Abstract
Background
Successful human embryo implantation requires the differentiation of endometrial stromal cells (ESCs) into decidual cells during a process called decidualization. ESCs express specific markers of decidualization, including prolactin, insulin-like growth factor-binding protein-1 (IGFBP-1), and connexin-43. Decidual cells also control of trophoblast invasion by secreting various factors, such as matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases. Preimplantation factor (PIF) is a recently identified, embryo-derived peptide with activities at the fetal-maternal interface. It creates a favorable pro-inflammatory environment in human endometrium and directly controls placental development by increasing the human trophoblastic cells’ ability to invade the endometrium. We hypothesized that PIF’s effects on the endometrium counteract its pro-invasive effects.
Methods
We tested sPIF effect on the expression of three decidualization markers by RT-qPCR and/or immunochemiluminescence assay. We examined sPIF effect on human ESC migration by performing an in vitro wound healing assay. We analyzed sPIF effect on endometrial control of human trophoblast invasion by performing a zymography and an invasion assay.
Results
Firstly, we found that a synthetic analog of PIF (sPIF) significantly upregulates the mRNA expression of IGFBP-1 and connexin-43, and prolactin secretion in ESCs - suggesting a pro-differentiation effect. Secondly, we showed that the HTR-8/SVneo trophoblastic cell line’s invasive ability was low in the presence of conditioned media from ESCs cultured with sPIF. Thirdly, this PIF’s anti-invasive action was associated with a specifically decrease in MMP-9 activity.
Conclusion
Taken as a whole, our results suggest that PIF accentuates the decidualization process and the production of endometrial factors that limit trophoblast invasion. By controlling both trophoblast and endometrial cells, PIF therefore appears to be a pivotal player in the human embryo implantation process.
Keywords: Preimplantation factor (PIF), Human endometrium, Decidualization, Embryo implantation, Trophoblastic invasion, Signal transduction
Background
Human embryo implantation is a multistep process that begins with the apposition of trophoblastic cells from a competent blastocyst against the maternal endometrium. This apposition occurs during the “implantation window” - a short period of uterine receptivity corresponding to the mid-secretory phase of the menstrual cycle [1]. After the blastocyst has attached to the endometrial epithelium, its extravillous trophoblasts (EVTs) penetrate into the endometrial stroma and acquire an invasive phenotype - resulting in the placenta anchoring in the endometrium [2].
During trophoblast invasion, EVTs secrete large amounts of matrix metalloproteinases (MMPs) [3]. The actions of MMP-2 and MMP-9 are crucial for trophoblast invasion [4]. Indeed, these gelatinases are able to degrade the major component of the endometrial extracellular matrix (ECM), i.e. collagen IV. The level of MMP activity is regulated by specific tissue inhibitors of metalloproteinases (TIMPs), which bind to and inactivate MMPs with a 1:1 stoichiometry [5]. Thus, correct regulation of the MMP/TIMP balance in EVTs is essential for successful embryo implantation [6]. Trophoblast invasion also requires adequate endometrial receptivity [7]. This is characterized by morphological, biochemical, and vascular changes, including (i) the appearance of epithelial pinopods, (ii) the modulated expression of cytokines, growth factors, and adhesion molecules (particularly the upregulation of αv- and β3-integrins and the disappearance of mucin-1 and mucin-12 in endometrial epithelial cells), and (iii) the decidualization of endometrial stromal cells (ESCs). Decidualization occurs in response to the ovarian hormones 17β-estradiol (E2) and progesterone (P4). It is accompanied by the secretion of prolactin and insulin-like growth factor-binding protein-1 (IGFBP-1), and the expression of the gap junction connexin-43 (CX-43) in human ESCs [8, 9]. Decidual ECM remodeling events have also been observed. These processes are associated with MMP-2,-9 secretion and the modulation of TIMP-1,-2 gene expression in human ESCs [10–12]. This type of ECM remodeling enhances ESC motility and thus facilitates trophoblastic infiltration into the endometrial stroma [13]. Decidual ECM remodeling is a complex process that is tightly controlled by intrinsic factors produced by both placental and decidual cells [14]. For example, human chorionic gonadotropin, tumor necrosis factor alpha (TNF-α), and adiponectin facilitate EVT migration by enhancing endometrial MMP-2,-9 activities and by inhibiting endometrial TIMP-1 secretion [12, 15, 16]. Conversely, the antifungal antibiotic trichostatin A limits trophoblast invasion by increasing the production of TIMP-1,-3 by ESCs and reducing the activity of endometrial MMP-2,-9 [11]. Hence, the local MMP/TIMP balance at the invasion site requires an appropriate interaction between trophoblasts and ESCs. Overall, the mechanisms underlying decidualization and decidual ECM remodeling are complex and have not yet been clearly elucidated.
Here, we focused on preimplantation factor (PIF) and its role in a successful, viable pregnancy. This factor is a small peptide secreted only by viable embryos and is present in the maternal circulation until term [17]. PIF is also expressed by the placenta [18]. Mondjie et al. have recently reported that PIF expression in trophoblastic cells is prominent in the earliest stages of pregnancy and then declines at term [19]. PIF’s major effects are essential for the initiation and maintenance of pregnancy by (i) promoting the development of cultured embryos, (ii) acting as a rescue factor against toxic-serum-induced embryo demise [20], (iii) regulating systemic immunity to promote embryo tolerance while preserving the mother’s ability to fight pathogens/disease and negating natural killer cell-induced toxicity [21], (iv) decreasing trophoblastic apoptosis through the p53 signaling pathway [22] and, (v) enhancing trophoblastic invasion [19]. Concerning this last point, it has been described that the pro-invasive effects of PIF in extravillous trophoblasts seemed to be mediated through different signaling pathways such as the mitogen-activated protein kinase (MAPK), phosphoinositide-3-kinase (PI3K), Janus-kinase signal transducer and transcription (JAK-STAT) signaling pathways [19]. Furthermore, two studies have shown that PIF can promote endometrial receptivity by regulating genes whose products are involved in inflammation, adhesion, and apoptosis, and by promoting the secretion of immune regulatory ligands and the phosphorylation of certain kinases as MAPK that actively condition the uterine environment [23, 24]. Taken as a whole, these data strongly suggest that PIF has a crucial role in embryo implantation. However, the molecular mechanisms underlying PIF’s effects on decidualization of human ESCs have not yet been fully characterized.
In this context, the primary objective of the current study was to specify PIF’s effect on human endometrium during the implantation window, in order to better understand the embryo–maternal dialog established during the early stages of pregnancy. To this end, we studied the in vitro effects of a synthetic analog of PIF (sPIF) at 50 nM on the human ESC decidualization program and the endometrial control of trophoblast invasion. This concentration was chosen since PIF is present at 50–150 ng/ml (30–100 nM) concentrations in the circulation of pregnant women [25] and sPIF is effective at modulating several decidual cell functions in the same concentration range [26–28].
Materials and methods
Materials
Dulbecco’s Modified Eagle’s Medium and Ham F-12 Nutrient Mix (DMEM/F12), Roswell Park Memorial Institute (RPMI) medium, progesterone, E2, penicillin, streptomycin, DNase type I, and bovine serum albumin were purchased from Sigma Chemical Co (Saint Louis, MO, USA). Fetal calf serum (FCS) was purchased from Invitrogen (Carlsbad, CA, USA). The sPIF (purity: 95%, as documented by HPLC and mass spectrometry) was produced by Biosynthesis (Lewisville, TX, USA). Superscript III RNase H-RT and primers were from Invitrogen, and RNase inhibitor was obtained from AMRESCO (Solon, OH, USA). The Nucleospin RNA II kit was obtained from Machery-Nagel (Düren, Germany). Trypsin was provided by Difco Laboratories (Detroit, MI, USA). Matrigel® was obtained from BD Biosciences (Le Pont-de-Claix, France) and collagenase A was from Boehringer (Mannheim, Germany). The selective, irreversible PI3K inhibitor wortmannin was purchased from Sigma Chemical Co. The suppliers of the various antibodies used here are described in the corresponding paragraphs below.
Study population and tissue collection
A total of 34 normally cycling women (mean ± standard deviation age: 33.6 ± 0.5) undergoing biopsies for fertility evaluation were included in the study. Endometrial tissue was obtained by aspiration with a Pipelle (Pipelle de Cornier®, France) during the implantation window (day 20–25 of a spontaneous ovarian cycle). Women with anatomical abnormalities such as the presence of submucous fibroma or polyps, or a low endometrial thickness (< 7 mm during the luteal phase) were excluded. The inclusion criteria also included a good hormonal reserve and normal responses to ovarian stimulation. Indeed, all our study population has hormonal assays of FSH ≤ 8 mUI/ml, E2 ≤ 40 pg/ml, AMH ≥ 1.5 ng/ml, and an antral follicular count ≥12 on day 3 of the follicular phase. The study was approved by the local investigational review board (Comité Consultatif de Protection des Personnes dans la Recherche Médicale, approval reference protocol 01–78), Paris, France; reference: 01–78). All participants provided their written, informed consent before tissue sampling.
Human ESC culture
Human ESCs were isolated and cultured as described by González and coworkers [29]. Briefly, tissue samples were minced into small pieces and digested in a two-step process. Tissues were incubated for 1 h at 37 °C in a phenol-red-free DMEM/F12 medium containing collagenase (0.1%), DNase type I (0.005%), penicillin (10 μg/mL), and streptomycin (100 U/mL). The supernatant was filtered through a 100 μm nylon screen and then centrifuged at 200 g for 10 min. A second enzymatic digestion was performed on undigested tissue for 10 min at 37 °C in DMEM/F12 medium containing trypsin (0.25%), DNase type I (0.1%), EDTA (0.03%), penicillin (10 μg/mL), and streptomycin (100 U/mL). The digested tissue was filtered through a 40 μm nylon screen and then centrifuged at 200 g for 10 min. Cell pellets from both digestions were pooled and centrifuged at 200 g for 10 min.
Cells were cultured in DMEM/F12 medium supplemented with streptomycin (10 μg/mL), penicillin (100 U/mL), and FCS (10%) at 37 °C in a 5% CO2 and 95% air atmosphere. When the cells reached confluence, they were cultured in the presence or absence of sPIF (50 nM or 100 nM) in differentiation medium containing DMEM/F12 with E2 (10− 8 M), P4 (10− 6 M), charcoal-stripped FCS (2%), penicillin (10 μg/mL), and streptomycin (100 U/mL) for 2 weeks. The medium was changed every 2 days, as described previously [16].
Invasion assay for HTR-8/SVneo cells co-cultured with ESC-conditioned culture medium
The invasiveness of trophoblastic cells in response to ESC-secreted molecules was assessed using the HTR-8/SVneo immortalized EVT cell line (kindly provided by Dr. Nadia Alfaidy (CEA Grenoble, France), in agreement with Dr. Charles H. Graham, Queen’s University, Ontario, Canada). HTR-8/SVneo cells were cultured in RPMI medium supplemented with HEPES 1 M (2%), penicillin (100 U/mL), streptomycin (100 μg/mL), and FCS (10%) until they reached confluence. Invasion assays were performed in 24-well plates containing Matrigel®-coated polycarbonate membrane (pore size: 8 μm) invasion chamber inserts (Greiner Bio-One SAS, Courtaboeuf, France), according to the modified protocol described by Tapia-Pizarro and coworkers. Next, the HTR-8/SVneo cells were suspended (5 × 104 cells per well) in 250 μL of conditioned medium (CM) from 15-day-decidualized ESCs treated (or not) with sPIF (50 nM). In order to validate our experimental conditions, media containing only E2 (10− 8 M) and P4 (10− 6 M) in the absence or presence of two positive controls i.e. FCS (10%) or adiponectin (250 ng/ml) were used as controls. RPMI medium supplemented with FCS (10%) was added to the lower well as a chemoattractant. After 48 h of incubation at 37 °C, medium containing non-invading cells was removed from the upper well. Invasive HTR-8/SVneo cells at the lower surface of the insert were washed and fixed with paraformaldehyde (4%) for 30 min. The nuclei were counterstained with 1 μg/mL Hoechst reagent and visualized with an inverted laser scanning confocal microscope (Leica white light laser TCS SP8-X, Leica Microsystems, Wetzlar, Germany). On each insert, invasive cells were counted on five randomly selected fields by using the post-imaging procedure in ImageJ software (National Institutes of Health, Bethesda, MD, USA). Invasive cells were defined as those whose nucleus exceeded 8 μm (i.e. the pore size).
In vitro wound healing assay
ESCs were seeded at 1 × 105 cells/cm2. After 15 days of differentiation (D15) in the presence or absence of sPIF (50 nM) in differentiation medium containing ovarian hormones (E2 at 10− 8 M and P4 at 10− 6 M), cell layers were wounded with a blade and washed three times with serum-free culture medium. The mark left by the blade on the plastic served as the migratory start line. Digital photographs of 6 different regions of each wound were taken before and 24 h after sPIF addition to serum-free culture medium. Cells that had migrated and repaired the wounded area were quantified using ImageJ software by measuring the width of the wound (mm/24 h) for each condition (in the presence of absence of sPIF).
Zymography
After 15 days of culture with differentiation medium in the absence or presence of sPIF (50 nM) in differentiation medium containing ovarian hormones (E2 at 10− 8 M and P4 at 10− 6 M), total gelatinase activities from ESCs were analyzed by zymography. Aliquots of CM containing 60 μg of protein were resolved under non-reducing conditions in 10% polyacrylamide gels containing 1 mg/mL gelatin (Difco). Gels were washed in Triton X-100 (2.5%) for 30 min to remove SDS and incubated overnight at 37 °C in renaturing buffer (50 mM Tris–HCl, pH 7.5, 5 mM CaCl2, 150 mM NaCl, and 0.02% sodium azide). Gels were stained with Coomassie Brilliant Blue and destained in methanol/acetic acid (20%/5% v/v). Proteolytic activity was identified as a clear band on a blue background. The images were scanned, and the band intensities were quantified using ImageJ software.
Reverse transcription - quantitative polymerase chain reaction (RT-qPCR)
ESCs were seeded into 12-well culture plates (3.5 × 105 cells per well). After 3, 8 or 15 days of differentiation (D3, D8, D15) in the presence or absence of sPIF (50 nM) in differentiation medium containing ovarian hormones (E2 at 10− 8 M and P4 at 10− 6 M), total RNA (0.1 μg) from ESCs was extracted and reverse-transcribed, as described previously. RT-qPCR was performed using the C1000 Thermal Cycler (CFX96 real-time system; BioRad, Hercules, CA, USA) and the primer sets indicated in Table 1. The final concentration of primers was 25 μM. Three reference genes coding for ribosomal protein L13A (RPL13A), TATA box-binding protein (TBP) and β2-microglobulin were chosen as described previously [30]. For each sample, the concentration ratios (target/three reference mRNAs) were calculated using BioRad CFX Manager software (version 3.0) and expressed in arbitrary units, as described previously [16, 31].
Table 1.
Primers used for RT-PCR
| Primer set | Sequence | PCR product (bp) |
|---|---|---|
| PROLACTIN | ||
| Sense | 5′ AGC CAG GTT CAT CCT GAA A 3′ | 99 |
| Antisense | 5′ TTC TCA GAG CGG AAA GAC GA 3′ | |
| IGFBP-1 | ||
| Sense | 5′ ATC ACA GCA GAC AGT GTG AGA 3′ | 71 |
| Antisense | 5′ CCA CGC AGA TGG GAA CCT TA 3′ | |
| CONNEXIN-43 | ||
| Sense | 5′ TTA AGC AAA AGA GTG GTG CCC 3’ | 179 |
| Antisense | 5′ GAC CCC TCT ACT CGT CAG AC 3’ | |
| TIMP-1 | ||
| Sense | 5′ GGG CTT CAC CAA GAC CTA CA 3’ | 71 |
| Antisense | 5′ TGC AGG GGA TGG ATA AAC AG 3’ | |
| TIMP-2 | ||
| Sense | 5′ GAA GAG CCT GAA CCA CAG GT 3’ | 85 |
| Antisense | 5′ GGG GGA GGA GAT GTA GCA C 3’ | |
| TBP | ||
| Sense | 5′ TGC ACA GGA GCC AAG AGT GAA 3’ | 132 |
| Antisense | 5′ CAC ATC ACA GCT CCC CAC CA 3’ | |
| B2-MICROGLOBULIN | ||
| Sense | 5′ TGC TGT CTC CAT GTT TGA TGT ATC T 3’ | 86 |
| Antisense | 5′ TCT CTG CTC CCC ACC TCT AAG T 3’ | |
| RPL13A | ||
| Sense | 5′ CCT GGA GGA GAA GAG GAA AGA GA 3’ | 125 |
| Antisense | 5′ TTG AGG ACC TCT GTG TAT TTG TCA A 3’ | |
Prolactin secretion
Prolactin secretion into the culture medium was measured using an automated immunochemiluminescence assay system (Alinity®, Abbott, Rungis, France). In order to compare the levels of prolactin secretion, the results were normalized per 1 μg of total protein. The protein concentration was measured according to Bradford’s method, with bovine serum albumin as the standard.
Statistical analysis
Statistical analyses were performed on the raw data from 5 to 13 separate experiments. A non-parametric, paired Wilcoxon test was used to compare (i) control situations (in the absence of sPIF) at D3 and D15, and (ii) the effect of 50 nM sPIF vs. the control situation at a given time point.
Results
Effect of sPIF on human ESC decidualization
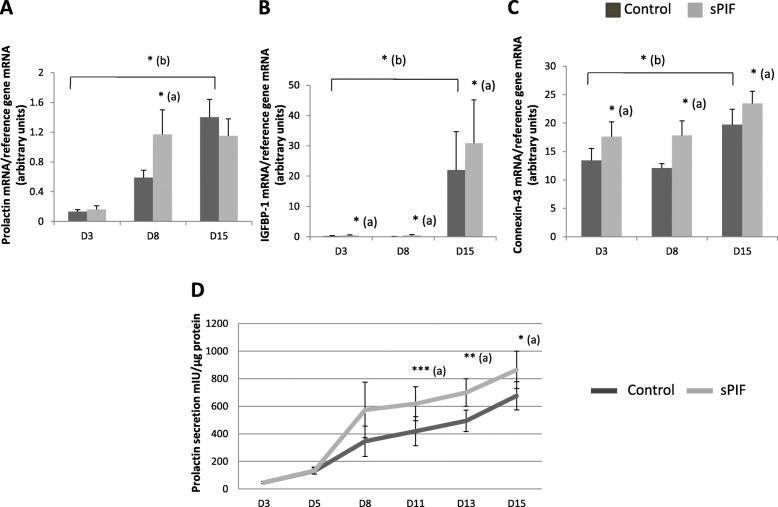
PRL, IGFBP-1, and CX-43 mRNA expression and prolactin secretion were used as biomarkers of sPIF’s effects on decidualization. After having validated our in vitro differentiation protocol (i.e. the observation of significant increases in the mRNA expression of PRL, IGFBP-1 and CX-43 during the ESC decidualization process; 11-, 100-, and 1.5-fold relative increases at D15, respectively) (Fig. 1A-C), we studied sPIF’s effect on this process. As shown in Fig. 1B-C, we found that IGFBP-1 and CX-43 mRNA expressions were significantly greater in the presence of sPIF at D3, D8 and D15. For PRL mRNA expression, this upregulatory effect was observed at D8 only (Fig. 1A). Furthermore, we observed significantly greater prolactin secretion in the presence of sPIF (50 nM) at D11, D13 and D15 (by a factor of 1.5, 1.4 and 1.3, respectively) (Fig. 1D). Similar results on prolactin secretion of ESCs were obtained with a higher sPIF concentration i.e. 100 nM (1.7 ± 0.4 fold-increase) (data not shown). So, we decided to use 50 nM of sPIF in the following experiments.
Fig. 1.
Effect of sPIF on human ESC decidualization. Human ESCs were cultured in DMEM/F12 medium supplemented with E2, P4 and (in some experiments) 50 nM sPIF for 15 days. A-C Total RNA was extracted after three (D3), eight (D8), and 15 days (D15) of cell decidualization. mRNA expression levels of prolactin (A), IGFBP-1 (B), and connexin-43 (C) were quantified by RT-qPCR, as described in the Materials and Methods section. The data are presented as the mean ± SEM of 6 to 10 separate experiments. D Prolactin secretion into the endometrial supernatants was measured after 3 days (D3), 8 days (D8), and 15 days (D15) of cell differentiation, as described in the Materials and Methods. The data are presented as the mean ± SEM of 8 to 13 separate experiments. The control values on D3, D5, D8, D11, D13, and D15 were 46.5 ± 8.7, 130.7 ± 22.5, 346 ± 110, 419 ± 105, 494.5 ± 77 and 676.4 ± 102 mIU/g protein, respectively. *: P < 0.05; **: P < 0.01; ***: P < 0.001 (Wilcoxon test); (a) Prolactin secretion in presence of sPIF vs. the control (lacking sPIF); (b) Prolactin secretion at D15 vs. D3 for control experimental condition (lacking sPIF)
PI3K pathway involved in sPIF’s pro-differentiation effect
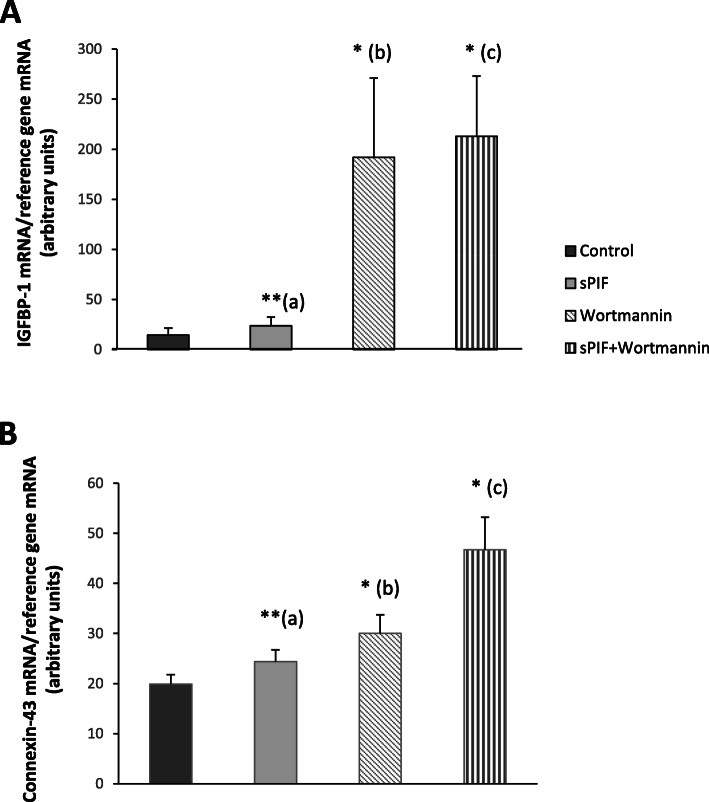
To gain insight into the molecular mechanisms underlying the PIF-induced decidualization of human ESCs, we performed further experiments with a selective inhibitor of the PI3K signaling pathway. As show in Fig. 2, wortmannin exerted a significant effect per se on IGFBP-1 and CX-43 mRNA expression levels. Furthermore, we showed that sPIF’s pro-differentiation effect was not abrogated when the PI3K pathway was inhibited.
Fig. 2.
Effect of a PI3K inhibitor on sPIF-enhanced ESC decidualization. Human ESCs were cultured in DMEM/F12 medium supplemented with E2, P4, and the PI3K inhibitor wortmannin (10 μM) in the presence or in the absence of sPIF (50 nM) for 15 days. A-B Total RNA was extracted after 15 days (D15) of cell decidualization. mRNA expression levels of IGFBP-1 (A), and connexin-43 (B) were quantified by RT-qPCR, as described in the Materials and Methods section. The data are presented as the mean ± SEM of 4 to 11 separate experiments. *: P < 0.05; **: P < 0.005 (Wilcoxon test). (a) sPIF vs. control (lacking sPIF); (b) wortmannin vs. control (lacking sPIF); (c) sPIF vs. sPIF + wortmannin
Involvement of sPIF in endometrial motility
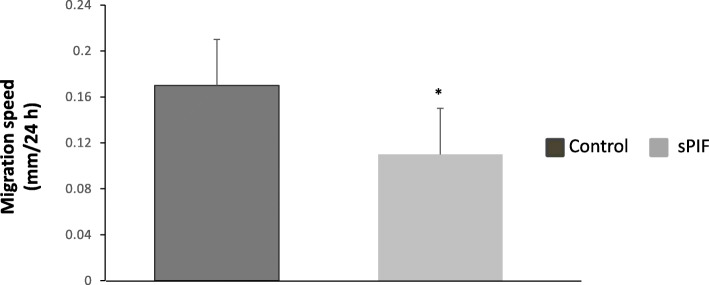
We used a wound repair assay to examine sPIF’s ability to modify the migratory properties of human ESCs. As shown in Fig. 3, cell motility into the denuded area under our experimental conditions was slightly decreased (by 18%) after exposure to sPIF for 15 days.
Fig. 3.
Effect of sPIF on human ESC motility. Human ESCs were cultured in DMEM/F12 medium supplemented with E2, P4 and (in some experiments) 50 nM sPIF for 15 days. Wound width was analyzed 0 and 24 h after wounding, as described in the Materials and Methods. The wound width data are expressed as the mean ± SEM of 5 separate experiments. *: P < 0.05 (Wilcoxon test) for sPIF vs. the control (lacking sPIF)
Involvement of sPIF in the endometrial control of trophoblast invasion
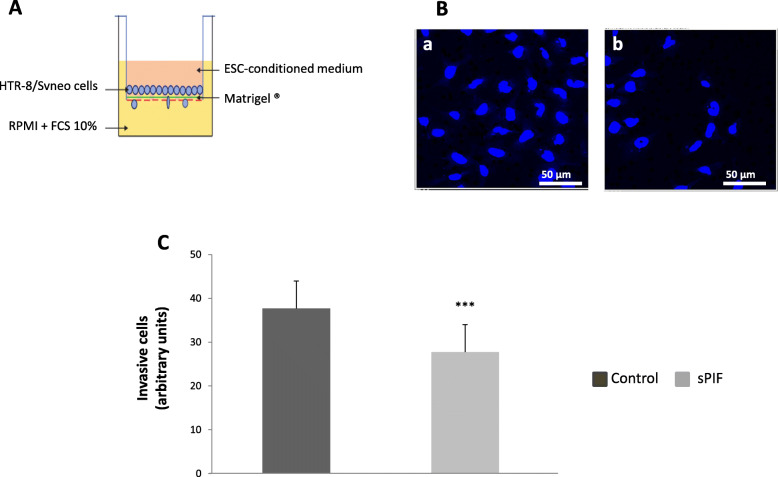
In order to determine how sPIF might be involved in the endometrial control of trophoblast invasion, we performed Matrigel® transwell invasion assays. We cultured the HTR-8/SVneo immortalized EVT cell line in the presence of CM conditioned by differentiating ESCs treated (or not) with sPIF (50 nM) for 15 days (Fig. 4A). As shown in Fig. 4B and C, we found that culture with CM from sPIF-treated ESCs was associated significantly lower invasive activity of HTR-8/SVneo cells (by 32%). In our experimental conditions, FCS (10%) and adiponectin (250 ng/ml) were used as positive control for EVT invasion.
Fig. 4.
Involvement of sPIF in the endometrial control of trophoblast invasion by ESCs. PIF improves the endometrial control of trophoblastic migration. A Transwell migration assays of HTR-8/SVneo cells were performed as described in the Materials and Methods. B Representative microphotographs of 12 separate experiments showing fixed and DAPI stained HTR-8/SVneo cells on the bottom side of the transwell membrane. HTR-8/SVneo cells were cultivated with conditioned medium (CM) from decidualized ESCs exposed for 15 days in control condition (a) or with 50 nM sPIF (b). C HTR-8/SVneo cells were suspended in the presence of CM from decidualized ESCs having been exposed for 15 days in presence or absence of sPIF (50 nM) or in the presence of control medium, supplemented or not with FCS 10% or adiponectin (250 ng/ml). The data are presented as the mean ± SEM of 3–12 separate experiments. ***: P < 0.001 (Wilcoxon test) for sPIF vs. the control (lacking sPIF)
Effect of sPIF on endometrial MMP activities
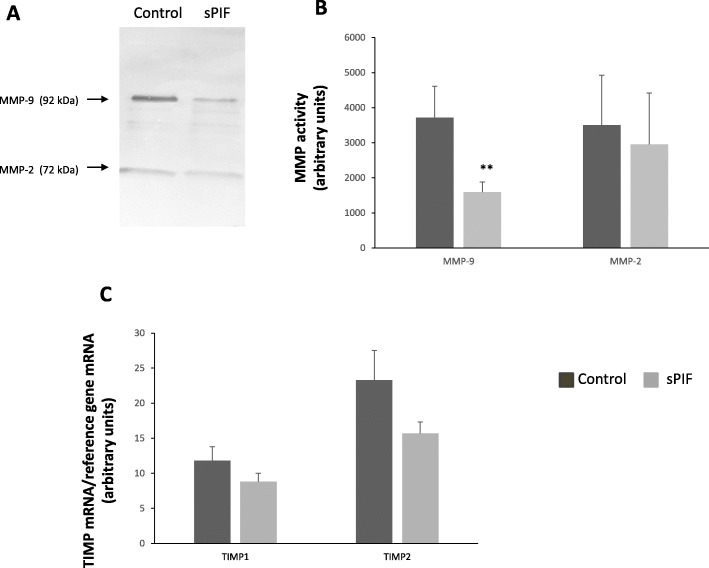
We next sought to specify the molecular mechanisms involved in sPIF’s anti-invasive action. Zymography assays revealed two bands, corresponding to the gelatinase activities of MMP-2 (72 kDa) and MMP-9 (92 kDa) in CM collected on D15 of cell culture. As shown in Fig. 5A and B, treatment with sPIF was associated with significantly lower MMP-9 activity on D15 (− 57%). However, under our experimental conditions, we did not observe any difference in MMP-2 activity in response to sPIF.
Fig. 5.
Effect of sPIF on endometrial TIMP mRNA expression and MMP activities. Human ESCs were cultured in DMEM/F12 medium supplemented with E2, P4 and (in some experiments) 50 nM sPIF for 15 days. A Activities of gelatinases (MMP-9 and MMP-2) in CM from decidualized ESCs having been exposed for 15 days in presence or absence of sPIF (50 nM), as described in the Materials and Methods. This figure shows one representative of 11 separate experiments. B Quantification of gelatin zymography results for MMP-2 and MMP-9 activities. The data are presented as the mean ± SEM of 5 to 8 separate experiments. C Total RNA was extracted after 15 days of cell differentiation. mRNA expression levels of TIMP-1 and TIMP-2 were quantified by RT-qPCR, as described in the Materials and Methods. The data are presented as the mean ± SEM of 11 separate experiments. **: P < 0.005 (Wilcoxon test) for sPIF vs. the control (lacking sPIF)
Effect of sPIF on mRNA expression of endometrial TIMPs
Lastly, we focused on the mRNA expression of the invasion inhibitors TIMP-1 and TIMP-2, both of which are strongly expressed in human endometrium. The RT-qPCR assay did not reveal any significant changes in TIMP-1 and TIMP-2 mRNA expression after 15 days of culture in the presence of sPIF (Fig. 5C).
Discussion
Early establishment of maternal-fetal crosstalk is critical for the progression of pregnancy. Many maternal and fetal factors have important roles during implantation and beyond. In the present study, we investigated the role of PIF - a small peptide exclusively secreted by competent embryos - on the decidualization process and on the endometrial control of trophoblast invasion.
We assessed ESC differentiation by measuring the mRNA expression of decidualization markers such as prolactin, IGFBP-1, and connexin-43. We observed significant upregulation of the expression of all three markers between D3 and D15 in response to the differentiation medium - indicating that ESCs are fully differentiated at D15. These results are in line with the literature data on in vitro ESC differentiation in the presence of E2 and P4 [32, 33]. We next studied the effect of sPIF on ESC decidualization. Our present results clearly show that sPIF increases biochemical ESC decidualization as evidenced by the significant increase in prolactin secretion by ESCs treated with sPIF. This upregulatory effect appears to be related to an effect of PIF on PRL gene transcription. However, PIF’s transitory effect on PRL mRNA expression is also associated with a longer-lasting effect on prolactin protein - suggesting that PIF influences the stabilization of PRL mRNA. The lack of correspondence between RNA and protein levels is not surprising and has been already described in the literature [34, 35]. More specifically, two different studies including a previous study of our laboratory clearly evidenced the mismatch between the prolactin mRNA expression and hormone release [16, 36]. We also demonstrated that sPIF upregulates IGFBP-1 and CX-43 mRNA expression during ESC differentiation and thus accentuated the decidualization of human ESCs. Our results are in line with previous genomic and proteomic studies in which PIF promoted endometrial receptivity by downregulating a gene specifically implicated in human endometrial receptivity i.e. the gene coding for phosphodiesterase 4B. Indeed, this enzyme specifically hydrolyses cAMP, a critical promotor of decidualization [23].
Many signaling pathways are known to be involved in human ESC decidualization. Furthermore, we previously reported that PIF’s role in trophoblast cells was mediated (at least in part) by the PI3K signaling pathway [19]. Hence, to gain insights into the molecular mechanisms involved in PIF-induced decidualization, we investigated the PI3K signaling pathway. We first showed that the specific PI3K inhibitor wortmannin per se upregulated IGFBP-1 and CX-43 transcription. These results were not unexpected because they are in line with a previous study of human stromal cells in which inhibition of the PI3K pathway was decisive in endometrial differentiation [37]. Secondly, our results revealed that sPIF’s prodifferentiation effect seems not be mediated by the PI3K pathway. We are currently seeking to determine the possible involvement of another signal transducer (Janus-kinase signal transducer and activator of transcription) in PIF’s control of the decidualization process. We effectively previously demonstrated in our laboratory that PIF promoted human trophoblast invasion by stimulating different signaling pathways including JAK-STAT transduction [19]. In addition, two genome-wide transcriptomic change studies revealed that decidualization of ESCs is a multiphasic process involving distinct transcriptional programs including JAK-STAT transduction [38, 39]. Finally, it has been described that the transcription factor STAT3 seems to play a critical role on decidualization process [40, 41].
Our experiments also demonstrated that sPIF downregulated the mobility of decidualized cells. Hence, PIF exerts a paracrine effect on endometrial stromal cells by promoting their differentiation and inhibiting their migration. A similar action has already been described for another secreted protein (prokineticin 1) in human ESCs [42, 43]. Thus, the present study highlights the involvement of the embryonic peptide PIF in endometrial receptivity.
Decidualized stromal cells also control trophoblast invasion [14, 33]. We therefore decided to determine whether sPIF could change the ability of decidualized ESCs to modulate this process. Our results clearly demonstrated that exposure to medium conditioned by ESCs cultured for 2 weeks in the presence of sPIF significantly decreased trophoblast invasion. In order to gain more information on the molecular level, we next investigated sPIF’s effect on MMP activities and TIMP mRNA expressions in human ESCs. Our data demonstrated that sPIF is significantly and specifically associated with low MMP-9 activity in ESCs but not with a difference in TIMP expression. These findings suggest that PIF could regulate the production of endometrial factors already described as negative regulators of human trophoblast invasion (e.g. transforming growth factor beta (TGFβ) family members and interferon-γ) [44]. At present, it is difficult to say whether PIF directly controls the secretion of endometrial factors or whether it has an indirect effect.
It has been previously described that sPIF exerted a pro-receptive effect by promoting the expression of α2β3 integrin, a critical implantation marker, in endometrial epithelial cells [24]. In this paper, we demonstrated that sPIF played a pro-differentiative role in ESCs. All these results suggest that PIF could be considered as a key regulator of endometrial functions.
Interestingly, we demonstrated in this paper that sPIF indirectly decreases the invasive capacities of human trophoblasts through secreted endometrial factors. In contrast, we previously showed that sPIF directly increases the invasive capacities of human trophoblasts [19]. So, the same molecule i.e. sPIF seems to exert a dual control of human trophoblast invasive capacities by maintaining pro-invasive and anti-invasive signals in placenta and endometrium, respectively. This dual control by PIF in decidual and trophoblast cells might limit cell migration and thus avoid excessive trophoblastic invasion. It is effectively well established that dysregulation of placental invasion is associated with several diseases of pregnancy, such as preeclampsia and intrauterine growth restriction [45, 46]. Our laboratory has previously reported that placental PIF protein levels in pregnancies affected by preeclampsia or intrauterine growth restriction were significantly lower than in gestational-age-matched controls [22]. Taken as a whole, our results suggest that PIF’s specific effects on human placenta and endometrium influence the onset of these pathologies by specifically modulating the trophoblast’s invasive capacities.
Conclusions
In conclusion, our data suggest that PIF has a critical role in the establishment of human embryo implantation by i) increasing decidualization of ESCs and ii) limiting trophoblastic invasion.
Acknowledgements
The authors thank the staff at the Department of Gynecology and Obstetrics at CHI de Poissy-Saint-Germain-en-Laye for kindly providing samples of human endometrial tissue. Immunofluorescence image acquisition and analysis were performed at the CYMAGES facility, funded by the Saint-Quentin-en-Yvelines Urban Community and the University of Versailles-Saint-Quentin-en-Yvelines. We thank Benoît Maury for technical assistance with the analysis of confocal microscopy data.
Abbreviations
- CM
Conditioned medium
- CX
Connexin
- DMEM/F12
Dulbecco’s modified Eagle’s medium and Ham F-12 nutrient mix
- E2
17β-estradiol
- ECM
Extracellular matrix
- ESC
Endometrial stromal cell
- EVT
Extravillous trophoblast
- FCS
Fetal calf serum
- IGFBP-1
Insulin-like growth factor-binding protein 1
- MAP kinase
Mitogen-activated protein kinase
- MMP
Matrix metalloproteinase
- P4
Progesterone
- PI3K
Phosphatidylinositol 3 kinase
- PIF
Preimplantation factor
- PRL
Prolactin
- RPMI
Roswell Park Memorial Institute
- TGFβ
Transforming growth factor beta
- TIMP
Tissue inhibitor of metalloproteinases
- TNF-α
Tumor necrosis factor alpha
Authors’ contributions
EDS and MND designed the experiments. KF provided human endometrial biopsies for experiments. PL, HM and LA performed the experiments. EDS and MND analyzed the data and wrote the manuscript. VS analyzed the RT-qPCR data. FV obtained research funding and critically revised the manuscript. sPIF was a gift from EB. The author(s) read and approved the final manuscript.
Funding
This work was funded by the Institut de Recherche en Santé de la Femme (based at the UFR des Sciences de la Santé, University of Versailles-Saint-Quentin-en-Yvelines) and the Maternité et Médecine de la Reproduction Association (based at the Centre Hospitalier de Poissy-Saint Germain).
Availability of data and materials
Literature search results are available from the authors on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the local investigational review board (Comité Consultatif de Protection des Personnes dans la Recherche Médicale, approval reference protocol 01–78), Paris, France; reference: 01–78). All participants provided their written, informed consent before tissue sampling.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Diedrich K, Fauser BCJM, Devroey P, Griesinger G. The role of the endometrium and embryo in human implantation. Hum Reprod Update. 2007;13:365–377. doi: 10.1093/humupd/dmm011. [DOI] [PubMed] [Google Scholar]
- 2.Lunghi L, Ferretti ME, Medici S, Biondi C, Vesce F. Control of human trophoblast function. Reprod Biol Endocrinol. 2007;5:1. doi: 10.1186/1477-7827-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burrows TD, King A, Loke YW. Trophoblast migration during human placental implantation. Hum Reprod Update. 1996;2:307–321. doi: 10.1093/humupd/2.4.307. [DOI] [PubMed] [Google Scholar]
- 4.Staun-Ram E, Goldman S, Gabarin D, Shalev E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod Biol Endocrinol. 2004;2:59. doi: 10.1186/1477-7827-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huppertz B, Kertschanska S, Demir AY, Frank HG, Kaufmann P. Immunohistochemistry of matrix metalloproteinases (MMP), their substrates, and their inhibitors (TIMP) during trophoblast invasion in the human placenta. Cell Tissue Res. 1998;291:133–148. doi: 10.1007/s004410050987. [DOI] [PubMed] [Google Scholar]
- 6.Nissi R, Talvensaari-Mattila A, Kotila V, Niinimäki M, Järvelä I, Turpeenniemi-Hujanen T. Circulating matrix metalloproteinase MMP-9 and MMP-2/TIMP-2 complex are associated with spontaneous early pregnancy failure. Reprod Biol Endocrinol. 2013;11:2. doi: 10.1186/1477-7827-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh M, Chaudhry P, Asselin E. Bridging endometrial receptivity and implantation: network of hormones, cytokines, and growth factors. J Endocrinol. 2011;210:5–14. doi: 10.1530/JOE-10-0461. [DOI] [PubMed] [Google Scholar]
- 8.Brar AK, Frank GR, Richards RG, Meyer AJ, Kessler CA, Cedars MI, Klein DJ, Handwerger S. Laminin decreases PRL and IGFBP-1 expression during in vitro decidualization of human endometrial stromal cells. J Cell Physiol. 1995;163:30–37. doi: 10.1002/jcp.1041630105. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Wu J, Bagchi IC, Bagchi MK, Sidell N, Taylor RN. Disruption of gap junctions reduces biomarkers of decidualization and angiogenesis and increases inflammatory mediators in human endometrial stromal cell cultures. Mol Cell Endocrinol. 2011;344:25–34. doi: 10.1016/j.mce.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Salamonsen LA. Tissue inhibitor of metalloproteinases (TIMP)-1, −2 and −3 in human endometrium during the menstrual cycle. Mol Hum Reprod. 1997;3:735–741. doi: 10.1093/molehr/3.9.735. [DOI] [PubMed] [Google Scholar]
- 11.Estella C, Herrer I, Atkinson SP, Quiñonero A, Martínez S, Pellicer A, Simón C. Inhibition of histone deacetylase activity in human endometrial stromal cells promotes extracellular matrix remodelling and limits embryo invasion. PLoS One. 2012;7:e30508. doi: 10.1371/journal.pone.0030508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tapia-Pizarro A, Argandoña F, Palomino WA, Devoto L. Human chorionic gonadotropin (hCG) modulation of TIMP1 secretion by human endometrial stromal cells facilitates extravillous trophoblast invasion in vitro. Hum Reprod. 2013;28:2215–2227. doi: 10.1093/humrep/det136. [DOI] [PubMed] [Google Scholar]
- 13.Grewal S, Carver JG, Ridley AJ, Mardon HJ. Implantation of the human embryo requires Rac1-dependent endometrial stromal cell migration. Proc Natl Acad Sci U S A. 2008;105:16189–16194. doi: 10.1073/pnas.0806219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma S, Godbole G, Modi D. Decidual control of trophoblast invasion. Am J Reprod Immunol. 2016;75:341–350. doi: 10.1111/aji.12466. [DOI] [PubMed] [Google Scholar]
- 15.Haider S, Knöfler M. Human tumour necrosis factor: physiological and pathological roles in placenta and endometrium. Placenta. 2009;30:111–123. doi: 10.1016/j.placenta.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duval F, Dos Santos E, Moindjie H, Serazin V, Swierkowski-Blanchard N, Vialard F, Dieudonné M-N. Adiponectin limits differentiation and trophoblast invasion in human endometrial cells. J Mol Endocrinol. 2017;59:285–297. doi: 10.1530/JME-17-0046. [DOI] [PubMed] [Google Scholar]
- 17.Ramu S, Stamatkin C, Timms L, Ruble M, Roussev RG, Barnea ER. PreImplantation factor (PIF) detection in maternal circulation in early pregnancy correlates with live birth (bovine model) Reprod Biol Endocrinol. 2013;11:105. doi: 10.1186/1477-7827-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnea ER, Vialard F, Moindjie H, Ornaghi S, Dieudonne MN, Paidas MJ. PreImplantation factor (PIF*) endogenously prevents preeclampsia: promotes trophoblast invasion and reduces oxidative stress. J Reprod Immunol. 2016;114:58–64. doi: 10.1016/j.jri.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Moindjie H, Dos Santos E, Loeuillet L, Gronier H, de Mazancourt P, Barnea ER, Vialard F, Dieudonne M-N. Preimplantation factor (PIF) promotes human trophoblast invasion. Biol Reprod. 2014;91:118. doi: 10.1095/biolreprod.114.119156. [DOI] [PubMed] [Google Scholar]
- 20.Stamatkin CW, Roussev RG, Stout M, Coulam CB, Triche E, Godke RA, Barnea ER. Preimplantation factor negates embryo toxicity and promotes embryo development in culture. Reprod BioMed Online. 2011;23:517–524. doi: 10.1016/j.rbmo.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Roussev RG, Dons’koi BV, Stamatkin C, Ramu S, Chernyshov VP, Coulam CB, Barnea ER. Preimplantation factor inhibits circulating natural killer cell cytotoxicity and reduces CD69 expression: implications for recurrent pregnancy loss therapy. Reprod BioMed Online. 2013;26:79–87. doi: 10.1016/j.rbmo.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Moindjie H, Dos Santos E, Gouesse R-J, Swierkowski-Blanchard N, Serazin V, Barnea ER, Vialard F, Dieudonné M-N. Preimplantation factor is an anti-apoptotic effector in human trophoblasts involving p53 signaling pathway. Cell Death Dis. 2016;7:e2504. doi: 10.1038/cddis.2016.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paidas MJ, Krikun G, Huang SJ, Jones R, Romano M, Annunziato J, Barnea ER. A genomic and proteomic investigation of the impact of preimplantation factor on human decidual cells. Am J Obstet Gynecol. 2010;202:459.e1–459.e8. doi: 10.1016/j.ajog.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnea ER, Kirk D, Paidas MJ. PreImplantation factor (PIF) promoting role in embryo implantation: increases endometrial integrin-α2β3, amphiregulin and epiregulin while reducing betacellulin expression via MAPK in decidua. Reprod Biol Endocrinol. 2012;10:50. doi: 10.1186/1477-7827-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duzyj CM, Barnea ER, Li M, Huang SJ, Krikun G, Paidas MJ. Preimplantation factor promotes first trimester trophoblast invasion. Am J Obstet Gynecol. 2010;203:402.e1–402.e4. doi: 10.1016/j.ajog.2010.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sbracia M, McKinnon B, Scarpellini F, Marconi D, Rossi G, Simmilion C, Mueller MD, Barnea ER, Mueller M. PreImplantation factor in endometriosis: a potential role in inducing immune privilege for ectopic endometrium. PLoS One. 2017;12:e0184399. doi: 10.1371/journal.pone.0184399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nash DM, Paddison J, Davies Morel MCG, Barnea ER. Preimplantation factor modulates acute inflammatory responses of equine endometrium. Vet Med Sci. 2018;4:351–356. doi: 10.1002/vms3.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wonfor RE, Natoli M, Rose MT, Nash DM. Effects of preimplantation factor on interleukin-6 and prostaglandin F2α and E2 in the bovine endometrium. Theriogenology. 2017;102:174–182. doi: 10.1016/j.theriogenology.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez RR, Palomino A, Boric A, Vega M, Devoto L. A quantitative evaluation of α1, α4, αV and β3 endometrial integrins of fertile and unexplained infertile women during the menstrual cycle. A flow cytometric appraisal. Hum Reprod. 1999;14:2485–2492. doi: 10.1093/humrep/14.10.2485. [DOI] [PubMed] [Google Scholar]
- 30.Benaitreau D, Dieudonné M-N, Dos Santos E, Leneveu M-C, de Mazancourt P, Pecquery R. Antiproliferative effects of adiponectin on human trophoblastic cell lines JEG-3 and BeWo. Biol Reprod. 2009;80:1107–1114. doi: 10.1095/biolreprod.108.070573. [DOI] [PubMed] [Google Scholar]
- 31.Duval F, Dos SE, Poidatz D, Sérazin V, Gronier H, Vialard F, Dieudonné M-N. Adiponectin inhibits nutrient transporters and promotes apoptosis in human villous cytotrophoblasts: involvement in the control of fetal growth. Biol Reprod. 2016;94:111. doi: 10.1095/biolreprod.115.134544. [DOI] [PubMed] [Google Scholar]
- 32.Kasahara K, Takakura K, Takebayashi K, Kimura F, Nakanishi K, Noda Y. The role of human chorionic gonadotropin on Decidualization of endometrial stromal cells in Vitro1. J Clin Endocrinol Metab. 2001;86:1281–1286. doi: 10.1210/jcem.86.3.7281. [DOI] [PubMed] [Google Scholar]
- 33.Godbole G, Suman P, Gupta SK, Modi D. Decidualized endometrial stromal cell derived factors promote trophoblast invasion. Fertil Steril. 2011;95:1278–1283. doi: 10.1016/j.fertnstert.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 34.Salari R, Wojtowicz D, Zheng J, Levens D, Pilpel Y, Przytycka TM. Teasing apart translational and transcriptional components of stochastic variations in eukaryotic gene expression. PLoS Comput Biol. 2012;8:e1002644. doi: 10.1371/journal.pcbi.1002644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beileke S, Claassen H, Wagner W, Matthies C, Ruf C, Hartmann A, Garreis F, Paulsen F, Schicht M, Bräuer L. Expression and localization of lung surfactant proteins in human testis. PLoS One. 2015;10:e0143058. doi: 10.1371/journal.pone.0143058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castano JP, Faught WJ, Glavé EE, Russell BS, Frawley LS. Discordance of prolactin gene transcription, mRNA storage, and hormone release in individual mammotropes. Am J Physiol - Endocrinol Metab. 1997;272:E390. doi: 10.1152/ajpendo.1997.272.3.E390. [DOI] [PubMed] [Google Scholar]
- 37.Fabi F, Grenier K, Parent S, Adam P, Tardif L, Leblanc V, Asselin E. Regulation of the PI3K/Akt pathway during decidualization of endometrial stromal cells. PLoS One. 2017;12:e0177387. doi: 10.1371/journal.pone.0177387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J-L, Wang T-S. Systematic analysis of the molecular mechanism underlying Decidualization using a text mining approach. PLoS One. 2015;10:e0134585. doi: 10.1371/journal.pone.0134585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rytkönen KT, Erkenbrack EM, Poutanen M, Elo LL, Pavlicev M, Wagner GP. Decidualization of human endometrial stromal fibroblasts is a multiphasic process involving distinct transcriptional programs. Reprod Sci. 2019;26:323–336. doi: 10.1177/1933719118802056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Y, Liao Y, He H, Xin Q, Tu Z, Kong S, Cui T, Wang B, Quan S, Li B, Zhang S, Wang H. FoxM1 directs STAT3 expression essential for human endometrial stromal Decidualization. Sci Rep. 2015;5:1–11. doi: 10.1038/srep13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JH, Kim TH, Oh SJ, Yoo J, Akira S, Ku BJ, Lydon JP, Jeong J. Signal transducer and activator of transcription-3 ( Stat3 ) plays a critical role in implantation via progesterone receptor in uterus. FASEB J. 2013;27:2553–2563. doi: 10.1096/fj.12-225664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macdonald LJ, Sales KJ, Grant V, Brown P, Jabbour HN, Catalano RD. Prokineticin 1 induces Dickkopf 1 expression and regulates cell proliferation and decidualization in the human endometrium. Mol Hum Reprod. 2011;17:626–636. doi: 10.1093/molehr/gar031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ujvari D, Jakson I, Oldmark C, Attarha S, Alkasalias T, Salamon D, Gidlöf S, Hirschberg AL. Prokineticin 1 is up-regulated by insulin in decidualizing human endometrial stromal cells. J Cell Mol Med. 2018;22:163–172. doi: 10.1111/jcmm.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knöfler M. Critical growth factors and signalling pathways controlling human trophoblast invasion. Int J Dev Biol. 2010;54:269–280. doi: 10.1387/ijdb.082769mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anin S, Vince G, Quenby S. Trophoblast invasion. Hum Fertil. 2004;7:169–174. doi: 10.1080/14647270400006911. [DOI] [PubMed] [Google Scholar]
- 46.Kadyrov M, Kingdom JCP, Huppertz B. Divergent trophoblast invasion and apoptosis in placental bed spiral arteries from pregnancies complicated by maternal anemia and early-onset preeclampsia/intrauterine growth restriction. Am J Obstet Gynecol. 2006;194:557–563. doi: 10.1016/j.ajog.2005.07.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Literature search results are available from the authors on reasonable request.