Abstract
C. elegans is a powerful model for studies of zinc biology. Here we review recent discoveries and emphasize the advantages of this model organism. Methods for manipulating and measuring zinc levels have been developed in or adapted to the worm. The C. elegans genome encodes highly conserved zinc transporters, and their expression and function are beginning to be characterized. Homeostatic mechanisms have evolved to respond to high and low zinc conditions. The pathway for high zinc homeostasis has been recently elucidated based on the discovery of the master regulator of high zinc homeostasis, HIZR-1. A parallel pathway for low zinc homeostasis is beginning to emerge based on the discovery of the Low Zinc Activation promoter element. Zinc has been established to play a role in two cell fate determination events, and accumulating evidence suggests zinc may function as a second messenger signaling molecule during vulval cell development and sperm activation. This article is part of a Special Issue entitled: Cell Biology of Metals.
Keywords: HIZR-1, sperm activation, LZA, vulval development, zinc transporters, lysosome-related organelles
1. C. elegans as a model system to study zinc biology.
Beginning with his landmark publication in 1974 titled “The Genetics of Caenorhabditis elegans”, Sydney Brenner established ‘the worm’ as a biomedical model organism [1]. Brenner chose C. elegans because it has intrinsic properties that are useful for experimental work: a small number of cells, simple anatomy, and transparency facilitate phenotypic and microscopic analyses; small size, rapid generation time, and a hermaphrodite/male sexual system facilitate genetic analysis; and a compact genome facilitates molecular analysis. Many followed Brenner’s lead, and since 1974 thousands of researchers have contributed to tens of thousands of articles using C. elegans to study fundamental biological processes.
1.1. Anatomy:
Like humans, C. elegans is a multicellular animal, but it is anatomically streamlined. Remarkably, the pattern of cell divisions during development is highly reproducible between different individuals, making it possible to map each cell division. The adult C. elegans hermaphrodite contains 959 somatic cell nuclei, and the full lineage diagram has been established [2–4] The simple anatomy of the animal can be described as a tube within a tube; the exterior tube consists of epithelial cells covered by a hard cuticle made of material reminiscent of a fingernail, and the interior tube consists of endothelial cells that compose the digestive tract, including the pharynx and intestine (Figure 1). Adult C. elegans has 20 intestinal cells comprising 30–34 intestinal nuclei, and these cells compose roughly one third of the total somatic mass [5]. The first control of zinc homeostasis occurs in intestinal cells, and one focus of this review is studies of zinc homeostasis in these cells.
Figure 1: The body plan of C. elegans.
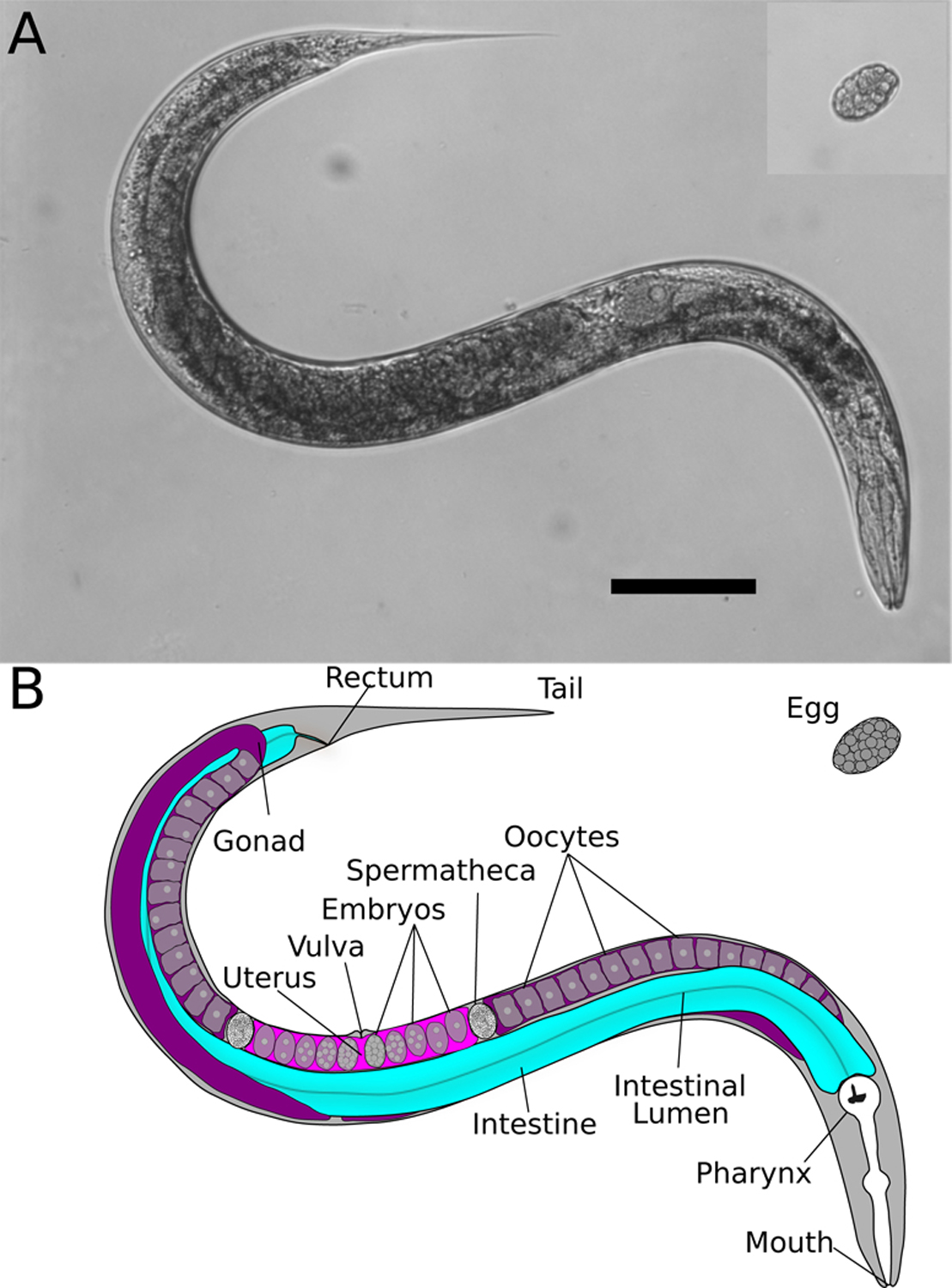
(A) Brightfield images of an adult C. elegans hermaphrodite and a recently laid egg. Scale bar = 0.1 millimeters. (B) Diagram highlighting major anatomical features relevant to zinc biology. The digestive tract begins at the mouth where food enters the pharynx (white). Bacterial food is ground in the pharynx and then enters the intestinal lumen (central line). Nutrients are absorbed from the lumen into intestinal cells (blue), and molecules can be excreted from intestinal cells into the lumen. Defecation occurs at the rectum. The germ line is composed of two gonad arms (purple). From the distal tips of the gonad arms, germ cells travel toward the vulva. During the fourth larval stage of development, hermaphrodite germ cells differentiate into spermatocytes. These spermatocytes are stored in the spermathecae in each gonad arm. Following the fourth larval stage, hermaphrodite germ cells differentiate into oocytes. As each oocyte passes through the spermatheca, the oocyte is fertilized by a spermatocyte and becomes a fertilized embryo. Embryos enter the uterus (fuchsia), where embryos develop a hardened shell and cell divisions progress rapidly. Developing embryos are laid into the environment, at which point they are termed eggs. Within 3 days of hatching, eggs develop into reproductively mature adults.
In the space between the epithelial cells and intestine, these worms contain muscles, neurons and the gonad (Figure 1). Hermaphrodites contain two gonad arms that are joined at the vulva near the middle of the worm on the ventral surface; the vulva is a specialized epithelial structure for egg laying and male mating. The hermaphrodite gonad initially produces sperm, which are stored in the spermatheca, and then switches to produce oocytes. In a self-fertile hermaphrodite, oocytes are fertilized as they pass through the spermatheca, producing about 300 progeny in their adult life. This remarkable quality makes C. elegans an exceptionally powerful animal for genetic analysis. One focus of this review is the emerging roles of zinc in the function of the gonad.
1.2. Genome and Genetics:
C. elegans was the first multicellular animal to have a completely sequenced genome [6]. This landmark accomplishment facilitated a wide range of genetic discoveries in worms and set the stage for the human genome project. The C. elegans genome is composed of 6 chromosomes: 5 autosomes and a sex chromosome, which yields XX hermaphrodites and XO hemizygous males. The genome contains about 20,000 genes, and many C. elegans genes have predicted orthology with human genes [7]. Due to the short generation time, simple culture techniques, and the power of hermaphrodite genetics, C. elegans has become a workhorse for genetic studies. Genetic discoveries are accelerated by the collegial community of C. elegans researchers who routinely share strains and reagents and the Caenorhabditis Genetics Center, based at the University of Minnesota, which stores and distributes tens of thousands of strains annually to researchers worldwide. The vast array of existing genetic reagents, coupled with many methods developed to create new genetic reagents, such as CRISPR-Cas 9 genome editing, makes it possible to use C. elegans to rapidly address a wide range of experimental questions.
1.3. Methods for studying zinc biology – manipulating dietary zinc and measuring zinc levels and distribution:
Zinc is a redox inactive di-cation in physiological conditions, and this chemical characteristic allows zinc to perform a wide range of essential biological functions. As such, the homeostasis of zinc is important for the basic biological functions of all organisms, including microbes, plants and animals such as C. elegans. C. elegans is a hardy soil nematode that thrives in the laboratory. Because C. elegans is surrounded by a cuticle, the predominant entry point for nutrients and chemicals is via the mouth, which is the entry point for bacterial food, followed by absorption by intestinal cells. The most common culture conditions are solid agar in a Petri dish with a lawn of bacteria as a food source. C. elegans can also be cultured in liquid medium using bacteria as a food source. These simple culture conditions are convenient and make it possible to manipulate environmental and dietary factors, but they also have limitations. The standard medium contains peptone and agar, which are both undefined sources of a variety of trace elements that are known to be essential for worms and humans, including iron, copper, manganese, and zinc. To address the uncertainties of an undefined mixture of nutrients, C. elegans Maintenance Media (CeMM), a fully-defined liquid medium that permits bacteria-free experiments has been developed. Because each essential metal must be added to CeMM, it is possible to prepare modified versions of this medium that have lower or higher concentrations of specific metals, including the complete absence of one or more metals [8, 9]. However, CeMM has limitations: it is expensive, prone to bacterial contamination, and although it permits indefinite culture, worms are thin and slow growing in this medium, suggesting it is less nutritious than bacteria.
The community of zinc biologists has developed an array of methods for studying zinc, and here we describe how some of these methods have been adapted or applied to C. elegans. The study of zinc biology in C. elegans requires the ability to manipulate dietary zinc, both by increasing and decreasing the concentration in the diet. In initial experiments designed to increase dietary zinc, supplemental zinc was added to Nematode Growth Medium, the standard solid agar medium [10]. However, the results were inconsistent because zinc tends to precipitate in this medium. To minimize precipitation, Noble Agar Minimal Media (NAMM) was developed [11]. NAMM lacks phosphate buffers, which tend to precipitate with zinc in solution. NAMM supplemented with zinc yields consistent results with increasing concentrations of zinc (Figure 2B). However, NAMM requires a bacterial food source, which is an undefined source of zinc. CeMM can be used to provide a defined amount of zinc in the medium, and Davis et al., (2009) manipulated the concentration of zinc in CeMM to demonstrate that zinc toxicity occurs at high concentrations [12] (Figure 2A). Two approaches have been used to decrease the level of dietary zinc. Davis et al., (2009) manipulated the concentration of zinc in CeMM to reduce dietary zinc, revealing that zinc is an essential nutrient in C. elegans [12] (Figure 2A). A more convenient but less well defined approach is to use the zinc chelator N,N,N′,N′-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine (TPEN), which can cross cellular membranes and is considered an intracellular zinc chelator [13–16]. This chelator can be added to NGM or NAMM medium, and it causes dose dependent zinc deficiency. For example, Figure 2C shows the effects of TPEN on oocyte cell divisions. Reducing the amount of zinc in the diet using defined medium is the gold standard approach for generating zinc deficiency. By contrast, chelators compete with endogenous molecules for binding to zinc that is inside the animal, and chelators are likely to have different concentrations in different cells and subcellular compartments, complicating the interpretation of chelators as a means to achieve zinc deficiency.
Figure 2: Methods to manipulate dietary zinc and quantify and visualize zinc in C. elegans.
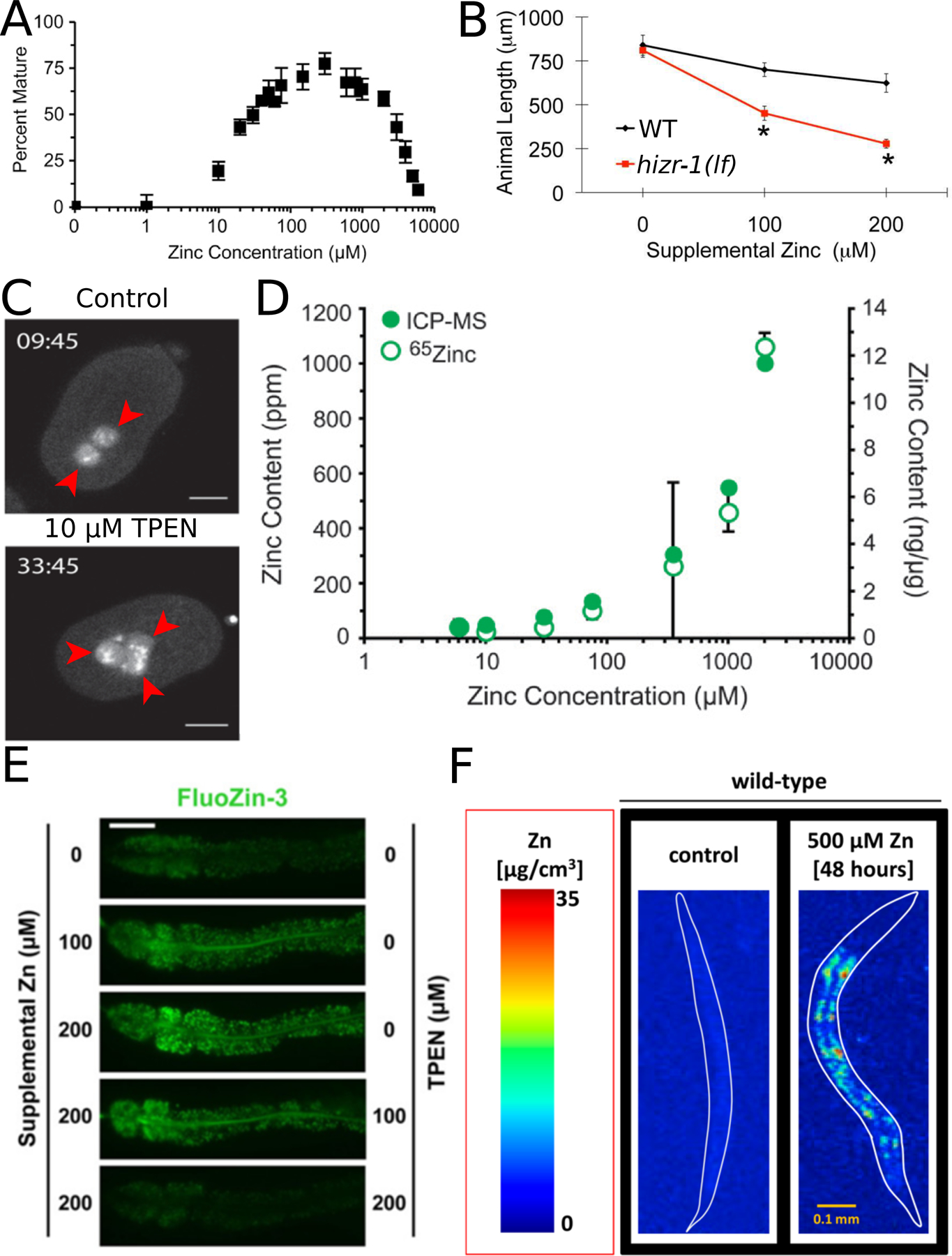
(A) Wild-type worms were cultured in CeMM with different concentrations of added zinc, and maturation was scored by visual inspection. Zinc is necessary for maturation, and high levels of zinc cause toxicity. Adapted from Davis et al., (2009) [12]. (B) Wild type and hizr-1 loss-of-function mutant worms were synchronized as embryos, cultured with the indicated concentrations of supplemental zinc on NAMM for 3 days, and the length of individuals was measured. High levels of zinc inhibit growth, and this is a sensitive method for identifying phenotypic differences in mutants. Adapted from Warnhoff et al., (2017) [33] (C) Embryos were dissected from untreated worms into medium containing 0 (control) or 10 μM TPEN; images are frames from time lapse movies of worms expressing GFP::tubulin and GFP::histone to visualize spindle dynamics. TPEN produces zinc deficient conditions and causes significant delays and abnormalities in cell division. Red arrowheads indicate pronuclei of prophase. Scale bar = 10 μm. Adapted from Mendoza et al., (2017) [15]. (D) Zinc content of mixed-stage wild-type animals. Worms were cultured in CeMM with a range of added zinc, shown on a logarithmic scale. The zinc content was determined by ICP-MS (ppm, closed green circles), or radiolabeled Zn-65 (average ng zinc/µg protein, open green circles). Animals cultured in CeMM with increasing concentrations of zinc display an increase in zinc content. Adapted from Davis et al., (2009) [12]. (E) Fluorescence images of live wild-type hermaphrodites cultured with FluoZin-3 and the indicated levels of supplemental zinc and TPEN. Panels display the anterior half of the intestine of a single animal with pharynx to the left and tail to the right. Scale bar = 50 µm. The FluoZin-3 signal increases with zinc supplementation and decreases with addition of TPEN. Adapted from Roh et al., (2012) [14]. (F) Wild-type animals cultured for 48 hours on NGM with 0 or 500 µM supplemental zinc and assayed for zinc accumulation with spatial resolution by X-ray fluorescence imaging (XFI) at the Stanford Synchrotron Radiation Laboratory. The false color scale indicates the levels of zinc at different positions in the animal. Scale bar = 100 µm. Adapted from Essig et al., (2016) [23].
A second critical technique for studies of zinc biology is the ability to measure the level and distribution of zinc. Several methods have been employed in C. elegans. The level of zinc can be quantified using inductively coupled plasma mass spectrometry (ICP-MS), a sensitive method that can measure the level of many different elements. This method requires a large sample size (thousands of worms), and it provides no spatial information (Figure 2D) [12, 17]. In addition, the level of zinc can be quantified using radioactive zinc, which also requires many worms and provides no spatial information. There are five stable isotopes of zinc, and many synthetic radioactive isotopes. The only experimentally useful radioactive isotope is Zinc-65, which has a half-life of 244 days. Zn-65 radioactivity can be measured in samples of Zn-65 treated worms, and the specific activity of the Zn-65 can be used to calculate the amount of zinc in the sample [12]. For both ICP-MS and radioactive Zn-65, the amount of zinc must be normalized to the amount of sample, which adds another measurement variable to the experiment. For the data shown in Figure 2D, the ICP-MS data is normalized to dry weight (parts per million), whereas the measurement with radioactive Zn-65 is normalized to the protein concentration of the sample (ng zinc per µg protein).
To determine the spatial distribution of zinc, the most useful tools are dyes that emit fluorescence when bound to zinc. The development of these dyes is reviewed in this special issue and previously [18] (Amy Palmer and Chris Chang reviews in this issue). Two fluorescent dyes have been used successfully in C. elegans: FluoZin-3 and Zinpyr-1. FluoZin-3 stains lysosome-related organelles in intestinal cells, which are also called gut granules [14] (Figure 2E). Zinpyr-1 stains the pseudocoelom and developing sperm cells [19–21]. Fluorescent dyes are easy to use and permit live imaging in transparent worms. However, they have significant limitations: they compete for binding with endogenous molecules, and thus only reveal “labile” zinc. The baseline distribution of the dye is not known, so a negative result can be interpreted as an absence of labile zinc or an absence of the dye. Finally, the sophisticated method of X-ray fluorescence allows both quantification and spatial resolution [22, 23] (Figure 2F). However, this technique requires specialized equipment that is only available at National laboratories and thus is not practical for routine analysis.
2. C. elegans contains conserved members of the ZnT/CDF/SLC30A and ZIP/SLC39 families of zinc transporters.
As a positively charged ion, zinc cannot diffuse across membranes. Thus, transporters play an essential role in zinc trafficking. In animals, zinc transporters belong to two main families: the ZnT/CDF/SLC30A family of zinc exporters, and the ZIP/SLC39 family of zinc importers [24] (and Eide, this issue). These families are highly conserved, and members have been identified in bacteria, yeast, fruit flies, zebrafish, plants, nematodes, mouse and humans. Members of these families have been documented to transport zinc, iron, and manganese, so experimental evidence is required to determine the specificity of any given family member. Members of the ZIP/SLC39 family generally possess eight transmembrane domains with a cytoplasmic region between transmembrane domains III and IV [25].These transporters increase the concentration of metal ions in the cytoplasm by importing it from the extracellular space or mobilizing it from inside organelles. The C. elegans genome encodes 14 ZIP transporters: ZIPT-1, ZIPT-2.1, ZIPT-2.2, ZIPT-2.3, ZIPT-2.4, ZIPT-3, ZIPT-7.1, ZIPT-7.2, ZIPT-9, ZIPT-11, ZIPT-13, ZIPT-15, ZIPT-16, and ZIPT-17 (Figure 3B). The ZnT/CDF/SLC30A family of transporters generally possess six transmembrane domains with cytoplasm facing N- and C- termini [26, 27]. These transporters decrease the concentration of metal ions in the cytoplasm by exporting it into the extracellular space or sequestering it in organelles. The C. elegans genome also encodes 14 CDF transporters: CDF-1, CDF-2, TTM-1, SUR-7, F19C6.5, SLC-30A9, F41C6.7, ZK185.5, K07G5.5, PDB1.1, R02F11.3, TOC-1, SLC-30A5, and F56C9.3 (Figure 3A). Many predicted ZIP and CDF transporters in C. elegans have not yet been studied, and here we describe only those family members that have been reported to be involved in zinc biology.
Figure 3: C. elegans CDF and ZIP transporters display orthology with zinc transporters from other species including Homo sapiens.
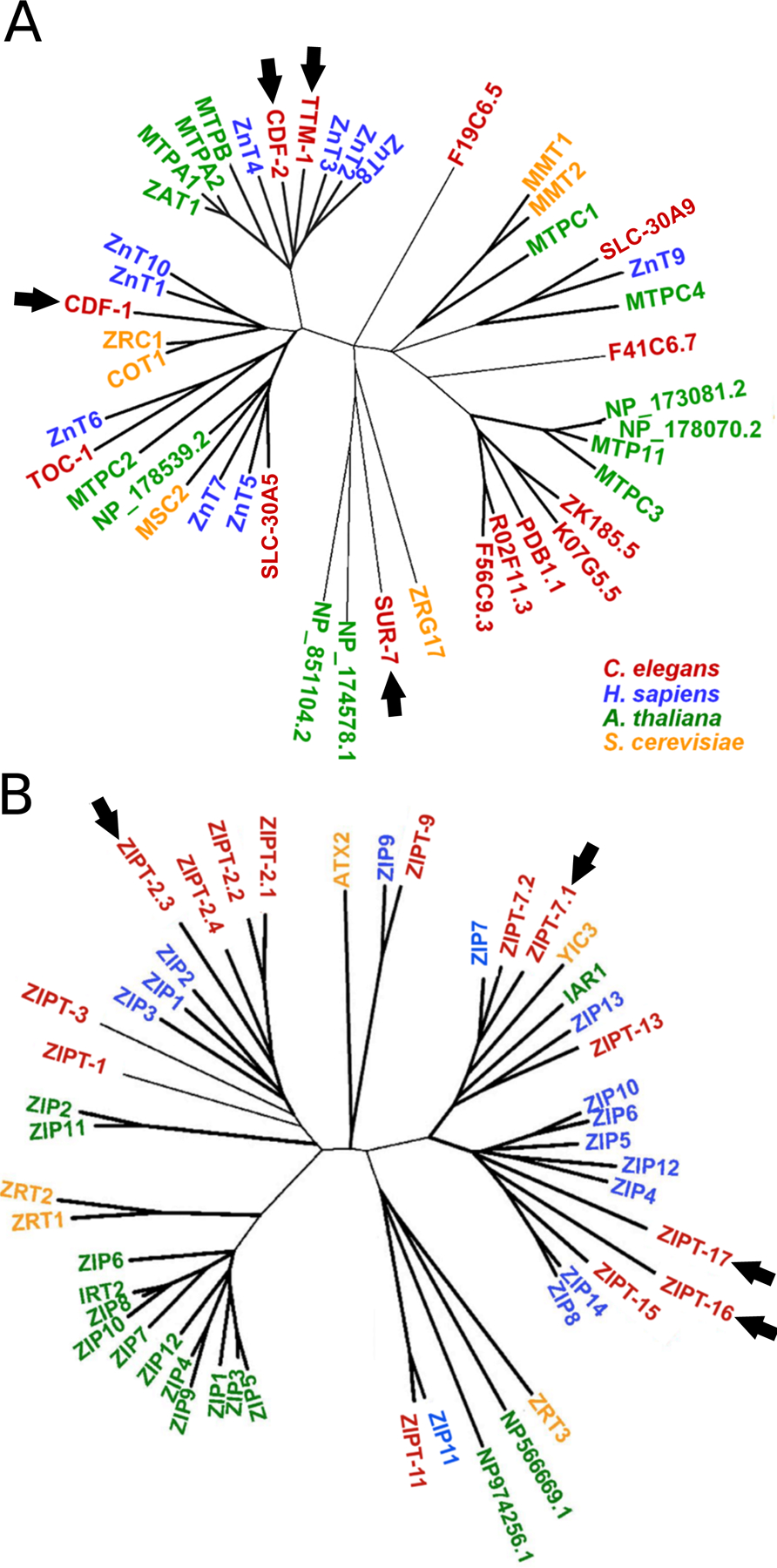
Phylogenetic trees of CDF (A) and ZIP (B) family members identified by PSI-BLAST from C. elegans (red), Homo sapiens (blue), Arabidopsis thaliana (green), and Saccharomyces cerevisiae (yellow). All ten human ZnT proteins in panel A and all 14 human ZIP proteins in panel B cluster with highly related C. elegans proteins. Transporters discussed in this review are indicated by arrows. Adapted from Roh et al., 2013 [20] (A) and Dietrich et al., (2017) [13] (B).
2.1. CDF-1:
cdf-1 is the first zinc transporter characterized in C. elegans. A loss-of-function mutation was identified in a forward genetic screen for suppressors of the multivulval (Muv) phenotype caused by constitutively active let-60 ras signaling, and the gene was identified by positional cloning [10, 28]. The CDF-1 protein has about 30% sequence identity with mouse ZnT-1 and yeast COT1. Mammalian ZnT-1 localizes to the plasma membrane and is a zinc exporter [26], and rat ZnT-1 expressed in C. elegans can rescue the cdf-1(lf) mutant phenotype, demonstrating evolutionary conservation of function. Genetic analysis indicated that cdf-1 is necessary for proper cell fate specification of the vulval precursor cells, and cdf-1 acts as a positive regulator of the Ras signaling pathway. The CDF-1 protein expression pattern was characterized using transgenic animals that express CDF-1::GFP fusion protein under the control of the endogenous cdf-1 promoter. CDF-1 is expressed in multiple cell types, including vulval cells and intestinal cells. In intestinal cells, CDF-1 is localized to the basolateral surface, suggesting it transports zinc from the cytoplasm of intestinal cells into the body cavity, called the pseudocoelom in worms [20].
To directly test the role of CDF-1 in zinc biology, Bruinsma et al., (2002) cultured cdf-1(lf) mutant animals in supplemental zinc [10]. cdf-1(lf) mutants displayed hypersensitivity to high zinc toxicity, including reductions of individual growth rate and population growth rate. The mutants displayed increased levels of zinc compared to wild type, indicating cdf-1 is necessary to limit zinc accumulation in the animal [12, 20]. Furthermore, cdf-1(lf) mutants displayed reduced zinc in the body cavity [20]. Thus, CDF-1 functions to transport zinc from the cytoplasm of intestinal cells into the body cavity to supply interior tissues with zinc. CDF-1 also appears to function in vulval precursor cells to control the concentration of zinc, as described below.
2.2. SUR-7:
Yoder et al., (2004) identified a loss-of-function mutation in sur-7 (Suppressor of Ras) in a forward genetic screen for suppressors of the Muv phenotype caused by constitutively active let-60 ras signaling, and the gene was identified by positional cloning [29]. This genetic screen was similar to the genetic screen used to identify cdf-1. The predicted SUR-7 protein has six predicted transmembrane domains, similar to other CDF proteins. Unlike other CDF proteins, it does not contain a histidine loop between predicted transmembrane domains IV and V. Instead SUR-7 possesses a cluster of five histidine residues between predicted transmembrane domains III and IV. SUR-7 is not closely related to any human CDF protein. A sur-7 partial loss-of-function mutation causes hypersensitivity to high zinc toxicity [12, 20, 29]. SUR-7 protein is localized to intracellular membranes, but the specific organelle has not been identified by colocalization with a known marker. Thus, sur-7 is necessary for high zinc resistance and it functions to promote Ras signaling during vulval development.
2.3. CDF-2:
The discoveries of cdf-1 and sur-7 in forward genetic screens initiated the analysis of the CDF family in worms and led to the appreciation that there are 12 additional CDF encoding genes in the C. elegans genome (Figure 3A). Two of these genes, CDF-2 and TTM-1, are similar to a cluster of human proteins–ZnT2, ZnT3, ZnT4 and ZnT8–that are localized to vesicular membranes in vertebrates. Based on this clustering, Davis et al., (2009) initiated a reverse genetic study of cdf-2 [12]. The predicted CDF-2 protein contains six predicted transmembrane-spanning domains and two histidine motifs (HX)3 in the loop between the fourth and fifth transmembrane segments and is most similar to ZnT2. To understand how CDF-2 is regulated by zinc, Davis et al., (2009) measured cdf-2 mRNA abundance in wild-type animals by RT-PCR [12]. cdf-2 mRNA abundance was increased by culturing animals in high dietary zinc, indicating that cdf-2 transcription is induced by high zinc [20]. The expression pattern of CDF-2 protein was analyzed using a CDF-2::GFP fusion protein in transgenic animals. CDF-2 is expressed only in intestinal cells, and it localized to membrane-bound vesicles in larvae and adults [12]. These abundant vesicles are called gut granules, and they are a type of lysosome-related organelles since they stain with the marker LysoTracker [14] (Figure 5A). This led to the model that CDF-2 transports zinc from the cytoplasm into the lumen of lysosome-related organelles (Figure 4C).
Figure 5: High Zinc Induces the Formation of Asymmetric Bilobed Gut Granules.
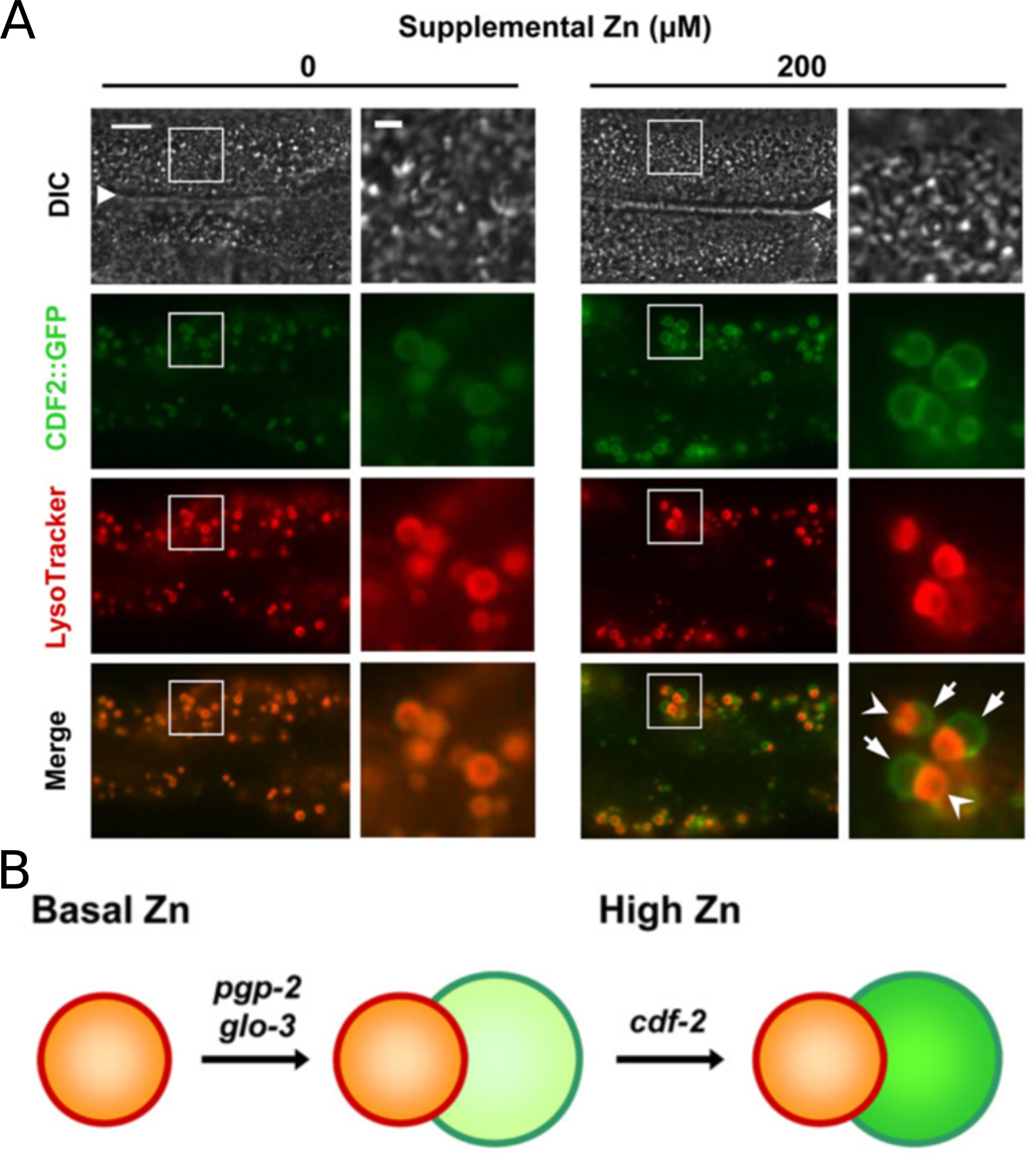
(A) Fluorescence images of live transgenic animals expressing CDF-2::GFP cultured with LysoTracker and the indicated levels of supplemental zinc. The differential interference contrast (DIC) images show the intestinal lumen (white triangle) and adjacent intestinal cells with pharynx to the left and tail to the right. Boxed regions are magnified in the right panels. With 200 µM supplemental zinc, many gut granules appear to be bilobed and asymmetric; one side is positive for CDF-2::GFP and LysoTracker (arrowhead), whereas the other side is positive for CDF-2::GFP and negative for LysoTracker (arrow). Scale bars: 10 µm and 2 µm (boxed regions). Adapted from Roh et al., (2012) [14]. (B) A genetic pathway for the formation of bilobed gut granules. The activity of granule biogenesis genes pgp-2 and glo-3 are necessary for the formation of bilobed granules. The zinc transporter cdf-2 is necessary for loading zinc into granules (indicated by dark green). Adapted from Roh et al., (2012) [14].
Figure 4: High zinc homeostasis in C. elegans.
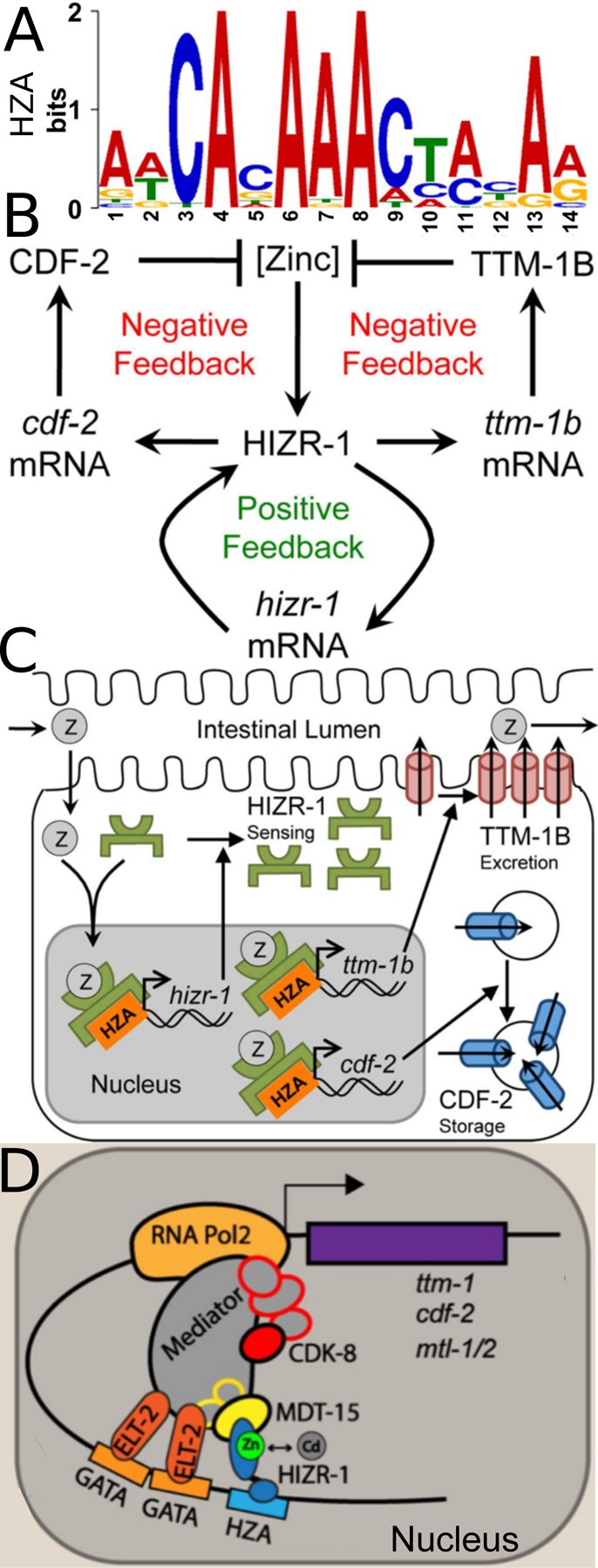
(A) A position weight matrix of the High Zinc Activation (HZA) promoter element based on 29 sequences. The height of the nucleotides at each position represents the frequency scaled in bits. Adapted from Roh et al., (2015) [36]. (B) A Genetic model of high zinc homeostasis in the C. elegans intestine. High levels of zinc promote HIZR-1 activity and transcriptional activation of multiple genes including cdf-2, ttm-1b and hizr-1. Increased levels of cdf-2 and ttm-1b mRNA promote increased levels of CDF-2 and TTM-1B protein, which reduce levels of cytoplasmic zinc in a parallel negative feedback circuit. Increased levels of hizr-1 mRNA promote increased levels of HIZR-1 protein, creating a positive feedback circuit that enhances the negative feedback system. Adapted from Warnhoff et al., (2017) [33]. (C) A molecular model. Dietary zinc (Z) enters intestinal cells, binds the ligand-binding domain of HIZR-1, and promotes nuclear accumulation, HZA enhancer binding, and transcriptional activation. The nuclear accumulation of HIZR-1 could result from increased HIZR-1 protein levels due to autoregulation and/ or translocation of HIZR-1 from the cytoplasm to the nucleus. Increased levels of CDF-2 and TTM-1B promote zinc detoxification by sequestration in lysosome-related organelles [14] and excretion into the intestinal lumen [20], respectively. Increased levels of HIZR-1 promote homeostasis by a positive feedback circuit. Adapted from Warnhoff et al., (2017) [33]. (D) A model of the transcriptional activation complex assembled on the cdr-1 promoter in response to high zinc or cadmium. In intestinal cells, MDT-15 cooperates with HIZR-1 bound to the HZA element to induce the metal-sequestering metallothioneins (mtl-1 and −2) and the transporter cdf-2. ELT-2 binds GATA promoter elements to promote intestinal expression of genes and may also contact Mediator to regulate cadmium or zinc responsive transcription. Adapted from Shomer et al., (2019) [37].
The function of cdf-2 was investigated using a deletion mutation that causes a strong loss of function. cdf-2(lf) mutant animals are hypersensitive to high zinc toxicity, indicating CDF-2 promotes resistance to high zinc. cdf-2(lf) animals displayed reduced total zinc in the body, indicating CDF-2 promotes zinc storage [12, 14, 20]. The zinc dye FluoZin-3 stains lysosome-related organelles in intestinal cells, indicating this is a site of zinc storage (Figure 2E). cdf-2(lf) mutant animals displayed reduced FluoZin-3 staining, consistent with the model that CDF-2 transports zinc into these organelles. Overexpression of CDF-2 increased FluoZin-3 staining, indicating CDF-2 is sufficient to promote zinc storage [14]. Thus, CDF-2 is specifically localized to the membrane of lysosome-related organelles, and it functions to sequester zinc in these organelles.
2.4. TTM-1:
Like CDF-2, the predicted TTM-1 protein is most similar to human ZnT2, ZnT3, ZnT4, and ZnT8 (Figure 3A) [20]. The ttm-1 gene contains two transcription initiation sites and encodes two transcripts: ttm-1a initiates at the upstream site, whereas ttm-1b initiates at the downstream site. Both TTM-1A and TTM-1B proteins possess the conserved six transmembrane motifs and a conserved zinc-binding histidine-rich motif (HX)n in the loop between transmembrane domains IV and V, like ZnT2 and CDF-2. However, TTM1-A and TTM1-B have different N-terminal amino acid sequences; TTM-1B contains an additional histidine-rich motif, raising the possibility that the N-terminus binds zinc. To understand how TTM-1 is regulated by zinc, Roh et al., (2013) measured ttm-1 mRNA abundance in wild type animals by RT-PCR [20]. ttm-1b mRNA abundance was increased by culturing animals in high dietary zinc, indicating that ttm-1b transcription is induced by high zinc, whereas ttm-1a was not regulated by dietary zinc [20]. The expression patterns of TTM-1A and TTM-1B proteins were analyzed using GFP fusion proteins in transgenic animals. TTM-1A::GFP was expressed in the intestine and hypodermis, whereas TTM-1B::GFP was expressed in the intestine, head neurons, seam cells, hypodermis and vulva [20]. Thus, both proteins are expressed in the intestine, but each was differentially expressed in other tissues. TTM-1B::GFP in the intestine is specifically localized to the apical side of the cell, facing the intestinal lumen. TTM-1B expression in the intestine increases in animals exposed to supplemental zinc and is barely detectible in control conditions. These results led to the model that TTM-1B transports zinc from the cytoplasm into the lumen of the intestine (Figure 4C). TTM-1A is localized to vesicles in intestinal cells that are distinct from lysosome-related organelles and have not been well characterized.
The function of ttm-1 was investigated using a deletion mutation that affects both transcripts and causes a strong loss of function. ttm-1(lf) mutant animals are hypersensitive to high zinc toxicity in sensitized genetic backgrounds lacking cdf-2 or genes involved in lysosome biogenesis such as pgp-2 and glo-1, indicating TTM-1 promotes resistance to high zinc in a manner that is redundant with these genes. ttm-1(lf) animals displayed increased total zinc in the body, indicating ttm-1 promotes zinc excretion. The zinc dye Zinpyr-1 stains zinc in the body cavity. ttm-1(lf) mutant animals displayed increased Zinpyr-1 staining, consistent with the model that TTM-1 transports zinc into the lumen of the intestine for excretion, and in the absence of ttm-1 excess zinc accumulates in the body cavity.
2.5. ZIPT-2.3:
Dietrich et al., (2017) identified zipt-2.3 as a gene that is transcriptionally activated by zinc deficiency and showed that the zipt-2.3 promoter has an Low Zinc Activation (LZA) enhancer (see section 4) that mediates this regulation [13]. The zipt-2.3 promoter fused to GFP displays expression in intestinal cells in transgenic animals, indicating this is the tissue where zipt-2.3 is expressed [13]. Chapman et al., (2019) used a translational fusion of ZIPT-2.3::GFP to show expression in intestinal cells with subcellular localization in puncta [30]. A genetic analysis using RNAi and a deletion mutation showed that zipt-2.3(lf) causes resistance to ionizing radiation-induced apoptosis in the germ line. Treatment with supplemental zinc inhibited ionizing radiation-induced apoptosis in the germ line, whereas treatment with the chelator TPEN enhanced this process. Chapman et al., (2019) conclude that zinc levels influence ionizing radiation-induced apoptosis in the germ line, and zipt-2.3 acts cell non-autonomously in intestinal cells to control apoptosis in the germ line [30].
2.6. ZIPT-7.1:
The predicted ZIPT-7.1 and ZIPT-7.2 proteins are highly related to human ZIP-7 (Figure 3B). Dietrich et al., (2017) identified zipt-7.1 as a transcript that is induced by zinc deficiency, suggesting it plays a role in zinc biology [13]. Zhao et al., (2018) conducted a reverse genetic study of zipt-7.1, which indicated this gene is involved in zinc biology during the process of sperm activation [21]. A strong loss-of-function mutation in zipt-7.1 causes fertility defects including small brood size, sterility and a large number of unfertilized oocytes. These phenotypes suggest that zipt-7.1(lf) mutant hermaphrodites generate normal oocytes and dysfunctional sperm. Male sperm also require zipt-7.1 function, since zipt-7.1(lf) males are partially sterile. Spermatids (inactive sperm) display a round morphology and are immotile until they are activated. They then extend a pseudopod which allows them to crawl towards the oocytes. Wild type spermatids extend pseudopods in response to activating signals, but zipt-7.1(lf) mutant spermatids are defective in pseudopod extensions [21].
The localization of ZIPT-7.1 was determined by analyzing zipt-7.1 RNA levels in mutants that lack a germline and ZIPT-7.1::GFP fusion protein localization in transgenic animals. ZIPT-7.1 is expressed in the germ line and appears to localize to intracellular membranes. When expressed in mammalian cells, ZIPT-7.1 promoted the uptake of zinc into the cells, demonstrating that ZIPT-7.1 is a zinc transporter [21].
2.7. ZIPT-16 and ZIPT-17:
Kumar et al., (2016) reported that zinc levels influence lifespan, with high dietary zinc shortening lifespan and zinc deficiency caused by the chelator TPEN lengthening lifespan [31]. These effects were observed in a variety of mutant backgrounds to varying degrees. To understand how lifespan might be affected by zinc levels, Novakovic et al., (2020) analyzed zipt genes and showed that zipt-16(lf) and zipt-17(lf) mutants displayed shorter lifespans compared to wild type [32]. However, it remains to be established if these transporters function by transporting zinc or another metal ion.
3. High Zinc Homeostasis
Because excess zinc is toxic, animals have evolved homeostatic mechanisms to respond to high zinc. C elegans has been an important model system for understanding this process. High zinc homeostasis is mediated by the intestine, which appears to control the level of zinc in other tissues by regulating excretion from the basolateral surface of intestinal cells into the body cavity of the animal.
3.1. The nuclear receptor HIZR-1 is the high zinc sensor:
Intestinal cells import zinc by a mechanism that has yet to be defined. While the ZIP family of importers seems like good candidates for the intestinal zinc importer, this has not been experimentally determined. In response to a high zinc diet, zinc levels increase in intestinal cells, and the HIZR-1 nuclear receptor becomes activated. The hizr-1 gene was discovered by Warnhoff et al., (2017) in a forward genetic screen for animals that do not correctly regulate the cdf-2 promoter in response to high dietary zinc [33]. To conduct this screen, the cdf-2 promoter was fused to GFP in transgenic animals; in standard medium these animals do not display significant GFP fluorescence. By contrast, in zinc excess conditions the cdf-2 promoter is activated, and these animals display strong GFP fluorescence, providing a visual readout of high zinc activated transcription. Warnhoff et al., (2017) identified five loss-of-function mutations that abrogated cdf-2 activation and one gain-of-function mutation that constitutively activated cdf-2 expression in zinc replete conditions. Positional cloning revealed that all six mutations affected the same gene, which was named High Zinc activated nuclear Receptor (hizr-1) [33]. The loss-of-function mutations resulted in truncated proteins or changed highly conserved residues in the DNA-binding domain. The gain-of-function mutation causes a single amino acid substitution in the ligand-binding domain. hizr-1(lf) mutants are defective in the transcriptional activation of multiple endogenous genes that are activated by high dietary zinc. Furthermore, hizr-1(lf) mutations cause hypersensitivity to the toxic effects of high zinc (Figure 2B). Thus, hizr-1 is necessary for the transcriptional response to high zinc and plays a critical role in high zinc resistance.
Nuclear receptors have two conserved domains: a ligand-binding domain and a zinc-finger DNA-binding domain. Nuclear receptors were first described for their role in endocrine signaling, where the ligand-binding domain directly interacts with large hydrophobic hormones such as estrogens and androgens [34, 35]. More recently, fatty acid molecules have been shown to function as ligands for nuclear receptors. Upon ligand binding, nuclear receptors typically translocate to the nucleus, interact with specific sequences through the DNA-binding domain, and activate transcription of target genes. Warnhoff et al., (2017) hypothesized that zinc directly bound the ligand-binding domain of HIZR-1 to activate the receptor [33]. Indeed, the ligand-binding domain of HIZR-1 was partially purified and demonstrated to bind radioactive Zn-65 with high affinity and specificity. Furthermore, Warnhoff et al., (2017) [33] showed that high dietary zinc causes a HIZR-1::GFP fusion protein to accumulate in intestinal nuclei. The analysis of HIZR-1 identified a nuclear receptor as a new class of zinc sensor and zinc as a new class of ligand for nuclear receptors.
3.2. HIZR-1 binds the HZA enhancer to mediate transcriptional activation in high zinc:
To investigate the mechanism of high zinc activated transcription, Roh et al., (2015) analyzed the promoters of several regulated genes searching for a common sequence element. This led to the identification of the High Zinc Activation (HZA) element [36]. The HZA motif spans 14 base pairs and contains five highly conserved positions (Figure 4A). The promoter regions of two CDF family zinc transporters (cdf-2 and ttm-1b) and the two metallothionein genes (mtl-1 and mtl-2) all contained this conserved promoter element. Mutational studies showed the HZA was necessary for transcriptional activation by high zinc. Furthermore, a short sequence containing the HZA was sufficient to confer activation by high zinc on a basal promoter. A second sequence element called GATA was also found in these promoters adjacent to the HZA; the GATA element promotes tissue specific expression in intestinal cells by interacting with ELT proteins such as ELT-2 [36–38]. Mutational studies showed the GATA element was also necessary for high zinc activated transcription. The HZA element and the GATA element function like enhancers, since they can work at a range of distances from the transcription start site and in either orientation. Shomer et al., (2019) recently extended this work by showing the HZA and GATA elements are necessary for high zinc regulation of the cdr-1 promoter [37]. Warnhoff et al., (2017) tested the hypothesis that HIZR-1 directly binds the HZA enhancer. Indeed, the partially purified DNA-binding domain of HIZR-1 interacted with high specificity with the HZA DNA element in an electrophoretic mobility shift assay [33]. Warnhoff et al., (2017) concluded that in response to high zinc HIZR-1 translocates to the nucleus and directly binds the HZA enhancer. Interestingly, the promoter of hizr-1 has an HZA enhancer, and the hizr-1 gene is transcriptionally activated by high zinc. Thus, HIZR-1 engages in a positive feedback loop by activating its own promoter, which leads to higher levels of HIZR-1 activity (Figure 4B,C).
The discovery of the HIZR-1/HZA interaction has led to a rapid advance in understanding the transcriptional complex that assembles on the promoters of zinc activated genes. The mediator complex contains multiple proteins that connect site-specific DNA binding transcription factors to RNA polymerase 2. Taubert et al., (2008) showed that mdt-15, a component of the mediator complex, is necessary for a wide range of transcriptional activation in response to exogenous chemicals, including cadmium [39]. Roh et al., (2015) showed that mdt-15 is necessary for a promoter with the HZA enhancer to respond to high zinc and cadmium [36]. Most recently Shomer et al., (2019) proposed a detailed molecular model of the transcriptional complex that integrates these different elements (Figure 4D) [37]. Using a cdr-1p::gfp promoter fusion, mdt-15, cdk-8, hizr-1, and elt-2 were shown to be necessary for cadmium- and zinc-induced transcription. Interestingly, MDT-15 and HIZR-1 proteins were shown to bind in the Yeast-2-hybrid system, and the strength of the interaction was enhanced by zinc or cadmium. Overall, the current model is that in response to high levels of zinc, the ligand-binding domain of HIZR-1 binds zinc, resulting in translocation to the nucleus. The DNA-binding domain of HIZR-1 binds the HZA enhancer, and ELT-2 binds the adjacent GATA element. HIZR-1 interacts directly with MDT-15, which recruits the mediator complex and RNA polymerase to the promoter to increase the rate of transcriptional initiation (Figure 4D).
3.3. The HIZR-1 target genes cdf-2 and ttm-1 promote homeostasis by storing zinc in lysosome-related organelles and excreting zinc into the intestinal lumen:
Two HIZR-1 target genes have been demonstrated to play important functional roles in high zinc detoxification: cdf-2 and ttm-1 [12, 14, 20]. The promoters of both genes contain HZA enhancers, and transcription is increased in response to high zinc [36]. The CDF-2 transporter is localized specifically to lysosome-related organelles in intestinal cells, which are also called gut granules, and CDF-2 functions to transport zinc into these organelles. These organelles are the major site of zinc storage in C. elegans, and they stain with the zinc dye FluoZin-3. Mutations in cdf-2 or glo genes that are essential for the biogenesis of lysosomes both cause hypersensitivity to high zinc. Thus, these organelles sequester and thereby detoxify excess zinc. Lysosome-related organelles undergo morphological changes in response to increasing concentrations of zinc. In zinc replete conditions, they appear to be spherical, and CDF-2 and LysoTracker colocalize (Figure 5A). In zinc excess conditions, they appear to have two compartments and are referred to as bilobed. CDF-2 is localized on both compartments, whereas LysoTracker only stains one of the lobes (Figure 5B). The function of this morphological change has yet to be determined and is an important goal of future work.
The CDF family transporter TTM-1B localizes specifically to the apical surface of intestinal cells, so it is positioned to excrete zinc into the lumen of the intestine. Genetic studies document that ttm-1 function promotes resistance to high zinc toxicity, but this phenotype is only observed in sensitized background such as the cdf-2;ttm-1 double mutant [20]. CDF-2 and TTM-1 function in a parallel negative feedback circuit (Figure 4B). Both genes are transcriptionally activated by high levels of cytoplasmic zinc, and both proteins function to reduce the level of cytoplasmic zinc, either through sequestration in lysosome-related organelles or excretion into the lumen of the intestine.
Metallothioneins in nematodes have been reviewed previously [40]. The C. elegans genome encodes two metallothionein genes: mtl-1 and mtl-2 [41–43]. Both genes are strongly induced by high levels of dietary zinc, and both promoters contain an HZA enhancer [36]. The biochemical activity of MTL-1 and MTL-2 as metal-binding proteins is well established [44, 45]. However, the function of these proteins in vivo remains obscure. Genetic analysis of mtl-1 and mtl-2 single mutants, as well as an mtl-1;mtl-2 double mutant, indicates that these strains are similar to wild type; in some experiments they display slight hypersensitivity to high zinc or cadmium toxicity, and in some experiments they are indistinguishable from wild type [46–48]. Recently, a gene family that encodes cysteine-rich proteins was discovered at a single locus of chromosome IV; four of these genes are expressed in the alimentary tract in response to zinc, and there is evidence that these proteins directly bind zinc and potentially other metals [49].
4. Low zinc homeostasis
Because zinc is an essential nutrient, it is critical for organisms to acquire adequate zinc for cellular and organismal function. Thus, mechanisms have evolved to mediate low zinc homeostasis. However, compared to high zinc homeostasis, much less is known about low zinc homeostasis in animals, probably because experimentally it is easier to add supplemental zinc than it is to create zinc deficiency. To begin to investigate the response to zinc deficiency in worms, Dietrich et al., (2017) analyzed the 14 predicted C. elegans zipt genes for transcriptional activation in response to zinc deficiency, reasoning that genes activated by zinc deficient conditions might play a role in low zinc homeostasis [13]. zipt-7.1, zipt-2.1 and zipt-2.3 each displayed significantly higher levels of transcripts in response to zinc deficiency caused by TPEN treatment. To understand the mechanism of regulation, Dietrich et al., (2017) searched for a common sequence element in the promoters of these three genes, resulting in the identification of the low zinc activation (LZA) element [13]. The LZA motif spans 19 base pairs and contains nine highly conserved positions (Figure 6A). Mutational analysis showed that the LZA enhancer is both necessary in the zipt-2.3 promoter and sufficient in a basal promoter to mediate transcriptional activation during zinc deficiency (Figure 6B,C). Remarkably, the zipt-2.3 promoter containing the LZA enhancer was activated by zinc deficiency in human cells, suggesting that this mechanism of low zinc homeostasis might be conserved in mammals [13]. By searching for LZA enhancers in the genome, Dietrich et al., (2017) identified four additional genes that are transcriptionally activated by zinc deficiency. The function of these genes during zinc deficiency has yet to be determined. Although no genes have been rigorously demonstrated to mediate low zinc homeostasis, Roh et al., (2012) showed that lysosome-related organelles release stored zinc during zinc deficient conditions, which is a mechanism of low zinc homeostasis [14].
Figure 6: Low zinc homeostasis in C. elegans.
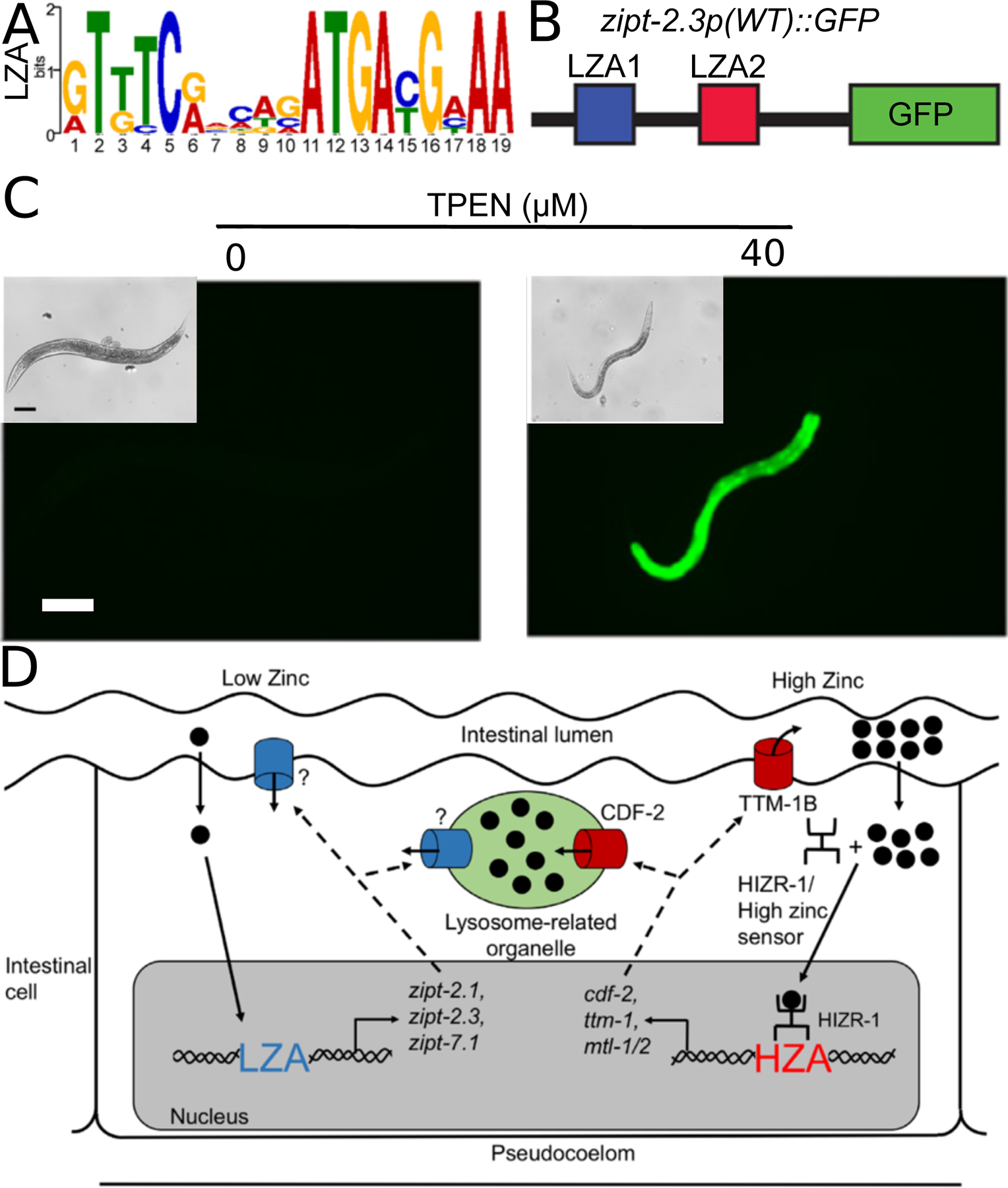
(A) A position weight matrix of the LZA element based on 12 sequences. The height of each nucleotide represents the frequency scaled in bits. Adapted from Dietrich et al., (2017) [13]. (B,C) Diagram of the zipt-2.3 promoter containing LZA1 (blue) and LZA2 (red) fused to GFP (green). Images show transgenic animals expressing this construct at the young adult stage cultured on the indicated concentration of TPEN. Bright field images (insets) show worm morphology, and fluorescent images show GFP fluorescence. GFP signals were captured with identical settings and exposure times. zipt-2.3 transcription is activated in response to zinc deficient conditions. Scale bars = 100 µm in brightfield (insets) and fluorescence images. Adapted from Dietrich et al., (2017) [13]. (D) Diagram of an intestinal cell flanked by the intestinal lumen (above) and the pseudocoelom (below). Two dietary zinc-conditions are illustrated: deficiency (left) and excess (right). In the presence of low dietary zinc (black circles), an undefined sensing mechanism causes activation of LZA element containing promoters. ZIP proteins (blue) might localize to the plasma membrane or lysosome related organelles (LRO, green), but the subcellular localization has not been established. In the presence of high dietary zinc, the HIZR-1 nuclear receptor causes activation of HZA element containing promoters. CDF-2 protein (red) localizes to the LRO where it functions to store zinc, and the TTM-1B protein (red) localizes to the apical plasma membrane where it functions to excrete zinc into the intestinal lumen. Adapted from Dietrich et al., (2017) [13]
A critical question is what transcription factor binds the LZA enhancer, and does this transcription factor function as the low zinc sensor? Although this remains an open question, Dietrich et al., (2017) demonstrated that the transcriptional activation of the zipt-2.3 promoter in zinc deficient conditions requires the mediator gene mdt-15 and the elt-2 gene. Thus, the LZA and the HZA may both recruit the mediator complex and both utilize the ELT-2/GATA element to achieve intestinal cell specificity. Overall, it appears there are parallel systems for high and low zinc homeostasis. High zinc is sensed by HIZR-1, which acts through the HZA enhancer. Low zinc is sensed by an unknown factor, that acts directly or indirectly through the LZA to activate transcription (Figure 6D).
5. Zinc signaling and cell fate determination in C. elegans
In addition to the well-established roles for zinc as a protein-binding cofactor, it has been proposed that zinc can also function as a signaling molecule [50–52]. One of the possible signaling mechanisms is zinc acting as an intracellular second messenger, similar to calcium [53]. These proposed mechanisms require dynamic changes in the concentration of zinc. In C. elegans, zinc transporters have been shown to be involved in at least two cell fate determination processes: vulval development [10] and sperm activation [21]. In both cases, manipulation of environmental zinc levels also affects the phenotype [10, 19]. These results suggest that zinc could function as a second messenger or have a specific and dynamic role in regulating the activity of a signaling protein. The alternative explanation for these types of results is that cell differentiation in these cases is sensitive to zinc levels in general, and these phenotypes represent a more general dysfunction caused by an abnormal zinc environment.
5.1. The Ras signaling pathway during vulval development:
The C. elegans vulva is a specialized epithelial opening on the ventral side of the organism that connects the worm gonad to the exterior (Figure 1). The vulva is required for both the transfer of the male sperm during copulation and laying fertilized embryos into the environment. The development of the vulva has been extensively studied as an example of organogenesis [54]. These studies have made significant contributions to the understanding of the signaling pathways that control the precise formation of the organ. One of the pathways involved in this process is the receptor tyrosine kinase/Ras/ERK MAP kinase signaling pathway. In the ventral epidermis of larval worms, six ectodermal blast cells (P3.p, P4.p, P5.p, P6.p, P7.p, and P8.p) are collectively known as the vulval precursor cells. In wild-type animals, the progeny of three of the vulval precursor cells adopt either the 1° (P6.p) or the 2° (P5.p and P7.p) cell fate and become the vulval cells, while the others adopt the 3° non-vulval cell fate and fuse with the hypodermal syncytium [55, 56]. The vulval cell fates depend on a LIN-3/ Epidermal growth factor inductive signal from the anchor cell of the somatic gonad. LIN-3 activates the EGF receptor LET-23 in the P6.p cell, which then leads to the activation of a Ras-mediated signaling pathway. As a consequence of Ras pathway activation, P6.p adopts the 1° cell fate and then signals to P5.p and P7.p instructing them to adopt the 2° cell fate [54]. In mutant animals with a gain-of-function of let-60/Ras that makes the protein constitutively active, other vulval precursor cells also adopt the vulval cell fate, resulting in a multivulva (Muv) phenotype [57].
The first two zinc transporters identified in C. elegans, CDF-1 and SUR-7, were discovered in forward genetic screens as suppressors of the gain-of-function let-60/Ras Muv phenotype [28, 29]. As described in section 2, both CDF-1 and SUR-7 are CDF transporters that likely reduce the level of cytoplasmic zinc. Thus, the finding that loss-of-function mutations in these genes reduce Ras signaling activity suggest that high cytoplasmic zinc levels function downstream of RAS to inhibit the Ras signaling pathway. This conclusion is supported by the finding that supplementing zinc in the growth medium also suppresses the let-60(gf) Muv phenotype, and that the suppression could be reversed by CDF-1 overexpression [10]. Further epistasis studies suggest that zinc inhibits the signaling pathway downstream of LET-60 RAS but upstream of MPK-1 ERK [10, 29]. The mechanism appears to be conserved during evolution, as mammalian ZnT1 can partially rescue the phenotype. In addition, expressing either the mammalian ZnT1 or the C. elegans CDF-1 can stimulate Ras signaling in Xenopus oocytes [10]. It is currently unknown precisely how zinc inhibits the Ras signaling pathway. Yoder et al., (2004) [29] proposed that the mechanism is related to the phosphorylation of KSR-1, while Jirakulaporn and Muslin [58] proposed that CDF-1 directly binds Raf-1 to regulate its activity.
As described above, Chapman et al., (2019) showed that ZIPT-2.3 activity modulates germ line apoptosis caused by ionizing radiation [30]. The germ cells utilize the same Ras/Raf/MEK/ERK signaling pathway as the vulval precursor cells. Chapman et al., (2019) proposed that zinc functions downstream of LET-60 RAS but upstream of MPK-1 ERK in the germ line to inhibit apoptosis. As with the studies of vulval development, the precise mechanism of zinc inhibition has not been established, and the possibility that zinc plays a more general role in inhibiting cellular function has not been rigorously excluded.
5.2. Sperm activation:
In C. elegans, sperm acquire motility through a process known as sperm activation (or spermiogenesis), in which the round spermatid is transformed into a spermatozoon that crawls with a pseudopod [59–61]. Both premature and delayed activation is detrimental to reproductive success, highlighting the importance of tightly controlling this differentiation process [62, 63]. While many genes have been identified to be involved in the sperm activation process, the extracellular signals that activate sperm are still not well defined. However, the transduction of the sperm activation signal must occur in the cytoplasm and cannot utilize changes in transcription, because the sperm DNA is transcriptionally silenced in preparation for delivery to the egg [64]. Various molecules have been shown to be able to activate sperm in vitro, one of which is zinc.
Liu et al., (2013) showed that extracellular zinc can activate dissected sperm in vitro; this effect is specific, since other divalent cations did not activate sperm [19]. The zinc-induced sperm activation depends on the spe-8 pathway, since sperm with mutations in the spe-8 pathway failed to be activated by zinc. The role of zinc in sperm activation is likely to be physiological, since Zhao et al., (2018) showed that the zinc transporter ZIPT-7.1 is involved in this process. zipt-7.1 mutant animals have a sterile phenotype that results from a sperm activation defect. In vitro activation experiments showed that zipt-7.1 mutant sperm are defective in responding to extracellular zinc [21]. Zhao et al., (2018) proposed that zinc is used as an intracellular second messenger during sperm activation, and extracellular zinc activates sperm by mimicking the intracellular zinc release [21]. The model proposes that in spermatids (non-activated sperm), zinc is stored in intracellular vesicles. This stored zinc is then released by the ZIPT-7.1 transporter upon receiving the sperm-activating signal. The intracellular zinc release increases the cytoplasmic zinc concentration and presumably binds to zinc-regulated proteins, leading to downstream activation processes that include the extension of the pseudopod and the fusion of the intracellular vesicles with the membrane (Figure 7) [21]. Because sperm biology requires a rapid response to an extracellular signal, and the signal cannot depend on a transcriptional response, it seems like an ideal setting to employ zinc as a second messenger in the cytoplasm. Furthermore, since zinc has the positive effect of activating sperm, rather than the negative effect of inhibiting a process, it seems less likely that these experimental results reflect a general dysfunction caused by an abnormal zinc environment. However, to firmly establish zinc signaling as a mechanism of sperm activation, it is important to visualize the dynamic change in zinc concentration and identify the target proteins that are affected by the released zinc. Another important goal is to identity the mechanism whereby the sperm activation signal triggers the activation of ZIPT-7.1, perhaps by a posttranslational modification that affects the activity of this zinc transporter.
Figure 7: ZIPT-7.1 mediates sperm activation.
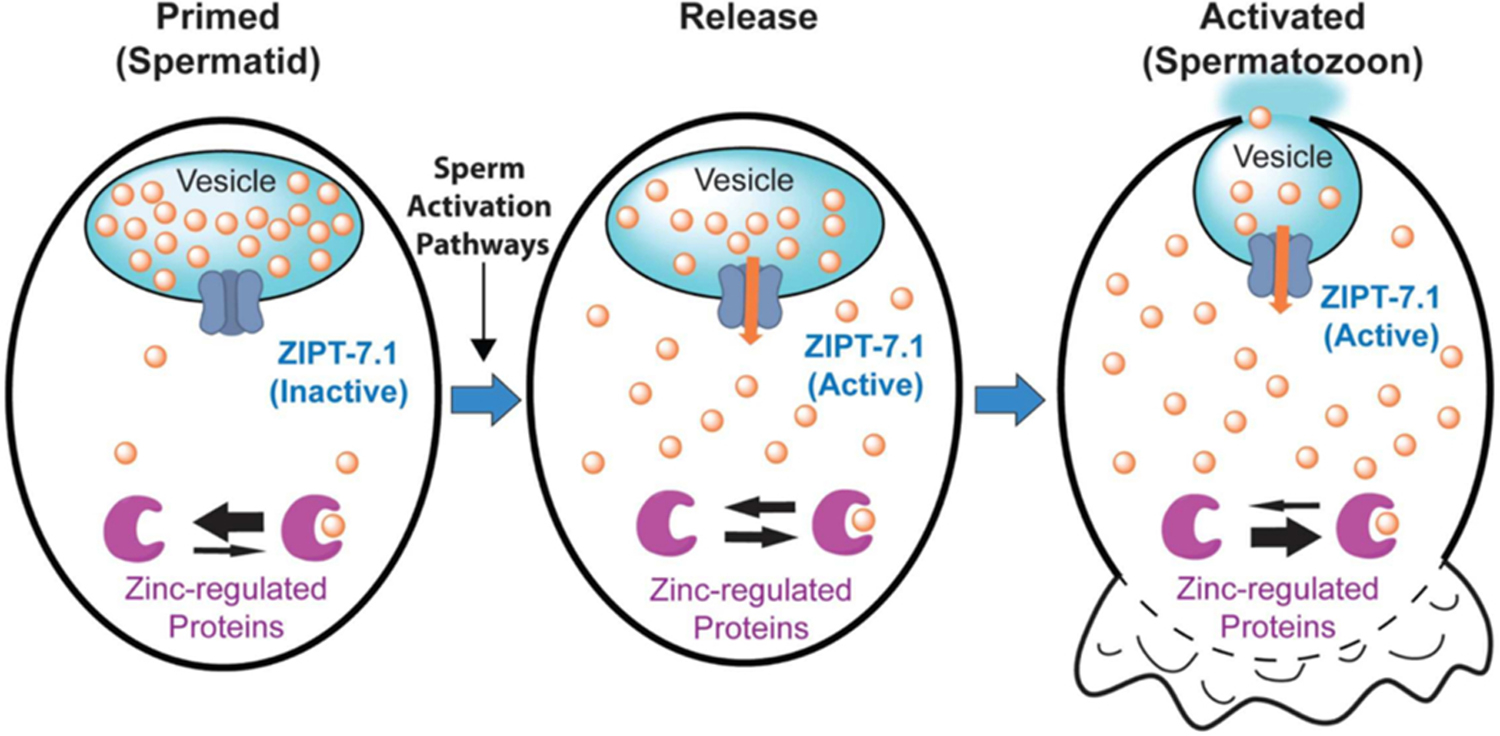
Model of the biochemical function of ZIPT-7.1 at three times during sperm activation: (1) Primed spermatid: prior to activation, spermatids are primed to respond; ZIPT-7.1 (dark blue) is inactive and localized to the membrane of a vesicle that has a high internal concentration of stored zinc (orange circles) that is presumably the membranous organelle (light blue). The cytoplasm has a low concentration of zinc, and the equilibrium of zinc-regulated proteins (purple) is shifted towards unbound. (2) Release: a sperm-activating signal results in activation of ZIPT-7.1 (orange arrow), zinc begins to flow into the cytoplasm, and zinc-regulated proteins begin binding zinc. (3) Activated spermatozoon: the cytoplasmic concentration of zinc is now high, and zinc-regulated proteins are bound to zinc. The pseudopod extension begins (lower), and the membranous organelles fuse with the plasma membrane. Adapted from Zhao et al., (2018) [21].
6. Outlook
6.1. The CDF and ZIP families of transporters:
The C. elegans genome encodes 14 CDF and 14 ZIP family transporters, and here we review evidence that four CDF transporters and two ZIP transporters play roles in zinc biology. Very little is known about the function of the other family members, and an important goal of future research is to determine the expression pattern, function, and metal selectivity of these 22 transporter proteins. The studies in C. elegans, similar to studies in mammals, have begun to provide a framework for understanding the specific roles of different members of these transporter families. One key aspect is tissue-specific expression. For example, ZIPT-2.3 is expressed in the intestine, whereas ZIPT-7.1 is expressed in the germ line. While all cells require zinc, different tissues may have specific needs that are met by expression of specific transporters. A second key aspect of specificity is subcellular localization. For example, in intestinal cells CDF-1 is localized to the basolateral membrane, TTM-1B is localized to the apical membrane, and CDF-2 is localized to the membrane of lysosome-related organelles. Presumably, each of these transporter proteins contains an epitope that targets it to the appropriate membrane compartment. However, the mechanism of specific subcellular localization has not been determined and is a promising area for future research. A third key aspect of specificity is metal selectivity, since some members of the CDF and ZIP family have been documented to transport iron and manganese [65–67]. In C. elegans, selectivity for zinc has been established using cell-based transport assays (ZIPT-7.1) and mutant phenotypes such as specific hypersensitivity to zinc toxicity (CDF-1, CDF-2). An important goal of future studies is to determine the metal selectivity of the remaining transporters. There are many examples of human diseases arising from mutations in ZIP and CDF transporters. For example, ZIP4 is associated with acrodermatitis enteropathica [68, 69], ZIP5 is associated with autosomal dominant myopia [70], ZIP8 is associated with congenital disorder of glycosylation type IIn [65, 66], ZIP13 is associated with Ehlers-Danlos syndrome spondylodysplastic type 3 [71, 72], ZIP 14 is associated with hyperostosis cranialis interna [73] and hypermanganesemia with dystonia type 2 [74, 75], ZnT2 is associated with transient neonatal zinc deficiency due to low zinc content in breast milk[76, 77], and ZnT10 is associated with hypermanganesemia with dystonia type 1[78]. Given that there are C. elegans proteins similar to each of these human proteins, C. elegans is a promising system to establish a fundamental understanding of these proteins that may be relevant to humans and suggest new strategies for treating human disease.
6.2. The high zinc sensor and high zinc homeostasis:
Homeostasis can be conceptualized as having two phases – sensing and response. In mammals and insects, the MTF1 transcription factor plays a critical role in the transcriptional response to high zinc; it has also been proposed to function as a sensor by binding zinc at one or more of its six zinc finger DNA binding domains [79] . Notably, MTF1 is also important for other stress responses including heat stress, oxidative stress, and hormonal stress. The analysis of HIZR-1 in C. elegans identified a nuclear receptor as a new class of high zinc sensor, and the phenotypic analysis in worms indicates it is specific for high zinc and does not respond to a wide range of stresses. The prototypical ligands for nuclear receptors are steroid hormones; more recently, some lipid molecules have been proposed as ligands for nuclear receptors [35]. The discovery that zinc is a ligand for the HIZR-1 nuclear receptor establishes transition metals as a new class of nuclear receptor ligands. Of the approximately 50 nuclear receptors in the human genome, about half have no defined ligand, and these nuclear receptors are called ‘orphan receptors’. An exciting possibility is that one or more of these orphan nuclear receptors may use zinc as a ligand. Another fascinating implication of the HIZR-1 discovery is that nuclear receptors may function as sensors for other transition metals, such as copper.
In C. elegans, a key aspect of the response to high zinc is zinc storage in lysosome-related organelles in intestinal cells; in high zinc conditions, about half of the total content of zinc is stored in these organelles [14]. Lysosome-related organelles are a morphologically diverse group of acidic, LAMP-containing membrane compartments [80, 81], and emerging evidence indicates these organelles store metals in a wide range or organisms [82]. In C. elegans, these organelles are restructured in high zinc to adopt a bilobed morphology, an interesting case of environmental conditions affecting lysosomal structure. These discoveries raise important questions for future research. What is the mechanism of forming bilobed granules in response to high zinc, and is this response coordinated by HIZR-1? How do bilobed granules function to promote high zinc storage? What is the metal selectivity of these organelles? Recent studies suggest copper is stored in lysosome-related organelles in C. elegans [83], and it will be important to determine if zinc and copper are stored in the same or separate organelles.
6.3. The low zinc sensor and low zinc homeostasis:
Compared to high zinc homeostasis, very little is known about low zinc homeostasis in animals. The first important insight from C. elegans is that the LZA enhancer activates transcription in response to zinc deficiency, indicating that there is an active homeostasis mechanism. A key objective of future research will be to identify the LZA binding factor and establish its relationship to the low zinc sensor. This may be relevant to mammals, since the LZA enhancer was sufficient to function in mammalian cells. A fascinating issue is the logic of the low zinc sensor – is it a zinc-binding protein that activates a response when it loses zinc binding – the conceptual opposite of HIZR-1, or is there a very different mechanism for an organism to sense that zinc levels are low. The second insight in worms is that zinc is released from the lysosome-related organelle storage sites in response to zinc deficiency, thus making more zinc available in the cytoplasm. An important question for future studies is determining the mechanism of zinc release from these storage depots.
6.4. Zinc as a second messenger signaling molecule:
While zinc has a well-established role as a protein binding cofactor, recent studies have suggested that zinc may act as a second messenger signaling molecule. A key issue is what evidence is required to prove that zinc is functioning as a signaling molecule? Three lines of evidence are important: (1) Visualizing a rapid change in zinc concentration in a specific cellular or extracellular compartment. (2) Characterizing the regulatory mechanism that causes the rapid change in concentration. (3) Defining the targets that respond to the increase in zinc with a change in activity. Thus far, no system has met this rigorous level of evidence. Here we describe studies in C .elegans vulval development and sperm activation that suggest zinc signaling may play a role in cell fate determination. The main evidence comes from loss-of-function genetic experiments. In cdf-1(lf) and sur-7(lf) mutants, the vulval precursor cell fates are abnormal. In zipt-7.1(lf) mutants, the sperm activation phenotype is abnormal. One interpretation of these observations is that zinc functions as a signaling molecule, and the transporters are necessary for a dynamic change in zinc concentration that influences cell fate decisions. An alternative explanation is that mutations in zinc transporters disrupt the baseline level of zinc, which is broadly necessary for cell fate determination. To distinguish these possibilities, future experiments will need to address the key questions: visualizing a dynamic change in zinc concentration, determining the mechanism of this change, and establishing the consequence at a molecular level. Sperm is the most promising system, because sperm cells use a signaling system that does not involve the nucleus and must act rapidly, which would be well suited to zinc signaling. Sperm are also experimentally tractable, since they can be dissected from animals and activated in vitro. However, their small size makes microscopic visualization challenging. A key technology that will facilitate these studies is better zinc sensors. While many zinc sensors have been developed for use in vitro and in cultured cells (see Amy Palmer and Chris Chang Reviews in this issue), only a handful have been used effectively in C. elegans. Current reliance on chemical sensors such as FluoZin-3 and Zinpyr-1 has clear limitations, and the development of sensors that can be targeted to specific subcellular compartments and detect dynamic changes in zinc will be a critical advance in understanding whether and how zinc functions as a second messenger.
Acknowledgements:
The authors are grateful to the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440), for providing strains that were integral to the reviewed literature. Funding from National Institutes of Health (grant number R01 GM068598 received by KK and F31 ES030622 received by BJE) supported this review.
Abbreviations:
- CDF
cation diffusion facilitator
- ZnT
Zinc Transporters
- SLC30A
Solute Carrier Family 30 (which includes ZnT and CDF transporters)
- ZIP
Zrt-/Irt-like Protein
- ZIPT
Zrt-/Irt-like Protein Transporter
- SLC39
Solute Carrier Family 39 (which includes ZIP transporters)
- LZA
Low Zinc Activation
- HZA
High Zinc Activation
- HIZR
1-HIgh Zinc activated nuclear Receptor
- C. elegans
Caenorhabditis elegans
- GFP
Green Fluorescent Protein
- CeMM
C. elegans Maintenance Media
- NAMM
Nobel Agar Minimal Media
- NGM
Nematode Growth Media
- TPEN
N,N,N’,N’-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine
- ICP-MS
Inductively Coupled Plasma Mass Spectrometry
- SUR
SUppressor of Ras
- TTM
Toxin-regulated Targets of MAPK
- RT-PCR
Real-Time quantitative Polymerase Chain Reaction
- mRNA
messenger RiboNucleic Acid
- RNAi
RiboNucleic Acid interference
- Muv
multivulval phenotype
- LRO
lysosome-related organelle
References
- [1].Brenner S, The genetics of Caenorhabditis elegans, Genetics, 77 (1974) 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sulston JE, Schierenberg E, White JG, Thomson JN, The embryonic cell lineage of the nematode Caenorhabditis elegans, Dev Biol, 100 (1983) 64–119. [DOI] [PubMed] [Google Scholar]
- [3].Sulston JE, Horvitz HR, Post-embryonic cell lineages of the nematode, Caenorhabditis elegans, Dev Biol, 56 (1977) 110–156. [DOI] [PubMed] [Google Scholar]
- [4].Kimble J, Hirsh D, The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans, Dev Biol, 70 (1979) 396–417. [DOI] [PubMed] [Google Scholar]
- [5].McGhee JD, The C. elegans intestine, WormBook, (2007) 1–36. [DOI] [PMC free article] [PubMed]
- [6].Waterston R, Ainscough R, Anderson K, Berks M, Blair D, Connell M, Cooper J, Coulson A, Craxton M, Dear S, et al. , The genome of the nematode Caenorhabditis elegans, Cold Spring Harb Symp Quant Biol, 58 (1993) 367–376. [DOI] [PubMed] [Google Scholar]
- [7].Kim W, Underwood RS, Greenwald I, Shaye DD, OrthoList 2: A New Comparative Genomic Analysis of Human and Caenorhabditis elegans Genes, Genetics, 210 (2018) 445–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Szewczyk NJ, Kozak E, Conley CA, Chemically defined medium and Caenorhabditis elegans, BMC Biotechnol, 3 (2003) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lu MC, Goetscu KM, Carbohydrate requirement of Caenorhabditis elegans and the final development of a hemically defined medium, Nematologica, 39 (1993) 8. [Google Scholar]
- [10].Bruinsma JJ, Jirakulaporn T, Muslin AJ, Kornfeld K, Zinc ions and cation diffusion facilitator proteins regulate Ras-mediated signaling, Dev Cell, 2 (2002) 567–578. [DOI] [PubMed] [Google Scholar]
- [11].Bruinsma JJ, Schneider DL, Davis DE, Kornfeld K, Identification of mutations in Caenorhabditis elegans that cause resistance to high levels of dietary zinc and analysis using a genomewide map of single nucleotide polymorphisms scored by pyrosequencing, Genetics, 179 (2008) 811–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Davis DE, Roh HC, Deshmukh K, Bruinsma JJ, Schneider DL, Guthrie J, Robertson JD, Kornfeld K, The cation diffusion facilitator gene cdf-2 mediates zinc metabolism in Caenorhabditis elegans, Genetics, 182 (2009) 1015–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dietrich N, Schneider DL, Kornfeld K, A pathway for low zinc homeostasis that is conserved in animals and acts in parallel to the pathway for high zinc homeostasis, Nucleic Acids Res, 45 (2017) 11658–11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roh HC, Collier S, Guthrie J, Robertson JD, Kornfeld K, Lysosome-related organelles in intestinal cells are a zinc storage site in C. elegans, Cell Metab, 15 (2012) 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mendoza AD, Woodruff TK, Wignall SM, O’Halloran TV, Zinc availability during germline development impacts embryo viability in Caenorhabditis elegans, Comp Biochem Physiol C Toxicol Pharmacol, 191 (2017) 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hester J, Hanna-Rose W, Diaz F, Zinc deficiency reduces fertility in C. elegans hermaphrodites and disrupts oogenesis and meiotic progression, Comp Biochem Physiol C Toxicol Pharmacol, 191 (2017) 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dietrich N, Tan CH, Cubillas C, Earley BJ, Kornfeld K, Insights into zinc and cadmium biology in the nematode Caenorhabditis elegans, Arch Biochem Biophys, 611 (2016) 120–133. [DOI] [PubMed] [Google Scholar]
- [18].Dean KM, Qin Y, Palmer AE, Visualizing metal ions in cells: an overview of analytical techniques, approaches, and probes, Biochim Biophys Acta, 1823 (2012) 1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu Z, Chen L, Shang Y, Huang P, Miao L, The micronutrient element zinc modulates sperm activation through the SPE-8 pathway in Caenorhabditis elegans, Development, 140 (2013) 2103–2107. [DOI] [PubMed] [Google Scholar]
- [20].Roh HC, Collier S, Deshmukh K, Guthrie J, Robertson JD, Kornfeld K, ttm-1 encodes CDF transporters that excrete zinc from intestinal cells of C. elegans and act in a parallel negative feedback circuit that promotes homeostasis, PLoS Genet, 9 (2013) e1003522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhao Y, Tan CH, Krauchunas A, Scharf A, Dietrich N, Warnhoff K, Yuan Z, Druzhinina M, Gu SG, Miao L, Singson A, Ellis RE, Kornfeld K, The zinc transporter ZIPT-7.1 regulates sperm activation in nematodes, PLoS Biol, 16 (2018) e2005069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jackson BP, Williams PL, Lanzirotti A, Bertsch PM, Evidence for biogenic pyromorphite formation by the nematode Caenorhabditis elegans, Environ Sci Technol, 39 (2005) 5620–5625. [DOI] [PubMed] [Google Scholar]
- [23].Essig YJ, Webb SM, Sturzenbaum SR, Deletion of Phytochelatin Synthase Modulates the Metal Accumulation Pattern of Cadmium Exposed C. elegans, Int J Mol Sci, 17 (2016) 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Eide DJ, Zinc transporters and the cellular trafficking of zinc, Biochim Biophys Acta, 1763 (2006) 711–722. [DOI] [PubMed] [Google Scholar]
- [25].Jeong J, Eide DJ, The SLC39 family of zinc transporters, Mol Aspects Med, 34 (2013) 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kambe T, Molecular architecture and function of ZnT transporters, Curr Top Membr, 69 (2012) 199–220. [DOI] [PubMed] [Google Scholar]
- [27].Kambe T, Tsuji T, Hashimoto A, Itsumura N, The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism, Physiol Rev, 95 (2015) 749–784. [DOI] [PubMed] [Google Scholar]
- [28].Jakubowski J, Kornfeld K, A local, high-density, single-nucleotide polymorphism map used to clone Caenorhabditis elegans cdf-1, Genetics, 153 (1999) 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yoder JH, Chong H, Guan KL, Han M, Modulation of KSR activity in Caenorhabditis elegans by Zn ions, PAR-1 kinase and PP2A phosphatase, EMBO J, 23 (2004) 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chapman EM, Lant B, Ohashi Y, Yu B, Schertzberg M, Go C, Dogra D, Koskimaki J, Girard R, Li Y, Fraser AG, Awad IA, Abdelilah-Seyfried S, Gingras AC, Derry WB, A conserved CCM complex promotes apoptosis non-autonomously by regulating zinc homeostasis, Nat Commun, 10 (2019) 1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kumar J, Barhydt T, Awasthi A, Lithgow GJ, Killilea DW, Kapahi P, Zinc Levels Modulate Lifespan through Multiple Longevity Pathways in Caenorhabditis elegans, PLoS One, 11 (2016) e0153513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Novakovic S, Molesworth LW, Gourley TE, Boag PR, Davis GM, Zinc transporters maintain longevity by influencing insulin/IGF-1 activity in Caenorhabditis elegans, FEBS Lett, 594 (2020) 1424–1432. [DOI] [PubMed] [Google Scholar]
- [33].Warnhoff K, Roh HC, Kocsisova Z, Tan CH, Morrison A, Croswell D, Schneider DL, Kornfeld K, The Nuclear Receptor HIZR-1 Uses Zinc as a Ligand to Mediate Homeostasis in Response to High Zinc, PLoS Biol, 15 (2017) e2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM, The nuclear receptor superfamily: the second decade, Cell, 83 (1995) 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sladek FM, What are nuclear receptor ligands?, Mol Cell Endocrinol, 334 (2011) 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Roh HC, Dimitrov I, Deshmukh K, Zhao G, Warnhoff K, Cabrera D, Tsai W, Kornfeld K, A modular system of DNA enhancer elements mediates tissue-specific activation of transcription by high dietary zinc in C. elegans, Nucleic Acids Res, 43 (2015) 803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shomer N, Kadhim AZ, Grants JM, Cheng X, Alhusari D, Bhanshali F, Poon AF, Lee MYY, Muhuri A, Park JI, Shih J, Lee D, Lee SV, Lynn FC, Taubert S, Mediator subunit MDT-15/MED15 and Nuclear Receptor HIZR-1/HNF4 cooperate to regulate toxic metal stress responses in Caenorhabditis elegans, PLoS Genet, 15 (2019) e1008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McGhee JD, Fukushige T, Krause MW, Minnema SE, Goszczynski B, Gaudet J, Kohara Y, Bossinger O, Zhao Y, Khattra J, Hirst M, Jones SJ, Marra MA, Ruzanov P, Warner A, Zapf R, Moerman DG, Kalb JM, ELT-2 is the predominant transcription factor controlling differentiation and function of the C. elegans intestine, from embryo to adult, Dev Biol, 327 (2009) 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Taubert S, Hansen M, Van Gilst MR, Cooper SB, Yamamoto KR, The Mediator subunit MDT-15 confers metabolic adaptation to ingested material, PLoS Genet, 4 (2008) e1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hockner M, Dallinger R, Sturzenbaum SR, Nematode and snail metallothioneins, J Biol Inorg Chem, 16 (2011) 1057–1065. [DOI] [PubMed] [Google Scholar]
- [41].Imagawa M, Onozawa T, Okumura K, Osada S, Nishihara T, Kondo M, Characterization of metallothionein cDNAs induced by cadmium in the nematode Caenorhabditis elegans, Biochem J, 268 (1990) 237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kondo M, Imagawa M, Maruyama K, Okada Y, Tsunasawa S, Nishihara T, Biochemical and immunochemical characterization of Caenorhabditis elegans metallothioneins I and II induced by cadmium, Biomed Environ Sci, 3 (1990) 315–325. [PubMed] [Google Scholar]
- [43].Slice LW, Freedman JH, Rubin CS, Purification, characterization, and cDNA cloning of a novel metallothionein-like, cadmium-binding protein from Caenorhabditis elegans, J Biol Chem, 265 (1990) 256–263. [PubMed] [Google Scholar]
- [44].Bofill R, Orihuela R, Romagosa M, Domenech J, Atrian S, Capdevila M, Caenorhabditis elegans metallothionein isoform specificity--metal binding abilities and the role of histidine in CeMT1 and CeMT2, FEBS J, 276 (2009) 7040–7056. [DOI] [PubMed] [Google Scholar]
- [45].Zeitoun-Ghandour S, Charnock JM, Hodson ME, Leszczyszyn OI, Blindauer CA, Sturzenbaum SR, The two Caenorhabditis elegans metallothioneins (CeMT-1 and CeMT-2) discriminate between essential zinc and toxic cadmium, FEBS J, 277 (2010) 2531–2542. [DOI] [PubMed] [Google Scholar]
- [46].Hall J, Haas KL, Freedman JH, Role of MTL-1, MTL-2, and CDR-1 in mediating cadmium sensitivity in Caenorhabditis elegans, Toxicol Sci, 128 (2012) 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hughes SL, Bundy JG, Want EJ, Kille P, Sturzenbaum SR, The metabolomic responses of Caenorhabditis elegans to cadmium are largely independent of metallothionein status, but dominated by changes in cystathionine and phytochelatins, J Proteome Res, 8 (2009) 3512–3519. [DOI] [PubMed] [Google Scholar]
- [48].Swain SC, Keusekotten K, Baumeister R, Sturzenbaum SR, elegans metallothioneins C: new insights into the phenotypic effects of cadmium toxicosis, J Mol Biol, 341 (2004) 951–959. [DOI] [PubMed] [Google Scholar]
- [49].Chaudhuri P, Imam HT, Essig Y, Krasauskas J, Webb SM, Blindauer CA, Sturzenbaum SR, Molecular genetic and biochemical characterization of a putative family of zinc metalloproteins in Caenorhabditis elegans, Metallomics, 10 (2018) 1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liang X, Dempski RE, Burdette SC, Zn(2+) at a cellular crossroads, Curr Opin Chem Biol, 31 (2016) 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Que EL, Bleher R, Duncan FE, Kong BY, Gleber SC, Vogt S, Chen S, Garwin SA, Bayer AR, Dravid VP, Woodruff TK, O’Halloran TV, Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks, Nat Chem, 7 (2015) 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tanimura N, Liao R, Wilson GM, Dent MR, Cao M, Burstyn JN, Hematti P, Liu X, Zhang Y, Zheng Y, Keles S, Xu J, Coon JJ, Bresnick EH, GATA/Heme Multi-omics Reveals a Trace Metal-Dependent Cellular Differentiation Mechanism, Dev Cell, 46 (2018) 581–594 e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K, Hirano T, Zinc is a novel intracellular second messenger, J Cell Biol, 177 (2007) 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sternberg PW, Vulval development, WormBook, (2005) 1–28. [DOI] [PMC free article] [PubMed]
- [55].Sternberg PW, Horvitz HR, Pattern formation during vulval development in C. elegans, Cell, 44 (1986) 761–772. [DOI] [PubMed] [Google Scholar]
- [56].Sulston JE, White JG, Regulation and cell autonomy during postembryonic development of Caenorhabditis elegans, Dev Biol, 78 (1980) 577–597. [DOI] [PubMed] [Google Scholar]
- [57].Beitel GJ, Clark SG, Horvitz HR, Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction, Nature, 348 (1990) 503–509. [DOI] [PubMed] [Google Scholar]
- [58].Jirakulaporn T, Muslin AJ, Cation diffusion facilitator proteins modulate Raf-1 activity, J Biol Chem, 279 (2004) 27807–27815. [DOI] [PubMed] [Google Scholar]
- [59].Chu DS, Shakes DC, Spermatogenesis, Adv Exp Med Biol, 757 (2013) 171–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].L’Hernault SW, The genetics and cell biology of spermatogenesis in the nematode C. elegans, Mol Cell Endocrinol, 306 (2009) 59–65. [DOI] [PubMed] [Google Scholar]
- [61].Smith HE, Nematode sperm motility, WormBook, (2014) 1–15. [DOI] [PMC free article] [PubMed]
- [62].Stanfield GM, Villeneuve AM, Regulation of sperm activation by SWM-1 is required for reproductive success of C. elegans males, Curr Biol, 16 (2006) 252–263. [DOI] [PubMed] [Google Scholar]
- [63].Ward S, Carrel JS, Fertilization and sperm competition in the nematode Caenorhabditis elegans, Dev Biol, 73 (1979) 304–321. [DOI] [PubMed] [Google Scholar]
- [64].Sadler PL, Shakes DC, Anucleate Caenorhabditis elegans sperm can crawl, fertilize oocytes and direct anterior-posterior polarization of the 1-cell embryo, Development, 127 (2000) 355–366. [DOI] [PubMed] [Google Scholar]
- [65].Boycott KM, Beaulieu CL, Kernohan KD, Gebril OH, Mhanni A, Chudley AE, Redl D, Qin W, Hampson S, Kury S, Tetreault M, Puffenberger EG, Scott JN, Bezieau S, Reis A, Uebe S, Schumacher J, Hegele RA, McLeod DR, Galvez-Peralta M, Majewski J, Ramaekers VT, Care4Rare Canada C, Nebert DW, Innes AM, Parboosingh JS, Abou Jamra R, Autosomal-Recessive Intellectual Disability with Cerebellar Atrophy Syndrome Caused by Mutation of the Manganese and Zinc Transporter Gene SLC39A8, Am J Hum Genet, 97 (2015) 886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Park JH, Hogrebe M, Gruneberg M, DuChesne I, von der Heiden AL, Reunert J, Schlingmann KP, Boycott KM, Beaulieu CL, Mhanni AA, Innes AM, Hortnagel K, Biskup S, Gleixner EM, Kurlemann G, Fiedler B, Omran H, Rutsch F, Wada Y, Tsiakas K, Santer R, Nebert DW, Rust S, Marquardt T, SLC39A8 Deficiency: A Disorder of Manganese Transport and Glycosylation, Am J Hum Genet, 97 (2015) 894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Rogers EE, Eide DJ, Guerinot ML, Altered selectivity in an Arabidopsis metal transporter, Proc Natl Acad Sci U S A, 97 (2000) 12356–12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kury S, Dreno B, Bezieau S, Giraudet S, Kharfi M, Kamoun R, Moisan JP, Identification of SLC39A4, a gene involved in acrodermatitis enteropathica, Nat Genet, 31 (2002) 239–240. [DOI] [PubMed] [Google Scholar]
- [69].Schmitt S, Kury S, Giraud M, Dreno B, Kharfi M, Bezieau S, An update on mutations of the SLC39A4 gene in acrodermatitis enteropathica, Hum Mutat, 30 (2009) 926–933. [DOI] [PubMed] [Google Scholar]
- [70].Guo H, Jin X, Zhu T, Wang T, Tong P, Tian L, Peng Y, Sun L, Wan A, Chen J, Liu Y, Li Y, Tian Q, Xia L, Zhang L, Pan Y, Lu L, Liu Q, Shen L, Li Y, Xiong W, Li J, Tang B, Feng Y, Zhang X, Zhang Z, Pan Q, Hu Z, Xia K, SLC39A5 mutations interfering with the BMP/TGF-beta pathway in non-syndromic high myopia, J Med Genet, 51 (2014) 518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Fukada T, Civic N, Furuichi T, Shimoda S, Mishima K, Higashiyama H, Idaira Y, Asada Y, Kitamura H, Yamasaki S, Hojyo S, Nakayama M, Ohara O, Koseki H, Dos Santos HG, Bonafe L, Ha-Vinh R, Zankl A, Unger S, Kraenzlin ME, Beckmann JS, Saito I, Rivolta C, Ikegawa S, Superti-Furga A, Hirano T, The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways, PLoS One, 3 (2008) e3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Giunta C, Elcioglu NH, Albrecht B, Eich G, Chambaz C, Janecke AR, Yeowell H, Weis M, Eyre DR, Kraenzlin M, Steinmann B, Spondylocheiro dysplastic form of the Ehlers-Danlos syndrome--an autosomal-recessive entity caused by mutations in the zinc transporter gene SLC39A13, Am J Hum Genet, 82 (2008) 1290–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hendrickx G, Borra VM, Steenackers E, Yorgan TA, Hermans C, Boudin E, Waterval JJ, Jansen IDC, Aydemir TB, Kamerling N, Behets GJ, Plumeyer C, D’Haese PC, Busse B, Everts V, Lammens M, Mortier G, Cousins RJ, Schinke T, Stokroos RJ, Manni JJ, Van Hul W, Conditional mouse models support the role of SLC39A14 (ZIP14) in Hyperostosis Cranialis Interna and in bone homeostasis, PLoS Genet, 14 (2018) e1007321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Rodan LH, Hauptman M, D’Gama AM, Qualls AE, Cao S, Tuschl K, Al-Jasmi F, Hertecant J, Hayflick SJ, Wessling-Resnick M, Yang ET, Berry GT, Gropman A, Woolf AD, Agrawal PB, Novel founder intronic variant in SLC39A14 in two families causing Manganism and potential treatment strategies, Mol Genet Metab, 124 (2018) 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tuschl K, Meyer E, Valdivia LE, Zhao N, Dadswell C, Abdul-Sada A, Hung CY, Simpson MA, Chong WK, Jacques TS, Woltjer RL, Eaton S, Gregory A, Sanford L, Kara E, Houlden H, Cuno SM, Prokisch H, Valletta L, Tiranti V, Younis R, Maher ER, Spencer J, Straatman-Iwanowska A, Gissen P, Selim LA, Pintos-Morell G, Coroleu-Lletget W, Mohammad SS, Yoganathan S, Dale RC, Thomas M, Rihel J, Bodamer OA, Enns CA, Hayflick SJ, Clayton PT, Mills PB, Kurian MA, Wilson SW, Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism-dystonia, Nat Commun, 7 (2016) 11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Chowanadisai W, Lonnerdal B, Kelleher SL, Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency, J Biol Chem, 281 (2006) 39699–39707. [DOI] [PubMed] [Google Scholar]
- [77].Lasry I, Seo YA, Ityel H, Shalva N, Pode-Shakked B, Glaser F, Berman B, Berezovsky I, Goncearenco A, Klar A, Levy J, Anikster Y, Kelleher SL, Assaraf YG, A dominant negative heterozygous G87R mutation in the zinc transporter, ZnT-2 (SLC30A2), results in transient neonatal zinc deficiency, J Biol Chem, 287 (2012) 29348–29361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Tuschl K, Clayton PT, Gospe SM Jr., Gulab S, Ibrahim S, Singhi P, Aulakh R, Ribeiro RT, Barsottini OG, Zaki MS, Del Rosario ML, Dyack S, Price V, Rideout A, Gordon K, Wevers RA, Chong WK, Mills PB, Syndrome of Hepatic Cirrhosis, Dystonia, Polycythemia, and Hypermanganesemia Caused by Mutations in SLC30A10, a Manganese Transporter in Man, Am J Hum Genet, 99 (2016) 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gunther V, Lindert U, Schaffner W, The taste of heavy metals: gene regulation by MTF-1, Biochim Biophys Acta, 1823 (2012) 1416–1425. [DOI] [PubMed] [Google Scholar]
- [80].Fukuda M, Lysosome-related organelles, Organizational Cell Biology, 2 (2016) 8. [Google Scholar]
- [81].Marks MS, Heijnen HF, Raposo G, Lysosome-related organelles: unusual compartments become mainstream, Curr Opin Cell Biol, 25 (2013) 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Blaby-Haas CE, Merchant SS, Lysosome-related organelles as mediators of metal homeostasis, J Biol Chem, 289 (2014) 28129–28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chun H, Sharma AK, Lee J, Chan J, Jia S, Kim BE, The Intestinal Copper Exporter CUA-1 Is Required for Systemic Copper Homeostasis in Caenorhabditis elegans, J Biol Chem, 292 (2017) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


