Abstract
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized by synovial inflammation and progressive joint destruction and is a primary cause of disability worldwide. Despite the existence of numerous anti-rheumatic drugs, a significant number of patients with RA do not respond or are intolerant to current treatments. Mesenchymal stem/stromal cell (MSCs) therapy represents a promising therapeutic tool to treat RA, mainly attributable to the immunomodulatory effects of these cells. This review comprises a comprehensive analysis of the scientific literature related to preclinical studies of MSC-based therapy in RA to analyse key aspects of current protocols as well as novel approaches which aim to improve the efficacy of MSC-based therapy.
Keywords: Rheumatoid arthritis, Mesenchymal stem/stromal cells, Animal models, Protocols, Improvements in MSC-based therapy
Abbreviations: A, allogeneic; AAV, adeno-associated virus; AD, adipose tissue; AD-MSCs, MSCs from adipose tissue; AhR, aryl hydrocarbon receptor; ALP, alkaline phosphatase; ANA, Anti-nuclear antibodies; APCP, α, β methylene selective A2A adenosine receptor competitive antagonist (CD73 inhibitor); AT, antithrombin; AS, arthritis score; AxLNs, axillar lymph nodes; BMZ, betamethasone; BM, bone marrow; BM-MSCs, MSCs from bone marrow; Bregs, regulatory B cells; Bzb, bortezomib; CAIA, collagen-antibody induced arthritis; CD, cluster of differentiation; CFA, Freund's complete adjuvant; CIA, collagen-induced arthritis; CII, collagen II; CM, conditioned medium; COMP, cartilage oligomeric matrix protein; Consec., consecutive; CRP, C reactive protein; CTLA4, cytotoxic T lymphocyte antigen; CXCR3, chemokine receptor 3-alternative; D, days; DAB, 3,3´-diaminobenzidine; DCs, dendritic cells; dLNs, draining lymph nodes; DMARDs, antirheumatic drugs; DM, dexamethasone; EMA, European medicine agency; ERDF, European Regional Development Fund; ESR, erythrocyte sedimentation rate; ETN, etanercept; EVs, extracellular vesicles; Exos, small vesicles/Exosomes; F, female; Flk, tyrosine kinase receptor for vascular endothelial growth factor; FOXP3, forkhead box P3; G, gingiva tissue; Gilz, glucocorticoid-induced leucine zipper; GIP, gastric inhibitory polypeptide; GITR, glucocorticoid-induced tumour necrosis factor receptor; GLP-1, glucagon-like peptide 1; G-MSCs, MSCs from gingiva tissue; GSH, glutathione; GSH-Px, glutathione peroxidase; HDL, high density lipoprotein; H/E, haematoxylin/eosin staining; HLA-II, human leukocyte antigen-II; HMGB-1, high-mobility group box-1; HSCs, hematopoietic stem cells; IA, intra-articular; IAA, indoleacetic acid; ID, intradermal; IDO, indoleamine 2,3-dioxygenase; IFA, incomplete Freund´s adjuvant; IFI, indirect immunofluorescence; IFN-γ, interferon gamma; Ig, immunoglobulin; IHC, immunohistochemical analysis; IL, interleukin; ILA, indole-3-lactic acid; iLNs, inguinal lymph nodes; IL-1RA, IL-1 receptor antagonist; IM, intramuscular; IN, intranodal; iNOS, inducible nitric oxide synthase; IP, intraperitoneal; IP10, IFN-γ-induced protein 10; ISCT, international society for cellular therapy; IV, intravenous; KC, keratinocyte chemo-attractant; LDL, low density lipoprotein; LP, lamina propria, LPS, lipopolysaccharide; M, male; mAIA, antigen-induced arthritis in mouse; mBSA, methylated bovine serum albumin; MCP-1, monocyte chemotactic protein 1; µCT, micro computed tomography imaging; MDA, malondialdehyde; MensMSCs, MSCs from menstrual fluid; MHC, major histocompatibility complex; MIP-2, macrophage inflammatory protein 2; miR, microRNA; mLNs, mesenteric lymph nodes; MMP, matrix metalloproteinase; MPO, myeloperoxidease; MPs, microparticles; MPCs, mesenchymal precursor cells; MRI, magnetic resonance imaging; MSCs, mesenchymal stem/ stromal cells; MTC, Masson´s trichrome; Mtx, methotrexate; MVs, microvesicles; NA, not available; NF-κB, nuclear factor-kappa B; NK, natural killer cells; NRP-1, neuropilin-1; NSAIDs, non-steroidal anti-inflammatory drugs; NuMa, nuclear mitotic apparatus; OCPs, osteoclast precursors; O-MSCs, MSCs from nasal tissue; OPG, osteoprotegerin; OVA, ovalbumin; OVAIA, ovalbumin-induced arthritis; P, placenta; PA, peri-articular; PB, peripheral blood; PBMCs, peripheral blood mononuclear cells; PG, proteoglycan; PGIA, proteoglycan-induced arthritis; PLGA, poly-lactic-co-glycolic acid; pLNs, popliteal lymph nodes; P-MSCs, MSCs from placenta tissue, POM-1, sodium polyoxotungstate (CD39 inhibitor); PP, peyer's patch; PPAR, peroxisome proliferator-activated receptor; PTPN22, protein tyrosine-phosphatase 22; RA, rheumatoid arthritis; RAGE, receptor for advanced glycation products; rAIA, adjuvant-induced arthritis in rats; RANKL, receptor activator of nuclear factor κB ligand; RANTES, regulated upon activation normal T cells expressed and presumably secreted; rCIA, collagen-induced arthritis in rats; RF, rheumatoid factor, RNA, ribonucleic acid; RORγT, retinoic acid-related orphan receptor; S, syngeneic; SAA, serum amyloid-A; SC, subcutaneous; sCIA, collagen-induced arthritis in sheep; SF, synovial fluid; SF-MSCs, MSCs from synovial fluid; sIL6R, soluble IL-6 receptor; SIRT1, sirtuin 1; SM, synovial membrane; SM-MSCs, MSCs from synovial membrane; SNP, sodium nitroprusside; SO, safranin; SP, spleen; SOD, superoxide dismutase; SSEA, severe spontaneous erosive arthritis; STAT3, signal transducer and activator of transcription 3; SVF, stromal vascular fraction; T-AOC, total antioxidant capacity; TBO, toluidine blue O; TCR, T cell receptor; TF, tissue factor; Tfh, follicular T helper cells; TGF-β, transforming growth factor β; Th, T helper cells, TLR, toll-like receptors; T-MSCs, MSCs from tonsil tissue; TNF-α, tumour necrosis factor α; TNFR, tumour necrosis factor receptor; Tol-DCs, tolerogenic dendritic cells; Tr1, IL-10-expressing T cells; TRACP, tartrate-resistant acid phosphatase; TRAF, tumour necrosis factor receptor-associated factor; Tregs, regulatory T cells; UC, umbilical cord; UC-MSCs, MSCs from umbilical cord; VEGF, vascular endothelial growth factor; WT, wild type; X, xenogeneic; >, better than; <, worse than; =, similar to or equal
1. Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease caused by loss of immunologic self-tolerance that generates chronic inflammation in the joints followed by cartilage and bone destruction with associated vascular, metabolic, bone and psychological comorbidities. Several loci related to immune system effector and regulatory genes such as human leukocyte antigen II (HLA-II), protein tyrosine-phosphatase 22 (PTPN22), signal transducer and activator of transcription 3 (STAT3), among others are involved in the aetiology of the disease. In addition, environmental stimuli and epigenetic changes can also contribute to the pathogenic profile of RA. During RA development, the synovium exhibits characteristics of chronic inflammation, including leukocyte infiltration of innate cells and adaptive immune responses mainly mediated by T and B cells [1,2]. The prevalence varies between 0.3 and 1% in western countries, increases with age and is more common in women than in men [3].
Current treatments for RA include non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, disease-modifying antirheumatic drugs (DMARDs) and, more recently, biologic DMARDs. However, there is still no cure for this disease, less than half of patients with RA are in remission, and 10%–15% are refractory or even develop severe adverse effects to the treatments [4], [5], [6]. In the last 30 years, mesenchymal stem/stromal cells (MSCs) have emerged in the field of regenerative medicine due to their capacity to differentiate into specific cell types [7]. Additionally, MSCs can secrete soluble factors and cytokines, exhibit haematopoiesis-supporting properties [8,9] and have immunomodulatory effects, thus representing a promising tool to treat immune-mediated disorders such as RA [9,10].
This review analyses the scientific literature regarding the characteristics of MSC therapy in preclinical studies of RA, focusing on experimental protocols that have paved the way for clinical translation of MSC-based therapy in RA.
2. Animal models of arthritis used in cell therapy protocols with MSCs
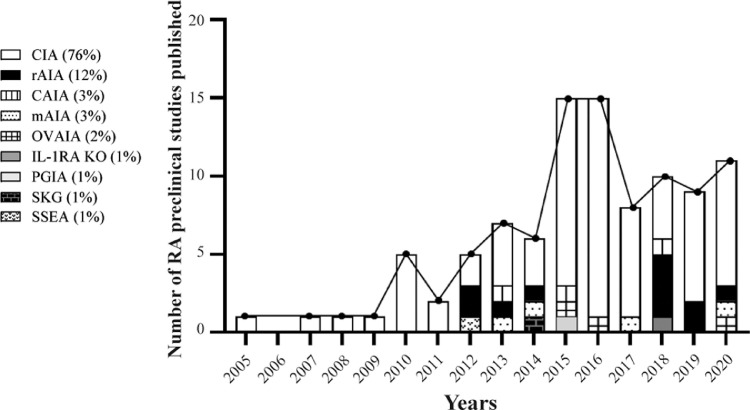
Among RA-like disease models, the collagen induced-arthritis (CIA) model is the most frequently-used experimental model of RA used to study MSC-based therapy (76% of the studies, Fig. 1 and Supplementary Tables). Regarding the species used, most of the studies using the CIA model with MSC-based therapy were conducted in mice (77%) followed by rats (20%, Supplementary Table 1) [11,12]. From 2005 to 2011, the number of studies published increased linearly each year and nearly quadrupled from 2010 to 2016. Since 2017, this tendency has dropped as a consequence of the clinical translation of MSC-based therapy for RA [13] (Fig. 1). In the CIA model, arthritis is induced by one/multiple subcutaneous (SC), intradermal (ID), intraperitoneal (IP) or intra-articular (IA) injections of type II collagen (CII; the major constituent collagen form of articular cartilage), either of chicken or bovine origin emulsified in complete Freund's adjuvant (CFA) containing Mycobacterium tuberculosis one, two or three weeks apart [14] (Table 1). Immunization with heterologous CII activates both CII-reactive T (mostly Th17) and B cells resulting in breach of immunological tolerance with systemic autoantibody-driven arthritis. The CIA model is widely acepted as the animal model that best resembles the systemic immune responses of human RA [15,16].
Fig. 1.
Published preclinical studies of RA with MSC-based therapy classified according to the arthritis models and publication date. Data are expressed as percentages (%).
Table 1.
Animal models of RA used in MSC-based therapy studies.
| RA model | Specie | Strains | Immunogen and protocol | Onset of arthritis from induction |
|---|---|---|---|---|
| Antigen-induced arthritis (mAIA) [96,97] | Mouse | C57BL/6 | SC/IP-injected mBSA in/or CFA with Bordetella pertussis toxin and IA-injected mBSA three weeks later | 21 days |
| Adjuvant-induced arthritis (rAIA) [17] | Rat | -Albino, Fisher-Lewis, Sprague dawley, Wistar | -SC-injected Mycobacterium butyricum in IFA -SC-injected mBSA in CFA & IA-injected mBSA -One or two IA injections of CFA 5 or 14 days apart | 6-15 days |
| Collagen antibody-induced arthritic mouse (CAIA) [98] | Mouse | DBA/1 | IV-injected antibodies against CII and IP-injected LPS 24, 48 or 72 hours later | 4 days |
| Collagen-induced arthritis (CIA) [14] | Mouse | -Albino, C57BL/6 DBA/1 | -One or two SC/ID/IV injections of mouse, chicken or bovine CII with CFA or IFA two or three weeks apart and, in some cases, IP LPS 1 week later | 21 days |
| Rat | -Lewis, Sprague-dawley Wistar | -One or two SC/ID/IA injections of chicken or bovine CII with CFA or IFA and other IP/IA injections one, two or three weeks apart | 7-12 days | |
| Sheep | Merino | -Two SC injections of bovine CII with CFA or IFA two weeks apart and other IA injection one week later | 29 days | |
| IL-1RA knockout mice [99] | Mouse | BALB/C | IL-1RA gene deleted | 5 weeks after birth |
| Ovalbumin (OVA)-induced arthritis (OVAIA) [100] | Rabbit | Japanese white | Three doses of SC-injected OVA in CFA weekly and IA-injected OVA two weeks later | 5 weeks |
| Proteoglycan-induced arthritis (PGIA) [101] | Mouse | BALB/C | Two IP injection of PG 3 weeks apart | 26 days |
| Severe arthritis spontaneously (SSEA) [102] | Mouse | K/BxN | Crossing KRN mice with NOD.Ea16 transgenic mice | 5 weeks after birth |
| SKG strain mice [103] | Mouse | BALB/C | IP injection of Curdlan | 14 days |
Although the CIA model is the archetypical model of RA and most of the studies with MSCs were done in this model, the high variability in terms of time required for the induction phase, variable incidence and the restriction of mouse strains used in this model have resulted in the development of alternative experimental models of RA in which MSC-based therapy has also been assessed (Fig. 1).
The adjuvant-induced arthritis model in rats (rAIA) and the antigen-induced arthritis model in mice (mAIA) are the second experimental models of arthritis most frequently-used to study MSC therapy (12% of the studies with rAIA and 3% of the studies with mAIA, Fig. 1 and Supplementary Tables). In these models, arthritis is induced by one or two subcutaneous injections of CFA or mycobacterium butyricum in incomplete Freund´s adjuvant (IFA) with or without methylated bovine serum albumin (mBSA) (Table 1) [17]. In the AIA models, arthritis is induced only locally with articular T cell-mediated damage [18].
The collagen antibody-induced arthritic model (CAIA), has been also used to study MSC-based therapy in 3% of the studies. In the CAIA model, arthritis is induced by injection of serum antibodies against endogenous CII (Table 1) resulting in immune complex formation and complement activation, but the induction phase of the disease is B cell and T cell independent. Therefore, this model does not completely recapitulate the complexity of immune and tissue remodelling responses during human RA [16].
In most of the RA preclinical studies, the efficacy of MSC-based therapy was evaluated by measuring paw swelling, histologic analysis, X-ray, and micro computed tomography (µCT) of joints. Moreover, quantification of the levels of auto-antibodies against CII, rheumatoid factor, erythrocyte sedimentation rate (ESR) and C reactive protein (CRP) in the sera in arthritic animals (Supplementary Table 1) were also evaluated similarly to the EULAR and ACR recommendations for the management of rheumatoid arthritis patient treatments [4,5].
Overall, MSC-based therapy can reduce arthritis inflammation by 30% in the majority of the published RA animal models (Supplementary table 1). The different RA-like disease models offer advantages for modelling different aspects of human disease although the CIA model is the most frequently used due to the fact that it shares local and systemic immunological aspects of human RA disease.
3. Mechanism of action of MSC-based therapy in preclinical studies of RA
Although different immune responses and mechanisms of action have been implicated in the immunomodulatory capacity of MSCs, nowadays the exact mechanism of action is still not fully defined. An increase in regulatory adaptive T cells (regulatory T cells (Treg) and IL10-expressed CD4+ T cells (Tr1)) and a decrease of inflammatory cells such as T helper 1 (Th1) and Th17 in peripheral blood (PB), spleen (SP), draining lymph nodes (dLNs) and in the joints have been amply described in preclinical studies of RA following MSC-based therapy (Supplementary table 1). Furthermore, a decrease of pro-inflammatory cytokines such as interferon (IFN) γ, tumour necrosis factor (TNF) α, interleukin (IL) 4 and IL17 levels together with an increase of anti-inflammatory cytokines such as transforming growth factor beta (TGF-β1) and interleukin 10 (IL10) in PB and joints are the most common mechanisms of action described in MSC-based studies (Supplementary Table 1). These mechanisms have also been analysed and most of them confirmed in clinical trials [13] emphasizing the importance of preclinical studies for the development of MSC-based therapies for RA.
4. Tissue sources for MSC isolation and expansion
In 2006, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) proposed a set of standards and minimal criteria to define human MSCs for cell-based therapy protocols. According to these criteria, MSCs must be plastic-adherent when maintained in standard culture conditions, must express CD105, CD73 and CD90 surface markers, and lack expression of hematopoietic and endothelial surface molecules such as CD45, CD34, CD14, CD11b, CD79α, CD19, HLA-DR and CD31. In vitro, under specific conditions, the expanded MSCs must differentiate to osteoblasts, adipocytes and chondroblasts to confirm their multipotency [19]. These criteria have been demonstrated suitable for laboratory-based and preclinical studies as well as clinical translation of MSC-based protocols, thus facilitating the comparison among laboratories and hospitals. Nevertheless, there is a great variety in the culture media for the in vitro expansion of the MSCs, the number of MSC passages and infusion vehicle of MSCs used in preclinical studies which hampers this comparison.
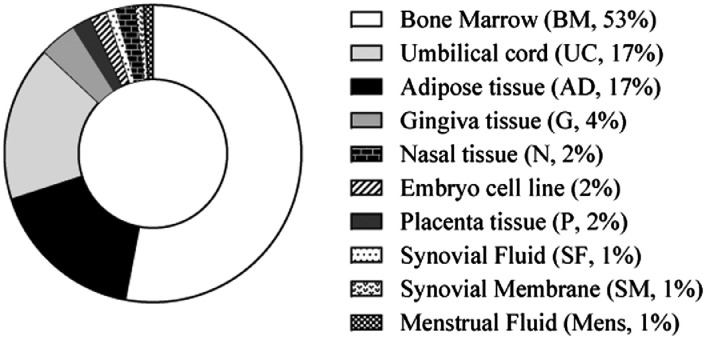
Bone marrow (BM) was the first tissue source used to isolate and expand MSCs (BM-MSCs) for cell-based therapy; consequently, it has been the most frequently used in animal models of RA [11] (53% of the studies, Fig. 2 and Supplementary Tables).
Fig. 2.
Published preclinical studies of RA with MSC-based therapy classified according to the tissue sources. Data are expressed as percentages (%).
Since the collection of BM tissue is a highly invasive procedure for the donor and the frequency of MSCs is extremely low, alternative tissue sources have been subjected to intensive investigation. The most frequent alternative sources of MSCs that have been used in RA preclinical studies are umbilical cord (UC, 17%) and adipose (AD, 17%) tissues (Fig. 2). The frequency of MSCs in adipose tissue is higher than in BM [20]. UC-MSCs can be obtained from Wharton's jelly, artery, vein, or cord lining but this requires time-consuming tissue dissection. Consequently, in general, MSCs from umbilical cord (UC-MSCs) are obtained easily by digesting the complete segments of the umbilical cord containing heterogeneous populations of MSCs. MSCs derived from adipose tissue (AD-MSCs) and UC-MSCs display similar functionality to BM-MSCs [21], [22], [23]. The use of adipose and umbilical cord tissues as sources of MSCs in preclinical studies of RA have been lesser-used sources than BM [11] (Fig. 2 and Supplementary Tables) since they have been identified more recently.
Several groups have isolated MSCs from alternative tissue sources such as gingiva (G-MSCs) [24], palatine tonsil (T-MSC) [25] and placenta tissues (P-MSCs) [26], as well as synovial fluid (SF-MSCs) and synovial membrane (SM-MSCs) [27], nasal (https://patentscope.wipo.int; patent number: WO2019022386 [28]) and embryo tissues [29]. The MSCs derived from these alternative sources have similar characteristics to BM-MSCs, AD-MSCs and UC-MSCs which make them potentially applicable for cell-based therapies in RA. In preclinical studies, these alternative tissue sources for MSCs have also shown amelioration of arthritis severity in RA animal models (Supplementary Tables), which warrants future translation into clinical trials for RA [13].
Interestingly, the stromal vascular fraction (SVF) from liposuction surgery contains MSCs apart from hematopoietic stem cells (HSCs) and elements directly capable of promoting tolerogenesis such as Tregs and inhibitory macrophages [30]. Although no RA preclinical studies with SVF therapy have been reported, SVF therapy has been used in five clinical trials for treatment of RA, one of which is currently ongoing [31].
Today, all tissue sources of MSCs have shown analogous efficacy in RA experimental models; consequently, any of them could be used for future treatments of patients with RA. Umbilical cord is the main source of MSCs used in RA clinical trials although BM-MSCs are the most frequently used in RA preclinical studies. This might be due to the fact that UC is more accessible and less aggressive for the donor compared to BM-derived MSCs.
5. Immunogenicity of MSCs
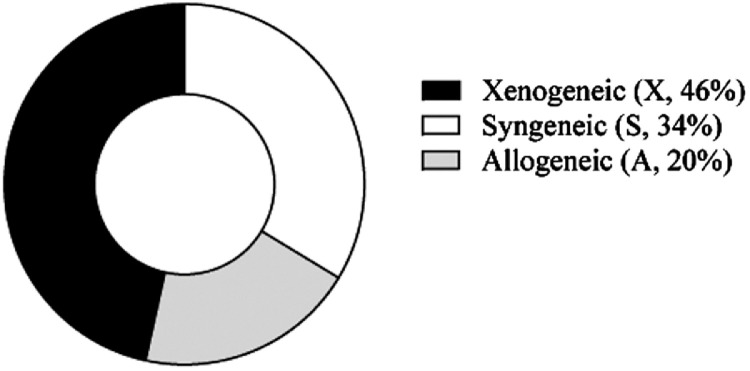
The large majority of RA preclinical studies for MSC-based therapy have been performed under xenogeneic conditions (46% of the studies) (Fig. 3 and Supplementary Tables), followed by the syngeneic major histocompatibility complex (MHC) context (34%) that mimics autologous settings in the clinic.
Fig. 3.
Published preclinical studies of RA with MSC-based therapy classified according to the MHC context. Data are expressed as percentages (%).
Several studies comparing different MHC contexts have been performed. All these studies claimed that xenogeneic, allogeneic, and syngeneic MSCs have similar beneficial effects [32], [33], [34], [35]. Despite these results and the number of studies claiming low immunogenicity of MSCs [8,36,37], Liu L et al.´s meta-analysis of RA preclinical studies pointed out that although MSCs from the different MHC contexts showed modulation in different RA models, more substantial benefit was observed under xenogeneic conditions rather than syngeneic or allogeneic contexts suggesting that an appropriate level of immune disparity between the donor and the host may benefit the efficacy of the cell therapy [11]. This is in line with the hypothesis that soon after infusion the MSCs undergo extensive caspase activation and apoptosis mainly induced by endogenous cytotoxic cells (CD8+ T and NK cells), in a process that is MHC independent. Apoptotic MSCs are then engulfed by phagocytic cells which in turn produce indoleamine 2,3-dioxygenase (IDO), thus triggering immunosuppression in a non-MHC-specific manner [38,39].
The allogeneic use of MSC-based therapy resolves the clinical need to obtain a sufficient number of cells to treat RA patients with MSC therapy and offers the possibility of establishing cell banks for immediate use when needed. Furthermore, some studies have pointed out that MSCs from RA patients may be functionally defective and could potentially contribute to the progression of RA, suggesting that their use in autologous context should be avoided [40,41]. Accordingly, most of the clinical trials in RA with MSC therapy, either completed or currently ongoing, are conducted with allogeneic MSCs [13].
6. Routes of administration and biodistribution of infused MSCs
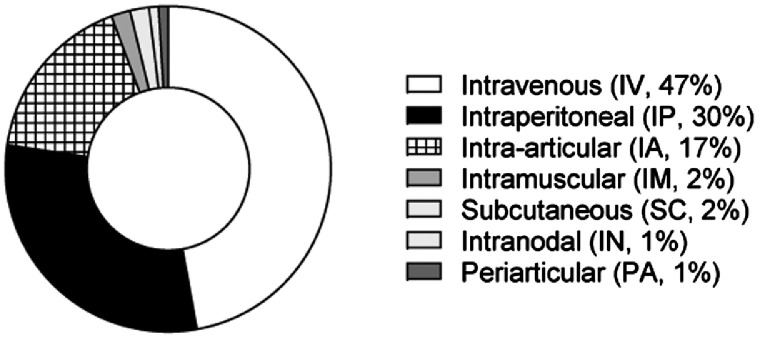
Intravenous (IV, 47% of the studies) and IP (30%) are the most frequent routes of administration in MSC-based therapy in preclinical models of RA. IA infusion of MSCs is the chosen route of administration in larger animals such as rats, rabbits and sheep (17%, Fig. 4). In general, MSC-based therapy has shown similar beneficial effects irrespective of the route of administration used (Supplementary Table 1). These findings are in line with the studies that have confirmed the systemic effects of MSCs [42], [43], [44]. The biodistribution of infused MSCs strongly depends on the route of administration used. The large majority of preclinical studies have shown that a very small number of infused MSCs migrate to the inflamed joints and in general no robust correlation between their biodistribution and their beneficial effect can be established. Numerous studies in preclinical and clinical trials have suggested that MSC-based therapy may generate a sustained beneficial effect in the long term [45], [46], [47], [48], [49], even though the infused MSCs are undetectable a few days following their systemic administration as a consequence of the MSCs being phagocytosed by host cells [38,39,50].
Fig. 4.
Published preclinical studies of RA with MSC-based therapy classified according to the route of administration. Data are expressed as percentages (%).
In preclinical studies, it is well documented that IV-infused MSCs can get entrapped in the lung, potentially inducing pulmonary embolism that may compromise the survival of MSC-treated arthritic mice. This is in part due to the larger cell size of murine MSCs with respect to human MSCs which limits their IV use under syngeneic or allogeneic conditions [51]. Based on studies demonstrating that MSCs, administered systemically, migrated to dLNs where they induced immune cells with a regulatory phenotype, we demonstrated that adipose derived-MSCs injected by intranodal (IN) route in a CIA mouse model was feasible [52].
Alternative routes of MSC administration for the purposes of prolonging their survival have been assessed in vivo. In this sense, local administration such as IA [35,43,[61], [62], [63], [64], [65], [66], [67], [68],[53], [54], [55], [56], [57], [58], [59], [60]], periarticular (PA) [64]; intramuscular (IM) [69] and SC [64,70] have also been used in MSC-based therapy in RA preclinical studies (Supplementary Tables 1 and 2).
IV route of administration is the chosen one in the clinical trials for RA conducted to date [13].
7. MSC dosage and time of infusion
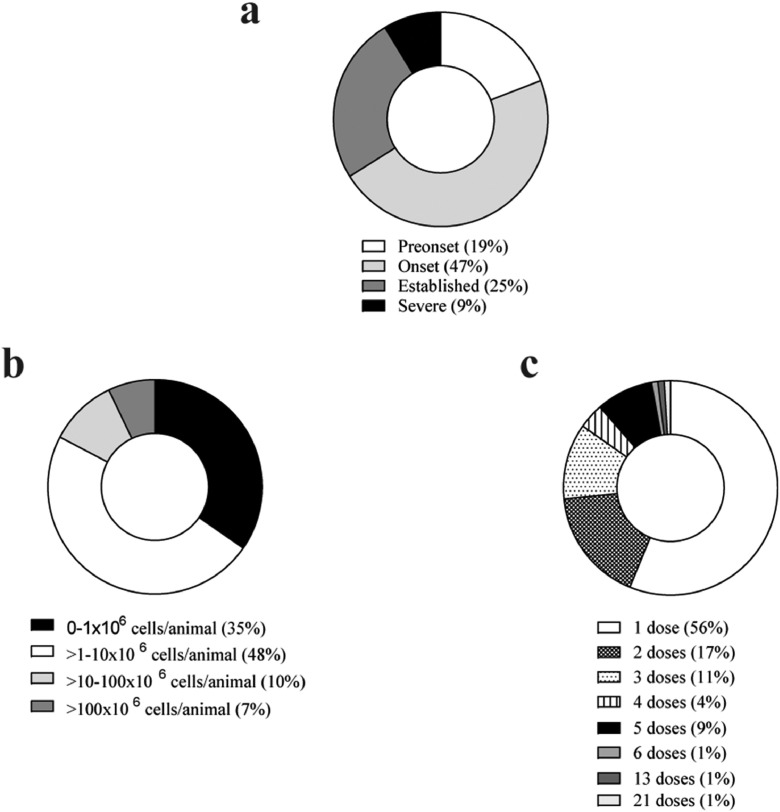
Numerous studies have observed improved efficacy when the MSC infusion was performed during the early phases of the disease (47% and 25% in the onset and in the established phase of the disease, respectively) rather than at pre-onset (19%) or during chronic phases (9%) of the disease, although promising results in terms of efficacy were observed in all instances (Fig. 5a, Supplementary Tables 1 and 2) [[33], [34], [35],[71], [72], [73], [74]]. In contrast, the completed RA clinical trials with MSC-based therapy have been conducted in very refractory RA patients with a long history of the disease which may have hindered the beneficial effects of MSC-based therapy in the clinic. Interestingly, RA patients during the early phases of the disease are now being recruited in currently ongoing clinical trials. This change in the inclusion criteria may increase the efficacy of MSC-based therapy [13].
Fig. 5.
Distribution of preclinical studies of RA with MSC therapy according to the time of treatment with MSCs related to the disease onset (a), MSC dose per mouse (b) and MSC dosage tested (c). Data are expressed in percentage (%).
A great range of cell doses have been tested, from 0.05 × 106 up to 1,700 × 106 MSCs per animal upon a single or multiple infusions (up to 21), although the most commonly used MSC dose has been in the range of 1 × 106 to 10 × 106 MSCs per mouse (Fig. 5b and c and Supplementary Table 1). No adverse effects have been reported in any of the MSC doses tested. Several studies observed better efficacy when multiple doses (2, 3 and even 5) of MSCs were infused, with respect to a single infusion of MSCs during the early phases of the disease [34,35,74,75]. MSC dosage escalating to human use should be between 50 × 106-500 × 106 MSCs/Kg based on mouse (≈20g) and human (≈70Kg) body weights. This is far above the actual MSCs dosage with beneficial effects used in RA clinical trials in MSC-based therapy [13]. This is a key issue in MSC-based therapy that needs careful consideration in translational studies.
8. Approaches for the improvement of MSC-based therapy in RA experimental models
At present, several studies aiming at improving the efficacy and longest-lasting beneficial effects of MSC-based therapy have emerged in RA experimental models (Supplementary Table 2). Different approaches such as pre-treatment of the MSCs with different compounds and inflammatory cytokines during the expansion of the MSCs or just before their infusion in vivo, combination of MSC-based therapy with other treatments for RA, the use of the MSC-conditioned medium (CM) or MSC-released microparticles as well as overexpression of molecules to increase their immunomodulatory properties by means of genetic modifications of native MSCs have been conducted to enhance their immunomodulatory properties. In addition to this, encapsulated MSCs in scaffolding structures have also been described to increase their persistence in vivo.
8.1. Pre-treatment of MSCs
It has been amply described that pro-inflammatory cytokines, toll-like receptor (TLRs) ligands and some molecules can increase the proliferation, survival, differentiation and immunomodulatory functions of MSCs, thus enhancing their therapeutic efficacy in vivo. Cosenza et al. observed that small vesicles isolated from IFN-γ-pretreated BM-MSCs decreased inflammation in CIA arthritic mice [76] suggesting that the therapeutic efficacy of MSCs is highly dependent on the IFN-γ levels. This was later confirmed by He X et al. where the authors observed enhanced synovial inflammation in mice treated with INF-γR−/− MSCs with respect to wild type MSCs [77].
Alternatively, pre-treatment of MSCs with a STAT3 inhibitor (Patent numbers KR101160698B1 and KR101723265B1) or with soluble IL-6 receptor (sIL6R) [55] can enhance the therapeutic effects of MSCs in arthritis inflammation.
All these results support the notion that the immunomodulatory properties of MSCs are favoured when the infused MSCs encounter an inflammatory environment.
8.2. MSC therapy in combination with other molecules or alternative cell-based therapies
Several investigators have used MSC therapy in combination with treatments used in RA such as TNF-α inhibitors [66,78], IL-4 [79] or hesperidin [80] in preclinical models of RA aiming to increase the beneficial effects of MSC-based therapy. These groups demonstrated that the combination of MSCs with these molecules was more efficient than either of the treatments alone.
Lim JY et al. observed improved beneficial effects of MSCs co-infused with Tr1 cells [81]. Li R et al. demonstrated that MSC-based therapy in combination with tolerogenic dendritic cells (Tol-DCs) [82] in the CIA mouse model was more efficient at modulating arthritis progression than either cell-based treatment alone.
All these results support the notion that the combination of MSC therapy with other treatments currently used in the clinic for inflammatory diseases could an adequate alternative to increase the therapeutic effects of MSC-based therapy.
8.3. Scaffolding methods for MSC therapy
To increase the beneficial immunomodulatory effects of MSC therapy, several investigators are developing methods aiming to increase the survival of MSCs when the cells are infused locally. Zhang X et al. [64] and Yamagata K et al. [55] developed a poly-lactic-co-glycolic acid scaffold and Liu H et al. developed fibrin gel-encapsulated MSCs [63] and thermogel-encapsulated MSCs [58] that when administered locally were retained at the implanted site longer, thus reducing their migration to other tissues and enhancing the modulation of inflammation thus resulting in the remission of local inflammatory conditions in comparison to scaffold-free infused MSCs.
8.4. MSC-derived vesicles and MSC-condition medium
Most of the beneficial effects of MSCs are thought to be mediated by paracrine mechanisms involving the secretion of factors or the packaging of these factors in membrane-bound vesicles including microvesicles (MVs). The therapeutic potential of conditioned medium from MSCs (CM-MSCs) and MSC-derived MVs are particularly attractive as a strategy to increase the clinical benefits of MSC therapy since they reduce the risks associated with potential immune reactions against MSCs [83]. Kay AG et al. [57] and Nazemian V et al. [84] observed that CM-MSCs ameliorated the severity of inflammatory arthritis. Cosenza S et al. observed that MSC-derived exosomes (exos, diameter below 150 nm) can decrease more efficiently the clinical signs of inflammation with respect to MVs (ranging from 150 to 1000 nm in diameter) in a CIA model [76]. In addition to this, MSC-conditioned medium and MSC-derived MVs would not suffer the size exclusion in the lungs upon IV administration. Additionally, the variety of proteins, peptides, ribonucleic acids (RNA) and lipid mediators that can be concentrated, frozen, or even lyophilized without loss of activity give them a certain advantage over cellular products that require liquid nitrogen storage and an adequate infrastructure to preserve and revive frozen cells. This suggests that the molecules released from the MSCs have an important role in their beneficial effects, thus increasing the safety and clinical applicability of MSC therapy.
8.5. Genetic modifications of MSCs
To increase the therapeutic effect of MSC therapy, some authors have genetically modified MSCs to express molecules with immunosuppressive properties such as IL-10 [85,86], TFG-β [87], IL-1RA [70], RAGE [88], cytotoxic T lymphocyte antigen (CTLA4Ig) [89], [90], [91] and etanercept (ETN) [92] or tumour necrosis factor receptor (TNFR) [93] which show an enhanced capacity to reduce inflammation compared to native MSCs. Additionally, the use of genetically-modified MSC-derived exosomes introduces the possibility of loading or changing the contents of the released extracellular vesicles (EVs) with factors with therapeutic interest such as miR-146a/miR-155 [94], miR-150-5p [95] and miR-192-5p [53].
All of these encouraging results in preclinical models of RA suggest that the use of modified MSCs may increase their beneficial therapeutic effects, thus overcoming potential limitations of MSC-based-therapy observed in the clinic. No clinical trials with genetically-modified MSCs in any disease have been conducted so far.
9. Discussion
MSC-based therapy modulates and delays arthritis inflammation independent of the tissue source, MHC context and route of administration in the majority of preclinical animal studies mimicking the pathogenesis of RA without adverse effects. This evidence provides a robust justification for the use of MSC-based therapy in the clinic. As a consequence of encouraging preclinical data, the use of MSC-based therapy in clinical trials of RA has experienced a rapid increase in the last decade. Overall, the CIA model is probably the best animal model to study MSC therapy due to similarities in the systemic immunological responses to human RA. Allogeneic context could be the best option for MSC treatment considering the possible impaired MSC function in RA patients that would also allow a cell bank establishment for immediate use. Furthermore, preclinical results suggest that the early phases of inflammation are the best moment for MSC infusion in contrast to most of the completed clinical trials with MSC-based therapy that have been conducted in RA patients with a long history of the disease which may have hidden the clinical benefit of the MSC-based therapy. Based on preclinical and clinical results, rational selection of subjects that would benefit from MSC-based therapy are indicating that recruiting RA patients during the early phases of the disease may increase of the efficacy of MSC therapy. A better understanding of the pathogenesis of the disease as well as the mechanisms of action underlying MSC therapy in RA would identify key biomarkers and would contribute to deployment of optimized protocols of MSC-based therapy for the benefit of RA patients with unmet medical needs.
Outstanding questions
Mesenchymal stem/stromal cell (MSC)-based therapy are undergoing a rapid development as a promising therapeutic tool to treat rheumatoid arthritis (RA) patients who do not respond or are intolerant to current treatments.
Based on the comprehensive analysis of the scientific literature related to preclinical studies of MSC-based therapy in RA, key aspects of currently used preclinical protocols have demonstrated:
-
1.
The ability of MSCs to modulate immune responses is a safe and feasible strategy to treat rheumatoid arthritis.
-
2.
MSC-based therapy has consistently exhibited therapeutic benefits in modulating arthritis inflammation despite the significant diversity in tissue sourcing, MHC contexts, routes of administration and the animal models used.
-
3.
The optimal timing of MSC infusion to achieve high efficacy seems to be during the early phases of inflammation. Further elucidation of this key aspect warrants future investigation.
-
4.
Assessment of long-term efficacy of MSC-based therapies in RA also needs to be addressed in the near future.
Despite the successful results achieved in preclinical studies, a better understanding of the pathogenesis of the rheumatoid arthritis disease as well as the in-depth mechanisms underlying the beneficial effects of MSCs will contribute to develop optimized protocols for improving the clinical outcomes of MSC-based therapies in RA.
Search strategy and selection criteria
The large majority of published data up to December 2020 that used MSC-based therapy to treat RA in animal models have been included in this review (106 articles). We have used the following terms: ´mesenchymal stromal cells´ or ´mesenchymal stem cells´ or ´mesenchymal´ and ´stromal´ and ´cells´ or ´mesenchymal´ and ´stem´ and ´cells´ and ´arthritis´ or ´arthritic´ and ´rheumatic´ or ´rheumatoid´. We searched in the following electronic databases: PubMed, Web of Science and patent registry (https://patentscope.wipo.int). The electronic search strategy excluded non-English articles, in vitro studies, human RA sample studies and RA clinical trials using MSC therapy.
Author contributions
Conceptualization, M.L-S and M.I.G.; writing-original draft preparation, M.L-S and M.I.G. writing—review and editing, M.L-S, J.A.B and M.I.G.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgments
This work was supported by Integrated Projects of Excellence funded by Instituto de Salud Carlos III and co-funded by the European Regional Development Fund (ERDF) [grant numbers PIE15/00048; PI17/01161].
All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103427.
Contributor Information
Mercedes Lopez-Santalla, Email: mercedes.lopezsantalla@externos.ciemat.es.
Marina I. Garin, Email: marina.garin@ciemat.es.
Appendix. Supplementary materials
References
- 1.Firestein G.S., McInnes I.B. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017 doi: 10.1016/j.immuni.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016 doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 3.Otón T., Carmona L. The epidemiology of established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2019;33 doi: 10.1016/j.berh.2019.101477. [DOI] [PubMed] [Google Scholar]
- 4.Singh J.A., Saag K.G., Bridges S.L., Akl E.A., Bannuru R.R., Sullivan M.C. 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016 doi: 10.1002/acr.22783. [DOI] [PubMed] [Google Scholar]
- 5.Smolen J.S., Landewé R.B.M., Bijlsma J.W.J., Burmester G.R., Dougados M., Kerschbaumer A. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 6.Winthrop K.L., Weinblatt M.E., Bathon J., Burmester G.R., Mease P.J., Crofford L. Unmet need in rheumatology: Reports from the Targeted Therapies meeting 2019. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2019-216151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;(80-) doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 8.Jiang W., Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020 doi: 10.1111/cpr.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deans R.J., Moseley A.B. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000 doi: 10.1016/S0301-472X(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 10.Galipeau J., Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018 doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L., Wong C.W., Han M., Farhoodi H.P., Liu G., Liu Y. Meta-analysis of preclinical studies of mesenchymal stromal cells to treat rheumatoid arthritis. EBioMedicine. 2019;47:563–577. doi: 10.1016/j.ebiom.2019.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hynes K., Bright R., Proudman S., Haynes D., Gronthos S., Bartold M. Immunomodulatory properties of mesenchymal stem cell in experimental arthritis in rat and mouse models: A systematic review. Semin Arthritis Rheum. 2016 doi: 10.1016/j.semarthrit.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Santalla M., Fernandez-Perez R., Garin M.I. Mesenchymal stem/stromal cells for rheumatoid arthritis treatment: an update on clinical applications. Cells. 2020 doi: 10.3390/cells9081852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brand D.D., Latham K.A., Rosloniec E.F. Collagen-induced arthritis. Nat Protoc. 2007 doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 15.Schinnerling K., Rosas C., Soto L., Thomas R., Aguillón J.C. Humanized mouse models of rheumatoid arthritis for studies on immunopathogenesis and preclinical testing of cell-based therapies. Front Immunol. 2019 doi: 10.3389/fimmu.2019.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caplazi P., Baca M., Barck K., Carano R.A.D., DeVoss J., Lee W.P. Mouse models of rheumatoid arthritis. Vet Pathol. 2015 doi: 10.1177/0300985815588612. [DOI] [PubMed] [Google Scholar]
- 17.van Eden W., Wagenaar-Hilbers J.P.A., Wauben M.H.M. Adjuvant arthritis in the rat. Curr Protoc Immunol. 1996 doi: 10.1002/0471142735.im1504s19. [DOI] [PubMed] [Google Scholar]
- 18.Jones G.W., Hill D.G., Sime K., Williams A.S. In vivo models for inflammatory arthritis. Methods Mol. Biol. 2018 doi: 10.1007/978-1-4939-7568-6_9. [DOI] [PubMed] [Google Scholar]
- 19.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F.C., Krause D.S. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006 doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 20.Gimble J.M., Bunnell B.A., Frazier T., Rowan B., Shah F., Thomas-Porch C. Adipose-derived stromal/stem cells: A primer. Organogenesis. 2013 doi: 10.4161/org.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mennan C., Brown S., McCarthy H., Mavrogonatou E., Kletsas D., Garcia J. Mesenchymal stromal cells derived from whole human umbilical cord exhibit similar properties to those derived from Wharton's jelly and bone marrow. FEBS Open Bio. 2016 doi: 10.1002/2211-5463.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valencia J., Blanco B., Yáñez R., Vázquez M., Herrero Sánchez C., Fernández-García M. Comparative analysis of the immunomodulatory capacities of human bone marrow– and adipose tissue–derived mesenchymal stromal cells from the same donor. Cytotherapy. 2016 doi: 10.1016/j.jcyt.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Mattar P., Bieback K. Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Front Immunol. 2015 doi: 10.3389/fimmu.2015.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrukhov O., Behm C., Blufstein A., Rausch-Fan X. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: implication in disease and tissue regeneration. World J Stem Cells. 2019 doi: 10.4252/wjsc.v11.i9.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho K.A., Lee H.J., Jeong H., Kim M., Jung S.Y., Park H.S. Tonsil-derived stem cells as a new source of adult stem cells. World J Stem Cells. 2019 doi: 10.4252/wjsc.v11.i8.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antoniadou E., David A.L. Placental stem cells. Best Pract Res Clin Obstet Gynaecol. 2016 doi: 10.1016/j.bpobgyn.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 27.De Sousa E.B., Casado P.L., Neto V.M., Duarte M.E.L., Aguiar D.P. Synovial fluid and synovial membrane mesenchymal stem cells: Latest discoveries and therapeutic perspectives. Stem Cell Res Ther. 2014 doi: 10.1186/scrt501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rui K., Zhang Z., Tian J., Lin X., Wang X., Ma J. Olfactory ecto-mesenchymal stem cells possess immunoregulatory function and suppress autoimmune arthritis. Cell Mol Immunol. 2016 doi: 10.1038/cmi.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sánchez L., Gutierrez-Aranda I., Ligero G., Rubio R., Muñoz-López M., García-Pérez J.L. Enrichment of human ESC-derived multipotent mesenchymal stem cells with immunosuppressive and anti-inflammatory properties capable to protect against experimental inflammatory bowel disease. Stem Cells. 2011 doi: 10.1002/stem.569. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez J.P., Murphy M.P., Hong S., Madrigal M., March K.L., Minev B. Autologous stromal vascular fraction therapy for rheumatoid arthritis: Rationale and clinical safety. Int Arch Med. 2012 doi: 10.1186/1755-7682-5-522313603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ichim T.E., Harman R.J., Min W.P., Minev B., Solano F., Rodriguez J.P. Autologous stromal vascular fraction cells: A tool for facilitating tolerance in rheumatic disease. Cell Immunol. 2010;264:7–17. doi: 10.1016/j.cellimm.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Parolini O., Souza-Moreira L., O'Valle F., Magatti M., Hernandez-Cortes P., Gonzalez-Rey E. Therapeutic effect of human amniotic membrane-derived cells on experimental arthritis and other inflammatory disorders. Arthritis Rheumatol. 2014 doi: 10.1002/art.38206. [DOI] [PubMed] [Google Scholar]
- 33.Papadopoulou A., Yiangou M., Athanasiou E., Zogas N., Kaloyannidis P., Batsis I. Mesenchymal stem cells are conditionally therapeutic in preclinical models of rheumatoid arthritis. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2011-200985. [DOI] [PubMed] [Google Scholar]
- 34.Bouffi C., Bony C., Courties G., Jorgensen C., Noël D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS One. 2010 doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González M.A., González-Rey E., Rico L., Büscher D., Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009 doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 36.Ankrum J.A., Ong J.F., Karp J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat Biotechnol. 2014 doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasmusson I., Ringdén O., Sundberg B., Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003 doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 38.de Witte S.F.H., Luk F., Sierra Parraga J.M., Gargesha M., Merino A., Korevaar S.S. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells. 2018 doi: 10.1002/stem.2779. [DOI] [PubMed] [Google Scholar]
- 39.Galleu A., Riffo-Vasquez Y., Trento C., Lomas C., Dolcetti L., Cheung T.S. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aam7828. [DOI] [PubMed] [Google Scholar]
- 40.Jones E., Churchman S.M., English A., Buch M.H., Horner E.A., Burgoyne C.H. Mesenchymal stem cells in rheumatoid synovium: enumeration and functional assessment in relation to synovial inflammation level. Ann Rheum Dis. 2010 doi: 10.1136/ard.2008.106435. [DOI] [PubMed] [Google Scholar]
- 41.Marinova-Mutafchieva L., Williams R.O., Funa K., Maini R.N., Zvaifler N.J. Inflammation is preceded by tumor necrosis factor-dependent infiltration of mesenchymal cells in experimental arthritis. Arthritis Rheum. 2002 doi: 10.1002/art.10126. [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Santalla M., Mancheño-Corvo P., Escolano A., Menta R., Delarosa O., Redondo J.M. Comparative analysis between the in vivo biodistribution and therapeutic efficacy of adipose-derived mesenchymal stromal cells administered intraperitoneally in experimental colitis. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19071853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swart J.F., De Roock S., Hofhuis F.M., Rozemuller H., Van Den Broek T., Moerer P. Mesenchymal stem cell therapy in proteoglycan induced arthritis. Ann Rheum Dis. 2015;74:769–777. doi: 10.1136/annrheumdis-2013-204147. [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Santalla M., Mancheño-Corvo P., Escolano A., Menta R., DelaRosa O., Abad J.L. Biodistribution and efficacy of human adipose-derived mesenchymal stem cells following intranodal administration in experimental colitis. Front Immunol. 2017 doi: 10.3389/fimmu.2017.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee H.J., Oh S.H., Jang H.W., Kwon J.H., Lee K.J., Kim C.H. Long-term effects of bone marrow-derived mesenchymal stem cells in dextran sulfate sodium-induced murine chronic colitis. Gut Liver. 2016 doi: 10.5009/gnl15229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serrero M., Grimaud F., Philandrianos C., Visée C., Sabatier F., Grimaud J.C. Long-term safety and efficacy of local microinjection combining autologous microfat and adipose-derived stromal vascular fraction for the treatment of refractory perianal fistula in Crohn's disease. Gastroenterology. 2019 doi: 10.1053/j.gastro.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 47.Alves V.B.F., de Sousa B.C., Fonseca M.T.C., Ogata H., Caliári-Oliveira C., Yaochite J.N.U. A single administration of human adipose tissue-derived mesenchymal stromal cells (MSC) induces durable and sustained long-term regulation of inflammatory response in experimental colitis. Clin Exp Immunol. 2019 doi: 10.1111/cei.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barnhoorn M.C., Wasser M.N.J.M., Roelofs H., Maljaars P.W.J., Molendijk I., Bonsing B.A. Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn's disease perianal fistulas. J Crohns Colitis. 2020 doi: 10.1093/ecco-jcc/jjz116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez-Santalla M., Hervas-Salcedo R., Fernandez-Garcia M., Bueren J.A., Garin M.I. Cell therapy with mesenchymal stem cells induces an innate immune memory response that attenuates experimental colitis in the long term. J Crohns Colitis. 2020 doi: 10.1093/ecco-jcc/jjaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee O.J., Luk F., Korevaar S.S., Koch T.G., Baan C.C., Merino A. The importance of dosing, timing, and (in)activation of adipose tissue-derived mesenchymal stromal cells on their immunomodulatory effects. Stem Cells Dev. 2020 doi: 10.1089/scd.2019.0225. [DOI] [PubMed] [Google Scholar]
- 51.Eggenhofer E., Benseler V., Kroemer A., Popp F.C., Geissler E.K., Schlitt H.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012 doi: 10.3389/fimmu.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mancheño-Corvo P., Lopez-Santalla M., Menta R., DelaRosa O., Mulero F., Rio B., del Intralymphatic administration of adipose mesenchymal stem cells reduces the severity of collagen-induced experimental arthritis. Front Immunol. 2017;8:1–12. doi: 10.3389/fimmu.2017.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng J., Zhu L., Iok In I., Chen Y., Jia N., Zhu W. Bone marrow-derived mesenchymal stem cells-secreted exosomal microRNA-192-5p delays inflammatory response in rheumatoid arthritis. Int Immunopharmacol. 2020;78 doi: 10.1016/j.intimp.2019.105985. [DOI] [PubMed] [Google Scholar]
- 54.Miranda J.P., Camões S.P., Gaspar M.M., Rodrigues J.S., Carvalheiro M., Bárcia R.N. The secretome derived from 3D-cultured umbilical cord tissue MSCS counteracts manifestations typifying rheumatoid arthritis. Front Immunol. 2019 doi: 10.3389/fimmu.2019.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamagata K., Nakayamada S., Zhang T., Zhang X T.Y. Soluble IL-6R promotes chondrogenic differentiation of mesenchymal stem cells to enhance the repair of articular cartilage defects using a rat model for rheumatoid arthritis. Clin Exp Rheumatol. 2019 [PubMed] [Google Scholar]
- 56.Yan M., Liu X., Dang Q., Huang H., Yang F., Li Y. Intra-Articular injection of human synovial membrane-derived mesenchymal stem cells in murine collagen-induced arthritis: assessment of immunomodulatory capacity in vivo. Stem Cells Int. 2017:2017. doi: 10.1155/2017/9198328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kay A.G., Long G., Tyler G., Stefan A., Broadfoot S.J., Piccinini A.M. Mesenchymal stem cell-conditioned medium reduces disease severity and immune responses in inflammatory arthritis. Sci Rep. 2017 doi: 10.1038/s41598-017-18144-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu H., Ding J., Wang J., Wang Y., Yang M., Zhang Y. Remission of collagen-induced arthritis through combination therapy of microfracture and transplantation of thermogel-encapsulated bone marrow mesenchymal stem cells. PLoS One. 2015 doi: 10.1371/journal.pone.0120596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kehoe O., Cartwright A., Askari A., El Haj A.J., Middleton J. Intra-articular injection of mesenchymal stem cells leads to reduced inflammation and cartilage damage in murine antigen-induced arthritis. J Transl Med. 2014 doi: 10.1186/1479-5876-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Markides H., Kehoe O., Morris R.H., El Haj A.J. Whole body tracking of superparamagnetic iron oxide nanoparticle-labelled cells - A rheumatoid arthritis mouse model. Stem Cell Res Ther. 2013;4:1–15. doi: 10.1186/scrt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu H., Ding J., Li C., Wang C., Wang Y., Wang J. Hydrogel is superior to fibrin gel as matrix of stem cells in alleviating antigen-induced arthritis. Polymers (Basel) 2016 doi: 10.3390/polym8050182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu C.C., Liu F.L., Sytwu H.K., Tsai C.Y., Chang D.M. CD146+ mesenchymal stem cells display greater therapeutic potential than CD146- cells for treating collagen-induced arthritis in mice. Stem Cell Res Ther. 2016 doi: 10.1186/s13287-016-0285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu H., Ding J., Wang C., Wang J., Wang Y., Yang M. Intra-articular transplantation of allogeneic BMMSCs rehabilitates cartilage injury of antigen-induced arthritis. Tissue Eng - Part A. 2015;21:2733–2743. doi: 10.1089/ten.tea.2014.0666. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X., Yamaoka K., Sonomoto K., Kaneko H., Satake M., Yamamoto Y. Local delivery of mesenchymal stem cells with poly-lactic-co-glycolic acid nano-fiber scaffold suppress arthritis in rats. PLoS One. 2014 doi: 10.1371/journal.pone.011462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santos J.M., Bárcia R.N., Simões S.I., Gaspar M.M., Calado S., Água-Doce A. The role of human umbilical cord tissue-derived mesenchymal stromal cells (UCX®) in the treatment of inflammatory arthritis. J Transl Med. 2013 doi: 10.1186/1479-5876-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu C.C., Wu T.C., Liu F.L., Sytwu H.K., Chang D.M. TNF-α inhibitor reverse the effects of human umbilical cord-derived stem cells on experimental arthritis by increasing immunosuppression. Cell Immunol. 2012 doi: 10.1016/j.cellimm.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 67.Greish S. Human umbilical cord mesenchymal stem cells as treatment of adjuvant rheumatoid arthritis in a rat model. World J Stem Cells. 2012 doi: 10.4252/wjsc.v4.i10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Djouad F., Fritz V., Apparailly F., Louis-Plence P., Bony C., Sany J. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor α in collagen-induced arthritis. Arthritis Rheum. 2005 doi: 10.1002/art.21012. [DOI] [PubMed] [Google Scholar]
- 69.Liu L.N., Wang G., Hendricks K., Lee K., Bohnlein E., Junker U. Comparison of drug and cell-based delivery: engineered adult mesenchymal stem cells expressing soluble tumor necrosis factor receptor II prevent arthritis in mouse and rat animal models. Stem Cells Transl Med. 2013 doi: 10.5966/sctm.2012-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu J., Li H., Chi G., Yang Z., Zhao Y., Liu W. IL-1RA gene-transfected bone marrow-derived mesenchymal stem cells in APA microcapsules could alleviate rheumatoid arthritis. Int J Clin Exp Med. 2015 [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou B., Yuan J., Zhou Y., Ghawji M., Deng Y.P., Lee A.J. Administering human adipose-derived mesenchymal stem cells to prevent and treat experimental arthritis. Clin Immunol. 2011 doi: 10.1016/j.clim.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 72.Zhao F.T., Yin J.W., Liu Q.F., Xu S.F. Effect of bone marrow mesenchymal stem cell transplant on synovial proliferation in rats with type II collagen-induced arthritis. Exp Clin Transplant. 2013 doi: 10.6002/ect.2012.0110. [DOI] [PubMed] [Google Scholar]
- 73.Kim J.H., Lee Y.T., Oh K., Cho J., Lee D.S., Hwang Y., il Paradoxical effects of human adipose tissue-derived mesenchymal stem cells on progression of experimental arthritis in SKG mice. Cell Immunol. 2014 doi: 10.1016/j.cellimm.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 74.Toupet K., Maumus M., Luz-Crawford P., Lombardo E., Lopez-Belmonte J., Van Lent P. Survival and biodistribution of xenogenic adipose mesenchymal stem cells is not affected by the degree of inflammation in arthritis. PLoS One. 2015;10:1–13. doi: 10.1371/journal.pone.0114962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gonzalo-Gil E., Pérez-Lorenzo M.J., Galindo M., Díaz de la Guardia R., López-Millán B., Bueno C. Human embryonic stem cell-derived mesenchymal stromal cells ameliorate collagen-induced arthritis by inducing host-derived indoleamine 2,3 dioxygenase. Arthritis Res Ther. 2016 doi: 10.1186/s13075-016-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cosenza S., Toupet K., Maumus M., Luz-Crawford P., Blanc-Brude O., Jorgensen C. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics. 2018 doi: 10.7150/thno.21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He X., Yang Y., Yao M., Yang L., Ao L., Hu X. Combination of human umbilical cord mesenchymal stem (stromal) cell transplantation with IFN-γtreatment synergistically improves the clinical outcomes of patients with rheumatoid arthritis. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217798. [DOI] [PubMed] [Google Scholar]
- 78.Zhao Y., Gao C., Liu H., Liu H., Feng Y., Li Z. Infliximab-based self-healing hydrogel composite scaffold enhances stem cell survival, engraftment, and function in rheumatoid arthritis treatment. Acta Biomater. 2021;121:653–664. doi: 10.1016/j.actbio.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 79.Haikal S.M., Abdeltawab N.F., Rashed L.A., Abd El-Galil T.I., Elmalt H.A., Amin M.A. Combination therapy of mesenchymal stromal cells and Interleukin-4 attenuates rheumatoid arthritis in a collagen-induced murine model. Cells. 2019 doi: 10.3390/cells8080823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abd-Elhalem S.S., Haggag N.Z., El-Shinnawy N.A. Bone marrow mesenchymal stem cells suppress IL-9 in adjuvant-induced arthritis. Autoimmunity. 2018 doi: 10.1080/08916934.2018.1428956. [DOI] [PubMed] [Google Scholar]
- 81.Lim J.Y., Il Im K., Lee E.S., Kim N., Nam Y.S., Jeon Y.W. Enhanced immunoregulation of mesenchymal stem cells by IL-10-producing type 1 regulatory T cells in collagen-induced arthritis. Sci Rep. 2016 doi: 10.1038/srep26851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li R., Zhang Y., Zheng X., Peng S., Yuan K., Zhang X. Synergistic suppression of autoimmune arthritis through concurrent treatment with tolerogenic DC and MSC. Sci Rep. 2017;7:1–11. doi: 10.1038/srep43188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akyurekli C., Le Y., Richardson R.B., Fergusson D., Tay J., Allan D.S. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Rev Reports. 2015 doi: 10.1007/s12015-014-9545-9. [DOI] [PubMed] [Google Scholar]
- 84.Nazemian V., Manaheji H., Sharifi A.M., Zaringhalam J. Long term treatment by mesenchymal stem cells conditioned medium modulates cellular, molecular and behavioral aspects of adjuvant-induced arthritis. Cell Mol Biol. 2018;64:19–26. doi: 10.14715/cmb/2018.64.2.5. [DOI] [PubMed] [Google Scholar]
- 85.Tian S., Yan Y., Qi X., Li X., Li Z. Treatment of type ii collagen-induced rat rheumatoid arthritis model by interleukin 10 (IL10)-mesenchymal stem cells (BMSCs) Med Sci Monit. 2019;25:2923–2934. doi: 10.12659/MSM.911184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choi J.J., Yoo S.A., Park S.J., Kang Y.J., Kim W.U., Oh I.H. Mesenchymal stem cells overexpressing interleukin-10 attenuate collagen-induced arthritis in mice. Clin Exp Immunol. 2008 doi: 10.1111/j.1365-2249.2008.03683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park M.J., Park H.S., La Cho M, Oh H.J., Cho Y.G., Min S.Y. Transforming growth factor β-transduced mesenchymal stem cells ameliorate experimental autoimmune arthritis through reciprocal regulation of Treg/Th17 cells and osteoclastogenesis. Arthritis Rheum. 2011 doi: 10.1002/art.30326. [DOI] [PubMed] [Google Scholar]
- 88.Park M.J., Lee S.H., Moon S.J., Lee J.A., Lee E.J., Kim E.K. Overexpression of soluble RAGE in mesenchymal stem cells enhances their immunoregulatory potential for cellular therapy in autoimmune arthritis. Sci Rep. 2016 doi: 10.1038/srep35933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sullivan C., Barry F., Ritter T., O'Flatharta C., Howard L., Shaw G. Allogeneic murine mesenchymal stem cells: Migration to inflamed joints in vivo and amelioration of collagen induced arthritis when transduced to express CTLA4Ig. Stem Cells Dev. 2013 doi: 10.1089/scd.2013.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi E.W., Yun T.W., Song J.W., Lee M., Yang J., Choi K.S. Preventive effects of CTLA4Ig-overexpressing adipose tissue-derived mesenchymal stromal cells in rheumatoid arthritis. Cytotherapy. 2015 doi: 10.1016/j.jcyt.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 91.Choi E.W., Shin I.S., Song J.W., Lee M.J., Yun T.W., Yang J. Effects of transplantation of CTLA4Ig-overexpressing adipose tissue-derived mesenchymal stem cells in mice with sustained severe rheumatoid arthritis. Cell Transplant. 2016 doi: 10.3727/096368915X688470. [DOI] [PubMed] [Google Scholar]
- 92.Park N., Rim Y.A., Jung H., Kim J., Yi H., Kim Y. Etanercept-synthesising mesenchymal stem cells efficiently ameliorate collagen-induced arthritis. Sci Rep. 2017 doi: 10.1038/srep39593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu L.N., Wang G., Hendricks K., Lee K., Bohnlein E., Junker U. Comparison of drug and cell-based delivery: engineered adult mesenchymal stem cells expressing soluble tumor necrosis factor receptor II prevent arthritis in mouse and rat animal models. Stem Cells Transl Med. 2013 doi: 10.5966/sctm.2012-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tavasolian F., Hosseini A.Z., Soudi S., Naderi M. miRNA-146a improves immunomodulatory effects of MSC-derived exosomes in rheumatoid arthritis. Curr Gene Ther. 2020 doi: 10.2174/1566523220666200916120708. [DOI] [PubMed] [Google Scholar]
- 95.Chen Z., Wang H., Xia Y., Yan F., Lu Y. Therapeutic potential of mesenchymal cell–derived miRNA-150-5p–expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J Immunol. 2018 doi: 10.4049/jimmunol.1800304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brackertz D., Mitchell G.F., Mackay I.R. Antigen-induced arthritis in mice. I. Induction of arthritis in various strains of mice. Arthritis Rheum. 1977 doi: 10.1002/art.1780200314. [DOI] [PubMed] [Google Scholar]
- 97.Brackertz D., Mitchell G.F., Vadas M.A., Mackay I.R., Miller J.F. Studies on antigen-induced arthritis in mice. II. Immunologic correlates of arthritis susceptibility in mice. J Immunol. 1977 [PubMed] [Google Scholar]
- 98.Nandakumar K.S., Svensson L., Holmdahl R. Collagen type II-specific monoclonal antibody-induced arthritis in mice: description of the disease and the influence of age, sex, and genes. Am J Pathol. 2003 doi: 10.1016/S0002-9440(10)63542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Horai R., Saijo S., Tanioka H., Nakae S., Sudo K., Okahara A. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin I receptor antagonist-deficient mice. J Exp Med. 2000 doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tiku M.L., Liu S., Weaver C.W., Teodorescu M., Skosey J.L. Class II histocompatibility antigen-mediated immunologic function of normal articular chondrocytes. J Immunol. 1985 [PubMed] [Google Scholar]
- 101.Glant T.T., Mikecz K. Proteoglycan aggrecan-induced arthritis: a murine autoimmune model of rheumatoid arthritis. Methods Mol Med. 2004 doi: 10.1385/1-59259-805-6:313. [DOI] [PubMed] [Google Scholar]
- 102.Miossec P., Jorgensen C., Lubberts E., Christensen A.D., Haase C., Cook A.D. K/BxN serum-transfer arthritis as a model for human inflammatory arthritis christensen et al. mechanisms in immune complex-driven arthritis. Front Immunol. 2016 doi: 10.3389/fimmu.2016.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sakaguchi N., Takahashi T., Hata H., Nomura T., Tagami T., Yamazaki S. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003 doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.