Abstract
Herlyn–Werner–Wunderlich syndrome (HWWS) is a rare congenital malformation characterized by uterus didelphys, unilateral blind hemivagina, and ipsilateral renal agenesis. The obstructed vagina affects menstrual flow, leading to related clinical symptoms after menarche. However, the age of onset, initial symptoms, and clinical complications differ among patients owing to the different types of vaginal septum. Herein, we report 2 cases. The first case is of a 20-year-old woman who presented with fever; she was diagnosed with vaginitis and pelvic inflammation due to the vaginal septum with ostiole. The second case is of a 12-year-old girl who complained of abdominal pain; she was diagnosed as having pelvic inflammation, omentitis, and suppurative appendicitis due to the atretic vaginal septum.
Keywords: Herlyn–Werner–Wunderlich syndrome, Hysteroscope, Laparoscope, complication, Computed Tomography, Magnetic Resonance Imaging
Abbreviations: HWWS, Herlyn–Werner–Wunderlich syndrome; CT, Computed Tomography; MRI, Magnetic Resonance Imaging; MDA, Müllerian Duct Anomaly; LAVA, liver acceleration volume acquisition; T2WFS, T2-weight fat-saturated
Introduction
Herlyn–Werner–Wunderlich syndrome (HWWS), also known as obstructed hemivagina and ipsilateral renal agenesis syndrome, is a rare, combined anomaly of malformation of the Müllerian duct and mesonephric duct of the female urogenital tract. HWWS is characterized by uterus didelphys, unilateral blind hemivagina, and ipsilateral renal agenesis [1]. The exact prevalence of HWWS is still unclear. Santos et al. [2] reported that 6% of patients with uterine duplication had a blocked hemivagina. Patients with HWWS are commonly asymptomatic until menarche [3]. Early and accurate diagnosis and treatment of this condition is important because of the possible associated complications including endometriosis, vaginitis, pelvic inflammation, fallopian tube adhesion, and future fertility issues [4,5]. Symptoms typically present after menarche, with regular advanced pelvic pain [4]. Hematocolpos is caused by the retention of menstrual blood in the obstructed hemivagina and is usually detected as a cystic pelvic mass [6]. Surgery is the main treatment, and it includes removal of the vaginal septum and drainage of the effusion [7]; however, complete removal of the vaginal septum in a single procedure may be difficult in case of local inflammation in the vagina and cervix. Herein, we present 2 cases of HWWS along with their complications. Written informed consent was obtained from the 2 patients or their guardians for publication of this case report and the accompanying images.
Case presentation
Case 1
A 20-year-old woman was referred to our hospital with slight vaginal bleeding, fever (38.6 °C), and a palpable abdominal mass in the hypogastric region for 6 days. The patient reported having irregular menstrual cycles since menarche, with mild to moderate bleeding lasting an average of 6 days. She also stated of experiencing recurrence of vaginal bleeding 2-3 days after stopping of her menses, which lasted for 1-2 days. Physical examination revealed a 6-cm × 6-cm mass on the right side of the posterior superior uterine area, without tenderness or rebound pain. Gynecologic examination detected reddish vaginal secretions.
Ultrasound examination revealed a bicornuate uterus, right renal agenesis, and a mucinous mass measuring 69 mm × 57 mm in the uterine cervix, suspected to be an abscess. Computed tomography (CT) revealed a smooth-edged cystic mass in the right side of the pelvic cavity, continuous with the uterus. Another uterus was observed at the upper left of the mass, continuous with the vagina (Fig. 1). Laboratory examination revealed no significant abnormalities other than elevated CA-125 level (68.99 U/mL).
Fig. 1.
Axial CT demonstrated cystic mass (white star, A & B) and enhancement of the cystic wall (A & B).
A uterine structure was observed in the left side of the pelvic cavity (white arrowhead, A & B & C). A duct opening was observed at the top of the cystic mass (white arrow, C & D). The lower margin of the left kidney was visible (black arrow, C & D) but the right kidney was not visible.
Under a suspected diagnosis of HWWS, laparoscopy and hysteroscopy were suggested. Intraoperative examination revealed the presence of approximately 50 mL of yellowish liquid in the pelvic cavity and 2 independent uteri measuring 4 cm × 3 cm × 3 cm. Bilateral ovaries and fallopian tubes were normal. A cystic mass located in the right uterus cervix compressed the stunted left uterine cervix; a septum with an ostiole originating from the left uterine cervix was obliquely attached to the right side of the vaginal wall. A yellowish purulent fluid and dark red blood clot flowed out from the cystic mass after removal of the membranous structure (Fig. 2). Pathologic examination revealed local ulceration and infection of the vaginal septum. The patient recovered well and was discharged from the hospital after 9 days of treatment with anti-infection agents, hemostasis, and rehydration supplementation. The final diagnosis was HWWS type I with secondary vaginitis and pelvic inflammation.
Fig. 2.
Laparoscopy showed 2 separate uteri (white arrow, A) and a mass (white arrowhead, B) beneath the right uterus. The mixture of blood and pus flowed out through the mass after the oblique septum was punctured (white arrow, C & D) in hysteroscopy.
However, the patient returned 49 day’s later, complaining of hypogastric discomfort. Gynecologic examination revealed a 1-cm wide vaginal septum covering the right uterine cervix in the vagina from the 11-o’-3-o’ clock position, with slightly yellowish purulent fluid oozing out of it. Hence, a second operation was performed with the patient's consent. The operation progressed smoothly, and the patient remained asymptomatic during the follow-up period of 3 years.
Case 2
A 12-year-old girl presented with acute right lower abdominal pain for 1 day. Based on her clinical symptom of persistent pain in the right lower abdomen, and tenderness and rebound pain at McBurney's point, the patient was diagnosed as having acute suppurative appendicitis and scheduled for emergency laparoscopic surgery. Intraoperative examination revealed appendicular abscess, and bloody secretions in the pelvis and omentum. As the procedure progressed, 2 uteri, a mass at the right subuterine side, and swelling of the right fallopian tube with pelvic adhesion were observed. A diagnosis of HWWS was established after emergency consultation with the gynecologist during the operation. A second operation was recommended after appendectomy and release of the right fallopian tube.
Two months’ later, the patient returned to the hospital for a second operation. Ultrasound revealed double uteri and double cervixes, right renal agenesis, fluid echo in the right cervix, and suspected hydrosalpinx on the right side. Magnetic resonance imaging (MRI) revealed abnormal development of the urogenital system as right renal agenesis; double uteri; double cervixes; and oblique vaginal septum; as well as hematocele in the right uterus, vagina, and fallopian tube (Fig. 3). Laboratory examination revealed no significant abnormalities except for elevated levels of CA-125 (127.6 U/mL) and CA-199 (89.04 U/mL). After excluding contraindications and obtaining informed consent from the patient, laparoscopy, and hysteroscopy were planned.
Fig. 3.
Coronal MRI of T2WFS demonstrated the cystic mass (A-C), 2 uterine structures (white arrowhead, A), and compressed vagina and cervix (white arrow, B). Coronal, sagittal, and axial MR of T2WFS showed the duct opening at the top of the cystic mass (white arrowhead, C & D & E). Coronal MRI enhanced scan with LAVA sequence of the upper abdomen showed agenesis of the right kidney.
Laparoscopic examination revealed a swollen right fallopian tube with fimbria atresia and adhesion to the pelvic wall, 2 separate uteri, and a mass measuring 6 cm × 8 cm × 7 cm below the right uterus. The adhesive tissue around the right fallopian tube was separated and the atretic tubal fimbria was opened, from which dark red blood flowed out. The opening of tubal fimbria was enlarged and the wound was sutured using an absorbable suture. Hysteroscopy revealed an immature cervix on the left side at the end of the vagina and a septum obliquely attached to the right side of the vaginal wall originating from the immature cervix. Incision of the vaginal oblique septum caused a dark red liquid to flow out (Fig. 4). Repeated washing revealed another small cervix behind the oblique septum; the endometrium of the right uterus was slightly thin and the right tubal orifice was unobstructed. The septum was completely excised using an electrotome. Pathologic examination revealed local inflammatory cell infiltration and squamous metaplasia of the vaginal septum. The final diagnosis was HWWS type II with secondary epiploitis, pelvic inflammation, and suppurative appendicitis. The patient recovered well and was discharged from the hospital after 9 days of treatment with anti-infection agents, hemostasis, and rehydration supplementation. The patient had no symptoms or complaints during the follow-up period of 2 years after being discharged from the hospital.
Fig. 4.
Hysteroscopy clearly showed the left uterine horn (white arrow, A) and oblique septum (white arrowhead, A). Blood flowed out when the vaginal oblique septum was incised (B & C), and residual blood was observed on the inner wall of the right vagina (D).
Discussion
Developmental abnormalities of the female genital tract include various disorders of the fallopian tubes, uterus, and vagina, with a mean prevalence of 7% [8]. They occur owing to maldevelopment of the Müllerian or paramesonephric ducts [7]. The development of Müllerian ducts is embryologically interlinked to the development of Wolffian or mesonephric ducts, which could explain the frequent association of urologic abnormalities and Müllerian malformations [3]. Renal agenesis is the most common among these concurrent abnormalities, accounting for up to 30% of cases [9].
HWWS is a rare Müllerian duct anomaly (MDA), with a reported prevalence of 2%-3% and a frequency of 1 in 200- 1 in 600 among fertile women [10,11]. The characteristic triad of HWWS comprises uterus didelphys, obstructed hemivagina, and ipsilateral renal agenesis [12]. HWSS was included in the most extensively used 1988 American Fertility Society classification of reproductive system malformations in women [13]. In China, on the basis of morphology and the degree of obstruction, HWWS could be divided into type I: Imperforated oblique septum, type II: Perforated oblique septum, and type III: Imperforated diagonal septum with a cervical fistula [14,15]. In this study, Case 1 was vaginal oblique septum type I and Case 2 was vaginal oblique septum type II. Type I HWWS generally develops earlier, whereas type II, and III HWWS develop later, which is consistent with that observed in our study.
Laboratory examinations have revealed that some patients have elevated levels of CA-199 [7] or CA-125 [16]. In this study, both patients showed elevated levels of CA-125 and 1 patient showed elevated level of CA-199, which returned to normal at the postoperative follow-up. The commonly used preoperative diagnostic methods for HWSS include ultrasound examination, hysterosalpingography, and MRI. CT imaging is not recommended for the screening and diagnosis of HWWS, as it is less accurate and subjects the patient to ionizing radiation [17]. Ultrasound examination is an inexpensive and convenient method of screening for HWWS; however, its diagnostic accuracy depends on the experience of the examiner. MRI is the imaging modality of choice [6,7] for the diagnosis and classification of HWWS, as it provides details about uterine morphology, including outline and intrauterine cavity shape and continuity with each vaginal lumen, and the character of the fluids in these cavities. It can also ascertain associated pathologies such as endometriosis, vaginitis, pelvic inflammation, and adhesion, as well as renal agenesis. However, it is important to note that if MRI or other imaging methods yield inconclusive results, hysteroscopy, laparoscopy, or laparoscopy combined with hysteroscopy may be performed [17].
The clinical symptoms of HWWS are often accompanied by complications including endometriosis, vaginitis, pelvic inflammation, and fallopian tube empyema, and adhesion. The menstrual blood of the patient in Case 1 could be slightly drained through the vaginal septum ostiole. However, this also allowed bacteria from the vagina to enter; thus, the vaginal septum mainly included a mixture of pus and blood, leading to symptoms of infection such as fever. The vaginal septum of the patient in Case 2 did not have an ostiole; hence, menstrual blood could be discharged only through the ipsilateral opening of the fallopian tube, which caused the pelvic inflammation, epiploitis, suppurative appendicitis, and right fallopian tube adhesion. The operation procedure was relatively straightforward, with an aim to remove the vaginal septum and drain the blood and/or pus. However, in case of inflammation and swelling of the vagina and cervix, complete removal of the vaginal septum in a single procedure may be difficult, and a second operation may be necessary. Early detection and treatment could help to provide pain relief and prevent further complications [18].
Conclusion
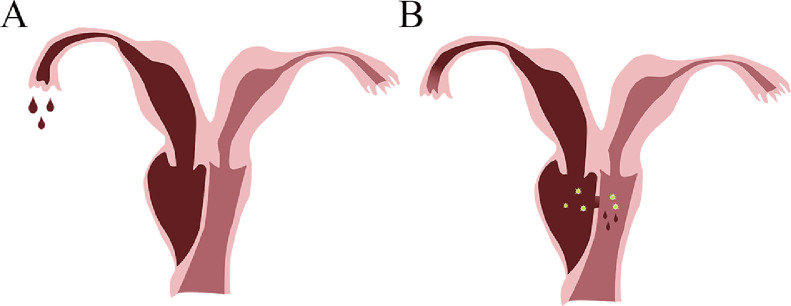
The clinical manifestations and age of onset may differ among the different types of Herlyn–Werner–Wunderlich syndrome (HWWS). Ultrasound is recommended for early screening and MRI, for further diagnosis. Early diagnosis and surgical treatment may help to avoid further complications; however, it should be noted that inflammation and swelling may render the surgery difficult and reduce its success rate (Fig. 5).
Fig. 5.
When the oblique vaginal septum is completely closed (A), menstrual blood is unable to flow out through the vagina and is forced to partially overflow from the ipsilateral fallopian tube, which may lead to pelvic inflammation, epiploitis, suppurative appendicitis, and right fallopian tube adhesion. When the vaginal oblique septum is not completely closed (B), the menstrual blood can flow out through the vagina; however, it may cause bacterial infection and suppuration.
Author contributions
Tianzhu Liu conceived the idea of the study; Xiaodan Li Performed examinations and operations; Xiaodan Li and Lina Li wrote the paper; all authors discussed the results and revised the manuscript.
Patient consent
Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Funding
No funding.
Footnotes
Acknowledgments: None.
Competing Interests: The authors declare that they have no competing interests.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2021.05.055.
Appendix. Supplementary materials
References
- 1.Acien P, Acien M. The presentation and management of complex female genital malformations. Hum Reprod Update. 2016;22(1):48–69. doi: 10.1093/humupd/dmv048. [DOI] [PubMed] [Google Scholar]
- 2.Santos XM, Dietrich JE. Obstructed hemivagina with ipsilateral renal anomaly. J Pediatr Adolesc Gynecol. 2016;29(1):7–10. doi: 10.1016/j.jpag.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Tuna T, Estevao-Costa J, Ramalho C, Fragoso AC. Herlyn-werner-wunderlich syndrome: Report of a prenatally recognised case and review of the literature. Urology. 2019;125:205–209. doi: 10.1016/j.urology.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Mittal R. Herlyn-werner-wunderlich syndrome. J Obstet Gynaecol India. 2016;66(2):128–130. doi: 10.1007/s13224-015-0707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dias JL, Jogo R. Herlyn-Werner-Wunderlich syndrome: pre- and post-surgical MRI and US findings. Abdom Imaging. 2015;40(7):2667–2682. doi: 10.1007/s00261-015-0421-0. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Ning G, Fu C, Bao L, Guo Y. Herlyn-Werner-Wunderlich syndrome: diverse presentations and diagnosis on MRI. Clin Radiol. 2020;75(6):480e417–480e425. doi: 10.1016/j.crad.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Xu S, Yang L, Songhong Y. MRI image features and differential diagnoses of Herlyn-Werner-Wunderlich syndrome. Gynecol Endocrinol. 2020;36(6):484–488. doi: 10.1080/09513590.2019.1680623. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich JE, Millar DM, Quint EH. Obstructive reproductive tract anomalies. J Pediatr Adolesc Gynecol. 2014;27(6):396–402. doi: 10.1016/j.jpag.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Hall-Craggs MA, Kirkham A, Creighton SM. Renal and urological abnormalities occurring with Mullerian anomalies. J Pediatr Urol. 2013;9(1):27–32. doi: 10.1016/j.jpurol.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Del Vescovo R, Battisti S, Di Paola V, Piccolo CL, Cazzato RL, Sansoni I. et al. Herlyn-Werner-Wunderlich syndrome: MRI findings, radiological guide (two cases and literature review), and differential diagnosis. BMC Med Imaging. 2012;12:4. doi: 10.1186/1471-2342-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gholoum S, Puligandla PS, Hui T, Su W, Quiros E, Laberge JM. Management and outcome of patients with combined vaginal septum, bifid uterus, and ipsilateral renal agenesis (Herlyn-Werner-Wunderlich syndrome) J Pediatr Surg. 2006;41(5):987–992. doi: 10.1016/j.jpedsurg.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Salastekar N, Coelho M, Majmudar A, Gupta S. Herlyn-Werner-Wunderlich syndrome: A rare cause of abdominal pain and dyspareunia. Radiol Case Rep. 2019;14(10):1297–1300. doi: 10.1016/j.radcr.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The american fertility society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, Müllerian anomalies and intrauterine adhesions. Fertil Steril. 1988;49(6):944–955. doi: 10.1016/s0015-0282(16)59942-7. [DOI] [PubMed] [Google Scholar]
- 14.SL J. Diagnosis and treatment of vaginal oblique septum syndrome. Chin J Appl Gynecol Obstet. 2018;34:374–377. [Google Scholar]
- 15.Robbins JB, Broadwell C, Chow LC, Parry JP, Sadowski EA. Mullerian duct anomalies: embryological development, classification, and MRI assessment. J Magn Reson Imaging. 2015;41(1):1–12. doi: 10.1002/jmri.24771. [DOI] [PubMed] [Google Scholar]
- 16.Tigga MP. An interesting case of Herlyn-Werner-Wunderlich syndrome. Ci Ji Yi Xue Za Zhi. 2020;32(2):216–218. doi: 10.4103/tcmj.tcmj_13_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girardi Fachin C, Aleixes Sampaio Rocha JL, Atuati Maltoni A, das Chagas Lima RL, Arias Zendim V, Agulham MA. et al. Herlyn-Werner-Wunderlich syndrome: Diagnosis and treatment of an atypical case and review of literature. Int J Surg Case Rep. 2019;63:129–134. doi: 10.1016/j.ijscr.2019.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang HI, Fu SC, Yin CH, Chang CC. Herlyn-Werner-Wunderlich syndrome: An unusual case with presentation of menorrhagia. Taiwan J Obstet Gynecol. 2020;59(6):948–951. doi: 10.1016/j.tjog.2020.09.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.