Abstract
Non-invasive ischemic cancer therapy requires reduced blood flow whereas drug delivery and radiation therapy require increased tumor perfusion for a better response. In this study we investigate the hypothesis that different dose models of antivascular ultrasound therapy (AVUS) can have opposite effects on hepatocellular carcinoma (HCC) tumor blood flow. HCC was induced in 22 Wistar rats by ingestion of diethylnitrosamine (DEN) for 12 weeks. Rats received AVUS treatment at low and high doses. Low dose group received 1 watt/cm2 ultrasound for 1 min with 0.2 mL microbubbles injected IV. High dose group received 2 watts/cm2 for 2 min with 0.7 mL microbubbles IV. A sham group did not receive any treatment. Tumor perfusion was measured before and after AVUS with contrast-enhanced ultrasound. Quantitative perfusion measures: perfusion index (PI) and peak enhancement (PE) were obtained from each AVUS dose. After high-dose AVUS, PE and PI decreased by an average of 58.1 ± 4.9% and 49.1 ± 6.5 % respectively. Conversely, following low dose AVUS, PE and PI increased from baseline by an average of 47.8 ± 4.5% % and 20.3 ± 2.4 %, respectively. The high-dose AVUS therapy decreased tumoral perfusion, an effect that could be used for noninvasive ischemic therapy. Conversely, low-dose therapy increased tumor perfusion, which may improve drug delivery or radiation therapy. These opposite therapy effects can support multiple roles for AVUS in cancer therapy by tunable modulation of blood flow in tumors.
Keywords: therapeutic ultrasound, hepatocellular carcinoma, contrast enhanced ultrasound, quantitative imaging, cancer therapy
I. Introduction
Hepatocellular carcinoma (HCC) is one of the major causes of cancer-related mortality [1]. It is particularly refractory to the available chemotherapeutic drugs used in other cancers [2]. Other therapeutic options including surgical resection and liver transplantation also encounter significant hurdles [3-5]. First, they involve major surgical intervention and high recurrence rates. Second, these therapies are effective only in small group of patients particularly non cirrhotic. Consequently, less invasive liver-directed therapies, including percutaneous ablative therapies, trans arterial chemoembolization (TACE), and trans arterial radioembolization (TARE), have increased in HCC management [6-8].
In our earlier studies we introduced antivascular ultrasound (AVUS) therapy as a non-invasive locoregional cancer therapy option [9-11]. AVUS uses low intensity ultrasound in the presence ultrasound contrast agents (microbubbles) to disrupt the tumor vasculature. Ultrasonication causes the microbubbles to oscillate at their resonance frequency generating heat and shear stress in the vasculature around the bubbles, and causing intracellular and architectural changes in the tumor vasculature.
In this study we evaluate the effectiveness of varying doses of AVUS on HCC tumor blood flow and viability. We hypothesized that different doses of antivascular ultrasound therapy (AVUS) can have differing effects on hepatocellular carcinoma (HCC) tumor blood flow. These effects were investigated in a realistic tumor model involving an orthotopic, autochthonous, chemically-induced HCC in rats that mimics the natural development, vasculature, and location of human HCC lesions.
II. Methods
Animal Model
Animal protocol was approved by the Institutional Animal Care and Use Committee. HCC was induced in 22 Wistar rats (Charles River Laboratories, Wilmington, MA) via orally-ingested diethylnitrosamine (DEN) (Sigma Aldrich, St. Louis, MO, USA) in accordance with the well-documented protocol. After one week of acclimatization, DEN was diluted into animals’ drinking water to a concentration 0.01% weekly and ingested by rats ad libitum for 12 weeks. DEN was stopped and normal drinking water resumed at the beginning of the 13th week. Tumors and liver echogenicity were documented by B-mode ultrasound at 13 weeks. After 13 weeks, tumors in the liver were selected for antivascular ultrasound therapy by the following criteria: size (5-15 mm) and location (anterior easier to treat; convenient trajectory avoiding lungs, bowel, and other non-target viscera).
Antivascular Ultrasound Therapy
Rats received AVUS therapy at low and high doses between weeks 13-18 (days 88-120). Pre-treatment grayscale ultrasound was used to select a tumor for treatment and find a trajectory to the tumor with a good window for imaging and therapy and secured in a holster. The therapeutic probe (Dynatronics D150 Plus) was then replaced for the imaging probe in the determined location. The low dose group (n=7) was treated at 1 W/cm2 for 3 min with 0.3 mL microbubbles IV (Definity, N. Billerica, MA) administered as boluses via a tail vein catheter. The high dose group (n=12) was treated at 2 W/cm2 for 6 min with 0.7 mL microbubbles IV. Sham group (n=3) did not receive any treatment. In each treatment, sonications were separated into 3 intervals of 2 minutes each, separated by 1 minute. The total dose of uB was distributed between sonications to ensure microbubbles were present in the circulation during therapeutic sonication.
Ultrasound imaging studies
Tumor perfusion was assessed before and after therapy using VisualSonics VevoLAZR [Fujifilm, Toronto, Canada] ultrasound scanner. Nonlinear contrast-enhanced ultrasound (NLC) was used for imaging tumor blood flow. 0.05 mL microbubbles were used in each contrast-enhanced imaging. Additionally, the liver was scanned using grayscale ultrasound,
Quantitative Image Analysis
The tumor margin was manually outlined using VisualSonics VevoLab [Fujifilm, Toronto, Canada] and a clip of 1000 frames was analyzed to produce time-intensity curves. The time-intensity curves were fitted to a perfusion model from which peak enhancement (PE) and perfusion index (PI) were measured. Peak enhancement represents the maximum amount of contrast agent in the cross-section during its transit through the tumor. Perfusion index, defined as the area under curve (AUC) divided by mean transit time, represents the average amount of contrast flow per unit time.
Data analysis
The mean and standard error of each perfusion measure were calculated pre and post AVUS therapy. A paired two-sided tailed t-test was used to compare post therapy changes to baseline. Percentage changes in perfusion parameters were calculated to evaluate the treatment effects.
III. Results
Animal model monitoring
All animals developed liver disease and by 15-17 weeks following DEN treatment multiple foci of hepatocellular carcinoma (HCC), were seen on B-mode ultrasound imaging.
Dose dependent effects of AVUS therapy on tumor perfusion
Contrast-enhanced ultrasound showed immediate changes in tumor perfusion following AVUS therapy. Following high dose AVUS therapy, the mean peak enhancement decreased 58.1 ± 4.9% from a baseline of 8.7± 1.9 to 3.8 ±1.0 (p = 0.0005). The mean perfusion index decreased 49.1 ± 6.5% from 8.4 ±2.5 to 4.0 (p = 0.0064). In Figure 1, an example of HCC tumor that received high dose AVUS demonstrates that post AVUS therapy tumor perfusion decrease compared to pretreatment baseline. The dilution curve shows less contrast flow after high dose therapy in this tumor.
Figure 1:
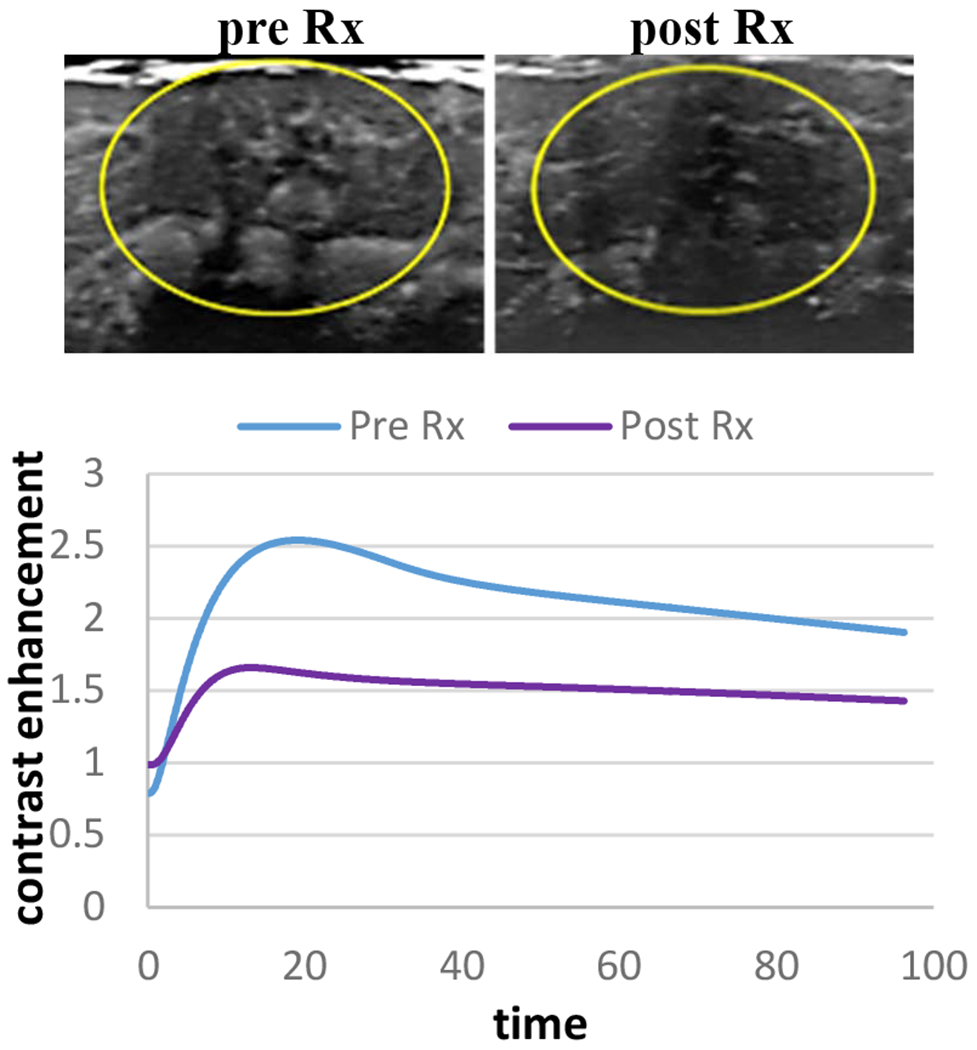
An example of HCC tumor that received high dose AVUS therapy. Maximum intensity projection (MIP) images derived from NLC shows a reduction in tumor perfusion after therapy. On the model fit dilution curve contrast flow decreased from baseline level after therapy.
Conversely, in low dose therapy cases the mean peak enhancement rose 47.8 ± 4.5% from 2.5 ± 0.7 to 4.0 ±1.2 and perfusion index rose 20.3 ± 2.4 from 3.1 ± 1. to 3.5 ± 1.2, (p=0.0304 and p=0.0391, respectively). Figure 2 shows an example of HCC tumor that received low dose AVUS where contrast flow increased after therapy compared to baseline.
Figure 2:
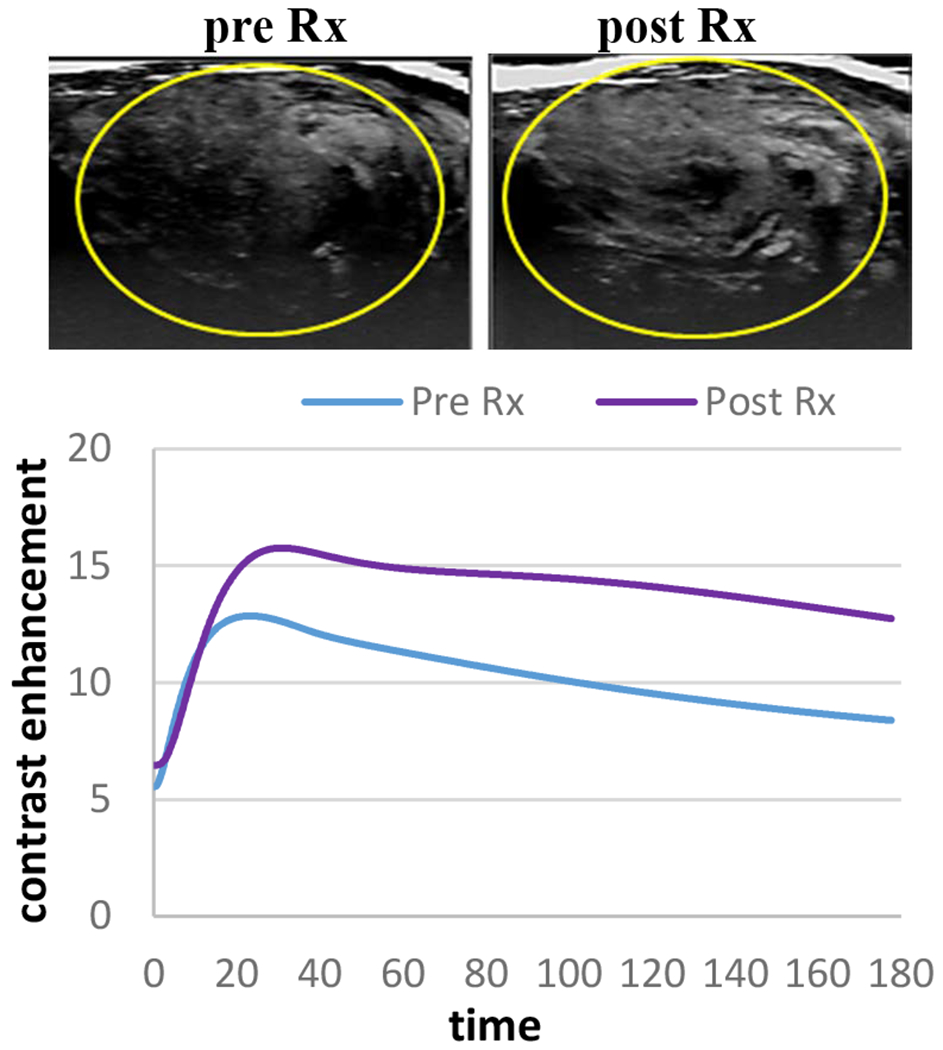
An example of HCC tumor that received low dose AVUS therapy. MIP illustrates an increase in tumor perfusion after therapy. On the contrast dilution curve flow rose following therapy compared to baseline level.
While in tumors that received sham therapy no or minimal change was observed. In figure 3 an example of HCC tumor that was used for sham therapy and only showed a minimal change in perfusion from the baseline.
Figure 3:
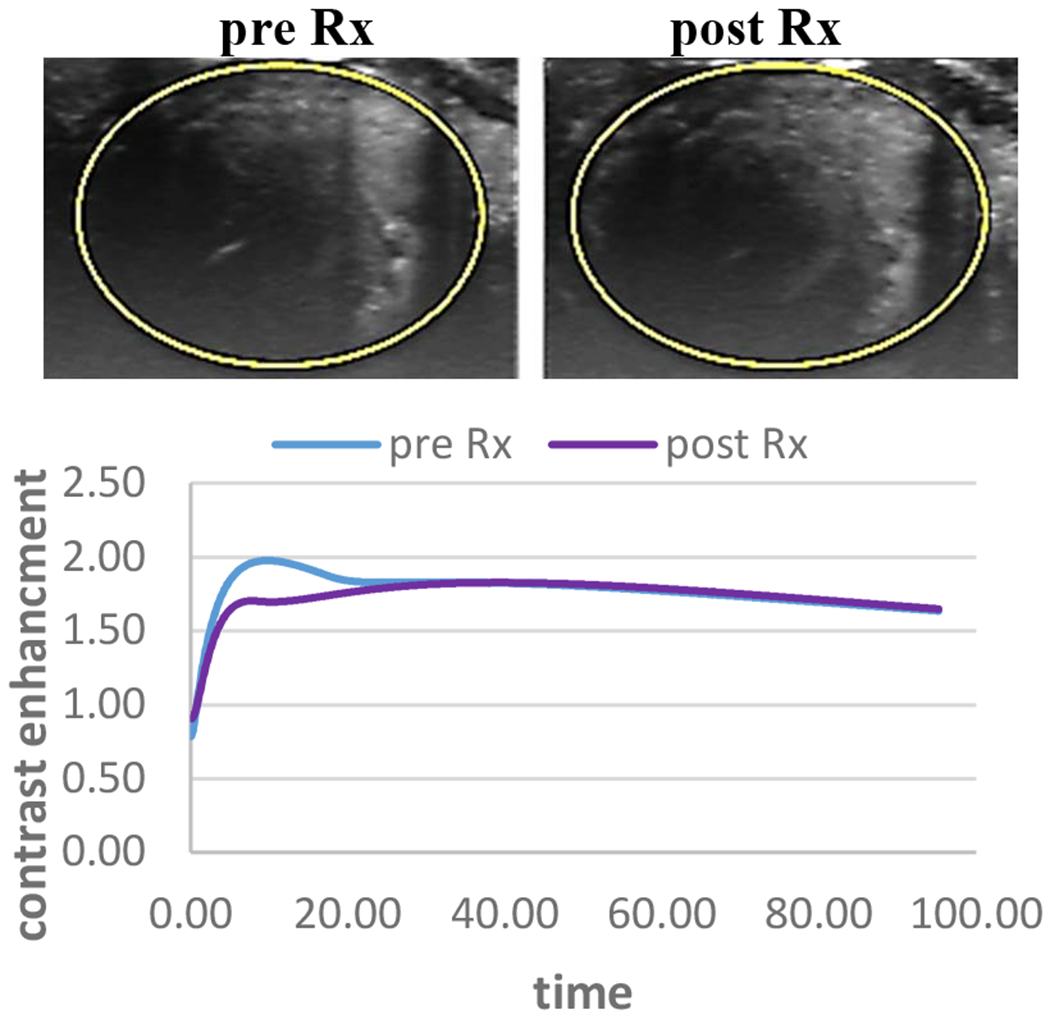
an example of HCC tumor that received sham therapy. MIP images derived from NLC shows that there is minimal change in tumor perfusion after therapy compared to baseline.
When the data were put together, an opposite trend of the percentage changes in tumor perfusion following low and high dose groups was observed (figure 4)
Figure 4:
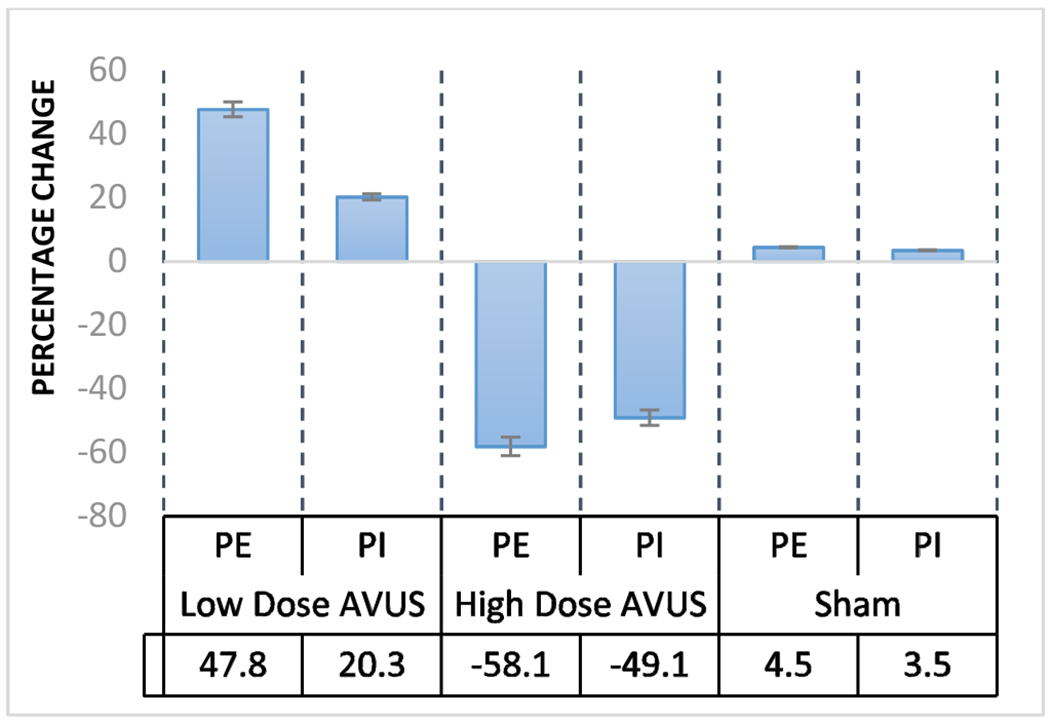
HCC tumor response to low, high and sham AVUS therapies. Opposite response are observed in low and high dose AVUS therapies.
IV. Discussion
The role of tumor perfusion in cancer therapies varies depending on the type of therapy. Certain therapies like drug delivery and radiation therapy require more perfusion to the treated tumor. For chemotherapy of solid tumors, the transport process of drug depends not only on the properties of the drug but also on physiological characteristics, including the structure and flow distribution in the system of microvessels supplying the tumor, and on the properties of extravascular tissue components, including the extracellular matrix (ECM) [12]. For radiation therapy oxygen is required to generate free radicals, that kill cancer cells [13]. When oxygen levels in a tumor are low, radiation therapy is less effective. In these two types of therapies an increase in tumor flow is needed to improve therapy outcomes. In contrast, lesser flow is desired for ischemic therapies. For this purpose, we investigated the hypothesis that antivascular ultrasound therapy can play multiple roles in cancer therapy through alteration of the therapy dose which ultimately lead to variable responses on tumor flow.
The study results showed that HCC tumors that received high dose of AVUS exhibited flow reduction, while tumors treated with lower doses demonstrated an increase in tumor perfusion compared to baseline. The findings related to high dose therapy support the efficacy of AVUS as a physical antivascular therapy for HCC. These results prove the ischemic effects of AVUS after translating to an autochthonous model versus subcutaneous tumors. The presence of circulating microbubbles damaged the HCC neovasculature leading to hemorrhage and necrosis.
The use of a lower dose of AVUS did not result in the proportional weaker disruptive effect. Instead, they showed an increase in flow for the group. This increase may be attributed increase in blood flow to dissipate thermal energy deposited by the low dose treatment. A similar effect was reported in earlier studies when low-intensity ultrasound used for physical therapy demonstrated an increase in muscle perfusion, in a study demonstrating an increase in blood flow after insonation with 1-W/cm2 US for 15 minutes [14]. Thus, with the reduced intensity, it is possible that hyperthermic effects involving increased flow dominates in this regimen compared to the higher intensity’s predominantly disruptive effects associated with stronger thermal and mechanical forces on the vascular endothelium.
The dose dependent effects of AVUS observed in this study could be a result of two factors: the concentration of microbubbles and ultrasound parameters. An increased microbubble concentration provides more sonosensitizers to generate local heat an affect the vasculature. Additionally, with higher concentrations, microbubbles are more tightly packed, placing more bubbles in closer proximity to the tumor’s endothelial cells. Previous studies have shown that the effects of microbubbles extend immediately around the microbubbles.
Therefore, better approximation of the bubbles to the endothelium could enhance the therapeutic effect. Secondly, the intensity of US used causes greater amplitudes of bubble oscillation leading to greater stable and transient cavitation generating more thermal and biochemical effects.
Limitation to this study is that the only two dosages were evaluated for a therapy involving multiple parameters including ultrasound power and frequency, time of administration, and microbubble concentration. A more controlled study investigating the role of each parameters influencing AVUS must be investigated to determine the transition from an increase in flow to disruption of vasculature.
In conclusion, differential effects have been observed at low and high doses of AVUS to treat autochthonous HCC. With high-dose therapy, a decrease in both peak enhancement and perfusion index were observed on CE-US, indicating vascular disruption, and leading to increased tumor necrosis on histology. This increase in flow with low-dose AVUS may allow AVUS to be potentially used in combination with drug delivery and radiation therapy. These therapies, may provide a non-invasive alternative to locoregional therapy for HCC and treatment of other tumors accessible by ultrasound.
Acknowledgment
We would like to thank Ms. Susan M Schultz, Ms. Brooke M Kirkham, Dr. Mustafa Mohammed for their help in data acquisition and analysis.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30J. [DOI] [PubMed] [Google Scholar]
- [2].Clerk Maxwell, De Lope CR A 4., Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. Journal of Hepatology. 2012:56 (SUPPL 1) [DOI] [PubMed] [Google Scholar]
- [3].Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: Outcomes and novel surgical approaches. In: Nature Reviews Gastroenterology and Hepatology. 2017 [DOI] [PubMed] [Google Scholar]
- [4].Bruix J, Sherman M. AASLD PRACTICE GUIDELINE Management of Hepatocellular Carcinoma: An Update 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].El-Serag HB, Marrero JA, Rudolph Rajender Reddy LK. Diagnosis and Treatment of Hepatocellular Carcinoma Diagnosis of Hepatocellular Carcinoma 2008;. [DOI] [PubMed] [Google Scholar]
- [6].Yu J, Yu X, Han Z. et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut. 2017;66:1172–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Forner A, Reig ME, Rodriguez De Lope C. et al. Current strategy for staging and treatment: The BCLC update and future prospects. In: Seminars in Liver Disease. 2010 [DOI] [PubMed] [Google Scholar]
- [8].Hunt SJ, Gade T, Soulen MC. et al. Antivascular ultrasound therapy: Magnetic resonance imaging validation and activation of the immune response in murine melanoma. In: Journal of Ultrasound in Medicine. 2015 [DOI] [PubMed] [Google Scholar]
- [9].Wood AKW, Ansaloni S, Ziemer LS. et al. The antivascular action of physiotherapy ultrasound on murine tumors. Ultrasound Med Biol. 2005;31:1403–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wood AKW, Sehgal CM. A review of low-intensity ultrasound for cancer therapy. In: Ultrasound in Medicine and Biology. 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].D’Souza JC, Sultan LR, Hunt SJ, Gade TP, Karmacharya MB, Schultz SM, Brice AK, Wood AKW, Sehgal CM. Microbubble-enhanced ultrasound for the antivascular treatment and monitoring of hepatocellular carcinoma. Nanotheranostics 2019;3(4):331–341. doi: 10.7150/ntno.39514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stylianopoulos T, Munn LL, Jain RK. Reengineering the Tumor Vasculature: Improving Drug Delivery and Efficacy. Trends Cancer. 2018;4(4):258–259. doi: 10.1016/j.trecan.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Eisenbrey JR, Shraim R, Liu JB, et al. Sensitization of Hypoxic Tumors to Radiation Therapy Using Ultrasound-Sensitive Oxygen Microbubbles. Int J Radiat Oncol Biol Phys. 2018;101(1):88–96. doi: 10.1016/j.ijrobp.2018.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Paul WD, Imig CJ. Temperature and blood flow studies after ultrasonic irradiation. Am J Phys Med. 1955 [PubMed] [Google Scholar]


