Abstract
Human mesenchymal stem cells (hMSCs) are an attractive source for cell therapies because of their multiple beneficial properties, i.e. via immunomodulation and secretory factors. Microfluidics is particularly attractive for cell encapsulation since it provides a rapid and reproducible methodology for microgel generation of controlled size and simultaneous cell encapsulation. Here, we report the fabrication of hMSC-laden microcarriers based on in situ ionotropic gelation of water-soluble chitosan in a microfluidic device using a combination of an antioxidant glycerylphytate (G1Phy) compound and tripolyphosphate (TPP) as ionic crosslinkers (G1Phy:TPP-microgels). These microgels showed homogeneous size distributions providing an average diameter of 104±12 μm, somewhat lower than that of control (127±16 μm, TPP-microgels). The presence of G1Phy in microgels maintained cell viability over time and upregulated paracrine factor secretion under adverse conditions compared to control TPP-microgels. Encapsulated hMSCs in G1Phy:TPP-microgels were delivered to the subcutaneous space of immunocompromised mice via injection, and the delivery process was as simple as the injection of unencapsulated cells. Immediately post-injection, equivalent signal intensities were observed between luciferase-expressing microgel-encapsulated and unencapsulated hMSCs, demonstrating no adverse effects of the microcarrier on initial cell survival. Cell persistence, inferred by bioluminescence signal, decreased exponentially over time showing relatively higher half-life values for G1Phy:TPP-microgels compared to TPP-microgels and unencapsulated cells. In overall, results position the microfluidics generated G1Phy:TPP-microgels as a promising microcarrier for supporting hMSC survival and reparative activities.
Keywords: Glycerylphytate, chitosan lactate, microgel, human mesenchymal stem cells encapsulation, microfluidics, secretome
1. Introduction
Therapies based on mesenchymal stem cells (MSCs) offer promising approaches for the treatment of diverse degenerative and inflammatory diseases. [1] Therapeutic interest in MSC originally focused on their self-renewal capacities and ability to differentiate into different cell lineages. [2] In recent years, paracrine signalling has been recognized to play an essential role in MSCs therapeutic efficacy via secretion of bioactive factors. [3–5] Secreted molecules influence tissue repair by enhancing processes such as angiogenesis, extracellular matrix (ECM) deposition, and by exerting immunoregulatory actions like macrophage activation and neutrophil recruitment, among others. [3] Nevertheless, the effective clinical application of MSCs-based therapies remains limited due to low cell survival and persistence in vivo, highlighting the need for developing new cell-carriers. Micron-scale hydrogels or microgels are attractive cell delivery platforms in comparison to bulk hydrogels because they can be delivered via minimally invasive techniques such as injection using small diameter needles. Microgels can be engineered to provide biological and physical support to enhance cell engraftment and sustain long-term release of paracrine molecules, enabling enhanced therapeutic actions. [6]
Cell microencapsulation requires a polymer network that ensures cell viability during microgel preparation and adequate crosslinking chemistry to form a polymer network under optimum gelation conditions (e.g. gelation time, pH, temperature). [2] Chitosan is a natural polysaccharide obtained from the alkaline deacetylation of chitin. It is composed of randomly distributed β-(1→4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit), [7, 8] which is similar to glycosaminoglycans present in natural ECMs. [9] Chitosan is biodegradable, non-toxic, and its origin makes it a renewable and eco-friendly material. [7] The use of chitosan microgels as a delivery vehicle for growth factors [10, 11] and drugs [12–20] has been described. However, their application as cell carriers has been limited because of its poor solubility at physiological pH, [21, 22] as well as the lack of cytocompatible crosslinking reactions and the necessity of aggressive purification methods (e.g. organic solvents, freeze-drying). [23, 24] In fact, few studies have reported fabrication approaches that allow simultaneous cell encapsulation and chitosan microgel preparation. [25–28] Generally, these reports focus on the combination of chitosan with a wide variety of polymers (e.g. collagen, chondroitin sulphate, dextran) or additives (e.g. hydroxyapatite), applying emulsification and precipitation methodologies. Daley et al. [27] fabricated chitosan-chondroitin sulfate polyelectrolyte microparticles for cartilage regeneration, applying a water/oil emulsification in a polydimethylsiloxane (PDMS) bath for MSCs embedding. Wise et al. [28] applied a water/oil emulsification in PDMS in combination with thermal gelation to fabricate encapsulated MSCs chitosan-collagen microgels for the enhancement of ectopic bone formation. Demir et al. [25] fabricated chitosan microgels by ionic gelation in a tripolyphosphate (TPP) bath, containing hydroxyapatite for studying osteogenic differentiation of encapsulated MSCs. As may be observed, none of the described studies make use of flow-focusing microfluidics, which is a particularly attractive methology for cell microcarriers fabrication as it provides a rapid and reproducible methodology for microgel generation of controlled size with simultaneous cell encapsulation, avoiding post-culture steps. [6, 29, 30] Although microfluidics generation of chitosan microgels have been explored, [20, 26, 31, 32] only one study has reported the in situ encapsulation of cells in oxidized dextran and N-carboxymethyl chitosan-based microgels using an asymmetric cross-junction microfluidic system. [26]
Despite this progress on chitosan-based microcarriers, further research on new cytocompatible crosslinkings and development of systems that provide additional biological properties would broaden cell encapsulation applications of chitosan microgels. Herein, chitosan-lactate microgels are fabricated in a flow-focusing microfluidic device via in situ gelation using the biologically active compound glycerylphytate (G1Phy) combined with TPP as ionic crosslinkers. G1Phy is a natural derivative compound with reduced cytotoxicity and powerful antioxidant activity [33] that could provide biological benefits to the microgels. Its crosslinking capacity has been applied in 3D printing technology, but it has never been applied before in MSCs encapsulation microfluidic processes. [34] The proposed microgel formulation and fabrication approach provide novelty at two different levels: G1Phy will act not only as a cytocompatible and natural-occurring crosslinker with powerful gelation properties, but also as a biologically active component of the developed microcarriers in comparison to other traditionally applied crosslinking agents that lack bioactivity (e.g. TPP, genipin) [35]. Thus, this is a straightforward approach to obtain in situ encapsulated human MSCs (hMSCs) in microcarriers that can be directly administered by minimally invasive injection, avoiding the need of post-culture protocols. Specifically, hMSCs encapsulation is realized via the use of (i) chitosan lactate (ChLA), a water-soluble chitosan derivative synthetized in our laboratory; and (ii) a reactive mixture of G1Phy combined with TPP as crosslinkers providing bioactivity and optimum gelation kinetics. The novel microgel composition (i.e. G1Phy:TPP-microgels) is studied and compared with the microgels formed with only TPP (i.e. TPP-microgels) as control to evaluate the effect exerted by G1Phy on microgels distributions and hMSCs encapasulation regarding viability, paracrine factor secretion, and in vivo persistence.
2. Experimental section
2.1. Materials
Chitosan with a degree of deacetylation of 90% and Mw = 300 kDa (medical grade and endotoxin free (<100 EU g−1), ChitoScience, Heppe Medical Chitosan GmbH, Halle, Germany) was used as received. Lactic acid, 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMTMM), TPP, mineral oil, SPAN80, and polydimethylsiloxane (PDMS) were all purchased from Sigma Aldrich and used as received. G1Phy was prepared as previously described by Mora-Boza et al. [33] using phytic acid and glycerol both supplied from Sigma Aldrich.
2.2. ChLA synthesis
ChLA was synthesized through a condensation reaction with lactic acid (Figure 1). Briefly, chitosan solution (2 wt-%, 1% v/v acetic acid) was stirred for 24 h to allow complete dissolution of the polymer. DMTMM (2-fold excess with respect to lactic acid) was dissolved in lactic acid solution (30 wt-% respect to chitosan) and stirred for 30 min. This solution was added dropwise to the chitosan solution and the reaction proceeded for 24 h. The final product was purified by precipitation in cold acetone, subsequently filtered, re-dissolved in distilled water, and dialysed (3500 Da cut off, Spectrum®) for 7 days. After freeze-drying, a white powder was obtained. Lactic conjugation was confirmed by 1H-Nuclear Magnetic Resonance (NMR). The degree of substitution (17.92%) of the chitosan amino groups was determined by formation of N-salicylidene, following the method reported by Qu et al. [36]
Figure 1:
a) Scheme of condensation reaction between chitosan and lactic acid activated by DMTMM in the synthesis of ChLA; b) 1H-NMR spectra of lactic acid, chitosan, and ChLA recorded in D2O; in ChLA spectrum red box denotes the chitosan conjugated lactate groups resonance signals at δ 1.6, 1.7, 1.85 and 1.90; c) Molecular structure of G1Phy and TPP, and schematic illustration of the network showing interactions that take place during chitosan lactate gelation with crosslinkers: (1) electrostatic and (2) hydrogen bond interactions.
2.3. Cell culture
Cells (hMSCs, bone marrow derived cells) were obtained from the Institute for Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White. Specifically, the used hMSCs corresponded to a 24-years old healthy male donor. The cell pehontype showed positive expression for CD90, CD105, CD73a (>95%) and negative for CD34, CD19, CD11b, CD45, CD79a, HLA-II, CD14 (<2%), and confirmed mycoplasma sp negative. Cells were cultured in α-Minimum Essential medium (α-MEM, Thermofisher/GIBCO) containing fetal bovine serum (FBS, 16.5%, Thermofisher/GIBCO), L-glutamine (2 mM, Thermofisher/GIBCO), penicillin (100 units mL−1, ThermoFisher) and streptomycin (100 μg mL−1, ThermoFisher). Cells were incubated at 37 °C and CO2 (5%), and subcultured at 70–80% confluence. Cells at passage 4 were used for all experiments. For in vivo experiments, luciferase-expressing hMSCs (hMSCsLuc) were generated by transducing hMSCs with lentivirus encoding for firefly luciferase as previously described. [37]
2.4. Microfluidic device fabrication
PDMS flow-focusing devices with a nozzle size of 300 μm were fabricated using soft lithography from silicon and SU8 masters. [29] Devices were plasma treated and then bonded directly to glass slides. Microfluidic devices were primed with light mineral oil containing 3% (v/v) SPAN80, which was also used as the continuous phase fluid.
2.5. ChLA microgel generation
ChLA microgels were obtained by in situ gelation in a flow-focusing microfluidic device (Figure 1a). ChLA phase consisted of ChLA solution (1.0 wt-%) in phosphate buffered saline (PBS, pH 7.4, ThermoFisher) and crosslinker phase contained a G1Phy:TPP mixture, whose composition is detailed in Table 1, or TPP as control. Both polymer and crosslinker solutions were firstly focused at a T-junction to obtain a pre-crosslinked mixture that was then emulsified by the continuous phase to generate microdroplets. The ionic crosslinking reaction was subsequently completed along the serpentine channel of the device. Finally, the microgels were collected in α-MEM and centrifuged at 29,300xg to remove continuous phase and crosslinker traces. For encapsulated-hMSCs microgels, cells were trypsinized, pelleted by centrifugation and resuspended in ChLA solution at a final density of 2×106 cells mL-1. Flow rates were adjusted to 1.5 μL min−1 for both polymer and crosslinker phases, and 20 μL min−1 for continuous phase.
Table 1:
Crosslinker phase compositions.
| Sample | TPP (wt-%) | G1Phy (wt-%) |
|---|---|---|
| TPP-microgel | 0.50 | 0 |
| G1Phy:TPP-microgel | 0.40 | 0.10 |
2.6. Morphological evaluation and microgel size distribution
Microgel morphology was evaluated by optical microscopy (EVOS Imaging System, ThermoFisher). Pictures of the different microgel formulations were taken and diameters of at least 100 microgels per condition were measured using ImageJ software. Size distributions were analyzed using GraphPad Prism software.
2.7. In vitro cell viability
Encapsulated hMSCs in TPP- and G1Phy:TPP-microgels were statically cultured for 10 days in complete α-MEM. At selected time points, microencapsulated cells were removed from culture and stained for 15 min with 2 μM Calcein-AM (live, Life Technologies) and 2 μM ethidium homodimer (dead, Life Technologies). At least 200 cells were imaged at each time point using confocal microscopy (Nikon Ti microscope equipped with C2+ confocal system). Viability over time was calculated by taking the ratio of live cells to total cells (mean ± standard deviation (sd)). Two-way ANOVA analysis (GraphPad Prism) was performed to study differences in cell viability percentages for G1Phy:TPP- and TPP-microgels.
2.8. In vitro paracrine secretory profile analysis
Paracrine factor secretion of encapsulated hMSCs in G1Phy:TPP- and TPP-microgels was evaluated under two conditions: oxidative stress and interferon-γ (IFN-γ) activation. For oxidative stress conditions, Fenton reaction was induced by adding Fe2SO4·7 H2O (in 0.5% v/v H2SO4) and H2O2 solutions to microgel culture to obtain final concentrations of 50 mM and 20 mM, respectively. After overnight culture, encapsulated cells were treated with oxidant agents for 4 h, following a similar procedure reported by Fuhrman et al. [38] Treatment with IFN-γ was carried for 30 h at a final concentration of 50 ng mL-1. For both experiments, encapsulated hMSCs were cultured in α-MEM overnight before treatment. After finishing their respective incubation periods, the cell supernatant was collected and centrifuged at 14,000xg for 20 min at 4 °C. Samples were analysed for 23 analytes using a custom Luminex® Assay (R&D Systems) following the manufacturer’s instructions. Secretory factor expression of encapsulated hMSCs in TPP and G1Phy:TPP-microgels was analysed via partial least squares discriminant analysis (PLS-DA) using a custom MATLAB script. Analytes expression heat maps and clustering analysis were performed using JMP software. Individual paracrine factor levels were plotted for each sample (average ± sd) for each condition, and unpaired t-tests were performed to detect differences between groups.
2.9. Pilot in vivo study: microgel injection and encapsulated hMSCs tracking
All animal experiments were performed with the approval of the Georgia Tech Animal Care and Use Committee with veterinary supervision and within the guidelines of the Guide for the Care and Use of Laboratory Animals. Cell persistence and survival were evaluated in vivo by tracking the bioluminescence of encapsulated hMSCsLuc that were injected into dorsal subcutaneous spaces of immunocompromised mice using a 15G needle. NSG male and female mice (5 weeks, Jackson Laboratories) were anesthetized under isoflurane, and 100 μL of microgel suspensions (containing 104 cells) of each group were injected subcutaneously in the dorsum. Unencapsulated hMSCs suspensions, referred as hMSC-only (100 μL containing 104 cells), were used as controls. Each mouse received 4 separate injections (randomized groups) in each quadrant of the dorsum. D-Luciferin salt (Promega) was dissolved in saline and sterile filtered through 0.22 μm pore membranes. Mice received a 150 mg kg−1 luciferin dose injected into the intraperitoneal cavity, and the bioluminescence signal was measured using the IVIS Spectrum CT System (Perkin Elmer) at specified time points. [37] Total flux was normalized to the obtained flux value of each group immediately after microgel suspension delivery in order to compare among samples. Data was fitted to a one-phase decay curve and plotted over time using GraphPad Prism. Half-life values for each group were obtained from a one-phase decay curve fit.
3. Results
3.1. ChLA synthesis and ionic crosslinking reaction
Chitosan is characterized by poor solubility at physiological pH, which limits its use for in situ cell encapsulation processes. [21, 22] Here, we synthetized a water-soluble derivative of chitosan, named chitosan lactate (ChLA), through a condensation reaction with lactic acid (Figure 1a), which showed improved solubility at pH 7.4. [23, 24] Lactate conjugation was confirmed by 1H-NMR by the appearance of new signals in the ChLA spectrum at 1.6, 1.7, 1.85 and 1.90 ppm (Figure 1b) due to lactate groups. The degree of substitution of chitosan amino groups in ChLA was 17.92% as indicated the formation of N-salicylidene compound, applying the method reported by Qu et al. [36] Interestingly, the developed derivative showed in situ gelation ability against ionic crosslinking agents containing phosphate groups at physiological conditions without the necessity of using strong bases or washing steps. This overcomes the lack of cytocompatibility of other crosslinking reactions applied for chitosan that usually need of aggressive purification methods (e.g. organic solvents, freeze-drying). Thus, the ionotropic gelation of chitosan is based on electrostatic interactions between phosphate groups present in the crosslinkers (G1Phy and TPP) and protonated amino groups of chitosan (Figure 1c). [32, 33, 39] As mentioned above, G1Phy is a novel natural-occurring compound obtained through the hydroxylic condensation reaction between phytic acid and glycerol, [33] which shows improved cytocompatibility than its precursor, phytic acid, and can provide the microgels with antioxidant and bioactive properties. [33] In other studies by the authors, this compound demonstrated its ability to crosslink natural polymers such as gelatin and chitosan. [34]
3.2. Generation of ChLA microgels using microfluidics
Flow-focusing devices with 3 independent flow inlets (ChLA, crosslinker, and continuous phases) were used (Figure 2a) to produce ChLA microgels with and without encapsulated hMSCs. First, ChLA (1.0 wt-% in PBS) and crosslinker phase (Table 1) were merged at a T-junction to enable polymer-crosslinker interaction inside the device. The reactive mixture was then focused to the continuous phase to allow water/oil emulsion and droplet generation. The distance between the two junctions of the device was optimized to reduce interaction time between ChLA and the crosslinkers before emulsion with continuous phase. Thus, we avoided the formation of hydrogel pieces that clogged the device due to the fast kinetics of the crosslinkers. Once microdroplets were generated, residence time along the device was increased by the incorporation of a serpentine channel to ensure full crosslinking of ChLA microgels. Finally, resulting microgels were collected in α-MEM through the outlet tubing and centrifuged to eliminate oil, surfactant, and crosslinker traces (Figure 2b). As mentioned above, crosslinker phase had to be optimized to give appropriate and homogeneous microgels. G1Phy by its own provided a very rapid crosslinking kinetics that impair the obtaining of microgels. In fact, G1Phy concentrations higher than 0.1 wt-% resulted in the formation of hydrogel fibers that clogged the device. It is important to note that G1Phy has at least double the amount of anion groups capable to interact with ChLA compared with TPP. However, when 0.1 wt-% G1Phy was mixed with 0.4 wt-% TPP, fully crosslinked ChLA microgels could be generated. This novel microgel formulation (G1Phy:TPP-microgels) was studied and compared with that using only TPP crosslinker as control (Table 1). The size distributions of the tested mixtures were analysed and presented in Figure 2c. Both microgels formulations showed relatively homogeneous size distributions. However, the novel microgel composition provided a decrease in the average diameter (104±12 μm) respect to control microgels (127±16 μm).
Figure 2:
a) Microfluidic device design that allowed: (i) the mixture of ChLA and crosslinker phases; and (ii) the subsequent water/oil emulsion with continuous phase to generate microdroplets. The device serpentine allowed for microdroplets to fully crosslink and form microgels; b) Light microscopy images of G1Phy:TPP-microgels at different magnifications after purification; c) Size distribution histograms for both microgel compositions (Table 1).
3.3. In vitro viability of encapsulated hMSCs
Cell viability of the encapsulated cells was evaluated by live/dead staining and confocal imaging at specified time points (Figure 3a). Confocal images show high encapsulation efficiency after synthesis, as the frequency of cells remaining outside the microgels was below 10%. Figure 3b shows cell viability percentages over time. A relatively high cell viability (79±2% and 67±2%, for TPP- and G1Phy:TPP-microgels, respectively) was observed immediately following microgel synthesis (0 days), demonstrating the suitability of encapsulation and fabrication methods regarding cell survival. For prolonged culture periods (from 1 to 10 days after encapsulation), encapsulated hMSCs showed a different survival profile in culture varying with microgel composition. For TPP-microgels, cell viability was significantly reduced (~ 40±8%) after 1 day in culture (p < 0.0001), remaining constant until day 3. Cell viability significantly decreased down to 13±4% from 3 to 10 days in culture (p < 0.0001). In contrast, G1Phy:TPP-microgels were more effective at supporting cell survival over time in comparison to TPP-microgels. G1Phy-based microgels exhibited an initial viability of 67±2% which decreased to 60±7% after 1 day culture (p < 0.01) and remained constant over time. These results show that the presence of G1Phy exerts a positive effect on the survival and maintenance of encapsulated hMSCs in ChLA microgels over time.
Figure 3:
a) Live /Dead staining and brighfield confocal images of G1Phy:TTP- and TPP-microgels after hMSCs microencapsulation. Live (green) and dead (red) cells were stained with Calcein-AM and ethidium homodimer; microgels are outlined with white dashed lines; scale bars correspond to 500 μm b) Cell viability percentages (mean ± sd) over time for G1Phy:TPP- and TPP-microgels calculated from confocal imaging analysis using ImageJ software. Two-way ANOVA analysis was performed to study significant differences between G1Phy:TPP- and TPP-microgels at each time point (**p < 0.001; ****p < 0.0001).
3.4. In vitro paracrine secretory profile of encapsulated hMSCs
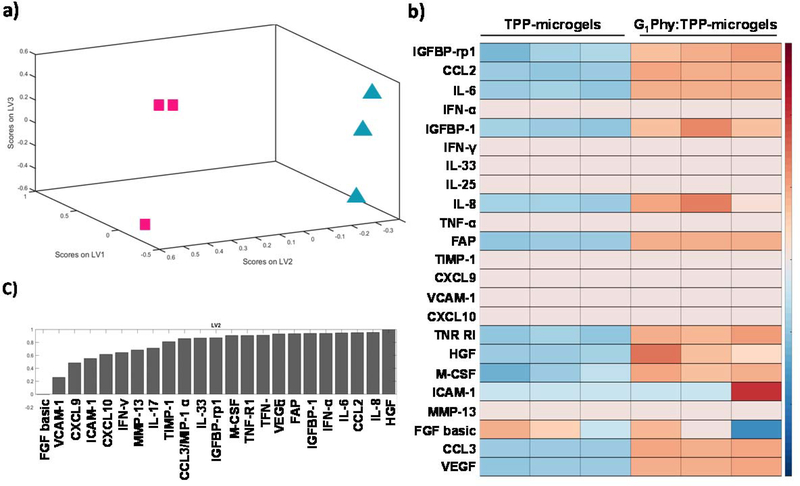
MSC secretome exerts powerful therapeutic actions on tissue regeneration processes. [3–5] Thus, we evaluated the effect of G1Phy:TPP-microgel on paracrine factors secretion of encapsulated hMSCs cultured under oxidative stress or IFN-γ activation. To analyse the effect of G1Phy on hMSC analyte secretion under oxidative stress, we induced lipid peroxidation on cells encapsulated in the microgels by triggering the Fenton reaction. [38] Antecedents on the in vitro activity of G1Phy to prevent lipid peroxidation of macrophages were previously reported [33]. Partial least squares-discriminant analysis (PLS-DA) of the complete collection of secreted factors showed clear clustering of the two experimental groups (Figure 4a), indicating that the crosslinker composition of the microgels had a considerable impact on secreted paracrine factors under oxidative stress. Consistent with PLS-DA, heatmap analysis revealed clustering for both microgel compositions (Figure 4b). PLS-DA also provided information about the analytes that accounted for the majority of variance between the experimental groups after oxidative stress induction (Figure 4c).
Figure 4:
Analysis of secreted analytes from encapsulated hMSCs in TPP-and G1Phy:TPP-microgels under oxidative stress conditions studied using Luminex® assay. a) PLS-DA of the total set of analyzed paracrine factors. Blue triangles and pink squares correspond to TPP-microgels and G1Phy:TPP-microgels groups, respectively; b) Heat maps analysis of the total set of analyzed paracrine factors; c) Normalized relative loading values given to each analyte during the PLS-DA.
Secreted paracrine factors were analysed individually in order to compare secretory profiles of G1Phy:TPP-microgels to those for TPP-microgels. Figure 5 shows the results of analytes that showed secretion values above the assay detection limit. The secretion of pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) for G1Phy-containing microgels was significantly higher than control microgels. Furthermore, hMSCs encapsulated in G1Phy:TPP-microgels showed a significant rise of interleukin-8 (IL-8) secretion which is related to monocyte activation and recruitment. [40] Other significantly up-regulated paracrine factors in encapsulated cells in G1Phy:TPP-microgels compared to control TPP-microgels were fibroblast activation protein (FAP), macrophage colony-stimulating factor (M-CSF), monocyte chemoattractant protein-1 (MCP-1), insulin-like growth factor binding protein (IGFBP), and TNF-R1, which belongs to the tumour necrosis factors (TNF) family. [41] These paracrine factors have different immunoregulatory roles in tissue repair. [4]
Figure 5:
Individual analysis of involved factors which were secreted from encapsulated hMSCs in function of microgel composition under oxidative stress conditions studied using Luminex® assay. n = 3 biologically independent samples, mean ± sd; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Unpaired t-test analysis was used to find significant differences between samples.
The hMSC secretome profile was also analysed after activation with IFN-γ, which induces inflammation-simulated conditions. [42, 43] PLS-DA revealed two well clustered groups corresponding to TPP- and G1Phy:TPP-microgels (Figure 6a). Heatmap analysis showed a similar clustering organization (Figure 6b), supporting the PLS-DA results. Figure 6c displays the influence of each analyte on the variability between the experimental groups after IFN-γ in PLS-DA.
Figure 6:
Analysis of secreted analytes from encapsulated hMSCs in TPP-and G1Phy:TPP-microgels activated with IFN-γ studied using Luminex® assay. a) PLS-DA of the total set of analyzed secretory factors. Blue triangles and pink squares correspond to TPP-microgels and G1Phy:TPP-microgels groups, respectively; b) Heat maps analysis of the total set of analyzed secretory factors; c) Normalized relative loading values given to each analyte during the PLS-DA.
Figure 7 presents results for individual factors whose secretion values were above the assay limit of detection. Several paracrine factors exhibited significantly elevated expression for encapsulated hMSCs in G1Phy:TPP-microgels in comparison to TPP-microgels (Figure 7). Hence, VEGF and basic FGF, which play key roles in tissue regeneration, [4, 44, 45] were significantly up-regulated in G1Phy:TPP-microgels compared to TPP-microgels. M-CSF, IL-8, and CXCL10 showed a significantly increased secretion in cells encapsulated in G1Phy:TPP-microgels. CXCL10 is a chemokine involved in diverse immunomodulatory roles such as chemoattraction for monocytes and macrophages and promotion of T cell adhesion to endothelial cells. [46]
Figure 7:
Individual analysis of factors secreted from encapsulated hMSCs in function of microgel composition that were activated with IFN-γ studied using Luminex® assay. n = 3 biologically independent samples, mean ± sd; *p < 0.05, **p < 0.01. Unpaired t-test analysis was used to find significant differences between samples.
Collectively, these results demonstrate that G1Phy presence into microgel composition had a modulatory effect in the secretome of encapsulated hMSCs by enhancing the secretion of different pro-survival and pro-angiogenic factors (e.g. VEGF, HGF, FBF basic, among others) and immunoregulatory factors (e.g. CXCL10, IL-8, MCP-1) when exposed to oxidative and inflammatory environments. Thus, G1Phy could be considered as a promising compound for enhancing paracrine signalling related to tissue repair capacities of encapsulated hMSCs in oxidative/inflammatory environments.
3.5. Pilot in vivo study: microgel-encapsulated hMSCs survival and persistence
Subcutaneous injection is an attractive route to evaluate the in vivo performance of microgels because it does not require invasive surgery and allows easy in vivo imaging. The survival and persistence of implanted microgel-encapsulated hMSCs were analysed via in vivo imaging. Thus, G1Phy:TPP- and TPP-microgels containing hMSCsLuc were subcutaneously injected in immunocompromised mice and bioluminescence was tracked (Figure 8a). Unencapsulated hMSCs (hMSC-only) were used as the control for comparison. At each time point (0, 3, 7, 10, 14, and 21 days) mice were injected with luciferin to measure bioluminescence signal. Microgel-encapsulated cells were easily delivered to the subcutaneous space via injection, and the delivery process was as simple as the injection of hMSC-only. Immediately post-injection, equivalent signal intensities were observed between microgel-encapsulated cells and unencapsulated cells in saline, demonstrating no adverse effects in initial cell survival. Normalized flux values (%) for each group are plotted over time in Figure 8b, and half-life values for each group were calculated from one-phase decay fitting. Similar bioluminescence profiles over time were observed for all samples, but differences in the decay rates were observed among groups (Figure 8b). Single-decay fit of the normalized total flux yielded half-life values of 3.7±1.9, 2.2±1.5, and 2.8±1.3 days for G1Phy:TPP-, TPP-microgels, and hMSCs-only, respectively. Although no statistical differences were found among groups, these results suggest an improved tendency since the found half-life values for G1Phy:TPP-microgels were relatively higher than those obtained for TPP-microgels and unencapsulated cells, opening the door to further experiments to optimize the output of this pilot study.
Figure 8:
a) Scheme of microfluidic synthesis of ChLA microgels containing hMCSsLuc that were subcutaneously injected in dorsal sites of immunocompromised mice. Bioluminescence signal emitted by hMCSLuc after luciferin injection was monitored by IVIS Spectrum CT; b) In vivo bioluminescence signal represented as normalized total flux (%) over time for TPP-, G1Phy:TPP-microgels, and hMSC-only. Photon flux at each time and sample was normalized to flux value obtained immediately after sample injection; n = 7 per group; mean ± sd.
4. Discussion
Microgels based on natural polymers are promising systems for cell delivery applications because they provide tissue-like structures that can support cell survival and enhance therapeutic activities of implanted cells [2]. Among their multiple advantages, microgels applications stand out due to their minimally invasive method of administration, which avoids surgical manipulations and complications associated with bulk hydrogel implantation (e.g. infection, trauma, or scarring). [2, 47] However, microencapsulation processes must ensure cell survival during fabrication, [2, 48] and the used materials should exhibit excellent crosslinking capacities to maintain their structure and retain alive cells at the site of administration, promoting their survival before delivery. [2] Thus, the preparation of microgels that can provide biological benefits to encapsulate hMSCs regarding viability, regenerative properties and paracrine mechanisms is of special interest for various biomedical applications [2, 49]. Although chitosan microgel preparation has been explored, [9, 10, 17, 24, 50–53] its use as a cell microcarrier has been significantly limited by: (i) its poor solubility at neutral pH; (ii) the use of toxic crosslinking agents, and (iii) the need of harsh purification methods. [21] Here, we developed a strategy for the fabrication of bioactive chitosan microgels by in situ ionotropic gelation in a flow-focusing microfluidic device. ChLA not only provided an adequate environment for cell encapsulation due to its excellent water-soluble properties, but the suitable amount of ionizable groups that can be successfully crosslinked at physiological conditions using phosphate-based agents. The microgels developed in this study incorporate the bioactive G1Phy crosslinker in combination with TPP. Crosslinker composition was adjusted to obtain optimum crosslinking kinetics. G1Phy provided rapid crosslinking kinetics due to its higher content of phosphate groups, whereas the addition of TPP slows down the gelation reaction, contributing to homogeneous microgel formation along the device. Thus, the blend G1Phy and TPP represents a new strategy to obtain stable microgels containing G1Phy that provided the microgel formulation with bioactive properties. Previous studies on the biological properties of G1Phy demonstrated that this compound is effective at concentrations in the order of μg/mL. [33] This strategy permitted the fabrication of ChLA microgels through ionic gelation that involved a one-step purification process by centrifugation without the necessity of using organic solvents. Furthermore, G1Phy incorporation enhanced hMSC survival and cytokine secretion. TPP-chitosan gelation has been previously applied for MSCs encapsulation by Demir et al. [25] In their work, they obtained chitosan microgels by precipitation of polymer droplets in a coagulation bath of TPP. Microcarriers obtained by precipitation-based methods generally show higher average diameter and polydispersity values than those obtained by microfluidic technology, which allowed better control over particle size. [54]
Encapsulated hMSCs with any microgel formulation showed relatively good cell viability after microencapsulation. G1Phy:TPP-microgels exhibited lower initial viability than TPP-microgels. This result could be related to the faster crosslinking kinetics when G1Phy was present. G1Phy exhibits 6 phosphate groups per phytate ring that are susceptible to crosslink polymers containing positive charges providing rapid crosslinking kinetics. In this work, G1Phy showed enhanced interaction ability against ChLA than TPP, giving rise to more robust microgels with lower average particle sizethan those obtained using only TPP as crosslinker. G1Phy:TPP-microgels supported good in vitro cell viability over 10 days of culture. This result may be explained by the stronger interaction between G1Phy and ChLA that contributed to maintain microgel shape and structure, providing a suitable matrix for prolonged cell culture. In this sense, G1Phy has previously shown to improve biological behaviour in terms of cell adhesion and proliferation in comparison to TPP; in particular, we applied G1Phy as an ionic crosslinker for 3D printed scaffolds of other natural polymers, finding out that G1Phy-crosslinked 3D scaffolds show better cellular adhesion and proliferation than when used only TPP. [34] Therefore, G1Phy can play an important role in cell viability, providing an amenable environment for prolonged cell survival.
The therapeutic potential of hMSC secretome on tissue regeneration has gained great interest in recent years [3–5]. Antioxidant compounds, such as G1Phy, are of great interest because injured tissues have elevated levels of reactive oxidative species (ROS). [4] In addition, although microgel injection is a minimally invasive method, it can prompt an adverse environment by the disruption of blood vessels and tissue damage. [2] In this work, G1Phy incorporation modulated important paracrine factors secretion in oxidative and inflammatory environments (Figure 5 and 7, respectively) in comparison to the TPP crosslinker. G1Phy-based microgels enhanced the secretion of vasculogenic factors like VEGF and HGF that could promote angiogenesis processes at similar oxidative conditions to those that can be found in injured tissues. In this sense, studies on its precursor, phytic acid, demonstrated to improve vascularization of endothelial cells by increasing VEGF expression when used as crosslinker. [55] The incorporation of G1Phy also promoted the expression of factors that play diverse immunomodulatory roles such as MCP-1 and M-CSF, which are related to the recruitment and proliferation and survival of monocytes; [5, 56, 57] and IL-8, an angiogenic promoter with a key role in the recruitment and activation of neutrophils. [40, 56] Moreover, TNF-RI (TNF-α family) secretion, was notably elevated in the presence of G1Phy. TNF-α production has previously demonstrated to promote VEGF, IL-8 and IGF1 expression, [56] and has been widely related to angiogenesis enhancement. [58] Finally, FAP, whose expression is closely related to tissue remodelling processes, [59] was also upregulated in the presence of G1Phy. Similar findings were observed after IFN-γ treatment, which is a common in vitro model to simulate the inflammatory environment of injured or diseased tissues [42, 43] and has been shown to activate hMSCs. G1Phy incorporation enhanced the secretion of pro-angiogenic factors such as VEGF, IL-8, and basic FGF. Basic FGF is an anti-inflammatory cytokine that belongs to the family of fibroblast growth factors, which regulates tissue repair signalling cascades. [44] Previous studies have shown polymeric microgel systems containing hMSCs that can actively participate in regeneration processes by modulating paracrine signaling and potentiate therapeutic activity of encapsulated cells. [60–62] Alginate is a biocompatible polymer widely applied for the preparation of cell-laden microgels. [61–63] Mao et al. reported a microfluidic-based method for encapsulating single cells in alginate microgels that maintained cell viability over a three-day period. These systems offered potential advantages in terms of cytokine secretion and enhanced the ability of encapsulated cells to respond to endogenous stimuli like IFN-γ. [62] Osteogenic capacity of encapsulated MSCs in polymeric microgels have been evaluated recently, [60, 61] resulting in attractive cell-laden matrices that can actively participate in regeneration processes through paracrine signalling. Collectively, the results showed in this work make G1Phy:TPP-microgels attractive for applications in the regenerative medicine field. The upregulation of paracrine signalling involved in tissue remodelling and healing by G1Phy supports the use of these microgels for MSC-based therapeutic applications.
Finally, we performed a pilot in vivo study examining hMSC survival and persistence for unencapsulated cells and microgel-encapsulated cells injected subcutaneously in immunocompromised mice. Microgel-encapsulated cells were easily delivered to the subcutaneous space via injection, and the delivery process was uneventful and as facile as injection of unencapsulated cells (hMSC-only). Immediately post-injection, equivalent signal intensities were observed between microgel-encapsulated cells and unencapsulated cells in saline, demonstrating no adverse effects in initial cell survival. For all groups, cell persistence, inferred by bioluminescence signal, decreased exponentially over time with average half-life values of 3 days. In contrast to in vitro observations, no statistically significant differences in cell persistence were observed among groups. Further analyses will be addressed in future studies to improve in vivo cell persistence in our formulations.
5. Conclusions
Herein, we present a microfluidics approach for hMSC encapsulation in bioactive chitosan microgels. ChLA microgels incorporating the bioactive G1Phy combined with TPP offer significant advantages as a hMSC delivery platform, including minimally invasive delivery by injection, cell viability maintenance over time and upregulation of paracrine signalling at adverse conditions (e.g. oxidative stress and inflammation). The as-obtained G1Phy-crosslinked microgels emerge as a suitable and novel cell delivery platform since its therapeutic effect is not only due to support of encapsulated hMSC viability but also modulation of hMSCs secretome. We envision that our G1Phy-crosslinked ChLA microgels will impact on the hMSC therapeutic field.
Funding
The authors thank the Spanish Ministry of Science and Innovation (project, MAT2017-84277-R) and U.S. National Institutes of Health (R01 AR062368) for financial support. B. Vázquez-Lasa and J. San Román are members of the SusPlast platform (Interdisciplinary Platform for Sustainable Plastics towards a Circular Economy) from the Spanish National Research Council (CSIC). Ana Mora-Boza was supported by “La Caixa” Foundation (ID 100010434, scholarship code LCF/BQ/ES16/11570018) and CIBER-BBN (Health Institute Carlos III) Travel grant.
Footnotes
Conflicts of interest
None of the authors have a conflict of interest to declare.
References
- [1].Facklam AL, Volpatti LR, Anderson DG, 32 (2020) 1902005. [DOI] [PubMed] [Google Scholar]
- [2].Choe G, Park J, Park H, Lee JY, Polymers (Basel), 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ascencio González D, Hernández Pando R, Ángel Gómez Lim M, Ayala Fraustro S, Torres Garcia A, Therapeutic Strategies of Secretome of Mesenchymal Stem Cell, Stromal Cells - Structure, Function, and Therapeutic Implications, 2019. [Google Scholar]
- [4].Gnecchi M, Ciuffreda MC, Mura M, Mesenchymal Stromal Cell Secretome for Tissue Repair, Cell Engineering and Regeneration, 2019, pp. 1–26. [Google Scholar]
- [5].Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V, Cells, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Headen DM, García JR, García AJ, Microsystems & Nanoengineering, 4 (2018) 17076. [Google Scholar]
- [7].Muxika A, Etxabide A, Uranga J, Guerrero P, de la Caba K, Int J Biol Macromol, 105 (2017) 1358–1368. [DOI] [PubMed] [Google Scholar]
- [8].Wu Q, Therriault D, Heuzey M-C, ACS Biomaterials Science & Engineering, 4 (2018) 2643–2652. [DOI] [PubMed] [Google Scholar]
- [9].Li K, Wang Y, Miao Z, Xu D, Tang Y, Feng M, Biotechnol Lett, 26 (2004) 879–883. [DOI] [PubMed] [Google Scholar]
- [10].Riederer MS, Requist BD, Payne KA, Way JD, Krebs MD, Carbohydr Polym, 152 (2016) 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Das A, Barker DA, Wang T, Lau CM, Lin Y, Botchwey EA, PLoS One, 9 (2014) e101276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang K, Lin S, Nune KC, Misra RD, J Biomater Sci Polym Ed, 27 (2016) 441–453. [DOI] [PubMed] [Google Scholar]
- [13].Chen C, Liu M, Lii S, Gao C, Chen J, J Biomater Sci Polym Ed, 23 (2012) 2007–2024. [DOI] [PubMed] [Google Scholar]
- [14].Yin R, Wang K, Du S, Chen L, Nie J, Zhang W, Carbohydr Polym, 103 (2014) 369–376. [DOI] [PubMed] [Google Scholar]
- [15].Isiklan N, Tokmak S, Carbohydr Polym, 218 (2019) 112–125. [DOI] [PubMed] [Google Scholar]
- [16].Liu H, Wei Z, Hu M, Deng Y, Tong Z, Wang C, RSC Adv, 4 (2014) 29344–29351. [Google Scholar]
- [17].Hong Y, Gong Y, Gao C, Shen J, J Biomed Mater Res A, 85 (2008) 628–637. [DOI] [PubMed] [Google Scholar]
- [18].Kim S-Y, Lee H, Cho S, Park J-W, Park J, Hwang J, Industrial & Engineering Chemistry Research, 50 (2011) 13762–13770. [Google Scholar]
- [19].Lee H, Jeong C, Ghafoor K, Cho S, Park J, Biomed Mater Eng, 21 (2011) 25–36. [DOI] [PubMed] [Google Scholar]
- [20].Yang CH, Huang KS, Chang JY, Biomed Microdevices, 9 (2007) 253–259. [DOI] [PubMed] [Google Scholar]
- [21].Chen X-G, Liu C-S, Liu C-G, Meng X-H, Lee CM, Park H-J, Biochemical Engineering Journal, 27 (2006) 269–274. [Google Scholar]
- [22].Li B, Wang L, Xu F, Gang X, Demirci U, Wei D, Li Y, Feng Y, Jia D, Zhou Y, Acta Biomater, 22 (2015) 59–69. [DOI] [PubMed] [Google Scholar]
- [23].Zou Q, Li J, Li Y, Int J Biol Macromol, 79 (2015) 736–747. [DOI] [PubMed] [Google Scholar]
- [24].Lu Z, Zhou Y, Liu B, J Biosci Bioeng, 128 (2019) 504–509. [DOI] [PubMed] [Google Scholar]
- [25].Koc Demir A, Elcin AE, Elcin YM, Cytotechnology, 70 (2018) 1375–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jang Y, Cha C, Jung J, Oh J, Macromolecular Research, 26 (2018) 1143–1149. [Google Scholar]
- [27].Daley EL, Coleman RM, Stegemann JP, J Mater Chem B, 3 (2015) 7920–7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wise JK, Alford AI, Goldstein SA, Stegemann JP, Connect Tissue Res, 57 (2016) 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Headen DM, Aubry G, Lu H, Garcia AJ, Adv Mater, 26 (2014) 3003–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Phelps EA, Enemchukwu NO, Fiore VF, Sy JC, Murthy N, Sulchek TA, Barker TH, Garcia AJ, Adv Mater, 24 (2012) 64–70, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yang CH, Lin YS, Huang KS, Huang YC, Wang EC, Jhong JY, Kuo CY, Lab Chip, 9 (2009) 145–150. [DOI] [PubMed] [Google Scholar]
- [32].Zamora-Mora V, Velasco D, Hernández R, Mijangos C, Optofluidics, Microfluidics and Nanofluidics, 1 (2014). [Google Scholar]
- [33].Mora-Boza A, López-Donaire ML, Saldaña L, Vilaboa N, Vázquez-Lasa B, San Román J, Scientific Reports, 9 (2019) 11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mora-Boza A, Włodarczyk-Biegun MK, del Campo A, Vázquez-Lasa B, Román JS, Biomaterials Science, 8 (2020) 506–516. [DOI] [PubMed] [Google Scholar]
- [35].Hennink WE, van Nostrum CF, Advanced drug delivery reviews, 54 (2002) 13–36. [DOI] [PubMed] [Google Scholar]
- [36].Qu X, Wirsén A, Albertsson A-C, Journal of Applied Polymer Science, 74 (1999) 3193–3202. [Google Scholar]
- [37].Clark AY, Martin KE, Garcia JR, Johnson CT, Theriault HS, Han WM, Zhou DW, Botchwey EA, Garcia AJ, Nat Commun, 11 (2020) 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fuhrman B, Oiknine J, Aviram M, Atherosclerosis, 111 (1994) 65–78. [DOI] [PubMed] [Google Scholar]
- [39].Cai Y, Lapitsky Y, J Colloid Interface Sci, 494 (2017) 242–254. [DOI] [PubMed] [Google Scholar]
- [40].Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, Klunker S, Meyer N, O’Mahony L, Palomares O, Rhyner C, Ouaked N, Schaffartzik A, Van De Veen W, Zeller S, Zimmermann M, Akdis CA, The Journal of allergy and clinical immunology, 127 (2011) 701–721.e701–770. [DOI] [PubMed] [Google Scholar]
- [41].Yan L, Zheng D, Xu RH, Front Immunol, 9 (2018) 1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Goedhart M, Cornelissen AS, Kuijk C, Geerman S, Kleijer M, van Buul JD, Huveneers S, Raaijmakers M, Young HA, Wolkers MC, Voermans C, Nolte MA, Stem Cells Dev, 27 (2018) 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Petinati NA, Kapranov NM, Bigil’deev AE, Popova MD, Davydova YO, Gal’tseva IV, Drize NI, Kuz’mina LA, Parovichnikova EN, Savchenko VG, Bulletin of experimental biology and medicine, 163 (2017) 230–234. [DOI] [PubMed] [Google Scholar]
- [44].Maddaluno L, Urwyler C, Werner S, Development, 144 (2017) 4047–4060. [DOI] [PubMed] [Google Scholar]
- [45].Carmeliet P, Jain RK, Nature, 473 (2011) 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhao Q, Kim T, Pang J, Sun W, Yang X, Wang J, Song Y, Zhang H, Sun H, Rangan V, Deshpande S, Tang H, Cvijic ME, Westhouse R, Olah T, Xie J, Struthers M, Salter-Cid L, Journal of Leukocyte Biology, 102 (2017) 1271–1280. [DOI] [PubMed] [Google Scholar]
- [47].Foster GA, Headen DM, Gonzalez-Garcia C, Salmeron-Sanchez M, Shirwan H, Garcia AJ, Biomaterials, 113 (2017) 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yeung TW, Ucok EF, Tiani KA, McClements DJ, Sela DA, Front Microbiol, 7 (2016) 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Headen DM, Aubry G, Lu H, García AJ, Adv Mater, 26 (2014) 3003–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xia P, Zhang K, Gong Y, Li G, Yan S, Yin J, ACS Appl Mater Interfaces, 9 (2017) 34751–34761. [DOI] [PubMed] [Google Scholar]
- [51].Zhang L, Pan J, Li J, Wu W, Yu Y, Artif Cells Blood Substit Immobil Biotechnol, 31 (2003) 293–301. [DOI] [PubMed] [Google Scholar]
- [52].Ye L, Ding S, Cui YL, Wang QS, Zhang Y, Advanced Materials Research, 282–283 (2011) 133–137. [Google Scholar]
- [53].Chui CY, Odeleye A, Nguyen L, Kasoju N, Soliman E, Ye H, J Biomed Mater Res A, 107 (2019) 122–133. [DOI] [PubMed] [Google Scholar]
- [54].Headen DM, García JR, García AJ, Microsystems & Nanoengineering, 4 (2018). [Google Scholar]
- [55].Wang X, Wen K, Yang X, Li L, Yu X, Journal of Materials Chemistry B, 5 (2017) 8115–8124. [DOI] [PubMed] [Google Scholar]
- [56].de Witte SFH, Franquesa M, Baan CC, Hoogduijn MJ, Frontiers in Immunology, 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wu AC, Morrison NA, Kelly WL, Forwood MR, Calcified tissue international, 92 (2013) 566–575. [DOI] [PubMed] [Google Scholar]
- [58].Lu ZY, Chen WC, Li YH, Li L, Zhang H, Pang Y, Xiao ZF, Xiao HW, Xiao Y, Mol Med Rep, 14 (2016) 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Chung K-M, Hsu S-C, Chu Y-R, Lin M-Y, Jiaang W-T, Chen R-H, Chen X, PloS one, 9 (2014) e88772–e88772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhao X, Liu S, Yildirimer L, Zhao H, Ding R, Wang H, Cui W, Weitz D, 26 (2016) 2809–2819. [Google Scholar]
- [61].An C, Liu W, Zhang Y, Pang B, Liu H, Zhang Y, Zhang H, Zhang L, Liao H, Ren C, Wang H, Acta Biomater, 111 (2020) 181–196. [DOI] [PubMed] [Google Scholar]
- [62].Mao AS, Shin J-W, Utech S, Wang H, Uzun O, Li W, Cooper M, Hu Y, Zhang L, Weitz DA, Mooney DJ, Nature Materials, 16 (2017) 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jin J, Ji Z, Xu M, Liu C, Ye X, Zhang W, Li S, Wang D, Zhang W, Chen J, Ye F, Lv Z, ACS Biomaterials Science & Engineering, 4 (2018) 2541–2551. [DOI] [PubMed] [Google Scholar]