Abstract
Introduction:
The limited cell number of primary trophoblasts and contamination of trophoblast cell lines promote us to develop a novel stable trophoblast cell line.
Method of study:
Primary trophoblast cells were isolated from first-trimester placenta and telomerase-induced immortalization was used to immortalize these cells. Subsets of cells were then evaluated by flow cytometry using CK7, HLA-G, CD45 and CD14, specific markers for trophoblast cells, extra-villous trophoblast, pan leucocyte and monocyte/macrophage, respectively. Immunofluorescence staining and immunocytochemistry were used to detect CK7 expression in trophoblast cells. The level of secreted human Chorionic Gonadotropin (hCG) was measured by electrochemiluminescence (ECL). The Bio-Plex MAGPIX System was used to analyze the cytokines and chemokines produced by AL07 cell line.
Results:
We were able to isolate primary trophoblast cells from several first-trimester placentas. One clone, AL07 trophoblast cells, isolated from a week 7 placenta, was morphologically stable and positive for the expression of CK7 by immunofluorescence and immunocytochemistry staining. Characterization of AL07 cells reveled that they are CD45 or CD14 negative and had constitutive secretion of hCG and low HLA-G expression. Furthermore, clone AL07 secret high levels of several cytokines and chemokines, including IL-6, IL-8 and VEGF, and moderately secreted MCP-1 IP-10 and RANTES.
Discussion:
We report the successful isolation, immortalization and characterization of AL07 cells, a novel cell clone isolated from first trimester human placenta. The clone is free of contamination of immune cells, and exhibits similar cytokine profile as other trophoblast cell lines. This new cytotrophoblast-like AL07 cell, can be a valuable tool for in-vitro trophoblast studies in the future.
Keywords: Primary trophoblasts, Cytokeratin 7, Trophoblast cell line, Pregnancy, hTERT
1. Introduction
Trophoblasts are cells form the outer layer of the blastocyst that facilitates embryonic implantation, and located next to the maternal endometrium and play important roles in diverse events at the maternal-fetal interface, including endocrinological and immune processes, placental-to-fetal nutrition and metabolism, and mother-to-fetus transmission [1]. They provide an important intermediate bridge at the maternal-fetal unit. Trophoblasts are originated from the outer layer of the blastocysts (also known as trophectoderm) and are the main cellular component responsible for the development of the placenta and fetal chorion [2]. There are three major subpopulations of trophoblasts: cytotrophoblasts (CTBs), syncytiotrophoblasts (STBs) and extravillous trophoblasts (EVTs) [3,4]. Recent studies showed that CTBs are undifferentiated and have characteristics of epithelial stem cells, since they can give rise to STBs and EVTs [4]. Indeed, proliferative CTBs are capable of fusing and forming STBs, a terminal differentiated multinucleated cell layer covering the placental villi and the main source of human chorionic gonadotrophin (hCG) in early pregnancy. CTBs can also assemble into the cell column at the tip of villi, where they differentiate into invasive EVTs and anchor in the decidua [2]. Due to their versatile characteristics, CTBs represent a useful tool to study trophoblasts biology in vitro.
The best source for the study of trophoblast biology are first trimester placenta obtained from elective terminations. However, there are a few drawbacks of using primary trophoblast cells from first trimester placenta. First of all, due to ethical and logistical difficulties limit the access to these type of placentas. Second, the available cells are often insufficient to perform experiments. In addition, the short lifespan of primary cells limits culture passages [5,6]. Another source of isolation of CTBs is from human term placenta; however, they also have their limitation since term placenta is an aging organ and cells isolated from third trimester could not recreate the developmental process observed during first trimester; therefore these cells are primarily used to study trophoblast invasion/migration [7–9]. Consequently, the access to novel first trimester trophoblast cell lines is fundamental to study early placentation. So far, the sources of trophoblastic cell lines have being generated from either normal tissues or choriocarcinoma, a trophoblastic malignant cancer [5,10], the former includes Swan 71 [6], ED27 [11] and HTR-8/SVneo lines [5], and the latter includes cell lines such as BeWo, JEG-3 and JAR [10,12]. Only limited trophoblast cell lines have been generated from the first trimester placenta, e.g. HTR-8/SVneo, which is the first established extravillous CTB (evCTB) cell line developed by Graham et al. [13] and the Swan 71 cell line immortalized with human telomerase reverse transcriptase (hTERT) [6]. Unfortunately, Abou-Kheir et al. [10] reported that HTR-8/SVneo cells were contaminated with stromal cells. As for the ED27 cell line, Kniss et al. found that the cells did not secrete detectable amount of hCG in culture and they expressed classical HLA-A and -B, which were thought not to be expressed by any trophoblast subpopulations and finally identified to be Hela cells [11]. These disadvantages were sometimes overlooked and potentially lead to incorrect and/or irrelevant scientific conclusions. Therefore, trophoblast cell lines possessing original genotypic and phenotypic characteristics are necessary when they are chosen for in vitro experiments. Establishment of new trophoblast cell lines is essential to meet the research needs.
Telomeres are repetitive DNA sequences at the end of chromosomes that are involved in DNA replication and stability. Human telomerase reverse transcriptase (hTERT) is the essential catalytic protein subunit of telomerase that maintain telomere length [14–16]. Adult somatic cells do not contain functional telomerase, therefore, their telomeres shorten with each mitotic division. This progressive shortening of telomere length is responsible of the loss of replication potential and the aging process [17,18]. The introduction of ectopic hTERT has being shown to restore telomerase activity sufficient for immortalization [19]. Indeed, our laboratory as well as others has successfully immortalized several cell lines by introducing exogenous hTERT without any effect on their karyotype or phenotype [20–24].
The objective of this study was to isolate trophoblast cells from first trimester placentas, and characterize immortalized clones for the establishment of new models of first trimester trophoblast cells. Herein, we report the establishment and characterization of a new trophoblast cell line, named AL07, using hTERT, which maintains the main phenotype of human first trimester trophoblast cells.
2. Materials and methods
2.1. Tissues
Tissues were obtained from the Maternal and Children’s Hospital of Hubei province, Wuhan, China. All the participants signed the informed consent, which was reviewed and approved by Huazhong University of Science and Technology Clinical Trial Ethics Committee (CTEC). The first-trimester placentas were collected from the healthy pregnant women, elective termination for non-medical reasons at weeks 6–8, aged 25–35 years old. The tissues were placed into pre-chilled sterile phosphate buffered saline (PBS, PH7.4) containing 1% penicillin–streptomycin (PS) and sent to the laboratory immediately for cell isolation.
2.2. Isolation of primary trophoblast cells
Primary trophoblast cells were isolated from first-trimester placentas according to the previous studies [6] with some modifications. Briefly, the tissue was washed with cold PBS containing 1% PS to remove excess blood. Then the tissue was transferred into a sterile dish and the extra liquid was removed. Trophoblast cells containing membrane was placed into a new 50 ml conical tube and washed with cold PBS, followed by careful removal of the supernatant. The membrane tissue was minced and incubated with equal volume of 0.25% trypsin-EDTA at 37 °C for 5–8 min on a shaker. After trypsinization, 5 volume of pre-warmed complete medium (high glucose DMEM+10% fetal bovine serum (FBS)+1% PS) was added to the tube to stop the process. The tube was vigorously vortexed for 20 s and digested tissue was settled to sediment. The supernatant was collected and centrifuged at 1500 rpm for 10 min at room temperature. The pellet was resuspended with complete medium and carefully laid on top of 5 ml of lymphocyte Separation Media in a new 15 ml conical tube. The tube was centrifuged at 800 g for 20 min with density gradient centrifugation, and the white layer of cells was collected and washed with complete medium. The cells were centrifuged again and resuspended with the medium so that the density of cells was about 1 × 105/ml. The cells were seeded onto a sterile dish and then cultured in 37 °C, 5% CO2 incubator. Then 50% medium was changed every two days.
2.3. Telomerase (hTERT) immortalization
The isolated primary trophoblast cells were immortalized using a retroviral system consisting of the pA317-hTERT cell line expressing human telomerase reverse transcriptase (hTERT), the essential catalytic protein subunit of telomerase, and the puromycin resistance gene as previously described [6]. Briefly, trophoblast cells were infected with the supernatant of pA317-hTERT cells overnight (16 h) in the presence of 10 μg/ml Polybrene (Sigma-Aldrich). The cells were allowed to recover in D-MEM media with 10% FBS for 48 h before selecting with 800 ng/ml of puromycin (Sigma-Aldrich). After 72 h of selection, the remaining cell clones were serially propagated and cultured in D-MEM media plus 10% FBS with puromycin.
2.4. RT-PCR
Primary trophoblast, AL07 cells and Swan71 cells were collected in 500 ml Trizol (Invitrogen, Carlsbad, CA, USA). Total RNA was isolated from these cells. Reverse transcriptase polymerase chain reaction (RT-PCR) was performed by using PrimeScript™RT reagent Kit with gDNA Eraser and TaKaRaCode:DRR820A SYBR PremixEx Taq TM II according to the manufacturer’s instructions (Takara Bio, Shiga, Japan). The primers of hTERT and β-actin and PCR conditions used during reactions were mentioned in Table 1. After the PCR reaction, products were electrophoresed by 2% agarose gel electrophoresis and stained with ethidium bromide.
Table 1.
Primers and conditions used in RT-PCR gene expression.
| Gene | Sequence (5′−3′) | cycles | Product size (bp) | Anneal. Temp. °C |
|---|---|---|---|---|
| hTERT | CGTACATGCGACAGTTCGTG AGTTCACCACTGTCTTCCGC |
35 | 408 | 62 |
| β-actin | CCTTCCTGGGCATGGAGTC TGATCTTCATTGTGCTGGGTG |
35 | 189 | 58 |
2.5. Flow cytometry
The isolated trophoblast cells were treated with the corresponding antibodies for flow cytometry analysis as previous described [25]. In detail, a total number of 1 × 105 cells were stained with the cell surface antibodies for 40 min in the dark, including PE-conjugated mAbs against HLA-G (BioLegend, San Diego, CA, USA), APC-conjugated mAbs against CD45 (BioLegend, San Diego, CA, USA), FITC-conjugated anti-human CD14 antibody (eBioscience, San Diego, CA, USA). After the cell surface staining, the cells were fixed with a Fix/Perm kit according to the manufacturer’s instructions (eBioscience, San Diego, CA, USA), and then labeled with the intracellular antibody FITC-conjugated mAbs against CK7 (Merck, Temecula, CA, USA) for 40 min in the dark. Then the cells were washed and resuspend with PBS. All the steps of centrifugation were performed at 300 g for 8 min. Then the flow cytometry was processed using the LSR II Flow Cytometer (BD Biosciences, San Jose, CA, USA), and the data was analyzed by the FlowJo software (Tree Star, Ashland, OR, USA).
2.6. Immunofluorescence staining
Ten thousand cells were seeded per well of the 24-well plate to make the cell slide and cultured at 37 °C in 5% CO2 incubator. When the cells reached more than 60% confluence, they were fixed with methanol at −80 °C for 7min. After fixation, the cells were incubated with 5% BSA in PBS for 30 min to block the non-specific binding and then incubated with the primary anti-Cytokeratin 7 antibody [EPR17078] (CK7, Cat#:ab181598, 1:200, abcam, USA) at 4 °C overnight. The primary antibody was removed the next day, and the cell slide was washed with PBS three times and then incubated with the DyLight 594 Goat anti-Rabbit IgG (H + L) (Cat#: A23420 abbkine, 1:1000) secondary antibody at room temperature for 2 h. The nuclei were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI) for 10 min. All the steps were performed in the dark. The images were taken at × 400 magnification using an Olympus BX51 microscope and Olympus DP70 manager (Japan).
2.7. Immunocytochemistry
The cell slides were prepared as described in 2.5. The endogenous peroxidase activity was blocked by 3% H2O2 in methanol, and non-specific bindings were blocked with 5% BSA. The primary antibody CK7 (Cat#: ab181598, 1:200, abcam, USA) was incubated at 4 °C overnight. The goat anti-rabbit IgG-HRP (Cat#:sc-2004, Stata Crus, USA, 1:400) secondary antibody was incubated at room temperature for 2 h. After the incubation, the color was developed by adding 3, 3′-Diaminobenzidine tetrahydrochloride kit according to the manufacturer’s instructions, followed by counterstaining the nuclei with hematoxylin. The cell slides were dehydrated with ethanol and xylene. The images were obtained from an Olympus BX51 microscope and Olympus DP70 manager (Japan).
2.8. Detection of the level of hCG
Ten thousand AL07 and Swan71 cells were seeded onto the 24-well plate, respectively, with 1 ml complete medium and cultured in the 37 °C, 5% CO2 incubator for 1, 2, 3, 4, 7 and 8 d. Then the supernatant was collected into the sterile micro tubes and stored at −20 °C for hCG measurement. The data was acquired using the electrochemiluminescence (ECL) in an automatic luminescence apparatus (Elecsyse 601, Roche, Basel, Switzerland).
2.9. Cytokines and chemokines analysis
Five thousand AL07 cells were plated onto the wells of a 12-well plate with 1 ml complete medium and cultured in 37 °C, 5% CO2 incubator for 4 and 8d, and the supernatants were collected. Then the profiles of cytokines and chemokines were obtained via Bio-Plex MAGPIX System (Bio-Rad, CA, USA) using the Bio-Plex Pro Human Cytokine GrpIPanel 27-plex kit (Cat:#M500KCAF0Y, Bio-Rad, CA, USA) according to the manufacturer’s instructions. Briefly, 50 μl standards, sample supernatants and the blank were loaded to the 96-well plate with diluted microbeads, mixed and vortexed at 850 rpm and incubated for 30 min in the dark. Each well was washed with PBS, and then incubated with diluted detection antibody at room temperature in the dark for 30 min. After three washes, 50 μl diluted Streptavidin-PE were added to the individual wells and incubated at room temperature in the dark for 10 min and resuspended with 125 μl Assay Buffer. Cytokines and chemokines were measured with Bio-Plex MAGPIX System (Bio-Rad, CA, USA).
2.10. Statistical analysis
The data was acquired from three independent experiments and presented as mean ± SEM for the statistical analysis. The Graphpad Prism software (Version 7.0a, GraphPad, San Diego, CA, USA) was used to analyze the difference. Statistical difference was processed by Student’s t-test. Significance was considered as P < 0.05.
3. Results
3.1. The preparation of primary trophoblast cells
Primary trophoblast cells were isolated from 6 to 8 gestational weeks placentas obtained from elective termination of healthy women. Isolated cells were cultured with high glucose DMEM for 15–20 days. Around three days in culture we can observe the presence of many irregular and polygon cells adhered to the bottom of the petri dish (Fig. 1A(i)), which revealed a morphology suggestive of cytotrophoblast cells phenotype. After 10 days of culture, we identify the presence of multinucleated cells suggesting the differentiation into syncytiotrophoblast-like cells (Fig. 1A(ii)).
Fig. 1.
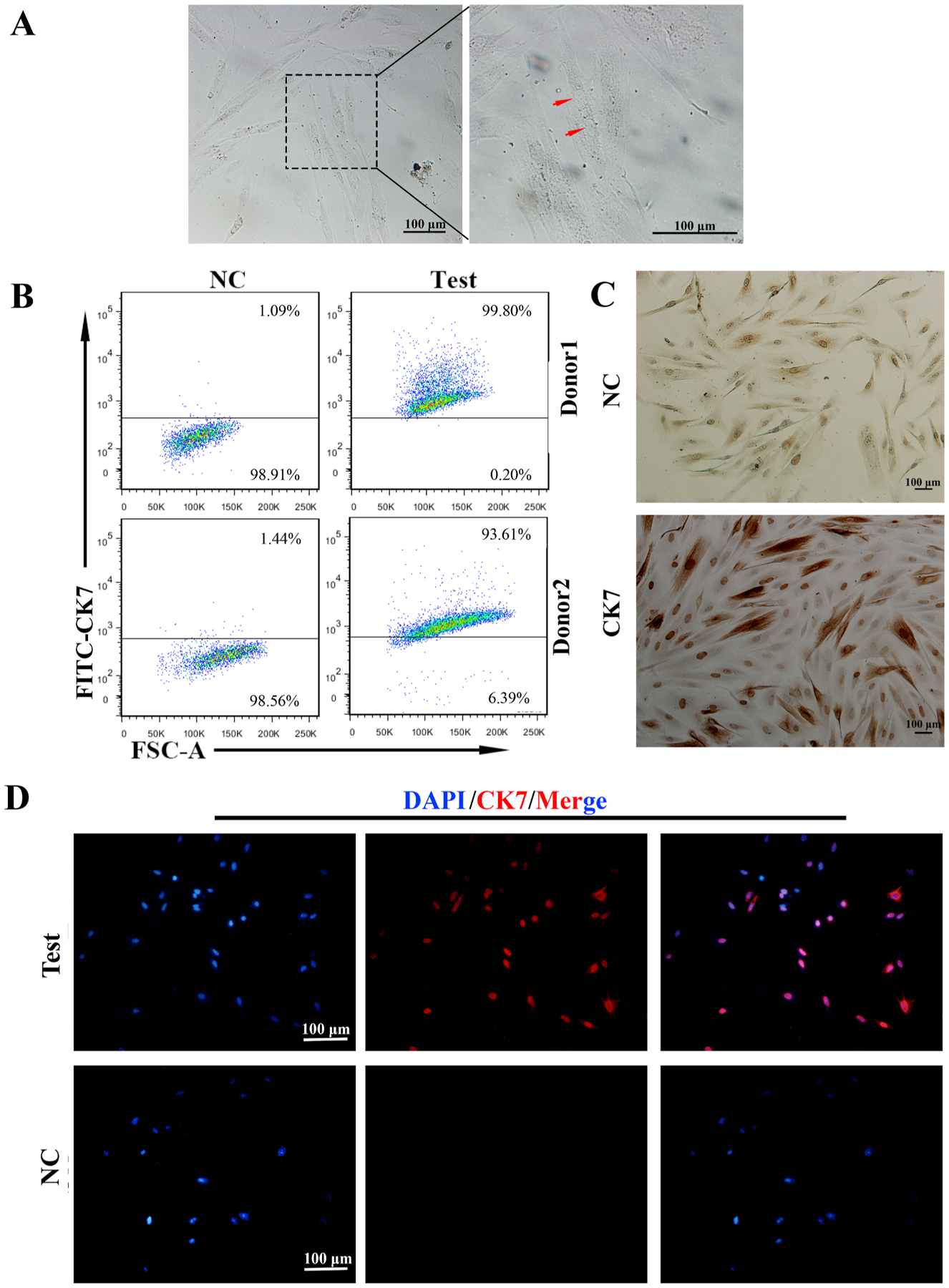
The preparation of highly purified primary trophoblasts.
A: The images of primary trophoblasts taken with different magnification. The left and middle ones showed the irregular and polygon cells in the field with 3 days cultures (i). The right one (iii) revealed three nuclei found in one cell (arrow) around 10 days cultures.B: The purity of primary trophoblasts by flow cytometry (FCM). The CK7+cells were trophoblasts, which reached 93% to 99% of cell populations.C: CK7 localization in primary trophoblasts by immunocytochemistry. (i)The upper panel was negative control. (ii)Almost all of the cells were stained with CK7 in these fields (lower panel). CK7 was localized in the cytoplasm and nucleus, especially deeply stained in the nucleus. Scale bars: 100 μm.D: Most of the primary trophoblast cells expressed trophoblast specific marker, CK7 in the field (upper panel). CK7 was mainly localized in the nucleus by Immunofluorescence (IF) staining. The lower panel was negative control (NC). Scale bars: 100 μm.
Our next objective was to characterize the phenotype of the isolated cells. Using flow cytometry (FCM), we determined the expression of CK7, a generally accepted marker for trophoblast cells [1] and found that almost 90% (93.6%–99.8%) of the cells in the culture were CK7 positive (Fig. 1B). In order to confirm the FCM findings, we stained the primary trophoblast cells for CK7 and evaluated the expression and intracellular localization by immunofluorescence (IF) and immunocytochemistry (ICC). As shown in Fig. 1C, CK7 expression was present in almost all the cells and positive staining was observed in the cytoplasm and nucleus. To confirm the nuclear localization of CK7, we used IF by labeling the nucleus with DAPI and CK7 with red signals. Interestingly, we observed a wide difference in the intensity of CK7 fluorescence as well as the intracellular localization. Although all the cells were CK7 positive in the cytoplasm, some cells displayed strong nuclear localization (confirmed by co staining with DAPI) (Fig. 1D upper panel), which suggest the presence of different trophoblast subsets [26]. Together, these results show that the isolated primary cells are CK7+ and consequently trophoblast cells.
3.2. Immortalization and characterization of clone AL07
Although primary trophoblast cells can proliferate during several passages, unfortunately with every passage the cultures will reveal a gradual decrease on their proliferative capacity, becoming senescent, which then will lead to cell death. In order to preserve the culture, trophoblast cells were infected with hTERT, the essential catalytic protein subunit of telomerase, overnight (16 h) followed by incubation in the presence of 800 ng/ml of puromycin (Sigma-Aldrich). Following 72 h of selection, the remaining cell clones were serially propagated and cultured in D-MEM media plus 10% FBS with puromycin. One surviving clone, which we have named AL07 was selected for further characterization. While the parental trophoblast cells reached senescence after 4 passages, the hTERT AL07 cells continue to proliferate and have been maintained in culture for over 33 passages without any features of senescence.
We then determined whether AL07 cells retained hTERT, by evaluating the expression of hTERT by PCR and found that while the primary cultures are negative for hTERT, clone AL07 shows a positive band in Fig. S1, confirming that AL07 are positive for telomerase. Furthermore, when we evaluated the morphological characteristics of AL07 cells during multiple passages we can observed that AL07 cells were morphologically stable after continue expansion. Fig. 2A shows light microscopy of AL07 cells at passage 19 (Fig. 2A(i)) and passage 33 (Fig. 2A(ii)) reveling similar morphologies.
Fig. 2.
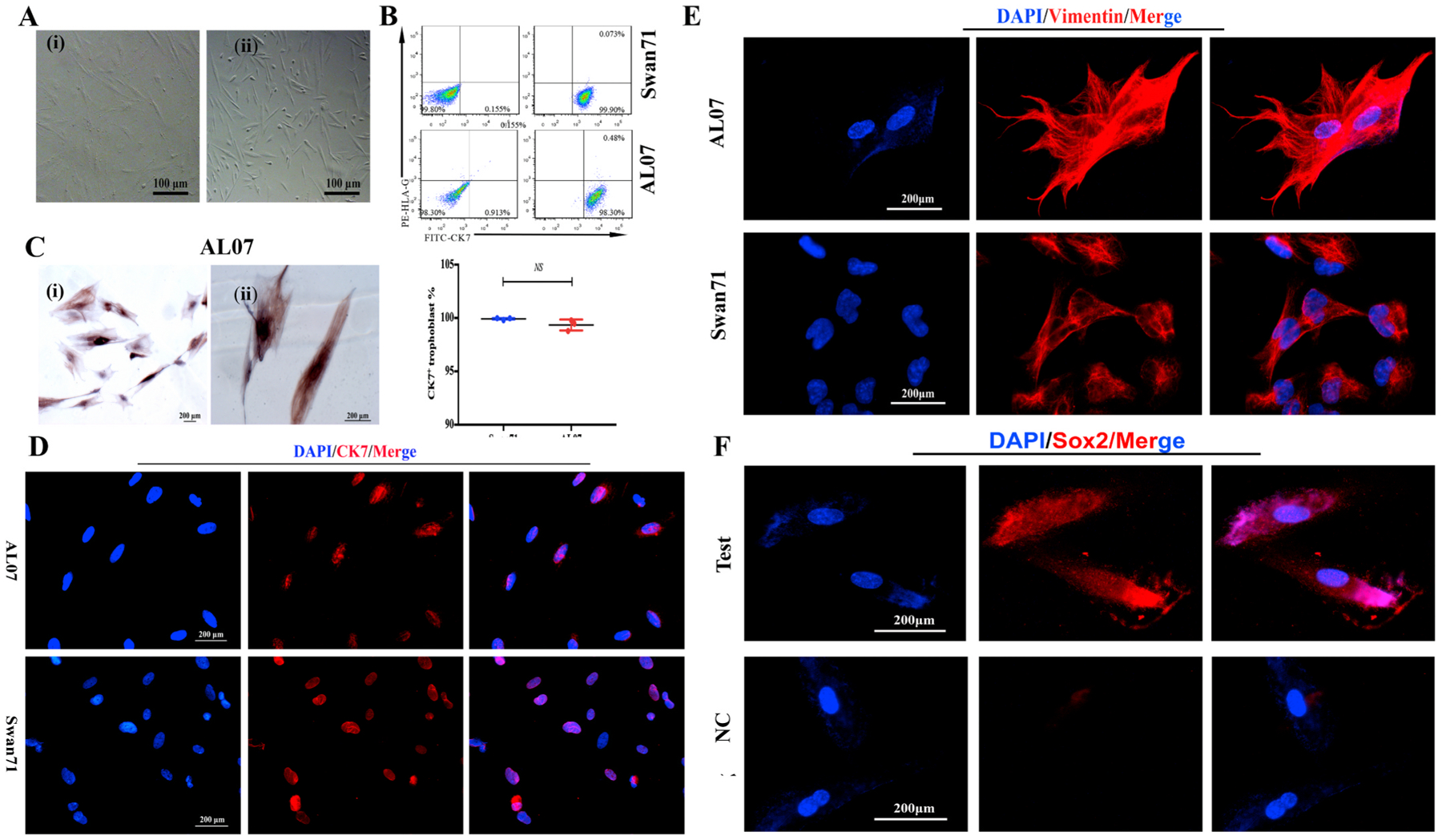
The purity of trophoblast cell lines AL07.
A: The images showed that AL07 cells were morphologically stable with passaging (i: passage 19 and ii: passage 33). B: The purity of the trophoblast cell line AL07 (lower panel) was evaluated by FCM using the specific marker CK7. Swan71 cell line served as positive controls (upper panel). HLA-G is a specific marker for extra-villous trophoblasts. Left panel was negative control(NC). CK7+cells were nearly 99% in AL07 cells and 100% in Swan71 cells. There were barely no CK7+HLA- G+cells in both cells lines. The purity of Swan71 and AL07 cell lines was identified by three independent experiments. The statistical result showed there was no significant difference between AL07 and Swan71 cells. Data are presented as the mean ± SD.The statistical result showed there was no significant difference between AL07 and Swan71 cells. C: CK7 was highly expressed in the cytoplasm and nucleus of AL07 cells, particularly in the nucleus by Immunocytochemistry. Original magnification ×200 (i) and ×400 (ii). Scale bars: 200 μm. D: Immunofluorescence staining was used to detect the trophoblast marker, CK7 expression in AL07 and Swan71 cell lines. Almost all the cells expressed CK7 with red signals in AL07 (upper panel) and Swan71(lower panel) cells, mainly in the nuclei. The Swan71 cell line was positive controls. Scale bars:200 μm. E: Immunofluorescence staining was performed to detect the expression of Vimentin in AL07 cells. Red signals of Vimentin was found in the cytoplasm of AL07 cells (upper panel), and similar to the positive control, Swan71 cells (lower panel). Scale bars: 200 μm. F: Immunofluorescence staining was performed to detect the expression of Sox2 in AL07 cells. Red signals of Sox2 was found in the nuclei and cytoplasm of AL07 cells (upper panel), and no signals was in negative control (lower panel). Scale bars: 200 μm.
In order to determine the purity of AL07 cell line, we evaluated the percentage of CK7+ cells using FCM with the gating strategy in Fig. S2. As shown in Fig. 2B, 98.7% of AL07 cells are CK7+ confirming the purity of the immortalized trophoblast clone. This finding were similar to Swan71 cells, another first trimester trophoblast cell line. We confirmed the FCM findings for CK7 by immunocytochemistry and showed that CK7 was localized in the cytoplasm and in the nucleus of the cells (Fig. 2C). Interestingly, we observed a high number of cells with positive CK7 staining in the nucleus (Fig. 2C). To confirm the nuclear localization of CK7 on AL07 cells we performed immunofluorescence and found that most of the cells showed positive signal for CK7 in the nucleus of these cells (Fig. 2D). Similar findings were found with Swan 71 cells used as positive control (Fig. 2D).
Next, we detected the vimentin expression in AL07 cells via IF. Vimentin is an intermediate filament (IF) protein. It was reported that novel roles of vimentin promote the migration of different cell types [27]. The IF results showed that AL07 cells expressed vimentin and were localized in the cytoplasm (Fig. 2E upper panel), which is similar to Swan71 cells (Fig. 2E lower panel). Existing studies showed that EVT may express vimentin as they have the characteristic of mesenchymal cells [28], suggesting that AL07 cells might have the migrative capacity and might be EVT-like trophoblast.
Next, to confirm whether AL07 cells are EVT origin, we evaluated HLA-G expression in the AL07 cells. Thus, we stained the cells with an anti- HLA-G specific antibodies and evaluated the expression by FCM. As shown in Fig. 2B, AL07 cells were negative for HLA-G further suggesting AL07 were a placental cytotrophoblast phenotype, since only extra-villi trophoblast cells (EVT) are known to express HLA-G [29].
Interestingly, IF results showed that AL07 cells also express the pluripotency marker, Sox2 [30] in the nuclear (Fig. 3F, lower panel), indicating AL07 cells may have the features of trophoblast stem cells.
Fig. 3.
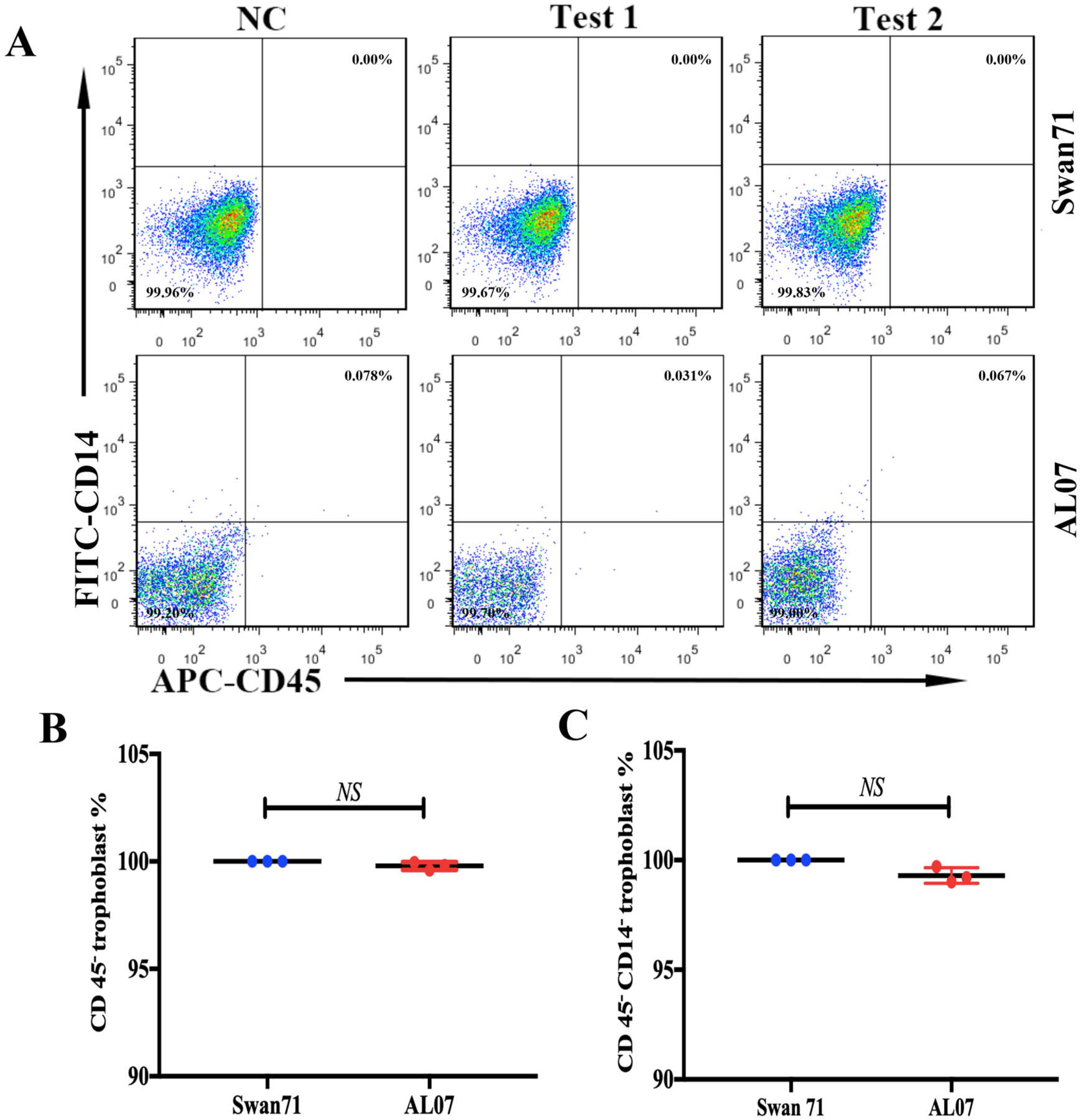
No expression of CD45 and CD14 in the AL07 cells.
A: No immune cells contamination in AL07 cells. CD45 and CD14 are pan lymphocyte and monocyte/macrophage markers, respectively. The Swan71 cell line was the positive controls. There were no CD45+ or CD14+or CD45+CD14+cells in the Swan71 and AL07 cells. B and C: the statistics of CD45− trophoblast % and CD45−CD14− trophoblast % in AL07 cells and Swan71 cells.
3.3. AL07 cells do not express CD45 or CD14
The potential factor affecting the purity of the trophoblast cells is contamination with immune cells, especially fetal macrophages present in the villi columns [6]. Although immune cells were not proliferative in vitro and might disappear with subcultures, we used flow cytometry (FCM) to detect if there were any leukocytes or macrophages present in AL07 cells. Therefore, we examined CD45 and CD14, pan lymphocyte and monocyte/macrophage markers using FCM to rule out the contamination of immune cells in AL07 cell line [31]. Compared to the negative control (NC) (Fig. 3A, left panel) and positive control (Swan71 cell line) (Fig. 3A upper panel test 1 and test 2), there were neither CD45+ nor CD45+ CD14+ cells in both AL07 and Swan71 cell line, which indicate that no macrophages even leukocytes were left in AL07 cells. These results suggest that AL07 cells are purified trophoblast cells and not mixed with immune cells.
3.4. The secretion of hCG by AL07 cells
Fusion of cytotrophoblast cells can lead to formation of the multicleated syncytiotrophoblast cells, which can produce large quantities of placental hormones, including hCG [32]. hCG is an early product of the placenta and it is generally accepted as a biochemical marker for pregnancy. Therefore, we examined if AL07 cells secreted hCG in their supernatants. Herein, we cultured AL07 cell line for different time points, 1, 2, 3, 4, 7 and 8 d to detect the levels of hCG in the supernatants. As shown in Fig. 4, although the hCG levels were very low initially, they gradually increased with the prolonged culture period (Fig. 4). There was no statistically significance in hCG secretion between AL07 and Swan71 cell lines.
Fig. 4.
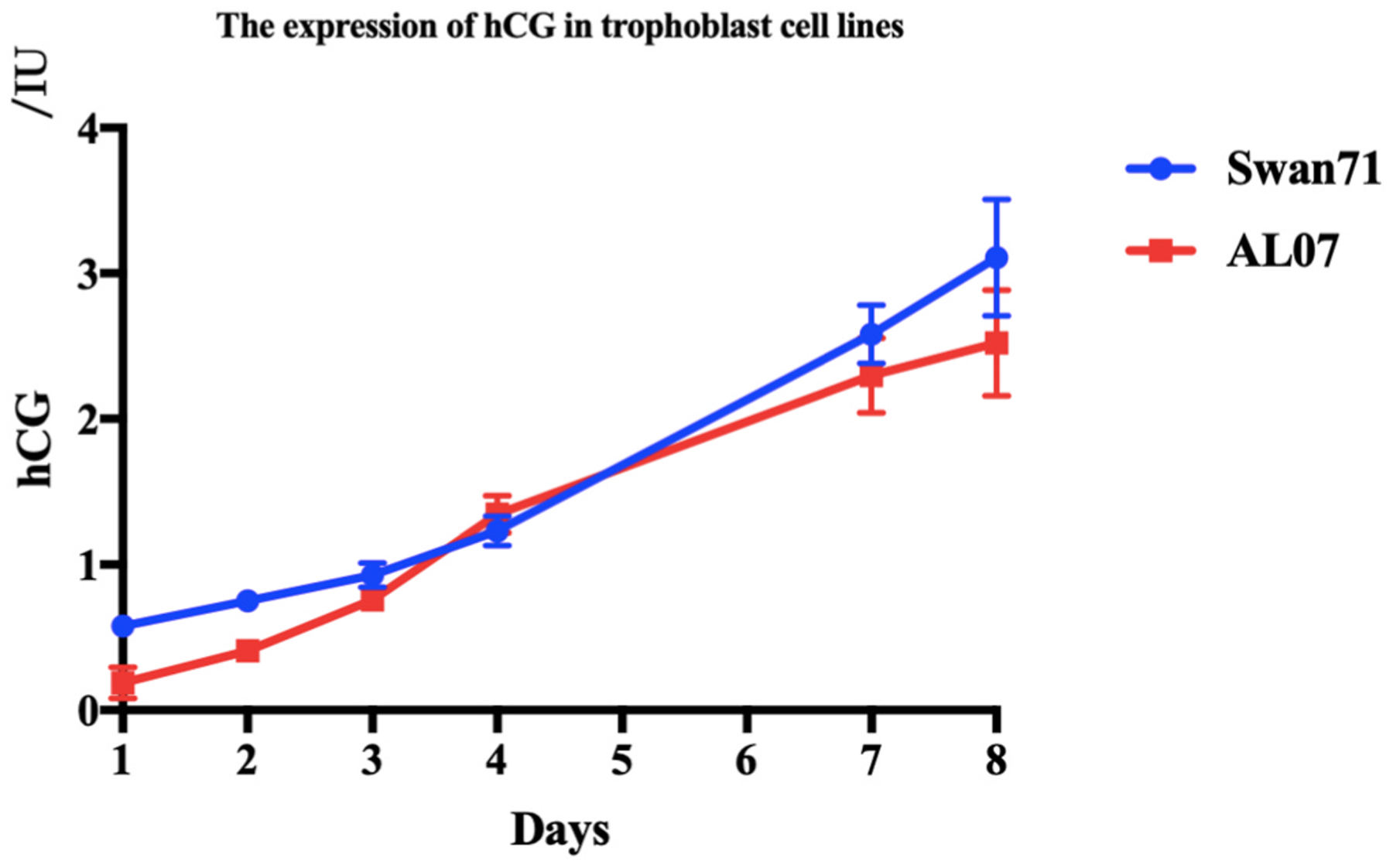
Low levels of hCG expressed by AL07 cells.
The supernatants were collected from the Swan71 and AL07 cell lines cultured for 1, 2, 3, 4, 7 and 8 d, respectively, in the 37°C, 5% CO2incubator. Elctrochemiluminescence (ECL) was used to determine the hCG levels. The Swan71 cell line was the positive control. Low levels of hCG were secreted by AL07 cell line.
3.5. The profiles of cytokines secreted by AL07 cell lines
Trophoblast cells can secret a lot of cytokines and chemokines to exert immune regulation to recruit and educate immune cells at the maternal-fetal interface [33]. Thus, we analyzed the profiles of cytokines and chemokines secreted by AL07 cell line in the supernatant cultured for 4 d (lanes 3–5) and 8 d (lanes 6–8) using Bio-Plex MAGPIX System compared with medium as blank control (Lanes 1 and 2), which contained 27 cytokines/chemokines. Compared with the cytokines profile of Swan71 in previous studies [6], AL07 cells showed similar expression pattern to Swan71. Interleukin (IL)-6, IL-8, IL-9, basic fibroblast growth factor (FGF), interferon gamma-induced protein 10 (IP-10), monocyte chemotactic protein-1 (MCP-1), regulated upon activation normal T cell expressed and secreted (RANTES)/chemokine (C–C motif) ligand 5 (CCL5), macrophage inflammatory proteins-1 beta (MIP-1beta) and vascular Endothelial Growth Factor (VEGF) were highly expressed in the supernatant of AL07 cells cultured for 8 days, which was consistent with that in Swan71 cells [6]. However, AL07 cells secreted low level of IL-1beta, which was in line with the HTR-8 trophoblast cell line [6]. In addition, other inflammatory cytokines were also evaluated in AL07 cell line, including IL-7, IL-17A, TNF-alpha, eotaxin (CCL11), granulocyte colony stimulating factor (G-CSF), granulocyte macrophage colony stimulating factor (GM-CSF). They were expressed at low levels (Fig. 5A). IL-15 was barely detected in the AL07 cell line (data not shown). The most highly produced cytokines/chemokines were shown in Fig. 5B, including IL-6, IL-8, VEGF, MCP-1, RANTES and IP-10 in the supernatants of AL07 cell line cultured for 8 d compared to those for 4 d.
Fig. 5.
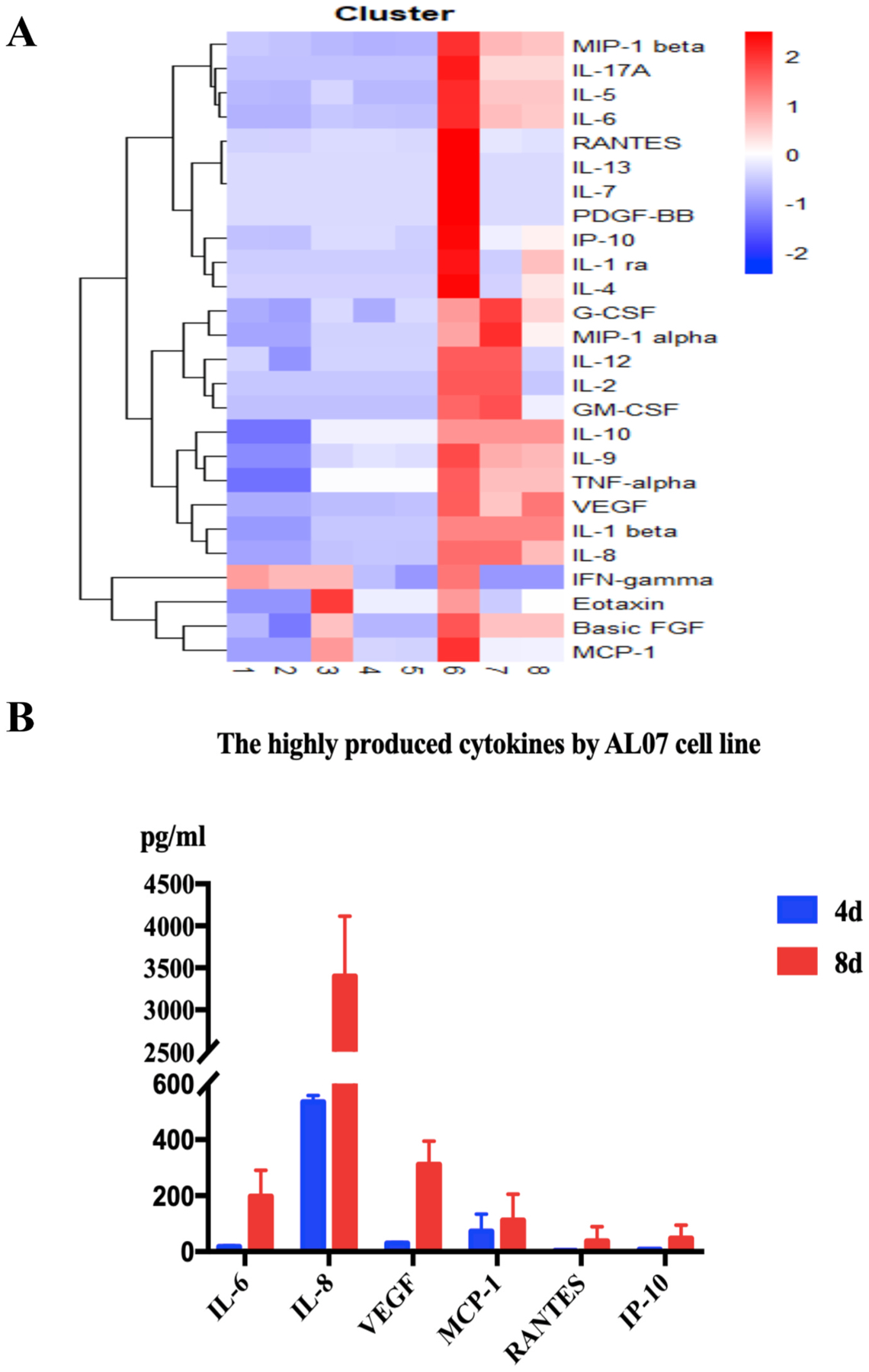
Cytokines/chemokines’ profiles in AL07 cell line.
A: Hot map of Cytokines/chemokines’ profiles secreted by AL07 cells There was an increase in secretion of IL-6, IL-8, IL-9, basic FGF, IP-10, MCP-1,RANTES/CCL5, MIP-1beta and VEGF in the supernatant of AL07 cells cultured for 8 d (Lanes 6–8), compared to those cultured for 4 d (Lanes 3–5). Lanes 1 and 2 were blank control. AL07 cells secreted low levels of IL-1beta. In addition, other inflammatory cytokines were also evaluated in the culture supernatants of AL07 cells, including IL-7, IL-17A, TNF-alpha, eotaxin (CCL11), G-CSF, and GM-CSF, although they were secreted at low levels. B: The most highly produced cytokines/chemokines were shown, including IL-6, IL-8, VEGF, MCP-1, RANTES and IP-10. Data were collected from three independent experiments and presented as the mean ± SD.
4. Discussion
Trophoblast cells, as the only fetal-derived cells at maternal-fetal interface, play dominant roles in the crosstalk between mother and fetus [34,35]. However, the individual difference of patients and the lack of sufficient first-trimester primary trophoblast cells limit further research to elucidate the role of trophoblast cells in normal and pathological pregnancy. A rapid increase of studies on trophoblast biology as well as immune regulation and function in recent years demands a reliable and stable in vitro model [6]. It has been reported that there are issues with trophoblast cell lines such as stromal cell contamination in the HTR-8/SVneo cell line [10]. In addition, the SV40 induced trophoblast cell lines were reported to exhibit karyotypic and/or phenotypic abnormalities [36,37]. On the other hand, the hTERT-immortalized trophoblast cells, Swan71 has been a valuable model for in vitro trophoblast cells studies [6]. Primary cells immortalized with telomerase were thought to maintain normal phenotypic properties and no chromosomal abnormalities as well as the cancer-associated changes compared with simian virus 40 tumor antigen (SV40 Tag) induced immortalization. However, the cytogenetic analysis of Swan71 cell line found that Swan71 cell line also contained multiple specific clonal rearrangements in 2017 [38]. Therefore, new immortalized trophoblast cell lines are needed to be established.
Herein, we developed a new first trimester trophoblast cell line induced by hTERT infection, named AL07. The trophoblast cell line was confirmed by expression of the trophoblast specific marker, CK7. To rule out the potential contamination of immune cells, the pan lymphocyte and monocyte/macrophage markers CD45 and CD14 were assessed and their expression in the AL07 cells was minimal. The secretion of hCG and an increase in its concentrations with extended culture period suggested that the AL07 cells are not syncytiotrophoblast-like cells.
Although AL07 cells express vimentin, a mesenchymal cell marker, but did not express HLA-G, suggesting AL07 cells are not EVT origin. Novel insights showed that expression of vimentin could promote the migration of different cell types [27]. Studied showed that cytotrophoblast cells also have the migrative capacity, which could support the expression of vimentin in AL07 cells and are evCTB-like trophoblast cells.
Nowadays, much progress has been made on the study of trophoblast stem cells, which showed that cell lines originated from human cytotrophoblast cells and blastocyst are trophoblast stem cells [4]. As AL07 cells were identified as evCTB -like cells, we detected that the expression of the transcription factor Sox2 was found in AL07 cells. Sox2, a pluripotency marker [30], is highly conserved in animal evolution and has functional relevance of stem cells [39], suggesting that AL07 cells may have the stem ability, but also need further investigations.
Interestingly, the previous study [6] reported that Swan71 cell line expressed soluble HLA-G5 (HLA-G1) and HLA-G6 (HLA-G2) detected by Western blot with the antibody which only recognized the two soluble isoforms (sHLA-G1and sHLA-G2). There are seven isoforms of HLA-G generated by alternative splicing, four are membrane bound (HLA-G1-4) and three are soluble (HLA-G5-7) [40,41]. In our study, we could not detect the expression of HLA-G membrane-bound isoforms in Swan71 cells by flow cytometry, which might be due to the allelic imbalance in the 3′-untranslated region (UTR) of the HLA-G gene that was associated with HLA-G surface expression on primary trophoblast cells isolated from first-trimester pregnancy placenta [41]. Therefore, the expression difference of HLA-G in Swan71 may be explained by the alternative splicing pattern and the instability of HLA-G caused by polymorphisms.
During early pregnancy, trophoblast cells serve as the first communication bridge between mother and blastocyst. Trophoblast cells act as immune regulators, which exert critical effects on remodeling the local immunological milieu by secreting various cytokines and chemokines to recruit and educate immune cells [33]. Thus we analyzed the cytokine and chemokine profiles of AL07 using Bio-Plex MAGPIX System and demonstrated that AL07 cells could produce high levels of IL-6, IL-8, and VEGF, but low levels of TNF-α and IFN-γ, similar to the primary trophoblast cells [6,42–44]. FGF, Platelet-derived growth factor subunit B (PDGF-B) and VEGF are critical cytokines in angiogenesis, cell proliferation, differentiation and migration [45–47], which participate in important biological processes in early pregnancy and placental development. Although AL07 cells expressed low levels of IFN- γ, they secreted high levels of IL-8, IP-10 and MCP-1, which may play important roles in the activation and recruitment of immune cells to the maternal-fetal interface to promote the blastocyst implantation [48–55]. Besides, it has been known that the trophoblast cells secreting MIP-1beta and MIP-1alpha could prevent the mother and fetus from being attacked by the viral infection [56–58]. However, in the current study, our findings showed that AL07 cells produced low levels of the cytokines required for B-cell activation and maturation, such as IL-2 [59], IL-5 [60], and IL-7 [61], thus induction of these cytokines in AL07 cells for stimulation of B cell functions may need other regulators.
The establishment and characterization of AL07 cell line reveal that this new first trimester trophoblast cell line behaves similarly to primary trophoblast and Swan71 cell line, therefore it provides a useful in vitro model for studying the role of trophoblast cells in early pregnancy.
Supplementary Material
Acknowledgments
We thank all the donors involved in the study. We are also thankful to the physicians of the Department of Obstetrics and Gynecology of Maternal and Children’s Hospital of Hubei Province for the help in collecting early pregnancy placenta. Funding: This work was supported by National Key Research & Developmental Program of China (2018YFC1003900; 2018YFC1003904), and National Natural Science Foundation of China (No. 81871186).
Footnotes
Declaration of competing interest
The authors declare there is no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.placenta.2020.08.013.
References
- [1].Maldonado-Estrada J, Menu E, Roques P, Barre-Sinoussi F, Chaouat G, Evaluation of Cytokeratin 7 as an accurate intracellular marker with which to assess the purity of human placental villous trophoblast cells by flow cytometry, J. Immunol. Methods 286 (2004) 21–34. [DOI] [PubMed] [Google Scholar]
- [2].Cierna Z, Varga I, Danihel L Jr., Kuracinova K, Janegova A, Danihel L, Intermediate trophoblast–A distinctive, unique and often unrecognized population of trophoblastic cells, Ann. Anat 204 (2016) 45–50. [DOI] [PubMed] [Google Scholar]
- [3].James JL, Carter AM, Chamley LW, Human placentation from nidation to 5 weeks of gestation. Part I: what do we know about formative placental development following implantation? Placenta 33 (2012) 327–334. [DOI] [PubMed] [Google Scholar]
- [4].Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K, Kabayama Y, Suyama M, Sasaki H, Arima T, Derivation of human trophoblast stem cells, Cell Stem Cell 22 (2018) 50–63 e6. [DOI] [PubMed] [Google Scholar]
- [5].Bischof P, Irminger-Finger I, The human cytotrophoblastic cell, a mononuclear chameleon, Int. J. Biochem. Cell Biol 37 (2005) 1–16. [DOI] [PubMed] [Google Scholar]
- [6].Straszewski-Chavez SL, Abrahams VM, Alvero AB, Aldo PB, Ma Y, Guller S, Romero R, Mor G, The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71, Placenta 30 (2009) 939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Daoud G, Amyot M, Rassart E, Masse A, Simoneau L, Lafond J, ERK1/2 and p38 regulate trophoblasts differentiation in human term placenta, J. Physiol 566 (2005) 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Daoud G, Rassart E, Masse A, Lafond J, Src family kinases play multiple roles in differentiation of trophoblasts from human term placenta, J. Physiol 571 (2006) 537–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF 3rd, Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae, Endocrinology 118 (1986) 1567–1582. [DOI] [PubMed] [Google Scholar]
- [10].Abou-Kheir W, Barrak J, Hadadeh O, Daoud G, HTR-8/SVneo cell line contains a mixed population of cells, Placenta 50 (2017) 1–7. [DOI] [PubMed] [Google Scholar]
- [11].Kniss DA, Xie Y, Li Y, Kumar S, Linton EA, Cohen P, Fan-Havard P, Redman CW, Sargent IL, ED(27) trophoblast-like cells isolated from first-trimester chorionic villi are genetically identical to HeLa cells yet exhibit a distinct phenotype, Placenta 23 (2002) 32–43. [DOI] [PubMed] [Google Scholar]
- [12].Weiss U, Cervar M, Puerstner P, Schmut O, Haas J, Mauschitz R, Arikan G, Desoye G, Hyperglycaemia in vitro alters the proliferation and mitochondrial activity of the choriocarcinoma cell lines BeWo, JAR and JEG-3 as models for human first-trimester trophoblast, Diabetologia 44 (2001) 209–219. [DOI] [PubMed] [Google Scholar]
- [13].Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK, Establishment and characterization of first trimester human trophoblast cells with extended lifespan, Exp. Cell Res 206 (1993) 204–211. [DOI] [PubMed] [Google Scholar]
- [14].Greider CW, Blackburn EH, The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity, Cell 51 (1987) 887–898. [DOI] [PubMed] [Google Scholar]
- [15].Wilkie AO, Lamb J, Harris PC, Finney RD, Higgs DR, A truncated human chromosome 16 associated with alpha thalassaemia is stabilized by addition of telomeric repeat (TTAGGG)n, Nature 346 (1990) 868–871. [DOI] [PubMed] [Google Scholar]
- [16].Morin GB, The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats, Cell 59 (1989) 521–529. [DOI] [PubMed] [Google Scholar]
- [17].Harley CB, Futcher AB, Greider CW, Telomeres shorten during ageing of human fibroblasts, Nature 345 (1990) 458–460. [DOI] [PubMed] [Google Scholar]
- [18].Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC, Telomere reduction in human colorectal carcinoma and with ageing, Nature 346 (1990) 866–868. [DOI] [PubMed] [Google Scholar]
- [19].Counter CM, Meyerson M, Eaton EN, Ellisen LW, Caddle SD, Haber DA, Weinberg RA, Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase, Oncogene 16 (1998) 1217–1222. [DOI] [PubMed] [Google Scholar]
- [20].Jiang XR, Jimenez G, Chang E, Frolkis M, Kusler B, Sage M, Beeche M, Bodnar AG, Wahl GM, Tlsty TD, Chiu CP, Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype, Nat. Genet 21 (1999) 111–114. [DOI] [PubMed] [Google Scholar]
- [21].Morales CP, Holt SE, Ouellette M, Kaur KJ, Yan Y, Wilson KS, White MA, Wright WE, Shay JW, Absence of cancer-associated changes in human fibroblasts immortalized with telomerase, Nat. Genet 21 (1999) 115–118. [DOI] [PubMed] [Google Scholar]
- [22].Krikun G, Mor G, Alvero A, Guller S, Schatz F, Sapi E, Rahman M, Caze R, Qumsiyeh M, Lockwood CJ, A novel immortalized human endometrial stromal cell line with normal progestational response, Endocrinology 145 (2004) 2291–2296. [DOI] [PubMed] [Google Scholar]
- [23].Alvero AB, Fishman DA, Qumsiyeh MB, Garg M, Kacinski BM, Sapi E, Telomerase prolongs the lifespan of normal human ovarian surface epithelial cells without inducing neoplastic phenotype, J. Soc. Gynecol. Invest 11 (2004) 553–561. [DOI] [PubMed] [Google Scholar]
- [24].Krikun G, Mor G, Huang J, Schatz F, Lockwood CJ, Metalloproteinase expression by control and telomerase immortalized human endometrial endothelial cells, Histol. Histopathol 20 (2005) 719–724. [DOI] [PubMed] [Google Scholar]
- [25].Hu X, Wang Y, Mor G, Liao A, Forkhead box P3 is selectively expressed in human trophoblasts and decreased in recurrent pregnancy loss, Placenta 81 (2019) 1–8. [DOI] [PubMed] [Google Scholar]
- [26].Muhlhauser J, Crescimanno C, Kasper M, Zaccheo D, Castellucci M, Differentiation of human trophoblast populations involves alterations in cytokeratin patterns, J. Histochem. Cytochem 43 (1995) 579–589. [DOI] [PubMed] [Google Scholar]
- [27].Battaglia RA, Delic S, Herrmann H, Snider NT, Vimentin on the move: new developments in cell migration, F1000Res 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].DaSilva-Arnold SC, Zamudio S, Al-Khan A, Alvarez-Perez J, Mannion C, Koenig C, Luke D, Perez AM, Petroff M, Alvarez M, Illsley NP, Human trophoblast epithelial-mesenchymal transition in abnormally invasive placenta, Biol. Reprod 99 (2018) 409–421. [DOI] [PubMed] [Google Scholar]
- [29].Ferreira LMR, Meissner TB, Tilburgs T, Strominger JL, HLA-G: at the interface of maternal-fetal tolerance, Trends Immunol. 38 (2017) 272–286. [DOI] [PubMed] [Google Scholar]
- [30].Weber M, Gohner C, San Martin S, Vattai A, Hutter S, Parraga M, Jeschke U, Schleussner E, Markert Ur, Fitzgerald JS, Unique trophoblast stem cell- and pluripotency marker staining patterns depending on gestational age and placenta-associated pregnancy complications, Cell Adhes. Migrat 10 (2016) 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bravo-Flores E, Mancilla-Herrera I, Espino YSS, Ortiz-Ramirez M, Flores-Rueda V, Ibarguengoitia-Ochoa F, Ibanez CA, Zambrano E, Solis-Paredes M, Perichart-Perera O, Sanchez-Martinez M, Medina-Bastidas D, Reyes-Munoz E, Estrada-Gutierrez G, Macrophage populations in visceral adipose tissue from pregnant women: potential role of obesity in maternal inflammation, Int. J. Mol. Sci 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Knofler M, Haider S, Saleh L, Pollheimer J, Gamage T, James J, Human placenta and trophoblast development: key molecular mechanisms and model systems, Cell. Mol. Life Sci 76 (2019) 3479–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mor G, Aldo P, Alvero AB, The unique immunological and microbial aspects of pregnancy, Nat. Rev. Immunol 17 (2017) 469–482. [DOI] [PubMed] [Google Scholar]
- [34].Du MR, Guo PF, Piao HL, Wang SC, Sun C, Jin LP, Tao Y, Li YH, Zhang D, Zhu R, Fu Q, Li DJ, Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal-fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells, J. Immunol 192 (2014) 1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Guo PF, Du MR, Wu HX, Lin Y, Jin LP, Li DJ, Thymic stromal lymphopoietin from trophoblasts induces dendritic cell-mediated regulatory TH2 bias in the decidua during early gestation in humans, Blood 116 (2010) 2061–2069. [DOI] [PubMed] [Google Scholar]
- [36].Khoo NK, Bechberger JF, Shepherd T, Bond SL, McCrae KR, Hamilton GS, Lala PK, SV40 Tag transformation of the normal invasive trophoblast results in a premalignant phenotype. I. Mechanisms responsible for hyperinvasiveness and resistance to anti-invasive action of TGFbeta, Int. J. Canc 77 (1998) 429–439. [DOI] [PubMed] [Google Scholar]
- [37].Aboagye-Mathiesen G, Zdravkovic M, Toth FD, Graham CH, Lala PK, Ebbesen P, Altered expression of the tumor suppressor/oncoprotein p53 in SV40 Tag-transformed human placental trophoblast and malignant trophoblast cell lines, Early Pregnancy 2 (1996) 102–112. [PubMed] [Google Scholar]
- [38].Reiter JL, Drendel HM, Chakraborty S, Schellinger MM, Lee MJ, Mor G, Cytogenetic features of human trophoblast cell lines SWAN-71 and 3A-subE, Placenta 52 (2017) 17–20. [DOI] [PubMed] [Google Scholar]
- [39].Feng R, Wen J, Overview of the roles of Sox2 in stem cell and development, Biol. Chem 396 (2015) 883–891. [DOI] [PubMed] [Google Scholar]
- [40].Avokpaho E, d’Almeida TC, Sadissou I, Tokplonou L, Adamou R, Sonon P, Milet J, Cottrell G, Mondiere A, Massougbodji A, Moutairou K, Donadi EA, Teixeira Mendes Junior C, Favier B, Carosella E, Moreau P, Rouas-Freiss N, Garcia A, Courtin D, HLA-G expression during hookworm infection in pregnant women, Acta Trop. 196 (2019) 52–59. [DOI] [PubMed] [Google Scholar]
- [41].Djurisic S, Teiblum S, Tolstrup CK, Christiansen OB, Hviid TV, Allelic imbalance modulates surface expression of the tolerance-inducing HLA-G molecule on primary trophoblast cells, Mol. Hum. Reprod 21 (2015) 281–295. [DOI] [PubMed] [Google Scholar]
- [42].Shore VH, Wang TH, Wang CL, Torry RJ, Caudle MR, Torry DS, Vascular endothelial growth factor, placenta growth factor and their receptors in isolated human trophoblast, Placenta 18 (1997) 657–665. [DOI] [PubMed] [Google Scholar]
- [43].Kameda T, Matsuzaki N, Sawai K, Okada T, Saji F, Matsuda T, Hirano T, Kishimoto T, Tanizawa O, Production of interleukin-6 by normal human trophoblast, Placenta 11 (1990) 205–213. [DOI] [PubMed] [Google Scholar]
- [44].Jokhi PP, King A, Loke YW, Cytokine production and cytokine receptor expression by cells of the human first trimester placental-uterine interface, Cytokine 9 (1997) 126–137. [DOI] [PubMed] [Google Scholar]
- [45].Mori S, Hatori N, Kawaguchi N, Hamada Y, Shih TC, Wu CY, Lam KS, Matsuura N, Yamamoto H, Takada YK, Takada Y, The integrin-binding defective FGF2 mutants potently suppress FGF2 signalling and angiogenesis, Biosci. Rep 37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vanlandewijck M, Lebouvier T, Andaloussi Mae M, Nahar K, Hornemann S, Kenkel D, Cunha SI, Lennartsson J, Boss A, Heldin CH, Keller A, Betsholtz C, Functional characterization of germline mutations in PDGFB and PDGFRB in primary familial brain calcification, PloS One 10 (2015), e0143407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Murphy JF, Fitzgerald DJ, Vascular endothelial growth factor induces cyclooxygenase-dependent proliferation of endothelial cells via the VEGF-2 receptor, Faseb. J 15 (2001) 1667–1669. [DOI] [PubMed] [Google Scholar]
- [48].Hebert CA, Luscinskas FW, Kiely JM, Luis EA, Darbonne WC, Bennett GL, Liu CC, Obin MS, Ma Gimbrone Jr., Baker JB, Endothelial and leukocyte forms of IL-8. Conversion by thrombin and interactions with neutrophils, J. Immunol 145 (1990) 3033–3040. [PubMed] [Google Scholar]
- [49].Romagnani P, Annunziato F, Lazzeri E, Cosmi L, Beltrame C, Lasagni L, Galli G, Francalanci M, Manetti R, Marra F, Vanini V, Maggi E, Romagnani S, Interferon-inducible protein 10, monokine induced by interferon gamma, and interferon-inducible T-cell alpha chemoattractant are produced by thymic epithelial cells and attract T-cell receptor (TCR) alphabeta+ CD8+ single-positive T cells, TCRgammadelta+ T cells, and natural killer-type cells in human thymus, Blood 97 (2001) 601–607. [DOI] [PubMed] [Google Scholar]
- [50].Smit MJ, Verdijk P, van der Raaij-Helmer EM, Navis M, Hensbergen PJ, Leurs R, Tensen CP, CXCR3-mediated chemotaxis of human T cells is regulated by a Gi- and phospholipase C-dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase, Blood 102 (2003) 1959–1965. [DOI] [PubMed] [Google Scholar]
- [51].Cheeran MC, Hu S, Sheng WS, Peterson PK, Lokensgard JR, CXCL10 production from cytomegalovirus-stimulated microglia is regulated by both human and viral interleukin-10, J. Virol 77 (2003) 4502–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Weber M, Uguccioni M, Baggiolini M, Clark-Lewis I, Dahinden CA, Deletion of the NH2-terminal residue converts monocyte chemotactic protein 1 from an activator of basophil mediator release to an eosinophil chemoattractant, J. Exp. Med 183 (1996) 681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chakravarty L, Rogers L, Quach T, Breckenridge S, Kolattukudy PE, Lysine 58 and histidine 66 at the C-terminal alpha-helix of monocyte chemoattractant protein-1 are essential for glycosaminoglycan binding, J. Biol. Chem 273 (1998) 29641–29647. [DOI] [PubMed] [Google Scholar]
- [54].Zhang YJ, Rutledge BJ, Rollins BJ, Structure/activity analysis of human monocyte chemoattractant protein-1 (MCP-1) by mutagenesis. Identification of a mutated protein that inhibits MCP-1-mediated monocyte chemotaxis, J. Biol. Chem 269 (1994) 15918–15924. [PubMed] [Google Scholar]
- [55].Li YS, Shyy YJ, Wright JG, Valente AJ, Cornhill JF, Kolattukudy PE, The expression of monocyte chemotactic protein (MCP-1) in human vascular endothelium in vitro and in vivo, Mol. Cell. Biochem 126 (1993) 61–68. [DOI] [PubMed] [Google Scholar]
- [56].Mouser EE, Pollakis G, Smits HH, Thomas J, Yazdanbakhsh M, de Jong Ec, Paxton WA, Schistosoma mansoni soluble egg antigen (SEA) and recombinant Omega-1 modulate induced CD4+ T-lymphocyte responses and HIV-1 infection in vitro, PLoS Pathog. 15 (2019), e1007924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hu L, Zhu Y, Zhang J, Chen W, Li Z, Li L, Zhang L, Cao D, Potential circulating biomarkers of circulating chemokines CCL5, MIP-1beta and HA as for early detection of cirrhosis related to chronic HBV (hepatitis B virus) infection, BMC Infect. Dis 19 (2019) 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Obregon-Perko V, Hodara VL, Parodi LM, Giavedoni LD, Baboon CD8 T cells suppress SIVmac infection in CD4 T cells through contact-dependent production of MIP-1alpha, MIP-1beta, and RANTES, Cytokine 111 (2018) 408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hromadnikova I, Li S, Kotlabova K, Dickinson AM, Influence of in vitro IL-2 or IL-15 alone or in combination with hsp 70 derived 14-mer peptide (TKD) on the expression of NK cell activatory and inhibitory receptors on peripheral blood T cells, B cells and NKT cells, PloS One 11 (2016), e0151535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yanagibashi T, Satoh M, Nagai Y, Koike M, Takatsu K, Allergic diseases: from bench to clinic - contribution of the discovery of interleukin-5, Cytokine 98 (2017) 59–70. [DOI] [PubMed] [Google Scholar]
- [61].Kulkarni U, Herrmenau C, Win SJ, Bauer M, Kamradt T, IL-7 treatment augments and prolongs sepsis-induced expansion of IL-10-producing B lymphocytes and myeloid-derived suppressor cells, PloS One 13 (2018), e0192304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


