Abstract
Persistent cannabis use among young adults with first episode psychosis (FEP), even those receiving early intervention services, has been associated with poor outcomes. In the United States (US), Coordinated Specialty Care (CSC) has been shown to be more effective at reducing symptoms, improving quality of life and increasing involvement in work or school, compared to typical care for FEP. However, little is known about the prevalence, course and outcomes for cannabis use in this real-world, clinical setting. This study examined the prevalence, course and outcomes of cannabis use categorized into three groups: no use, reduced use, and persistent use, among a sample of 938 CSC participants enrolled for at least 1 year. Prevalence of cannabis use was 38.8% at admission and 32.8% of the sample had persistent cannabis use at 1 year. At baseline, persistent cannabis users were more likely to be male (p < .001), white, non-Hispanic and black non-Hispanic (p = .001), have worse symptoms as measured by the GAF (p < .001), increased suicidality (p = .024), violent ideation (p = .008), and legal trouble (p = .006) compared with non-users. At 1 year, persistent users maintained worse symptoms compared with non-users (p = .021) while those who reduced use had significant improvement in symptoms compared with persistent users (p = .008). This study suggests that cannabis use is common among young adults enrolled in a CSC program in the US and that persistent cannabis users may have worse outcomes while reducing cannabis use may improve outcomes. These findings highlight the potential impact of secondary prevention in this population through reduction in cannabis use.
Keywords: First episode psychosis, Early psychosis, Cannabis use, Substance use, Coordinated specialty care
1. Introduction
Cannabis use has been implicated in both the development and progression of psychotic disorders (Leeson et al., 2012; Moore et al., 2007; Myles et al., 2016), based on evidence that demonstrates an increased risk of developing a psychotic disorder related to age at first use, frequency of use and potency of cannabis used (Compton et al., 2009; Di Forti et al., 2013; Large et al., 2011; Marconi et al., 2016). Despite this risk, cannabis is consistently reported as the most frequent and commonly used illicit substance among young people with early psychosis (Baeza et al., 2009; Lange et al., 2014; Van Mastrigt et al., 2004; Harrison et al., 2008). Within some first episode psychosis (FEP) samples, the prevalence of cannabis use is as high as 60–70% (Carr et al., 2009; Schimmelmann et al., 2012; Wade et al., 2005). Persistent cannabis use following onset of psychosis has been associated with poor outcomes including lower antipsychotic medication adherence, more symptoms, increased hospitalizations, and heightened risk of relapse (Mazzoncini et al., 2010; Patel et al., 2016; Schoeler et al., 2017; Seddon et al., 2016). Early intervention services for individuals with FEP, which is considered standard of care in this population (Malla and McGorry, 2019), have produced decreased cannabis use in a subgroup of individuals, however, many individuals with FEP maintain persistent use despite treatment (Addington and Addington, 2007; Carr et al., 2009; Schimmelmann et al., 2012).
Most of the research focusing on cannabis use in FEP has examined European, Canadian or Australian cohorts receiving some type of early intervention services (Schoeler et al., 2016a, 2016b; Zammit et al., 2008). In the United States, Coordinated Specialty Care (CSC) has been shown to be more effective at reducing symptoms, improving quality of life and increasing involvement in work or school, compared to treatment as usual (Kane et al., 2015) and is being implemented across the country (https://www.nimh.nih.gov/health/topics/schizophrenia/raise/what-is-coordinated-specialty-care-csc.shtml, accessed 12/2/2019). Little is known about the prevalence, course and outcomes for cannabis use in this setting. Of the few studies conducted in the U.S., The Recovery After an Initial Schizophrenia Episode-Early Treatment Program study (RAISE-ETP) tested a CSC model (NAVIGATE) for early psychosis (Kane et al., 2015) and used a combination of motivational, educational, and cognitive-behavioral strategies (Cather et al., 2018) for those participants who used substances as part of the treatment model. At baseline, almost half of the participants (48.8%) reported drug or alcohol use, and over half (51.7%) met the criteria for a lifetime SUD, with cannabis use disorder accounting for 34.7% (Cather et al., 2018). The RAISE-ETP study observed no reduction in cannabis use among patients during the two-year period, with substance use rates remaining stable over time (Cather et al., 2018). In a separate analysis, baseline cannabis use was found to be associated with higher scores on the PANSS positive subscale and on the Clinical Global Impressions (CGI) scale during treatment, but cannabis use over time was not examined (Oluwoye et al., 2019).
To address this gap in the literature, this paper aims to better understand the course of cannabis use and the impact of persistent use on symptoms and functioning in a cohort of young adults with FEP enrolled in a CSC program in the United States called OnTrackNY.
This paper had several aims:
To describe the prevalence and course of cannabis use over a one-year follow up period among a sample of young adults with early psychosis;
To examine differences in baseline characteristics between courses of cannabis use over one-year follow up (persistent and reduced use) compared with non-users; and
To examine associations between courses of cannabis use and concurrent clinical outcomes.
2. Methods and materials
2.1. Participants and study design
OnTrackNY, a CSC program, consists of a recovery-oriented, multidisciplinary team delivering evidence-based psychosocial interventions and medication to young people with the recent onset of a non-affective psychotic disorder (Bello et al., 2017; Dixon et al., 2015). Teams work with participants and families on individual goals related to school, work and relationships. OnTrackNY sites are located in licensed outpatient clinics at community agencies, state-operated facilities, and community and academic hospitals in urban and suburban areas throughout New York State (NYS). Eligibility criteria for OnTrackNY enrollment includes individuals ages 16–30 years who experienced non-affective psychosis for less than two years. The research sample was limited to those who were enrolled at one of 19 OnTrackNY sites from October 2013 through December 2017 and had at least one year of possible follow up (N = 938).
OnTrackNY clinicians submit client-level data to the NYS Office of Mental Health (OMH) at admission to OnTrackNY, quarterly, and at discharge for quality improvement and fidelity monitoring. Data are collected using standardized admission forms which clinicians complete through report of participants and their families, and chart review. For research purposes, all data are deidentified and protected health information was removed from the dataset by OMH prior to data sharing with research teams. The New York State Psychiatric Institute (NYSPI) Institutional Review Board reviewed the study procedures and did not consider this secondary data analysis human subjects research, therefore it was exempt from approval.
2.2. Measures
Domains assessed included demographics, family/social characteristics, and clinical characteristics. All measures are considered “current” or “recent” when assessed at baseline or in the 90 days prior to the assessment, unless otherwise stated. This cutoff was determined from a programmatic perspective by OnTrackNY program staff and OMH. Demographics included age, gender, race/ethnicity, health insurance status, current employment or education status. Current employment or education was defined as those who were enrolled in an education program (full or part-time, including high school, vocational training, college, or graduate study) or had any paid employment (including competitive or non-competitive work, self-employment, or internship) at the time of admission to OnTrackNY. Family and social characteristics included homelessness, family contact, and legal issues. Homelessness was defined as any client who spent ANY time sleeping in a homeless shelter, on the street, public place (e.g., subway), place not meant for sleeping, or temporary place that is not the client’s residence (e.g., “couch surfing”) in the 90-days prior to admission. Family involvement included who the client lived with and frequency of contact with family at time of admission. Legal issues were defined as having any legal issues, including being on parole or probation in the 90 days prior to admission. This measure was only analyzed for a subset of participants for whom the data was available.
Baseline clinical characteristics included MIRECC Global Assessment of Functioning (GAF) (Niv et al., 2007) symptom, occupational and social functioning scales at the time of admission; suicidal or violent ideation or behavior; tobacco, alcohol or other drug use at admission; age at onset of psychosis; time from onset to enrollment in OnTrackNY. Scores on the MIRECC GAF range from 0 to 100, with scores below 40 considered in the impaired range and scores of 70 and above considered normal range. Suicidal and violent ideation or behavior included any report of suicidal ideation or attempts or of violent or aggressive ideation or behavior in the 90 days prior to admission. Tobacco, alcohol or other drug use at admission was defined as “any” use in the 90 days prior to admission for each of the substances based on clinician report. Team clinicians assessed the time of onset of qualifying psychotic symptoms based on participant and/or family member report as well as collateral information from medical records or other sources as part of the initial evaluation of each participant. Age at onset of psychosis was calculated based upon the date of onset of qualifying psychotic symptoms and date of birth of the participant.
Current cannabis use was defined as “any” use in the 90 days prior to admission and each quarterly assessment, based on clinician report. In addition to GAF scores and education/employment (defined above), other longitudinal clinical outcomes included hospitalizations, medication adherence, and early discharge. Hospitalizations were defined as any psychiatric hospitalizations, excluding substance use rehabilitation or detoxification admissions, in the 90 days prior to assessment. Medication prescription and adherence was defined as having been prescribed an antipsychotic medication, and for those being prescribed, whether they had adherence of at least 80% in the month prior (Haynes, 1976) to each assessment based on clinician report. Early discharge was defined as any individual who left the program within 12 months of being enrolled for any reason.
2.3. Statistical analyses
The first aim was to describe the prevalence and course of cannabis use at admission and through follow-up periods. The course of cannabis use was categorized into three groups: no use, reduced use, and persistent use. The ‘no-use’ category included participants whose clinicians reported they never used cannabis at admission or through their follow-up periods. The ‘reduced use’ category included participants whose clinicians reported they used cannabis at admission, but then discontinued use at some time during the follow-up period. The ‘persistent use’ category included those who used continuously from admission through follow-up, those who used on and off through follow-up, and those who were not using at admission but began using during follow-up.
For the second aim, descriptive summaries of baseline characteristics were calculated stratified by course of cannabis use with means and standard deviations for normally distributed continuous measures, medians and interquartile ranges for skewed measures, and proportions for categorical measures. Associations between each baseline measure and the three groups (i.e. no use, reduced use, and persistent use) were tested using one-way ANOVAs, non-parametric Kruskal-Wallis test, or chi-square tests depending on the distribution of the characteristic. Pairwise comparisons between groups were computed when the overall test was significant at p < .05.
The third aim included examining the association between course of cannabis use and clinical outcomes. Longitudinal mixed effects models were run using an identity link function for continuous clinical outcomes (GAF scores) and a logit link function for dichotomous clinical outcomes (education/employment, psychiatric hospitalizations, medication adherence). Each model included an autoregressive covariance structure for the errors over time to account for within-subject correlation. The continuous outcome models additionally included a random effect for site, but for dichotomous outcome models, including site did not allow the models to converge and it was not included. Each model included as predictors the course of cannabis use (no use, reduced use, and persistent use), follow-up time (baseline, 3, 6, 9, and 12 months), and their interaction. Pre-specified contrasts were computed to assess the pairwise and overall effect of course of cannabis use at baseline, at 12-month follow-up, and of the change from baseline to 12-month follow-up. Each model additionally controlled for age, gender, race, and an indicator of whether the person had an early discharge (discharge prior to 1-year of admission). Finally, a Cox proportional hazard model was fit to assess the effect of course of cannabis use on early discharge controlling for the same covariates of age, gender, and race. This model included a 2-way interaction between course of cannabis use by time to estimate the pairwise effects specifically at 12-month follow-up.
All analyses were done using SAS version 9.4, and all hypothesis tests were two-sided with 5% significance level. Due to the exploratory nature of these analyses, pre-specified clinically meaningful contrasts below 10% significance level are presented as well. Missing data was limited in this dataset due to data collection and quality procedures, with a range of 0.5% to 6% missing for the MIRECC GAF, employment/education, and psychiatric outcomes, and a range between 3.4% to 9.3% for medication adherence. Data was assumed to be missing at random in statistical analyses.
3. Results
3.1. Sample characteristics
Demographics, social and clinical characteristics of the sample are presented in Table 1. This sample included all participants that were enrolled prior to 2018, and therefore had at least one-year of possible follow-up. At admission, the participants were on average 21 years old, were mostly male (74%), with 27% white non-Hispanic, 36% black non-Hispanic, 28% Hispanic, and 10% other races (Asians, American Indian/Alaskan Native, and Native Hawaiian/Other Pacific Islander). About 40% were either employed or in school, only 5% were uninsured, 46% had public insurance and 41% had private insurance. The majority lived with parents (84%) and had daily family contact (91%), while 6% reported being homeless.
Table 1.
Participant characteristics at admission of the overall sample by course of cannabis use (N = 938).
| Course of cannabis use |
Group comparisons |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall (N = 938) |
Group 1: no use (N = 475) |
Group 2: reduced (N = 155) |
Group 3: persistent (N = 308) |
Overall difference |
Pairwise: 1 vs 2 |
Pairwise: 1 vs 3 |
Pairwise: 2 vs 3 |
||||||
| Measures | N | %, M (SD), Med (IQR) | N | %, M (SD), Med (IQR) | N | %, M (SD), Med (IQR) | N | %, M (SD), Med (IQR) | Test Statistic | p | p | p | p |
| Demographic measures | |||||||||||||
| Age (years) | 938 | 21.0 (3.3) | 475 | 21.1 (3.5) | 155 | 21.0 (2.9) | 308 | 20.9 (2.9) | F(2, 935) = 0.39 | 0.676 | |||
| Gender | χ2(4) = 21.72 | <0.001 | 0.014 | <0.001 | 0.839 | ||||||||
| Female | 236 | 25.2% | 150 | 31.6% | 30 | 19.4% | 56 | 18.2% | |||||
| Male | 697 | 74.3% | 322 | 67.8% | 124 | 80.0% | 251 | 81.5% | |||||
| Other | 5 | 0.5% | 3 | 0.6% | 1 | 0.6% | 1 | 0.3% | |||||
| Race | χ2(6) = 24.39 | <0.001 | 0.006 | 0.001 | 0.916 | ||||||||
| White (non-Hispanic) | 253 | 27.0% | 116 | 24.4% | 46 | 29.7% | 91 | 29.5% | |||||
| Black (non-Hispanic) | 335 | 35.7% | 158 | 33.3% | 62 | 40.0% | 115 | 37.3% | |||||
| Hispanic | 258 | 27.5% | 133 | 28.0% | 40 | 25.8% | 85 | 27.6% | |||||
| Other | 92 | 9.8% | 68 | 14.3% | 7 | 4.5% | 17 | 5.5% | |||||
| Insurance status | χ2(6) = 7.13 | 0.309 | |||||||||||
| Uninsured | 48 | 5.1% | 30 | 6.3% | 7 | 4.5% | 11 | 3.6% | |||||
| Public | 430 | 45.8% | 214 | 45.1% | 71 | 45.8% | 145 | 47.1% | |||||
| Private | 383 | 40.8% | 190 | 40.0% | 70 | 45.2% | 123 | 39.9% | |||||
| Other | 77 | 8.2% | 41 | 8.6% | 7 | 4.5% | 29 | 9.4% | |||||
| Social/family measures | |||||||||||||
| Homelessness | χ2(2) = 4.99 | 0.083 | |||||||||||
| No | 885 | 94.3% | 456 | 96.0% | 143 | 92.3% | 286 | 92.9% | |||||
| Yes | 53 | 5.7% | 19 | 4.0% | 12 | 7.7% | 22 | 7.1% | |||||
| Lives with family | χ2(6) = 7.63 | 0.267 | |||||||||||
| Parents | 787 | 83.9% | 408 | 85.9% | 132 | 85.2% | 247 | 80.2% | |||||
| Other family (not parents) | 63 | 6.7% | 28 | 5.9% | 8 | 5.2% | 27 | 8.8% | |||||
| Alone | 41 | 4.4% | 15 | 3.2% | 7 | 4.5% | 19 | 6.2% | |||||
| Other | 47 | 5.0% | 24 | 5.1% | 8 | 5.2% | 15 | 4.9% | |||||
| Family contact | χ2(4) = 2.26 | 0.688 | |||||||||||
| Daily | 842 | 90.8% | 430 | 90.9% | 141 | 91.6% | 271 | 90.3% | |||||
| Weekly | 60 | 6.5% | 31 | 6.6% | 7 | 4.5% | 22 | 7.3% | |||||
| Monthly or less | 25 | 2.7% | 12 | 2.5% | 6 | 3.9% | 7 | 2.3% | |||||
| Legal issues | χ2(2) = 7.68 | 0.021 | 0.287 | 0.006 | 0.223 | ||||||||
| No | 454 | 88.7% | 233 | 92.1% | 93 | 88.6% | 128 | 83.1% | |||||
| Yes | 58 | 11.3% | 20 | 7.9% | 12 | 11.4% | 26 | 16.9% | |||||
| Clinical measures | |||||||||||||
| Time to OTNY (days) | 937 | 170.0 (82.0–341.0) | 474 | 175.5 (85.0–337.0) | 155 | 149.0 (75.0–286.0) | 308 | 180.0 (85.5–351.0) | χ2(2) = 1.78 | 0.412 | |||
| Age at onset of psychosis | 937 | 20.9 (3.2) | 474 | 21.0 (3.5) | 155 | 20.9 (2.9) | 308 | 20.8 (2.9) | F(2, 934) = 0.39 | 0.680 | |||
| Violent ideation/attempt | χ2(2) = 8.17 | 0.017 | 0.053 | 0.008 | 0.851 | ||||||||
| No | 722 | 77.0% | 384 | 80.8% | 114 | 73.5% | 224 | 72.7% | |||||
| Yes | 216 | 23.0% | 91 | 19.2% | 41 | 26.5% | 84 | 27.3% | |||||
| Suicide ideation/attempt | χ2(2) = 6.19 | 0.045 | 0.079 | 0.024 | 0.964 | ||||||||
| No | 669 | 71.3% | 356 | 74.9% | 105 | 67.7% | 208 | 67.5% | |||||
| Yes | 269 | 28.7% | 119 | 25.1% | 50 | 32.3% | 100 | ||||||
| Baseline tobacco use | χ2(2) = 56.37 | <0.001 | <0.001 | <0.001 | 0.079 | ||||||||
| No | 802 | 85.5% | 445 | 93.7% | 127 | 81.9% | 230 | 74.7% | |||||
| Yes | 136 | 14.5% | 30 | 6.3% | 28 | 18.1% | 78 | 25.3% | |||||
| Baseline alcohol use | χ2(2) = 87.04 | <0.001 | <0.001 | <0.001 | 0.012 | ||||||||
| No | 703 | 74.9% | 415 | 87.4% | 84 | 54.2% | 204 | 66.2% | |||||
| Yes | 235 | 25.1% | 60 | 12.6% | 71 | 45.8% | 104 | 33.8% | |||||
| Baseline other drug use | χ2(2) = 44.85 | <0.001 | <0.001 | <0.001 | 0.073 | ||||||||
| No | 884 | 94.2% | 470 | 98.9% | 133 | 85.8% | 281 | 91.2% | |||||
| Yes | 54 | 5.8% | 5 | 1.1% | 22 | 14.2% | 27 | 8.8% | |||||
| Baseline outcome measures | |||||||||||||
| GAF symptoms | 937 | 30.7 (14.9) | 474 | 32.5 (15.9) | 155 | 29.7 (13.1) | 308 | 28.3 (13.7) | F(2, 934) = 7.81 | <0.001 | 0.053 | <0.001 | 0.282 |
| GAF SF | 934 | 56.3 (15.9) | 472 | 56.9 (16.0) | 155 | 55.9 (14.9) | 307 | 55.7 (16.4) | F(2, 931) = 0.60 | 0.548 | |||
| GAF OC | 933 | 36.2 (20.0) | 472 | 37.9 (21.4) | 155 | 35.3 (18.3) | 306 | 34.1 (18.3) | F(2, 930) = 3.62 | 0.027 | 0.170 | 0.010 | 0.510 |
| Education/employment | χ2(2) = 1.45 | 0.483 | |||||||||||
| No | 553 | 59.0% | 271 | 57.1% | 95 | 61.3% | 187 | 60.7% | |||||
| Yes | 385 | 41.0% | 204 | 42.9% | 60 | 38.7% | 121 | 39.3% | |||||
| Any psychiatric hospitalization | χ2(2) = 5.34 | 0.069 | |||||||||||
| No | 244 | 26.0% | 136 | 28.6% | 30 | 19.4% | 78 | 25.3% | |||||
| Yes | 694 | 74.0% | 339 | 71.4% | 125 | 80.6% | 230 | 74.7% | |||||
| Medication adherence | χ2(6) = 14.54 | 0.024 | 0.003 | 0.391 | 0.167 | ||||||||
| Not med adherent | 139 | 14.8% | 56 | 11.8% | 36 | 23.2% | 47 | 15.3% | |||||
| Med adherent | 651 | 69.4% | 346 | 72.8% | 96 | 61.9% | 209 | 67.9% | |||||
| Not prescribed meds | 61 | 6.5% | 27 | 5.7% | 12 | 7.7% | 22 | 7.1% | |||||
| Missing | 87 | 9.3% | 46 | 9.7% | 11 | 7.1% | 30 | 9.7% | |||||
| Early discharge prior to | χ2(2) = 0.404 | ||||||||||||
| 1 year No | 634 | 67.6% | 328 | 69.1% | 98 | 63.2% | 208 | 67.5% | |||||
| Yes | 304 | 32.4% | 147 | 30.9% | 57 | 36.8% | 100 | 32.5% | |||||
3.2. Course of cannabis use (aim 1)
At admission, 38.8% of participants reported cannabis use within the prior 90 days. (Data not shown) The prevalence decreased from baseline to 3 months and then remained steady across time at approximately 25% at months 3, 6, 9, and 12. About half (50.64%, n = 475) of the participants reported no use at admission and throughout all follow-up visits (i.e., no use group) and 16.52% (n = 155) reported use at admission, and then no longer used by end of follow-up (i.e. reduced use group). The remaining participants (32.84%, 308) had either mixed use or continued use from enrollment and through follow-up (i.e., persistent group).
3.3. Characteristics associated with course of cannabis use (aim 2)
The association of baseline characteristics with course of cannabis use is shown in Table 1.
Gender and race/ethnicity was significantly associated with course of cannabis use (both p < .001) with the no use group having significantly greater proportion of females compared to the reduced group (31.6% vs 19.4% female) and to the persistent group (31.6% vs 18.2% female), and having a greater proportion of Hispanics and other races compared to the reduced and persistent groups. GAF occupational (OC) and GAF symptom scores at admission also were significantly associated with courses of cannabis use (p = .027 and p < .001, respectively) with the persistent group having significantly lower scores (ie., worse) in both the OC (Mean (SD) = 34.1 (18.3) vs 37.9 (21.4)) and symptoms (Mean (SD) = 28.3 (13.7) vs 32.5 (15.9)) domains compared to the no use group. Violent ideation/behavior, suicidal ideation/behavior, and legal issues were also significantly related to course of cannabis use (p = .017, p = .045, p = .021, respectively) with the persistent use group having significantly higher proportions of participants with baseline violent ideation/behavior (27.3% vs 19.2%), suicidal ideation/behavior (32.5% vs 25.1%), and legal issues (16.9% vs 7.9%) compared to the no use group. Baseline tobacco, alcohol, and other drug use were significantly related to course of cannabis use (all p < .001). The cannabis no use group had significantly lower baseline use of tobacco, alcohol, and other drugs (6.3%, 12.6%, and 1.1%, respectively) compared to the reduced use (18.1%, 45.8%, and 14.2, respectively) and persistent use (25.3%, 33.8%, and 8.8%, respectively) groups. Additionally, baseline alcohol use was significantly higher in the reduced use group than the persistent use group (45.8% vs 14.2%). Medication prescription and adherence at admission was significantly related to course of cannabis use, with the no use group more likely to be medication adherent when prescribed anti-psychotic medication compared to the reduced group (72.8% vs 61.9%). No differences were found on other variables.
3.4. Course of cannabis use and concurrent clinical outcomes (aim 3)
Observed mean GAF scores along with 1-standard error bars during one-year follow-up by course of cannabis use are shown in Fig. 1, and results of linear models are presented in Table 2. The course of cannabis use was significantly associated with GAF symptoms at 12-month follow-up (p = .013). At 12-months, those with persistent cannabis use had significantly lower GAF symptoms compared to those with reduced cannabis use (b = −3.39, p = .021), but this effect was not different than the effect seen at baseline (baseline: b = −3.06, p = .012; change from BL to 12 months: b = −0.32, p = .858). At 12 months compared to baseline, those with reduced use tended to have a greater improvement in symptoms compared to both persistent users and nonusers (change from baseline to 12 months: b = 4.20, p = .093; b = 3.87, p = .100, respectively), with the reduced users achieving significantly higher symptom scores (i.e., better) at 12-months than those with persistent use (b = 5.33, p = .008). When adjusting for covariates, course of cannabis use was not significantly associated with GAF social functioning (SF) or GAF OC scores at 12-month follow-up (p = .683 and p = .612, respectively).
Fig. 1.
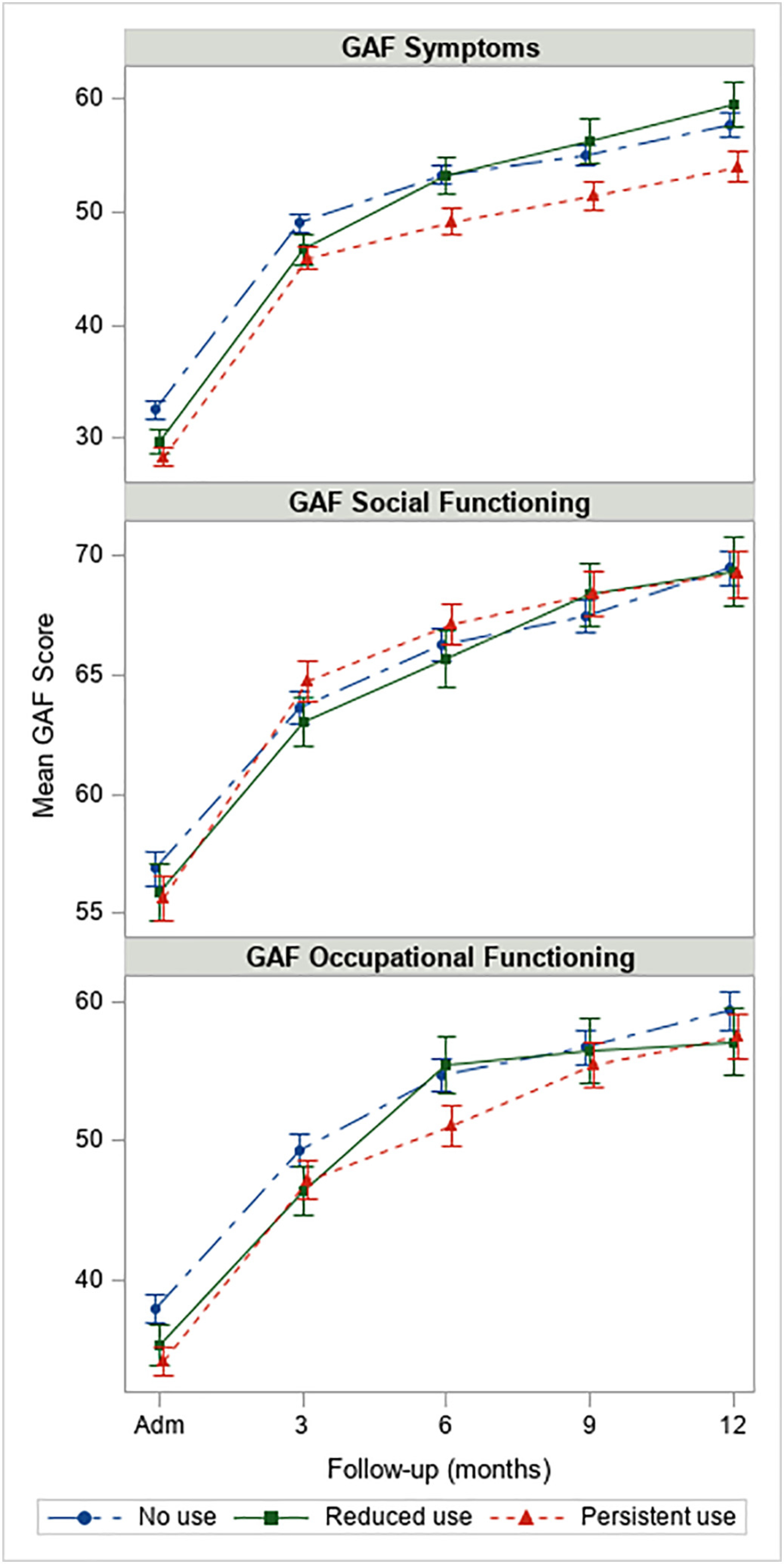
Observed mean GAF scores along with 1 standard error bars among participants in OTNY with 1-year eligibility (N = 938). Note: y-axis varies by outcome.
Table 2.
Results of longitudinal linear models on continuous clinical outcomes. Pairwise differences between course of cannabis use classes are shown at baseline, 12-month follow-up, and the change between baseline and 12 months. Overall joint tests were also computed for each set of pairwise comparisons.
| Pairwise differences | GAF Symptoms |
GAF SF |
GAF OC |
||||||
| b | 95% CI | p | b | 95% CI | p | b | 95% CI | p | |
| At baseline | |||||||||
| Reduced vs persistent | 1.14 | (−2.05,4.33) | 0.485 | 0.45 | (−2.25, 3.14) | 0.746 | 0.58 | (−3.72, 4.87) | 0.792 |
| Reduced vs no use | −1.93 | (−4.95,1.09) | 0.211 | 0.55 | (−2.01, 3.10) | 0.675 | −2.10 | (−6.16,1.96) | 0.311 |
| Persistent vs no use | −3.06 | (−5.45, −0.68) | 0.012 | 0.10 | (−1.92, 2.12) | 0.921 | −2.68 | (−5.90, 0.54) | 0.103 |
| At 12 months | |||||||||
| Reduced vs persistent | 5.33 | (1.38, 9.29) | 0.008 | 0.41 | (−2.85, 3.68) | 0.803 | −0.29 | (−5.56,4.97) | 0.914 |
| Reduced vs no use | 1.95 | (−1.81,5.70) | 0.310 | 1.20 | (−1.90, 4.30) | 0.448 | −1.94 | (−6.93, 3.06) | 0.447 |
| Persistent vs no use | −3.39 | (−6.26, −0.52) | 0.021 | 0.79 | (−1.59, 3.16) | 0.517 | −1.65 | (−5.48, 2.19) | 0.400 |
| Change from BLto12 months | |||||||||
| Reduced vs persistent | 4.20 | (−0.69, 9.09) | 0.093 | −0.03 | (−3.89,3.83) | 0.988 | −0.87 | (−7.32, 5.59) | 0.792 |
| Reduced vs no use | 3.87 | (−0.74,8.49) | 0.100 | 0.65 | (−2.99, 4.30) | 0.725 | 0.16 | (−5.93, 6.25) | 0.959 |
| Persistent vs no use | −0.32 | (−3.89, 3.24) | 0.858 | 0.68 | (−2.13, 3.50) | 0.634 | 1.03 | (−3.68, 5.74) | 0.668 |
| GAF Symptoms |
GAF SF |
GAF OC |
|||||||
| Overall effects | F | df | p | F | df | p | F | df | p |
| Overall test at baseline | 3.28 | (2,3771) | 0.038 | 0.09 | (2,3763) | 0.915 | 1.46 | (2,3754) | 0.232 |
| Overall test at 12 months | 4.35 | (2,3771) | 0.013 | 0.38 | (2,3763) | 0.683 | 0.49 | (2,3754) | 0.612 |
| Overall test of change (12 m-BL) | 1.61 | (2,3771) | 0.200 | 0.14 | (2,3763) | 0.872 | 0.10 | (2,3754) | 0.909 |
The observed prevalence of categorical clinical outcomes along with 1-standard error bars are presented in Fig. 2, and results of logistic regression models are presented in Table 3. Course of cannabis use was not significantly associated with education/ employment at 12-month follow-up (p = .735), but course of cannabis use was related to psychiatric hospitalization at 12-month follow-up where persistent users had higher odds of hospitalizations compared to those with no use (log-odds = 0.72, p = .020). Additionally, from baseline to 12-months, those with reduced use tended to have a reduction in hospitalizations compared to persistent users (log-odds = −0.93, p = .060). Course of cannabis use was significantly associated with medication adherence at 12 months. Reduced users had lower odds of medication adherence compared to non-users (log-odds = −0.67, p = .006), but this was not different than the effect at baseline (baseline: log-odds = −0.60, p = .008; change from baseline to 12 months: b = −0.07, p = .823).
Fig. 2.
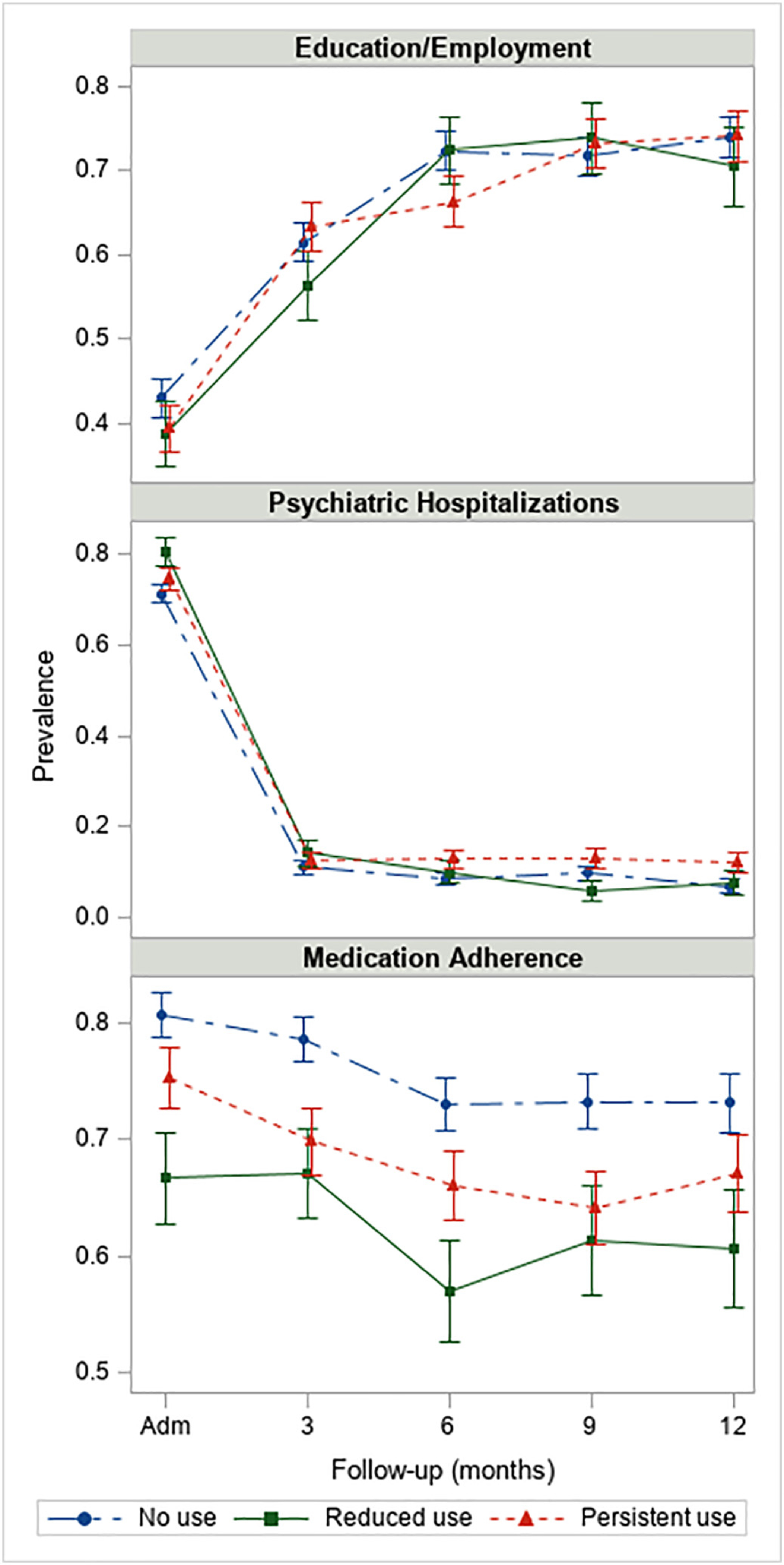
Observed rates of psychiatric hospitalizations, education/employment, and medication adherence along with 1-standard error bars among participants in OTNY with 1-year eligibility (N = 938). Note: y-axis varies by outcome.
Table 3.
Results of longitudinal logistic models on categorical clinical outcomes and of Cox-proportional hazards model on time to early discharge. Pairwise differences between course of cannabis use classes are shown at baseline, 12-month follow-up, and the change between baseline and 12 months. Overall joint tests were also computed for each set of pairwise comparisons.
| Education/employment |
Psychiatric hospitalizations |
Medication adherence |
Time to early discharge |
|||||||||
| Pairwise differences | Log- odds | 95% CI | p | Log- odds | 95% CL | p | Log- odds | 95% CI | p | HR | 95% CI | p |
|
At baseline | ||||||||||||
| Reduced vs persistent | −0.04 | (−0.45, 0.36) | 0.841 | 0.39 | (−0.10, 0.87) | 0.117 | −0.34 | (−0.79, 0.12) | 0.149 | – | – | – |
| Reduced vs no use | −0.12 | (−0.51, 0.26) | 0.533 | 0.57 | (0.12,1.03) | 0.014 | −0.60 | (−1.04, −0.16) | 0.008 | – | – | – |
| Persistent vs no use | −0.08 | (−0.38, 0.22) | 0.602 | 0.19 | (−0.14, 0.52) | 0.263 | −0.26 | (−0.64, 0.11) | 0.167 | – | – | – |
| At 12 months | ||||||||||||
| Reduced vs persistent | −0.17 | (−0.70, 0.36) | 0.528 | −0.54 | (−1.37, 0.29) | 0.204 | −0.36 | (−0.86, 0.14) | 0.157 | 1.92 | (0.99, 3.72) | 0.052 |
| Reduced vs no use | −0.20 | (−0.70, 0.30) | 0.438 | 0.18 | (−0.67, 1.02) | 0.683 | −0.67 | (−1.15, −0.20) | 0.006 | 1.68 | (0.92, 3.08) | 0.090 |
| Persistent vs no use | −0.03 | (−0.43, 0.37) | 0.892 | 0.72 | (0.11,1.32) | 0.020 | −0.31 | (−0.69, 0.07) | 0.109 | 0.88 | (0.51, 1.50) | 0.629 |
| Change from BLto12 months | ||||||||||||
| Reduced vs persistent | −0.13 | (−0.78, 0.52) | 0.693 | −0.93 | (−1.89, 0.04) | 0.060 | −0.02 | (−0.69, 0.64) | 0.946 | – | – | – |
| Reduced vs no use | −0.08 | (−0.69, 0.53) | 0.805 | −0.40 | (−1.36, 0.56) | 0.415 | −0.07 | (−0.71, 0.56) | 0.823 | – | – | – |
| Persistent vs no use | 0.05 | (−0.43,0.53) | 0.829 | 0.53 | (−0.16,1.21) | 0.132 | −0.05 | (−0.57,0.47) | 0.853 | – | – | – |
| Education/employment |
Psychiatric hospitalizations |
Medication adherence |
Time to early discharge |
|||||||||
| Overall effects | F | df | p | F | df | p | F | df | p | X2 | df | p |
| Overall test at baseline | 0.25 | (2, 2903) | 0.777 | 3.14 | (2,3014) | 0.043 | 3.67 | (2, 2804) | 0.026 | – | – | – |
| Overall test at 12 months | 0.31 | (2, 2903) | 0.735 | 2.83 | (2,3014) | 0.059 | 4.06 | (2, 2804) | 0.017 | 4.12 | 2 | 0.128 |
| Overall test of change (12 m-BL) | 0.08 | (2, 2903) | 0.924 | 2.17 | (2,3014) | 0.115 | 0.03 | (2, 2804) | 0.969 | – | – | – |
The cumulative incidence of early-discharge by course of cannabis use is presented in Fig. 3, and results of Cox-proportional hazard model on time to early discharge are presented in Table 3. At 12-month follow-up, course of cannabis use was not significantly related to time to early discharge (p = .128), however, those with reduced cannabis use tended to be more likely to discharge earlier than persistent users and non-users at 12-month follow-up (HR = 1.92, p = .052 and HR = 1.68, p = .090, respectively).
Fig. 3.
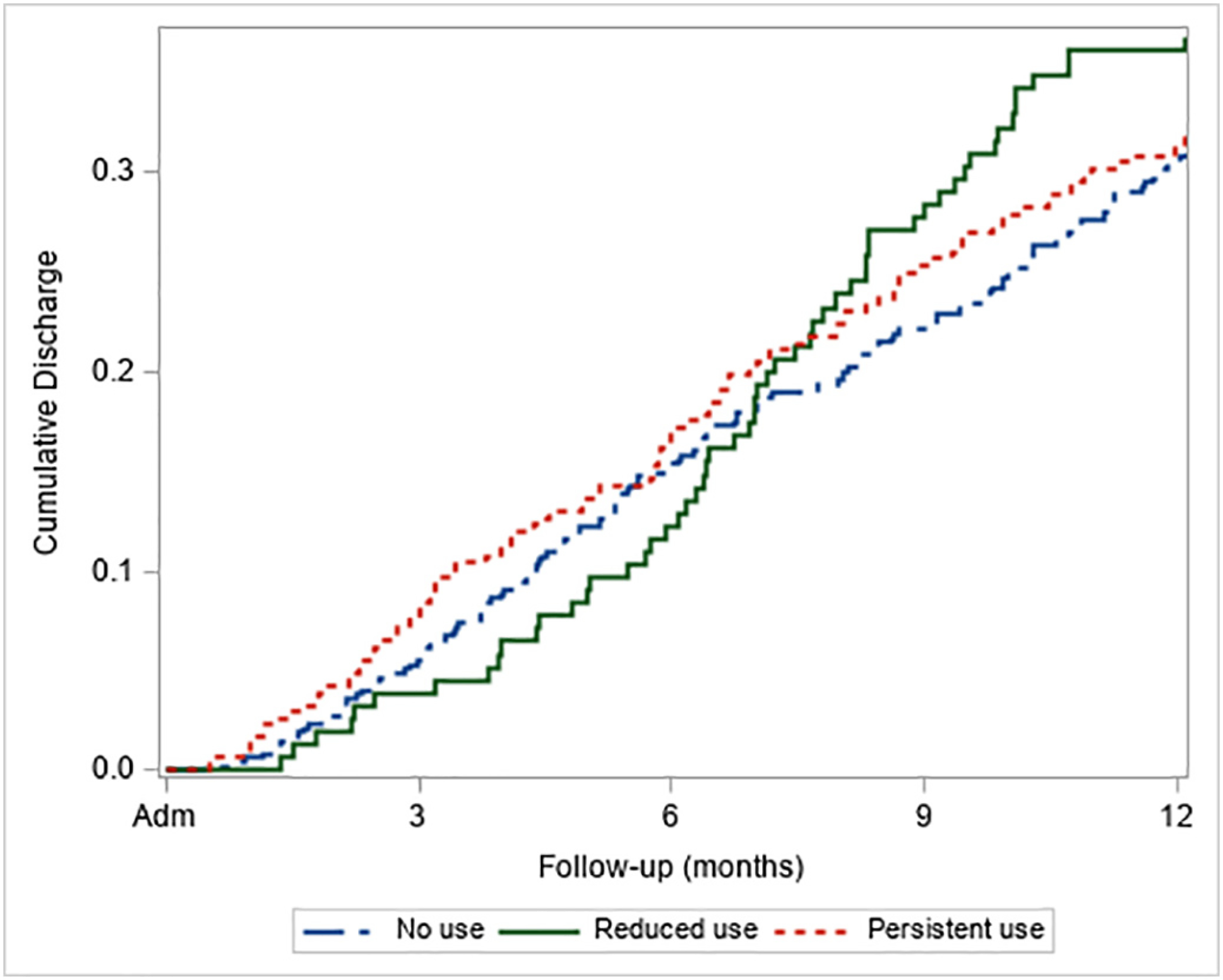
Cumulative incidence of early discharge by course of cannabis use among participants in OTNY with 1-year eligibility (N = 938).
4. Discussion
To our knowledge, this is the first study to examine prevalence, baseline characteristics and outcomes associated with cannabis use over time in a US-based FEP cohort enrolled in a coordinated specialty care program. We found a moderately high prevalence rate of recent cannabis use (38.8% in the prior 90 days) at baseline which is higher than prevalence rates found in the RAISE-ETP trial (23.6% past month use of cannabis) and lower than that of the EPICENTER trial (48% in the prior 6 months), but the time course over which cannabis use was measured varied (Cather et al., 2018; Breitborde et al., 2015). One year after enrollment, 16.5% of those who were using cannabis at admission stopped while roughly one-third had persistent use. While we cannot draw conclusions given the lack of a control group, these data suggest that the substance use treatment component of OnTrackNY, which utilizes at stage-wise motivational approach may help a subset of cannabis users reduce their use, but persistent use remains high and problematic and may require a more effective intervention. In the RAISE-ETP trial, self-reported cannabis use did not differ over 2 years in the NAVIGATE treatment condition compared with community care and the rate of heavy cannabis use was twice as high in the treatment arm compared with community care, even after controlling for baseline heavy cannabis use (Cather et al., 2018; Alcover et al., 2019). These studies and our findings support the need for further research into development, adaptation and implementation of effective interventions to reduce cannabis use among young adults with early psychosis. Several trials have examined interventions employing motivational interviewing and/or cognitive behavioral therapy treatments to reduce cannabis use in this population, with poor results (Bonsack et al., 2011; Edwards et al., 2006; Madigan et al., 2013).
Demographic characteristics found to be associated with cannabis use include gender and race/ethnicity. A significantly higher proportion of reduced and persistent users were males compared with non-users, consistent with other FEP cohorts (Donoghue et al., 2014; Arranz et al., 2015; Setien-Suero et al., 2017; Seddon et al., 2016). The sex differences in cannabis use among FEP are not well understood. Studies suggest that an interaction between gender and substance use in FEP may impact the age of onset of psychosis and possibly outcomes (Donoghue et al., 2014; Arranz et al., 2015; Lange et al., 2014). Further research is needed to examine sex differences among cannabis and substance users more generally in FEP to better clarify the etiology of the differences and potential impact on treatment outcomes. A higher proportion of non-users were also Hispanic or other race/ethnicity compared with reduced or persistent use groups. These findings mirror those of the general population which demonstrate lower overall rates of cannabis use among Hispanics and Asians, who make up the majority of the “other” race/ethnicity category within OnTrackNY (Hasin et al., 2019). It is important to note that prevalence rates among these minority groups are on the rise, and as the prevalence of marijuana use increases in the general population (Hasin et al., 2015) we may see the differences in prevalence by gender and race/ethnicity in FEP populations begin to decrease also.
At baseline, both the persistent cannabis use group and reduced use group were found to have worse symptoms and lower functioning compared with non-users, consistent with findings from the RAISE-ETP study (Oluwoye et al., 2019) though the differences for the reduced use group did not reach statistical significance, likely due to a smaller sample size. We also found that cannabis users (persistent and reduced) had higher rates of recent suicidal ideation, violent ideation, and legal issues at baseline, compared with non-users, which may be driven by an overall increase in positive psychotic or more depressive symptoms as demonstrated by lower GAF symptom scores. Increased risk of suicide attempts has been associated with co-morbid substance use at baseline in FEP (Togay et al., 2015) and violence and legal issues have also been found to be associated with cannabis use in cross-sectional studies (Rolin et al., 2019). Overall, this sample had similar baseline prevalence of alcohol and other drug use compared with RAISE ETP (Cather et al., 2018), but substantially lower prevalence compared with the EPICENTER study (Breitborde et al., 2015). It is possible these differences are due to different screening/eligibility criteria, with OnTrackNY and RAISE-ETP screening out individuals with more significant substance use, compared with EPICENTER. The finding of higher baseline alcohol use among reduced cannabis users is interesting, and to our knowledge, has not been previously reported. Further research examining the interaction between alcohol and cannabis use, particularly among those who reduce or stop use is warranted. Contrary to other findings (Leeson et al., 2012), cannabis use in this sample was not associated with better social functioning. Given the cross-sectional nature of the data at baseline and lack of data regarding onset of use and premorbid functioning, we cannot determine the direction of the relationship between these clinical characteristics and cannabis use. What the data does demonstrate from a clinical perspective is that at entry into CSC, individuals who are using cannabis are more symptomatic and it is difficult to differentiate those individuals who will ultimately reduce or stop using and those who will persist and potentially require more effective substance use interventions.
There were no differences between the three groups at one year in GAF occupational functioning scores or achievement of work or school. OnTrackNY participants achieve high rates of employment and education overall based on findings from previous studies (Nossel et al., 2018; Humensky et al., 2019). OnTrackNY uses a supported employment and education approach based on the Individual Placement and Support (IPS) model that supports individual client goals for work or education with zero exclusion and ongoing support from the team’s full-time supported employment and education specialist. This model has been shown to improve rates of competitive employment for individuals with severe mental illness (Marino and Dixon, 2014) and this approach may contribute to the lack of difference in employment and education outcomes seen among cannabis users versus non-users in OnTrackNY.
Persistent cannabis use was associated with lower GAF symptom scores and increased likelihood of hospitalization compared with nonusers, while those who reduced or stopped use appear to have greater improvement than both groups and achieve outcomes similar to nonusers. In a meta-analysis conducted by Schoeler et al. (2016a), continued cannabis users showed similar levels of functioning compared to non-users, but those who discontinued use had higher levels of functioning than non-users. In a prospective study by Schoeler et al. (2016b), those with persistent cannabis use had greatest risk of relapse (defined as hospitalization), while those who were former users had the lowest risk. In addition, we found that those who reduced/stopped use had lower medication adherence compared with non-users over time, but there was no significant difference in medication adherence over time among persistent users compared with non-users, which decreased slightly at six months but then remained stable in both groups. In contrast, Schoeler et al., 2017 found that medication adherence is worse among persistent users and may mediate some of the risk of relapse. Faridi et al., 2012 also found that medication adherence mediated the impact of persistent cannabis use on symptoms, but over time persistent users had substantially greater medication adherence compared with those who stop using. The authors suggest that these inconsistent findings may be due to participation in an intensive early intervention program which provides counseling and education around the importance of medication adherence and also offers the opportunity for shared decision making with patients which allows patients the room to make decisions about taking medication, as well as their substance use (Faridi et al., 2012), similar to the CSC model. Further research is needed to examine the interaction between cannabis use, medication adherence and symptoms over time in this setting.
Finally, those who reduced their cannabis use also trended toward being more likely to have an early discharge compared with non-users and persistent users. Taken together, the findings in this study suggest that reducing and/or stopping cannabis use may result in improved symptoms and decreased likelihood of hospitalization which could explain lower medication adherence and early drop-out. One hypothesis is that some of the symptomatology in the cannabis users may be related to the effects of cannabis itself and once they reduce or stop using cannabis, their symptoms improve, they stop taking medications and may be more likely to leave the program; however, more research is needed to investigate these associations.
The study is limited by the constraints of data collection in a clinical rather than research context. All assessments were developed for clinical use by OnTrackNY and assessments are conducted quarterly. With the exception of the MIRECC GAF, many of the measures used for data collection within OnTrackNY are not research measures. Quarterly data collection is performed and submitted to OMH by clinical staff and may be subject to site differences in how clinical assessment data is obtained. For example, our measure for cannabis use is based on the clinician report of “any cannabis use in the prior 90 days” and the clinic-level assessment of cannabis use that informs this measure may vary by clinic or by clinician. We recognize this variable is limited and may likely result in underreporting of cannabis use and an underestimate of the actual prevalence and scope of cannabis use in this sample. In addition, we do not know the pattern of cannabis use of those individuals with early drop-out which limits the interpretation of longitudinal findings. This dataset also does not include measures of lifetime cannabis use, age at onset of cannabis use, frequency or severity of use or other premorbid functioning measures, which may be factors relevant to the outcomes which are unable to be examined in this data. Finally, this is an observational study with no control group and caution should be used when interpreting findings.
The findings of this study suggest that cannabis use is common among young adults enrolled in a CSC program in the US and that individuals with persistent cannabis use may have worse outcomes while reducing cannabis use may improve outcomes. Given the high prevalence of cannabis use in this population and the changing landscape of legalization in the US, it is imperative to develop and test interventions to reduce cannabis use in the CSC setting.
Acknowledgments
Role of the funding source
There was no funding source for this research.
Footnotes
Declaration of competing interests
The authors of no conflicts of interest to disclose.
Data availability
The data is available upon reasonable request from the corresponding author.
References
- Addington J, Addington D, 2007. Patterns, predictors and impact of substance use in early psychosis: a longitudinal study. Acta Psychiatr. Scand. 115 (4), 304–309. 10.1111/j.1600-0447.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- Alcover K, Oluwoye O, Kriegel L, McPherson S, McDonell M, 2019. Impact of first episode psychosis treatment on heavy cannabis use: Secondary analysis of RAISE-ETP study. Schizophr. Res 211, 86–87. 10.1016/j.schres.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arranz B, Safont G, Corripio I, Ramirez N, Duenas RM, Perez V, et al. , 2015. Substance use in patients with first-episode psychosis: is gender relevant? J. Dual Diagn. 11 (3±4), 153–60. 10.1080/15504263.2015.1113761. [DOI] [PubMed] [Google Scholar]
- Baeza I, Graell M, Moreno D, Castro-Fornieles J, Parellada M, González-Pinto A, ... Arango C, 2009. Cannabis use in children and adolescents with first episode psychosis: influence on psychopathology and short-term outcome (CAFEPS study). Schizophr. res 113 (2‐3), 129–137. 10.1016/j.schres.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Bello I, Lee R, Malinovsky I, Watkins L, Nossel I, Smith T, Dixon LB, 2017. OnTrackNY: the development of a coordinated specialty care program for individuals experiencing early psychosis. Psychiatr. Serv 68, 318–320. 10.1176/appi.ps.201600512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsack C, Manetti SG, Favrod J, Montagrin Y, Besson J, Bovet P, Conus P, 2011. Motivational intervention to reduce cannabis use in young people with psychosis: a randomized controlled trial. Psychother. Psychosom 80 (5), 287–297. 10.1159/000323466. [DOI] [PubMed] [Google Scholar]
- Breitborde NJ, Bell EK, Dawley D, Woolverton C, Ceaser A, Waters AC, ... Harrison-Monroe P, 2015. The Early Psychosis Intervention Center (EPICENTER): development and six-month outcomes of an American first-episode psychosis clinical service. BMC Psychiatry 15, 266. 10.1186/s12888-015-0650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr JA, Norman RM, Manchanda R, 2009. Substance misuse over the first 18 months of specialized intervention for first episode psychosis. Early Interv. in Psychiatry 3 (3), 221–225. 10.1111/j.1751-7893.2009.00136.x. [DOI] [PubMed] [Google Scholar]
- Cather C, Brunette MF, Mueser KT, Babbin SF, Rosenheck R, Correll CU, Kalos-Meyer P, 2018. Impact of comprehensive treatment for first episode psychosis on substance use outcomes: a randomized controlled trial. Psychiatry Res. 268, 303–311. 10.1016/j.psychres.2018.06.055. [DOI] [PubMed] [Google Scholar]
- Compton MT, Kelley ME, Ramsay CE, Pringle M, Goulding SM, Esterberg ML, ... Walker EF, 2009. Association of pre-onset cannabis, alcohol, and tobacco use with age at onset of prodrome and age at onset of psychosis in first-episode patients. American Journal of Psychiatry 166 (11), 1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Forti M, Sallis H, Allegri F, Trotta A, Ferraro L, Stilo SA, ... Dazzan P, 2013. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophrenia bull 40 (6), 1509–1517. 10.1093/schbul/sbt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon LB, Goldman HH, Bennett ME, Wang Y, McNamara KA, Mendon SJ, Essock SM, 2015. Implementing coordinated specialty care for early psychosis: the RAISE connection program. Psychiatr. Serv. 66, 691–698. 10.1176/appi.ps.201400281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue K, Doody GA, Murray RM, Jones PB, Morgan C, Dazzan P, et al. , 2014. Cannabis use, gender and age of onset of schizophrenia: data from the AESOP study. Psychiatry Res. 215 (3), 528–532. 10.1016/j.psychres.2013.12.038. [DOI] [PubMed] [Google Scholar]
- Edwards J, Elkins K, Hinton M, Harrigan SM, Donovan K, Athanasopoulos O, McGorry PD, 2006. Randomized controlled trial of a cannabis-focused intervention for young people with first-episode psychosis. Acta Psychiatr. Scand. 114 (2), 109–117. 10.1111/j.1600-0447.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- Faridi K, Joober R, Malla A, 2012. Medication adherence mediates the impact of sustained cannabis use on symptom levels in first-episode psychosis. Schizophrenia Res 141, 78–82. 10.1016/j.schres.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Harrison I, Joyce EM, Mutsatsa SH, Hutton SB, Huddy V, Kapasi M, Barnes TRE, 2008. Naturalistic follow-up of co-morbid substance use in schizophrenia: the West London first-episode study. Psychol. Med 38 (1), 79–88. 10.1017/S0033291707000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, ... Huang B, 2015. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA psychiatry 72 (12), 1235–1242. 10.1001/jamapsychiatry.2015.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Shmulewitz D, Sarvet AL, 2019. Time trends in US cannabis use and cannabis use disorders overall and by sociodemographic subgroups: a narrative review and new findings. The Am. J. of Drug and Alcohol Abus. 165, 181–190. 10.1080/00952990.2019.1569668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes RB, 1976. A critical review of the “determinants” of patient compliance with therapeutic regimens. In: Sackett DL, Haynes RB (Eds.), Compliance with Therapeutic Regimens. Johns Hopkins University Press, Baltimore, MD, pp. 26–39. [Google Scholar]
- Humensky JL, Nossel I, Bello I, Dixon LB, 2019. Supported education and employment services for young people with early psychosis in OnTrackNY. J.L of Men. Health Policy and Econ.S 22 (3), 95–108. [PMC free article] [PubMed] [Google Scholar]
- Kane JM, Robinson DG, Schooler NR, Mueser KT, Penn DL, Rosenheck RA, ... Marcy P, 2015. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am. J. of Psychiatry 173 (4), 362–372. 10.1176/appi.ajp.2015.15050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange EH, Nesvåg R, Ringen PA, Hartberg CB, Haukvik UK, Andreassen OA, ... Agartz I, 2014. One year follow-up of alcohol and illicit substance use in first-episode psychosis: does gender matter? Compr. psychiatry 55 (2), 274–282. 10.1016/j.comppsych.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Large M, Sharma S, Compton MT, Slade T, Nielssen O, 2011. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch. of gen.psychiatry 68 (6), 555–561. 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Harrison I, Ron MA, Barnes TR, Joyce EM, 2012. The effect of cannabis use and cognitive reserve on age at onset and psychosis outcomes in first-episode schizophrenia. Schizophrenia bull 38 (4), 873–880. 10.1093/schbul/sbq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan K, Brennan D, Lawlor E, Turner N, Kinsella A, O’Connor JJ, ... O’Callaghan E, 2013. A multi-center, randomized controlled trial of a group psychological intervention for psychosis with comorbid cannabis dependence over the early course of illness. Schizophrenia res 143 (1), 138–142. 10.1016/j.schres.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Malla A, McGorry P, 2019. Early intervention in psychosis in young people: a population and public health perspective. AJPH 109 (S3), S181–S184. 10.2105/AJPH.2019.305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi A, Di Forti M, Lewis CM, Murray RM, Vassos E, 2016. Meta-analysis of the association between the level of cannabis use and risk of psychosis. Schizophrenia bull 42 (5), 1262–1269. 10.1093/schbul/sbw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino LA, Dixon LB, 2014. An update on supported employment for people with severe mental illness. Curr. opin. in psychiatry 27 (3), 210–215. 10.1097/YCO10.1097/YCO.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoncini R, Donoghue K, Hart J, Morgan C, Doody GA, Dazzan P, ... Fearon P, 2010. Illicit substance use and its correlates in first episode psychosis. Acta Psychiatrica Scandinavica 121 (5), 351–358. 10.1111/j.1600-0447.2009.01483.x. [DOI] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G, 2007. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 370 (9584), 319–328. 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Myles H, Myles N, Large M, 2016. Cannabis use in first episode psychosis: meta-analysis of prevalence, and the time course of initiation and continued use. Aust. & N.Z. J. of Psychiatry 50 (3), 208–219. 10.1177/0004867415599846. [DOI] [PubMed] [Google Scholar]
- Niv N, Cohen AN, Sullivan G, Young AS, 2007. The MIRECC version of the global assessment of functioning scale: reliability and validity. Psychiatr. Serv 58, 529–535. 10.1176/ps.2007.58.4.529. [DOI] [PubMed] [Google Scholar]
- Nossel I, Wall MM, Scodes J, Marino L, Zilkha S, Bello I, ... Dixon LB, 2018. Outcomes and predictors in OnTrackNY, a coordinated specialty care program for early psychosis. Psychiatric Serv 69 (8), 863–870. [DOI] [PubMed] [Google Scholar]
- Oluwoye O, Monroe-DeVita M, Burduli E, Chwastiak L, McPherson S, McClellan JM, McDonell MG, 2019. Impact of tobacco, alcohol and cannabis use on treatment outcomes among patients experiencing first episode psychosis: data from the national RAISE-ETP study. Early Interv Psychiatry 13 (1), 142–146. 10.1111/eip.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Wilson R, Jackson R, Ball M, Shetty H, Broadbent M, ... Bhattacharyya S, 2016. Association of cannabis use with hospital admission and antipsychotic treatment failure in first episode psychosis: an observational study. BMJ open 6 (3), e009888. 10.1136/bmjopen-2015-009888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolin SA, Marino LA, Pope LG, Compton MG, Lee RJ, Rosenfeld B, Dixon LB, 2019. Recent violence and legal involvement among young adultswith early psychosis enrolled in coordinated specialty care. Early Interv Psychiatry 13, 832–840. 10.1111/eip.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmelmann BG, Conus P, Cotton S, Kupferschmid S, McGorry PD, Lambert M, 2012. Prevalence and impact of cannabis use disorders in adolescents with early onset first episode psychosis. Eur. Psychiatry 27 (6), 463–469. 10.1016/j.eupsy.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Schoeler T, Monk A, Sami MB, Klamerus E, Foglia E, Brown R, ... Bhattacharyya S, 2016a. Continued versus discontinued cannabis use in patients with psychosis: a systematic review and meta-analysis. The Lancet Psychiatry 3 (3), 215–225. 10.1016/S2215-0366(15)00363-6. [DOI] [PubMed] [Google Scholar]
- Schoeler T, Petros N, Di Forti M, Klamerus E, Foglia E, Anjnakina O, ... Bhattacharyya S, 2016b. Effects of continuation, frequency, and type of cannabis use on relapse in the first 2 years after onset of psychosis: an observational study. The Lancet Psychiatry 3, 947–953. 10.1016/S2215-0366(16)30188-2. [DOI] [PubMed] [Google Scholar]
- Schoeler T, Petros N, Di Forti M, Klamerus E, Foglia E, Murray R, Bhattacharyya S, 2017. Poor medication adherence and risk of relapse associated with continued cannabis use in patients with first-episode psychosis: a prospective analysis. Lancet Psychiatry 4 (8), 627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JL, Birchwood M, Copello A, Everard L, Jones PB, Fowler D, ... Singh SP, 2016. Cannabis use is associated with increased psychotic symptoms and poorer psychosocial functioning in first-episode psychosis: a report from the UK national EDEN study. Schizophrenia bull 42 (3), 619–625. 10.1093/schbul/sbv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setien-Suero E, Neergaard K, RamõÂrez-Bonilla M, Correa-Ghisays P, FañanaÂs L, Crespo-Facorro B, Ayesa-Arriola R, 2017. Cannabis use in male and female first episode of non-affective psychosis patients: long-term clinical, neuropsychological and functional differences. PLoS One 12 (8), e0183613. 10.1371/journal.pone.0183613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togay B, Noyan H, Tasdelen R, Ucok A, 2015. Clinical variables associated with suicide attempts in schizophrenia before and after the first episode. Psychiatric Res 229 (1–2), 252–256. 10.1016/j.psychres.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Van Mastrigt S, Addington J, Addington D, 2004. Substance misuse at presentation to an early psychosis program. Soc. psychiatry and psychiatr. epidemiol 39 (1), 69–72. 10.1007/s00127-004-0713-0. [DOI] [PubMed] [Google Scholar]
- Wade D, Harrigan S, Edwards J, Burgess PM, Whelan G, McGorry PD, 2005. Patterns and predictors of substance use disorders and daily tobacco use in first-episode psychosis. Aust. and N.Z. J. of Psychiatry 39 (10), 892–898. [DOI] [PubMed] [Google Scholar]
- Zammit S, Moore TH, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G, 2008. Effects of cannabis use on outcomes of psychotic disorders: systematic review. The Br. J. of Psychiatry 193 (5), 357–363. 10.1192/bjp.bp.107.046375. [DOI] [PubMed] [Google Scholar]


