Abstract
Background
Social anxiety disorder (SAD) is a serious psychiatric condition with a high prevalence, and a typical onset during childhood/adolescence. The condition runs in families, but it is largely unknown which neurobiological characteristics transfer this genetic vulnerability (‘endophenotypes’). Using data from the Leiden Family Lab study on SAD, including two generations of families genetically enriched for SAD, we investigated whether social anxiety (SA) co-segregated with changes in intrinsic functional connectivity (iFC), and examined heritability.
Methods
Functional MRI data were acquired during resting-state in 109 individuals (56 males; mean age: 31·5, range 9·2-61·5 years). FSL's tool MELODIC was used to perform independent component analysis. Six networks of interest (default mode, dorsal attention, executive control, frontoparietal, limbic and salience) were identified at the group-level and used to generate subject-specific spatial maps. Voxel-wise regression models, with SA-level as predictor and voxel-wise iFC as candidate endophenotypes, were performed to investigate the association with SA, within masks of the networks of interest. Subsequently, heritability was estimated.
Findings
SA co-segregated with iFC within the dorsal attention network (positive association in left middle frontal gyrus and right postcentral gyrus) and frontoparietal network (positive association within left middle temporal gyrus) (cluster-forming-threshold z>2·3, cluster-corrected extent-threshold p<0·05). Furthermore, iFC of multiple voxels within these clusters was at least moderately heritable.
Interpretation
These findings provide initial evidence for increased iFC as candidate endophenotype of SAD, particularly within networks involved in attention. These changes might underlie attentional biases commonly present in SAD.
Funding
Leiden University Research Profile ‘Health, Prevention and the Human Lifecycle’.
Keywords: Social anxiety disorder, Family study, Functional brain connectivity, Resting state, Magnetic resonance imaging
Research in context.
Evidence before this study
Social anxiety disorder (SAD) is a serious psychiatric condition with a high prevalence, and a typical onset during childhood or adolescence. The condition runs in families, but it is largely unknown which neurobiological characteristics transfer this genetic vulnerability. Studying endophenotypes, i.e., heritable characteristics on the pathway from genotype to phenotype, could inform us about the genetic susceptibility to develop the disorder. Previous work indicated changes in functional brain connectivity related to SAD, for example in patients with the disorder as well as in children at risk for developing SAD. Specifically, alterations were present in brain networks involved in attention and emotion processing, such as the default mode network, dorsal attention network, executive control network, frontoparietal network, limbic network and the salience network. It is, however, unknown whether these alterations qualify as SAD endophenotypes.
Added value of this study
Using data from the unique Leiden Family Lab study on Social Anxiety Disorder, in which patients with SAD as well as their family members of two generations were included, we were able to examine two endophenotype criteria, within six pre-defined brain networks of interest. First, we investigated the co-segregation of social anxiety with changes in intrinsic functional connectivity (iFC) within families genetically enriched for the disorder; second, we estimated the heritability of iFC. Our findings indicate, for the first time, that alterations in iFC in the dorsal attention network and the frontoparietal network meet both endophenotype criteria, making them promising candidate endophenotypes.
Implication of all available evidence
These results provide initial evidence that increased iFC within the dorsal attention network and frontoparietal network are genetically linked with social anxiety. This way, the findings provide new insight in the genetic susceptibility to develop SAD.
Alt-text: Unlabelled box
1. Introduction
Social anxiety disorder (SAD) is a prevalent anxiety disorder [1], [2], [3], with serious and often life-long consequences for patients, their families and society [4], [5], [6]. Patients with SAD fear a negative evaluation by others and avoid social situations as much as possible. With its typical onset during childhood and early adolescence [7], [8], [9], followed by a chronic course [10], suboptimal treatment [11,12] and high rates of comorbid psychopathology [13], [14], [15], the disorder is very incapacitating. This stresses the need for insight in the neurobiology underlying the development of SAD, in order to advance preventive interventions [16,17].
In the present work, we focus on the innate vulnerability to develop SAD, as previous research demonstrated that SAD runs in families: several studies involving families or twins indicated that being ‘genetically close’ to a patient with SAD leads to an enhanced risk to develop the disorder [18], [19], [20], and heritability estimates of SAD around 50 % have been reported [21]. Little is known, however, about the neurobiological variations underlying the genetic risk to SAD. A promising method to investigate the innate neurobiological susceptibility to SAD is the endophenotype approach, as endophenotypes are heritable, measurable characteristics on the pathway from genotype to phenotype [22], [23], [24], [25]. Endophenotypes should be associated with the disorder of interest (criterion 1) and are supposed to be stable, state-independent traits, already present in a preclinical state (criterion 2). Furthermore, an endophenotype should be heritable (criterion 3), and an endophenotype typically co-segregates with the disorder within a family, with nonaffected family members showing altered levels of the endophenotype when compared to the general population (criterion 4) – in other words: ‘the endophenotype is more prevalent among the ill relatives of ill probands compared with the well relatives of the ill probands’ [26], which can be examined by exploring associations between symptoms and the hypothesized endophenotype within families. As recently discussed [27], the application of the endophenotype-approach in psychiatry takes into account the notion that genes are ‘the biological bedrock of mental illness’, and as such they provide an important starting point to delineate the often complex pathophysiology of psychiatric disorders. This is of particular importance in SAD, given the high prevalence of the disorder already in adolescence [28] and the struggle to treat SAD effectively [29].
The Leiden Family Lab study on Social Anxiety Disorder (LFLSAD), which involved participants of two generations from families genetically enriched for SAD, was especially designed to examine neurobiological SAD endophenotypes [30]. In previous work on this sample, we reported on several promising SAD endophenotypes [31], involving characteristics of brain structure [32] and brain function [33], [34], [35], as neuroimaging data from the LFLSAD supported the endophenotype criterion of co-segregation of the endophenotype with social anxiety within the families, and provided evidence for heritability.
In addition to these structural and functional brain characteristics, the question whether connectivity of the socially-anxious brain meets the criteria for being a candidate endophenotype warrants attention. Brain connectivity can be determined by outlining the density of white matter tracts between brain regions using diffusion tensor imaging (DTI; structural connectivity), or by detecting correlations in brain activation patterns across regions, using functional Magnetic Resonance Imaging (fMRI; functional connectivity) [36,37]. Within the LFLSAD, data to establish both types of connectivity were collected; while the present work focuses on functional connectivity [38], results of endophenotype analyses on structural connectivity are reported elsewhere [39,40]. Investigating connectivity is important, as brain regions do not function in isolation, but are tightly connected and part of large-scale networks; moreover, changes in connectivity could play a role in the development, expression and course of psychopathology [41], [42], [43], [44], [45], [46]. Notably, genetic influences on brain connectivity are repeatedly established [47,48] and microscale alterations, for example in gene expression, are thought to underlie macroscale networks [49]. Moreover, multiple studies have indicated that functional brain networks have unique characteristics for each individual, which are stable over months to years [50,51], and that temperamental traits can be predicted based on functional connectivity networks [52]. These findings were supported by a recent paper which used vector machine classifiers to demonstrate the stability and similarity of functional brain networks within pediatric and adult samples; this paper also pointed out that the genetic influences on functional brain connectivity are present already early in life [53]. Other studies have investigated the relationship between connectivity and trait anxiety; for example, Takagi et al. reported on a functional network underlying state, trait, and pathological anxiety [54]. Furthermore, a recent paper used connectome-based predictive modelling and showed that trait anxiety could be reliably predicted based on whole-brain functional connectivity, especially connectivity in limbic and prefrontal networks. These results were replicated in independent datasets adding to its validity [55]. Taken together, these observations provide initial support for the endophenotype criteria of heritability and trait-stability over time.
In addition, several studies revealed associations between functional brain networks and SAD (endophenotype criterion 1): SAD-related alterations in functional connectivity have been reported in multiple networks, including the default mode network, the dorsal attention network, executive control network, frontoparietal network, limbic network and salience network [56], [57], [58], [59], [60], [61], [62], [63], [64], [65]. Furthermore, changes in functional connectivity have been reported in children at risk for developing SAD [66]. In addition, a meta-analysis on > 800 individuals with different levels of anxiety or anxiety disorders revealed hypo-connectivity between the executive control network and the limbic network; furthermore, hypo-connectivity within the salience network was associated with anxiety and anxiety disorders [67]; see also multiple reviews summarizing findings in anxiety disorders [45,68,69].
To the best of our knowledge, no study to date has explored functional brain connectivity as a candidate endophenotype of SAD, although the evidence summarized here and elsewhere [31] suggests that indices of functional connectivity have good potential to qualify as candidate endophenotypes. Given the heritable background of SAD, investigating whether measurements of functional connectivity qualify as endophenotypes could provide important additional knowledge to improve prevention and intervention for children and adolescents who are vulnerable to developing SAD due to their genetic make-up [70].
Here, we investigated intrinsic functional connectivity (iFC) within six networks of interest, being the default mode [56,57,71], dorsal attention [71], executive control [61,66,71], frontoparietal [71], limbic [60] and salience network [56,60,66], based on the findings reported in previous work. We explored whether iFC within these networks co-segregated with social anxiety within the families; next, we estimated heritability. We hypothesized, based on previous work as summarized above, that characteristics of these networks would meet these two criteria for endophenotypes.
2. Methods
2.1. Participants
Data originated from the Leiden Family Lab study on Social Anxiety Disorder (LFLSAD), a multiplex, multigenerational study in which families genetically enriched for SAD are included [30].
Families were recruited through media exposure, like interviews in Dutch newspapers, on television and radio; furthermore, the study was brought to the attention of patient organizations, to clinical psychologists, general practitioners and mental health care organizations. Recruitment was targeted at families in which multiple family members experienced ‘extreme shyness’ and took place between Summer 2013 and Summer 2015. Families were invited for participation when a parent (aged 25 - 55 years old; ‘proband’) had a primary diagnosis of SAD; secondly, a child within this nuclear family was supposed to suffer from clinical or subclinical SAD (‘proband's SA-child’; age 8 – 21 years). The proband's SA-child should live at home with the proband; comorbidity other than internalizing disorders or substance abuse was an exclusion criterion for the proband and proband's SA-child. In addition to these two SAD-cases, first- and second-degree family members of two generations were invited to participate, being the proband's partner and other children of the nuclear family (age 8 years), as well as the proband's sibling(s), with their partners and children (age 8 years). These family members were included independent from the presence of psychopathology. Insufficient comprehension of the Dutch language was an exclusion criterion for all participants, and general MRI contraindications led to exclusion of the MRI experiment.
2.2. Screening and inclusion of families
The inclusion of families consisted of several steps and has been described previously in a dedicated design paper [30]. First, potential probands were screened for eligibility by a telephone call or an email, depending on their preference. This screening consisted of questions with respect to the presence of social anxiety in the proband and the proband's SA‐child, the age of the proband and his or her child(ren), and the potential number of family members that could be invited for the study. In addition, probands were further informed about the study. When they passed the screening and showed interest in participation, an information letter was sent to the proband and his or her nuclear family members, containing detailed information about the study. Two weeks later, participants were contacted by telephone and any questions about the study were answered. Next, the proband, the proband's spouse, and the proband's SA‐child were invited to come to the Leiden University Medical Center for an introductory meeting and structured clinical interview by an experienced clinician, in order to confirm the presence of a primary diagnosis of SAD (proband) and (sub)clinical social anxiety (proband's SA‐child). Furthermore, a screening was performed to exclude the presence of autism in the proband and the proband's SA‐child, and developmental disorders like attention deficit hyperactivity disorder. When the inclusion criteria were met, the proband and his or her nuclear family were included in the study. In addition, we asked the proband to contact his or her sibling(s), in order to confirm that they were interested to be informed about participation in the study. Given a positive response, these siblings, together with their partner and/or children, were invited to participate by the investigators. Given the inherent characteristic of socially anxious people to avoid new situations and their tendency to stay out of the spotlights, we encouraged participants to visit the lab together with their family members, in order to make them feel more comfortable. Although we emphasized the importance of including as many family members as possible within the study, we also indicated that each individual was free to decide whether or not to participate.
In line with this procedure, the LFLSAD sample (total: n = 132, nine families; MRI sample: n = 110, eight families) consists of family members of two generations (Fig. 1). Participants completed a number of measurements, such as a diagnostic interview, self-report questionnaires and an MRI scan [30].
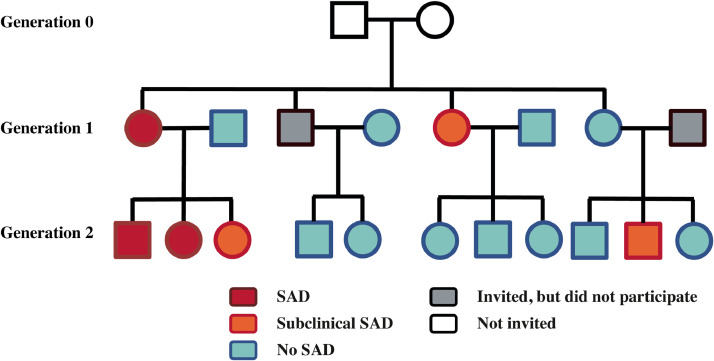
Fig. 1.
Family composition within the LFLSAD.
Families were included based on the combination of a parent with SAD (‘proband’; depicted in red) and a proband's child with SAD (red) or subclinical SAD (orange). In addition, family members of two generations were invited, independent from the presence of SAD within these family members (no SAD: light blue; did not participate: grey). Grandparents (generation 0; white) were not invited for participation. This family is slightly modified to guarantee anonymity; however, the number of family members and the frequency of (sub)clinical SAD are depicted truthfully. Squares and circles represent men and women, respectively. Reprint of the figure published in [30,35]. SAD: social anxiety disorder.
2.3. Ethics and sample-size estimation
The study was approved by the Medical Ethical Committee of the Leiden University Medical Center and all participants provided informed consent according to the Declaration of Helsinki: both parents signed the informed consent form for their children, and children between 12 and 18 years of age signed the form themselves as well. Participants received a financial compensation. Confidentiality of the data was maintained by the use of a unique research ID number for each family member.
Detailed information about the LFLSAD and an a priori power-calculation for the study are outlined in [30]; furthermore, the study was preregistered online [72,73] (osf.io/E368H and osf.io/AQ3SV).
2.4. Phenotyping
In order to facilitate extensive phenotyping, the LFLSAD protocol consisted of several measurements [30] . The following assessments are relevant for the present work.
Experienced clinicians determined the presence of DSM-IV diagnoses using the Mini-International Neuropsychiatric Interview (M.I.N.I.)-Plus (version 5·0·0) [74,75] or the M.I.N.I.-Kid interview
(version 6·0) [76,77]. Given the nature of the LFLSAD sample, special attention was paid to the presence of (sub)clinical SAD. Clinical SAD was established using the DSM-IV-TR criteria for the generalized subtype of SAD, but the clinician verified whether the DSM-5 criteria for SAD were also met. A diagnosis of subclinical SAD was established when participants met the criteria for SAD as described in the DSM-5, but did not show impairing limitations in important areas of functioning (criterion G) [78]. The interviews were recorded to enable a considerate evaluation of psychopathology.
Furthermore, participants completed age-appropriate questionnaires on the level of social anxiety (SA) symptoms, being the Liebowitz Social Anxiety Scale for adults (LSAS) [79] or the Social Anxiety Scale for adolescents (SAS-A) [80], as well as on the level of depressive symptoms (Beck Depression Inventory (BDI) [81] or the Children's Depression Inventory (CDI) [82]). After verifying that values were indeed missing completely at random [83] by carefully inspecting which specific items lacked an answer (for example, whether particular participants failed to answer both the ‘fear’ and ‘avoidance’ question with respect to a social situation described in the LSAS, or whether multiple participants did not reply to one particular item of the questionnaire, which was not the case), incidental missing values were replaced by the average value of the completed items (LSAS: 3 participants with missing values, missing on average over the whole sample: 0·11 % of the items; SAS-A: 1 participant with missing value, missing on average over the whole sample: 0·16 %; BDI: no missing values; CDI: 1 participant with missing values, missing on average over the whole sample: 0·26 %). We used this approach as only a few participants had missing values and for these participants, the number of missing values was limited, making other approaches to handle missing data (for example, multiple imputation or the full information maximum likelihood method) less suitable [83] (i.e., only 29 participants completed the SAS-A, which contains 22 items, and 30 participants filled out the CDI (26 items)). To enable interpreting the scores of the age-specific questionnaires over the whole sample, z-scores were computed [30].
In order to obtain a comprehensive characterization of the sample (cf. [30]), participants completed multiple questionnaires on anxiety-related constructs. The intensity of fear of negative evaluation was assessed using the revised Brief Fear of Negative Evaluation (BFNE) – II scale [84,85]. The State-Trait Anxiety Inventory (STAI) [86] (see [87] for psychometric properties) was used to determine self-reported trait anxiety, as well as state anxiety before and after the MRI scan. The sensitivity for the temperamental traits ‘behavioral inhibition’ and ‘behavioral activation’ was assessed using the self-report BIS/BAS [88,89] or the BIS/BAS scales for children (BIS/BAS-C) [90].
Furthermore, two subscales of the Wechsler Adult Intelligence Scale-IV (WAIS-IV) [91] or Wechsler Intelligence Scale for Children-III (WISC) [92], the similarities (verbal comprehension) and block design (perceptual reasoning) subtests, were administered to obtain an estimate of cognitive functioning.
2.5. Acquisition MRI data
Scanning was performed using a 3·0 T Philips Achieva MRI scanner (Philips Medical Systems, Best, The Netherlands), equipped with a 32-channel Sensitivity Encoding head coil. Prior to the MRI scan, participants were informed about the safety procedures and they were told that they could refrain from continuing the experiment at any time. Children and adolescents were familiarized with the MRI scanner using a mock scanner [93], and all participants received instructions about the task paradigms presented during the scan session [32], [33], [34], [35].The MRI experiment consisted of several structural scans [32,39] and functional task paradigms [[33], [34], [35],94], and the total duration of the MRI scan protocol was 54 min 47 s.
Participants were instructed to keep their eyes closed and to stay awake during the resting-state scan; meanwhile, functional (f)MRI scans were acquired using T2*-weighted echo-planar imaging (EPI). These scans had the following characteristics: 200 volumes, 38 axial slices, 2·75 mm x 2·75 mm x 2·75 mm + 10 % interslice gap, field of view (FOV) = 220 mm x 115 mm x 220 mm, repetition time (TR) = 2200 ms, echo time (TE) = 30 ms. The first six volumes of each fMRI scan were dummy volumes; these volumes were removed to allow for equilibration of T1 saturation effects.
In addition, a high-resolution EPI scan (84 axial slices, 1·964 mm x 1·964 mm x 2 mm, FOV = 220 mm x 168 mm x 220 mm, TR = 2200 ms, TE = 30 ms) and a high-resolution T1-weighted scan (140 slices, resolution 0·875 mm × 0·875 mm × 1·2 mm, FOV = 224 mm × 168 mm × 177·333 mm, TR = 9·8 ms, TE = 4·59 ms, flip angle = 8◦) were acquired and used for within-subject registration purposes. Furthermore, the structural T1-scans were inspected by a neuroradiologist, but no clinically relevant abnormalities were present in any of the participants.
2.5.1. Statistics: characteristics of the participants
Scripts and data supporting this work are available at osf.io/q4hsr.
Participants with and without (sub)clinical SAD (predictor) were compared on demographic variables and on the level of self-reported symptoms by performing chi-square tests in SPSS (v25; dependent variables: gender distribution; distribution of (sub)clinical SAD cases over the two generations; clinical diagnoses) and by fitting multi-level regression models in R (dependent variables: age; estimated IQ; social anxiety symptoms; level of fear of negative evaluation; level of depressive symptoms; level of trait anxiety; level of behavioral inhibition; level of behavioral activation) [RRID: SCR_003005] [95]. Within these multi-level regression models, we modelled genetic correlations between family members by including random effects; a kinship matrix was built using the lmekin function within the coxme package.
2.5.2. Resting-state data
2.5.2.1. General processing steps
FMRI data were denoised using FIX (FMRIB's ICA-based X-noiseifier), a publicly available plugin for FSL (FMRIB Software Library, version 5·0·9) [96], which provides an automatic solution for denoising fMRI data via accurate classification of ICA components [97,98]. This step removed a scanner-related artefact from the data, as well as signals from nuisance variables like cerebrospinal fluid and white matter. Next, data underwent several preprocessing steps using FEAT (FMRI Expert Analysis Tool; v6·00) [96,99], including motion correction using MCFLIRT [100], spatial smoothing using a Gaussian kernel of full-width half-maximum (FWHM) 6·0 mm and grand-mean intensity normalization of the entire 4D dataset by a single scaling factor in order to enable higher-level analyses and registration. Scans were first registered to high-resolution EPI images, which were registered to T1 images, which in turn were registered to the Montreal Neurological Institute (MNI) T1-template brain (resolution 2 mm) using FNIRT nonlinear registration (warp resolution 10 mm) [100], [101], [102]. Next, ICA-AROMA (ICA-based Automatic Removal Of Motion Artifacts) was used to remove motion-related artefacts [103,104]. Data were then submitted to FEAT to perform non-brain removal using BET [105], high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, cutoff 0·01 Hz), and registration. Resting-state fMRI data of each participant were registered to their individual 3D T1-weighted anatomical scan using FLIRT [100,101] and subsequently to the MNI T1-template brain. We checked whether the individual scans were registered correctly and confirmed that relative motion parameters did not exceed 2·5 mm.
2.5.2.2. Extraction of functional networks: group level
Group-level resting-state networks were determined using Probabilistic Independent Component Analysis [106] as implemented in MELODIC (Multivariate Exploratory Linear Decomposition into Independent Components; v3·15), part of FSL. This method separates 4D functional data into spatial maps, each with an associated time-course.
First, data were pre-processed, by masking of non-brain voxels, voxel-wise de-meaning of the data, and normalization of the voxel-wise variance. Next, the pre-processed data were concatenated in time and decomposed into sets of independent vectors describing signal variation across the temporal (time-courses) and spatial domain (maps) by optimizing for non-Gaussian spatial source distributions using a fixed-point iteration technique [107]. Estimated component maps were divided by the standard deviation of the residual noise and thresholded by fitting a mixture model to the histogram of intensity values [106]. We chose to decompose the data into 20 spatial maps, in line with previous work in a large dataset, showing that this threshold results in a representative set of functional connectivity networks, with a good balance between clustering and splitting of networks [108].
2.5.2.3. Extraction of functional networks: individual level
Dual regression was used to generate individual-specific versions of the 20 group-level spatial maps, and the associated time-courses [109]. For each subject, the group-average set of spatial maps was regressed (as spatial regressors in a multiple regression) into the individual's 4D space-time dataset. This results in a set of subject-specific timeseries, one per group-level spatial map. Those timeseries were regressed (as temporal regressors, again in a multiple regression) into the same 4D dataset, resulting in a set of 20 subject-specific spatial maps, one for each of the group-level spatial maps.
2.5.2.4. Identification of networks
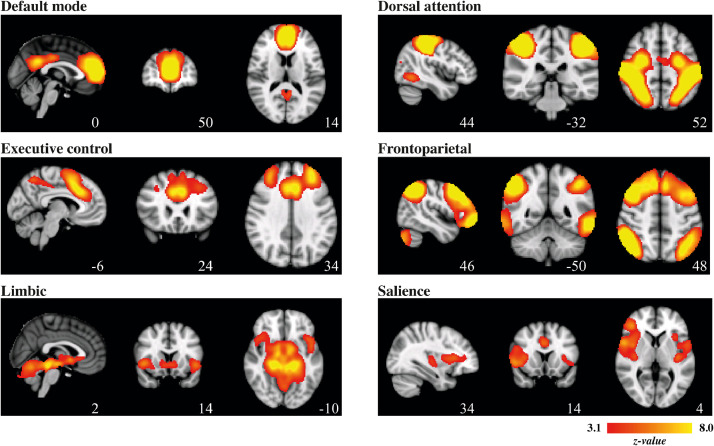
The 20 spatial maps at group-level were visually inspected, and based on descriptions of functional networks in previous work [108,110], we selected components with spatial similarity to the six functional networks of interest: the default mode, dorsal attention, executive control, frontoparietal (separated in a left and right-sided spatial map), limbic and salience network. The dorsal attention, executive control, and frontoparietal network were, given the often inconsistent naming conventions of these networks in the current literature, validated using Fig. 1 of the recent work by Witt and colleagues [111], and we checked that these networks were topographically separable. Next, we created binary masks of these networks by using the FSL-tool easythresh (cluster-forming threshold z > 3·1, cluster-corrected extent threshold p < 0·05) [112]. The networks of interest are illustrated in Fig. 2 and their topographical properties are summarized in Table 1.
Fig. 2.
Functional connectivity networks of interest (group-level).
Networks are superimposed on the template MNI_T1_152_2mm_brain. Images are displayed according to radiological convention: right in image is left in the brain.
Table 1.
Characteristics of functional connectivity networks (group-level).
| Peak coordinates (MNI space) |
|||||||
|---|---|---|---|---|---|---|---|
| Network | Clusters | Z-score | x | y | z | Cluster size | Regions within cluster |
| Default mode | 1 | 11·3 | 0 | 50 | 14 | 10389 | Paracingulate gyrus, frontal pole |
| 2 | 8·1 | -4 | 48 | 28 | 2503 | Posterior cingulate gyrus, precuneus | |
| Dorsal attention | 1 | 19·7 | 44 | -32 | 52 | 10870 | Post- and precentral gyrus, superior parietal lobule, middle / superior frontal gyrus, lateral occipital cortex (right) |
| 2 | 16·2 | -42 | -36 | 50 | 9783 | Post- and precentral gyrus, superior parietal lobule, middle / superior frontal gyrus, lateral occipital cortex (left) | |
| 3 | 7·4 | 52 | -60 | -8 | 1252 | Lateral occipital cortex, middle temporal gyrus (right) | |
| 4 | 6·4 | -48 | -66 | -4 | 786 | Lateral occipital cortex, middle temporal gyrus (left) | |
| Executive control | 1 | 8·8 | 10 | 8 | 60 | 15950 | Supplementary motor cortex, superior frontal gyrus, middle frontal gyrus |
| 2 | 5·1 | -8 | -60 | 54 | 1394 | Precuneus | |
| 3 | 6·3 | 44 | -58 | -32 | 1311 | Cerebellum (right) | |
| 4 | 6·0 | -38 | -58 | -30 | 820 | Cerebellum (left) | |
| Frontoparietal - left | 1 | 9·7 | -46 | 10 | 36 | 12493 | Middle frontal gyrus, frontal pole |
| 2 | 10·1 | 36 | -76 | -46 | 3588 | Cerebellum | |
| 3 | 9·2 | -32 | -70 | 44 | 3492 | Lateral occipital cortex, angular gyrus | |
| 4 | 9·4 | -58 | -50 | -12 | 3143 | Inferior / middle temporal gyrus | |
| Frontoparietal - right | 1 | 12·2 | 44 | 16 | 44 | 16425 | Middle frontal gyrus, frontal pole |
| 2 | 14·4 | 46 | -50 | 48 | 4335 | Angular gyrus, lateral occipital cortex | |
| 3 | 11·4 | -12 | -82 | -28 | 3910 | Cerebellum | |
| 4 | 9·6 | 66 | -28 | -10 | 3190 | Inferior / middle temporal gyrus | |
| Limbic | 1 | 9·7 | -14 | -26 | -14 | 15136 | Parahippocampal gyrus, bilateral amygdala, bilateral hippocampus |
| 2 | 5·4 | -46 | 14 | -6 | 1405 | Frontal operculum cortex, insula | |
| Salience | 1 | 7·0 | 60 | -28 | 32 | 7187 | Supramarginal gyrus, parietal operculum cortex (right) |
| 2 | 6·4 | -62 | -26 | 22 | 4057 | Supramarginal gyrus, parietal operculum cortex (left) | |
| 3 | 5·7 | 10 | -34 | 46 | 3614 | Posterior cingulate cortex | |
Cluster-forming threshold z > 3·1, cluster-corrected extent threshold p < 0·05.
2.5.2.5. Statistics: neurobiological candidate endophenotypes within networks
The subject-specific spatial maps, belonging to the networks of interest, were used to examine whether characteristics of iFC could serve as candidate endophenotypes of SAD. We investigated the ‘co-segregation of the candidate endophenotype with the disorder within families’ using multi-level regression models in R [RRID: SCR_003005] [95], with self-reported SA-level (z-score; centered) as independent variable and individual voxel-wise iFC within the specified networks as dependent variables. Correlations between family members were modelled by including random effects (lmekin function within the coxme package of R); age and gender (both centered) were included as covariates of no interest (cf. [33], [34], [35]). The regression models can be found online at osf.io/q4hsr.
Within each network of interest, the analysis was run for each voxel separately and results (z-scores) were transformed into a nifti-image with the dimensions of the MNI T1-template brain. Results were corrected for multiple comparisons using the FSL-tool easythresh (cluster-forming threshold z > 2·3, cluster-corrected extent threshold p < 0·05, minimum of 10 voxels) [112], within the binary masks of the networks of interest.
Next, we determined the heritability of iFC for voxels in the significant clusters: voxelwise heritability estimates were obtained using the statistical model developed by Tissier et al. (2017). This method uses a multivariate mixed probit model in which the ascertainment of the families (based on SAD in the proband and (sub)clinical SAD in the proband's SA-child) and the familial relationship are taken into account by jointly modelling SAD status in these participants and brain activation. To adjust for age and gender, these variables were included as covariates (both centered) in the marginal regression models. Variance of the random effects was determined using maximum likelihood estimates; subsequently, heritability was computed [113].
Significant findings within networks were followed by analyses with (sub)clinical SAD as a discrete predictor. Furthermore, sensitivity analyses were performed to investigate whether the results of the association analyses were driven by the severity of depressive symptoms as measured by the BDI-II or the CDI, or by (comorbid) psychopathology other than SAD (cf. [32,34]). To this aim, we added the z-score of the level of depressive symptoms as a covariate in the voxelwise analyses (sensitivity analysis 1) or excluded all family members with past and/or present psychopathology other than SAD and repeated the association analyses (sensitivity analysis 2). Note however that this latter analysis may yield biased and weaker results, as the majority of the probands, on which the selection of the families was based, had comorbid psychopathology and were thus excluded. We used the same statistical threshold as for the main analyses (cluster-forming threshold z > 2·3, cluster-corrected extent threshold p < 0·05).
3. Role of the funding source
The LFLSAD and Janna Marie Bas-Hoogendam were funded by Leiden University Research Profile ‘Health, Prevention and the Human Life Cycle’. This funding source had no involvement in writing this paper nor in the decision to submit this work for publication.
4. Results
4.1. Data availability and quality checking
The LFLSAD sample consisted of 132 family members, and we collected MRI data from 113 participants (nine families) [30]. Reasons for this data-reduction were the following: MRI contraindication due to medical condition (n = 3); claustrophobia (n = 2); preferred to fill out questionnaires at home only (n = 8); preferred not to take part in the MRI experiment (n = 6). For the present analysis, we had to exclude data from one family (n = 3 family members) as this family's proband was not able to participate in the MRI experiment due to an MRI contraindication. Therefore, 110 resting-state data sets (from 8 families) were available for fMRI pre-processing and quality control. One dataset could not be used because the relative motion parameters exceeded 2·5 mm. As a result, 109 resting-state fMRI datasets were available for further analysis. Furthermore, data on the presence of subclinical SAD were lost for eight family members.
4.2. Sample characteristics
Characteristics of the sample (n = 109 for the resting-state analyses, data on subclinical SAD available for 101 participants) are presented in Table 2. In line with the design of the study, participants originated from two generations, which differed significantly in age (β ± SE = -30·2 ± 0·7, p < 0·001), but not in male/female ratio (χ2(1) = 0·72, p = 0·45). Family members with (sub)clinical SAD did not differ from family members without SAD with respect to male/female ratio, age and estimated IQ (all p > 0·3), but they reported higher levels of social anxiety and more depressive symptoms. Furthermore, groups did differ in comorbidity rates: family members with (sub)clinical SAD were more often diagnosed with depression (past) and dysthemia (present). These differences were, however, only significant at an uncorrected significance level. In addition, family members with (sub)clinical SAD reported higher levels of fear of negative evaluation, higher levels of trait anxiety and behavioral inhibition (BIS), as well as lower levels of behavioral activation (BAS).
Table 2.
Sample characteristics.
| (Sub)clinical SAD (n = 39) | No SAD (n = 62) | Statistical analysis | |
|---|---|---|---|
| Demographics | |||
| Male / Female (n) | 20 / 19 | 31/31 | χ2(1) = 0·02, p = 1·00 |
| Generation 1 / Generation 2 (n) | 19 / 20 | 27 / 35 | χ2(1) = 0·26, p = 0·68 |
| Age in years (mean ± SD) | 30·3 ± 15·5 | 31·3 ± 15·2 | β (± SE) = -1·0 ± 3·1, p = 0·76 |
| Estimated IQ (mean ± SD) | 104·3 ± 12·2 | 105·6 ± 10·5 | β (± SE) = -2·1 ± 2·2, p = 0·33 |
| Diagnostic information (n) | |||
| Clinical SAD | 17 | 0 | χ2(1) = 32·5, p < 0·001 |
| Depressive episode present | 1 | 1 | χ2(1) = 0·15, p = 1·00 |
| Depressive episode past | 12 | 9 | χ2(1) = 4·8, p = 0·04 |
| Dysthymia present | 3 | 0 | χ2(1) = 5·3, p = 0·05 |
| Dysthymia past | 1 | 1 | χ2(1) = 0·2, p = 1·00 |
| Panic disorder lifetime | 5 | 2 | χ2(1) = 3·9, p = 0·10 |
| Agoraphobia present | 3 | 2 | χ2(1) = 1·2, p = 0·35 |
| Agoraphobia past | 0 | 2 | χ2(1) = 1·2, p = 0·53 |
| Separation anxiety | 0 | 1 | χ2(1) = 0·8, p = 1·00 |
| Specific phobia | 2 | 3 | χ2(1) = 0·02, p = 1·00 |
| Generalized anxiety disorder present | 1 | 0 | χ2(1) = 1·7, p = 0·37 |
| Obsessive compulsive disorder | 1 | 0 | χ2(1) = 1·7, p = 0·37 |
| Alcohol dependency present | 1 | 1 | χ2(1) = 0·2, p = 1·00 |
| Alcohol dependency lifetime | 1 | 3 | χ2(1) = 0·3, p = 1·00 |
| Attention deficit hyperactivity disorder | 1 | 3 | χ2(1) = 0·3, p = 1·00 |
| Self-report measures | |||
| Social anxiety symptoms (z-score; mean ± SD) | 3·0 ± 3·3 | 0·6 ± 1·5 | β ± SE = 2·6 ± 0·5, p < 0·001 |
| Fear of negative evaluation (mean ± SD) | 23·3 ± 12·3 | 12·8 ± 8·0 | β ± SE = 10·4 ± 2·0, p < 0·001 |
| Depressive symptoms (z-score; mean ± SD) | 0·0 ± 0·9 | -0·5 ± 0·7 | β ± SE = 0·5 ± 0·2, p < 0·001 |
| STAI - trait (mean ± SD) | 38·8 ± 9·4 | 33·1 ± 8·5 | β ± SE = 5·5 ± 1·2, p = 0·002 |
| BIS (z-score; mean ± SD) | 0·4 ± 1·3 | -0·4 ± 0·9 | β ± SE = 0·8 ± 0·2, p = 0·0004 |
| BAS (z-score; mean ± SD) | -0·9 ± 1·0 | -0·6 ± 1·0 | β ± SE = -0·5 ± 0·2, p = 0·02 |
aDue to technical reasons, data on the presence of subclinical SAD were lost for eight family members. Data from these participants were, however, included in the endophenotype analyses using SA-level (z-score) as a predictor. BAS = behavioral activation system; BIS = behavioral inhibition system; SD = standard deviation; SE = standard error; STAI = state trait anxiety inventory;
4.3. Neurobiological candidate endophenotypes within iFC networks
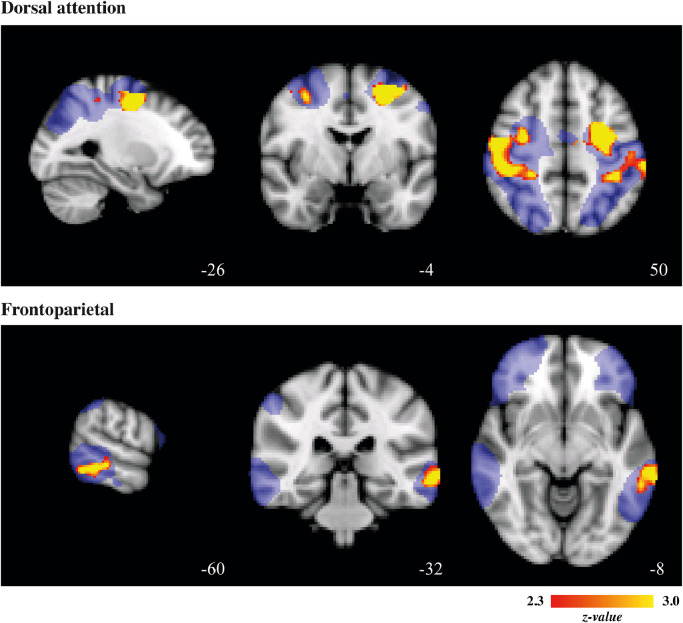
Voxel-wise association analyses within the functional brain networks revealed that the level of social anxiety symptoms (z-score SA) co-segregated with iFC within the dorsal attention network (positive association within two clusters, the first located in the left middle frontal gyrus extending into the superior parietal lobule, the second encompassing the right postcentral gyrus, extending into the supramarginal gyrus, middle frontal gyrus and superior parietal lobule) and frontoparietal network (positive association within left middle temporal gyrus) (Fig. 3; Table 3; cluster-forming threshold z > 2.3, cluster-corrected extent threshold p < 0.05). Results within the dorsal attention network even survived when a more stringent threshold (cluster-forming threshold z > 3·1, cluster-corrected extent threshold p < 0·05) was applied. Furthermore, iFC of multiple voxels within these clusters was at least moderately heritable (h2 > 0·20) (Table 3).
Fig. 3.
Associations between SA and iFC in networks.
Significant associations between level of social anxiety (SA) and intrinsic functional connectivity (iFC) within the dorsal attention and frontoparietal network. Cluster-forming threshold z > 2•3, cluster-corrected extent threshold p < 0•05. Clusters are superimposed on the template MNI_T1_152_2mm_brain; masks of the networks at group-level are displayed in blue. Images are displayed according to radiological convention: right in image is left in the brain.
Table 3.
Associations between social anxiety (z-score SA) and intrinsic functional connectivity; heritability within the clusters.
| Peak (MNI space) |
Heritability |
|||||||
|---|---|---|---|---|---|---|---|---|
| Network | Clusters | Z-score | x | y | z | Cluster size | Number of voxels with h2 > 0·20 | Mean h2, range |
| Dorsal attention | 1* | 4.55 | -26 | -4 | 50 | 1885 | 327 | 0·35, 0·20 – 0·95 |
| 2 | 4.23 | 54 | -14 | 50 | 1356 | |||
| Frontoparietal | 1 | 3.49 | -66 | -32 | -6 | 527 | 195 | 0·42, 0·20 – 0·90 |
Cluster-forming threshold z > 2·3, cluster-corrected extent threshold p < 0·05.
This cluster survived even a more stringent threshold (cluster-forming threshold z > 3·1, cluster-corrected extent threshold p < 0·05).
The follow-up regression analyses within the frontoparietal and dorsal attention network using discrete (sub)clinical SAD as a predictor did not yield clusters surviving the predefined threshold. So, although we did find an association between iFC in these networks and self-reported SA (continuous predictor), there was no relation with (sub)clinical SAD (discrete predictor). We speculate that this lack of a correlation is power-related, as the fMRI sample only contained 39 (sub)clinical SAD cases. This indicates the need for replication of the present findings in a larger sample.
Results of the first sensitivity analysis, with the level of depressive symptoms as an additional covariate, confirmed the relationship between SA and iFC in the dorsal attention network (Table 4), while the relationship between SA and iFC in the frontoparietal network was not significant.
Table 4.
Sensitivity analysis with level of depressive symptoms as covariate.
| Peak (MNI space) |
||||||
|---|---|---|---|---|---|---|
| Network | Clusters | Z-score | x | y | z | Cluster size |
| Dorsal attention | 1* | 4·91 | -24 | -8 | 50 | 1339 |
| 2 | 3·64 | 48 | -8 | 52 | 973 | |
| Frontoparietal | - | |||||
Cluster-forming threshold z > 2·3, cluster-corrected extent threshold p < 0·05.
This cluster survived even a more stringent threshold (cluster-forming threshold z > 3·1, cluster-corrected extent threshold p < 0·05).
In the second sensitivity analysis, we excluded all participants with past and/or present comorbid psychopathology other than SAD; this resulted in a sample of 60 participants, of which only 13 in the (sub)clinical SAD group. Next, we repeated the association analysis with self-reported social anxiety as predictor; this analysis confirmed the relation between SA level and increased iFC in the dorsal attention network (Table 5); no significant clusters were present in the frontoparietal network.
Table 5.
Sensitivity analysis in sample without comorbidity.
| Peak (MNI space) |
||||||
|---|---|---|---|---|---|---|
| Network | Clusters | Z-score | x | y | z | Cluster size |
| Dorsal attention | 1* | 4·50 | -22 | -2 | 46 | 948 |
| Frontoparietal | - | |||||
Cluster-forming threshold z > 2·3, cluster-corrected extent threshold p < 0·05.
This cluster survived even a more stringent threshold (cluster-forming threshold z > 3·1, cluster-corrected extent threshold p < 0·05).
5. Discussion
Here, we investigated whether intrinsic functional connectivity (iFC) within six brain networks of interest (default mode, dorsal attention, executive control, frontoparietal, limbic and salience; Fig. 2) met the endophenotype criterion of co-segregation with social anxiety within families genetically enriched for social anxiety disorder (SAD) and, subsequently, the criterion of heritability. Networks of interest were extracted using independent component analysis (ICA) at the group-level, and results of voxel-wise analyses provided evidence that increased iFC in the dorsal attention and frontoparietal (left) network qualifies, according to the tested criteria, as candidate endophenotypes of the disorder.
Both the dorsal attention and the frontoparietal network are implicated in attentional processing, and increased activation within these networks has been shown to be related to decreased activation within the default mode network [114]. The dorsal attention network is a distributed cortical network and has strong functional connections to extra-striate sensory regions and premotor regions; the network includes, among others, the superior parietal, middle and superior frontal areas, intraparietal sulcus and frontal eye-fields (Fig. 2) and plays a role in voluntary top-down orienting and selecting attention in accordance with goals, as well as in emotion regulation [110,[115], [116], [117]]. The clusters of increased iFC in the present work were located in the left and right middle frontal gyrus, extending into the bilateral superior parietal lobule (Fig. 3); alterations within this network were also present in two sensitivity analyses, being a sensitivity analysis accounting for the level of depressive symptoms (Table 4) and an analysis in which participants with comorbid psychopathology were excluded (Table 5). These findings underscore the robustness of the results within this network.
One previous study showed changes in iFC related to SAD within the dorsal attention network, and these alterations were located within the inferior frontal gyrus (increased connectivity) and superior parietal gyrus (decreased connectivity) [71], while others implicated reduced iFC in the dorsal attention network in participants with high levels of social inhibition [118].
Interestingly, several studies on structural characteristics in SAD, as well as research on functional brain reactivity in socially-anxious participants, revealed alterations within regions of the dorsal attention network. For example, a voxel-based meta-analysis showed increased gray matter volume in the left superior parietal gyrus in SAD patients without comorbidity [119]; cf. the accompanying commentary [120]. Moreover, Brühl et al. described increased cortical thickness in the superior parietal cortex in SAD [121], a finding confirmed by Zhao and colleagues [122], although we and others could not replicate this finding in multiple samples of SAD patients [32,123,124]; cf. [125]. At the functional level, Kreifelts et al. linked increased brain activation within the dorsal attention network to a negative attention bias towards socially-rejecting laughter [126], while other studies involving patients with SAD implicated the superior parietal lobule in emotion processing and cognitive control [127], in viewing disorder-related pictures [128], and in processing social threat (study in healthy participants: enhanced activity during watching negative videos) [129].
The frontal parietal network, which is often divided into a left- and right lateralized component, typically involves the pars opercularis and pars triangularis of the inferior frontal gyrus (Brodmann area (BA) 44/45), parts of the parietal lobe (angular gyrus and supramarginal gyrus), extending into the temporal gyrus (BA 22/39/40); the left-lateralized component of the network involves Broca's and Wernicke's areas supporting the role of this network in several language and cognition paradigms [108,130]. Our data revealed increased iFC in a cluster located in the left middle temporal gyrus (MTG), a region implicated in the implicit detection of social environmental signals [131] and processing emotional faces [132]. Several previous studies on resting-state functional connectivity networks, using various analysis methods, reported alterations in this area related to SAD. First of all, Yun and colleagues showed alterations in connectivity of the left MTG in SAD patients using graph theory analysis (reduced within-module degree z-score), which was related to the subjective degree of functional impairment [133], while others reported increased functional connectivity between the right hippocampal gyrus and the left MTG [65], hyperconnectivity between subcortical nodes and the left MTG [63], and increased negative functional connectivity between the amygdala and left MTG [60]. Two other resting-state studies from the same research group, focusing on the amplitude of low-frequency fluctuations (ALFF) in brain activation, revealed altered regional baseline brain function in, among others, the left MTG, in SAD patients, but the results were inconsistent as one study reported decreased ALFF and the other increased ALFF [134,135]. Although the differences in methodology prevent a direct comparison of these findings, for example with respect to the direction of the changes (increased vs. decreased connectivity), these results clearly implicate the left MTG as an important network hub in the socially-anxious brain.
In addition to these connectivity studies, neuroimaging research on the structure and function of the socially-anxious brain revealed larger gray matter volume of the MTG [119], as well as increased MTG activation during the processing of external threats (i.e. detecting an angry face in a crowd), positively related to the level of social anxiety [136]. Furthermore, greater symptom reduction after cognitive behavioral therapy (CBT) was associated with the baseline level of MTG activation in a sample of 14 patients with SAD [137]. It should be noted however that the findings in the frontoparietal network were not replicated in the sensitivity analysis when depressive symptoms were taken into account (Table 4) or when accounting for comorbid psychopathology in the sample (Table 5). We cautiously speculate that this points towards the influence of depressive symptoms on the left MTG network, cf. the findings described by [138].
Together, these neurobiological alterations within the dorsal attention and frontoparietal network could underlie the negative attention- and interpretation biases which are commonly present in SAD patients [139], [140], [141], [142]. Furthermore, by demonstrating heritability within these SA-related clusters (Table 3), the present data extend previous work by provided novel evidence for increased iFC within these networks as candidate endophenotypes of the disorder. Therefore, we hypothesize that the alterations in iFC within these networks reflect the innate vulnerability to the development and maintenance of these biases [143]. As such, it is important to establish the cerebral structures and networks underlying these information processing biases, as they offer potential targets for treatment. For example, it deserves to be investigated whether effective CBT or attention bias modification (ABM) training, which are commonly used treatments for anxiety [144], [145], [146], [147], [148], [149], [150], would alter iFC within these networks, specifically in the areas revealed by the present work. Previous work demonstrated changes in structural connectivity after active ABM [151] as well as alterations in brain structure, function and connectivity after CBT [152], [153], [154] and a combination of ABM and CBT [155], but, to the best of our knowledge, changes in attentional networks (like the dorsal attention and frontoparietal networks) due to these treatments have not been specifically examined. Furthermore, the brain networks revealed by the present work could be targets for brain stimulation like transcranial magnetic stimulation (TMS) [156,157] or transcranial direct current stimulation ((TDCS) [158], [159], [160]; cf. [161].
Contrary to our expectations, iFC within four other networks of interest (default mode, executive control, limbic and salience; Fig. 2) did not show a significant association with the level of social anxiety. Previous work did reveal alterations within these networks related to SAD, although there is little consistency in these findings, probably due to small samples which vary in characteristics and differences in methodology (cf. the discussion provided in [162]). Liao et al., for example, reported on increases as well as decreases in functional connectivity within the default mode network in a sample of young adult SAD patients (n = 20; mean age 22·9 years) compared to matched healthy control participants (n = 20), although these alterations were not related to the level of symptoms when analyses were corrected for multiple comparisons [71]; Pannekoek and colleagues, on the other hand, were unable to replicate these SAD-related alterations within the default mode network (sample: n =12 patients with SAD but without comorbidity, mean age 34·8 years vs. n = 12 matched healthy controls), but they did report on abnormalities in connectivity within the limbic and salience network [60]. In addition, Geiger and colleagues described SAD-related changes in connectivity within the executive control network, as well as between this network and the amygdala (n = 18 SAD patients vs 15 control participants), but they did not investigate other functional networks. Together, these findings stress the need for large-scale studies on functional connectivity in SAD, using a comprehensive approach encompassing multiple functional networks, in order to determine reliable characteristics of functional connectivity in SAD.
The LFLSAD, from which the data of the present work originated, is a unique neuroimaging study involving two generations of family members, including patients with clinical SAD, participants with subclinical SAD, and family members without social anxiety [30]. This design enabled investigating the co-segregation of social anxiety with iFC within families, and establishing heritability. However, due to the cross-sectional design of the study, the trait stability of iFC (endophenotype criterion 2) could not be examined, nor could we compare iFC between non-affected family members and the general population (second part of endophenotype criterion 4). Longitudinal studies, involving control families from the general population as well as socially-anxious families, are essential to study these criteria. In addition, studies comparing patients and unrelated healthy control participants could provide further evidence for the association with the disorder (endophenotype criterion 1).
Furthermore, although we corrected the analyses for age, we were, due to the complexity of the regression models (accounting for familial relationships), unable to take specific neurodevelopmental changes in functional connectivity into account. Previous work indicated changes within resting-state networks across the human lifespan [163], [164], [165], while another study including typically developing individuals and patients with internalizing psychopathologies (age 7-29 years) indicated differential age-related alterations in iFC between the groups [169]. These findings stress the need for incorporating the neurodevelopmental perspective into endophenotype research (cf. [27]). In addition, it should be noted that the findings of the present study are probably not specific for (social) anxiety, although sensitivity analyses, accounting for the effect of depressive symptoms and comorbid psychopathology within the sample, replicated the findings within the dorsal attention network. Interestingly, two recently published papers provided evidence for transdiagnostic alterations in functional connectivity, in networks underlying cognitive performance [166] and networks supporting executive control and self-referential processes [167]. Therefore, endophenotype studies dedicated to SAD, as well as large-scale transdiagnostic studies on the connectivity of the human brain are needed to explore which alterations in brain connectivity increase the genetic vulnerability to social anxiety and internalizing psychopathology in general. Lastly, due to the family-structure of the dataset, we were unable to analyze the neuroimaging data using permutation tests (cf. the recommendation by [168]). As methodological and technical advances are constantly being made, future studies will most likely be able to perform more advanced analyses using further sophisticated analysis methods.
To conclude, the results of the present work point at increased iFC in the dorsal attention and frontoparietal network as candidate endophenotypes of SAD, using data from a unique sample of families genetically enriched for social anxiety. These findings have relevance for preventative and therapeutic interventions for youth at risk for developing SAD.
Contributors
Janna Marie Bas-Hoogendam: study design, data collection, data analysis, data interpretation, writing, preparing the manuscript, preparing the figures
Henk van Steenbergen: study design, data analysis, data interpretation, verifying the underlying data, reviewing the manuscript
Kathrin Cohen Kadosh: data analysis, data interpretation, reviewing the manuscript
P. Michiel Westenberg: study design, data collection, data analysis, data interpretation, reviewing the manuscript
Nic J. A. van der Wee: study design, data collection, data analysis, data interpretation, reviewing the manuscript
Data sharing statement
Janna Marie Bas-Hoogendam has full access to all the data in the study. A preregistration of the LFLSAD is available at osf.io/e368h [72] and the design of the study is extensively outlined in [30]. The scripts and data that support the findings of this particular study are available at osf.io/q4hsr.
Declaration of Competing Interest
Dr. Bas-Hoogendam has nothing to disclose. Dr. van Steenbergen has nothing to disclose. Dr. Cohen Kadosh has nothing to disclose. Dr. Westenberg has nothing to disclose. Dr. van der Wee has nothing to disclose.
Acknowledgments
The LFLSAD and Janna Marie Bas-Hoogendam were funded by Leiden University Research Profile ‘Health, Prevention and the Human Life Cycle’.
Contributor Information
Janna Marie Bas-Hoogendam, Email: j.m.hoogendam@fsw.leidenuniv.nl.
Henk van Steenbergen, Email: HvanSteenbergen@fsw.leidenuniv.nl.
Kathrin Cohen Kadosh, Email: k.cohenkadosh@surrey.ac.uk.
P. Michiel Westenberg, Email: westenberg@fsw.leidenuniv.nl.
Nic J.A. van der Wee, Email: N.J.A.van_der_Wee@lumc.nl.
References
- 1.Stein D.J., Ruscio A.M., Lee S., Petukhova M., Alonso J., Andrade L.H.S.G. Subtyping social anxiety disorder in developed and developing countries. Depress Anxiety. 2010;27:390–403. doi: 10.1002/da.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler R.C., Petukhova M., Sampson N.A., Zaslavsky A.M., Wittchen H.-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandelow B., Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 2015;17:327–335. doi: 10.31887/DCNS.2015.17.3/bbandelow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendriks S.M., Spijker J., Licht C.M.M., Hardeveld F., de Graaf R., Batelaan N.M. Long-term work disability and absenteeism in anxiety and depressive disorders. J Affect Disord. 2015;178:121–130. doi: 10.1016/j.jad.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Russell G., Topham P. The impact of social anxiety on student learning and well-being in higher education. J Ment Heal. 2012;21:375–385. doi: 10.3109/09638237.2012.694505. [DOI] [PubMed] [Google Scholar]
- 6.Aderka I.M., Hofmann S.G., Nickerson A., Hermesh H., Gilboa-Schechtman E., Marom S. Functional impairment in social anxiety disorder. J Anxiety Disord. 2012;26:393–400. doi: 10.1016/j.janxdis.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Ormel J., Raven D., van Oort F., Hartman C.A., Reijneveld S.A., Veenstra R. Mental health in Dutch adolescents: a TRAILS report on prevalence, severity, age of onset, continuity and co-morbidity of DSM disorders. Psychol Med. 2014:1–16. doi: 10.1017/S0033291714001469. [DOI] [PubMed] [Google Scholar]
- 8.Burstein M., He J.-P., Kattan G., Albano A.M., Avenevoli S., Merikangas K.R. Social phobia and subtypes in the national comorbidity survey-adolescent supplement: prevalence, correlates, and comorbidity. J Am Acad Child Adolesc Psychiatry. 2011;50:870–880. doi: 10.1016/j.jaac.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bas-Hoogendam J.M., Roelofs E.F., Westenberg P.M., van der Wee N.J.A. Pathogenesis of SAD. In: Simon N.M., Hollander E., Rothbaum B.O., Stein D.J., editors. Textb. Anxiety, Trauma OCD-related Disord. 3rd ed. The American Psychiatric Association Publishing; Washington DC: 2020. pp. 429–444. [Google Scholar]
- 10.Beesdo-Baum K., Knappe S., Fehm L., Höfler M., Lieb R., Hofmann S.G. The natural course of social anxiety disorder among adolescents and young adults. Acta Psychiatr Scand. 2012;126:411–425. doi: 10.1111/j.1600-0447.2012.01886.x. [DOI] [PubMed] [Google Scholar]
- 11.Chapdelaine A., Carrier J.-D., Fournier L., Duhoux A., Roberge P. Treatment adequacy for social anxiety disorder in primary care patients. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alonso J., Liu Z., Evans-Lacko S., Sadikova E., Sampson N., Chatterji S. Treatment gap for anxiety disorders is global: results of the World Mental Health Surveys in 21 countries. Depress Anxiety. 2018;35:195–208. doi: 10.1002/da.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehm L., Pelissolo A., Furmark T., Wittchen H.-U. Size and burden of social phobia in Europe. Eur Neuropsychopharmacol. 2005;15:453–462. doi: 10.1016/j.euroneuro.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Ohayon M.M., Schatzberg A.F. Social phobia and depression: prevalence and comorbidity. J Psychosom Res. 2010;68:235–243. doi: 10.1016/j.jpsychores.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Beesdo K., Bittner A., Pine D.S., Stein M.B., Höfler M., Lieb R. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch Gen Psychiatry. 2007;64:903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- 16.Beauchaine T.P., Neuhaus E., Brenner S.L., Gatzke-Kopp L. Ten good reasons to consider biological processes in prevention and intervention research. Dev Psychopathol. 2008;20:745–774. doi: 10.1017/S0954579408000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craske M.G., Zucker B.G. Prevention of anxiety disorders: a model for intervention. Appl Prev Psychol. 2001;10:155–175. doi: 10.1016/S0962-1849(01)80012-3. [DOI] [Google Scholar]
- 18.Isomura K., Boman M., Rück C., Serlachius E., Larsson H., Lichtenstein P. Population-based, multi-generational family clustering study of social anxiety disorder and avoidant personality disorder. Psychol Med. 2015;45:1581–1589. doi: 10.1017/S0033291714002116. [DOI] [PubMed] [Google Scholar]
- 19.Merikangas K.R., Lieb R., Wittchen H.-U., Avenevoli S. Family and high-risk studies of social anxiety disorder. Acta Psychiatr Scand Suppl. 2003:28–37. doi: 10.1034/j.1600-0447.108.s417.5.x. [DOI] [PubMed] [Google Scholar]
- 20.Stein M.B., Chartier M.J., Hazen A.L., Kozak M.V., Tancer M.E., Lander S. A Direct-Interview Family Study of Generalized Social Phobia. Am J Psychiatry. 1998;155:90–97. doi: 10.1176/ajp.155.1.90. [DOI] [PubMed] [Google Scholar]
- 21.Bandelow B., Baldwin D., Abelli M., Altamura C., Dell’Osso B., Domschke K. Biological markers for anxiety disorders, OCD and PTSD – a consensus statement. Part I: neuroimaging and genetics. World J Biol Psychiatry. 2016;17:321–365. doi: 10.1080/15622975.2016.1181783. [DOI] [PubMed] [Google Scholar]
- 22.Glahn D.C., Thompson P.M., Blangero J. Neuroimaging endophenotypes: strategies for finding genes influencing brain structure and function. Hum Brain Mapp. 2007;28:488–501. doi: 10.1002/hbm.20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puls I., Gallinat J. The concept of endophenotypes in psychiatric diseases meeting the expectations? Pharmacopsychiatry. 2008;41(Suppl 1):S37–S43. doi: 10.1055/s-2008-1081462. [DOI] [PubMed] [Google Scholar]
- 24.Lenzenweger M.F. Thinking clearly about the endophenotype-intermediate phenotype-biomarker distinctions in developmental psychopathology research. Dev Psychopathol. 2013;25:1347–1357. doi: 10.1017/S0954579413000655. [DOI] [PubMed] [Google Scholar]
- 25.Gottesman I.I., Gould T.D. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 26.Lenzenweger M.F. Endophenotype, intermediate phenotype, biomarker: definitions, concept comparisons, clarifications. Depress Anxiety. 2013;30:185–189. doi: 10.1002/da.22042. [DOI] [PubMed] [Google Scholar]
- 27.Roffman J.L. Endophenotype Research in Psychiatry—The Grasshopper Grows Up. JAMA Psychiatry. 2019 doi: 10.1001/jamapsychiatry.2019.2194. [DOI] [PubMed] [Google Scholar]
- 28.Merikangas K.R., He J.-P., Burstein M., Swanson S.A., Avenevoli S., Cui L. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kodal A., Fjermestad K., Bjelland I., Gjestad R., Öst L.-G., Bjaastad J.F. Long-term effectiveness of cognitive behavioral therapy for youth with anxiety disorders. J Anxiety Disord. 2018;53:58–67. doi: 10.1016/j.janxdis.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Bas-Hoogendam J.M., Harrewijn A., Tissier R.L.M., van der Molen M.J.W., van Steenbergen H., van Vliet I.M. The Leiden Family Lab study on Social Anxiety Disorder: a multiplex, multigenerational family study on neurocognitive endophenotypes. Int J Methods Psychiatr Res. 2018;27:e1616. doi: 10.1002/mpr.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bas-Hoogendam J.M., Blackford J.U., Brühl A.B., Blair K.S., van der Wee N.J.A., Westenberg P.M. Neurobiological candidate endophenotypes of social anxiety disorder. Neurosci Biobehav Rev. 2016;71:362–378. doi: 10.1016/j.neubiorev.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 32.Bas-Hoogendam J.M., van Steenbergen H., Tissier R.L.M., Houwing-Duistermaat J.J., Westenberg P.M., van der Wee N.J.A. Subcortical brain volumes, cortical thickness and cortical surface area in families genetically enriched for social anxiety disorder - a multiplex multigenerational neuroimaging study. EBioMedicine. 2018:410–428. doi: 10.1016/j.ebiom.2018.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bas-Hoogendam J.M., van Steenbergen H., Blackford J.U., Tissier R.L.M., van der Wee N.J.A., Westenberg P.M. Impaired neural habituation to neutral faces in families genetically enriched for social anxiety disorder. Depress Anxiety. 2019;36:1143–1153. doi: 10.1002/da.22962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bas-Hoogendam J.M., van Steenbergen H., Tissier R.L.M., van der Wee N.J.A., Westenberg P.M. Altered neurobiological processing of unintentional social norm violations: a multiplex, multigenerational fMRI study on social anxiety endophenotypes. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:981–990. doi: 10.1016/j.bpsc.2019.03.003. in press. [DOI] [PubMed] [Google Scholar]
- 35.Bas-Hoogendam J.M., van Steenbergen H., van der Wee N.J.A., Westenberg P.M. Amygdala hyperreactivity to faces conditioned with a social-evaluative meaning - a multiplex, multigenerational fMRI study on social anxiety endophenotypes. NeuroImage Clin. 2020 doi: 10.1016/j.nicl.2020.102247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fornito A., Bullmore E.T. Connectomics: a new paradigm for understanding brain disease. Eur Neuropsychopharmacol. 2015;25:733–748. doi: 10.1016/j.euroneuro.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Park H.-J., Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342 doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- 38.Bas-Hoogendam J.M., van Steenbergen H., Cohen Kadosh K., van der Wee N.J.A., Westenberg P.M. Increased Intrinsic Functional Connectivity in Families Genetically Enriched for Social Anxiety. Biol Psychiatry. 2020;87:S296–S297. doi: 10.1016/j.biopsych.2020.02.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roelofs E.F., Bas-Hoogendam J.M., van Ewijk H., Ganjgahi H., van der Werff S.J.A., Barendse M.E.A. Investigating microstructure of white matter tracts as candidate endophenotypes of Social Anxiety Disorder – findings from the Leiden Family Lab study on Social Anxiety Disorder (LFLSAD) NeuroImage Clin. 2020 doi: 10.1016/j.nicl.2020.102493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roelofs E., Bas-Hoogendam J.M., van Ewijk H., van der Werff S.J.A., Vermeiren R.R.J.M., Westenberg P.M. Alterations in White Matter Integrity as Candidate Endophenotypes of Social Anxiety Disorder: Findings From the Leiden Family Lab on Social Anxiety Disorder (LFLSAD) Biol Psychiatry. 2020;87:S248. doi: 10.1016/j.biopsych.2020.02.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buckholtz J.W. Meyer-Lindenberg A. Psychopathology and the Human Connectome: Toward a Transdiagnostic Model of Risk For Mental Illness. Neuron. 2012;74:990–1004. doi: 10.1016/j.neuron.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Bassett D.S., Sporns O. Network neuroscience. Nat Neurosci. 2017;20:353–364. doi: 10.1038/nn.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan S.E., White S.R., Bullmore E.T., Vértes P.E. A Network Neuroscience Approach to Typical and Atypical Brain Development. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:754–766. doi: 10.1016/j.bpsc.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bassett D.S., Xia C.H., Satterthwaite T.D. Understanding the Emergence of Neuropsychiatric Disorders With Network Neuroscience. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:742–753. doi: 10.1016/j.bpsc.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sylvester C.M., Corbetta M., Raichle M.E., Rodebaugh T.L., Schlaggar B.L., Sheline Y.I. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35:527–535. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Thompson P.M., Ge T., Glahn D.C., Jahanshad N., Nichols T.E. Genetics of the connectome. Neuroimage. 2013;80:475–488. doi: 10.1016/j.neuroimage.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao H., Gillihan S.J., Wang J., Korczykowski M., Sankoorikal G.M.V., Kaercher K.A. Genetic variation in serotonin transporter alters resting brain function in healthy individuals. Biol Psychiatry. 2007;62:600–606. doi: 10.1016/j.biopsych.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 49.Scholtens L.H., van den Heuvel M.P. Multimodal Connectomics in Psychiatry: Bridging Scales From Micro to Macro. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:767–776. doi: 10.1016/j.bpsc.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Horien C., Shen X., Scheinost D., Constable R.T. The individual functional connectome is unique and stable over months to years. Neuroimage. 2019;189:676–687. doi: 10.1016/j.neuroimage.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seitzman B.A., Gratton C., Laumann T.O., Gordon E.M., Adeyemo B., Dworetsky A. Trait-like variants in human functional brain networks. Proc Natl Acad Sci. 2019 doi: 10.1073/pnas.1902932116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang R., Calhoun V.D., Zuo N., Lin D., Li J., Fan L. Connectome-based individualized prediction of temperament trait scores. Neuroimage. 2018;183:366–374. doi: 10.1016/j.neuroimage.2018.08.038. [DOI] [PubMed] [Google Scholar]
- 53.Demeter D.V., Engelhardt L.E., Mallett R., Gordon E.M., Nugiel T., Harden K.P. Functional Connectivity Fingerprints at Rest Are Similar across Youths and Adults and Vary with Genetic Similarity. IScience. 2020;23 doi: 10.1016/j.isci.2019.100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takagi Y., Sakai Y., Abe Y., Nishida S., Harrison B.J., Martínez-Zalacaín I. A common brain network among state, trait, and pathological anxiety from whole-brain functional connectivity. Neuroimage. 2018;172:506–516. doi: 10.1016/j.neuroimage.2018.01.080. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z., Goerlich K.S., Ai H., Aleman A., Luo Y., Xu P. Connectome-Based Predictive Modeling of Individual Anxiety. Cereb Cortex. 2021 doi: 10.1093/cercor/bhaa407. [DOI] [PubMed] [Google Scholar]
- 56.Kim Y.-.K., Yoon H.-K. Common and distinct brain networks underlying panic and social anxiety disorders. Prog Neuro-Psychopharmacology Biol Psychiatry. 2018;80:115–122. doi: 10.1016/j.pnpbp.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 57.Ergül C., Ulasoglu-Yildiz C., Kurt E., Koyuncu A., Kicik A., Demiralp T. Intrinsic Functional Connectivity in Social Anxiety Disorder with and without Comorbid Attention Deficit Hyperactivity Disorder. Brain Res. 2019 doi: 10.1016/j.brainres.2019.146364. [DOI] [PubMed] [Google Scholar]
- 58.Cui Q., Vanman E.J., Long Z., Pang Y., Chen Y., Wang Y. Social anxiety disorder exhibit impaired networks involved in self and theory of mind processing. Soc Cogn Affect Neurosci. 2017;12:1284–1295. doi: 10.1093/scan/nsx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang X., Liu J., Meng Y., Xia M., Cui Z., Wu X. Network analysis reveals disrupted functional brain circuitry in drug-naive social anxiety disorder. Neuroimage. 2019;190:213–223. doi: 10.1016/j.neuroimage.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 60.Pannekoek J.N., Veer I.M., van Tol M.-J., van der Werff S.J.A., Demenescu L.R., Aleman A. Resting-state functional connectivity abnormalities in limbic and salience networks in social anxiety disorder without comorbidity. Eur Neuropsychopharmacol. 2013;23:186–195. doi: 10.1016/j.euroneuro.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 61.Geiger M.J., Domschke K., Ipser J., Hattingh C., Baldwin D.S., Lochner C. Altered executive control network resting-state connectivity in social anxiety disorder. World J Biol Psychiatry. 2016;17:47–57. doi: 10.3109/15622975.2015.1083613. [DOI] [PubMed] [Google Scholar]
- 62.Liu F., Zhu C., Wang Y., Guo W., Li M., Wang W. Disrupted cortical hubs in functional brain networks in social anxiety disorder. Clin Neurophysiol. 2015;126:1711–1716. doi: 10.1016/j.clinph.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 63.Arnold Anteraper S., Triantafyllou C., Sawyer A.T., Hofmann S.G., Gabrieli J.D., Whitfield-Gabrieli S. Hyper-connectivity of Subcortical Resting State Networks in Social Anxiety Disorder. Brain Connect. 2014;4:81–90. doi: 10.1089/brain.2013.0180. [DOI] [PubMed] [Google Scholar]
- 64.Manning J., Reynolds G., Saygin Z.M., Hofmann S.G., Pollack M., Gabrieli J.D.E. Altered resting-state functional connectivity of the frontal-striatal reward system in social anxiety disorder. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao W., Xu Q., Mantini D., Ding J., Machado-de-Sousa J.P., Hallak J.E.C. Altered gray matter morphometry and resting-state functional and structural connectivity in social anxiety disorder. Brain Res. 2011;1388:167–177. doi: 10.1016/j.brainres.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 66.Taber-Thomas B.C., Morales S., Hillary F.G., Pérez-Edgar K.E. Altered topography of intrinsic functional connectivity in childhood risk for social anxiety. Depress Anxiety. 2016;33:995–1004. doi: 10.1002/da.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu J., Van Dam N.T., Feng C., Luo Y., Ai H., Gu R. Anxious brain networks: a coordinate-based activation likelihood estimation meta-analysis of resting-state functional connectivity studies in anxiety. Neurosci Biobehav Rev. 2019;96:21–30. doi: 10.1016/j.neubiorev.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 68.MacNamara A., DiGangi J., Phan K.L. Aberrant spontaneous and task-dependent functional connections in the anxious brain. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:278–287. doi: 10.1016/j.bpsc.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peterson A., Thome J., Frewen P., Lanius R.A. Resting-State Neuroimaging Studies: a New Way of Identifying Differences and Similarities among the Anxiety Disorders? Can J Psychiatry. 2014;59:294–300. doi: 10.1177/070674371405900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dick D.M. Mapping Risk from Genes to Behavior: The Enduring and Evolving Influence of Irving Gottesman's Endophenotype Concept. Twin Res Hum Genet. 2018;21:306–309. doi: 10.1017/thg.2018.35. [DOI] [PubMed] [Google Scholar]
- 71.Liao W., Chen H., Feng Y., Mantini D., Gentili C., Pan Z. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage. 2010;52:1549–1558. doi: 10.1016/j.neuroimage.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 72.Bas-Hoogendam J.M., Harrewijn A., van der Molen M.J.W., van Steenbergen H., van Vliet I., Houwing-Duistermaat J. Preregistration: General Background and Key Question of Project, part of “Profiling Endophenotypes in Social Anxiety Disorder: a neurocognitive approach. Database Open Sci Framew. 2014 doi: 10.17605/OSF.IO/E368H. [DOI] [Google Scholar]
- 73.Bas-Hoogendam J.M., Harrewijn A., van der Molen M.J.W., van Steenbergen H., van Vliet I., Houwing-Duistermaat J. Preregistration: Methods: study Design and Sample. part of “Profiling Endophenotypes in Social Anxiety Disorder: a neurocognitive approach. Database Open Sci Framew. 2014 doi: 10.17605/OSF.IO/AQ3SV. [DOI] [Google Scholar]
- 74.Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 2):22–33. [PubMed] [Google Scholar]
- 75.van Vliet I.M., de Beurs E. The MINI-International Neuropsychiatric Interview. A brief structured diagnostic psychiatric interview for DSM-IV en ICD-10 psychiatric disorders. Tijdschr Psychiatr. 2007;49:393–397. [PubMed] [Google Scholar]
- 76.Bauhuis O., Jonker K., Verdellen C., Reynders J., Verbraak M. De introductie van een Nederlandstalig instrument om DSM-IV-Tr-diagnoses bij kinderen te stellen. Kind Adolesc Prakt. 2013;12:20–26. doi: 10.1007/s12454-013-0005-5. [DOI] [Google Scholar]
- 77.Sheehan D.V., Sheehan K.H., Shytle R.D., Janavs J., Bannon Y., Rogers J.E. Reliability and Validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) J Clin Psychiatry. 2010;71:313–326. doi: 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- 78.2013. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition (DSM-5) [Google Scholar]
- 79.Fresco D.M., Coles M.E., Heimberg R.G., Liebowitz M.R., Hami S., Stein M.B. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychol Med. 2001;31:1025–1035. doi: 10.1017/S0033291701004056. [DOI] [PubMed] [Google Scholar]
- 80.La Greca A.M., Lopez N. Social Anxiety Among Adolescents: Linkages with Peer Relations and Friendships. J Abnorm Child Psychol. 1998;26:83–94. doi: 10.1023/A:1022684520514. [DOI] [PubMed] [Google Scholar]
- 81.Beck A.T., Steer R., Brown G. Psychological Corporation; San Antonio, TX: 1996. Manual for the Beck Depression Inventory-II. [Google Scholar]
- 82.Kovacs M. The Children's Depression Inventory (CDI) Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 83.van Ginkel J.R., Linting M., Rippe R.C.A., van der Voort A. Rebutting Existing Misconceptions About Multiple Imputation as a Method for Handling Missing Data. J Pers Assess. 2020;102:297–308. doi: 10.1080/00223891.2018.1530680. [DOI] [PubMed] [Google Scholar]
- 84.Carleton R.N., McCreary D.R., Norton P.J., Asmundson G.J.G. Brief fear of negative evaluation scale-revised. Depress Anxiety. 2006;23:297–303. doi: 10.1002/da.20142. [DOI] [PubMed] [Google Scholar]
- 85.Leary M.R. A Brief Version of the Fear of Negative Evaluation Scale. Personal Soc Psychol Bull. 1983;9:371–375. doi: 10.1177/0146167283093007. [DOI] [Google Scholar]
- 86.Spielberger C.D., Gorsuch R.L., Lushene R.E. Consulting Psychologists Press; Palo Alto, CA: 1970. STAI manual for the State-Trait Anxiety Inventory. [Google Scholar]
- 87.Spielberger C.D., Vagg P.R. Psychometric Properties of the STAI: A Reply to Ramanaiah, Franzen, and Schill. J Pers Assess. 1984;48:95–97. doi: 10.1207/s15327752jpa4801_16. [DOI] [PubMed] [Google Scholar]
- 88.Carver C.S., White T.L. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J Pers Soc Psychol. 1994;67:319–333. doi: 10.1037/0022-3514.67.2.319. [DOI] [Google Scholar]
- 89.Franken I.H.A., Muris P., Rassin E. Psychometric Properties of the Dutch BIS/BAS Scales. J Psychopathol Behav Assess. 2005;27:25–30. doi: 10.1007/s10862-005-3262-2. [DOI] [Google Scholar]
- 90.Muris P., Meesters C., de Kanter E., Timmerman P.E. Behavioural inhibition and behavioural activation system scales for children: relationships with Eysenck's personality traits and psychopathological symptoms. Pers Individ Dif. 2005;38:831–841. doi: 10.1016/j.paid.2004.06.007. [DOI] [Google Scholar]
- 91.Wechsler D., Coalson D., Raiford S. Pearson; San Antonio, TX: 2008. WAIS-IV technical and interpretive manual. [Google Scholar]
- 92.Wechsler D. The Psychological Corporation; San Antonio, TX: 1991. Manual for the Wechsler Intelligence Scale for Children - Third Edition (WISC-III) [Google Scholar]
- 93.Galván A. Neural plasticity of development and learning. Hum Brain Mapp. 2010;31:879–890. doi: 10.1002/hbm.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bas-Hoogendam J.M., van Steenbergen H., Kreuk T., van der Wee N.J.A., Westenberg PM. How embarrassing! The behavioral and neural correlates of processing social norm violations. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Core Team R. 2016. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 96.Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M.F.S.L. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 97.Griffanti L., Salimi-Khorshidi G., Beckmann C.F., Auerbach E.J., Douaud G., Sexton C.E. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage. 2014;95:232–247. doi: 10.1016/J.NEUROIMAGE.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salimi-Khorshidi G., Douaud G., Beckmann C.F., Glasser M.F., Griffanti L., Smith S.M. Automatic denoising of functional MRI data: Combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 2014;90:449–468. doi: 10.1016/J.NEUROIMAGE.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 100.Jenkinson M., Bannister P., Brady M., Smith S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. Neuroimage. 2002;17:825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- 101.Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/S1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 102.Andersson J.L., Jenkinson M., Smith S. 2007. Non-linear registration aka Spatial normalisation. FMRIB Tech Rep TR07JA2 from WwwFmribOxAcUk/Analysis/Techrep.www.fmrib.ox.ac.uk/analysis/techrep [Google Scholar]
- 103.Pruim R.H.R., Mennes M., van Rooij D., Llera A., Buitelaar J.K., Beckmann C.F. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. doi: 10.1016/J.NEUROIMAGE.2015.02.064. [DOI] [PubMed] [Google Scholar]
- 104.Pruim R.H.R., Mennes M., Buitelaar J.K., Beckmann C.F. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage. 2015;112:278–287. doi: 10.1016/J.NEUROIMAGE.2015.02.063. [DOI] [PubMed] [Google Scholar]
- 105.Smith S.M. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 107.Hyvarinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Networks. 1999;10:626–634. doi: 10.1109/72.761722. [DOI] [PubMed] [Google Scholar]
- 108.Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci. 2009;106 doi: 10.1073/pnas.0905267106. 13040 LP –13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beckmann C.F., Mackay C.E., Filippini N., Smith S.M. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. OHBM. 2009 [Google Scholar]
- 110.Barkhof F., Haller S., Rombouts S.A.R.B. Resting-State Functional MR Imaging: a New Window to the Brain. Radiology. 2014;272:29–49. doi: 10.1148/radiol.14132388. [DOI] [PubMed] [Google Scholar]
- 111.Witt S.T., van Ettinger-Veenstra H., Salo T., Riedel M.C., Laird A.R. What executive function network is that? An image-based meta-analysis of network labels. BioRxiv. 2020 doi: 10.1101/2020.07.14.201202. 2020.07.14.201202. [DOI] [PubMed] [Google Scholar]
- 112.Worsley KJ., Jezzard P., Matthews P.M., Smith S.M. Funct. MRI An Introd. to Methods. Oxford University Press; 2001. Statistical analysis of activation images; pp. 1–23. [DOI] [Google Scholar]
- 113.Tissier R., Tsonaka R., Mooijaart S.P., Slagboom E., Houwing-Duistermaat J.J. Secondary phenotype analysis in ascertained family designs: application to the Leiden longevity study. Stat Med. 2017;36:2288–2301. doi: 10.1002/sim.7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hellyer P.J., Shanahan M., Scott G., Wise R.J.S., Sharp D.J., Leech R. The Control of Global Brain Dynamics: opposing Actions of Frontoparietal Control and Default Mode Networks on Attention. J Neurosci. 2014;34 doi: 10.1523/JNEUROSCI.1853-13.2014. 451 LP –461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thomas Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sripada C., Angstadt M., Kessler D., Phan K.L., Liberzon I., Evans G.W. Volitional regulation of emotions produces distributed alterations in connectivity between visual, attention control, and default networks. Neuroimage. 2013;89:110–121. doi: 10.1016/j.neuroimage.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 118.Blackford J.U., Clauss J.A., Avery S.N., Cowan R.L., Benningfield M.M., Vanderklok R.M. Amygdala-cingulate intrinsic connectivity is associated with degree of social inhibition. Biol Psychol. 2014;99:15–25. doi: 10.1016/j.biopsycho.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang X., Cheng B., Luo Q., Qiu L., Wang S. Gray Matter Structural Alterations in Social Anxiety Disorder: a Voxel-Based Meta-Analysis. Front Psychiatry 2018;9:449. https://doi.org/10.1093/scan/nsy035. [DOI] [PMC free article] [PubMed]
- 120.Bas-Hoogendam J.M. Commentary: Gray Matter Structural Alterations in Social Anxiety Disorder: A Voxel-Based Meta-Analysis. Front Psychiatry. 2019;10:1–3. doi: 10.3389/fpsyt.2019.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brühl A.B., Hänggi J., Baur V., Rufer M., Delsignore A., Weidt S. Increased cortical thickness in a frontoparietal network in social anxiety disorder. Hum Brain Mapp. 2014;35:2966–2977. doi: 10.1002/hbm.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao Y., Chen L., Zhang W., Xiao Y., Shah C., Zhu H. Gray Matter Abnormalities in Non-comorbid Medication-naive Patients with Major Depressive Disorder or Social Anxiety Disorder. EBioMedicine. 2017;21:228–235. doi: 10.1016/j.ebiom.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bas-Hoogendam J.M., van Steenbergen H., Pannekoek J.N., Fouche J.-.P., Lochner C., Hattingh C.J. Voxel-based morphometry multi-center mega-analysis of brain structure in social anxiety disorder. NeuroImage Clin. 2017;16:678–688. doi: 10.1016/j.nicl.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang X., Luo Q., Wang S., Qiu L., Pan N., Kuang W. Dissociations in cortical thickness and surface area in non-comorbid never-treated patients with social anxiety disorder. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2020.102910. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bas-Hoogendam J.M. Gray matter matters: the structure of the socially-anxious brain. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kreifelts B., Brück C., Ritter J., Ethofer T., Domin M., Lotze M. They are laughing at me: cerebral mediation of cognitive biases in social anxiety. PLoS One. 2014;9 doi: 10.1371/journal.pone.0099815. e99815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brühl A.B., Herwig U., Delsignore A., Jäncke L., Rufer M. General emotion processing in social anxiety disorder: Neural issues of cognitive control. Psychiatry Res Neuroimaging. 2013;212:108–115. doi: 10.1016/j.pscychresns.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 128.Heitmann C.Y., Feldker K., Neumeister P., Zepp B.M., Peterburs J., Zwitserlood P. Abnormal brain activation and connectivity to standardized disorder-related visual scenes in social anxiety disorder. Hum Brain Mapp. 2016;37:1559–1572. doi: 10.1002/hbm.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goldin P.R., Manber T., Hakimi S., Canli T., Gross J.J. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Laird A.R., Fox P.M., Eickhoff S.B., Turner J.A., Ray K.L., McKay D.R. Behavioral Interpretations of Intrinsic Connectivity Networks. J Cogn Neurosci. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sugiura M., Yomogida Y., Mano Y., Sassa Y., Kambara T., Sekiguchi A. From social-signal detection to higher social cognition: an fMRI approach. Soc Cogn Affect Neurosci. 2014;9:1303–1309. doi: 10.1093/scan/nst119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fusar-Poli P., Placentino A., Carletti F., Landi P., Allen P., Surguladze S. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- 133.Yun J.-Y., Kim J.-C., Ku J., Shin J.-E., Kim J.-J., Choi S.-H. The left middle temporal gyrus in the middle of an impaired social-affective communication network in social anxiety disorder. J Affect Disord. 2017;214:53–59. doi: 10.1016/j.jad.2017.01.043. [DOI] [PubMed] [Google Scholar]
- 134.Qiu C., Feng Y., Meng Y., Liao W., Huang X., Lui S. Analysis of Altered Baseline Brain Activity in Drug-Naive Adult Patients with Social Anxiety Disorder Using Resting-State Functional MRI. Psychiatry Investig. 2015;12:372–380. doi: 10.4306/pi.2015.12.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yuan M., Zhu H., Qiu C., Meng Y., Zhang Y., Ren Z. Altered regional and integrated resting-state brain activity in general social anxiety disorder patients before and after group cognitive behavior therapy. Psychiatry Res Neuroimaging. 2018;272:30–37. doi: 10.1016/j.pscychresns.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 136.Choi S.-H., Shin J.-E., Ku J., Kim J.-J. Looking at the self in front of others: Neural correlates of attentional bias in social anxiety. J Psychiatr Res. 2016;75:31–40. doi: 10.1016/j.jpsychires.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 137.Klumpp H., Fitzgerald D.A., Phan K.L. Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder. Prog Neuro-Psychopharmacology Biol Psychiatry. 2013;45:83–91. doi: 10.1016/j.pnpbp.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cheng C., Dong D., Jiang Y., Ming Q., Zhong X., Sun X. State-Related Alterations of Spontaneous Neural Activity in Current and Remitted Depression Revealed by Resting-State fMRI. Front Psychol. 2019;10:245. doi: 10.3389/fpsyg.2019.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Miers A.C., Sumter S., Leigh E., Clark D.M. Interpretation bias in online and offline social environments and associations with social anxiety, peer victimization, and avoidance behavior. Cogn Ther Res OP. 2020 doi: 10.1007/s10608-020-10097-1. [DOI] [Google Scholar]
- 140.Mohlman J., Carmin C.N., Price R.B. Jumping to interpretations: Social anxiety disorder and the identification of emotional facial expressions. Behav Res Ther. 2007;45:591–599. doi: 10.1016/j.brat.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 141.Heimberg R.G., Brozovich F.A., Rapee R.M. A Cognitive Behavioral Model of Social Anxiety Disorder: Update and Extension. In: Hofmann S.G., DiBartolo P.M.B.T., editors. Soc. Anxiety. Second Ed. Academic Press; San Diego: 2010. pp. 395–422. [DOI] [Google Scholar]
- 142.Clark D.M., McManus F. Information processing in social phobia. Biol Psychiatry. 2002;51:92–100. doi: 10.1016/S0006-3223(01)01296-3. [DOI] [PubMed] [Google Scholar]
- 143.Spence S.H., Rapee R.M. The etiology of social anxiety disorder: an evidence-based model. Behav Res Ther. 2016;86:50–67. doi: 10.1016/j.brat.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 144.Tobon J.I., Ouimet A.J. Dozois DJAVO-25. Attentional Bias in Anxiety Disorders Following Cognitive Behavioral Treatment. J Cogn Psychother. 2011;25:114–129. doi: 10.1891/0889-8391.25.2.114. [DOI] [Google Scholar]
- 145.Linke J.O., Jones E., Pagliaccio D., Swetlitz C., Lewis K.M., Silverman W.K. Efficacy and mechanisms underlying a gamified attention bias modification training in anxious youth: protocol for a randomized controlled trial. BMC Psychiatry. 2019;19:246. doi: 10.1186/s12888-019-2224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fodor L.A., Georgescu R., Cuijpers P., Szamoskozi Ş., David D., Furukawa T.A. Efficacy of cognitive bias modification interventions in anxiety and depressive disorders: a systematic review and network meta-analysis. The Lancet Psychiatry. 2020;7:506–514. doi: 10.1016/S2215-0366(20)30130-9. [DOI] [PubMed] [Google Scholar]
- 147.Bar-Haim Y. Research Review: attention bias modification (ABM): a novel treatment for anxiety disorders. J Child Psychol Psychiatry. 2010;51:859–870. doi: 10.1111/j.1469-7610.2010.02251.x. [DOI] [PubMed] [Google Scholar]
- 148.Hakamata Y., Lissek S., Bar-Haim Y., Britton J.C., Fox N.A., Leibenluft E. Attention Bias Modification Treatment: A Meta-Analysis Toward the Establishment of Novel Treatment for Anxiety. Biol Psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.MacLeod C., Clarke PJF. The Attentional Bias Modification Approach to Anxiety Intervention. Clin Psychol Sci. 2015;3:58–78. doi: 10.1177/2167702614560749. [DOI] [Google Scholar]
- 150.Naim R., Kivity Y., Bar-Haim Y., Huppert J.D. Attention and interpretation bias modification treatment for social anxiety disorder: a randomized clinical trial of efficacy and synergy. J Behav Ther Exp Psychiatry. 2018;59:19–30. doi: 10.1016/j.jbtep.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 151.Abend R., Rosenfelder A., Shamai D., Pine D.S., Tavor I., Assaf Y. Brain structure changes induced by attention bias modification training. Biol Psychol. 2019;146 doi: 10.1016/j.biopsycho.2019.107736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Månsson K.N.T., Salami A., Frick A., Carlbring P., Andersson G., Furmark T. Neuroplasticity in response to cognitive behavior therapy for social anxiety disorder. Transl Psychiatry. 2016;6:e727. doi: 10.1038/tp.2015.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yuan M., Zhu H., Qiu C., Meng Y., Zhang Y., Shang J. Group cognitive behavioral therapy modulates the resting-state functional connectivity of amygdala-related network in patients with generalized social anxiety disorder. BMC Psychiatry. 2016;16:198. doi: 10.1186/s12888-016-0904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Goldin P.R., Ziv M., Jazaieri H., Hahn K., Heimberg R., Gross J.J. Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA Psychiatry. 2013;70:1048–1056. doi: 10.1001/jamapsychiatry.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.White L.K., Sequeira S., Britton J.C., Brotman M.A., Gold A.L., Berman E. Complementary Features of Attention Bias Modification Therapy and Cognitive-Behavioral Therapy in Pediatric Anxiety Disorders. Am J Psychiatry. 2017;174:775–784. doi: 10.1176/appi.ajp.2017.16070847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hoogendam J.M., Ramakers G.M.J., Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3:95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 157.Paes F., Baczynski T., Novaes F., Marinho T., Arias-Carrión O., Budde H. Repetitive Transcranial Magnetic Stimulation (rTMS) to Treat Social Anxiety Disorder: Case Reports and a Review of the Literature. Clin Pract Epidemiol Ment Heal. 2013;9:180–188. doi: 10.2174/1745017901309010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Heeren A., Billieux J., Philippot P., De Raedt R., Baeken C., de Timary P. Impact of transcranial direct current stimulation on attentional bias for threat: a proof-of-concept study among individuals with social anxiety disorder. Soc Cogn Affect Neurosci. 2017;12:251–260. doi: 10.1093/scan/nsw119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nitsche M.A., Cohen L.G., Wassermann E.M., Priori A., Lang N., Antal A. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 160.Ironside M., Browning M., Ansari T.L., Harvey C.J., Sekyi-Djan M.N., Bishop S.J. Effect of prefrontal cortex stimulation on regulation of amygdala response to threat in individuals with trait anxiety: A randomized clinical trial. JAMA Psychiatry. 2019;76:71–78. doi: 10.1001/jamapsychiatry.2018.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vicario C.M., Salehinejad M.A., Felmingham K., Martino G., Nitsche M.A. A systematic review on the therapeutic effectiveness of non-invasive brain stimulation for the treatment of anxiety disorders. Neurosci Biobehav Rev. 2019;96:219–231. doi: 10.1016/j.neubiorev.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 162.Brühl A.B., Delsignore A., Komossa K., Weidt S. Neuroimaging in Social Anxiety Disorder–a meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev. 2014;47:260–280. doi: 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 163.Betzel R.F., Byrge L., He Y., Goñi J., Zuo X.-N., Sporns O. Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage. 2014;102:345–357. doi: 10.1016/j.neuroimage.2014.07.067. [DOI] [PubMed] [Google Scholar]
- 164.van Duijvenvoorde A.C.K., Westhoff B., de Vos F., Wierenga L.M., Crone E.A. A three-wave longitudinal study of subcortical–cortical resting-state connectivity in adolescence: Testing age- and puberty-related changes. Hum Brain Mapp. 2019;40:3769–3783. doi: 10.1002/hbm.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Solé-Padullés C., Castro-Fornieles J., de la Serna E., Calvo R., Baeza I., Moya J. Intrinsic connectivity networks from childhood to late adolescence: effects of age and sex. Dev Cogn Neurosci. 2016;17:35–44. doi: 10.1016/j.dcn.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sha Z., Wager T.D., Mechelli A., He Y. Common Dysfunction of Large-Scale Neurocognitive Networks Across Psychiatric Disorders. Biol Psychiatry. 2019;85:379–388. doi: 10.1016/j.biopsych.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 167.Elliott M.L., Romer A., Knodt A.R., Hariri A.R. A Connectome-wide Functional Signature of Transdiagnostic Risk for Mental Illness. Biol Psychiatry. 2018;84:452–459. doi: 10.1016/j.biopsych.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci. 2016 doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Burkhouse K., Stange J.P., Jacobs R.H., Bhaumik R., Bessette K.L., Peters A.T. Developmental changes in resting-state functional networks among individuals with and without internalizing psychopathologies. Depression and Anxiety. 2019;36(2):141–152. doi: 10.1002/da.22864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The LFLSAD sample consisted of 132 family members, and we collected MRI data from 113 participants (nine families) [30]. Reasons for this data-reduction were the following: MRI contraindication due to medical condition (n = 3); claustrophobia (n = 2); preferred to fill out questionnaires at home only (n = 8); preferred not to take part in the MRI experiment (n = 6). For the present analysis, we had to exclude data from one family (n = 3 family members) as this family's proband was not able to participate in the MRI experiment due to an MRI contraindication. Therefore, 110 resting-state data sets (from 8 families) were available for fMRI pre-processing and quality control. One dataset could not be used because the relative motion parameters exceeded 2·5 mm. As a result, 109 resting-state fMRI datasets were available for further analysis. Furthermore, data on the presence of subclinical SAD were lost for eight family members.