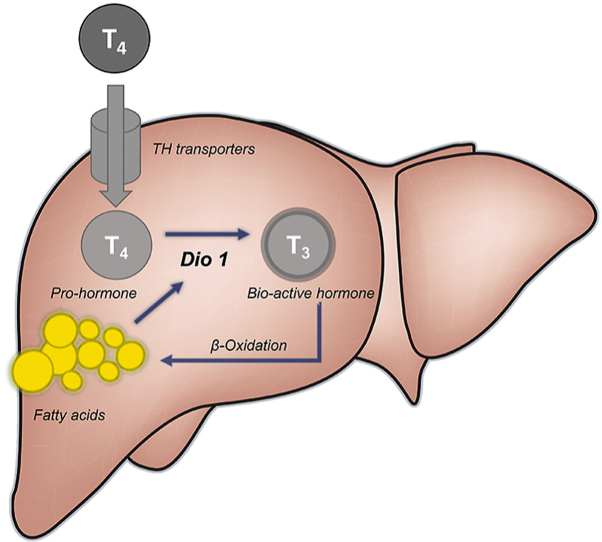
Abstract
Objective
Nonalcoholic fatty liver disease (NAFLD) comprises a spectrum ranging from hepatosteatosis to progressive nonalcoholic steatohepatitis that can lead to cirrhosis. Humans with low levels of prohormone thyroxine (T4) have a higher incidence of NAFLD, and thyroid hormone treatment is very promising in all patients with NAFLD. Deiodinase type 1 (Dio1) is a hepatic enzyme that converts T4 to the bioactive T3 and therefore regulates thyroid hormone availability within hepatocytes. We investigated the role of this intrahepatic regulation during the progression of NAFLD.
Methods
We investigated hepatic thyroid hormone metabolism in two NAFLD models: wild-type mice fed a Western diet with fructose and Leprdb mice fed a methionine- and choline-deficient diet. AAV8-mediated liver-specific Dio1 knockdown was employed to investigate the role of Dio1 during the progression of NAFLD. Intrahepatic thyroid hormone levels, deiodinase activity, and metabolic parameters were measured.
Results
Dio1 expression and activity were increased in the early stages of NAFLD and were associated with an increased T3/T4 ratio. Prevention of this increase by AAV8-mediated liver-specific Dio1 knockdown increased hepatic triglycerides and cholesterol and decreased the pACC/ACC ratio and acylcarnitine levels, suggesting there was lower β-oxidation. Dio1 siRNA KD in hepatic cells treated with fatty acids showed increased lipid accumulation and decreased oxidative phosphorylation.
Conclusion
Hepatic Dio1 gene expression was modulated by dietary conditions, was increased during hepatosteatosis and early NASH, and regulated hepatic triglyceride content. These early adaptations likely represent compensatory mechanisms that reduce hepatosteatosis and prevent NASH progression.
Keywords: Liver, Steatosis, Thyroid, NAFLD, Deiodinase, NASH
Graphical abstract
Highlights
-
•
Low thyroid hormone action is implicated in the pathogenesis of NAFLD.
-
•
Intracellular thyroid hormone concentrations are regulated by deiodinases.
-
•
Increased deiodinase 1 was found in early NAFLD increasing T3/T4 ratio.
-
•
AAV8 mediated liver specific knockdown of deiodinase 1 increases steatosis.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) comprises a spectrum of diseases ranging from simple steatosis in the liver to steatohepatitis (NASH) with inflammation and fibrosis. NAFLD affects approximately 25% of the adult population worldwide, and its rise has been associated with the recent pandemic of obesity and diabetes [1]. Currently, there are no approved drugs for the treatment of NAFLD. Thus, there is an urgent need for the development of new therapies. Recently, thyroid hormone (TH) and TH-analogs (thyromimetics) have shown to be effective therapies for hepatosteatosis and NASH [[2], [3], [4], [5], [6]]. However, the physiological basis of their effects on NAFLD is not well understood at present.
TH stimulates lipophagy, β-oxidation of fatty acids, and oxidative phosphorylation in the liver [4,7]. Previous studies demonstrated that both hypothyroidism and thyroid hormone receptor β mutations in mice and humans increase NAFLD risk [8,9]. Further, lower serum levels of prohormone thyroxine (T4), including those within the normal range, increase NAFLD prevalence [10]. However, serum levels of T4 are not the only factor determining intrahepatic concentrations of the bioactive triiodothyronine (T3), which binds to the nuclear hormone receptor β (TRβ) and causes transcriptional activation of T3 target genes. Intrahepatic concentrations of the prohormone T4 and the bioactive form of TH (i.e., T3) are tightly regulated by deiodinases. There are three deiodinases: deiodinase type1 (Dio1), deiodinase type 2 (Dio2) and deiodinase type 3 (Dio3). These are all selenoenzymes of which Dio1 and Dio3 are expressed in hepatocytes. Dio1 is responsible for outer and inner ring deiodination of the thyroid hormone and is therefore involved in T3 production and rT3 clearance. Dio3 regulates T3 and T4 conversion to the inert metabolites T2 and rT3, respectively. The expression of hepatic Dio1 is influenced by cytokines and nutritional status, and it is markedly up-regulated by T3 [2,11]. Previous research has found low levels of Dio1 expression in the livers of mice after acute and chronic inflammation, as well as rodents and patients with NASH [2,12,13]. In this study, we examined the role of intrahepatic regulation of the thyroid hormone by Dio1 during the different phases of NAFLD progression.
2. Materials and methods
2.1. General
All mice were maintained according to the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH) publication 1.0.0; revised 2011], and experiments were approved by the Singhealth Institutional Animal Care and Use Committee (2015/SHS/1104).
2.2. Western diet and fructose model
Ten-week-old male C57Bl/6J mice were fed a Western diet (D12079B; Research Diets), supplemented with 15% weight/volume fructose (Sigma–Aldrich, 57-48-7) in drinking water for 8 or 16 weeks, whereas the control mice received normal chow and tap water for 16 weeks [14].
2.3. Leprdb with MCD diet model
Male BKS.Cg-Dock7m+/+LeprdbJ (db/db) mice (Jackson Laboratory 009659) at 12 weeks of age were fed a normal chow diet or an MCD (A02082002BRi, Research Diets) diet for 2, 4, and 8 weeks to produce NASH stages. C57Bl/6J mice (NUSCARE C57BL/6 JInv) fed a normal chow diet served as the control.
2.4. Liver-specific Dio1 knockdown
Ten-week-old male C57Bl/6J mice were injected via tail vein with AAV8-ALB-eGFP-mDio1-shRNAmir or AAV8-ALB-eGFP-ctrl-shRNAmir (Lot 181231#13, Vector Biolabs) and fed with NCD for two weeks, followed by a Western diet with fructose in the drinking water or NCD for the following 12 weeks. A small group of mice (n = 3) injected with the control shRNA were fed with NCD for reference purposes only and not used for statistical purposes.
2.5. Cell culture
AML12 cells were passaged in DMEM/F12 (cat. 11320-033), 10% FBS, and 1× pen/strep, insulin transferrin selenium. 24 h after plating the cells, a mix of oleic acid 0.6 M and palmitic acid (OAPA) in the above media (with 1% BSA as carrier or only 1% BSA) was added for 24, 48, and 36 h. For siRNA transfection, AML12 cells were trypsinized and mixed with opti-MEM medium (Invitrogen, 31,985,070), containing Lipofectamine RNAimax (Invitrogen, 13,778,150) and Dio1 (ON-TARGET plus Mouse Dio1 (13370) siRNA SMARTPOOL (Dharmacon), or control siRNA (10 nM)) according to the manufacturer's recommendations. 24 h later, OAPA was added for 24 h. The neutral lipid was stained with fluorescent dye BODIPY 493/503 (5 μg/ml) for 10 min. Oxygen consumption was measured at 37 °C using an XF24 extracellular analyzer (Seahorse Bioscience Inc., North Billerica, MA, USA) [15].
2.6. Analysis
Triglyceride concentrations in the liver and serum (10010303; Cayman Chemical Company, Ann Arbor, MI) and total cholesterol (ab65390, abcam) were measured with colorimetric kits according to the manufacturer's instructions after chloroform/methanol lipid extraction. Total RNA isolation was performed using an InviTrap Spin Universal RNA kit (Stratec Biomedical), and RT-qPCR was performed as previously described [15], using a QuantiTect SYBR Green PCR kit (Table primers in supplementary methods). Liver TH concentrations (T4 and bioactive T3) were measured by LC–MS/MS. Deiodinase 1 (Dio1) and 3 (Dio3) activity were measured by the conversion of 125I-labeled rT3 and T3, respectively, as previously described [12]. For western blot analysis, proteins were separated by SDS–PAGE under reducing conditions and transferred to nitrocellulose membranes. Membranes were blocked with 5% nonfat milk in phosphate-buffered saline with 0.1% Tween 20 (Sigma–Aldrich, P9416; PBST). The blots were incubated overnight at 4 °C with primary antibodies. Immunoblot analysis was performed using an enhanced chemiluminescence procedure (GE Healthcare, RPN2106).
2.7. Statistical analysis
For the WDF model, the groups were compared using a one-way ANOVA with a post-hoc Dunnett's multiple comparison test to establish significance between the groups. For the Leprdb with MCD model, the wild-type mice with an NCD diet were compared with the Leprdb mice on an NCD diet with an unpaired t-test to establish the effect of the genotype. We investigated the effect of the MCD diet by comparing the Leprdb on an NCD diet and 2, 4, and 8 weeks of MCD with a one-way ANOVA with a post-hoc Dunnett's multiple comparison test to establish significance between the groups. Dio1LKD-WDF were compared to the control-WDF with an unpaired t-test. Data points lesser than Q1 − 1.5 × IQR or greater than Q3 + 1.5 × IQR were considered outliers and removed from further analysis. Prism 8 was used for the statistical analysis. Data are expressed as mean ± SEM. Significance was established at p < 0.05.
3. Results and discussion
3.1. Dio1 expression and intrahepatic TH concentrations in mice fed a Western diet and fructose (WDF)
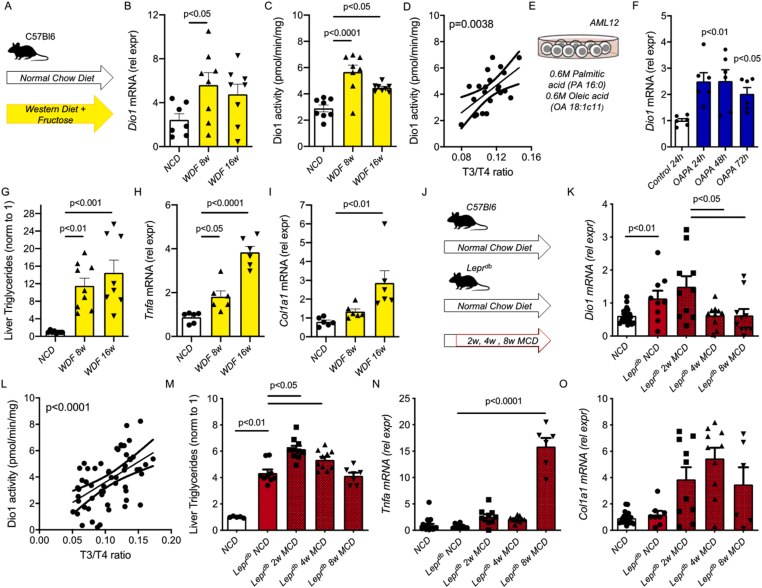
To examine intrahepatic TH regulation during the progression of NAFLD, we employed two different NAFLD models to induce hepatosteatosis and NASH. In the first model, we fed mice a Western diet with 15% fructose water (WDF) for 8 and 16 weeks to induce steatosis and early-stage NASH and then compared them with mice fed a normal chow diet (NCD) (Figure 1A) [14]. We observed that hepatic T4 decreased in mice fed WDF for 8 (15.3 pmol/g) and 16 (15.1 pmol/g) weeks compared to mice fed NCD (26.0 pmol/g) (Table 1). This decrease in hepatic T4 was not explained by a decreased expression of the thyroid hormone transporters Mct8 and Mct10, as we found an increased expression of both transporters in mice fed WDF for 8 weeks, followed by a return to levels similar to those found in mice fed NCD (Table 1) when measured again at 16 weeks. In contrast to the prohormone T4, hepatic T3 was not significantly different in mice fed WDF for 8 weeks (2.1 pmol/g) and slightly decreased in mice fed WDF for 16 weeks (1.7 pmol/g) compared to mice fed with NCD (2.7 pmol/g) (Table 1). These data suggest that intrahepatic regulation of T3 levels during NAFLD progression could be mediated by deiodinases.
Figure 1.
Dio1 increases early during the progression of NAFLD (A) Western Diet with 15% fructose in the drinking water (WDF) for 8 and 16 weeks compared to Normal Chow Diet (NCD), (n = 7–8 per group) (B, C) Dio1mRNA, Dio1 Activity in the WDF model, (D) Association between Deiodinase 1 activity and liver T3/T4 ratio in the WDF model (E) Mouse AML12 cell line with oleic acid and palmitic acid (OAPA) (6 wells per group) (F) Dio1 mRNA in the AML12 OAPA cell model (G–I) Liver triglycerides, Tnfa mRNA and Col1a1 mRNA in the WDF model (J) Leprdb model with normal chow diet (NCD) or methionine and choline deficient diet (MCD) compared to C57Bl6 with NCD diet (n = 6–10 per group) (K) Dio1 mRNA in the Leprdb model (K) Association between Deiodinase 1 activity and liver T3/T4 ratio in the Leprdb model (L–O) Liver triglycerides, Tnfa mRNA and Col1a1 mRNA in the Leprdb model. Data is depicted in mean ± SEM.
Table 1.
Liver parameters during the progression of NAFLD in the Western Diet with 15% Fructose in the drinking water (left) and the Leprdb model with a methionine and choline deficient diet (MCD) or normal chow diet (NCD) (left). Significance of post-hoc analysis WDF 8w vs. NCD and WDF 16 w vs. NCD (left panel), Leprdb NCD vs. C57Bl6 NCD, Leprdb 2w, 4w and 8w post-hoc analysis vs. Leprdb NCD (right panel). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Data is depicted in mean ± SEM.
| WDF |
Leprdb + MCD |
|||||||
|---|---|---|---|---|---|---|---|---|
| NCD | WDF 8w | WDF 16w | Control NCD | Leprdb NCD | Leprdb MCD 2w | Leprdb MCD 4w | Leprdb MCD 8w | |
| Liver T4 (pmol/g) | 25.96 (2.20) | 15.29∗∗∗∗ (1.02) | 15.08∗∗∗∗ (0.82) | 29.11 (1.03) | 17.00∗∗ (1.26) | 11.22∗∗ (0.72) | 12.22∗∗∗ (0.71) | 12.46∗∗∗ (1.31) |
| Liver T3 (pmol/g) | 2.66 (0.23) | 2.09 (0.32) | 1.69∗ (0.06) | 2.25 (0.18) | 1.99∗∗ (0.09) | 1.48∗∗ (0.09) | 1.3∗∗∗∗ (0.09) | 1.55∗ (0.16) |
| Liver T3/T4 ratio | 0.10 (0.01) | 0.14 (0.02) | 0.11 (0.00) | 0.08 (0.00) | 0.12∗∗∗∗ (0.01) | 0.14 (0.00) | 0.11 (0.01) | 0.11 (0.01) |
| Dio3mRNA | 1.90 (0.42) | 2.55 (0.71) | 2.36 (0.39) | 2.09 (0.28) | 1.20 (0.29) | 2.93∗∗∗ (0.33) | 1.64 (0.23) | 1.16 (0.27) |
| Dio3 activity (fmol/min/mg) | 0.20 (0.03) | 0.14 (0.03) | 0.15 (0.01) | 0.13 (0.01) | 0.05∗∗∗∗ (0.01) | 0.07 (0.01) | 0.11 (0.03) | 0.06 (0.02) |
| Thrb mRNA | 0.97 (0.14) | 0.94 (0.18) | 0.67 (0.10) | 3.21 (0.23) | 2.46 (0.27) | 2.66 (0.30) | 1.94 (0.21) | 3.86∗ (0.57) |
| Mct8 mRNA | 1.07 (0.27) | 2.72∗∗ (0.43) | 1.94 (0.22) | 2.95 (0.32) | 2.35 (0.44) | 4.89∗ (0.88) | 2.94 (0.42) | 6.82∗∗ (1.34) |
| Mct10 mRNA | 1.31 (0.36) | 3.75∗ (1.01) | 1.20 (0.26) | 2.28 (0.38) | 1.16 (0.19) | 1.63 (0.34) | 1.31 (0.37) | 1.57 (0.33) |
Next, we examined Dio1 gene expression, which converts T4 to T3 in liver cells. Dio1 mRNA increased more than 2-fold at both 8 and 16 weeks (Figure 1B) in WDF mice. Dio1 activity was measured by the conversion of 125I-labeled rT3; it increased significantly and was most pronounced at WDF 8 weeks compared to NCD mice (Figure 1C). This increased Dio1 mRNA and activity was not attributable to increased intrahepatic T3, a known inducer of Dio1 mRNA expression. Increased Dio1 activity was associated with an increased T3/T4 ratio for all mice, indicating that it regulated the conversion of the prohormone T4 to T3 (Figure 1D). We also measured Dio3 mRNA and Dio3 activity, known to metabolize T3 to its inert metabolites, and found they were not significantly different in mice fed WDF vs. NCD (Table 1). To further examine Dio1 mRNA induction during hepatosteatosis, we treated the mouse hepatic cell line, AML12, with a combination of 0.6 M oleic acid and 0.6 M palmitic acid (OAPA) and observed increases in Dio1 mRNA expression at 24, 48, and 72 h, suggesting that this combination of saturated and monounsaturated fatty acids could induce Dio1 mRNA expression acutely in a cell autonomous manner (Figure 1E, F). Oleic acid and palmitic acid comprise the most abundant fatty acids in a western diet and, when applied together, cause the greatest lipid accumulation and protect against palmitic acid-induced apoptosis [16]. Nonesterified fatty acids bind to the ligand-binding domain of several nuclear receptors expressed in the liver, including PPAR (α, β, γ), HNF4 (α,γ), retinoid X-receptor (RXR) α, and liver X receptor (LXR) (α,β) [17]. The induction of these transcription factors possibly induces Dio1 mRNA by a combination of saturated and unsaturated fatty acids.
We measured hepatic triglyceride content and found that it increased more than 10-fold in mice fed WDF for 8 and 16 weeks compared to mice fed NCD (Figure 1G). Hepatic tumor necrosis factor alpha (Tnfa) was slightly increased in mice fed WDF for 8 weeks and more than 3-fold in mice fed WDF for 16 weeks (Figure 1H). Alpha-1 type I collagen (Col1a1) mRNA significantly increased in mice fed WDF for 16 weeks (Figure 1I). These data suggest that mice fed WDF for 8 weeks had hepatosteatosis and slight inflammation, whereas mice fed WDF for 16 weeks developed early-stage NASH with induction of inflammation and fibrosis marker mRNAs. These changes occurred parallel to increased Dio1 mRNA expression and activity and were correlated with the T3/T4 ratio. When taken together, these data suggest that although a previous report determined that Dio1 decreased in late-stage NASH [2], Dio1 gene expression and activity were increased in hepatosteatosis and early-stage NASH to maintain intrahepatic T3 concentration.
3.2. Dio1 expression and intrahepatic TH concentrations in Leprdb mice fed a methionine and choline-deficient diet
To validate this observation in a second NAFLD model, we used Leprdb mice that previously developed severe steatosis with only mild inflammation when fed a normal chow diet (NCD) [18]. To induce the NASH phenotype, Leprdb were switched after 12 weeks of age to a methionine- and choline-deficient diet (MCD) for 2, 4, and 8 weeks (Figure 1J) [18]. Leprdb mice that continued to consume an NCD diet had hepatic T4 levels that were 42% lower than wild-type mice on NCD (Leprdb-NCD T4: 17 vs. control-NCD 29.11 pm/g). In this model, increases of the thyroid hormone transporter Mct8 mRNA were found after 2 and 8 weeks in Leprdb mice fed an MCD diet. Next, we investigated intrahepatic T3, which decreased by only 12% in Leprdb-NCD compared to control-NCD (T3: 1.99 vs. 2.25 pmol/g). In this model, we also observed an increase in Dio1 mRNA expression in steatotic livers (Leprdb-NCD vs. control-NCD) (Figure 1K). Dio1 mRNA decreased below the basal level in Leprdb mice fed MCD for 4 and 8 weeks, similar to NASH in rats previously observed by us [2]. The enzyme activity of Dio1 was positively correlated with the T3/T4 ratio (Figure 1L) again, demonstrating a regulatory role of deiodinases in liver T3 availability.
Triglyceride content in livers from Leprdb-NCD mice increased more than 4-fold compared to control-NCD (Figure 1M). Triglyceride content was further increased in Leprdb fed MCD for 2 and 4 weeks. Tnfa and Col1a1 mRNA were not significantly different in Leprdb-NCD and control-NCD mice. However, there was an increased Tnfa mRNA expression starting at 2 weeks and continuing to 6 weeks, suggesting ongoing inflammation after the initiation of MCD (Figure 1N). Col1a1 mRNA was increased more than two-fold at 2 and 4 weeks and increased 16-fold at 6 weeks (Figure 1). These findings suggest that fibrosis associated with gene expression became more prominent by 8 weeks in the Leprdb mice fed MCD. Taken together in this second NAFLD model, an early increase in Dio1 mRNA was also found to be associated with the T3/T4 ratio.
3.3. Liver-specific Dio1 shRNA knockdown in mice fed WDF
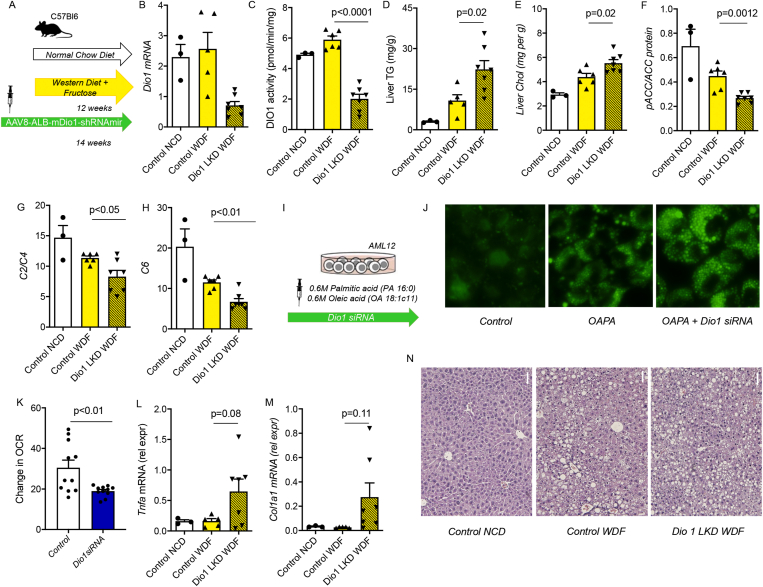
As T3 stimulates fatty acid β-oxidation, we investigated whether Dio1 increases during hepatosteatosis and early-stage NASH were protective mechanisms used to maintain an intrahepatic T3 concentration to reduce triglyceride accumulation in the liver during nutritional overload. Accordingly, we prevented the early induction of Dio1 by performing a liver-specific knockdown with shRNA against mouse Dio1, cloned under the control of a mouse albumin promoter in an adeno-associated viral vector (AAV8-Albumin-eGFP-mDio1-shRNAmir). Two weeks after tail vein injection of shRNA, mice began consuming WDF or NCD for 12 weeks before sacrifice, which is a transitional period when mice typically convert from hepatosteatosis to early NASH (Figure 2A). The control-WDF group had increased body weight and fat mass compared to control mice fed NCD (i.e., control-NCD). However, there were no significant differences in body weight or fat mass measured by MRI, and food intake was similar for Dio1LKD-WDF and control-WDF mice (data not shown).
Figure 2.
Dio1 KD increases liver triglycerides and cholesterol. (A) WDF model with injection of AAV8-Albumin-eGFP-mDio1-shRNAmir (WDF + Dio1 LKD) (n = 7) or AAV8-Albumin-eGFP-ctrl-shRNAmir (WDF + control) (n = 6). For reference, a group of NCD + control shRNA was included (n = 3). (B–G) Dio1 mRNA expression (B) DIO1 activity (C), liver triglycerides (TG; D), liver cholesterol (E), densitometric quantification of western blots analyzing pACC/ACC (F), C2/C4 acylcarnitines (G), C6 acylcarnitines (H) in the WDF Dio1 KD model. (I) Schematic representation of in vitro experiment utilizing mouse AML12 cell line treated with oleic acid and palmitic acid (OAPA) combined with Dio1 siRNA knockdown. (J) BODIPY staining of the AML12 cell line combined with OAPA and OAPA with Dio1 siRNA. (K) Change in oxygen consumption rate (OCR) after Dio1 siRNA in AML12 cells. (L–M) Tnfa mRNA (L), Col1a1mRNA (M) in the WDF Dio1 KD model. (N) Histology of control NCD, control WDF, and Dio1 LKD WDF with TG content on average. Data is depicted in mean ± SEM.
We observed that hepatic Dio1 mRNA and Dio1 activity decreased by 72% and 66%, respectively, in Dio1 KD mice fed WDF (Dio1LKD-WDF) compared to control mice fed WDF (control-WDF) 14 weeks after shRNA treatment (Figure 2B–C). Hepatic T4 levels were higher in Dio1LKD-WDF (40.6 pmol/g) than control-WDF as would be expected if Dio1 expression/activity were decreased (Table 2). Interestingly, hepatic T3 concentrations were not significantly different among the three groups of mice. When we analyzed sera from the mice, we observed increased T4 levels in Dio1LKD-WDF (72.4 nmol/l), as compared to the control-WDF group (52.3 nmol/l), which was consistent with findings in the whole-body knockout of Dio1 [19]. Thus, our data showed that a decreased hepatic Dio1 expression and activity from liver-specific KD was sufficient to exert this serum TH profile in mice. Of note, serum T3 levels were not significantly different among the three groups of mice. In this experiment, we did not find any differences in Mct8 and Mct10 mRNA levels among the three groups of mice (Table 2). Taken together, the Dio1LKD-WDF showed a decrease in Dio1 gene expression and Dio1 activity, resulting in reduced metabolism of intrahepatic T4 to T3.
Table 2.
Liver and serum parameters in normal chow diet (NCD) with a control shRNA (control-NCD) and western diet with 15% fructose in the drinking water (WDF) with a control shRNA (control-WDF) compared to Dio1 liver-specific knockdown (Dio1LKD-WDF). Significance is shown for WDF control vs. WDF Dio1 LKD. ∗p < 0.05, ∗∗p < 0.01. Data is depicted in mean ± SEM.
| Control-NCD | Control-WDF | Dio1LKD-WDF | |
|---|---|---|---|
| T4 liver (pmol/g) | 41.40 (4.71) | 28.12 (2.40) | 40.59 (2.81)∗ |
| T3 liver (pmol/g) | 6.07 (1.01) | 6.82 (1.06) | 8.86 (2.58) |
| T4 serum (nmol/l) | 62.00 (4.04) | 52.33 (3.73) | 72.43 (3.09)∗∗ |
| T3 serum (nmol/l) | 1.63 (0.05) | 1.68 (0.15) | 1.56 (0.10) |
| Thrb mRNA | 2.74 (0.55) | 1.13 (0.10) | 2.15 (0.31)∗ |
| Mct8 mRNA | 3.67 (1.19) | 3.15 (1.41) | 4.36 (1.07) |
| Mct10 mRNA | 1.23 (0.18) | 0.64 (0.09) | 1.20 (0.41) |
Interestingly, Dio1LKD-WDF showed increased hepatic triglyceride and cholesterol content compared with control-WDF (Figure 2D, E). We thus analyzed hepatic fatty acid metabolism and found Dio1LKD-WDF had a lower pACC/ACC protein ratio than the control-WDF group (Fig. 2F), suggesting increased fatty acid synthesis and decreased β-oxidation of fatty acids. We performed metabolomics of hepatic acylcarnitines as a measure of fatty acid β-oxidation. When comparing control-WDF mice and control-NCD mice, a pattern of decreased short-chain acylcarnitines (C2, C3, C4, C6) and increased medium- and long-chain acylcarnitines (C10:1, C10:2, C12:1, C14:2) emerged, suggesting lower β-oxidation of fatty acids (Supplementary Figure 1). There was a further decrease in the C2/C4 ratio and C6 in Dio1LKD-WDF compared to the control-WDF group (Figure 2G–H). We only observed an increase of the very long acylcarnitine C22:5 (Supplemental Figure 1). We further examined the effects of Dio1 KD by siRNA in vitro combined with OAPA treatment in AML12 cells. We measured fat content by BODIPY staining in KD cells and observed increased fat content compared to control cells (Figure 2I–J). Additionally, Dio1 KD cells exhibited decreased oxidative consumption rate by Seahorse analysis, consistent with lower fatty acid β-oxidation (Figure 2K).
Lastly, we noticed a trend toward an increased hepatic expression of Tnfa and Col1a1 mRNA in Dio1LKD-WDF compared to control-WDF (Figure 2L, M), with several individual Dio1LKD-WDF showing significant expression of these inflammation and fibrosis markers during this transitional period. In contrast, none of the control-NCD or control-WDF mice had any increases in Tnfa and Col1a1 mRNA at 12 weeks. This finding represents differences between experiments in which inflammation is usually induced between 8 and 16 weeks. Histology showed increased fat droplets visible in Dio1LKD-WDF and increased ballooning of hepatocytes (Figure 2N). Serum triglyceride, cholesterol and glucose levels were not significantly altered by Dio1LKD-WDF compared to control-WDF (data not shown).
4. Conclusions
Our study demonstrated that hepatic Dio1 expression and activity are increased in early NAFLD, and blocking Dio1 induction by shRNA increased hepatic triglyceride and cholesterol content. These findings may have significant physiological ramifications, as they suggest induction of Dio1 expression and activity during hepatosteatosis, and early NASH may play a preventive role in NAFLD progression. These results help explain our previous finding that levothyroxine (T4) can be an effective treatment for hepatosteatosis, as it can convert to T3 intrahepatically [2]. It is also possible that patients with a lower Dio1 expression or function may be at a higher risk for developing hepatosteatosis and NASH more rapidly. Decreased Dio1 activity is observed in older age, certain medications (e.g., propranolol and propylthiohuracil), and those with a selenium deficiency. Recently, the first loss-of-function human Dio1 mutation causing changes in thyroid hormone metabolism was described [20]. A Dio1 polymorphism with increased Dio1 activity may protect against hepatosteatosis [21]. Thus, epigenetic and genetic factors could alter Dio1 expression and/or activity, modulating the risk for NAFLD progression. Future studies must determine whether Dio1 overexpression and/or medication-inducing Dio1 activity will have therapeutic potential.
In conclusion, our findings show that hepatic Dio1 expression during early NAFLD is sensitive to nutritional conditions and serves as a metabolic regulator to help reduce hepatosteatosis in early NAFLD.
Author contributions
Eveline Bruinstroop: Conceptualization, Methodology, Formal analysis, Investigation, Writing – Original Draft, Funding acquisition. Jin Zhou: Conceptualization, Methodology, Investigation, Writing – Review & Editing. Madhulika Tripathi: Investigation, Writing – Review & Editing. Winifred W. Yau: Investigation, Writing – Review & Editing. Anita Boelen: Conceptualization, Investigation, Writing – Review & Editing. Brijesh Kumar Singh: Conceptualization, Methodology, Investigation, Supervision, Writing – Original Draft. Paul M. Yen: Conceptualization, Methodology, Supervision, Writing – Original Draft, Funding acquisition.
Acknowledgments
The authors would like to acknowledge Jia Pei, Keziah Tikno, and An de Ruiter for their technical assistance and Eric Fliers for his critical appraisal of the manuscript. E.B. was funded by a Khoo Postdoctoral Fellowship Award, Niels Stensen Fellowship, Ter Meulen Grant of the Royal Netherlands Academy of Arts and Sciences, and Catherine van Tussenbroekfonds (A3-2). The authors would also like to acknowledge the funding from National Medical Research Council, Ministry of Health and A∗STAR Singapore NMRC/OFYIRG/0002/2016 and MOH-000319 to B.K.S.; NMRC/OFYIRG/077/2018 to M.T.; and Clinician Scientist Award MOH-000306 to P.M.Y. The funding source had no involvement in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2021.101266.
Conflicts of interest
The authors have declared that no conflict of interest exists.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Bruinstroop E., Dalan R., Cao Y., Bee Y.M., Chandran K., Cho L.W. Low-dose levothyroxine reduces intrahepatic lipid content in patients with type 2 diabetes mellitus and NAFLD. The Journal of Clinical Endocrinology and Metabolism. 2018;103(7):2698–2706. doi: 10.1210/jc.2018-00475. [DOI] [PubMed] [Google Scholar]
- 3.Harrison S.A., Bashir M.R., Guy C.D., Zhou R., Moylan C.A., Frias J.P. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394(10213):2012–2024. doi: 10.1016/S0140-6736(19)32517-6. [DOI] [PubMed] [Google Scholar]
- 4.Sinha R.A., Bruinstroop E., Singh B.K., Yen P.M. Nonalcoholic fatty liver disease and hypercholesterolemia: roles of thyroid hormones, metabolites, and agonists. Thyroid. 2019;29(9):1173–1191. doi: 10.1089/thy.2018.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cable E.E., Finn P.D., Stebbins J.W., Hou J., Ito B.R., van Poelje P.D. Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology. 2009;49(2):407–417. doi: 10.1002/hep.22572. [DOI] [PubMed] [Google Scholar]
- 6.Finan B., Parlee S.D., Yang B. Nuclear hormone and peptide hormone therapeutics for NAFLD and NASH. Molecular Metabolism. 2020:101153. doi: 10.1016/j.molmet.2020.101153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha R.A., You S.H., Zhou J., Siddique M.M., Bay B.H., Zhu X. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. Journal of Clinical Investigation. 2012;122(7):2428–2438. doi: 10.1172/JCI60580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaves C., Bruinstroop E., Refetoff S., Yen P.M., Anselmo J.D. Increased hepatic fat content in patients with resistance to thyroid hormone beta. Thyroid. 2021 doi: 10.1089/thy.2020.0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araki O., Ying H., Zhu X.G., Willingham M.C., Cheng S.Y. Distinct dysregulation of lipid metabolism by unliganded thyroid hormone receptor isoforms. Molecular Endocrinology. 2009;23(3):308–315. doi: 10.1210/me.2008-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu C., Xu L., Yu C., Miao M., Li Y. Association between thyroid function and nonalcoholic fatty liver disease in euthyroid elderly Chinese. Clinical Endocrinology. 2011;75(2):240–246. doi: 10.1111/j.1365-2265.2011.04016.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu J., Hernandez-Ono A., Graham M.J., Galton V.A., Ginsberg H.N. Type 1 deiodinase regulates ApoA-I gene expression and ApoA-I synthesis independent of thyroid hormone signaling. Arteriosclerosis, Thrombosis, and Vascular Biology. 2016;36(7):1356–1366. doi: 10.1161/ATVBAHA.116.307330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boelen A., van der Spek A.H., Bloise F., de Vries E.M., Surovtseva O.V., van Beeren M. Tissue thyroid hormone metabolism is differentially regulated during illness in mice. Journal of Endocrinology. 2017;233(1):25–36. doi: 10.1530/JOE-16-0483. [DOI] [PubMed] [Google Scholar]
- 13.Bohinc B.N., Michelotti G., Xie G., Pang H., Suzuki A., Guy C.D. Repair-related activation of hedgehog signaling in stromal cells promotes intrahepatic hypothyroidism. Endocrinology. 2014;155(11):4591–4601. doi: 10.1210/en.2014-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widjaja A.A., Singh B.K., Adami E., Viswanathan S., Dong J., D’Agostino G.A. Inhibiting interleukin 11 signaling reduces hepatocyte death and liver fibrosis, inflammation, and steatosis in mouse models of nonalcoholic steatohepatitis. Gastroenterology. 2019;157(3):777–792. doi: 10.1053/j.gastro.2019.05.002. e14. [DOI] [PubMed] [Google Scholar]
- 15.Singh B.K., Sinha R.A., Zhou J., Tripathi M., Ohba K., Wang M.E. Hepatic FOXO1 target genes are co-regulated by thyroid hormone via RICTOR protein deacetylation and MTORC2-AKT protein inhibition. Journal of Biological Chemistry. 2016;291(1):198–214. doi: 10.1074/jbc.M115.668673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricchi M., Odoardi M.R., Carulli L., Anzivino C., Ballestri S., Pinetti A. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. Journal of Gastroenterology and Hepatology. 2009;24(5):830–840. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- 17.Jump D.B., Tripathy S., Depner C.M. Fatty acid-regulated transcription factors in the liver. Annual Review of Nutrition. 2013;33:249–269. doi: 10.1146/annurev-nutr-071812-161139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witek R.P., Stone W.C., Karaca F.G., Syn W.K., Pereira T.A., Agboola K.M. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50(5):1421–1430. doi: 10.1002/hep.23167. [DOI] [PubMed] [Google Scholar]
- 19.Schneider M.J., Fiering S.N., Thai B., Wu S.Y., St Germain E., Parlow A.F. Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology. 2006;147(1):580–589. doi: 10.1210/en.2005-0739. [DOI] [PubMed] [Google Scholar]
- 20.França M.M., German A., Fernandes G.W., Liao X.H., Bianco A.C., Refetoff S. Human type 1 iodothyronine deiodinase (DIO1) mutations cause abnormal thyroid hormone metabolism. Thyroid. 2021;31(2):202–207. doi: 10.1089/thy.2020.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panicker V., Cluett C., Shields B., Murray A., Parnell K.S., Perry J.R. A common variation in deiodinase 1 gene DIO1 is associated with the relative levels of free thyroxine and triiodothyronine. The Journal of Clinical Endocrinology and Metabolism. 2008;93(8):3075–3081. doi: 10.1210/jc.2008-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.