a single musculoskeletal radiologist who had >10 years of experience in joint MRI interpretation and who was blinded to patient data, intraoperative findings, and postoperative periods. To minimize variability, each image was evaluated twice. All patients underwent physical examination and imaging studies, including radiographs and MRI.
Preparation of Alginate Gel
The medical device candidate used in the study (dMD-001; Mochida Pharmaceutical Co Ltd) is composed of an original in situ–forming material based on UPAL gel (molecular weight of 1700 kDa) and 0.1 mol/L CaCl2 solution. The materials were sterilized by ultrafiltration (0.22-mm pore size filter). The UPAL gel exhibited a very low endotoxin level of 5.76 EU/g, as opposed to 75,950 EU/g for commercial-grade alginate (sodium alginate 500, 199-09961; Wako). 13 We used 2% (wt/vol) sodium alginate solution dissolved in distilled water.
Surgical Technique
All procedures were performed with the patient in the supine position under general anesthesia, with the arm brought across the chest, by 1 of 3 surgeons (M.K. [28 years of experience], T.F. [24 years of experience], or D.M. [13 years of experience]). After the extent of the lesion was confirmed by arthroscopy and instability was determined by palpation using an arthroscopic probe, a 4- to 6-cm incision was made on the posterior aspect of the radiocapitellar joint. The fascia over the anconeus muscle was divided sharply, and the muscle was split. The capsule was incised from the posterior edge of the lateral epicondyle to the proximal edge of the annular ligament. The OCD lesion on the capitellum was exposed. When required, the inflamed synovium of the radiohumeral joint was resected. For lateral lesions identified preoperatively on radiographs, a more posterior dissection of the extensor compartment was performed to expose the lesion. The displaced or dislocated fragments were removed, and the fibrous tissue around the osteochondral lesion was carefully curetted. The size of the capitellar lesion was calculated using the short and long axes of an oval template placed for direct measurement. For the BMST, 5 to 10 holes (1.0 mm in diameter and 10.0 mm in depth) were drilled into the defect (Figure 1A) and filled with 2% sodium alginate solution. Because of its high viscosity, the alginate solution did not flow out from the defect. For gelation of the superficial layer of alginate solution, CaCl2 was injected onto the surface of the alginate for 5 minutes (Figure 1B), and the CaCl2 solution was then washed away by irrigating the elbow with normal saline. To confirm the stability of the implanted UPAL gel, the elbow was flexed and extended 10 times intraoperatively. Without any additional fixation of the augmentation site, the capsule and skin were closed.
Figure 1.
(A) The radiohumeral joint is exposed posteriorly by splitting the anconeus muscle. A capitellar osteochondritis dissecans lesion is exposed with extreme elbow flexion, and the blood supply is confirmed after bone marrow stimulation technique. (B) Ultrapurified alginate gel is implanted into the prepared osteochondral defect (arrow).
Postoperative Management
After surgery, the elbow was immobilized at 90° of flexion with neutral forearm rotation for 2 weeks. The patients began active and assisted passive motion exercise of the elbow and forearm immediately after removal of the splint. After 8 to 12 weeks, we encouraged patients to engage in exercises to strengthen the trunk, scapulothoracic joint, and rotator cuff. Pitching activity was permitted 2 to 3 months after surgery, and patients were permitted to return to their previous activity level by 3 to 4 months postoperatively.
Postoperative Assessments
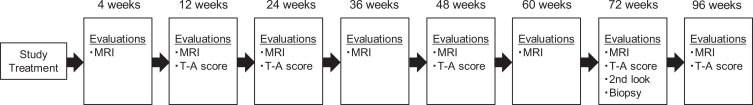
Figure 2 shows the postoperative assessments that were conducted at each time point, including MRI evaluations 32 and Timmerman-Andrews scoring. 39 Although the Timmerman-Andrews score has not been validated, it has been used as an outcome measure in previous studies on treatment of capitellar OCD. 9 The preoperative clinical score of the elbow was compared with that at each follow-up examination. Standardized MRI scans were obtained at 8 postoperative time points in each patient. The MRI evaluation focused on evidence of graft migration or displacement and the presence of fluid surrounding the graft on T2-weighted images. Cartilage surface characteristics, highlighted fluid in the joint, and edema in the subchondral bone were evaluated using fat-saturated proton density–weighted images. The quality of the joint surface reconstruction was evaluated based on the Roberts score 32 and Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score; descriptions of scoring are available in Appendix Tables A1 and A2. 38 After informed consent was obtained, second-look arthroscopy was performed in 4 patients at 72 weeks after surgery, and tissues were examined histologically by biopsy at the second-look surgery. The samples for histologic evaluation were obtained from the center portion of each reparative area using a biopsy needle of 1.8-mm diameter and 10-mm depth. The obtained samples were then prepared for histologic and immunohistochemical analyses. The samples were stained with safranin O and hematoxylin and eosin. Immunohistochemical staining was performed with anti–type II collagen antibodies (Kyowa Pharma Chemical Co Ltd). To quantitatively evaluate the reparative tissue, macroscopic and histologic findings were scored using the International Cartilage Regeneration and Joint Preservation Society (ICRS) Visual Assessment Scale II histologic score. 22 All scoring was performed by a single blinded observer (T.O.). To avoid observer bias, the samples were randomized and coded before analysis.
Figure 2.
Postoperative assessments at each time point. MRI, magnetic resonance imaging; T-A, Timmerman-Andrews.
Statistical Analyses
After normal data distribution was determined, a paired t test was used to compare the preoperative and final follow-up data regarding elbow range of motion and Timmerman-Andrews scores. To investigate changes in clinical and MRI-based outcomes over time, 1-way repeated-measures analysis of variance was used. P values <.05 were considered significant.
Results
All 5 study patients were male, with a mean age of 19.6 years (range, 13-35 years) at the time of operation; the mean cartilage defect size was 1.4 cm2 (range, 1.1-1.9 cm2) (Table 1). The mean follow-up period was 97.2 weeks (range, 96-99 weeks). No findings indicating material-derived side effects were observed during the follow-up period. At the final follow-up, 4 patients were free of elbow pain, and the remaining patient reported occasional mild pain. At the final follow-up, the total arc of elbow motion had significantly improved compared with preoperatively, from a mean ± SD of 100.0° ± 12.7° to 131.0° ± 12.4°, and the mean Timmerman-Andrews score significantly increased, from 100 ± 7.1 to 194 ± 10.8 (Table 1).
Table 1.
Characteristics of the Study Participants a
| Patient No. | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Mean | |
| Age, y | 18 | 35 | 18 | 13 | 14 | 19.6 |
| Dominant side | Right | Right | Right | Right | Right | |
| Sport | Self-defense | Baseball | Table tennis | Baseball | Baseball | |
| Height, cm | 176.4 | 177.5 | 176.5 | 163 | 173.6 | 173.4 |
| Weight, kg | 70 | 78.4 | 100 | 77.1 | 72.8 | 79.7 |
| Minami classification | Displaced | Displaced | Detached | Detached | Displaced | |
| Defect size, cm2 | 1.1 | 1.3 | 1.9 | 1.1 | 1.4 | 1.4 |
| ICRS grade | 3 | 4 | 4 | 3 | 4 | |
| 2nd-look surgery, wk postoperative | NA | 72 | 96 | 87 | 86 | 85 |
| ICRS grade at 2nd-look surgery | NA | 2 | 2 | 2 | 1 | |
| Duration of follow-up, wk | 96 | 96 | 98 | 99 | 97 | 97.2 |
| Total arc of elbow motion, deg | ||||||
| Preoperative | 100 | 100 | 80 | 105 | 115 | 100 |
| Postoperative | 140 | 130 | 110 | 135 | 140 | 131 |
| Timmerman-Andrews score | ||||||
| Preoperative | 90 | 105 | 105 | 105 | 95 | 100 |
| Postoperative | 200 | 195 | 175 | 200 | 200 | 194 |
a ICRS, International Cartilage Regeneration and Joint Preservation Society; NA, not available.
In total, 4 of the 5 patients exhibited excellent clinical results (Timmerman-Andrew score range, 180-200), and the remaining patient exhibited good clinical results (Timmerman-Andrews score range, 160-179) at the final follow-up. The changes in postoperative Timmerman-Andrews scores are shown in Figure 3. The mean value significantly increased from preoperatively to 184 ± 11.4 by 12 weeks postoperatively and to 196 ± 8.9 by 24 weeks postoperatively (P < .0001).
Figure 3.
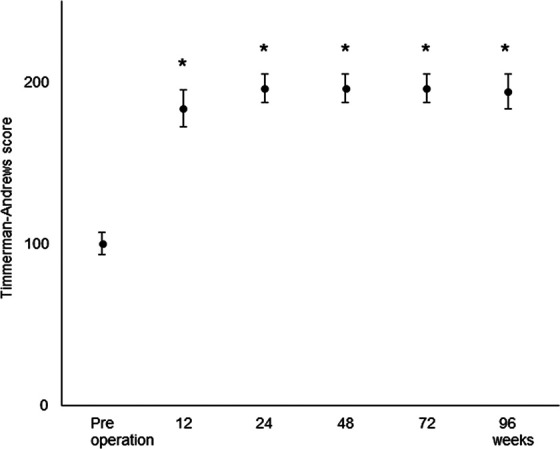
Mean Timmerman-Andrews scores plotted versus time. Error bars indicate standard deviations. *P < .0001 versus preoperative score.
Radiographically, all patients exhibited graft incorporation and normal contour of the subchondral cortex. MRI demonstrated that the preoperative heterogeneity of the lesions had disappeared, and the signal intensity returned to the normal range by the final follow-up (Figure 4A). The mean Roberts score was 0 at 4 weeks postoperatively, but it increased to 0.4 ± 0.5 by 12 weeks, 2.0 ± 1.9 by 24 weeks, and 3.4 ± 1.3 by 36 weeks. The 36-week score was a significant improvement from the scores at 4 weeks (P = .0038) and 12 weeks (P = .0143). Improvements in the Roberts scores were maintained up to 96 weeks postoperatively (Figure 4B). The mean MOCART score was 38 ± 16.0 at 4 weeks postoperatively, but it increased to 59 ± 13.9 by 12 weeks and 85 ± 3.5 by 24 weeks. The 24-week value was a significant improvement compared with scores at 4 weeks (P < .0001) and 12 weeks (P = .0032). At the final follow-up, the mean MOCART score was 84 ± 6.5 (Figure 4C).
Figure 4.
(A) Preoperative and postoperative MRI scans of the same patient depicted in Figure 1 (B) Mean Roberts score plotted versus time. (C) Mean MOCART score plotted versus time. Error bars indicate standard deviations.
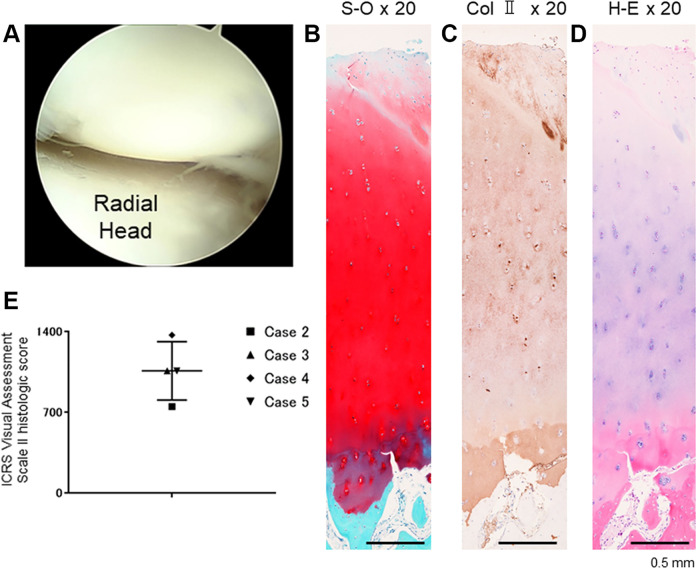
Of the 5 patients, 4 patients underwent second-look surgery beginning at 72 weeks postoperatively (range, 72-96 weeks). Arthroscopic examination consistently demonstrated improvement in the ICRS grade of the lesions from 3 or 4 to 1 or 2 (Table 1). No adverse effects such as synovitis or inflammation were observed during second-look surgery in any of the 4 patients (Figure 5A). Histologic analysis of biopsy specimens revealed hyaline-like cartilage repair in 3 of the 4 patients (Figure 5, B-D). The mean total ICRS Visual Assessment Scale II histologic score (maximum score 1400) was 1060 ± 253 (Figure 5E).
Figure 5.
(A) Second-look arthroscopy of the same patient depicted in Figure 1, at 86 weeks after treatment. Repair biopsy specimen stained for (B) safranin O (S-O), (C) collagen type II (Col II), and (D) hematoxylin and eosin (H-E). All scale bars: 0.5 mm. (E) Plot of International Cartilage Regeneration and Joint Preservation Society (ICRS) Visual Assessment Scale II histologic scores.
By the end of the follow-up period, all patients had returned to competitive-level sports.
Discussion
To the best of our knowledge, this is the first prospective study to evaluate a material-based acellular cartilage repair technique for advanced capitellar OCD. The present study demonstrated that BMST augmented by UPAL gel implantation significantly improved clinical outcomes in patients with advanced capitellar OCD. The imaging and histologic findings of this study suggested hyaline-like cartilage repair of the OCD lesion. These results indicate that our acellular cartilage repair technique using UPAL gel promotes the cartilage repair process, leading to satisfactory clinical outcomes in patients with advanced capitellar OCD.
Adverse tissue reactions and rejection represent important potential side effects of material-based therapies. The safety of xenograft products has been justifiably questioned since the study by Iannotti et al, 12 in which a porcine product used to supplement rotator cuff repair produced severe tissue reactions and rejection. To overcome these adverse effects, we developed a highly purified biocompatible alginate that exhibits reduced endotoxicity. 13 Initial studies of implantation of this alginate reported no infections or allergic reactions in rabbit and canine models. 19,40 The low endotoxin level enables safe clinical application of the UPAL gel because it does not induce immunologic reactions to the implanted material. 11,14 The current clinical and radiographic findings suggested that no infections or allergic reactions occurred in any of the 5 patients. Additionally, second-look arthroscopy and biopsy provided visual confirmation that there were no adverse effects derived from the material in the soft tissues of the elbow joint and cartilage, thereby indicating a reasonable safety profile.
Previous studies have advocated several surgical options for patients with advanced capitellar OCD lesions. 20,23,30,37 Among these options, BMST has been widely performed for relatively small unstable lesions. 33,34 However, BMSTs are thought to involve fibrous or fibrocartilaginous repair, which can lead to unsatisfactory long-term clinical results. 4 We speculate that this limited repair is due to insufficient chondrocyte proliferation and differentiation in conjunction with a lack of scaffold matrix formation. Previous studies have demonstrated that UPAL gel administration without exogenous cell implantation induces hyaline-like cartilage repair, possibly because this hydrogel retains migrated cells and enhances cellular proliferation and chondrogenic differentiation. 2,11 Using a canine model, Baba et al 1 reported that the BMST augmented by UPAL gel elicited hyaline-like cartilage repair characterized by rich glycosaminoglycan and matrix immunostaining by anti–type II collagen antibody. The histologic assessments in the current study demonstrated acceptable safranin O and type II collagen staining of reparative tissue, indicating hyaline-like cartilage repair in 3 of the 4 patients who underwent biopsy at second-look surgery.
Recent studies have demonstrated that mosaicplasty provides favorable clinical outcomes in patients with large capitellar OCD lesions. 16,17 Mosaicplasty is based on the concept that osteochondral grafts harvested from the articular cartilage of the knee joint provide an adequate biomechanical environment and friction pattern similar to that of the normal elbow joint. 15,16 A considerable limitation of mosaicplasty, however, is the possibility of adverse effects on donor sites in the knee. 15,16 Most patients with advanced capitellar OCD for which mosaicplasty is indicated are young athletes. Therefore, a less invasive technique that allows for early rehabilitation and prevents postoperative adverse effects attributed to surgery is desired. In a study of teenage athletes with mean OCD lesion size of 15.6 × 14.4 mm, Shimada et al 35 reported that mosaicplasty produced satisfactory clinical outcomes. Funakoshi et al 9 reported that MRI findings after mosaicplasty confirmed the smoothness and integrity of the cartilage surface in lateral as well as central OCD lesions. The results of our study demonstrated satisfactory MRI findings and short-term clinical outcomes after treatment with UPAL gel for advanced capitellar OCD in athletes, without invasion of the knee. Regarding lesion size and MRI findings, the current results are comparable to previous reports on mosaicplasty. Hence, this less invasive technique may become an alternative treatment for mosaicplasty of unstable OCD lesions.
There are several limitations to this study. First, the present study investigated relatively short-term clinical outcomes, and the study group was small. Second, we did not compare BMST augmented by UPAL gel with other surgical procedures. Third, there were 3 surgeons for 5 cases. Fourth, the treatment was performed by open surgery. Patients with advanced capitellar OCD for which surgical treatment is indicated are mostly young athletes. A less invasive, arthroscopic technique should be developed. Further multicenter, comparative, and long-term investigations should attempt to confirm the effectiveness and appropriate indication of our treatment using UPAL gel.
Conclusion
The current study suggests that the BMST augmented by UPAL gel administration provides satisfactory clinical outcomes for athletes with advanced capitellar OCD. Moreover, second-look arthroscopy and biopsy provided visual and histologic confirmation of hyaline-like cartilage repair. These results indicate the practicality of our novel cartilage repair technique using UPAL gel for treating advanced capitellar OCD in athletes.
Acknowledgment
The authors are especially thankful to Nozomu Watanabe, Masaki Higano, and Mochida Pharmaceutical Co Ltd, a participating company in NexTEP, for material preparations.
APPENDIX
Table A1.
Roberts Score Description
| Feature | Score |
|---|---|
| Surface integrity and contour | 1 = normal or near normal, 0 = abnormal |
| Cartilage signal in graft region | 1 = normal or near normal, 0 = abnormal |
| Cartilage thickness | 1 = normal or near normal, 0 = abnormal |
| Changes in underlying bone | 1 = normal or near normal, 0 = abnormal |
| Maximum total possible | 4 |
Table A2.
MOCART Score Description
| Variable | Class | Score |
|---|---|---|
| Degree of defect repair and defect filling | Complete (on a level with adjacent) | 20 |
| Hypertrophy (over the level of the adjacent cartilage) | 15 | |
| Incomplete (under the level of the adjacent cartilage: underfilling) | ||
| >50% of the adjacent cartilage | 10 | |
| <50% of the adjacent cartilage | 5 | |
| Subchondral bone exposed (complete delamination or dislocation and/or loose body) | 0 | |
| Integration to border zone | Complete (complete integration with adjacent cartilage) | 15 |
| Incomplete (incomplete integration with adjacent cartilage), demarcation border visible (split-like) | 10 | |
| Defect visible | ||
| <50% of the length of the repair tissue | 5 | |
| >50% of the length of the repair tissue | 0 | |
| Surface of the repair tissue | Surface intact (lamina splendens intact) | 10 |
| Surface damaged (fibrillations, fissures, and ulcerations) | ||
| <50% of repair tissue depth | 5 | |
| >50% of repair tissue depth or total degeneration | 0 | |
| Structure of the repair tissue | Homogeneous | 5 |
| Inhomogeneous of cleft formation | 0 | |
| Signal intensity of the repair tissue | Isointense | 30 |
| Moderately hyperintense | 10 | |
| Markedly hyperintense | 0 | |
| Subchondral lamina | Intact | 5 |
| Not intact | 0 | |
| Subchondral bone | Intact | 5 |
| Edema, granulation tissue, cysts, sclerosis | 0 | |
| Adhesions | No | 5 |
| Yes | 0 | |
| Effusion | No effusion | 5 |
| Effusion | 0 | |
| Maximum score | 100 |
Footnotes
Final revision submitted September 14, 2020; accepted October 16, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: This work was supported in part by the Newly Extended Technology Transfer Program (NexTEP) of the Japan Science and Technology Agency for the development of the sclerosing gel for the treatment of articular cartilage lesions. Mochida Pharmaceutical Co Ltd, which provided some of the materials used in the study, is a participating company in NexTEP. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Hokkaido University Institutional Review Board (No. 15056).
References
- 1. Baba R, Onodera T, Matsuoka M, et al. Bone marrow stimulation technique augmented by an ultrapurified alginate gel enhances cartilage repair in a canine model. Am J Sports Med. 2018;46(8):1970–1979. [DOI] [PubMed] [Google Scholar]
- 2. Baba R, Onodera T, Momma D, et al. A novel bone marrow stimulation technique augmented by administration of ultrapurified alginate gel enhances osteochondral repair in a rabbit model. Tissue Eng Part C Methods. 2015;21(12):1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker CL III, Romeo AA, Baker CL, Jr. Osteochondritis dissecans of the capitellum. Am J Sports Med. 2010;38(9):1917–1928. [DOI] [PubMed] [Google Scholar]
- 4. Bexkens R, van den Ende KIM, Ogink PT, et al. Clinical outcome after arthroscopic debridement and microfracture for osteochondritis dissecans of the capitellum. Am J Sports Med. 2017;45(10):2312–2318. [DOI] [PubMed] [Google Scholar]
- 5. Bodo G, Hangody L, Szabo Z, et al. Arthroscopic autologous osteochondral mosaicplasty for the treatment of subchondral cystic lesion in the medial femoral condyle in a horse. Acta Vet Hung. 2000;48(3):343–354. [DOI] [PubMed] [Google Scholar]
- 6. Byram IR, Kim HM, Levine WN, Ahmad CS. Elbow arthroscopic surgery update for sports medicine conditions. Am J Sports Med. 2013;41(9):2191–2202. [DOI] [PubMed] [Google Scholar]
- 7. Curtis AS, Deshmukh R. Throwing injuries: diagnosis and treatment. Arthroscopy. 2003;19(suppl 1):80–85. [DOI] [PubMed] [Google Scholar]
- 8. Feczko P, Hangody L, Varga J, et al. Experimental results of donor site filling for autologous osteochondral mosaicplasty. Arthroscopy. 2003;19(7):755–761. [DOI] [PubMed] [Google Scholar]
- 9. Funakoshi T, Momma D, Matsui Y, et al. Autologous osteochondral mosaicplasty for centrally and laterally located, advanced capitellar osteochondritis dissecans in teenage athletes: clinical outcomes, radiography, and magnetic resonance imaging findings. Am J Sports Med. 2018;46(8):1943–1951. [DOI] [PubMed] [Google Scholar]
- 10. Hennrikus WP, Miller PE, Micheli LJ, Waters PM, Bae DS. Internal fixation of unstable in situ osteochondritis dissecans lesions of the capitellum. J Pediatr Orthop. 2015;35(5):467–473. [DOI] [PubMed] [Google Scholar]
- 11. Hishimura R, Onodera T, Hontani K, et al. Osteochondral autograft transplantation technique augmented by an ultrapurified alginate gel enhances osteochondral repair in a rabbit model. Am J Sports Med. 2019;47(2):468–478. [DOI] [PubMed] [Google Scholar]
- 12. Iannotti JP, Codsi MJ, Kwon YW, et al. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears: a randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238–1244. [DOI] [PubMed] [Google Scholar]
- 13. Igarashi T, Iwasaki N, Kasahara Y, Minami A. A cellular implantation system using an injectable ultra-purified alginate gel for repair of osteochondral defects in a rabbit model. J Biomed Mater Res A. 2010;94(3):844–855. [DOI] [PubMed] [Google Scholar]
- 14. Igarashi T, Iwasaki N, Kawamura D, et al. Repair of articular cartilage defects with a novel injectable in situ forming material in a canine model. J Biomed Mater Res A. 2012;100(1):180–187. [DOI] [PubMed] [Google Scholar]
- 15. Iwasaki N, Kamishima T, Kato H, Funakoshi T, Minami A. A retrospective evaluation of magnetic resonance imaging effectiveness on capitellar osteochondritis dissecans among overhead athletes. Am J Sports Med. 2012;40(3):624–630. [DOI] [PubMed] [Google Scholar]
- 16. Iwasaki N, Kato H, Ishikawa J, et al. Autologous osteochondral mosaicplasty for osteochondritis dissecans of the elbow in teenage athletes. J Bone Joint Surg Am. 2009;91(10):2359–2366. [DOI] [PubMed] [Google Scholar]
- 17. Iwasaki N, Kato H, Ishikawa J, Saitoh S, Minami A. Autologous osteochondral mosaicplasty for capitellar osteochondritis dissecans in teenaged patients. Am J Sports Med. 2006;34(8):1233–1239. [DOI] [PubMed] [Google Scholar]
- 18. Kida Y, Morihara T, Kotoura Y, et al. Prevalence and clinical characteristics of osteochondritis dissecans of the humeral capitellum among adolescent baseball players. Am J Sports Med. 2014;42(8):1963–1971. [DOI] [PubMed] [Google Scholar]
- 19. Kim W, Onodera T, Kondo E, et al. Effects of ultra-purified alginate gel implantation on meniscal defects in rabbits. Am J Sports Med. 2019;47(3):640–650. [DOI] [PubMed] [Google Scholar]
- 20. Kosaka M, Nakase J, Takahashi R, et al. Outcomes and failure factors in surgical treatment for osteochondritis dissecans of the capitellum. J Pediatr Orthop. 2013;33(7):719–724. [DOI] [PubMed] [Google Scholar]
- 21. Lyons ML, Werner BC, Gluck JS, et al. Osteochondral autograft plug transfer for treatment of osteochondritis dissecans of the capitellum in adolescent athletes. J Shoulder Elbow Surg. 2015;24(7):1098–1105. [DOI] [PubMed] [Google Scholar]
- 22. Mainil-Varlet P, Van Damme B, Nesic D, et al. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med. 2010;38(5):880–890. [DOI] [PubMed] [Google Scholar]
- 23. Maruyama M, Harada M, Satake H, et al. Bone-peg grafting for osteochondritis dissecans of the humeral capitellum. J Orthop Surg (Hong Kong). 2016;24(1):51–56. [DOI] [PubMed] [Google Scholar]
- 24. Maruyama M, Takahara M, Harada M, Satake H, Takagi M. Outcomes of an open autologous osteochondral plug graft for capitellar osteochondritis dissecans: time to return to sports. Am J Sports Med. 2014;42(9):2122–2127. [DOI] [PubMed] [Google Scholar]
- 25. Maruyama M, Takahara M, Satake H. Diagnosis and treatment of osteochondritis dissecans of the humeral capitellum. J Orthop Sci. 2018;23(2):213–219. [DOI] [PubMed] [Google Scholar]
- 26. Matsuura T, Kashiwaguchi S, Iwase T, Takeda Y, Yasui N. Conservative treatment for osteochondrosis of the humeral capitellum. Am J Sports Med. 2008;36(5):868–872. [DOI] [PubMed] [Google Scholar]
- 27. Matsuura T, Suzue N, Iwame T, Nishio S, Sairyo K. Prevalence of osteochondritis dissecans of the capitellum in young baseball players: results based on ultrasonographic findings. Orthop J Sports Med. 2014;2(8):2325967114545298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mihara K, Suzuki K, Makiuchi D, et al. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31–37. [DOI] [PubMed] [Google Scholar]
- 29. Miyake J, Masatomi T. Arthroscopic debridement of the humeral capitellum for osteochondritis dissecans: radiographic and clinical outcomes. J Hand Surg Am. 2011;36(8):1333–1338. [DOI] [PubMed] [Google Scholar]
- 30. Nishinaka N, Tsutsui H, Yamaguchi K, et al. Costal osteochondral autograft for reconstruction of advanced-stage osteochondritis dissecans of the capitellum. J Shoulder Elbow Surg. 2014;23(12):1888–1897. [DOI] [PubMed] [Google Scholar]
- 31. Reddy S, Pedowitz DI, Parekh SG, Sennett BJ, Okereke E. The morbidity associated with osteochondral harvest from asymptomatic knees for the treatment of osteochondral lesions of the talus. Am J Sports Med. 2007;35(1):80–85. [DOI] [PubMed] [Google Scholar]
- 32. Roberts S, McCall IW, Darby AJ, et al. Autologous chondrocyte implantation for cartilage repair: monitoring its success by magnetic resonance imaging and histology. Arthritis Res Ther. 2003;5(1):R60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ruch DS, Cory JW, Poehling GG. The arthroscopic management of osteochondritis dissecans of the adolescent elbow. Arthroscopy. 1998;14(8):797–803. [DOI] [PubMed] [Google Scholar]
- 34. Schoch B, Wolf BR. Osteochondritis dissecans of the capitellum: minimum 1-year follow-up after arthroscopic debridement. Arthroscopy. 2010;26(11):1469–1473. [DOI] [PubMed] [Google Scholar]
- 35. Shimada K, Tanaka H, Matsumoto T, et al. Cylindrical costal osteochondral autograft for reconstruction of large defects of the capitellum due to osteochondritis dissecans. J Bone Joint Surg Am. 2012;94(11):992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shimada K, Yoshida T, Nakata K, Hamada M, Akita S. Reconstruction with an osteochondral autograft for advanced osteochondritis dissecans of the elbow. Clin Orthop Relat Res. 2005;435:140–147. [DOI] [PubMed] [Google Scholar]
- 37. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205–1214. [DOI] [PubMed] [Google Scholar]
- 38. Takazawa K, Adachi N, Deie M, et al. Evaluation of magnetic resonance imaging and clinical outcome after tissue-engineered cartilage implantation: prospective 6-year follow-up study. J Orthop Sci. 2012;17(4):413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Timmerman LA, Andrews JR. Arthroscopic treatment of posttraumatic elbow pain and stiffness. Am J Sports Med. 1994;22(2):230–235. [DOI] [PubMed] [Google Scholar]
- 40. Tsukuda Y, Onodera T, Ito M, et al. Therapeutic effects of intra-articular ultra-purified low endotoxin alginate administration on an experimental canine osteoarthritis model. J Biomed Mater Res A. 2015;103(11):3441–3448. [DOI] [PubMed] [Google Scholar]
- 41. Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714–720. [DOI] [PubMed] [Google Scholar]