Abstract
BACKGROUND:
Large-scale, multisite studies in which researchers evaluate patient- and systems-level factors associated with pediatric asthma exacerbation outcomes are lacking. We sought to investigate patient-level risks and system-level practices related to physiologic readiness for discharge (PRD) in the prospective Ohio Pediatric Asthma Repository.
METHODS:
Participants were children ages 2 to 17 years admitted to an Ohio Pediatric Asthma Repository hospital for asthma exacerbation. Demographics, disease characteristics, and individual hospital practices were collected. The primary outcome was PRD timing (hours from admission or emergency department [ED] presentation until the first 4-hour albuterol spacing).
RESULTS:
Data for 1005 participants were available (865 ED presentations). Several nonstandard care practices were associated with time to PRD (P < .001). Continuous pulse oximetry was associated with increased time to PRD (P = .004). ED dexamethasone administration was associated with decreased time to PRD (P < .001) and less ICU admittance and intravenous steroid use (P < .0001). Earlier receipt of chest radiograph, antibiotics, and intravenous steroids was associated with shorter time to PRD (P < .05). Care practices associated with shorter time to PRD varied markedly by hospital.
CONCLUSIONS:
Substantial variation in care practices for inpatient asthma treatment exists among children’s hospital systems in Ohio. We found several modifiable, system-level factors and therapies that contribute to PRD that warrant further investigation to identify the best and safest care practices. We also found that there was no standardized measure of exacerbation severity used across the hospitals. The development of such a tool is a critical gap in current practice and is needed to enable definitive comparative effectiveness studies of the management of acute asthma exacerbation.
Asthma is the most pervasive chronic childhood condition, affecting more than 7 million US children.1 Hospital asthma admissions are costly,2 and longer time to physiologic readiness for discharge (PRD) coincides with increased length of stay and cost.3 Determinants of PRD for asthma exacerbations are multifactorial, reflecting in part disease heterogeneity, the presence of comorbid conditions,4 and the rapidity of response to exacerbation therapies.5–7 In addition to patient-level factors, the systems in which care occurs have the potential to influence PRD timing. System-level care practices may dictate the timing or duration of therapies, the use of diagnostic testing, preferred methods of medication administration, patient monitoring practices, discharge criteria, and other aspects of acute exacerbation care. System-level practices in asthma, particularly with regard to treatment standardization, guideline implementation,8 and albuterol weaning protocols,9 are associated with patient outcomes. However, there is a need for multisite studies in which researchers address the distinct and combined impact of patient-level risks and system-level care practices on PRD in acute asthma.
Our goal in this study was to investigate patient-level risks and system-level practices as they relate to PRD for asthma exacerbations using a unique, multicenter, statewide database called the Ohio Pediatric Asthma Repository (OPAR).10 OPAR is a comprehensive, statewide, prospective study in which researchers link clinical, demographic, environmental, and health outcomes data from the 6 major children’s hospitals in Ohio. By using OPAR, we are uniquely positioned to address how variability in patient- and system-level factors at each site impact PRD during acute asthma exacerbation.
METHODS
Subject Identification and Eligibility
OPAR was initiated and funded by the state of Ohio to better understand and optimize asthma care practices.10 Children aged 2 to 17 years who were admitted for asthma, wheezing, or reactive airway disease at 1 of 6 Ohio children’s hospitals were eligible. Each participant’s parent or legal guardian provided informed consent, and assent was obtained from children >7 years old. For this analysis, participants recruited between December 2012 and September 2013 were included. As previously reported, the mean proportion of eligible participants enrolled was 68% (53%–81%). Nonparticipants did not differ from participants by age or sex.10 This study was approved by the Institutional Review Board.
Questionnaires and Clinical Data Collection
Participants completed questionnaires that were used to capture demographics, symptom history, and health care use for wheezing and/or asthma. Information from admission, emergency department (ED) course, and ICU course (including respiratory assessment tools used, drugs administered, and providers) was collected from the medical record.
Outcome Definition: PRD
Medical criteria for PRD varied. Four hospitals used albuterol spacing every 4 hours (q4h), and 2 hospitals used albuterol spacing every 6 hours.10 To account for these differences, we defined PRD as the first time point at which the patient demonstrated sufficient clinical improvement while receiving albuterol inhalation treatments no more frequently than q4h. We measured the time from inpatient (IP) or ICU admission until the patient reached the first 4-hour albuterol spacing (PRDIP) (Fig 1). If a patient was discharged before meeting q4h, then PRDIP was the time from IP admission to discharge. We also measured PRDTOT as the time from emergency department presentation to first 4-hour albuterol spacing (Fig 1). If a patient was discharged before meeting q4h, then PRDTOT was defined as the time from ED presentation to discharge. Because of skewing, PRDIP and PRDTOT were log-transformed.
FIGURE 1.
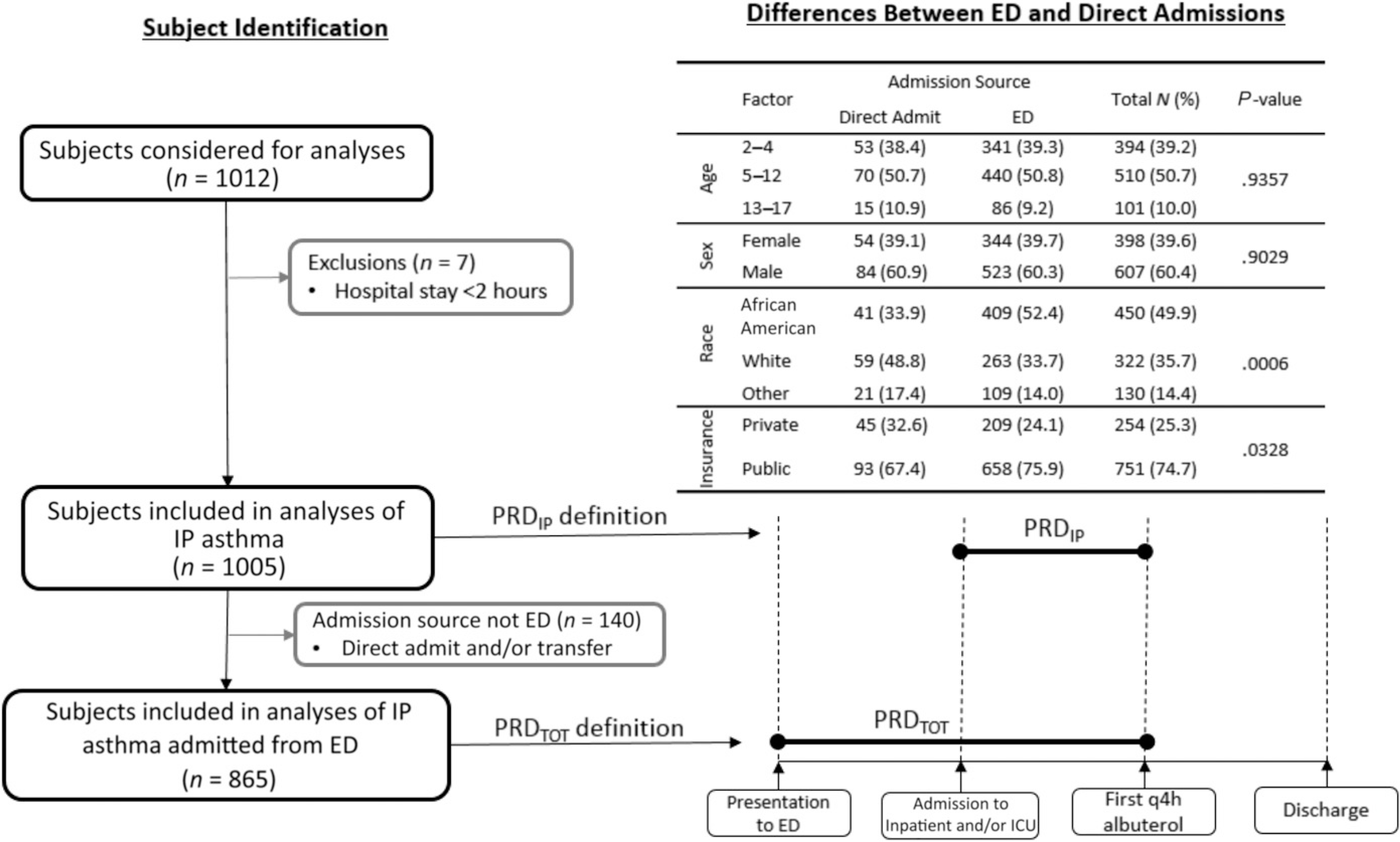
Subject identification and PRD definitions.
Covariate Definitions
Chronic Severity and Risk
Chronic asthma severity classification was assigned as intermittent, mild persistent, moderate persistent, or severe persistent on the basis of baseline symptom frequencies according to Expert Panel Report 3.11 High risk for asthma readmission was defined as parental report of hospitalization for asthma in the past 12 months. We combined asthma severity and risk into 4 categories: intermittent, mild and low risk; moderate, severe and low risk; intermittent, mild and high risk; and moderate, severe and high risk.10
Nonstandard Care Practices
We defined nonstandard care practices as intravenous (IV) steroid use, IP ipratropium administration, ICU admission, chest radiograph, continuous albuterol, and antibiotic administration.
Statistical Analyses
Our primary goal was to identify factors associated with PRD. Because demographics and care practices varied across hospitals,10 analyses were performed with mixed linear models in which hospital was included as a random effect. Because the factors associated with PRD may be correlated, we used a multistep strategy. First, we determined the relationships between PRDIP and each factor individually. These analyses reveal information about individual-level associations; however, they do not account for correlation between variables. Therefore, we next generated a multivariate model, considering variables exhibiting a marginal level of significance (P < .2) in the individual screens. Variables that were significant in the individual but not the multivariable analyses were evaluated for collinearity by using contingency tables. When collinearity existed, only 1 variable was included. The final multivariable model eliminated variables at P > .2. We used a similar strategy to evaluate factors associated with PRDTOT but also evaluated ED practices. For antibiotic use, steroid administration route, and chest radiograph, we created combined variables with 4 categories (ED only, IP only, both, and none), which replaced the dichotomous variables. Because ED dexamethasone was used primarily at a single hospital, analyses using only that hospital were evaluated. Additionally, we modeled PRDIP in the ED subpopulation to ensure consistency of results (data not shown).
To determine if hospital differences persisted after accounting for demographics, nonstandard care practices, and hospital practices, we performed multivariate modeling for hospital association. We included a fixed and a random effect for the number of nonstandard care practices received. We included demographic and IP practices that were significant in the multivariable PRDIP model as covariates. We generated a second multivariable PRDIP model that incorporated ED practices and location-dependent practices as covariates. These models allowed for the comparison of PRD among hospitals. Statistical analyses were performed by using SAS 9.4 (SAS Institute, Inc, Cary, NC).
RESULTS
Characteristics of the Study Population
The study population included 1005 children who were primarily boys, African American, and on public insurance (Fig 1). Because not all enrollees presented to an OPAR hospital-affiliated ED (direct admit), analyses of PRDIP include fewer children (n = 865; Fig 1). PRDIP and PRDTOT, as well as the proportion of patients who received nonstandard care practices, varied markedly among hospitals (Table 1).
TABLE 1.
Prevalence of IP and ED Practices Associated With Shorter Time to PRD by Hospital
| Dayton | Akron | Cincinnati | Toledo | Cleveland | Columbus | |
|---|---|---|---|---|---|---|
| n, IP (ED) | 115 (114) | 103 (72) | 365 (356) | 58 (35) | 196 (121) | 168 (168) |
| PRDIP, mean ± SD | 9.74 ± 0.06 | 18.06 ± 0.07 | 19.13 ± 0.04 | 22.46 ± 0.09 | 33.57 ± 0.05 | 38.66 ± 0.05 |
| PRDTOT, mean ± SD | 13.77 ± 0.05 | 20.77 ± 0.07 | 24.71 ± 0.03 | 26.09 ± 0.10 | 39.01 ± 0.05 | 44.60 ± 0.04 |
| Receipt of nonstandard care, % | 20.0 | 68.0 | 45.2 | 70.7 | 93.1 | 63.1 |
| Intervention Pulse oximetry, % spot only (n) |
77.4 (89) | 72.8 (75) | 8.8 (32) | 67.3 (39) | 39.3 (77) | 4.8 (8) |
| ED dexamethasone % (n in ED) | 0 (0) | 1.4 (1) | 0.2 (1) | 0 (0) | 72.7 (88) | 0.6 (1) |
| ED antibioticsa % (n in ED) | 6.1 (7) | 13.8 (10) | 3.9 (14) | 11.4 (4) | 9.1 (11) | 8.3 (14) |
| ED chest radiographb % (n in ED) | 56.1 (64) | 44.4 (32) | 47.2 (168) | 45.7 (16) | 51.2 (62) | 43.3 (73) |
| ED IV steroidsa % (n in ED) | 13.2 (15) | 37.5 (27) | 11.5 (41) | 14.3 (5) | 12.4 (15) | 31.5 (53) |
| ED intervention among those receiving intervention Antibioticsa, % (n in ED/n ever) |
38.9 (7/18) | 47.6 (10/21) | 30.4 (14/46) | 26.7 (4/15) | 28.2 (11/39) | 30.4 (14/46) |
| Chest radiographb, % (n in ED/n ever) | 90.1 (64/71) | 69.6 (32/46) | 78.1 (168/215) | 55.1 (16/29) | 63.3 (62/98) | 69.5 (73/105) |
| IV steroidsc, % (n in ED/n ED and IP) | 77.8 (7/9) | 64.7 (11/17) | 18.1 (17/94) | 12.5 (1/8) | 8.9 (4/45) | 28.1 (9/32) |
Initiated in ED.
Received only in ED.
Includes individuals who received during IP hospital course but excludes individuals receiving in both the ED and IP.
Factors Influencing PRDIP
In the individual screen, adolescent age, injected steroid route, oral steroids, ipratropium, ICU admission, chest radiograph, continuous albuterol, antibiotics, specialist overseeing physician, continuous pulse oximetry, and albuterol weaning by multiple providers were associated with increased PRDIP (Supplemental Table 2).
Because factors associated with PRDIP may correlate, we considered factors jointly. Demographics contributed to increased PRDIP after accounting for nonstandard care and routine care practices. There was a 2-hour increase in PRDIP for children with public compared with private insurance (P = .02) and a 2.7-hour increase for adolescents (aged 13–17 years) compared with younger children (P = .04; Fig 2).
FIGURE 2.
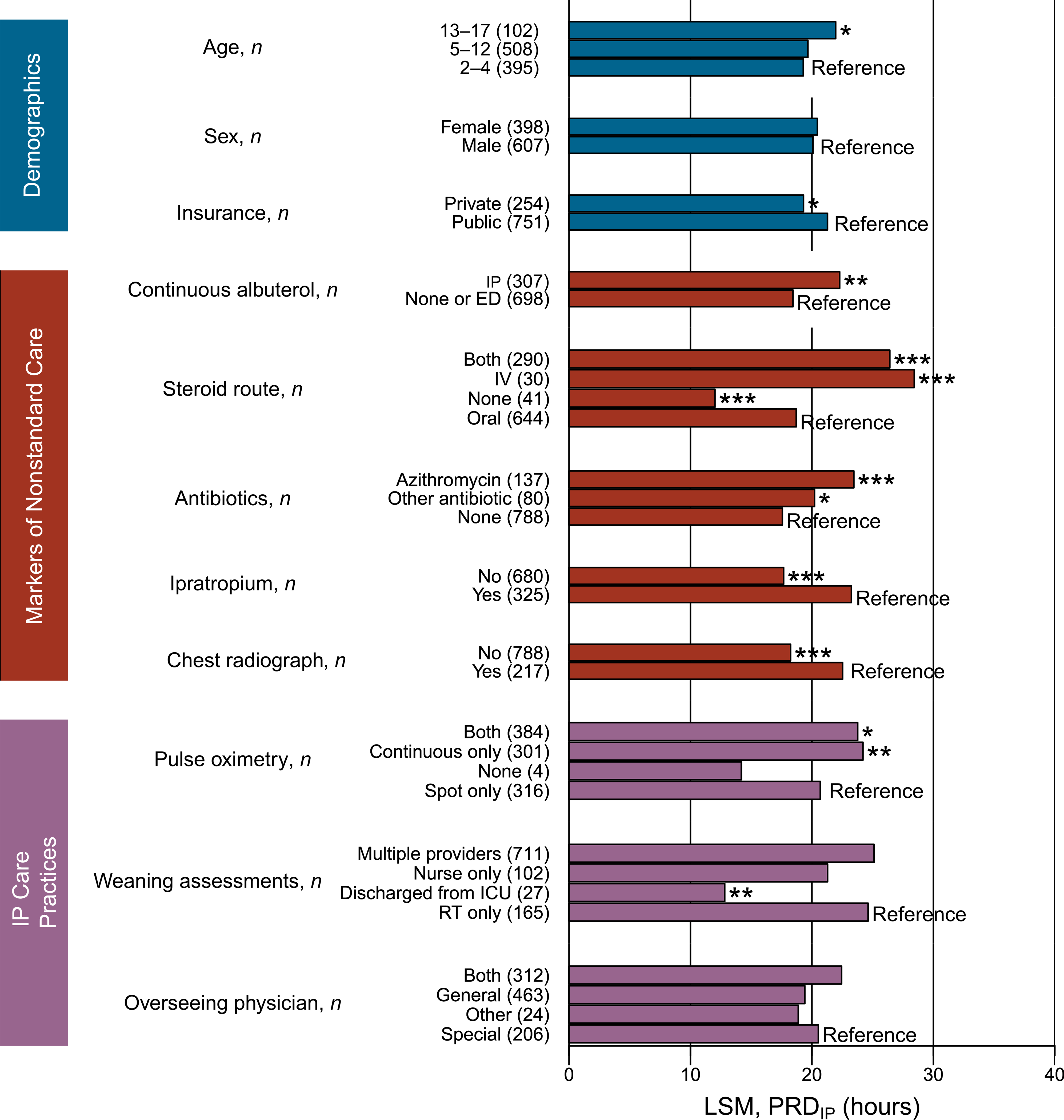
Demographic, severity indicator practices, and IP practices are associated with PRDIP in a multivariable model. LSM, least square mean; RT, respiratory therapist. * P < .05; ** P < .01; *** P < .001.
Nonstandard care practices were also associated with PRDIP in the multivariable model (Fig 2). The receipt of continuous albuterol increased PRDIP by almost 4 hours (P < .001). Children receiving injected steroids had a 9.7-hour longer PRDIP than those who only received oral steroids (P < .001). Receiving azithromycin increased PRDIP by 5.9 hours (P < .001). The administration of ipratropium and receipt of a chest radiograph increased PRDIP by 5.6 and 4.3 hours, respectively (P < .001 for both). ICU admission was not associated with PRDIP (P = .82) because of its high correlation with continuous albuterol (Spearman’s ρ = 0.80; P < .0001). These results support that nonstandard care practice use is associated with PRDIP.
IP care practices also contributed to PRDIP in the multivariable model (Fig 2). Children receiving continuous pulse oximetry, alone or in combination with spot checks, had a 3- to 3.5-hour longer PRDIP than children receiving only spot checks (P < .05). Other than the small number of children discharged from the ICU, the provider performing weaning assessments did not markedly alter PRDIP. PRDIP was not associated with the managing physician (P = .08; Fig 2); however, children who were seen only by a generalist were less likely to receive nonstandard care practices (odds ratio = 0.20; confidence interval = 0.15–0.26; P < .0001; data not shown). These results reveal that continuous pulse oximetry is associated with longer PRDIP after accounting for other factors.
Factors Influencing PRDTOT
ED-specific and location-dependent factors (performed in the ED, IP, or both) that were individually associated with increased PRDTOT included injected steroids outside the ED, not receiving ED dexamethasone, receiving ED magnesium sulfate, chest radiograph not performed in the ED, and the administration of IP and antibiotics outside of the ED (Supplemental Table 3). Because dexamethasone was used mainly at the Cleveland hospital, we tested the association between PRDTOT and dexamethasone for Cleveland and found that it was strongly associated with shorter PRDTOT (P < .001; data not shown).
In the multivariable model, location-dependent practices were associated with PRDTOT (Fig 3). Receiving antibiotics only in IP was associated (P ≤ .001) with a 6- to 6.7-hour longer PRDTOT than receiving antibiotics in the ED and IP, ED only, and not receiving antibiotics. Furthermore, having chest radiographs in the IP only or in both the IP and ED was associated with 5.6- and 7.9-hour longer PRDTOT, respectively, than not having a chest radiograph (P < .001). Individuals receiving a chest radiograph only in the ED did not have a significantly increased PRDTOT (P = .06). Receiving IV steroids only in IP was associated with 8.4- and 7.1-hour longer PRDTOT than receiving no IV steroids or only in the ED, respectively (P < .001).
FIGURE 3.
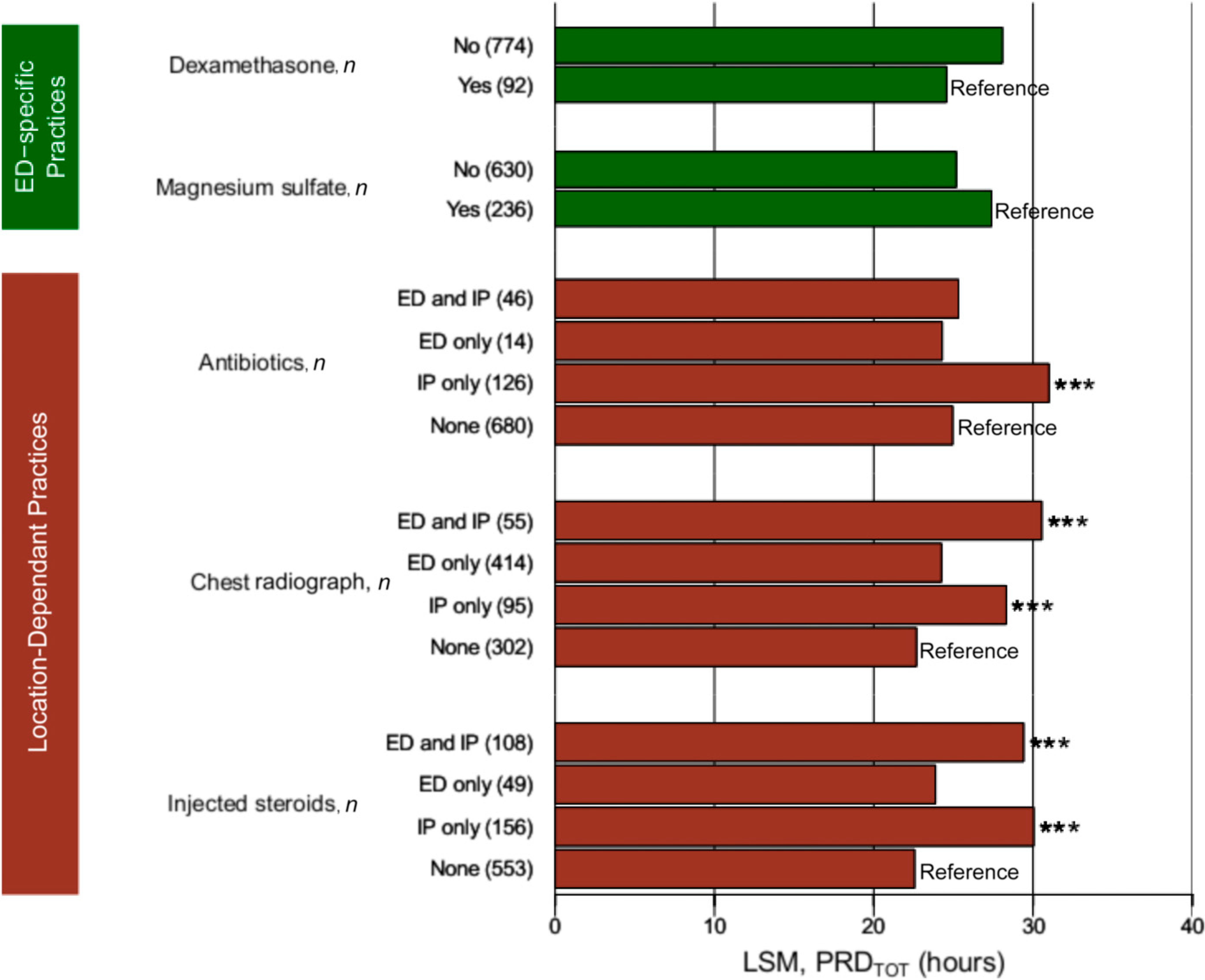
ED practices and time-dependent practices associated with PRDTOT in a multivariate model. LSM, xxx. * P < .05; ** P < .01; *** P < .001.
Dexamethasone and magnesium administration in the ED were marginally significant in the multivariate model (P ≤ .14), although they were highly significant individually (Supplemental Table 3). Individuals receiving magnesium sulfate were more likely to receive nonstandard care (odds ratio = 5.53; confidence interval = 3.87–8.05; P < .001; data not shown). To evaluate the effect of dexamethasone on PRDTOT, we restricted the analyses to Cleveland. Those receiving dexamethasone were less likely to receive nonstandard care (60.2% vs 87.9%; P = .0041; data not shown), be admitted to the ICU (36.0% vs 69.7%; P < .0001), and to receive injected steroids (31.8% vs 72.7%; P < .0001). In a multivariate model that was not adjusted for nonstandard care, those receiving dexamethasone stayed for 11.7 hours less (P = .007; data not shown) than those who did not.
Variation in PRDIP Among Hospitals is Due in Part to Variation in Hospital-Level Factors
After accounting for demographics, nonstandard care, and IP practices or demographics, nonstandard care, IP practices, and ED practices, the differences in PRD among the hospitals substantially decreased compared with what was seen in the unadjusted model (Fig 4). In the adjusted models, the longer PRDIP at the Cleveland site observed in the unadjusted model decreased. Thus, hospital-level factors contributed substantially to PRDIP. It is important to note that even after adjustment for system-level variation, differences in PRDIP among the sites persisted, suggesting that other, unaccounted factors play a role.
FIGURE 4.
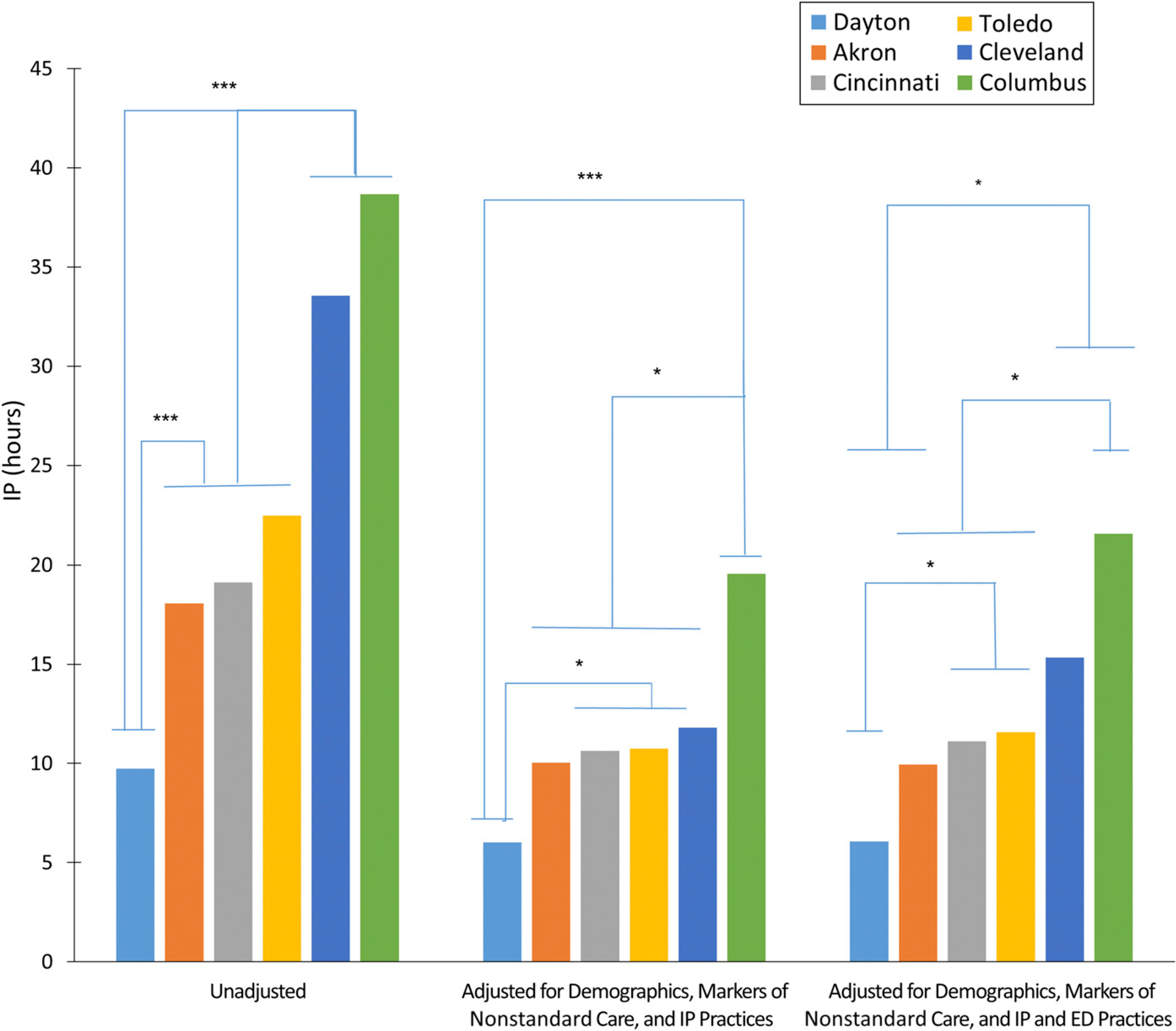
Variation in timing to PRD by hospital is partially explained by demographics, exacerbation severity, and IP and ED practices. * P < .05; ** P < .01; *** P < .001.
Opportunities for Process Improvement by Site
To determine if there are opportunities for improvement within each hospital, we evaluated hospital practices that were associated with shorter PRDIP by hospital (Table 1). The use of spot-only pulse oximetry monitoring was >72% at Dayton and Akron, but it is rarely exclusively performed in Cincinnati or Columbus. Dayton and Akron also had higher rates of the initiation of antibiotics and IV steroids in the ED compared with the other sites. ED dexamethasone use was limited almost exclusively to the Cleveland site but was associated with a significantly shorter PRDIP when that site was evaluated alone.
DISCUSSION
When children are admitted for an acute asthma exacerbation, the goal is to use guideline-level care to bring the patient to PRD in the shortest amount of time. However, treatment guidelines for IP asthma exacerbation translate into practice differently, leading to wide variation in hospitals. In this article, using a prospective study of 6 major hospitals in Ohio (OPAR), we demonstrate that PRD timing is multifactorial and includes a substantial contribution from hospital-level factors. Although clearly influenced by patient factors, the differences in hospital practices identified herein may offer an opportunity for improvement. Specifically, our results reveal that when clinically indicated, using spot-only pulse oximetry may shorten time to PRD. Furthermore, because those who did not receive nonstandard care had markedly lower PRD, minimizing the use of nonstandard care practices unless medically warranted is critical. These results reveal that the use of standardized and evidence-based severity assessments to identify those who warrant nonstandard care is essential.
We found that continuous pulse oximetry use was associated with increased time to PRD. It has been recommended to limit continuous pulse oximetry in children with severe illness, who likely need intensive monitoring (eg, in the ICU), but those with mild and moderate respiratory disease can be managed with spot checks.12 Furthermore, although pulse oximetry offers a noninvasive method for measuring arterial oxygen saturation, a major limitation is that of motion artifacts.13 Continuous monitoring likely increases the observation of artifacts and other nonclinically significant, transient decreases in oxygen saturation that may lead to more aggressive care and increase time to PRD. In several of the OPAR hospitals, the use of continuous pulse oximetry is routine. Our results suggest that limiting the use of continuous pulse oximetry to only when it is clinically indicated may decrease time to PRD. Additional studies are required to verify this finding.
We also found that nonstandard care practice use is associated with increased PRD. One reason for nonstandard care practices use is the more severe or complex exacerbation. Previous researchers have found comorbid respiratory infections14,15 and respiratory failure16 to be associated with increased length of stay. In addition, the use of nonstandard care practices that contribute to delaying PRD may be related to physician preference or a lack of use of evidence-based practices across providers. Interestingly, in our previous article,10 we noted a high rate of IP ipratropium use at the Cleveland hospital. It was determined that this high rate had no relation to exacerbation, but rather it was due to a standard order set. The routine use of ipratropium on the IP units in Cleveland has since been discontinued, but our data reveal the need to minimize nonstandard care unless it is medically warranted.
Notably, dexamethasone use in the ED was associated with shorter time to PRD. Previous studies have revealed potential benefits of dexamethasone, including decreased vomiting and improved adherence.17 Furthermore, dexamethasone has been associated with shorter length of stay and comparable readmission rates.18 We found that individuals receiving ED dexamethasone were less likely to be admitted to the ICU or receive IV steroids, suggesting that receiving ED dexamethasone may circumvent the need for more aggressive IP interventions. Because this finding was driven by nearly exclusive use in Cleveland, it requires confirmation. Early (within 1 hour of arrival) administration of systemic corticosteroid in the ED has been associated with fewer admissions and shorter length of stay.19 Although the early administration of dexamethasone in the Cleveland ED could be an alternative explanation for the differential outcome, this was not the practice at this site. As part of process improvement, an evaluation of the use of dexamethasone in the ED has been implemented at Cincinnati on the basis of our findings, and data on readmission and hospital PRD are being prospectively collected.
The timing of certain care practices (chest radiographs, antibiotics, and/or IV steroids in the ED rather than IP) was also associated with shorter PRD timing among those who received the practice. There are 2 possible reasons for such an association. First, an earlier initiation of clinically important and indicated specific treatments may shorten PRD. When indicated by symptoms or physical findings (eg, fever, localized adventitious lung sounds, hypoxemia, or vomiting), initiating such interventions in the ED may shorten length of stay by 6 to 10 hours.19,20 If this is the case, then there could be opportunities for improvement by incorporating evidence-based care algorithms in the ED, given the marked variations among the OPAR hospitals. Indeed, the use of validated assessment tools or development of more sensitive scores could expedite the identification of children with more severe illnesses who would benefit from an earlier initiation of treatments. It is also possible that nonstandard interventions may be used more indiscriminately in the ED on patients with milder exacerbations, and then these care practices are discontinued during the IP stay. However, we are unable to determine if certain nonstandard care practices, such as chest radiographs or antibiotics, were definitely indicated. Clearly, additional studies are warranted to determine guidelines for ED physicians; however, the routine use of such practices should be discouraged because children who do not require nonstandard care have shorter PRD.
Not surprisingly, patient-level factors, such as public insurance, were also associated with increased time to PRD. Our finding is consistent with previous studies in which researchers have found that children with public insurance had longer length of stay21 and higher hospital charges.22 This may be because socioeconomic status (for which insurance is a proxy) is considered a rough marker of environmental and/or behavioral exposures that may contribute to exacerbation severity.23 Indeed, once matched for severity, researchers in a multisite study failed to find differences in length of stay by insurance.24
A major limitation of our study is that the 6 OPAR hospitals do not use consistent measures to evaluate exacerbation severity, such as the use of an established scoring system.25–27 This is a critical gap in current practice and makes multisite quality improvement challenging. Although the nonstandard care practices that we examined herein may be indicators of a more severe course (confounding by indication), they are not exacerbation severity measures and cannot be used as such. Furthermore, it is important to recognize that simply shortening PRD may not result in optimal patient outcomes. Patients who fail standard ED therapy and then also fail to respond to or deteriorate with standard IP treatment will also require nonstandard care practices and longer time to PRD. It is important to note that even after adjustment for hospital-level practice variation, significant differences in PRD among the sites persisted, suggesting that other, unaccounted factors play a role. This suggests that factors such as adherence and underlying differences in disease biology (eg, treatment response phenotypes, immunologic phenotypes, and genetic variation) may also contribute.
CONCLUSIONS
Variation in care practices exists within and among children’s hospital systems in Ohio, and specific, system-level factors and therapies contribute to PRD. We found several modifiable factors that warrant further investigation. Furthermore, the assessment of exacerbation severity is critical to ensure that those who require nonstandard care receive it in a timely manner while minimizing nonessential care for those who are not likely to need it.
Supplementary Material
Acknowledgments
We thank the staff at each of the participating sites who helped recruit the patients for this study as well as the participants and their families.
FINANCIAL DISCLOSURE:
Dr Simmons reports grants from the Patient-Centered Outcomes Research Institute outside the submitted work. Drs Hershey and Martin report grants from the National Institutes of Health outside the submitted work. Dr Kercsmar reports personal fees from GlaxoSmithKline outside the submitted work; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING:
Funded by the Ohio Department of Job and Family Services grant G-1 213-07-0561.
Footnotes
POTENTIAL CONFLICT OF INTEREST: Dr Kercsmar reports personal fees from GlaxoSmithKline outside the submitted work; the other authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
- 1.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Rep. 2011;(32):1–14 [PubMed] [Google Scholar]
- 2.Hasegawa K, Tsugawa Y, Brown DF, Camargo CA Jr. Childhood asthma hospitalizations in the United States, 2000–2009. J Pediatr. 2013;163(4):1127–1133.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wazeka A, Valacer DJ, Cooper M, Caplan DW, DiMaio M. Impact of a pediatric asthma clinical pathway on hospital cost and length of stay. Pediatr Pulmonol. 2001;32(3):211–216 [DOI] [PubMed] [Google Scholar]
- 4.Shanley LA, Lin H, Flores G. Factors associated with length of stay for pediatric asthma hospitalizations. J Asthma. 2015;52(5):471–477 [DOI] [PubMed] [Google Scholar]
- 5.Rowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CA Jr. Intravenous magnesium sulfate treatment for acute asthma in the emergency department: a systematic review of the literature. Ann Emerg Med. 2000;36(3):181–190 [DOI] [PubMed] [Google Scholar]
- 6.Vézina K, Chauhan BF, Ducharme FM. Inhaled anticholinergics and short-acting beta(2)-agonists versus short-acting beta2-agonists alone for children with acute asthma in hospital. Cochrane Database Syst Rev. 2014;(7):CD010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manser R, Reid D, Abramson M. Corticosteroids for acute severe asthma in hospitalised patients. Cochrane Database Syst Rev. 2001;(1):CD001740. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham S, Logan C, Lockerbie L, Dunn MJ, McMurray A, Prescott RJ. Effect of an integrated care pathway on acute asthma/wheeze in children attending hospital: cluster randomized trial. J Pediatr. 2008;152(3):315–320 [DOI] [PubMed] [Google Scholar]
- 9.Johnson KB, Blaisdell CJ, Walker A, Eggleston P. Effectiveness of a clinical pathway for inpatient asthma management. Pediatrics. 2000;106(5):1006–1012 [PubMed] [Google Scholar]
- 10.Biagini Myers JM, Simmons JM, Kercsmar CM, et al. Heterogeneity in asthma care in a statewide collaborative: the Ohio Pediatric Asthma Repository. Pediatrics. 2015;135(2):271–279 [DOI] [PubMed] [Google Scholar]
- 11.National Asthma Education and Prevention Program; NHLBI. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. NIH Publication No. 07–4051. Bethesda, MD: National Institutes of Health, National Heart, Lung and Blood Institute; 2007 [Google Scholar]
- 12.Martin S, Martin J, Seigler T. Evidence-based protocols to guide pulse oximetry and oxygen weaning in inpatient children with asthma and bronchiolitis: a pilot project. J Pediatr Nurs. 2015; 30(6):888–895 [DOI] [PubMed] [Google Scholar]
- 13.Fouzas S, Priftis KN, Anthracopoulos MB. Pulse oximetry in pediatric practice. Pediatrics. 2011;128(4):740–752 [DOI] [PubMed] [Google Scholar]
- 14.Soyiri IN, Reidpath DD, Sarran C. Asthma length of stay in hospitals in London 2001–2006: demographic, diagnostic and temporal factors. PLoS One. 2011;6(11):e27184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin HC, Kao S, Wen HC, Wu CS, Chung CL. Length of stay and costs for asthma patients by hospital characteristics–a five-year population-based analysis. J Asthma. 2005;42(7):537–542 [DOI] [PubMed] [Google Scholar]
- 16.Pendergraft TB, Stanford RH, Beasley R, Stempel DA, Roberts C, McLaughlin T. Rates and characteristics of intensive care unit admissions and intubations among asthma-related hospitalizations. Ann Allergy Asthma Immunol. 2004;93(1):29–35 [DOI] [PubMed] [Google Scholar]
- 17.Meyer JS, Riese J, Biondi E. Is dexamethasone an effective alternative to oral prednisone in the treatment of pediatric asthma exacerbations? Hosp Pediatr. 2014;4(3):172–180 [DOI] [PubMed] [Google Scholar]
- 18.Parikh K, Hall M, Mittal V, et al. Comparative effectiveness of dexamethasone versus prednisone in children hospitalized with asthma. J Pediatr. 2015;167(3):639–644.e1 [DOI] [PubMed] [Google Scholar]
- 19.Davis SR, Burke G, Hogan E, Smith SR. Corticosteroid timing and length of stay for children with asthma in the emergency department. J Asthma. 2012; 49(8):862–867 [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa K, Brenner BE, Nowak RM, et al. Association of guideline-concordant acute asthma care in the emergency department with shorter hospital length of stay: a multicenter observational study. Acad Emerg Med. 2016;23(5): 616–622 [DOI] [PubMed] [Google Scholar]
- 21.Samuels BN, Novack AH, Martin DP, Connell FA. Comparison of length of stay for asthma by hospital type. Pediatrics. 1998;101(4). Available at: www.pediatrics.org/cgi/content/full/101/4/e13 [DOI] [PubMed] [Google Scholar]
- 22.Todd J, Armon C, Griggs A, Poole S, Berman S. Increased rates of morbidity, mortality, and charges for hospitalized children with public or no health insurance as compared with children with private insurance in Colorado and the United States. Pediatrics. 2006;118(2):577–585 [DOI] [PubMed] [Google Scholar]
- 23.Forno E, Celedon JC. Asthma and ethnic minorities: socioeconomic status and beyond. Curr Opin Allergy Clin Immunol. 2009;9(2):154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silber JH, Rosenbaum PR, Wang W, et al. Practice patterns in Medicaid and non-Medicaid asthma admissions. Pediatrics. 2016;138(2):e20160371. [DOI] [PubMed] [Google Scholar]
- 25.Gorelick MH, Stevens MW, Schultz TR, Scribano PV. Performance of a novel clinical score, the Pediatric Asthma Severity Score (PASS), in the evaluation of acute asthma. Acad Emerg Med. 2004; 11(1):10–18 [DOI] [PubMed] [Google Scholar]
- 26.Ducharme FM, Chalut D, Plotnick L, et al. The Pediatric Respiratory Assessment Measure: a valid clinical score for assessing acute asthma severity from toddlers to teenagers. J Pediatr. 2008; 152(4):476–480, 480.e1 [DOI] [PubMed] [Google Scholar]
- 27.Smith SR, Baty JD, Hodge D III. Validation of the pulmonary score: an asthma severity score for children. Acad Emerg Med. 2002;9(2):99–104 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


