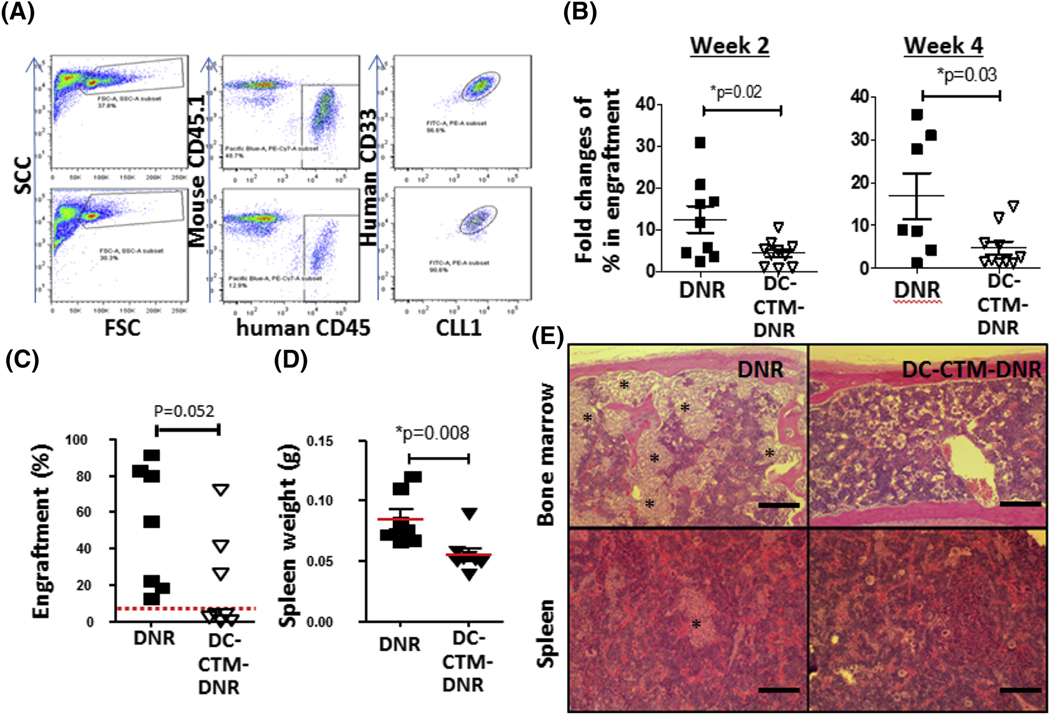
Figure 4. In vivo efficacy, pharmacokinetic and toxicity studies of DC-CTM-DNR.
(a) Representative flow cytometry gating and plots in a de novo AML PDX model (TM00346) at 4 weeks. (b) Anti-AML efficacy study of free DNR and DC-CTM-DNR in AML model. AML carrying mice were treated with 1 mg/kg free DNR or DC-CTM-DNR on day 1, 2, and 3 early after establish of leukemia (5–10% engraftment). Leukemia burden was evaluated by flow cytometry to determine the fold changes of AML cells from bone marrow. t-test, *p<0.05. (c) Evaluation of anti-LSC activity of free DNR and DC-CTM-DNR treatment after secondary transplantation. Mice were treated as in Fig 2b, and 2 weeks later, mice were sacrificed and 5 × 105 total bone marrow nucleated cells were injected into another naïve NSG mouse. After 8 weeks, AML cell engraftment was analyzed by flow cytometry, with 5% used as the cut-off for establishment of leukemia. (d) Spleen weight from mice 8 weeks after secondary transplantation. *t-test, p<0.05. (e) Histopathologic evaluation of AML burden in the bone marrow and spleen from free DNR and DC-CTM-DNR treated secondary transplantation groups (Stars: leukemia cell patches).