Abstract
Patients with acute myeloid leukemia have a very poor prognosis related to a high rate of relapse and drug-related toxicity. The ability of leukemia stem cells (LSCs) to survive chemotherapy is primarily responsible for relapse, and eliminating LSCs is ultimately essential for cure. We developed novel disulfide-crosslinked CLL1-targeting micelles (DC-CTM), which can deliver high concentrations of daunorubicin (DNR) into both bulk leukemia cells and LSCs. Compared to free DNR, DC-CTM-DNR had a longer half-life, increased DNR area under the curve concentration by 11-fold, and exhibited a superior toxicity profile. In patient-derived AML xenograft models, DC-CTM-DNR treatment led to significant decreases in AML engraftment and impairment of secondary transplantation compared to control groups. Collectively, we demonstrate superior anti-LSC/AML efficacy, and preferable pharmacokinetic and toxicity profiles of DC-CTM-DNR compared to free DNR. DC-CTM-DNR has the potential to significantly improve treatment outcomes and reduce therapy-related morbidity and mortality for patients with AML.
Leukemia stem cells (LSC) could repopulate the leukemia after chemotherapy resulting recurrence. CLL1 only expressed on LSC but not hematopoietic stem cells, and thus we developed CLL1 targeting micelles to deliver high dose of chemotherapeutic cells to eradicate LSC and bulk leukemia. This strategy cure leukemia from the root.
Keywords: Leukemia stem cells, nanoparticle, daunorubicin, CLL1, AML
Introduction:
Acute myeloid leukemia (AML) is a highly aggressive hematological malignancy. The standard “7+3” induction chemotherapy regimen of cytarabine and daunorubicin(DNR) leads to complete remissions in up to 70–80% of patients(1, 2). Unfortunately, treatment-related mortality can approach 30% in some patient subpopulations and more than 50% of patients experience relapse(3). Leukemia stem cells (LSCs) are relatively resistant to and can survive conventional chemotherapy to cause relapse. Therefore, LSC serve as an attractive therapeutic target. Up to 70–92% of AML samples, including LSCs, express C-type lectin-like molecule-1 (CLL1), whereas normal hematopoietic stem cells (HSCs) do not express CLL1(1). Monoclonal antibodies, bi-specific T cell engagers, and a peptide (amino acid sequence: CDLRSAAVC) targeting CLL1 have been developed with the goal of specifically targeting LSCs to eliminate the source of AML(2). Our group previously showed non-crosslinked micelles (CTM) could only deliver minimal payload to CLL1+ cells and LSCs from patient samples(2). By decorating the surface of CTM loaded with DNR with a CLL1-targeting peptide (CTM-DNR), we demonstrated delivery of DNR into LSCs, but not HSCs, ex vivo(2). Herein, we further modified CTM-DNR by introducing disulfide crosslinks (DC-CTM-DNR) to improve stability and prevent premature drug release during circulation(4). Compared to free DNR, DC-CTM-DNR showed a superior in vivo anti-bulk AML and LSC efficacy and improved pharmacokinetic (PK) and toxicity profiles in patient-derived xenograft (PDX) models of AML.
Methods:
Synthesis of DC-CTM-DNR
Two types of telodendrimers, CLL1-peptide-PEG5K-CA8 and PEG5K-Cys4-L8-CA8 (Figure S1a,b) were synthesized as previously described and mixed with a 1:1 ratio(3, 4). After DNR loading, disulfide crosslinks were formed from cysteine residues under oxidative conditions and the properties of micelles and encapsulated efficiency were characterized (Supplementary Methods) (2–4).
Pharmacokinetic (PK) and toxicity studies
PK studies and toxicity studies were performed in rats and balb/c mice, respectively, as previously described (Supplementary Methods)(3).
Anti-leukemia efficacy and secondary transplantation studies in PDX models of AML
Animal studies and collection of patient samples were approved (UC Davis IACUC protocol #18386 and IRB protocols #218204 and 1204087). PDX models were established from patient AML samples transplanted into immunodeficient NSG (NOD-Scid-IL2Rgcnull) (Jackson lab, Sacramento, CA). After establishment of engraftment, mice were treated with DNR or DC-CTM-DNR and leukemia burden was evaluated by flow cytometry (supplementary methods). Two weeks after initial treatment, bone marrow cells were harvested for secondary transplantation studies.
Result & Discussion:
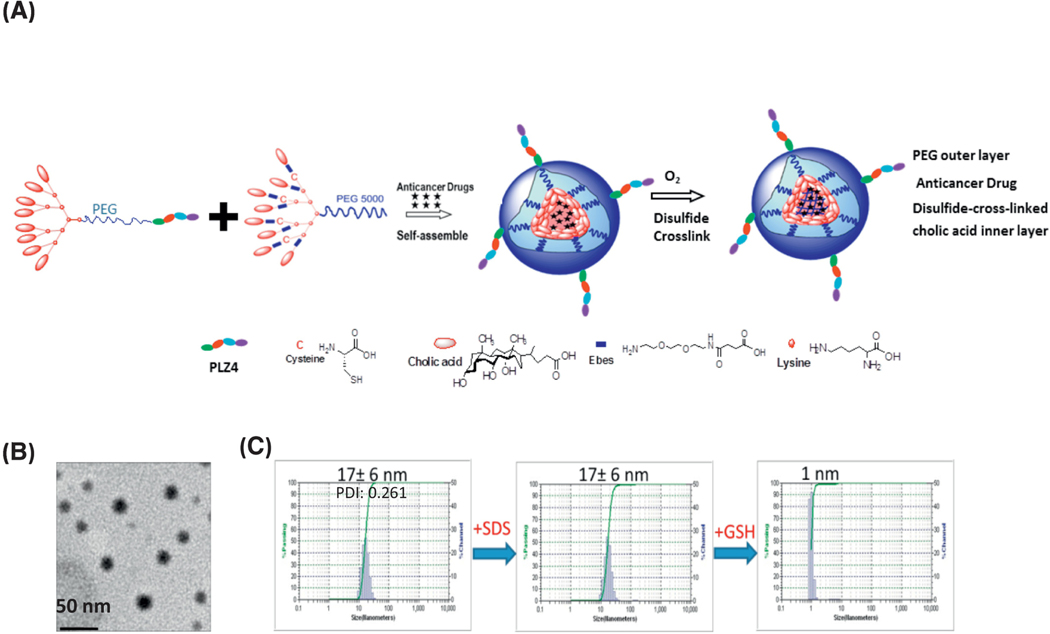
As illustrated in Figure 1a, two types of telodendrimers, CLL1-peptide-PEG5K-CA8 and PEG5K-Cys4-L8-CA8 were synthesized and mixed with a 1:1 ratio (Figure. 1a). After DNR loading, disulfide crosslinks were formed from cysteine residues under oxidative conditions(2–4). DC-CTM-DNR were spherically-shaped (Figure 1b) with a size distribution of 17+/−6 nm (Figure 1b-c). The loading capacity for DNR was 2 mg/mL with 99% encapsulated efficiency. DC-CTM-DNR were stable in the presence of a strong detergent, sodium dodecyl sulfate (SDS) but dissolved upon addition of a physiological intracellular concentration of the reducing agent glutathione (Figure 1c). These features can prevent premature release during blood circulation and facilitate target-specific delivery upon uptake by target cells.
Figure 1. Synthesis and characterization of disulfide-crosslinked CLL1-targeting nanomicelles (DC-CTM).
(a) Diagram of telodendrimer and DC-CTM-DNR. Each telodendrimer (PEG5K-Cys4-L8-CA8) contains a PEG (molecular weight 5 KDa) backbone with four cysteine (C4), eight linker (L8) and eight cholic acid (CA8) units conjugated at the distal end of PEG. CLL1-L can be conjugated at the other terminus of PEG for synthesis of DC-CTM. After DNR is loaded in the core formed by cholic acid, cysteine residues can form disulfide crosslinks under oxidative conditions. These disulfide bonds are reversible and can be broken open under the reducing intracellular environment to release the drug load locally. (b) Morphologic analysis with electron microscopy. Bar = 50 nm. (c) Size distribution of DC-CTM was analyzed by dynamic light scattering (DLS). The same sample was treated with SDS for 10 min and particle size was analyzed again. Later, this sample was treated with 10 mM glutathione (GSH) for 30 min followed by particle size analysis.
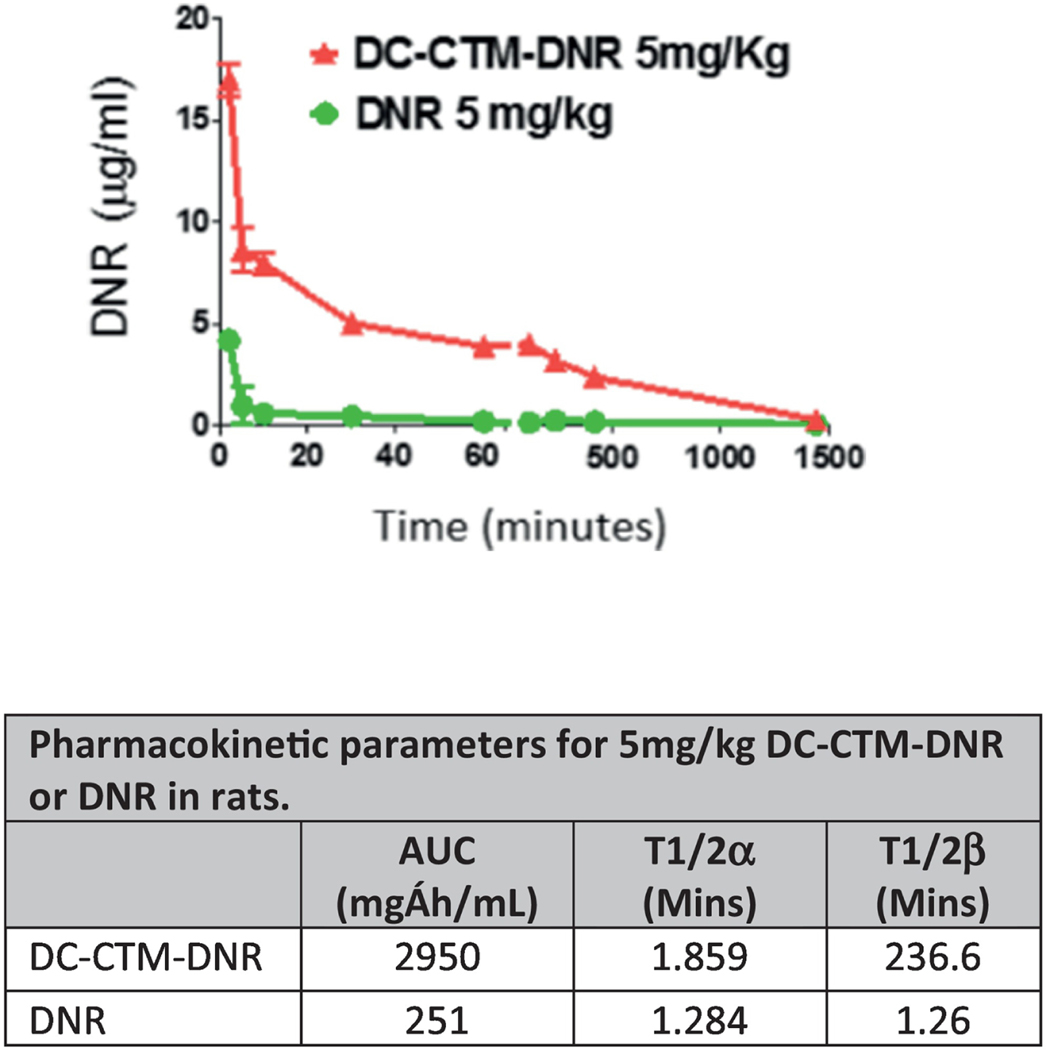
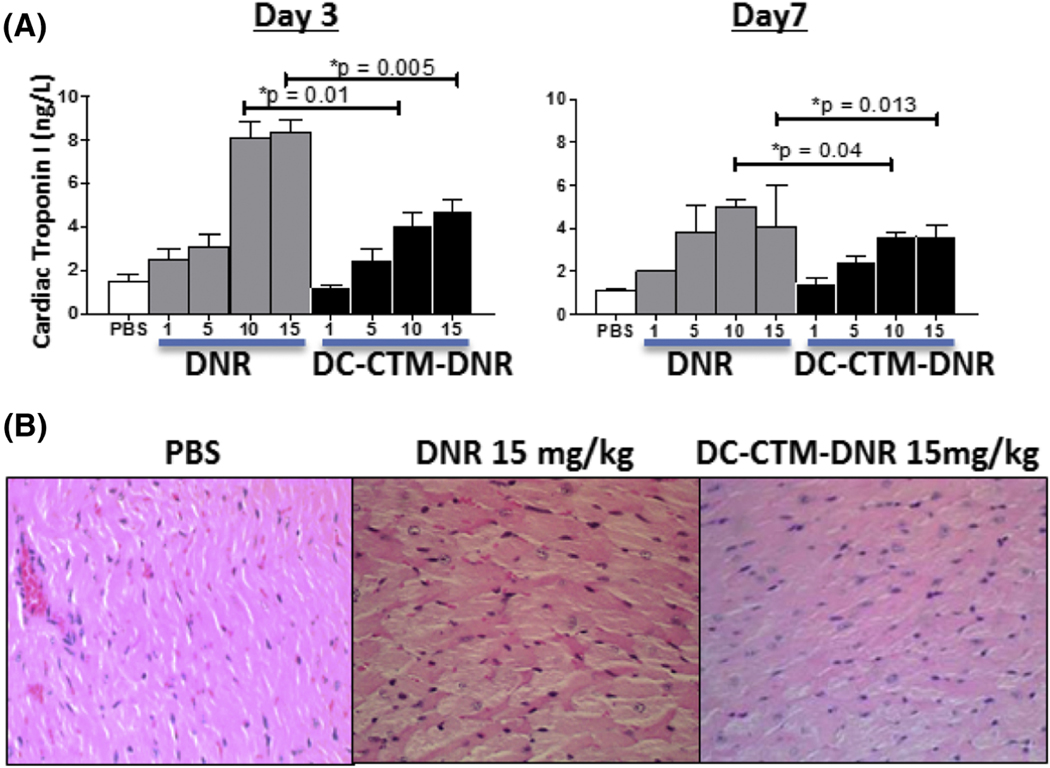
Consistent with the stability conferred by disulfide crosslinking, DC-CTM-DNR demonstrated an 11-fold increased area under curve with prolonged T1/2 compared to free DNR in cannulated Sprague-Dawley rats (Figure 2 and Supplementary Methods). This result suggests a longer drug exposure to both bulk AML and LSCs, potentially enabling more cell death. In contrast to the rapid diffusion into normal tissue after administration of free DNR, retention of DNR by DC-CTM-DNR was associated with less major organ damage, especially cardiotoxicity. Both free DNR and DC-CTM-DNR caused a dose-dependent increase in serum cardiac troponin I (cTnI), a specific biomarker for acute cardiac tissue damage, in balb/c mice (Figure 3); however, the DC-CTM-DNR group had significantly lower serum cTnI concentrations that correlated with less cardiomyolysis on histopathologic evaluation (Figure 3c). No other obvert toxicity identified in other major organs (Figure S2).
Figure 2. Pharmacokinetic studies of free DNR and DC-CTM-DNR in jugular vein cannulated Sprague-Dawley rats.
Rats were treated with 5 mg/kg DC-CTM-DNR or free DNR. Blood was collected at different time points and DNR concentrations were measured based on fluorescence (n=3).
Figure 3. Toxicity profile of DC-CTM-DNR.
(a) Serum cardiac troponin I (cTnI) changes after 3 and 7 days post DNR and DC-CTM-DNR treatment. (n=3; *t-test, p<0.05 was considered as significant) (b) Histopathologic evaluation of heart tissue from balb/c mice treated with PBS, 15 mg/kg DNR, or 15 mg/kg DC-CTM-DNR at 72 hours. Cardiac tissue from mice treated with free DNR showed massive disarray of cardiomyocytes and disruption of myofibrils, while DC-CTM-DNR treatment caused much milder change (H&E stain, Olympus, 40X).
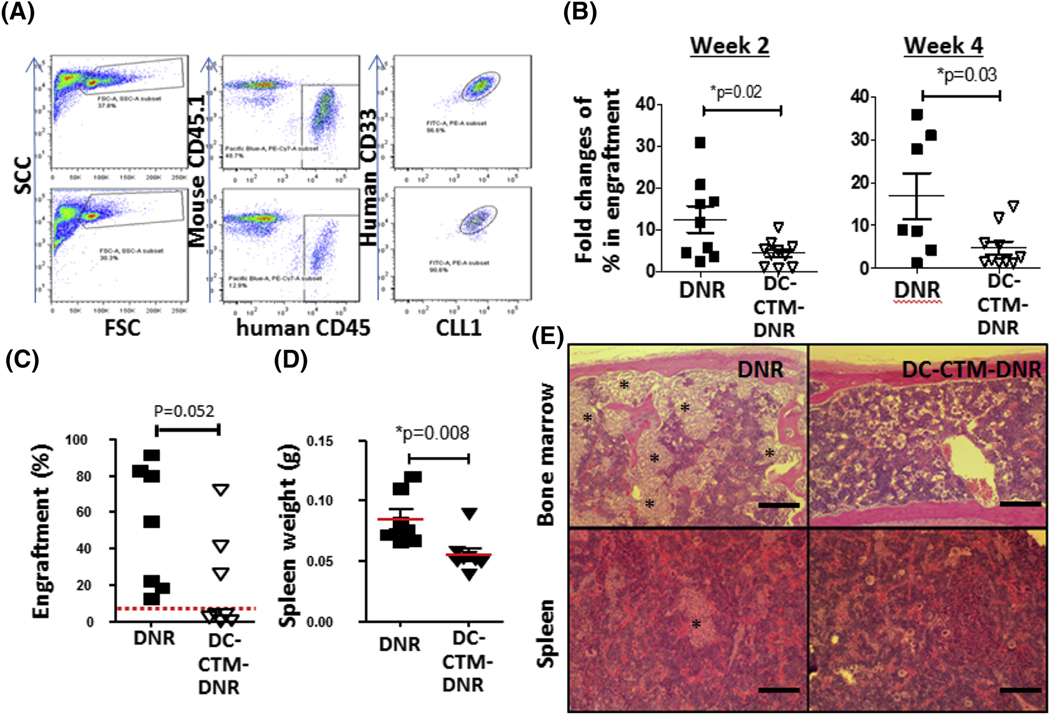
CLL1-expressing AML patient sample with de novo FLT3-ITD mutated AML (Table S1) was used to establish AML PDX models (Figure S3) and evaluate the anti-AML and anti-LSC activity of DC-CTM-DNR. Immunodeficient NSG (NOD-Scid-IL2Rgcnull) (Jackson lab, Sacramento, CA) mice were intravenously injected with human leukemia cells after receiving 0.2 Gray of radiation. After leukemia burden reached 5–15% in the bone marrow, mice were treated with PBS, 1 mg/kg free DNR or DC-CTM-DNR on day 1, 2, and 3. Two and four weeks post-treatment, DC-CTM-DNR-treated mice had significantly less AML engraftment compared to the groups treated with free DNR (Figure 4a-b). Secondary transplantation of bone marrow cells from primary treated mice was performed to determine presence of residual functional LSCs two weeks after treatment. Eight weeks after post-secondary transplantation from free DNR- and DC-CTM-DNR-treated mice, the free DNR and DC-CTM-DNR groups had 100% (7/7) and 37.5% (3/8) secondary transplantation rates, respectively (Figure 4 c). Furthermore, the DNR group had significantly larger spleens with increased AML cell infiltration and had more bone marrow involvement (Figure 4 d-e). Of note, unlike balb/c or NRG mice, NSG mice are extremely sensitive to DNA damaging agents, including DNR (Figure S4), and toxicity limited us to giving 1/10th the equivalent human therapeutic dose. Nevertheless, our results strongly suggested a significantly greater anti-bulk AML cell and anti-LSC efficacy for DC-CTM-DNR compared to free DNR.
Figure 4. In vivo efficacy, pharmacokinetic and toxicity studies of DC-CTM-DNR.
(a) Representative flow cytometry gating and plots in a de novo AML PDX model (TM00346) at 4 weeks. (b) Anti-AML efficacy study of free DNR and DC-CTM-DNR in AML model. AML carrying mice were treated with 1 mg/kg free DNR or DC-CTM-DNR on day 1, 2, and 3 early after establish of leukemia (5–10% engraftment). Leukemia burden was evaluated by flow cytometry to determine the fold changes of AML cells from bone marrow. t-test, *p<0.05. (c) Evaluation of anti-LSC activity of free DNR and DC-CTM-DNR treatment after secondary transplantation. Mice were treated as in Fig 2b, and 2 weeks later, mice were sacrificed and 5 × 105 total bone marrow nucleated cells were injected into another naïve NSG mouse. After 8 weeks, AML cell engraftment was analyzed by flow cytometry, with 5% used as the cut-off for establishment of leukemia. (d) Spleen weight from mice 8 weeks after secondary transplantation. *t-test, p<0.05. (e) Histopathologic evaluation of AML burden in the bone marrow and spleen from free DNR and DC-CTM-DNR treated secondary transplantation groups (Stars: leukemia cell patches).
In this project, we presented a unique CLL1-targeting nano-scale DNR delivery formulation with improved PK and toxicity profiles and anti-bulk leukemia and LSC efficacy in AML PDX models. Recently, CLL1 has become a promising and attractive target for LSC targeted therapy with various approaches, such as CLL1 antibody-drug conjugate(5, 6), anti-CD3/anti-CLL-1 bispecific antibody(7), and CLL1 targeting CAR-T cells(8). Decreased cardiotoxicity is clinically significant as many elderly AML patients have pre-existing cardiac diseases that preclude them from being treated with DNR. Even though liposomal doxorubicin and DNR formulations also have improved cardiotoxicity and improved PK(9–11), they do not have LSC-specific targeting features and are larger (>100nm) in size, potentially limiting penetration into LSC niches. Collectively, DC-CTM-DNR have improved PK and toxicity profiles and can potentially improve complete remission rates and decrease relapse through eradication of both LSCs and bulk AML cells, even in difficult-to-treat relapsed or FLT3-ITD mutation positive AML patients.
Supplementary Material
Acknowledgments
This project was supported by R01 CA176803 (PI: Pan), K12CA138464 (PI: Lara), R01CA199668, R01CA232845, R01HD086195 (PI: Li), NCI contract grant HHSN261201400042C (PI: Lin), and Cancer Center Support Grant (CCSG) IRG grant (PI: Lin).
Footnotes
Potential conflicts of interest: Y.L and K.L. are the inventors of a pending patent on disulfide cross-linked nanoparticles (US patent application US 14/117,570, filed on May 14, 2012). H. Z., K.S.L. and C.P. are the inventors of CLL1-P (US 14/130,909). K.S.L. and C.P. are co-founder and T.L and Y.L. are shareholders for the LP Therapeutics Inc that has exclusively licensed the CLL1-P patent from University of California Davis. The remaining authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.van Rhenen A, van Dongen GA, Kelder A, Rombouts EJ, Feller N, Moshaver B, et al. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood. 2007;110(7):2659–66. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H, Luo J, Li Y, Henderson PT, Wang Y, Wachsmann-Hogiu S, et al. Characterization of high-affinity peptides and their feasibility for use in nanotherapeutics targeting leukemia stem cells. Nanomedicine. 2012;8(7):1116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H, Li Y, Lin TY, Xiao K, Haddad AS, Henderson PT, et al. Nanomicelle formulation modifies the pharmacokinetic profiles and cardiac toxicity of daunorubicin. Nanomedicine. 2014;9(12):1807–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Xiao K, Luo J, Xiao W, Lee JS, Gonik AM, et al. Well-defined, reversible disulfide cross-linked micelles for on-demand paclitaxel delivery. Biomaterials. 2011;32(27):6633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang YP, Liu BY, Zheng Q, Panuganti S, Chen R, Zhu J, et al. CLT030, a leukemic stem cell-targeting CLL1 antibody-drug conjugate for treatment of acute myeloid leukemia. Blood advances. 2018;2(14):1738–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng B, Yu SF, del Rosario G, Leong SR, Lee GY, Vij R, et al. An Anti-CLL-1 Antibody-Drug Conjugate for the Treatment of Acute Myeloid Leukemia. Clinical Cancer Research. 2019;25(4):1358–68. [DOI] [PubMed] [Google Scholar]
- 7.Leong SR, Sukumaran S, Hristopoulos M, Totpal K, Stainton S, Lu E, et al. An anti-CD3/anti-CLL-1 bispecific antibody for the treatment of acute myeloid leukemia. Blood. 2017;129(5):609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang JH, Chen SY, Xiao W, Li WD, Wang L, Yang S, et al. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. Journal of hematology & oncology. 2018;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, et al. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2000;11(8):1029–33. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2004;15(3):440–9. [DOI] [PubMed] [Google Scholar]
- 11.Guaglianone P, Chan K, DelaFlor-Weiss E, Hanisch R, Jeffers S, Sharma D, et al. Phase I and pharmacologic study of liposomal daunorubicin (DaunoXome). Investigational new drugs. 1994;12(2):103–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.