Abstract
Avian influenzas, Ebola, Nipah, Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is an RNA virus covered by a lipid bilayer, are directly affecting people worldwide. On the other hand, in addition to the main spread source (human contact) of SARS-CoV-2, consumers have started to think about whether foods are dangerous in terms of SARS-CoV-2 spread. The consumption of wild animals as well as the possible contamination of SARS-CoV-2 in fresh and frozen foods have caused concern and increased awareness among consumers. A heating process >70°C is being suggested to eliminate viral contamination risk. Cutting tools, slicing machines, and food-contact surfaces including stainless steel, aluminum, or glass must be regularly sanitized. The sous vide cooking method, which is based on cooking under vacuum and with pH treatments in the range of 3 and 10, could be advised in this risky period for decreasing contamination risk in food. Also, recent studies have shown that nanotechnology applications such as nanoparticles could be used to combat the SARS-CoV-2 spread, which is 50–200 nm in size. Another suggested technique is cold plasma technology that could damage the protein structure of the virus. Besides these techniques, it is important to boost the immune system. In this regard, recent researches have revealed the importance of honey consumption (1 g/kg per person/day), intake of vitamins, minerals like selenium, and ω-3 fatty acids.
Keywords: Cold plasma, COVID-19, Food safety, Immune-boosting, Nanotechnology
12.1. Introduction
Humankind struggles with many health matters, particularly those caused by bacteria and viruses. Bacteria-based illnesses have gained notoriety globally. Studies have mainly focused on pathogenic bacteria in food. In other words, other potential hazardous effects were mostly ignored. In this respect, today, humans are learning how to fight viruses.1, 2, 3
A typical virus has RNA or DNA and is mostly 300 nm in size. Unlike bacteria, viruses may not reproduce in a food environment because viruses are obligate intracellular parasites. In other words, they can only survive by moving their genetic material from one host cell to another. However, water and food may act as vectors for the transmission of viruses to humans via the fecal-oral route.4, 5, 6 A virus can spread to food in several ways: (1) contamination during food processing through infected food personnel; (2) direct contamination of human feces without soil or water treatment; (3) ingestion of food animal origin with zoonotic viruses; and (4) inadequate treatment of wastewater.7, 8, 9, 10, 11
Ebola, avian influenzas, Nipah, MERS-CoV, SARS-CoV, and currently the most dangerous one, SARS-CoV-2, are the main zoonotic viruses that affect human health directly.12, 13, 14 CoVs can easily pass to humans from different sources like animals. According to previous studies, CoVs can transfer by consumption of infected animals.15, 16, 17, 18, 19, 20, 21 Especially in the case of foods not being heated or cooked, these viruses can cause diseases in humans. Also, it has been observed that some food materials such as chicken or some fish samples are contaminated with SARS-CoV-2.22, 23, 24 Pneumonia, diarrhea, fever, loss of odor and taste, and organ failures are common symptoms of SARS-CoV-2 and its infection.25 Because of its long incubation stage, SARS-CoV-2 can have more infectious effects on society. In terms of food, the aforementioned viruses can be related with the consumption of wild animals. In China, in the first step, as can be seen from various news sources, the main source of spread of SARS-CoV-2 is also associated with some animals such as bats, pangolins, and civets. Furthermore, the relationship between animal-based food consumption and coronavirus disease (COVID-19) infections was associated with Wuhan’s Seafood Wholesale Market, which was immediately closed at the beginning of January 2020.8 , 26 , 27 A graphical illustration of the possible routes of SARS-CoV-2 transmission from foods to humans is presented in Fig. 12.1 . Following the initial stages of COVID-19 spread in China, World Health Organization (WHO) and some leader organizations such as Food and Drug Administration (FDA) and European Food Safety Authority (EFSA) began preparing reports, which consisted of frequently asked questions related to the COVID-19 outbreak and food safety.28 , 29 At that moment, consumers were thinking that foods could be contaminated by COVID-19. Restaurants, markets, and manufacturing facilities were seen as potential areas of risk.11 , 30, 31, 32, 33, 34 Consumers have observed a lack of information regarding such risks. After the initial period of the COVID-19 outbreak, new studies related to food safety and COVID-19 have revealed the worry and awareness among consumers.8 Also, it has emphasized the Hazard Analysis and Critical Control Point (HACCP) principles for the food industry.35
Figure 12.1.
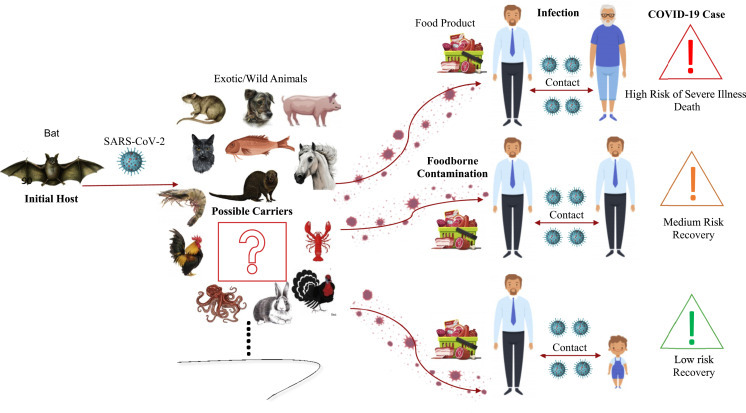
Graphical abstract of emerging SARS-CoV-2 that shows the probable main source, possible intermediate carriers, contamination to food products, infection in humans, and finally, COVID-19 cases, respectively.
The International Commission on Microbiological Specifications for Foods (ICMSF) claimed that it is highly improbable that the ingestion of SARS-CoV-2 will result in illness, and there is still no evidence that food is a major source of transmission.25 Oral transmission via food consumption has not been reported. One of the ways in which COVID-19 can be spread is through cross-contamination of surfaces. It has been reported that CoV particles may survive for hours to days on surfaces.11 , 34 , 36 , 37 However, the possibility of transmission through inanimate surfaces has been observed to be lower.25 , 38
Food preservation methods and hygiene practices, already in use for food safety, could be important in eliminating different microbiological risks at the onset of a COVID-19 outbreak. In this respect, to improve consumer safety, recently different certificate systems have been applied.14 , 33 , 39 Besides novel approaches like nanotechnology applications or packaging applications, some minor preventions for industrial kitchens and households are gaining importance.8 , 34 , 40, 41, 42, 43 On the other hand, consumption of vegetables and dairy products, as well as foods rich in protein such as fish and red meat, will boost the human immune system. In addition, from their harvesting to the plate, food safety should be enhanced.33 , 44
This study aims to highlight the probable risks based on food material contaminated with potential COVID-19 infection from food surfaces, food packaging materials, food processing equipment, and other items in the food processing industry. With this study, novel perspectives including nanoscience and cold plasma treatment have been presented to society for the elimination or prevention of COVID-19.
12.2. Food safety and COVID-19
Considering risk management, there is currently no evidence that food is a source or route of transmission of SARS-CoV-2 food or food packaging, according to Centers for Disease Control and Prevention (CDC), U.S. Department of Agriculture (USDA), and EFSA.23 , 45 , 46 However, there are a few reports that SARS-CoV-2 is found on food packaging materials. These reports show the presence of an RNA virus which may exist as a hazard to human health. As is known, the first case related to food-contact material (a salmon cutting board) with SARS-CoV-2 was determined in the wholesale market in Beijing.11 , 23 , 24 , 47, 48, 49 Since July 2020, some cases of food contamination have been reported in China, where SARS-CoV-2 was detected on imported frozen food packaging materials such as shrimp.23 , 24 SARS-CoV-2 was also detected in a food shipping container.23 Food contamination that occurred with COVID-19 may be observed via the farming activity, processing, storage, transport, and retailing process in which foods might come in contact with staff and atmospheric conditions.23 , 50 Ferretti et al. (2020) emphasized the importance of environmental conditions. In this sense, SAR-CoV-2 can transfer from symptomatic people at a 90% rate. On the other hand, 10% defines the environment, surfaces, and other sources.51 Therefore, food safety related to COVID-19 should never be ignored.
12.2.1. Vegetables and fruits
According to Mullis et al., a kind of coronavirus called bovine on lettuce samples was stable for 14 days when it was stored at 4°C.52 In addition, another type (CoV 229E) was found on lettuce after two days of storage at 4°C. On the other hand, this virus surprisingly could not survive on the surface because of the acidity in strawberry samples.53
Significant records related to COVID-19 in fruit and vegetables have not been directly detected yet. On the contrary, recently published articles have revealed that indirect contamination can affect vegetables and fruits. For example, irrigation with sewage is able to infect vegetables and fruit with COVID-19.4 Also, if plants or fruit trees are irrigated by sewage constituting SARS-CoV-2, there would be a risk in terms of contamination in vegetables and fruits. In another study, to remove or decline the virus load on the surface of them, a good washing is suggested but the fruit or vegetable’s surface structure would play a key role.1 In this sense, the pH value of the fruit could play a significant role in the elimination of COVID-19.54 , 55
12.2.2. Animal resources
Breeders play a major role in animal production. With socioeconomic concerns, instead of small-sized family businesses, farms that are formed by the gathering of thousands of animals in closed areas have increased. Diseases transmitted through animal origin have increased because of industrial animal production. CoVs are responsible for respiratory and intestinal infections in animals.56 , 57 For example, the host animal for MERS-CoV was dromedary camels. Foodborne transmission through consumption of unpasteurized camel milk and raw camel meat was an infection source for humans.58 Like MERS-CoV, SARS-CoV-2 is also a zoonotic virus and has a genome similarity of 96% to a SARS-related bat CoV.56 In fact, a zoonotic transmission from animals to humans has been reported in a Dutch mink farm during the COVID-19 pandemic.56 Around 20 million mink in Europe have been culled in case of further zoonotic transmission possibility.59 , 60 On November 12, 2020 the Food and Agriculture Organization (FAO) announced guidelines about the exposure of humans or animals to SARS-CoV-2 from wild, livestock, companion, and aquatic animals.56
Determining disease-resistant animal species by epigenetic studies may be beneficial for future applications. Genomic selection applications for animals that have resistance to diseases, will make a significant contribution to preventing the spread of COVID-19. In this way breeding studies could be carried out for the ones that are resistant to COVID-19.61 , 62 Animals that are the sources of infection are a concern. To avoid the SARS-CoV-2 that causes disease, while planning animal shelters, attention should be paid to natural ventilation, adequate living space, controlled production and hygiene. All these data show that further investigation is needed for livestock, and precautions need to be taken against COVID-19 on an industrial scale.
12.2.3. Meat and meat products
CoVs can be transmitted from an infected person to meat samples by contaminated hands, or via sneezing or coughing if hygiene rules are not followed. Although the oral/alimentary transmission by consuming meat products does not play a role, according to new research, it was revealed that SARS-CoV-2 lost only a little of its infectivity on frozen meat even after three weeks.63 Also, Liji (2020) has stated that SARS-CoV-2 may survive on frozen or cold storage salmon and meat samples for more than three weeks.64 To the best of our knowledge, on August 12, 2020, the first known case of SARS-CoV-2 was detected on a chicken wing sample that had been imported from Brazil to China.22 , 23
The CDC requested data related to the number of poultry and meat plants (where beef, bison, lamb, pork, and poultry meats are processing) negatively affected, how many workers were affected in these plants, and the number of deaths based on COVID-19 in these facilities of the United States.65 According to the report, 115 poultry or meat processing facilities, in which 4913 cases and 20 worker deaths were reported between April 9–27, 2020, were affected by this pandemic outbreak. It has been assumed that difficulties at workplaces regarding physical distancing, hygiene, and crowded living and transportation conditions were the factors that potentially affected infection risk. Other data revealed that a slaughterhouse in Germany was shut down on June 18, 2020 because COVID-19 test results of 1550 of 7000 workers were came out positive.66 These data imply that the number of workers who have to work in crowded places in these facilities increased SARS-CoV-2 transmission risk.65 Moreover, salmon from Europe was banned and Ecuadorean shrimp were suspended because of the SARS-CoV-2 outbreak.24 , 64
European Federation of Food Agriculture and Tourism Trade Unions stated that, according to a report from IUF consultation with Imperial College in London, the meat sector greatly relies on immigrants and cross-border workers in almost all European countries. In some meat factories, especially the old ones, the spread of the disease is 20 times more likely where there is insufficient ventilation. On the other hand, certain parts of meat processing plants are very cold and the virus spread faster in colder temperatures.66
All these data prove that during meat processing, SARS-CoV-2 transmission risk should be considered. The recognized risk to poultry and meat facility operation requires action plans to decrease risks to workers, preserve facility function, and maintain the food supply. Cleaning and disinfection, screening staff for symptoms, and providing multilingual disinfection training might slow the virus spread among workers in meat and poultry processing plants. Temperature monitoring and symptom screening are important to avoid the introduction of COVID-19 into a facility from infected persons.65 Inactivation of SAR-CoV-2 in artificially contaminated chicken, pork, and salmon samples reveal that three weeks’ storage at 4, −20, and −80°C, respectively, did not decrease infection level.11 , 24 Above all, precautions in the meat sector should be improved and updated.
12.2.4. Dairy products
Some natural milk preservative components like lactoferrin (LCF), lactadherin and glycomacropeptide in milk serve an antiviral role against some viruses, particularly HIV,67 and may also have antiviral activity for SARS-CoV−2. It is known that LCF can have an interaction with heparin glycosaminoglycan (HSPG) cell receptors. Also, the aforementioned receptors play a significant role in cells of the first term of the virus infection from CoV. These relationships contain the angiotensin-converting enzyme 2 (ACE2) receptor, a metallopeptidase that can bind virus terminals and also facilitate cell entry. All mechanisms mentioned above were revealed in the SARS-CoV epidemic in 2002. In this regard, similar interactions and mechanisms in ACE2 have been observed in SARS-CoV-2.68
Besides SARS-CoV, SARS-CoV-2 and MERS-CoV were determined in 41.9% of camel milk samples.69 In this sense, the experiment indicated that MERS-CoV inoculated to camel milk could survive up to 72 h at 4 and 22°C, respectively. As stated by van Doremalen et al., the virus survived for more than two days in unpasteurized milk samples.70 However, the consumption of milk by pasteurization prevents such foodborne contamination. In order to inactivate a virus like SARS-CoV, it is evaluated as sufficient to heat the milk to ∼60°C for 30 min.71 In addition to the heating processes, Ultraviolet (UV) light, alkaline pH (>12), or acidic pH (<3) are the other tools to inactive SARS-CoV.72 , 73 They may be recommended for the elimination of dairy products with SARS-CoV-2. Another widely consumed dairy product is yogurt, which is fermented by lactic acid bacteria following heat treatment. The last product possesses about 4.5 pH, but Chin et al. (2020) has reported that SARS-CoV-2 is evaluated as stable from 3 to 10 pH ranges.54 Contrary to these aforementioned issues, some metabolites such as lactic acid, hydrogen peroxide (H2O2), and bacteriocins shaped by lactic acid bacteria at the end of fermentation possess antiviral properties.74 So, the last product could provide a good opportunity to support the immune systems of humans in pandemic terms.
Yekta et al. noted that for dairy products, SARS-CoV-2 at −20°C for 2 years could be found as stable, so ice cream, frozen yogurt, and dairy desserts may be defined to be SARS-CoV-2 carriers.1 Moreover, many more studies on dairy products should be revealed for potential contamination risk.
12.3. Food packaging applications and SARS-CoV-2
Food packaging applications are gaining importance. In the case of outbreaks, all worldwide consumers by time are getting much more worried because of the possible contamination risk of SARS-CoV-2. In this sense, we already know that packaging materials play a key role. Also, if possible, to decrease contamination risks with SARS-CoV-2 on food, different kinds of food products should be processed, preheated, and pasteurized or sterilized. Moreover, following the mentioned applications, food packaging material can be stored. For example, sous vide technology can provide a lower contamination risk for food samples because food material can be placed into the food packaging material then vacuum packaged, sealed, cooked, cooled, and stored (Fig. 12.2 ). When food products are cooked with sous vide technology, they could be directly consumed or heated, for instance, in a microwave oven. In addition to sous vide technology, intelligent and active packaging enriched with nanosensors could be used with basic food packaging materials used widely in the food industry.75 , 76 The average risk of contamination with food or food packaging is evaluated to be lower.77
Figure 12.2.
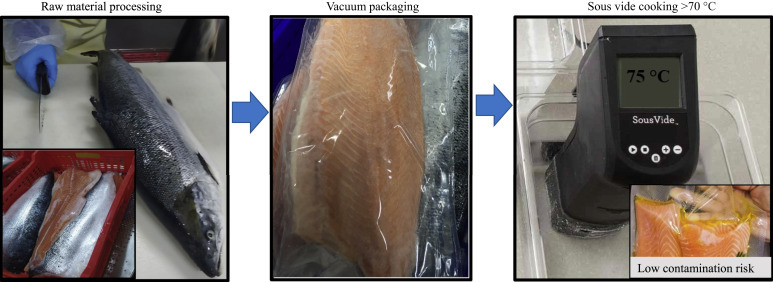
Main steps of sous vide cooking to lower viral contamination risks for food products.
Zhang et al. revealed that fecal-oral contamination of the virus is an important issue.78 In the case of handling food packages and containers, SARS-CoV-2 could be transmitted from the food packaging material surface to the eyes, nose, and mouth. But the aforementioned contamination way is not evaluated to be a separate route for this pandemic virus.79 So, packaged food samples and canned foods may be preferred in this risky period.
12.4. Limitation of SARS-CoV-2 spread
12.4.1. Nanotechnological approach
The particle size of SARS-CoV-2 ranges from 50 to 200 nm.77 Ignatov has reported the exact size as 120 nm.80 The use of nanomaterials based on antiviral activity provided inactivation of some viruses in chicken embryo models and shrimp samples.42 , 81 , 82 These studies have shown that nanotechnological approaches should be considered for the purpose of developing resistance against SARS-CoV-2.41, 42, 43 , 83 Antimicrobial coatings by metal oxide nanostructures and nanopolymers may inhibit the transmission of COVID-19.83 , 84 Silver (Ag) nanoparticles and zinc (Zn) nanomaterials indicated inhibition in viral replication, virus inactivation, viral protein translation, and polyprotein processing.40 , 85 Nanoform of Ag and copper (Cu) cations could affectively damage the nucleotides of some viruses.43 In this respect, TiO2, ZnO, and Cu nanoparticles are suggested to control the disease by surface coating and disinfection. In one study, the use of nanosilver colloids was investigated for therapeutic purposes in the early stages of COVID-19. As a result of the study, it was determined that antimicrobial doses of nanosilver colloids also show antiviral effects. In the same study, it was stated that nanosilver colloids can be applied both at home and in the hospital through inhalation.80 In another study, 2–15 nm size Ag nanoparticles provided a successful inhibition of SARS-CoV-2 at concentrations above 1 ppm.86 In this regard, a relevance between the intake of SARS-CoV-2 contaminated food and the possibility of infections as well as the advancement of antiviral packaging using nanotechnology ought to be searched.79 The use of nanomaterial coatings or films (containing Cu, Ag, and ZnO nanoparticles) has a potential against SARS-CoV-2 to avoid contamination of food packaging surfaces, and thus this approach may reduce its rapid transmission.41 Also, nanosized materials can be integrated with other technologies for obtaining much safer materials, which can be contacted by foods. In this respect, packaging material enriched with nanoparticles in active and intelligent applications could be advised in order to provide food safety against SARS-CoV-2. Moreover, nanoparticles, nanofibers, nanoencapsulation technologies, nanoemulsions, and nanocomposites providing a larger contact area on the surface of the material could be used to increase food safety.
12.4.2. Cold plasma
Cold plasma has demonstrated great potential for inactivation of SARS-CoV-2.10 , 87, 88, 89, 90 Since it does not generate waste or toxic chemicals, this method is seen as environmentally friendly. This technique also has potential with extensive applications in the disinfection of food surfaces, equipment, and food packages.88 , 91 Furthermore, the in-package plasma treatment can be combined with the bacteriostatic nanophotocatalysts onto food packing films.91 , 92
The mechanism is attributed to free radicals that diffuse through the capsid to reach and damage the RNA of the virus.93 Although Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) are considered the main contributors to the inactivation of viruses,88 plasma does not fully damage the virus but it can delay its ability to infect.94 Photons, positive and negative ions, neutral atoms, UV photons, and free radicals could play a key role.95 , 96 The occurrence and role of ozone and UV photons in cold plasma species can affect the protein structure and damage RNA in a virus.97 The electrostatic forces related to plasma technology may affect the disruption of cell membranes that lead to cell death. In this respect, gas composition, flow rate, voltage parameters, and food composition are the other main factors to inactivate microbial load in food.98 , 99
In terms of SARS-CoV-2, cold plasma applications have been discussed to degrade the RNA virus.100 , 101 Cold plasma with Argon gas to deactivate SARS-CoV-2 on various surfaces, including plastic, metal, and cardboard, was also employed. These studies showed that cold plasma could be an effective method to avoid transmission and infections on various surfaces.89 , 101 Also, Verma (2020) has reported that application parameters such as duration should be optimized for the elimination of viruses on the contaminated material.94 In terms of food products, inactivation of the Tulane virus on spinach,102 murine norovirus-1 and hepatitis A virus on fresh meats,95 norovirus surrogates on blueberries97 and some other food systems,103 Tulane virus on romaine lettuce,104 and Newcastle disease virus on poultry105 was effectively applied by cold plasma treatment.
Major foodborne viruses on surfaces such as stainless steel106 were also studied. These studies demonstrated that cold plasma is effective for reducing microbial load in surface areas such as stainless steel or other industry materials that may have close contact with food products.107 For these reasons, cold plasma application can be considered for decontamination of food processing equipment. Furthermore, cold plasma usage can decrease the rapid contamination of COVID-19 in water samples.88 , 108 It is known that most food or food products, such as a different kind of seafood and meat, contain high levels of water. So, in further studies, cold plasma treatment can be combined with food samples that are widely consumed.
12.5. Suggestions for consumers
Suggestions will be evaluated from two perspectives. The main aim is to explain how to decrease the risk in food, food packaging, or kitchen equipment contaminated with SARS-CoV-2. After purchasing foods, packaging materials including food should be washed or cleaned by using different materials having higher acidic pH value. For example, canned sample surfaces should be washed with disinfectants. Cooking temperature or heating processes are highly important to eliminate the potential risk. If possible, as can be seen from the present study, the midpoint temperature of food products such as fish and meat should be controlled before consumption. Also, especially fruits with lower acidity should be washed. Recently, studies reported that freezing or chilling is not meant to eliminate the virus; therefore, it is not thought that they are safer for COVID-19. Moreover, this sort of sample must be well cooked like fresh food samples. Kitchen equipment (knives, cutting board, etc.) must be continually disinfected by using hot water.
CoVs are thermolabile and do not withstand normal cooking temperatures.109 SARS-CoV was found to be inactivated after 15 min incubation at >75°C. Similarly, SARS-CoV-2 can be inactivated after incubation for 5 min at 70°C.54 These findings recommend that cooking at >70°C is adequate for virus inactivation, but transmission from frozen food should still be considered; therefore, thorough hand washing after handling raw food is necessary.33 , 73 , 110
Besides the aforementioned hygienic precautions, in order to support the immune system in humans, some recently published articles have presented some suggestions. Ashraf et al. has suggested that consumption of honey (1 g/kg per person/day) will gain a powerful resistance toward COVID-19.111 Adequate and balanced nutrition is important for boosting the immune system. In this respect, consuming foods rich in vitamins, minerals, and ω-3 fatty acids may have a potential role to help fight COVID-19. Vitamin B plays a role in cell functioning and energy metabolism. Vitamin D decreases rates of infection. The use of vitamin C can help minimize the symptoms caused by COVID-19, while vitamin E and some minerals like selenium may assist in the recovery stage.48 , 112, 113, 114 Also, an increase in immunomodulatory effects can be provided by using ω-3 fatty acids.112 , 113 According to recent studies, polyphenols such as resveratrol and flavonoids have potential inhibitors.48 , 115 , 116 There is also indefinite proof supporting the future use of β-glucan to address COVID-19 due in part to variability in the immune response resulting from heterogeneity in the polysaccharide branch.44 Nanoencapsulation improves bioavailability through controlled-release properties of substances in food with more health-promoting properties. This contribution summarizes the development of effective antioxidants such as coenzyme Q10, quercetin, curcumin, and probiotics that influences body immune systems in a positive way. In addition to these, micronutrients, functional food ingredients like probiotics, herbs, flavonoids, and carotenoids are also evaluated to be natural immune boosters.48 , 116, 117, 118 As could be seen in several studies, a balanced diet enriched with different vitamins, minerals, and also probiotics may be playing a key role in this fight.
12.6. Conclusion
The potential effect of COVID-19 related to the safety of food is an important issue for the food industry. Further investigation is needed for livestock, and precautions need to be taken against COVID-19 on an industrial scale. Because SARS-CoV-2 is a zoonotic virus, in regards to animal resources, identifying disease-resistant animal genotypes and including them in breeding programs through genetic mapping is necessary. Furthermore, potential viral contamination risks on meat and dairy food products should be further investigated. Novel approaches, such as nanoscience (nanofibers, nanocapsules, and nanocomposites), cold plasma, and ozone applications can be considered as safe and effective methods toward SARS-CoV-2 prevention. The mentioned applications could be combined or integrated with sous vide, active, and intelligent packaging applications to improve food safety against future viral outbreaks. Consumers must take hygienic precautions to decrease the SARS-CoV-2 spread risk for frozen or chilled foods. The heating/cooking process, pH value, and temperature duration for all foods must be controlled. Packaging materials and food-contact surfaces should be cleaned to prevent contamination. Also, a balanced diet with intake of vitamins, minerals, phenolic compounds, and ω-3 fatty acids from various sources, consumption of dairy products, and honey-based products such as bee propolis and other supplementary materials play key roles in supporting the immune system.
References
- 1.Yekta R., Vahid-Dastjerdi L., Norouzbeigi S., Mortazavian A.M. Food products as potential carriers of SARS-CoV-2. Food Contr. 2020:107754. doi: 10.1016/j.foodcont.2020.107754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knorr D., Khoo C.-S.H. COVID-19 and food: challenges and research needs. Front Nutr. 2020;7:598913. doi: 10.3389/fnut.2020.598913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO and FAO . 2020. Viruses in Food: scientific Advice to Support Risk Management Activities: meeting Report. Viruses in food: scientific advice to support risk management activities: meeting report (who.int) Accessed 25.12.20. [Google Scholar]
- 4.Rusiñol M., Hundesa A., Cárdenas-Youngs Y., et al. Microbiological contamination of conventional and reclaimed irrigation water: evaluation and management measures. Sci Total Environ. 2020;710:136298. doi: 10.1016/j.scitotenv.2019.136298. [DOI] [PubMed] [Google Scholar]
- 5.Miranda R.C., Schaffner D.W. Virus risk in the food supply chain. Curr Opin Food Sci. 2019;30:43–48. doi: 10.1016/j.cofs.2018.12.002. [DOI] [Google Scholar]
- 6.Öksüztepe G., Demir P. Gıda Güvenliği ve Virüsler. Turkiye Klinikleri J Food Hyg Technol-Special Topics. 2016;2(3):49–55. [Google Scholar]
- 7.Alp D., Kuleaşan H. Gıda kaynaklı viral gastroenteritler. TURJAF. 2018;6:1592–1598. doi: 10.24925/turjaf.v6i11.1592-1598.2054. [DOI] [Google Scholar]
- 8.Ceylan Z., Meral R., Cetinkaya T. Relevance of SARS-CoV-2 in food safety and food hygiene: potential preventive measures, suggestions and nanotechnological approaches. Virus Dis. 2020;31:154–160. doi: 10.1007/s13337-020-00611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duizer E., Koopmans M. In: Food-borne viruses progress and challenges. Marion P.G.K., Dean O.C., Albert B., Michael P.D., editors. ASM Press; Washington DC: 2008. Emerging food-borne viral diseases; pp. 117–145. [Google Scholar]
- 10.Roos Y.H. Water and pathogenic viruses inactivation—food engineering perspectives. Food Eng Rev. 2020:1–17. doi: 10.1007/s12393-020-09234-z. [DOI] [Google Scholar]
- 11.Mardones F.O., Rich K.M., Boden L.A., et al. The COVID-19 pandemic and global food security. Front Vet Sci. 2020;7 doi: 10.3389/fvets.2020.578508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Vir. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koonin E v, Senkevich T.G., Dolja V. The ancient Virus World and evolution of cells. Biol Direct. 2006;1:29. doi: 10.1186/1745-6150-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galanakis C.M. The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods. 2020;9:1–10. doi: 10.3390/foods9040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L.F., Shi Z., Zhang S., Field H., Daszak P., Eaton B.T. Review of bats and SARS. Emerg Infect Dis. 2006;12:1834–1840. doi: 10.3201/eid1212.060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chhikara B.S., Rathi B., Singh J. Corona virus SARS-CoV-2 disease COVID-19: infection, prevention and clinical advances of the prospective chemical drug therapeutics. Chem Biol Let. 2020;7(1):63–72. [Google Scholar]
- 17.Yuan J., Lu Y., Cao X., Cui H. Regulating wildlife conservation and food safety to prevent human exposure to novel virus. Ecosyst Health Sustain. 2020;6:1741325. doi: 10.1080/20964129.2020.1741325. [DOI] [Google Scholar]
- 18.Monchatre-Leroy E., Boué F., Boucher J.-M., et al. Identification of alpha and beta coronavirus in wildlife species in France: bats, rodents, rabbits, and hedgehogs. Viruses. 2017;9:364. doi: 10.3390/v9120364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhama K., Pawaiya R.V.S., Chakrabort S., Tiwari R., Saminathan M., Verma A.K. Coronavirus infection in equines: a review. Asian J Anim Vet Adv. 2014;9:164–176. doi: 10.3923/ajava.2014.164.176. [DOI] [Google Scholar]
- 20.van der Hoek L., Pyrc K., Jebbink M.F., et al. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oakenfull R.J., Wilson A.J. 2020. Qualitative risk assessment: what is the risk of food or food contact materials being a source or transmission route of SARS-CoV-2 for UK consumers?https://www.food.gov.uk/sites/default/files/media/document/web-version-qualitative-risk-assessment-risk-of-food-or-food-contact-materials-as-transmission-route-of-sars-cov-2-002.pdf Accessed 28.11.20. [Google Scholar]
- 22.Shenzhen Municipal Health Commission (SMHC) 2020. Detection of SARS-CoV-2 on an imported chicken wing sample.http://wjw.sz.gov.cn/yqxx/content/post_7998108.html Accessed 26.11.20. [Google Scholar]
- 23.Han J., Zhang X., He S., Jia P. Can the coronavirus disease be transmitted from food? A review of evidence, risks, policies and knowledge gaps. Environ Chem Lett. 2020:1–12. doi: 10.1007/s10311-020-01101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher D., Reilly A., Kang A., Zheng E., Cook A.R., Anderson D.E. bioRxiv; 2020. Seeding of outbreaks of COVID-19 by contaminated fresh and frozen food. 2020.08.17.255166. [DOI] [Google Scholar]
- 25.ICMSF . 2020. ICMSF opinion on SARS-CoV-2 and its relationship to food safety.https://www.icmsf.org/publications/papers/ Accessed 25.11.20. [Google Scholar]
- 26.Li X., Song Y., Wong G., Cui J. Bat origin of a new human coronavirus: there and back again. Sci China Life Sci. 2020;63:461–462. doi: 10.1007/s11427-020-1645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui D.S., I Azhar E., Madani T.A., et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health — the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO . 2020. Novel coronavirus (2019-NCoV) SITUATION REPORT - 3.https://apps.who.int/iris/bitstream/handle/10665/330762/nCoVsitrep23Jan2020-eng.pdf Accessed 25.11.20. [Google Scholar]
- 29.EFSA . 2020. COVID-19 and food safety-questions and answers. Accessed 26.11.20. [Google Scholar]
- 30.Jalava K. First respiratory transmitted food borne outbreak? Int J Hyg Environ Health. 2020;226:113490. doi: 10.1016/j.ijheh.2020.113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song H.J., Yeon J., Lee S. Impact of the COVID-19 pandemic: evidence from the U.S. restaurant industry. Int J Hosp Manag. 2021;92:102702. doi: 10.1016/j.ijhm.2020.102702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Freitas R.S.G., Stedefeldt E. COVID-19 pandemic underlines the need to build resilience in commercial restaurants’ food safety. Food Res Int. 2020;136:109472. doi: 10.1016/j.foodres.2020.109472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizou M., Galanakis I.M., Aldawoud T.M.S., Galanakis C.M. Safety of foods, food supply chain and environment within the COVID-19 pandemic. Trends Food Sci Tech. 2020;102:293–299. doi: 10.1016/j.tifs.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuber S., Brüssow H. Covid 19: challenges for virologists in the food industry. Microb Biotechnol. 2020;13:1689–1701. doi: 10.1111/1751-7915.13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tayar M. Gıda Güvenliği ve COVID-19. Bull Vet Pharm Tox. 2020;11:61–71. doi: 10.38137/vetfarmatoksbulten.765700. [DOI] [Google Scholar]
- 36.van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akram M.Z. Inanimate surfaces as potential source of 2019-nCoV spread and their disinfection with biocidal agents. Virus Dis. 2020;31:94–96. doi: 10.1007/s13337-020-00603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldman E. Exaggerated risk of transmission of COVID-19 by fomites. Lancet Infect Dis. 2020;20:892–893. doi: 10.1016/S1473-3099(20)30561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Djekic I., Nikolić A., Uzunović M., et al. Covid-19 pandemic effects on food safety - multi-country survey study. Food Contr. 2021;122:107800. doi: 10.1016/j.foodcont.2020.107800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imani S.M., Ladouceur L., Marshall T., Maclachlan R., Soleymani L., Didar T.F. Antimicrobial nanomaterials and coatings: current mechanisms and future perspectives to control the spread of viruses including SARS-CoV-2. ACS Nano. 2020;14:12341–12369. doi: 10.1021/acsnano.0c05937. [DOI] [PubMed] [Google Scholar]
- 41.Sportelli M.C., Izzi M., Kukushkina E.A., et al. Can nanotechnology and materials science help the fight against SARS-CoV-2? Nanomaterials. 2020;10:802. doi: 10.3390/nano10040802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vazquez-Munoz R., Lopez-Ribot J.L. Nanotechnology as an alternative to reduce the spread of COVID-19. Challenges. 2020;11:15. doi: 10.3390/challe11020015. [DOI] [Google Scholar]
- 43.Ruiz‐Hitzky E., Darder M., Wicklein B., et al. Nanotechnology responses to COVID‐19. Adv Healthcare Mater. 2020;9:2000979. doi: 10.1002/adhm.202000979. [DOI] [PubMed] [Google Scholar]
- 44.Galanakis C.M., Aldawoud T.M.S., Rizou M., Rowan N.J., Ibrahim S.A. Food ingredients and active compounds against the coronavirus disease (COVID-19) pandemic: a comprehensive review. Foods. 2020;9:1701. doi: 10.3390/foods9111701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.U.S. Food & Drug Administration . 2020. COVID-19 frequently asked questions.https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-frequently-asked-questions Accessed 27.11.20. [Google Scholar]
- 46.FDA . 2020. Food safety and the coronavirus disease 2019 (COVID-19)https://www.fda.gov/food/food-safety-during-emergencies/food-safety-and-coronavirus-disease-2019-covid-19 Accessed 27.11.20. [Google Scholar]
- 47.Aday S., Aday M.S. Impact of COVID-19 on the food supply chain. Food Qual Saf. 2020;4:167–180. doi: 10.1093/fqsafe/fyaa024. [DOI] [Google Scholar]
- 48.Ayseli Y.I., Aytekin N., Buyukkayhan D., Aslan I., Ayseli M.T. Food policy, nutrition and nutraceuticals in the prevention and management of COVID-19: advice for healthcare professionals. Trends Food Sci Technol. 2020;105:186–199. doi: 10.1016/j.tifs.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Global Times . 2020. Beijing supermarkets stop selling salmon after wholesalers test positive for coronavirus - global Times.https://www.globaltimes.cn/content/1191462.shtml Accessed 26.11.20. [Google Scholar]
- 50.Lacombe A., Quintela I., Liao Y., Wu V.C.H. Food safety lessons learned from the COVID‐19 pandemic. J Food Saf. 2020:e12878. doi: 10.1111/jfs.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferretti L., Wymant C., Kendall M., et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368:eabb6936. doi: 10.1126/science.abb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mullis L., Saif L.J., Zhang Y., Zhang X., Azevedo M.S.P. Stability of bovine coronavirus on lettuce surfaces under household refrigeration conditions. Food Microbiol. 2012;30:180–186. doi: 10.1016/j.fm.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yépiz-Gómez M.S., Gerba C.P., Bright K.R. Survival of respiratory viruses on fresh produce. Food Environ Virol. 2013;5:150–156. doi: 10.1007/s12560-013-9114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chin A.W.H., Chu J.T.S., Perera M.R.A., et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1:e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Brien B., Goodridge L., Ronholm J., Nasheri N. Exploring the potential of foodborne transmission of respiratory viruses. Food Microbiol. 2021;95:103709. doi: 10.1016/j.fm.2020.103709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.el Masry I., von Dobschuetz S., Plee L., et al. Exposure of Humans or Animals to SARS-CoV-2 from wild, livestock, Companion and aquatic animals: qualitative exposure assessment. FAO Animal Product Health. 2020:181. doi: 10.4060/ca9959en. [DOI] [Google Scholar]
- 57.Hernández M., Abad D., Eiros J.M., Rodríguez-Lázaro D. Are animals a neglected transmission route of SARS-CoV-2? Pathogens. 2020;9:480. doi: 10.3390/pathogens9060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gossner C., Danielson N., Gervelmeyer A., et al. Human–dromedary camel interactions and the risk of acquiring zoonotic Middle East respiratory syndrome coronavirus infection. Zoonoses Public Health. 2016;63:1–9. doi: 10.1111/zph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Azkur A. Covid-19 and animals. Bull Vet Pharm Tox. 2020;11:49–60. doi: 10.38137/vetfarmatoksbulten.768811. [DOI] [Google Scholar]
- 60.Koopmans M. SARS-CoV-2 and the human-animal interface: outbreaks on mink farms. Lancet Infect Dis. 2020:1–2. doi: 10.1016/S1473-3099(20)30912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koyun H., Karakuş K., Taş A. Impacts of climite changes in animal genetic diversity. TJSR. 2016;9:01–02. doi: 10.1098/rspb.2011.2363. [DOI] [Google Scholar]
- 62.Ekinci M.S., Akyol İ., Karaman M., Özköse E. Recent Advances in Applications of Animal Biotechnology. KSU J Sci Eng. 2005;8(2):89–95. [Google Scholar]
- 63.BfR. German Federal Institute for Risk Assessment . 2020. Can the new type of coronavirus Be transmitted via food and objects? [DOI] [Google Scholar]
- 64.Liji T. 2020. Fresh and frozen food can harbor SARS-CoV-2 for at least 21 days.https://www.news-medical.net/news/20200819/Fresh-and-frozen-food-can-harbor-SARS-CoV-2-for-at-least-21-days.aspx Accessed 27.11.2020. [Google Scholar]
- 65.Dyal J.W., Grant M.P., Broadwater K., et al. COVID-19 among workers in meat and poultry processing facilities ― 19 states, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:557–561. doi: 10.15585/mmwr.mm6918e3. [DOI] [PubMed] [Google Scholar]
- 66.EFFAT . 2020. Covid-19 outbreaks in slaughterhouses and meat processing plants state of affairs and proposals for policy action at EU level. EFFAT-Report-Covid-19-outbreaks-in-slaughterhouses-and-meat-packing-plants-State-of-affairs-and-proposals-for-policy-action-at-EU-level-30.06.2020.pdf; Accessed 26.11.20. [Google Scholar]
- 67.Giansanti F., Panella G., Leboffe L., Antonini G. Lactoferrin from milk: nutraceutical and pharmacological properties. Pharmaceuticals. 2016;9:61. doi: 10.3390/ph9040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peroni D.G., Fanos V. Lactoferrin is an important factor when breastfeeding and COVID‐19 are considered. Acta Paediatr. 2020;109:2139–2140. doi: 10.1111/apa.15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reusken C.B., Farag E.A., Jonges M., et al. Middle East respiratory syndrome coronavirus (MERS-CoV) RNA and neutralising antibodies in milk collected according to local customs from dromedary camels, Qatar, April 2014. Euro Surveill. 2014;19:20829. doi: 10.2807/1560-7917.ES2014.19.23.20829. [DOI] [PubMed] [Google Scholar]
- 70.van Doremalen N., Bushmaker T., Karesh W.B., Munster V.J. Stability of Middle East respiratory syndrome coronavirus in milk. Emerg Infect Dis. 2014;20:1263–1264. doi: 10.3201/eid2007.140500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rabenau H.F., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H.W. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Darnell M.E.R., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J Virol Met. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pressman P., Naidu A.S., Clemens R. COVID-19 and food safety. Nutr Today. 2020;55:125–128. doi: 10.1097/NT.0000000000000415. [DOI] [Google Scholar]
- 74.Tachedjian G., Aldunate M., Bradshaw C.S., Cone R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol. 2017;168:782–792. doi: 10.1016/j.resmic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 75.Zafer C., Ünal Şengör G.F. Determination of some quality changes of sous vide-sea bass fillets (Dicentrarchus labrax, linnaeus, 1758) treated with dried basil, fresh garlic, and dill weed. Acta Aquatica Turcica. 2019;15:126–134. doi: 10.22392/actaquatr.577330. [DOI] [Google Scholar]
- 76.Gonzalez-Fandos E., Laorden A.M. In: Innovative technologies in seafood processing. Ozogul Y., editor. CRC Press; Boca Raton, Florida: 2019. Sous vide technology. [ chapter 13] [Google Scholar]
- 77.Anelich L.E.C.M., Lues R., Farber J.M., Parreira V.R. SARS-CoV-2 and risk to food safety. Front Nutr. 2020;7 doi: 10.3389/fnut.2020.580551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang W., Du R.-H., Li B., et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microb Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Olaimat A.N., Shahbaz H.M., Fatima N., Munir S., Holley R.A. Food safety during and after the era of COVID-19 pandemic. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ignatov I. Global congress on infectious diseases. Sofia, Bulgaria. 2020. Antiviral effects of nano colloidal silver, water catholyte, oxidal with methylene blue. Possible effects of influence over coronavirus SARS-CoV and SARS-CoV-2 with disease COVID-19. [Google Scholar]
- 81.Nazaktabar A., Lashkenari M.S., Araghi A., Ghorbani M., Golshahi H. In vivo evaluation of toxicity and antiviral activity of polyrhodanine nanoparticles by using the chicken embryo model. Int J Biol Macromol. 2017;103:379–384. doi: 10.1016/j.ijbiomac.2017.05.069. [DOI] [PubMed] [Google Scholar]
- 82.Ufaz S., Balter A., Tzror C., et al. Anti-viral RNAi nanoparticles protect shrimp against white spot disease. Mol Syst Des Eng. 2018;3:38–48. doi: 10.1039/C7ME00092H. [DOI] [Google Scholar]
- 83.Abd Elkodous M., El-Sayyad G.S., Abdel-Daim M.M. Engineered nanomaterials as fighters against SARS-CoV-2: the way to control and treat pandemics. Environ Sci Pollut Res. 2020:1–7. doi: 10.1007/s11356-020-11032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rai P.K., Usmani Z., Thakur V.K., Gupta V.K., Mishra Y.K. Tackling COVID-19 pandemic through nanocoatings: Confront and exactitude. Curr Res Green Sustain Chem. 2020;3:100011. doi: 10.1016/j.crgsc.2020.100011. [DOI] [Google Scholar]
- 85.Lara H.H., Ayala-Nuñez N v, Ixtepan-Turrent L., Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnol. 2010;8:1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jeremiah S.S., Miyakawa K., Morita T., Yamaoka Y., Ryo A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem Biophys Res Commun. 2020;533:195–200. doi: 10.1016/j.bbrc.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo L., Yao Z., Yang L., et al. Plasma-activated water: An alternative disinfectant for S protein inactivation to prevent SARS-CoV-2 infection. Chem Eng J. 2020:127742. doi: 10.1016/j.cej.2020.127742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Filipić A., Gutierrez-Aguirre I., Primc G., Mozetič M., Dobnik D. Cold plasma, a new hope in the field of virus inactivation. Trends Biotechnol. 2020 doi: 10.1016/j.tibtech.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bekeschus S., Kramer A., Suffredini E., von Woedtke T., Colombo V. Gas plasma technology—an asset to healthcare during viral pandemics such as the COVID-19 crisis? IEEE Trans Radiat Plasma Med Sci. 2020;4:391–399. doi: 10.1109/trpms.2020.3002658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohan S.V., Hemalatha M., Kopperi H., Ranjith I., Kumar A.K. SARS-CoV-2 in environmental perspective: Occurrence, persistence, surveillance, inactivation and challenges. Chem Eng J. 2021;405:126893. doi: 10.1016/j.cej.2020.126893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peng P., Chen P., Zhou N., et al. In: Advances in cold plasma applications for food safety and preservation. Daniela B.A., editor. Academic Press; Richland, WA: 2020. Packed food and packaging materials disinfected by cold plasma; pp. 269–286. [Google Scholar]
- 92.Ostrikov K., Neyts E.C., Meyyappan M. Plasma nanoscience: from nano-solids in plasmas to nano-plasmas in solids. Adv Phys. 2013;62:113–224. doi: 10.1080/00018732.2013.808047. [DOI] [Google Scholar]
- 93.Pradeep P., Chulkyoon M. Non-thermal plasmas (NTPs) for inactivation of viruses in abiotic environment. Res J Biotechnol. 2016;11(6):91–96. [Google Scholar]
- 94.Verma S.S. Fighting COVID-19 with non-thermal plasma technology. Indian Pract. 2020;73(7):42–45. [Google Scholar]
- 95.Bae S.C., Park S.Y., Choe W., Ha S do. Inactivation of murine norovirus-1 and hepatitis A virus on fresh meats by atmospheric pressure plasma jets. Food Res Int. 2015;76:342–347. doi: 10.1016/j.foodres.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 96.Bermudez-Aguirre D. In: Advances in cold plasma applications for food safety and preservation. Daniela B.A., editor. Academic Press; Richland, WA: 2020. Advances in the inactivation of microorganisms and viruses in food and model systems using cold plasma; pp. 49–91. [Google Scholar]
- 97.Lacombe A., Niemira B.A., Gurtler J.B., et al. Nonthermal inactivation of norovirus surrogates on blueberries using atmospheric cold plasma. Food Microbiol. 2017;63:1–5. doi: 10.1016/j.fm.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 98.Feizollahi E., Misra N., Roopesh M.S. Factors influencing the antimicrobial efficacy of dielectric barrier discharge (DBD) atmospheric cold plasma (ACP) in food processing applications. Crit Rev Food Sci Nutr. 2020:1–24. doi: 10.1080/10408398.2020.1743967. [DOI] [PubMed] [Google Scholar]
- 99.Zhao Y.M., de Alba M., Sun D.W., Tiwari B. Principles and recent applications of novel non-thermal processing technologies for the fish industry—a review. Crit Rev Food Sci Nutr. 2019;59:728–742. doi: 10.1080/10408398.2018.1495613. [DOI] [PubMed] [Google Scholar]
- 100.Bisag A., Isabelli P., Laurita R., et al. Cold atmospheric plasma inactivation of aerosolized microdroplets containing bacteria and purified SARS‐CoV‐2 RNA to contrast airborne indoor transmission. Plasma Process Polym. 2020;17:2000154. doi: 10.1002/ppap.202000154. [DOI] [Google Scholar]
- 101.Chen Z., Garcia G., Arumugaswami V., Wirz R.E. Cold atmospheric plasma for SARS-CoV-2 inactivation. Phys Fluids. 2020;32:111702. doi: 10.1063/5.0031332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ailavadi S., Davidson P.M., Morgan M.T., D’Souza D.H. Thermal inactivation kinetics of Tulane virus in cell-culture medium and spinach. J Food Sci. 2019;84:557–563. doi: 10.1111/1750-3841.14461. [DOI] [PubMed] [Google Scholar]
- 103.Ahmed H., Maunula L., Korhonen J. Reduction of norovirus in foods by nonthermal treatments. J Food Protect. 2020;83:2053–2073. doi: 10.4315/JFP-20-177. [DOI] [PubMed] [Google Scholar]
- 104.Min S.C., Roh S.H., Niemira B.A., Sites J.E., Boyd G., Lacombe A. Dielectric barrier discharge atmospheric cold plasma inhibits Escherichia coli O157:H7, Salmonella, Listeria monocytogenes, and Tulane virus in Romaine lettuce. Int J Food Microbiol. 2016;237:114–120. doi: 10.1016/j.ijfoodmicro.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 105.Schiappacasse C., Peng P., Zhou N., et al. Inactivation of aerosolized Newcastle disease virus with non-thermal plasma. Appl Eng Agric. 2020;36:55–60. doi: 10.13031/aea.13699. [DOI] [Google Scholar]
- 106.Park S.Y., Ha S do. Assessment of cold oxygen plasma technology for the inactivation of major foodborne viruses on stainless steel. J Food Eng. 2018;223:42–45. doi: 10.1016/j.jfoodeng.2017.11.041. [DOI] [Google Scholar]
- 107.Yepez Xv., Misra N.N., Keener K.M. In: Food safety engineering. Food engineering series. Demirci A., Feng H., Krishnamurthy K., editors. Springer; 2020. Nonthermal plasma technology; pp. 607–628. [Google Scholar]
- 108.Ghernaout D., Elboughdiri N. Disinfecting water: plasma discharge for removing coronaviruses. OALib. 2020;07:1–29. doi: 10.4236/oalib.1106314. [DOI] [Google Scholar]
- 109.FAO . 2020. Food safety in the time of COVID-19. [DOI] [Google Scholar]
- 110.Nakat Z., Bou-Mitri C. COVID-19 and the food industry: readiness assessment. Food Contr. 2021;121:107661. doi: 10.1016/j.foodcont.2020.107661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ashraf S., Ashraf S., Ashraf M., et al. medRxiv; 2020. Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK): a multi-center placebo-controlled randomized clinical trial. 2020.10.30.20217364. [DOI] [PubMed] [Google Scholar]
- 112.Shakoor H., Feehan J., al Dhaheri A.S., et al. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19? Maturitas. 2021;143:1–9. doi: 10.1016/j.maturitas.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Junaid K., Qasim S., Yasmeen H., et al. Potential inhibitory effect of vitamins against COVID-19. Comput Mater Continua (CMC) 2020;66:707–714. doi: 10.32604/cmc.2020.012976. [DOI] [Google Scholar]
- 114.Shakoor H., Feehan J., Mikkelsen K., et al. Be well: a potential role for vitamin B in COVID-19. Maturitas. 2021 doi: 10.1016/j.maturitas.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Annunziata G., Sanduzzi Zamparelli M., Santoro C., et al. May polyphenols have a role against coronavirus infection? An Overview of in vitro evidence. Front Med. 2020;7 doi: 10.3389/fmed.2020.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iddir M., Brito A., Dingeo G., et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12:1562. doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singh P., Tripathi M.K., Yasir M., Khare R., Tripathi M.K., Shrivastava R. Potential inhibitors for SARS-CoV-2 and functional food components as nutritional supplement for COVID-19: a review. Plant Foods Hum Nutr. 2020;75:458–466. doi: 10.1007/s11130-020-00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jampilek J., Kralova K. Potential of nanonutraceuticals in increasing immunity. Nanomaterials. 2020;10:2224. doi: 10.3390/nano10112224. [DOI] [PMC free article] [PubMed] [Google Scholar]


