Abstract
Background:
Immune checkpoint inhibitors (ICIs) have changed the treatment paradigm of advanced-stage non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC). The aim of this study was to evaluate the effectiveness and tolerance of ICIs in a real-world patient population and to investigate the predictive factors associated with survival outcomes.
Methods:
Medical records of patients with advanced lung cancer who started ICI monotherapy were reviewed for data collection. Treatment outcomes included objective response rate, progression-free survival (PFS), and overall survival (OS). Immune-related adverse events (irAEs) were assessed. Multiple Cox regression models were fit to investigate the predictive factors for survival outcomes.
Results:
We included 220 patients (median 66.5 years). Seventy-nine (35.9%) patients had Eastern Cooperative Oncology Group (ECOG) performance-status (PS) score ⩾2. Median follow-up was 11.4 months. In NSCLC, median PFS was 3.8 months (4.7 months for first line and 3.7 months for subsequent line). Median OS was 12.4 months (15.6 months for first line therapy and 11.5 months for subsequent line). In SCLC, median PFS was 1.8 months, and median OS was 4.6 months. A quarter of patients developed irAEs. There was 1 disease flare among 17 patients with pre-existing autoimmune diseases. ECOG PS of 0 to 1 and body mass index (BMI) ⩾ 25 kg/m2 (but not occurrence of irAE) were independently associated with improved OS in NSCLC, with a hazard ratio of 0.41 (95% confidence interval [CI], 0.29-0.59) and 0.62 (95% CI, 0.44-0.87), respectively.
Conclusions:
The clinical benefit of ICIs appears to persist in a real-world population of relatively older age, including those with poor PS and pre-existing autoimmune diseases. ECOG PS of 0 to 1 and BMI ⩾ 25 kg/m2 were independently associated with improved OS.
Keywords: Non-small-cell lung cancer (NSCLC), small-cell lung cancer (SCLC), immunotherapy, anti-PD-1, anti-PD-L1
Introduction
Lung cancer is the leading cause of cancer-related deaths in both men and women, with a 5-year survival rate of around 19%.1 Historically, the treatment of advanced lung cancer has been limited to radiotherapy, chemotherapy, or a combination of both depending on the stage of the disease and performance status (PS) of the patient. The advent of immune checkpoint inhibitors (ICIs) has significantly changed how advanced lung cancer is managed. Immune checkpoint inhibitors are monoclonal antibodies directed against cytotoxic T-lymphocyte antigen 4, programmed death 1 (PD-1), or its ligand, programmed death ligand 1 (PD-L1). Blockage of these proteins removes inhibitory signals of T cell activation and produces T-cell-mediated anti-tumor responses. In a series of randomized phase 3 clinical trials (KEYNOTE-010, KEYNOTE-024, KEYNOTE-042, CheckMate 017, CheckMate 057, and OAK),2–7 these agents have consistently demonstrated superior outcomes compared with cytotoxic chemotherapy in many patients with advanced non-small-cell lung cancer (NSCLC). However, the participants in these studies were limited to those with good PS (Eastern Cooperative Oncology Group [ECOG] PS score 0 or 1) and those without autoimmune diseases. Also, the median age of patients in these clinical trials tended to be younger than that of patients in routine oncology practice (“real world”). There have been concerns regarding the efficacy of these agents in the elderly due to age-associated decline in the immune system termed “immunosenescence.”8 Although there is preliminary evidence suggesting little difference in efficacy of ICIs in older patients compared with younger patients, the impact on the elderly is largely unknown.9 To bridge this knowledge gap, we conducted a retrospective study of the effectiveness and safety of ICI monotherapy in 220 individuals with advanced lung cancer in a real-world setting, where patients tend to be older, have poorer PS, and have pre-existing autoimmune diseases in some instances. In addition, we investigated the predictive factors for survival outcomes of NSCLC with a multivariable Cox regression model.
Methods
Data collection
After approval from the University of Minnesota Institutional Review Board (Study Number: 1606M88925), we retrospectively collected pathological, radiological, and clinical data on consecutive lung cancer patients at the University of Minnesota/Fairview Health System, who were started on single-agent ICI (atezolizumab, nivolumab, or pembrolizumab) therapy between March 2015 and August 2018. Patients on clinical trials involving ICIs were excluded. Patients treated with combination ICIs (eg, ipilimumab + nivolumab) or ICI plus cytotoxic chemotherapy were not included in this study.
Outcome measures
Disease responses were evaluated using the Response Evaluation Criteria in Solid Tumors (version 1.1). Progression-free survival (PFS) was defined as time from the start of ICI treatment to radiologically confirmed progressive disease or death due to any cause. Overall survival (OS) was defined as time from the start of ICI treatment to death due to any cause.
Statistical analysis
Predictive models for OS and PFS were fit using multiple Cox regression. Predictive factors were chosen based on prior published results or hypothesized associations. Sex, ECOG PS score (0-1 vs ⩾2), and smoking status (never vs current/former) were modeled as binary variables. Body mass index (BMI) was modeled both as a binary variable (⩾ 25 kg/m2 vs <25 kg/m2) and as a continuous variable using a 4-knot restricted cubic spline. Splines are flexible parameterizations used to model smooth but non-linear associations between the predictor and outcome.10 A hazard ratio (HR) and confidence interval (CI) can be calculated at any 2 points within the variable’s range; for convenience, we chose round values corresponding to approximately the first and third quartiles of the variable’s distribution. Age was similarly modeled using a 3-knot restricted cubic spline. Immune-related adverse events (irAEs) could occur any time during the follow-up period and were therefore modeled as a time-varying binary variable. If an irAE occurs at time t, the patient belongs to the no irAE group before time t, then moves to the irAE group.11 Wald null hypothesis tests for each variable are reported. Kaplan-Meier curves were calculated for OS and PFS. Analysis was performed using R software, version 3.412,13 and IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp, Armonk, NY, USA).
Results
Patient and disease characteristics
A total of 220 patients with advanced lung cancer who received at least 1 dose of single-agent atezolizumab, nivolumab, or pembrolizumab were included in this analysis. Of those, 100 (45.5%) patients were male; most (93.2%) patients were white. Median age of patients was 66.5 years (range 36-92); 32.7% of the patients were 65 years and older, and 22.3% were 75 years and older. At the time of staring ICIs, 125 (56.8%) patients were overweight or obese (BMI ⩾ 25 kg/m2); 79 (35.9%) patients had an ECOG PS score ⩾2; and 26 (11.8%) patients were never smokers. Seventeen (7.7%) patients had pre-existing autoimmune disease. The most common histology was NSCLC (90.9%), with 24.5% squamous and 66.4% nonsquamous NSCLC; 95.5% were treated at stage IV disease and 4.5% at stage III disease; 25.0% had brain metastases at baseline. Median number of previous lines of systemic therapy was 1 (range 0-10). Atezolizumab, nivolumab, and pembrolizumab were given to 9 (4.1%), 152 (69.1%), and 59 (26.8%) patients, respectively (Table 1).
Table 1.
Patient demographic and disease characteristics.
| Characteristic | N = 220 |
|---|---|
| Age (y) | |
| Median | 66.5 |
| Range | 36-92 |
| Age category—n (%) | |
| <65 y | 99 (45.0) |
| ⩾65 to <75 y | 72 (32.7) |
| ⩾75 y | 49 (22.3) |
| Male sex—n (%) | 100 (45.5) |
| Race—n (%) | |
| White | 205 (93.2) |
| Other | 15 (6.8) |
| Body mass index | |
| <25 kg/m2 | 95 (43.2) |
| ⩾25 kg/m2 | 125 (56.8) |
| Disease stage—n (%) | |
| III | 10 (4.5) |
| IV | 210 (95.5) |
| ECOG performance-status score—n (%) † | |
| 0-1 | 141 (64.1) |
| ⩾2 | 79 (35.9) |
| Smoking status—n (%) | |
| Current or former smoker | 194 (88.2) |
| Never smoker | 26 (11.8) |
| Pre-existing autoimmune diseases—n (%) | 17 (7.7) |
| Histology—n (%) | |
| Squamous NSCLC | 54 (24.5) |
| Nonsquamous NSCLC | 146 (66.4) |
| SCLC | 20 (9.1) |
| Presence or history of brain metastases—n (%) | 55 (25.0) |
| Previous line of systemic therapy—n (%) | |
| 0 | 43 (19.5) |
| 1 | 102 (46.4) |
| ⩾2 | 75 (34.1) |
| ICI drug name—n (%) | |
| Atezolizumab | 9 (4.1) |
| Nivolumab | 152 (69.1) |
| Pembrolizumab | 59 (26.8) |
Abbreviations: ICI, immune checkpoint inhibitor; NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer.
Programmed death ligand 1 expression status was known in 94 (42.7%) patients: 48 (21.8%) with PD-L1 tumor proportion score (TPS) ⩾50% and 46 (20.9%) with PD-L1 TPS <50%. Other molecular characteristics including epidermal growth factor receptor (EGFR) mutation, anaplastic lymphoma kinase (ALK) rearrangement, proto-oncogene receptor tyrosine kinase ROS proto-oncogene 1 (ROS1) rearrangement, and Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation are summarized in Table 2.
Table 2.
Molecular characteristics.
| Characteristic | Entire cohort (N = 220) | NSCLC (n = 200) | SCLC (n = 20) |
|---|---|---|---|
| PD-L1 TPS—n (%) | |||
| ⩾50% | 48 (21.8) | 48 (24.0) | 0 |
| <50% | 46 (20.9) | 43 (21.5) | 3 (15.0) |
| Unknown | 126 (57.3) | 109 (54.5) | 17 (85.0) |
| EGFR mutation—n (%) | |||
| Positive | 15 (6.8) | 15 (7.5) | 0 |
| Negative | 145 (65.9) | 141 (70.5) | 4 (20.0) |
| Unknown | 60 (27.3) | 44 (22.0) | 16 (80.0) |
| ALK rearrangement—n (%) | |||
| Positive | 2 (0.9) | 2 (1.0) | 0 |
| Negative | 154 (70.0) | 150 (75.0) | 4 (20.0) |
| Unknown | 64 (29.1) | 48 (24.0) | 16 (80.0) |
| ROS1 rearrangement—n (%) | |||
| Positive | 2 (0.9) | 2 (1.0) | 0 |
| Negative | 148 (67.3) | 144 (72.0) | 4 (20.0) |
| Unknown | 70 (31.8) | 54 (27.0) | 16 (80.0) |
| KRAS mutation—n (%) | |||
| Positive | 33 (15.0) | 33 (16.5) | 0 |
| Negative | 58 (26.4) | 54 (27.0) | 4 (20.0) |
| Unknown | 129 (58.6) | 113 (56.5) | 16 (80.0) |
Abbreviations: ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; KRAS, Kirsten rat sarcoma viral oncogene homolog; NSCLC, non-small-cell lung cancer; PD-L1, programmed death ligand 1; ROS1, proto-oncogene receptor tyrosine kinase ROS proto-oncogene 1; SCLC, small-cell lung cancer; TPS, tumor proportion score.
Treatment outcomes
Median follow-up from initiating ICI therapy was 11.4 (interquartile range [IQR] 3.8-23.3) months with data cutoff in March 2020. Median number of cycles of therapy was 4 (IQR 2-11.5). In the NSCLC cohort, the objective response rate (ORR) was 32.0% (95% CI, 25.6%-39.0%). Ten (5.0%) patients had complete response, and 54 (27.0%) had partial response (Table 3). Median PFS was 3.8 months (4.7 months for first-line therapy and 3.7 months for subsequent-line therapy). The estimated rate of PFS was 38.8% at 6 months, 27.8% at 12 months, and 18.2% at 24 months (Figure 1). Median OS was 12.4 months (15.6 months for first-line therapy and 11.5 months for subsequent-line therapy). The estimated rate of OS was 33.6% at 24 months, 26.7% at 36 months, and 22.9% at 48 months.
Table 3.
Summary of response.
| Variable | NSCLC (n = 200) | SCLC (n = 20) |
|---|---|---|
| Objective response—n (% [95% CI]) | 64 (32.0 [25.6-39.0]) | 5 (25.0 [8.7-49.1]) |
| Complete response—n (% [95% CI]) | 10 (5.0 [2.4-9.0]) | 0 |
| Partial response—n (% [95% CI]) | 54 (27.0 [21.0-33.7]) | 5 (25.0 [8.7-49.1]) |
| Stable disease—n (% [95% CI]) | 40 (20.0 [14.7-26.2]) | 1 (5.0 [0.1-24.8]) |
| Progressive disease—n (% [95% CI]) | 95 (47.5 [40.4-54.7]) | 14 (70.0 [45.7-88.1]) |
| Could not be determined—n (% [95% CI]) | 1 (0.5 [0-2.8]) | 0 |
Abbreviations: CI, confidence interval; NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer.
Figure 1.
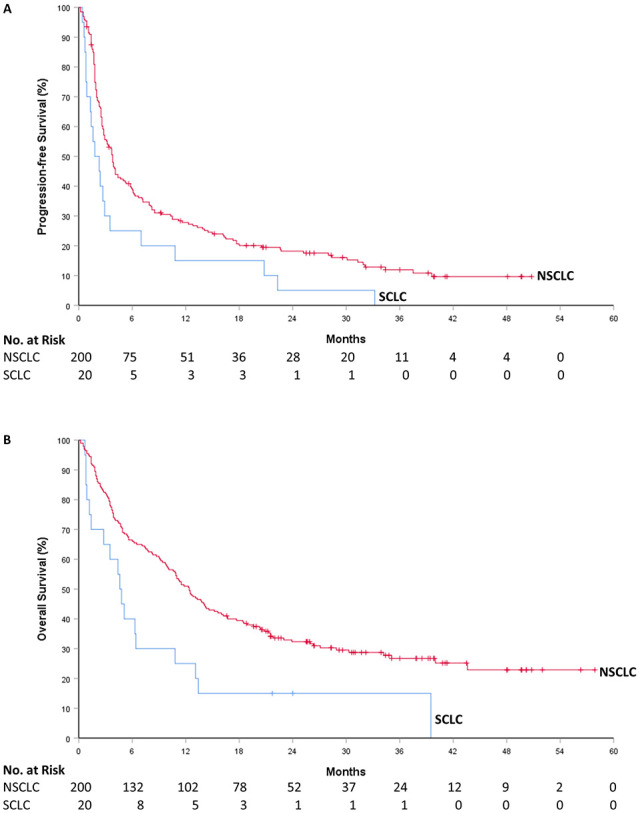
Progression-free survival and overall survival: (A) Kaplan-Meier estimates of progression-free survival and (B) overall survival. Tick marks indicate censoring of data. NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer.
Of the 20 patients with SCLC, the ORR was 25.0% (95% CI, 8.7%-49.1%). There were no complete responses. Median PFS was 1.8 months. The estimated rate of PFS was 30.0% at 3 months and 15.0% at 12 months. Median OS was 4.6 months. The estimated rate of OS was 65.0% at 3 months and 25.0% at 12 months.
Immune-related adverse events
In the entire cohort, 55 patients experienced irAEs (Table 4), with an incidence rate of 25.0% (95% CI, 19.4%-31.3%). The most common irAEs were hypothyroidism (6.4%) and colitis (5.5%). Median time to irAE onset was 3.3 (IQR 1.6-6.7) months. Thirty-three (15.7%) patients required systemic corticosteroids for irAEs. Discontinuation of treatment because of irAEs occurred in 7 (3.2%) patients. There was no death related to irAEs.
Table 4.
Immune-related adverse events.
| Adverse event | N = 220 |
|---|---|
| Any irAE—n (%) | 55 (25.0) |
| Endocrine disorder | 20 (9.1) |
| Hypothyroidism | 14 (6.4) |
| Hyperthyroidism | 2 (0.9) |
| Hypophysitis | 2 (0.9) |
| Thyroiditis | 1 (0.5) |
| Diabetes | 1 (0.5) |
| Respiratory disorder | 4 (1.8) |
| Pneumonitis | 4 (1.8) |
| Skin disorder | 9 (4.1) |
| Skin rash/dermatitis | 8 (3.6) |
| Psoriasis | 1 (0.5) |
| Gastrointestinal disorder | 19 (8.6) |
| Colitis | 12 (5.5) |
| Hepatitis | 3 (1.4) |
| Gastritis | 2 (0.9) |
| Pancreatitis/pancreatic insufficiency | 2 (0.9) |
| Musculoskeletal disorder | 4 (1.8) |
| Arthritis | 3 (1.4) |
| Myopathy | 1 (0.5) |
| Other | 12 (5.5) |
| Fever | 2 (0.9) |
| Immune-mediated cytopenias | 2 (0.9) |
| Nephritis | 2 (0.9) |
| Neuritis | 1 (0.5) |
| Mucositis | 1 (0.5) |
| Dry mouth | 1 (0.5) |
| Dry eye | 1 (0.5) |
| Encephalitis | 1 (0.5) |
| Hemolysis | 1 (0.5) |
Immune checkpoint inhibitors in patients with pre-existing autoimmune diseases
Among the 17 patients with pre-existing autoimmune diseases, 1 (5.9%) patient with rheumatoid arthritis had flare of disease while receiving ICI therapy. Four (23.5%) patients developed an irAE that was not attributed to the pre-existing autoimmune disease. The ORR in these 17 patients was 23.5% (95% CI, 6.8%-49.9%).
Therapy discontinuation
Ten patients (all with NSCLC) were taken off therapy after being treated with ICI monotherapy for 2 years or more (Supplementary Table 1). With a median follow-up of 3.6 (IQR 3.1-5.5) months from the last dose of ICI, no patient had evidence of disease progression.
Predictive factors associated with survival outcomes in NSCLC
Based on our multiple Cox regression model, ECOG PS 0-1 was independently associated with a longer PFS (HR = 0.54, 95% CI, 0.39-0.75, P < .01), whereas never smoking was independently associated with a shorter PFS (HR = 1.92, 95% CI, 1.18-3.12, P < .01) in the NSCLC cohort. There was a trend toward longer PFS in patients with BMI ⩾ 25 kg/m2 (HR = 0.76, 95% CI, 0.55-1.04, P = .08). We did not find strong evidence of association between PFS and age, sex, or the occurrence of an irAE (time-varying) (Table 5).
Table 5.
Cox proportional hazard regression analysis of the effect of various parameters on progression-free survival and overall survival.
| Variable | Progression-free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (75 vs 60)† | 0.87 | 0.68-1.11 | .29 | 0.85 | 0.65-1.11 | .41 |
| Sex (male vs female) | 1.21 | 0.89-1.65 | .23 | 1.22 | 0.88-1.71 | .24 |
| ECOG PS (0-1 vs ⩾2) | 0.54 | 0.39-0.75 | <.01 | 0.41 | 0.29-0.59 | <.01 |
| BMI (⩾25 vs <25) | 0.76 | 0.55-1.04 | .08 | 0.62 | 0.44-0.87 | <.01 |
| Smoking (never vs current/former) | 1.92 | 1.18-3.12 | <.01 | 1.35 | 0.79-2.31 | .28 |
| irAE (time-varying) | 1.20 | 0.81-1.78 | .36 | 0.69 | 0.45-1.07 | .10 |
Abbreviations: BMI, body mass index; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; irAE, immune-related adverse event; PS, performance status.
In the NSCLC cohort, ECOG PS 0 to 1 and BMI ⩾ 25 kg/m2 were independently associated with a longer OS, with a HR of 0.41 (95% CI, 0.29-0.59, P < .01) and 0.62 (95% CI, 0.44-0.87, P < .01), respectively. We did not find strong evidence of association between OS and age, sex, smoking status (never vs current/former), or the occurrence of an irAE (time-varying). BMI was also fit as a continuous variable in a separate model (Supplementary Table 2). We found that expected OS was longest for BMI values between 27 and 32 kg/m2, and gradually diminished for BMI values both above and below that range (Supplementary Figure 1). For example, the estimated HR for a BMI of 30 kg/m2 relative to 20 kg/m2 was 0.44 (95% CI, 0.28-0.71, P < .01).
Because of the high amount of missing data (not done or not accessible) for the molecular markers, separate exploratory models were fit to assess the association of PD-L1 expression, EGFR mutation, and KRAS mutation with PFS and OS. Eastern Cooperative Oncology Group PS was included in the exploratory models because it was the strongest predictor in the full model. Programmed death ligand 1 TPS ⩾ 50% did not have a significant association with prolonged PFS or OS as compared with PD-L1 TPS < 50%. Epidermal growth factor receptor mutation was associated with shorter PFS (HR = 2.41, 95% CI, 1.35-4.30, P < .01), but not OS (HR = 0.85, 95% CI, 0.42-1.72, P = .65). There was no significant association between KRAS mutation and PFS (HR = 1.21, 95% CI, 0.77-1.90, P = .41) or OS (HR = 1.33, 95% CI, 0.82-2.17, P = .25) (Supplementary Tables 3–5).
Cox regression analysis was not performed for SCLC given the small number of patients.
Discussion
To the best of our knowledge, this is one of the largest studies with access to individual patient data to assess the real-world outcomes of single-agent ICI therapy in advanced lung cancer, particularly NSCLC. Youn et al14 recently published real-world survival outcomes of ICI therapy in 1256 older adults (⩾65 years) with NSCLC based on the Surveillance, Epidemiology, and End Results (SEER)–Medicare linked database. The advantage of our study is that we have access to each patient’s electronic medical record (EMR), which allows us to have more comprehensive and accurate data collection. With each individual’s EMR available, we were able to evaluate the ORR, PFS, molecular markers, and other characteristics, which were not examined in Youn’s study given the limitations of SEER-Medicare linked database.
The median age (66.5 years) of our patient population was noted to be older than the landmark phase 3 trials, which ranged from 61 years in the CheckMate 057 trial6 to 64.5 years in the OAK trial.7 Around 36% of our patients had an ECOG PS ⩾ 2, whereas those patients with poor PS were excluded from all major landmark trials. While caution should be exercised when comparing the results of different studies, the median OS (15.6 months) in our NSCLC cohort was comparable with that reported in the KEYNOTE-042 trial (16.7 months)4 in the first-line setting. In the non-first-line setting, the median OS (11.5 months) in our NSCLC population was also comparable with that reported in some of the landmark trials (from 9.2 to 13.8 months depending on the studies).2,5–7 It should be kept in mind that the treatment option in our cohort was more heterogeneous with 3 different ICI monotherapies (atezolizumab, nivolumab, and pembrolizumab). Given the high amount of missing data for PD-L1 expression in our population, median OS was not calculated based on PD-L1 TPS.
A quarter of patients in our study experienced at least 1 irAE, with endocrine and gastrointestinal disorders being the most common. The incidence (23.5%) of irAEs in patients with pre-existing autoimmune diseases was similar to that in our entire cohort. There was only 1 disease flare (rheumatoid arthritis) in the 17 patients with underlying autoimmune diseases. These findings suggest the relative safety of ICIs in this unique patient population, which was excluded from the phase 3 landmark trials. Further studies with large numbers of patients in prospective randomized controlled trials (RCTs) are needed to evaluate the safety of ICIs in patients with pre-existing autoimmune diseases.
Little is known regarding when to stop ICIs in patients who have had prolonged responses. Ten patients with NSCLC discontinued ICI therapy after at least 2 years of treatment; none of them had disease progression during the relatively short median follow-up of 3.6 months after the last dose of ICI. Gettinger et al reported long-term outcomes of the phase 1 nivolumab trial in previously treated advanced NSCLC. Nivolumab was discontinued in 18 responders for reasons other than progressive disease (7 completed the maximum 96 weeks of therapy according to the study protocol); half of the patients had responses for more than 9 (range 9.2-16.4) months after the last dose of nivolumab.15 CheckMate 153 prospectively evaluated the safety/efficacy with continuous versus 1-year fixed duration nivolumab in advanced NSCLC as an exploratory endpoint. Continuous nivolumab increased PFS (HR = 0.42, 95% CI, 0.25-0.71) and led to a trend toward prolonged OS (HR = 0.63, 95% CI, 0.33-1.20), as compared with stopping nivolumab at 1 year.16 In our practice, we discuss the pros and cons of discontinuing ICI with patients at the 2-year mark and make a shared decision. Further studies are warranted to determine the optimal duration of ICI therapy in advanced NSCLC.
Using multiple Cox regression models, we attempted to identify predictive factors for better survival outcomes in advanced NSCLC. Our finding that an ECOG PS of 0 or 1 was independently associated with significant improvement in PFS and OS compared with ECOG PS of 2 or greater is consistent with outcomes reported in prior studies.17,18 Individuals with ECOG PS ⩾ 2 constituted 36% of the entire cohort; however, this population is usually excluded or underrepresented in clinical trials. Caution should be exercised when treating this frail population with ICIs. In our study, never smoking was independently associated with significantly shorter PFS, but not OS in NSCLC patients treated with ICI monotherapy. Smoking has been shown to strongly correlate with tumor mutational burden, thus potentially increasing ICI efficacy in NSCLC.19 However, results from phase 3 RCTs have been inconsistent, and it requires further study.20 Overweight and obesity (BMI ⩾ 25 kg/m2) were found to be independently associated with improved OS and a trend toward improved PFS in our NSCLC cohort, which is consistent with a large study by Kichenadasse and colleagues.21 It is postulated that obesity increases immune aging and leads to PD-1-mediated T cell dysfunction through leptin.22 Interestingly, when BMI was fit in a separate Cox regression model as a continuous variable, the expected OS was longest in the BMI range of 27 to 32 kg/m2 and gradually diminished for BMI values both below and above that range. In a small study of 70 patients with metastatic NSCLC who received nivolumab monotherapy, significant increase in ORR and PFS was observed in the 28 patients who developed irAEs.23 However, the result of this study was confounded because the longer the PFS was, the more time the patient had to develop irAEs. Haratani et al24 recognized the time-dependent nature of irAEs and did a landmark study to examine the association of irAEs with survival outcomes in 134 patients with advanced NSCLC who were treated with nivolumab. Immune-related adverse events were observed in 69 patients and associated with significant increase in ORR, PFS, and OS in a 6-week landmark analysis. In contrast, we fit our multivariable Cox regression model using irAEs as a time-varying binary variable, similar to a recent analysis in melanoma.25 We found no significant associations between irAEs and PFS or OS in advanced NSCLC. The different methodologies in Cox regression analysis could be one of the causes of the contradicting results as compared with Haratani’s study.
The published data on ICIs in real-world lung cancer patients are limited to (1) small studies of less than 100 patients, focusing on particular subgroups such as patients with autoimmune disease, individuals with advanced age, or those experiencing irAEs or (2) large studies based on certain database (eg, SEER-Medicare linked database) that did not have access to detailed individual patient records. Our study is one of the largest studies to date with more comprehensive data collection. Our findings will help clinicians assess the safety and effectiveness of ICIs in advanced lung cancer patients in routine oncology practice, particularly in those who would be excluded from completed RCTs. This study has a few limitations. First, this is a retrospective study at a single institution, providing a lower level of evidence compared with RCTs. Second, with the vast majority of our patients being white, other races were underrepresented. Third, the ICI monotherapy is heterogeneous as we included patients treated with single-agent atezolizumab, nivolumab, or pembrolizumab, and these 3 medications were not evenly distributed in the cohort with only 4.1% receiving atezolizumab. Fourth, the large amount of missing data on molecular markers makes it impossible to perform multivariable Cox regression analysis using these markers as preplanned variables. Caution should be exercised when interpreting the results from this study.
Conclusions
In conclusion, our study supports data from the major landmark trials of ICI monotherapy in advanced lung cancer (particularly NSCLC) with comparable outcomes, although we included patients with poor PS and pre-existing autoimmune diseases in the real-world setting. Eastern Cooperative Oncology Group PS of 0 to 1 and BMI ⩾ 25 kg/m2 were independently associated with improved OS in NSCLC patients treated with ICI monotherapy.
Supplemental Material
Supplemental material, sj-pdf-1-onc-10.1177_11795549211004489 for Real-World Outcomes and Clinical Predictors of Immune Checkpoint Inhibitor Monotherapy in Advanced Lung Cancer by Shijia Zhang, Daniel F Pease, Amit A Kulkarni, Manoj Kumar, Ryan M Shanley, Beibei Xu, Shilvi P Joshi and Manish R Patel in Clinical Medicine Insights: Oncology
Acknowledgments
Research reported in this publication was also supported by NIH Grant P30 CA77598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota, and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Michael Franklin for reviewing the manuscript.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded, in part, by Minnesota Masonic Charities.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Patel reports having served on Nektar Therapeutics Advisory board and receiving research funding from Merck, Vyriad, and FATE therapeutics, outside the submitted work. No other disclosures were reported.
Author Contributions: SZ, DFP, AAK, and MRP contributed to the conception and design; acquisition of data was performed by SZ, DFP, AAK, MK, and SPJ; interpretation of data was made by SZ, SPJ, DFP, AAK, MK, RMS, BX, and MRP; and manuscript writing and editing was done by SZ, AAK, BX, and MRP.
Ethical Approval: This study was approved by the Institutional Review Board of the University of Minnesota. Study # 1606M88925.
ORCID iD: Manish R Patel  https://orcid.org/0000-0003-2752-945X
https://orcid.org/0000-0003-2752-945X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA. 2019;69:7-34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540-1550. doi: 10.1016/S0140-6736(15)01281. [DOI] [PubMed] [Google Scholar]
- 3. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823-1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 4. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819-1830. doi: 10.1016/S0140-6736(18)32409. [DOI] [PubMed] [Google Scholar]
- 5. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123-135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. The Lancet. 2017;389:255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elias R, Karantanos T, Sira E, et al. Immunotherapy comes of age: immune aging & checkpoint inhibitors. J Geriatric Oncol. 2017;8:229-235. [DOI] [PubMed] [Google Scholar]
- 9. Elias R, Hartshorn K, Rahma O, Lin N, Snyder-Cappione JE. Aging, immune senescence, and immunotherapy: a comprehensive review. Semin Oncol. 2018;45:187-200. [DOI] [PubMed] [Google Scholar]
- 10. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed. New York: Springer International Publishing; 2015. [Google Scholar]
- 11. Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. 2nd ed. New York: Springer-Verlag; 2003. [Google Scholar]
- 12. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. https://www.R-project.org. [Google Scholar]
- 13. Harrell FE. RMS: Regression Modeling Strategies. R Package Version 5.1-3.1; 2019. https://CRAN.R-project.org/package=rms.
- 14. Youn B, Trikalinos NA, Mor V, et al. Real-world use and survival outcomes of immune checkpoint inhibitors in older adults with non-small cell lung cancer. Cancer. 2020;126:978-985. doi: 10.1002/cncr.32624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004-2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spigel DR, McLeod M, Hussein MA, et al. Randomized results of fixed-duration (1-yr) vs continuous nivolumab in patients (pts) with advanced non-small cell lung cancer (NSCLC). Ann Oncol. 2017;28:v460-v496. [Google Scholar]
- 17. Muchnik E, Loh KP, Strawderman M, et al. Immune checkpoint inhibitors in real-world treatment of older adults with non-small cell lung cancer. J Am Geriatr Soc. 2019;67:905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spigel DR, McCleod M, Jotte RM, et al. Safety, efficacy, and patient-reported health-related quality of life and symptom burden with nivolumab in patients with advanced non-small cell lung cancer, including patients aged 70 years or older or with poor performance status (CheckMate 153). J Thorac Oncol. 2019;14:1628-1639. [DOI] [PubMed] [Google Scholar]
- 19. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124-128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pirker R. Is smoking history the truly best biomarker for immune checkpoint inhibitor treatment in advanced non-small cell lung cancer. ESMO Open. 2018;3:e000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kichenadasse G, Miners JO, Mangoni AA, et al. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. 2020;6:512-518. doi: 10.1001/jamaoncol.2019.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25:141-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toi Y, Sugawara S, Kawashima Y, et al. Association of immune-related adverse events with clinical benefit in patients with advanced non-small-cell lung cancer treated with nivolumab. Oncologist. 2018;23:1358-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4:374-378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020:6:519-527. doi: 10.1001/jamaoncol.2019.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-onc-10.1177_11795549211004489 for Real-World Outcomes and Clinical Predictors of Immune Checkpoint Inhibitor Monotherapy in Advanced Lung Cancer by Shijia Zhang, Daniel F Pease, Amit A Kulkarni, Manoj Kumar, Ryan M Shanley, Beibei Xu, Shilvi P Joshi and Manish R Patel in Clinical Medicine Insights: Oncology


