Abstract
The plant extracts are known for their anti-inflammatory, antifungal, antiviral and antibacterial properties. The use of plant extracts in the preparation of bio-materials increases their biological application. In this concern, herein reporting an eco-friendly procedure which is also a simple and cost effective, for the synthesis of Zinc Oxide nanoparticles (ZnONPs) using Saussurea lappa plant root (rhizome) extract as a fuel. The prepared nanoparticles were confirmed using various characterization techniques. The Dynamic light scattering data showed 123.5 nm particle size with -99.9 mv zeta potential which indicates excellent stability of the particles. The peak at 541 cm−1 in the IR spectrum is assigned to the stretching frequency of the zinc-binding to oxygen. The X-ray diffraction peaks confirm the close association with JCPDS Data Card No: 36-1451. The FESEM data revealed a hexagonal wurtzite structure with a hexagonal shape of synthesized ZnO nanoparticles. The antibacterial studies indicate the gram-negative strains showed better inhibition activity than gram-positive strains. Among Fungal strains, Aspergillus niger and flavus, Fusarium oxysporum, and Rhizopus oryzae showed good inhibition activity at higher concentrations. The cytotoxic data indicates the 5 μg/mL of the ZnO particles showed cytotoxicity on the CHO cell line and with IC50 value 3.164 ± 0.8956 μg/mL.
Keywords: ZnO nanoparticles, Saussurea lappa, Antimicrobial activity, Cytotoxic activity
ZnO nanoparticles; Saussurea lappa; Antimicrobial activity; Cytotoxic activity.
1. Introduction
Today nanotechnology plays a vital role in the fields of science and technology due to their chemical, physical, and biological properties of the nanoparticles than the bulk [1, 2]. This leads to enhance the importance of nanoparticles (NPs) to a greater extent in various industries such as chemical, biological, medicinal, and environmental. In addition, nanoparticles also using in the purification of water [3], packaging and processing of food [4], in the storage devices [5, 6], fuel cells to catalytic converters and in biosensors [7, 8], photocatalytic studies [9], in drug delivery [10, 11] magnetic hyperthermia [12, 13] magnetofection [14], antimicrobial agents, cytotoxic agents, bone regeneration, proliferation, and cell adhesion capabilities [15], tissue engineering [16], bio-labelling [17], etc.
In the light of the above, the ZnO NPs were synthesized using different approaches for various applications. For example, Rania et.al reported the preparation of the ZnO nanoparticles by co-precipitation method for solar-driven photodegradation of Congo red dye [18], Mohd Farhan Khan. et.al reported by sol-gel method for antimicrobial activities [19], Hyeonhan Lim et.al reported by hydrothermal method photodegradation studies [20], Mosalagae. et.al reported by ultrasonic spray pyrolysis method [21], Anh Thi Le.et.al reported by solid precipitation method for removal of heavy metal ions [22], Yan Xiu Liu et.al reported microwave-assisted zinc oxide nanoparticles for photodegradation of CTMAB [23], Dapeng Wu et.al. prepared by the solvothermal method for dye-sensitized solar cells [24], Jen-Chieh Lin et.al synthesized by microemulsion technique for dye-sensitized solar cells [25], Raghu and Ram synthesized by supercritical water method [26] and Giri. et.al used a mechanical method for the preparation of ZnO materials [27]. Among, the most popular chemical synthetic methods usually require high levels of temperature and pressure. Then, also using toxic chemicals like sodium borohydride, citrate as reducing agents and capping agents. The use of these toxic materials may associate with environmental-related issues. Hence, a chemist searches for a method that is an easy, inexpensive, non-toxic method to synthesize bio-nanoparticles. To overcome these difficulties and reduce environmental issues, the alternative is the use of different plant extracts as a source of reducing agent in the preparation of nanoparticles. Hence green chemistry gained more interest in research due to eco-friendly property.
Several metal oxides such as oxides of silver, copper, gold, etc have been widely used for the green synthesis of NPs using different plant extracts [28, 29, 30]. As zinc is found in cells throughout the human body and is essential for the immune system, zinc oxide nanoparticles (ZnO-NPs) have gained more focus in biomedical applications. Even though, ZnO NPs are firstly used in the rubber industry for wearproof of the rubber composite to enhance the properties of the polymers such as toughness and intensity and antiaging, etc. [31, 32]. Thereafter ZnO-NPs holds greater potential to use as a photocatalyst in water purification [33, 34], light-emitting devices, solar cells, lasers, piezoelectric transducers, chemical sensors, photocatalysts, and transparent electronics are led in nanotechnologies [35, 36], Crop production, nutrient management [37], biomedical applications [38, 39, 40, 41, 42, 43, 44, 45, 46], anti-microbial preservative for wood and food products [47], etc.
For the various applications, the green synthesized ZnO-NPs reported in the literature by using many plant extracts [33, 34, 39, 42, 43, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57] to get a flower, cube, rod, wires shaped particles [58, 59, 60, 61]. The advantage of plant extracts in the green route of ZnONPs is due to the intrinsic bio-features of the plant extract help in exhibiting the distinctive properties of nanoparticles in a colloidal solution. In addition, the chemical constituents of the plant extract such as organic compounds (alkaloids, steroids, glycosides, resins, tannins, organic acids, volatile oils, sugars, enzymes) and inorganic ingredients (trace elements) [62] will be used as a reducing agent. Based on these properties they can be applied in medicinal and industrial fields. In the current study, the ZnO nanoparticles were prepared from the extract of the medicinal plant Saussurea plant.
Saussurea lappa (Costus or Kushta) plant belongs to the Asteraceae family, usually grows in Himalayan ranges at 8000–12000 feet, Western Ghats, in China, Korea, and Japan [63]. For many years this herb has been using in cosmetics and also in the treatment of gastric ulcers, arthritis, inflammation, skin healing, as a supplement for infertility, menstrual disorders, and many more. From the ancient days, this herb has been used as an anti-angiogenesis, antiarthritic, anti-asthmatic, antidysentery, anti-inflammatory, anti-ulcerative [64, 65].
Best of our knowledge, Saussurea lappa plant extract used for the synthesis of the oxides of the Silver [66], Magnesium [67], and Selenium [68] but no literature available on a synthesis of ZnO-NPs. In the light of the above, herein reporting the preparation of ZnO nanoparticles from roots (rhizome) of Saussurea lappa plant methanol extract and its anti-bacterial, anti-fungal activities and cytotoxicity effect of ZnO nanoparticles against Chinese Hamster Ovary (CHO) cell lines.
2. Experimental
2.1. Materials
Zinc nitrate hexahydrate (98% AR), EDTA, Ammonium hydroxide were purchased from Merck, India Pvt. Ltd. The Chinese Hamster Ovary (CHO) cells were obtained from National Centre for Cell Sciences (NCCS), Pune, India. All other solvents used were analytical grade and distilled water used throughout the work.
2.2. Physical measurements
The electronic spectrum of the material was measured in the range of 200–800nm using Spectra 450 SHIMADZU UV-Visible Spectrophotometer. Dynamic Light Scattering technique was used in order to measure the size and distribution of NPs (Nanopartica, HORIBA, SZ-100). The ATR (Attenuated Total Reflectance) Fourier–Transform Infrared (FT-IR) spectrum of the material was recorded between 500–4000cm−1 with the KBr pellet method. The crystallinity of the nanoparticles was determined by using Rigaku Miniflex 600, Japan X-Ray Diffraction (XRD) tool. The shape and external morphology of the NPs were characterized by using the Field Emission Scanning Electron Microscope (FESEM) for Elemental and chemical analysis of NPs was done by Energy Dispersive X-ray (EDX) of the model (FEI-Quanta FEG 200F) of IIT- Madras, Chennai.
2.3. Extraction of the plant
The root extract of the Saussurea lappa was used in the preparation of the Zinc oxide NPs and the extract was collected using the following procedure: The roots (rhizome) of Saussurea lappa were gathered from the Srinagar and the surrounding forest region, Jammu and Kashmir, India. Dr. K. Madhava Chetty authenticated the same and sample is deposited in herbarium, Botany department, S.V. University, Tirupati, India. The roots of the plant were grinded to get powder using a pulverizing machine and extracted with methanol on soaking for 72 h.
2.4. Preparation of ZnO nanoparticles from methanol extract of the Saussurea lappa
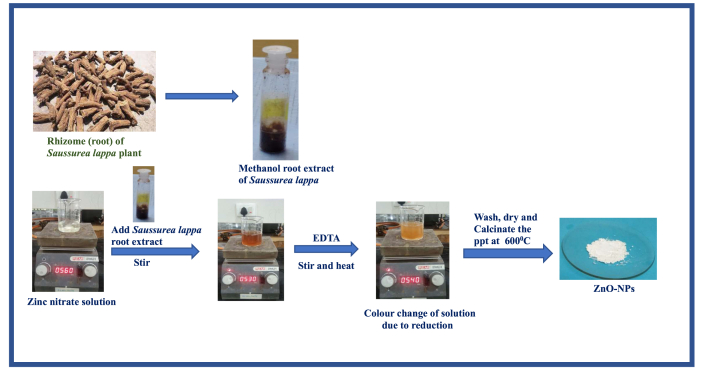
The ZnO nanoparticles were prepared by the Co-precipitation method using plant extract (Figure 1). To a 50 ml of the plant extract added 50 ml of the 0.1 M aqueous solution of hexahydrate zinc nitrate. The resultant mixture was stirred for about 15 min and continued the stirring at 60 °C for about 20 min more. Then added EDTA (0.1 g) and also a few drops of ammonium hydroxide solution to maintain pH 12 and the mixture was vigorously stirred at 1000 rpm for 2 h at 70–80 °C. The precipitation formed was cooled and washed with distilled water and ethanol 3–5 times and centrifuged the solution with ethanol solution. Thereafter, the supernatant solution was discarded and dried the obtained precipitate. The dried precipitate was grinded with a mortar and pestle to make a fine powder. Then calcinated the powder at 600 °C for 4 h to clean and pure nanoparticles.
Figure 1.
Synthetic route of ZnO-NPs using Saussurea lappa plant extract.
2.5. Biological studies
2.5.1. Antimicrobial activity
The disk diffusion agar analysis has been followed to test the effectiveness of the antimicrobial activity of biosynthesized ZnO nanoparticles against gram-positive (Streptococcus aureus, Bacillus subtilis) gram-negative (Sphingobacterium thalpophilum, Staphylococcus aureus, E. coli, Pseudomonas aeruginosa, Sphingobacterium sp, Acinetobacter sp, Ochrobactrum sp) bacteria and also on fungi. Three different concentrations of (170, 100, 50 ppm) ZnO-NPs were prepared and introduced an array of bacterial species.
2.5.2. Cytotoxicity effect of ZnO nanoparticles
The invitro-cytotoxic studies of the ZnO-NPs were evaluated using the MTT [(3-(4, 5-dimethylthiozol-2-yl)-2,5-diphenyltetrazolium bromide)] assay method on Chinese Hamster Ovary (CHO) cell lines, a widely employed for the expression of recombination proteins. The cells were grown at 37 °C in a humidified incubator under 5% CO2/95% air in DMEM with 10% Fetal Bovine Serum (FBS). The CHO cells have been planted in 96 well tissue culture plates at the quantity of 0.2 × 106 cells per well. After that the corresponding CHO cells were kept in a humidified CO2 incubator to treat with test compounds for 24 h. Then the cells were treated with 20 μL of 5 mg/mL MTT and incubated for 4 h in a humidified atmosphere. Then added 200 μL of DMSO to the wells so as to dissolve the MTT formazan crystals. Further in the control experiment, the cells were grown without test compounds, in the same media. Immediately after the development of purple colour, the absorbance of the wells was measured at 570 nm. In the control cells, the formazan generated was picked up in order to represent 100 % viability. Relative cell viability was computed in relation to the extent of MTT converted into formazan salt. Three independent experiments were executed and the average ± S.E.M was calculated. Cell viability (%) was calculated using the following Eq. (1) and reported as cell viability (%) Vs concentration (μM).
| (1) |
Inhibition Concentration (IC50) means the concentration of ZnONPs inhibiting 50% of cell growth and the same was calculated from the graph of visible cells against nanoparticle concentration.
2.5.3. Statistical analysis
All the data reported were examined repeatedly for three trials. The data were analysed using SPSS version 16.0 and computed mean ± SD. Further, one way ANOVA was carried followed by Duncan's Multiple Range Test (DMRT) (P < 0.05).
3. Results and discussion
3.1. Synthesis and characterization of ZnO-NPs
As shown in Figure 1, the zinc oxide nanoparticles were prepared by adding Saussurea lappa plant extract as reducing agent to an aqueous solution of hexahydrate zinc nitrate by the co-precipitation method. The ingredients flavonoid glycosides, chlorogenic acid, shikokiols, chrysophanol, etc [69] may be caused to change zinc(II) ion to zinc oxide NPs. The physicochemical changes during the preparation of zinc oxide nanoparticles were observed in previously reported literature [70]. The formation of ZnO-NPs was further investigated by following analytical techniques.
3.1.1. UV visible spectral analysis
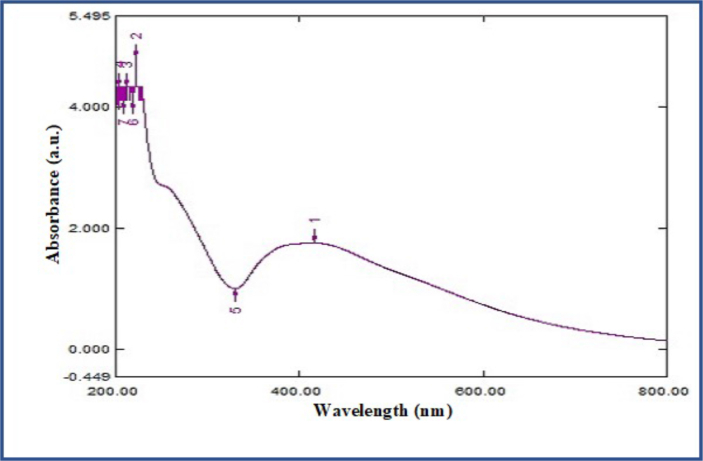
A UV-Visible spectrum of the prepared sample was recorded in the range of 200–800 nm at different time intervals using Spectra 450, SHIMADZU Spectrophotometer. In an aqueous colloidal solution, the formation and the stability of the synthesized ZnO NPs were confirmed by the absorption maximum peak at 430 nm (Figure 2) and this peak occurs due to the phenomenon of redshift.
Figure 2.
UV-visible spectrum analysis of Saussurea lappa mediated synthesized ZnONPs.
3.1.2. Dynamic light scattering analysis
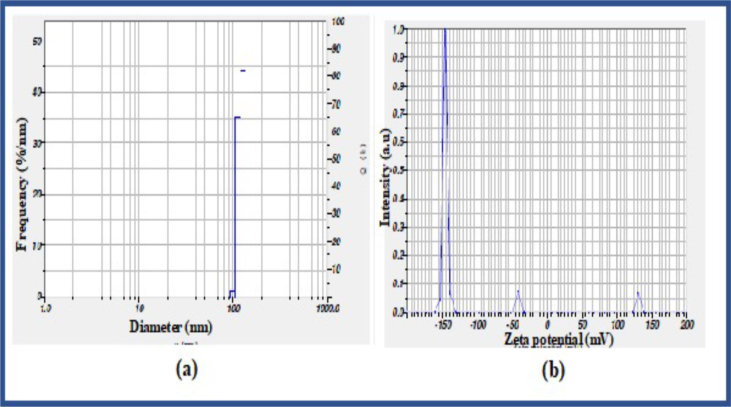
Dynamic light scattering is the most useful technique to determine particle size. The formed ZnO nanoparticles are well distributed concerning volume and intensity. Zeta potential analysis determines the surface charge of synthesized nanoparticles. The Nanopartica SZ-100 instrument was used to know the particle size and zeta potential. The particle size distribution spectrum was recorded by plotting the diameter (nm) on the X-axis and frequency (%/nm) on Y-axis. The zeta potential spectra of ZnO nanoparticles were taken by plotting the zeta potential (mV) and intensity (a.u) on the X and Y-axis respectively. The nanoparticle's stability is directly proportional to magnitude of the charge. From Table 1, the synthesized nanoparticle showed 123.5 nm particle size with -99.9 mv zeta potential which indicates excellent stability (Figure 3a and b). The higher value of zeta potential (either positive or negative) implies excellent physical colloidal stability [71]. This is because of the electrostatic repulsions among the respective particles. In another way, assembling the particles may show the Zeta potential of a lower magnitude.
Table 1.
Dynamic light scattering and Zeta potential data of synthesized ZnO nanoparticles.
| Particle Size |
Zeta Potential |
||||||
|---|---|---|---|---|---|---|---|
| Peak No | S P area ratio | Mean nm | SD nm | Mode nm | Peak No | Zeta Potential mV | Electrophoretic Mobility mean cm2/Vs |
| 1 | 1.00 | 124.0 | 11.4 | 123.5 | 1 | -146.1 | -0.001131 |
| 2 | - | - | - | - | 2 | -41.6 | -0.000322 |
| 3 | - | - | - | - | 3 | 130.7 | 0.001012 |
| Total | 1.00 | 124.0 | 11.4 | 123.5 | -99.9 | -0.000774 | |
Figure 3.
(a) Histogram of synthesized Zinc oxide nanoparticles using Dynamic light scattering. (b) Zeta potential of synthesized ZnO nanoparticles.
3.1.3. Fourier transform infrared (FT-IR) spectroscopy
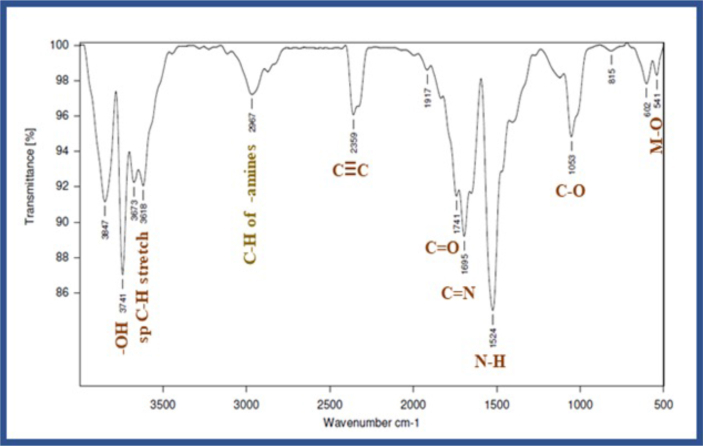
The FT-IR spectrum of synthesized nanoparticles from Saussurea lappa was recorded in the range from 500– 4000 cm−1 by using the ATR technique and representative spectrum shown in Figure 4. In the FT-IR spectrum, the peaks at 3847 cm−1, 3741 cm−1, and 3673 cm−1 observed correspond to O–H stretching absorption of zinc nitrate hexahydrate. Peaks at 3618 cm−1 related to the stretching vibrations of C–H bonds of alkynes which may present in alkaloids of plant extract. The peak at 2967 cm−1 is for the strong C–H stretching vibrations of amines. The peak at 2359 cm−1 corresponds to the C≡C stretching vibrations of alkynes present in the plant extract. The peak at 1917 cm−1 related to the strong C=O stretching. The peak at 1741 cm−1 shows the C=O stretching vibrations of carboxylic acids. The peak at 1695 cm−1 was assigned to the C–N stretching. The peak at 1524 cm−1 shows the N–H bending and -C-O-H in-plane band. The peak at 1053 cm−1 shows the strong C–O stretching. The peaks at 815 cm−1 and 602 cm−1 are assigned to the strong stretching and bending of C–H bonds. The peak at 541 cm−1 corresponds to a standard peak of (ZnO) due to the stretching frequency of the metal binding to oxygen. Similar observations were reported in the literature [50, 72, 73, 74, 75].
Figure 4.
IR spectrum of the synthesized ZnO nanoparticles.
3.1.4. X-ray diffraction (XRD) analysis
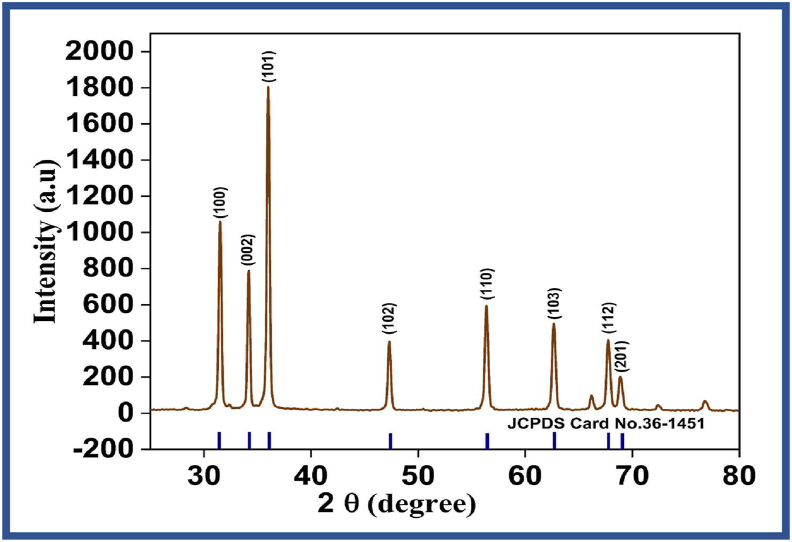
Powder X-ray diffraction (XRD) was used to investigate the structural information, identify the phase and crystallinity of the prepared ZnO-NPs, and Figure 5 shows the XRD patterns of synthesized ZnO nanoparticles using S. lappa plant extract. The intensity data were obtained in the range of 200–800. A definite peak broadening indicates that the synthesized material contains the particles in the nanoscale range was obtained at λ = 1.54 A0 by using X-Ray Diffractometer. The diffraction peaks (2θ) at 31.54°, 34.30°, 36.11°, 47.28°, 56.47°, 62.77°, 67.89° and 69.03° correspond to the planes (100), (002), (101), (102), (110), (103), (112) and (201) confirmed the close association with JCPDS Data Card No: 36-1451 (Figure 5). There was the similarity of the lattice planes reported in previous literature of ZnO nanoparticles synthesis from Ixora Coccinea leaf extract [72], Cinnamomum Tamala [76], Laurus nobilis [77], and Costus pictus leaf extracts [56]. Therefore, it was confirmed that the synthesized ZnO nanoparticles exhibit a hexagonal wurtzite structure with slight agglomeration. The average size of the synthesized ZnO nanoparticles obtained was around 26±1nm. Apart from the Bragg peaks of FCC ZnO nanoparticles, unassigned peaks were also observed in the spectrum implying the crystallization of the bio-organic state formed on the surface of the NPs.
Figure 5.
XRD spectrum of synthesized ZnO nanoparticles.
3.1.5. Morphology studies of zinc oxide nanoparticles
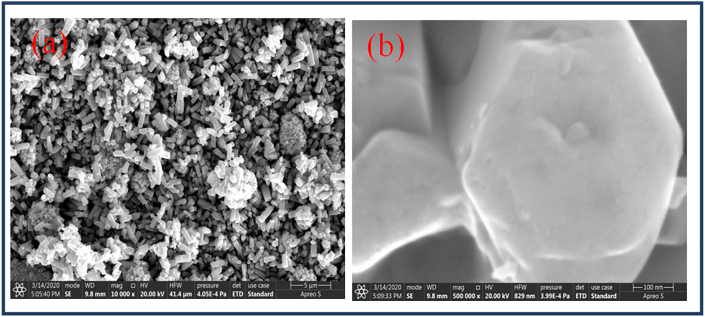
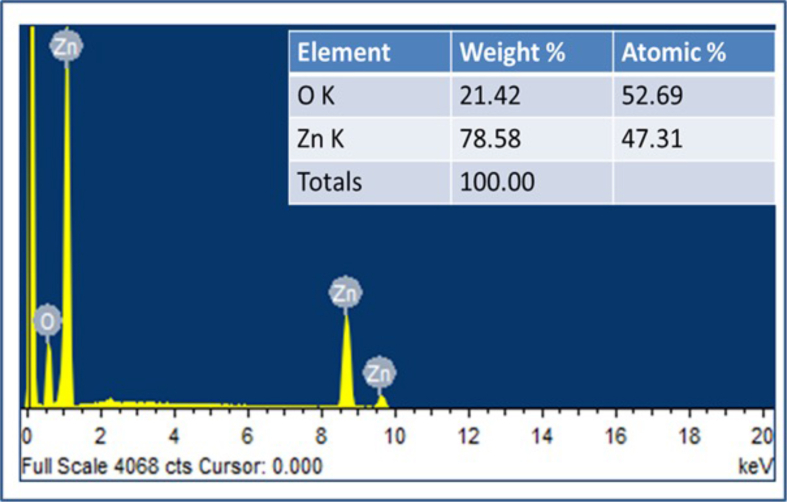
The FESEM images of prepared ZnO NPs analysis helps to determine the structure of the nanoparticles. The FESEM images (Figure 6a and b) revealed a hexagonal wurtzite structure with hexagonal shape of synthesized ZnONPs. The results of the JCPDS data of XRD and FESEM have correlated the shape of the nanoparticles. The elemental composition of ZnO nanoparticles was confirmed by Energy Dispersive X-ray (EDX) analysis (Figure 7) of the NPs and composed of zinc, oxygen. In the synthesized nanoparticles strong signals of ZnO atoms were observed, it revealed that the ZnO nanoparticles have Zn rich environment in the lattice.
Figure 6.
FESEM images of synthesized ZnO nanoparticles (a) at 5 μm and (b) at 100 nm.
Figure 7.
EDX graph and elemental analysis of ZnO nanoparticles.
3.2. Biological activities of the prepared zinc oxide nanoparticles
3.2.1. Antimicrobial activity
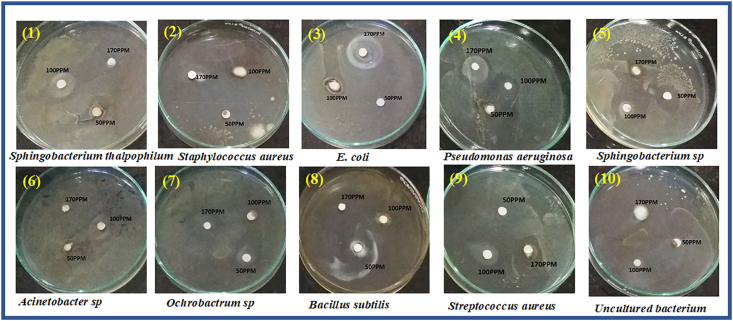
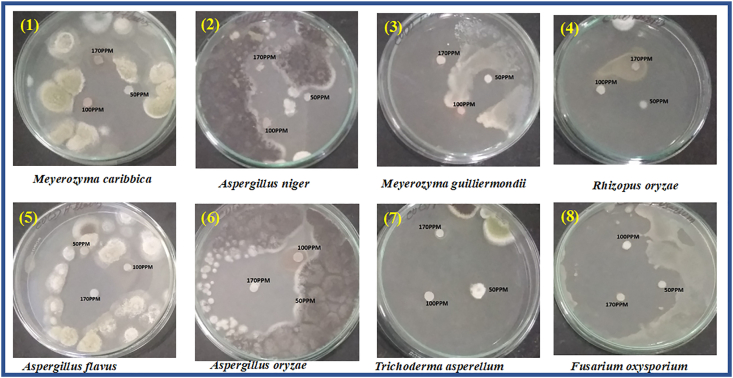
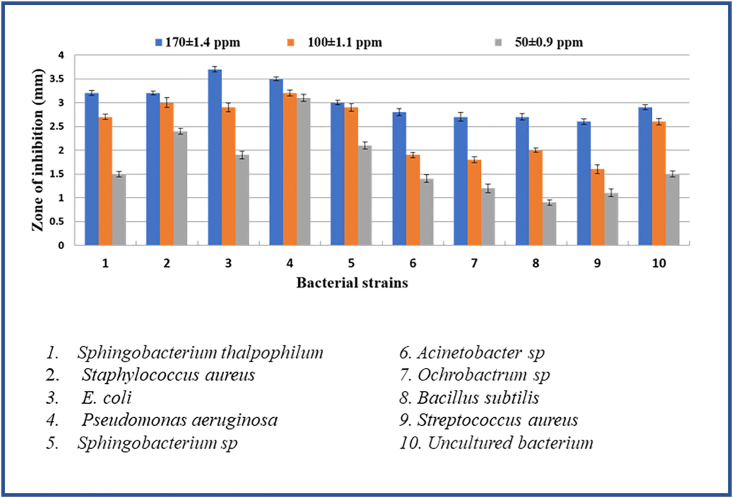
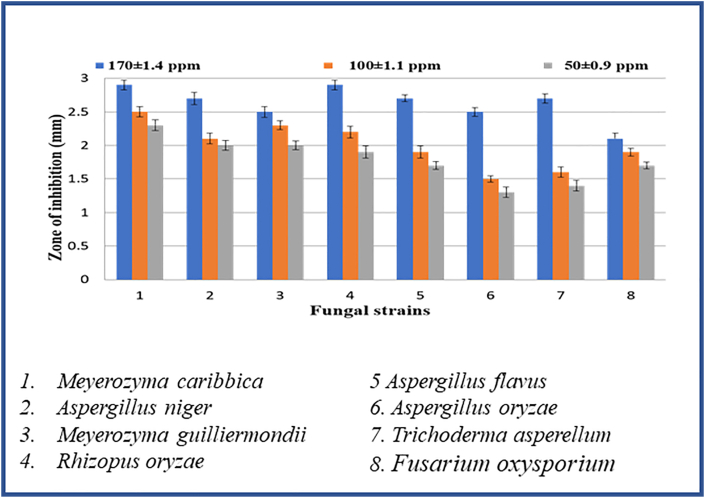
The mechanism of the ZnONPs antimicrobial activity even though not clear, the literature suggests that the activity due to various mechanisms [78, 79, 80, 81, 82, 83]. In present studies, the antimicrobial activity was investigated by disk diffusion analysis for biosynthesized ZnO nanoparticles performed for three different concentrations (50, 100, 170 ppm) against gram-positive (Streptococcus aureus, Bacillus subtilis), and gram-negative (Sphingobacterium thalpophilum, Staphylococcus aureus, E. coli, Pseudomonas aeruginosa, Sphingobacterium sp, Acinetobacter sp, Ochrobactrum sp) bacteria and also on fungi. At 170 ppm concentration, ZnO nanoparticles exhibited a notable antimicrobial effect compared over the concentrations at 50 and 100 ppm. A similar observation was made in reported literature [83, 84, 85]. From Table 2, Figure 8 and Figure 9, Among the tested pathogenic strains, the gram-negative strains showed better inhibition activity than gram-positive strains [86]. This may be due to the nature of the structure of the cell wall, cell physiology, metabolism or degree of contact, etc. But when compared to E. Coli and S. Aureus (Gram-positive) P. Aeruginosa (Gram-negative) and Bacillus (Gram-positive) showed an effective zone of inhibition in all concentrations. In the case of anti-fungal activity, ZnO NPs showed good inhibition activity at higher concentrations (170ppm) than the lower concentration (50 and 100ppm). Among, Aspergillus Niger and Aspergillus Flavus, Fusarium Oxysporum and Rhizopus showed very good inhibition activity at higher concentrations (170 and 100 ppm). The higher activity of ZnO nanoparticles was due to their well-developed chemical stability, surface chemistry, and smaller size, enables to interact with the microorganisms easily. Also, the particles interact with the building blocks of the outer membrane and might cause structural changes, degradation, and finally leads to cell lysis. The inhibitory action of the microbes may be due to the loss of DNA replication ability upon treatment with the metal ion. All this is due to the inactivation of ribosomal subunit proteins expression, as well as inactivation of other cellular proteins. Mainly the inactivation of enzymes essential for ATP production occurs due to the release of ROS (Reactive oxygen species) [87]. The results of the antifungal assay of ZnO nanoparticles were given in (Table 3), Figure 10 and Figure 11. The mechanism of inhibition of metallic nanoparticles on microorganisms is not well known. Nanoparticles bind with cytoplasmic membrane and killed bacterial and fungal cells. This comes out of the electrostatic interaction between positively charged nanoparticles and negatively charged cell membranes of microorganisms.
Table 2.
In-vitro antibacterial studies of synthesized ZnO nanoparticles.
| S. No | Bacteria | Zinc oxide Nanoparticles Zone of inhibition (mm) |
||
|---|---|---|---|---|
| 170 ± 2 ppm | 100 ± 1 ppm | 50 ± 1 ppm | ||
|
Gram-negative | ||||
| 1 | Sphingobacterium thalpophilum | 3.2 ± 0.04bc | 2.7 ± 0.02d | 1.5 ± 0.05cd |
| 2 | Staphylococcus aureus | 3.2 ± 0.03a | 3.0 ± 0.05a | 2.4 ± 0.06a |
| 3 | E. coli | 3.7 ± 0.05a | 2.9 ± 0.04abc | 1.9 ± 0.07c |
| 4 | Pseudomonas aeruginosa | 3.5 ± 0.08bc | 3.2 ± 0.08ab | 3.1 ± 0.05cd |
| 5 | Sphingobacterium sp | 3.0 ± 0.06a | 2.9 ± 0.03a | 2.1 ± 0.16a |
| 6 | Acinetobacter sp | 2.8 ± 0.04b | 1.9 ± 0.04d | 1.4 ± 0.06b |
| 7 |
Ochrobactrum sp |
2.7 ± 0.18de |
1.8 ± 0.13a |
1.2 ± 0.08d |
|
Gram-positive | ||||
| 8 | Bacillus subtilis | 2.7 ± 0.07d | 2.0 ± 0.04b | 0.9 ± 0.08d |
| 9 | Streptococcus aureus | 2.6 ± 0.02d | 1.6 ± 0.06bc | 1.1 ± 0.07cd |
| 10 | Uncultured bacteriumFigure 8 | 2.9 ± 0.17d | 2.6 ± 0.06abc | 1.5 ± 0.09d |
| Completely Randomized Design CRD (P ≤ 0.05) |
0.295 | 0.259 | 0.248 | |
The data presented is ± SE of three measurements.
Data followed by the same letter are not significantly different at P ≤ 0.05, whereas those followed by different letters are significantly different at P ≤ 0.05.
Figure 8.
In-vitro antibacterial studies of ZnO nanoparticles with bacterial strains: 1. Sphingobacterium thalpophilum 2. Staphylococcus aureus 3. E. coli 4. Pseudomonas aeruginosa 5. Sphingobacterium sp 6. Acinetobacter sp 7. Ochrobactrum sp 8. Bacillus subtilis 9. Streptococcus aureus 10. Uncultured bacterium.
Figure 9.
In-vitro antifungal studies of ZnO nanoparticles with strains: 1. Meyerozyma caribbica 2. Aspergillus niger 3. Meyerozyma guilliermondii 4. Rhizopus oryzae 5. Aspergillus flavus 6. Aspergillus oryzae 7. Trichoderma asperellum 8. Fusarium oxysporium.
Table 3.
In-vitro antifungal studies of synthesized ZnO nanoparticles.
| S No | Fungi | Zinc oxide Nanoparticles Zone of inhibition (mm) |
||
|---|---|---|---|---|
| 170 ± 2 ppm | 100 ± 1 ppm | 50 ± 1 ppm | ||
| 1 | Meyerozyma caribbica | 2.9 ± 0.12b | 2.5 ± 0.08cd | 2.3 ± 0.02ab |
| 2 | Aspergillus niger | 2.7 ± 0.14c | 2.1 ± 0.22c | 2.0 ± 0.05a |
| 3 | Meyerozyma guilliermondii | 2.5 ± 0.05b | 2.3 ± 0.14d | 2.0 ± 0.07a |
| 4 | Rhizopus oryzae | 2.9 ± 0.08a | 2.2 ± 0.02ab | 1.9 ± 0.08c |
| 5 | Aspergillus flavus | 2.7 ± 0.06a | 1.9 ± 0.08ab | 1.7 ± 0.06a |
| 6 | Aspergillus oryzae | 2.5 ± 0.04c | 1.5 ± 0.06c | 1.3 ± 0.04a |
| 7 | Trichoderma asperellum | 2.7 ± 0.08a | 1.6 ± 0.05a | 1.4 ± 0.12b |
| 8 | Fusarium oxysporium | 2.1 ± 0.02a | 1.9 ± 0.04d | 1.7 ± 0.14c |
| C.R.D (P < 0.05) The data |
0.27 | 0.23 | 0.22 | |
The data presented is ± SE of three measurements.
Data followed by the same letter are not significantly different at P ≤ 0.05, whereas those followed by different letters are significantly different at P ≤ 0.05.
Figure 10.
Bar diagram of in-vitro antibacterial studies of ZnO nanoparticles.
Figure 11.
Bar diagram of in-vitro antifungal studies of ZnO nanoparticles.
3.2.2. Cytotoxicity effect of ZnO nanoparticles
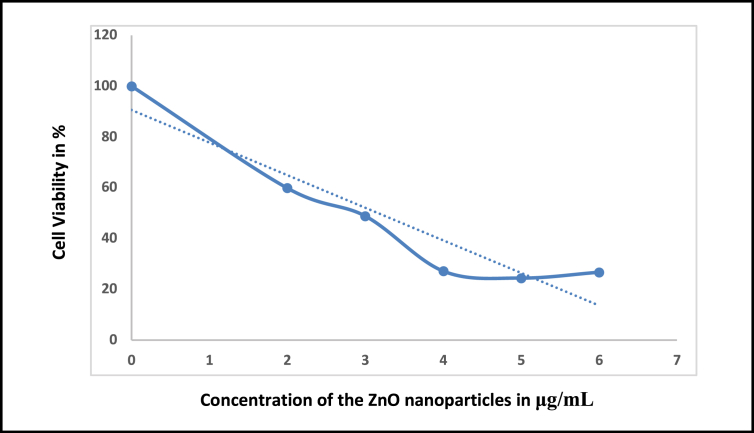
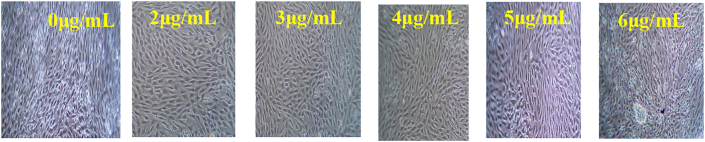
In vitro cytotoxic effect of ZnO-NPs on Chinese Hamster Ovary (CHO) cells were investigated using different concentrations of ZnO-NPs and the data provided in Table 4. In a control experiment, cells were cultured same as in media without ZnO NPs. At 570 nm the absorbance was recorded preceded by the development of purple colour. Three separate experiments were executed and mean ± SEM was determined and dose dependent graph was plotted between cell viability in % Vs concentration (μg/mL) (Figure 12). A concentration of 2, 3, 4, 5, 6 μg/mL nanoparticles were used to investigate the cytotoxic studies on dosage effect. From Figure 13, on increasing the concentration of the NPs up to 5μg the % of the cell viability decreases then again increases for 6μg. Hence 5μg concentration cells showed cytotoxicity on the CHO cell line and its IC50 value is 3.164 ± 0.8956 μg/mL. Further size and shape dependent uptake of ZnO nanoparticles into mammalian cells have been reported and point to the need for in-depth study of antimicrobial and cytotoxic effects of nanoparticles.
Table 4.
Cytotoxic data of the ZnO nanomaterials on CHO cell lines.
| Sample Concentration in μg/mL | Optical Density |
Mean | SD | SE | % of Control | ||
|---|---|---|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 3 | |||||
| Control | 1.49 | 1.50 | 1.39 | 1.46 | 0.06 | 0.04 | 100.00 |
| 2 | 0.88 | 0.89 | 0.85 | 0.87 | 0.02 | 0.01 | 59.82 |
| 3 | 0.71 | 0.78 | 0.65 | 0.71 | 0.07 | 0.04 | 48.86 |
| 4 | 0.42 | 0.36 | 0.41 | 0.40 | 0.03 | 0.02 | 27.17 |
| 5 | 0.37 | 0.36 | 0.34 | 0.36 | 0.02 | 0.01 | 24.43 |
| 6 | 0.37 | 0.39 | 0.41 | 0.39 | 0.02 | 0.01 | 26.71 |
Figure 12.
Dose dependent graph for cytotoxic studies for ZnO nanoparticles.
Figure 13.
Cytotoxic effect of synthesized ZnO nanoparticles on CHO cell lines.
4. Conclusion
In the present work, ZnO nanoparticles were prepared using methanolic extract of roots (rhizome) of the Saussurea lappa plant by the co-precipitate method. The formation of NPs confirmed using UV–Vis, FT-IR, XRD, DLS, and FESEM techniques. In the IR spectrum, the peak at 541 cm−1 assigned to the stretching frequency of the Zn–O vibration. The FESEM data indicates a hexagonal wurtzite structure with hexagonal shape of the ZnO nanoparticles. The antibacterial data shows the gram-negative strains better inhibition activity than gram positive strains. Among tested Fungal strains, Aspergillus niger and Aspergillus flavus, Fusarium oxysporum, and Rhizopus showed very good inhibition activity at higher concentrations. The cytotoxic data indicates the 5 μg/mL of the ZnO showed cytotoxicity on the CHO cell line and with an IC50 value of 3.164 ± 0.8956 μg/mL.
Declarations
Author contribution statement
Lalitha A. Kolahalam: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
K. R. S. Prasad: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
P. Murali Krishna: Analyzed and interpreted the data; Wrote the paper.
N. Supraja: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
Lalitha A. K was supported by Koneru Lakshmaiah Education Foundation, Vaddeswaram (A.P) India.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thankful to Koneru Lakshmaiah Education Foundation (KLEF), Vaddeswaram (A.P) India, for providing necessary laboratory facilities to carry out research work.
Contributor Information
K.R.S. Prasad, Email: krsprasad_fed@kluniversity.in.
P. Murali Krishna, Email: muralikp@msrit.edu.
References
- 1.Zak A.K., Razali R., Majid W.H., Darroudi M. Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int. J. Nanomed. 2011;6:1399–1403. doi: 10.2147/IJN.S19693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaudhury K., Kumar V., Kandasamy J., Roy Choudhury S. Regenerative nano- medicine: current perspectives and future directions. Int. J. Nanomed. 2014;9:4153–4167. doi: 10.2147/IJN.S45332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaur P., Thakur R., Malwal H., Manuja A., Chaudhury A. Biosynthesis of biocompatible and recyclable silver/iron and gold/iron core-shell nanoparticles for water purification technology. Biocatal. Agricult. Biotechnol. 2018;14:189–197. [Google Scholar]
- 4.Sepulveda J., Villegas C., Torres A., Vargas E., Rodriguez F., Baltazar S., Prada A., Rojas A., Romero J., Faba S., Galotto M.J. Effect of functionalized silica nanoparticles on the mass transfer process in active∖PLA nanocomposite films obtained by supercritical impregnation for sustainable food packaging. J. Supercrit. Fluids. 2020;161:104844. [Google Scholar]
- 5.Shiri H.M., Ehsani A., Behjatmanesh-Ardakani R., Hajghani S. Electrosynthesis of Y2O3 nanoparticles and its nanocomposite with ∖ POAP as high efficient electrode materials in energy storage device: surface, density of state and electrochemical investigation. Solid State Ionics. 2019;338:87–95. [Google Scholar]
- 6.Bose P., Bhattacharya S. Influence of anion structure on thermal, mechanical, dielectric and electrochemical properties of ∖ ZrO 2 nanoparticle-pyrrolidinium ionic liquid hybrid electrolytes for the application in energy storage devices. Int. J. Hydrogen Energy. 2017;43:4090–4100. [Google Scholar]
- 7.Yadav N., Chhillar A.K., Pundir C.S. Preparation, characterization and application of haemoglobin nanoparticles for detection of acrylamide in processed foods. Int. J. Biol. Macromol. 2018;107A:1000–1013. doi: 10.1016/j.ijbiomac.2017.09.070. [DOI] [PubMed] [Google Scholar]
- 8.Majdi H., Salehi R., Pourhassan-Moghaddam M., Mahmoodi S., Poursalehi Z., Vasilescu S. Antibody conjugated green synthesized chitosan-gold nanoparticles for optical biosensing. Colloid Interf. Sci. Commun. 2019;33:100207. [Google Scholar]
- 9.Nagaraju G., Karthik K., Shashank M. Ultrasound-assisted Ta2O5 nanoparticles and their photocatalytic and biological applications. Microchem. J. 2019;147:749–754. [Google Scholar]
- 10.Liang J., Dong X., Yang A., Zhu D., Kong D., Lv F. A dual fluorescent reverse targeting drug delivery system based on curcumin-loaded ovalbumin nanoparticles for allergy treatment. Nanomed. Nanotechnol. Biol. Med. 2018;16:56–68. doi: 10.1016/j.nano.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Tonbul H., Sahin A., Tavukcuoglu E., Esendagli G., Capan Y. Combination drug delivery with actively-targeted ∖ PLGA nanoparticles to overcome multidrug resistance in breast cancer. J. Drug Deliv. Sci. Technol. 2019;54:101380. [Google Scholar]
- 12.Daboin V., Briceño S., Suárez J., Carrizales-Silva L., Alcalá O., Silva P., Gonzalez G. Magnetic∖SiO 2-Mn1 - xCoxFe 2 O 4 nanocomposites decorated with Au @ Fe 3 O 4 nanoparticles for hyperthermia. J. Magn. Magn Mater. 2019;479:91–98. [Google Scholar]
- 13.Sharma S.K., Shrivastava N., Rossi F., Tung L.D., Thanh N.T.K. Nanoparticles-based magnetic and photo induced hyperthermia for cancer treatment. Nano Today. 2019;29:100795. [Google Scholar]
- 14.Yapici M.K., Al Nabulsi A., Rizk N., Boularaoui S.M., Christoforou N., Lee S. Alternating magnetic field plate for enhanced magnetofection of iron oxide nanoparticle conjugated nucleic acids. J. Magn. Magn Mater. 2019;469:598–605. [Google Scholar]
- 15.Coluccio M.L., De Vitis S., Strumbo G., Candeloro P., Perozziello G., Di Fabrizio E., Gentile F. Inclusion of gold nanoparticles in meso-porous silicon for the ∖ SERS analysis of cell adhesion on nano-structured surfaces. Microelectron. Eng. 2016;158:102–106. [Google Scholar]
- 16.Augustine R., Dalvi Y.B., Yadu Nath V.K., Varghese R., Raghuveeran V., Hasan A., Thomas S., Sandhyarani N. Yttrium oxide nanoparticle loaded scaffolds with enhanced cell adhesion and vascularization for tissue engineering applications. Mater. Sci. Eng. C. 2019;103:109801. doi: 10.1016/j.msec.2019.109801. [DOI] [PubMed] [Google Scholar]
- 17.MubarakAli D., Arunkumar J., Nag K.H., SheikSyedIshack K.A., Baldev E., Pandiaraj D., Thajuddin N. Gold nanoparticles from Pro and eukaryotic photosynthetic microorganisms-Comparative studies on synthesis and its application on biolabelling. Colloids Surf. B Biointerfaces. 2013;103:166–173. doi: 10.1016/j.colsurfb.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Rania E. Adam, Gallia Pozina, Magnus Willander, and Omer Nur, Synthesis of ZnO nanoparticles by co-precipitation method for solar driven photodegradation of Congo red dye at different pH. Photon. Nanostruct. Fundament. Appl. 2018;32:11–18. [Google Scholar]
- 19.Khan Mohd Farhan, Ansari Akhter H., Hameedullah M., Ahmad Ejaz, Mabood Husain Fohad, Zia Qamar, Baig Umair, Rehan Zaheer Mohd, Mezbaul Alam Mohammad, Mustafa Khan Abu, AlOthman Zeid A., Ahmad Iqbal, Ashraf Ghulam Md, Aliev Gjumrakch. sol-gel synthesis of thorn-like ZnO nanoparticles endorsing mechanical stirring effect and their antimicrobial activities: potential role as nano-antibiotics. Sci. Rep. 2016;6:27689. doi: 10.1038/srep27689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim Hyeonhan, Yusuf Mohammad, Song Sehwan, Park Sungkyun, Hyun Park Kang. Efficient photocatalytic degradation of dyes using photo-deposited Ag nanoparticles on ZnO structures: simple morphological control of ZnO. RSC Adv. 2021;11:8709–8717. doi: 10.1039/d0ra10945b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosalagae K., Murape D.M., Lepodise L.M. Effects of growth conditions on properties of CBD synthesized ZnO nanorods grown on ultrasonic spray pyrolysis deposited ZnO seed layers. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anh Thi Le, Pung Swee-Yong, Sreekantan Srimala, Matsuda Atsunori, Huynh Dai Phu. Mechanisms of removal of heavy metal ions by ZnO particles. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Yanxiu, Song Hua, Zhu Kenan, Li Feng. Microwave-assisted synthesis of zinc oxide and its performance in photodegradation of CTMAB. Res. Chem. Intermed. 2017;43:971–982. [Google Scholar]
- 24.Wu Dapeng, Gao Zhiyong, Xu Fang, Chang Jiuli, Tao Wenguang, He Jinjin, Gao Shuyan, Jiang Kai. Hierarchical ZnO Aggregates assembled by orderly Aligned nanorods for dye-sensitized solar cells. Crystal Eng. Commun. 2013;15:1210–1217. [Google Scholar]
- 25.Lin Jen-Chieh, Lee Chuan-Pei, Ho Kuo-Chuan. Zinc oxide synthesis via a microemulsion technique: morphology control with application to dye-sensitized solar cells. J. Mater. Chem. 2012;22(4):1270–1273. [Google Scholar]
- 26.Raghu V., Gupta Ram B. Formation of zinc oxide nanoparticles in supercritical water. J. Supercrit. Fluids. 2004;148(1):15–19. [Google Scholar]
- 27.Giri P.K., Bhattacharyya S., Singh D.K., Kesavamoorthy R., Panigrahi B.K., Nair K.G.M. Correlation between microstructure and optical properties of ZnO nanoparticles synthesized by ball milling. J. Appl. Phys. 2007;102(9) [Google Scholar]
- 28.Mittal A.K., Chisti Y., Banerjee U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013;31(2):346–356. doi: 10.1016/j.biotechadv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Rajan R., Chandran K., Harper S.L., Yun S.I., Kalaichelvan P.T. Plant extract synthesized silver nanoparticles: an ongoing source of novel biocompatible materials. Ind. Crop. Prod. 2015;70:356–373. [Google Scholar]
- 30.Ahmed S., Ahmad M., Swami B.L., Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. Adv. Res. 2016;7:17–28. doi: 10.1016/j.jare.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolodziejczak-Radzimska A., Jesionowski T. Zinc oxide–from synthesis to application: a review. Materials. 2014;7(4):2833–2881. doi: 10.3390/ma7042833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahoo S., Maiti M., Ganguly A., George J.J., Bhowmick A.K. Effect of zinc oxide nanoparticles as cure activator on the properties of natural rubber and nitrile rubber. J. Appl. Polym. Sci. 2007;105(4):2407–2415. [Google Scholar]
- 33.Golmohammadi Morteza, Honarmand Moones, Ghanbari Saeed. A green approach to synthesis of ZnO nanoparticles using jujube fruit extract and their application in photocatalytic degradation of organic dyes. Spectrochim. Acta Mol. Biomol. Spectrosc. 2020;229:117961. doi: 10.1016/j.saa.2019.117961. [DOI] [PubMed] [Google Scholar]
- 34.Chen Ling, Batjikh Indra, Hurh Joon, Han Yaxi, Huo Yue, Ali Hashmoonah, Feng Li Jin, Jahan Rupa Esrat, Chan Ahn Jong, Mathiyalagan Ramya, Yang Deok Chun. Green synthesis of zinc oxide nanoparticles from root extract of Scutellaria baicalensis and its photocatalytic degradation activity using methylene blue. Optik-Int. J. Light Electron Optics. 2019;184:324–329. [Google Scholar]
- 35.Kumar Neeraj, Mittal Hemant, Reddy Leelakrishna, Nair Padmanabhan, Catherine Ngila Jane, Parashar Vyom. Morphogenesis of ZnO nanostructures: role of acetate (COOH-) and nitrate (NO-) ligand donors from zinc salt precursors in synthesis and morphology dependent photocatalytic properties. RSC Adv. 2015;5(48):38801–38809. [Google Scholar]
- 36.Xu J., Pan Q., Shun Y., Tian Z. Grain size control and gas sensing properties of ZnO gas sensor. Sensor. Actuator. B Chem. 2000;66:277–279. [Google Scholar]
- 37.Dimkpa Christian O., Singh Upendra, Bindraban Prem S., Elmer Wade H., Gardea-Torresdey Jorge L., White Jason C. Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 2019;688:926–934. doi: 10.1016/j.scitotenv.2019.06.392. [DOI] [PubMed] [Google Scholar]
- 38.Mishra P.K., Mishra H., Ekielski A., Talegaonkar S., Vaidya B. Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug Discov. Today. 2017;22(12):1825–1834. doi: 10.1016/j.drudis.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Punnoose Alex, Kelsey Dodge, Rasmussen John W., Chess Jordan, Wingett Denise, Anders Catherine. Cytotoxicity of ZnO nanoparticles can Be tailored by modifying their surface structure: a green chemistry approach for safer nanomaterials. ACS Sustain. Chem. Eng. 2014;2(7):1666–1673. doi: 10.1021/sc500140x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Premanathan M., Karthikeyan K., Jeyasubramanian K., Manivannan G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine. 2011;7(2):184–192. doi: 10.1016/j.nano.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Hatamie A., Khan A., Golabi M. Zinc oxide nanostructure-modified textile and its application to bio- sensing, photocatalysis, and as antibacterial material. Langmuir. 2015;31(39):10913–10921. doi: 10.1021/acs.langmuir.5b02341. [DOI] [PubMed] [Google Scholar]
- 42.Ambika Subramanian, Sundrarajan Mahalingam. Antibacterial behaviour of Vitex negundo extract assisted ZnO nanoparticles against pathogenic bacteria. J. Photochem. Photobiol. B Biol. 2015;146:52–57. doi: 10.1016/j.jphotobiol.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 43.Nagajyothi P.C., Cha Sang Ju, Yang In Jun, Sreekanth T.V.M. Kwang Joong Kim, Heung Mook Shin, Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015;146:10–17. doi: 10.1016/j.jphotobiol.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Selim Y.A., Azb M.A., Ragab I. Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-60541-1. article ID 3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bisht G., Rayamajhi S. ZnO nanoparticles: a promising anticancer agent. Nanobiomedicine. 2016;3(9) doi: 10.5772/63437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonia S., Linda Jeeva Kumari H., Ruckmanib K., Sivakumara M. Antimicrobial and antioxidant potentials of biosynthesized colloidal zinc oxide nanoparticles for a fortified cold cream formulation: a potent nanocosmeceutical application. Mater. Sci. Eng. C: Mater. Biol. Appl. 2017;79:581–589. doi: 10.1016/j.msec.2017.05.059. [DOI] [PubMed] [Google Scholar]
- 47.Schilling K., Bradford B., Castelli D., Dufour E., Frank Nash J., Pape W., Schulte S., Tooley I., van den Bosch J., Schellauf F. Human safety review of “nano” titanium diox- ide and zinc oxide. Photochem. Photobiol. Sci. 2010;9(4):495–509. doi: 10.1039/b9pp00180h. [DOI] [PubMed] [Google Scholar]
- 48.Rajiv P., Rajeshwari Sivaraj, Venckatesh Rajendran. Bio-Fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectrochim. Acta Mol. Biomol. Spectrosc. 2013;112:384–387. doi: 10.1016/j.saa.2013.04.072. [DOI] [PubMed] [Google Scholar]
- 49.Nagajyothi P.C., Sreekanth T.V.M., Tettey Clement O. Yang in Jun, shin Heung Mook, characterization, antibacterial, antioxidant, and cytotoxic activities of ZnO nanoparticles using Coptidis rhizoma. Bioorg. Med. Chem. Lett. 2014;24:4298–4303. doi: 10.1016/j.bmcl.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 50.Bhuyan Tamanna, Mishra Kavita, Khanuja Manika, Prasad Ram, Varma Ajit. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process. 2015;32:55–61. [Google Scholar]
- 51.Thema F.T., Manikandan E., Dhlamini M.S., Maaza M. Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Mater. Lett. 2015;161:124–127. [Google Scholar]
- 52.Sundrarajan M., Ambika S., Bharathi K. Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Adv. Powder Technol. 2015;26:1294–1299. [Google Scholar]
- 53.Jafarirad Saeed, Mehrabi Meysam, Divband Baharak, Kosari-Nasab Morteza. Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: a mechanistic approach. Mater. Sci. Eng. C: Mater. Biol. Appl. 2016;59:296–302. doi: 10.1016/j.msec.2015.09.089. [DOI] [PubMed] [Google Scholar]
- 54.Agarwal Happy, Venkat Kumar S., Rajeshkumar S. A review on green synthesis of zinc oxide nanoparticles-An eco-friendly approach. Res. Effic. Technol. 2017;3(4):406–413. [Google Scholar]
- 55.Kalpana V.N., Devi Rajeswari V. 2018. A Review on Green Synthesis, Biomedical Applications, and Toxicity Studies of ZnO NPs, Bioinorganic Chemistry and Applications. Article ID 3569758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suresh Joghee, Pradheesh Ganeshan, Vincent Alexramani, Sundrarajan Mahalingam, Sun Ig Hong. Green synthesis and characterization of zinc oxide nanoparticle using insulin plant (Costus pictus D. Don) and investigation of its antimicrobial as well as anticancer activities. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018;9(1) [Google Scholar]
- 57.Zangeneh M.M., Ghaneialvar H., Akbaribazm M., Ghanimatdan M., Abbasi N., Goorani S., Pirabbasi E., Zangeneh A. Novel synthesis of Falcaria vulgaris leaf extract conjugated copper nanoparticles with potent cytotoxicity, antioxidant, antifungal, antibacterial, and cutaneous wound healing activities under in vitro and in vivo condition. J. Photochem. Photobiol. B Biol. 2019;197:111556. doi: 10.1016/j.jphotobiol.2019.111556. [DOI] [PubMed] [Google Scholar]
- 58.Chen L., Batjikh I., Hurh J., Han Y., Huo Y., Ali H., Li J.F., Rupa E.J., Ahn J.C., Mathiyalagan R., Yang D.C. Green synthesis of zinc oxide nanoparticles from root extract of Scutellaria baicalensis and its photocatalytic degradation activity using methylene blue. Optik. 2019;184:324–329. [Google Scholar]
- 59.Król A., Railean-Plugaru V., Pomastowski P., Buszewski B. Phytochemical investigation of Medicago sativa L. extract and its potential as a safe source for the synthesis of ZnO nanoparticles: the proposed mechanism of formation and antimicrobial activity. Phytochem. Lett. 2019;31:170–180. [Google Scholar]
- 60.Soto-Robles C.A., Luque P.A., Gómez-Gutiérrez C.M., Nava O., Vilchis-Nestor A.R., Lugo-Medina E., Ranjithkumar R., Castro-Beltrán A. Study on the effect of the concentration of Hibiscus sabdariffa extract on the green synthesis of ZnO nanoparticles. Result Phys. 2019;15:102807. [Google Scholar]
- 61.Zare M., Namratha K., Thakur M.S., Byrappa K. Biocompatibility assessment and photocatalytic activity of bio-hydrothermal synthesis of ZnO nanoparticles by Thymus vulgaris leaf extract. Mater. Res. Bull. 2019;109:49–59. [Google Scholar]
- 62.Hao D., Xiao P. Pharmaceutical resource discovery from traditional medicinal plants: pharmacophylogeny and pharmacophylogenomics. Chin. Herbal Med. 2020;12(2):104–117. doi: 10.1016/j.chmed.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zahara K., Tabassum S., Sabir S., Arshad M., Qureshi R., Amjad M.S., Chaudhari S.K. A review of therapeutic potential of Saussurea lappa-An endangered plant from Himalaya. Asian Pac. J. Trop. Med. 2014;7:S60–S69. doi: 10.1016/S1995-7645(14)60204-2. [DOI] [PubMed] [Google Scholar]
- 64.Singh B. Springer India; New Delhi: 2016. Medicinal Plants and Phytomedicines: Herbal Insecticides, Repellents and Biomedicines: Effectiveness and Commercialization; pp. 127–145. [Google Scholar]
- 65.Jung J.H., Kim Y., Lee C.O., Kang S.S., Park J.H., Im K.S. Cytotoxic constituents of Saussurea lappa. Arch Pharm. Res. (Seoul) 1998;21(12):153–156. doi: 10.1007/BF02974020. [DOI] [PubMed] [Google Scholar]
- 66.Ashwin Sheetal Batakurki, Kumar Jyotsna. Biogenic synthesis, antibacterial and antioxidant studies of prepared silver nano particles using root extract of Saussurea lappa. Int. J. New Technol. Sci. Eng. 2019;6(1):11–21. [Google Scholar]
- 67.Amina Musarat, Nawal M., Al Musayeib, Alarfaj Nawal A., El-Tohamy Maha F., Oraby Hesham F., Al Hamoud Gadah A., Bukhari Sarah I., Nadine M., Moubayed S. Biogenic green synthesis of MgO nanoparticles using Saussurea costus biomasses for a comprehensive detection of their antimicrobial, cytotoxicity against MCF-7 breast cancer cells and photocatalysis potentials. PloS One. 2020;15(8) doi: 10.1371/journal.pone.0237567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Al-Saggaf Mohammed S., Tayel Ahmed A., Ghobashy Madeha O.I., Alotaibi Maeidh A., Alghuthaymi Mousa A., Moussa Shaaban H. Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens. Green Process. Synth. 2020;9:477–487. [Google Scholar]
- 69.Wei Hua, Yan Lihua, Feng Weihong, Guoxu M.A., Peng Yong, Wang Zhimin, Xiao Peigen. Research progress on active ingredients and pharmacologic properties of Saussurea lappa. Curr. Opin. Complement. Altern. Med. 2014;1(1) [Google Scholar]
- 70.Kundu T.K., Karak N., Barik P., Saha S. Optical properties of ZnO nanoparticles prepared by chemical method using poly (vinyl alcohol) (PVA) as capping agent. Int. J. Soft Comput. Eng. 2011;1:19–24. [Google Scholar]
- 71.Kumar A., Dixit C.K. Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids. Elsevier; 2017. Methods for characterization of nanoparticles; pp. 43–58. [Google Scholar]
- 72.Yedurkar S., Maurya C., Mahanwar P. Biosynthesis of zinc oxide nanoparticles using Ixora Coccinea leaf extract-A green approach. Open J. Synth. Theor. Appl. 2016;5:1–14. [Google Scholar]
- 73.Ambika S., Sundrarajan M. Green biosynthesis of ZnO nanoparticles using vitexnegundoL. extract: spectroscopic investigation of interaction between ZnO nanoparticles and human serum albumin. J. Photochem. Photobiol. B Biol. 2015;149:143–148. doi: 10.1016/j.jphotobiol.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 74.Elumalai K., Velmurugan S., Ravi S., Kathiravan V., Ashokkumar S. Green synthesis of zinc oxide nanoparticles using Moringa oleifera leaf extract and evaluation of its antimicrobial activity. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015;143:158–164. doi: 10.1016/j.saa.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 75.Ramesh M., Anbuvannan M., Viruthagiri G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015;136:864–870. doi: 10.1016/j.saa.2014.09.105. [DOI] [PubMed] [Google Scholar]
- 76.Agarwal H., Nakara A., Menon S., Shanmugam V. Eco-friendly synthesis of zinc oxide nanoparticles using Cinnamomum Tamala leaf extract and its promising effect towards the antibacterial activity. J. Drug Deliv. Sci. Technol. 2019;53:101212. [Google Scholar]
- 77.Fakhari S., Jamzad M., Kabiri Fard H. Green synthesis of zinc oxide nanoparticles: a comparison. Green Chem. Lett. Rev. 2019;12:19–24. [Google Scholar]
- 78.Wang L., Hu C., Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomed. 2017;12:1227–1249. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gurunathan S., Han J.W., Abdal Dayem A., Eppakayala V., Kim J.H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012;7:5901–5914. doi: 10.2147/IJN.S37397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nagy A., Harrison A., Sabbani S., Munson R.S., Dutta P.K., Waldman J. Silver nanoparticles embedded in zeolite membranes: release of silver ions and mechanism of antibacterial action. Int. J. Nanomed. 2011;6:1833–1852. doi: 10.2147/IJN.S24019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leung Y.H., Ng A.M.C., Xu X., Shen Z., Gethings L.A., Wong M.T., Chan C.M.N., Guo M.Y., Ng Y.H., Djuri A.B., Lee P.K.H., Chan W.K., Yu L.H., Phillips D.L., Ma A.P.Y., Leung F.C.C. Mechanisms of antibacterial activity of MgO: non-ROS mediated toxicity of MgO nanoparticles towards Escherichia coli. Small. 2014;10:1171–1183. doi: 10.1002/smll.201302434. [DOI] [PubMed] [Google Scholar]
- 82.Espitia P.J.P., deF N., Soares F., dos R.Coimbra J.S., de Andrade N.J., Cruz R.S., Medeiros E.A.A. Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012;5:1447–1464. [Google Scholar]
- 83.Raghupathi K.R., Koodali R.T., Manna A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir. 2011;27:4020–4028. doi: 10.1021/la104825u. [DOI] [PubMed] [Google Scholar]
- 84.Emami-Karvani Zarrindokht, Chehrazi Pegah. Antibacterial activity of ZnO nanoparticle on gram- positive and gram-negative bacteria. Afr. J. Microbiol. Res. 2011;5(12):1368–1373. [Google Scholar]
- 85.Rizwan W., Young-Soon K., Amrita M., Soon-Il Y., Hyung-ShikSh Formation of ZnOmicro-flowers prepared via solution process and their antibacterial activity. Nanoscale Res. Lett. 2010;5(10):1675–1681. doi: 10.1007/s11671-010-9694-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reddy K.M., Kevin F., Jason B., Denise G.W., Cory H., Alex P. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007;90(21):1–3. doi: 10.1063/1.2742324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu K.N., Yoon T.-J., Minai-Tehrani A., Kim J.-E., Park S.J., Jeong M.S., Ha S.-W., Lee J.-K., Kim J.S., Cho M.-H. Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation. Toxicol. Vitro. 2013;27:1187–1195. doi: 10.1016/j.tiv.2013.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.