Abstract
The high cytotoxic activity of Vγ9Vδ2 T lymphocytes against tumor cells makes them useful candidates in anticancer therapies. However, the molecular mechanism of their activation by phosphoantigens (PAgs) is not completely known. Many studies have depicted the mechanism of Vγ9Vδ2 T-cell activation by PAg-sensed accessory cells, such as immune presenting cells or tumor cells. In this study, we demonstrated that pure resting Vγ9Vδ2 T lymphocytes can self-activate through exogenous PAgs, involving their TCR and the butyrophilins BTN3A1 and BTN2A1. This is the first time that these three molecules, concurrently expressed at the plasma membrane of Vγ9Vδ2 T cells, have been shown to be involved together on the same and unique T cell during PAg activation. Moreover, the use of probucol to stimulate the inhibition of this self-activation prompted us to propose that ABCA-1 could be implicated in the transfer of exogenous PAgs inside Vγ9Vδ2 T cells before activating them through membrane clusters formed by γ9TCR, BTN3A1 and BTN2A1. The self-activation of Vγ9Vδ2 T cells, which leads to self-killing, can therefore participate in the failure of γδ T cell-based therapies with exogenous PAgs and should be taken into account.
Keywords: Vγ9Vδ2 T cells, Phosphoantigen, Butyrophilins, T-cell receptor
Subject terms: Cell biology, Gammadelta T cells
Introduction
The mediation of cytotoxicity against tumor cells by Vγ9Vδ2 T lymphocytes [1, 2] has been considered as an interesting candidate cancer immunotherapy for several years. Even if these cells represent only 1–3% of blood mononuclear cells, they can proliferate in vitro and in vivo upon activation and infiltrate the tumor site [2–4]. However, their effects have not been consistent across different studies and different types of malignancies, and their use has resulted in both good [5, 6] and bad prognoses [7, 8]. In addition, no substantial antitumoral activity was detected in clinical trials with Vγ9Vδ2 T lymphocyte-based immunotherapies, although weak tumor regression associated with significant amplification of these lymphocytes in the blood was found in a few cases (all reviewed in ref. [9]). These failures can be attributed to the resistance of tumor cells to Vγ9Vδ2 T-cell killing and/or the limited understanding of Vγ9Vδ2 T-cell receptor (TCR) diversity and the mode of action of receptor-ligand interactions.
Vγ9Vδ2 T cells are activated by nonpeptide phosphoantigens (PAgs), which are metabolites of the methyl erythritol phosphate pathway in microbial pathogens [10] and the eukaryotic mevalonate (MVA) pathway in tumor cells [11, 12]. This PAg activation was clearly shown to be TCR-dependent and possibly modulated by immune checkpoint inhibitors and natural killer (NK) receptors also expressed by Vγ9Vδ2 T cells [13–17]. Moreover, the upregulation of endogenous biosynthesis of PAgs in tumor cells with inhibitors of the MVA pathway, such as aminobisphophonate (ABP), can exacerbate antitumor Vγ9Vδ2 T-cell functions. Even if novel pathways to potentiate the clinical effects of Vγ9Vδ2 T cells were proposed [9], the clarification of some gray areas of PAg activation of these cells remains essential. Although the details of tumor cell recognition can be controversial, members of the butyrophilin A (BTNA) family, BTN3A1 and BTN2A1, were shown to be essential for this recognition, as have the ABCA1 transporter and the intracellular RHOB and periplakin molecules [18–24].
However, direct PAg activation in Vγ9Vδ2 T cells, i.e., without an accessory cell and without any cell contact, has never been described. A few years ago, some researchers proposed direct activation of Vγ9Vδ2 T cells by exogenous PAg, while others argued that the small size of these molecules precludes direct activation of the TCR [25, 26], but experimental evidence was missing to explain the exact mechanism. In this study, we have shown that pure resting Vγ9Vδ2 T cells from the blood can be directly activated by exogenous PAg but not by ABP in a cell contact-independent manner with a mechanism involving γ9TCR, BTN2A1, and BTN3A1.
Materials and methods
Vγ9Vδ2 T cells
Untouched fresh γδ T cells were obtained from fresh PBMCs from healthy donors using the TCRγδ+ T-Cell Isolation Kit, a MidiMACS™ Separator, and an LS Column, according to the manufacturer’s instructions (Miltenyi Biotec, Germany).
Reagents and antibodies
Reagents
BrHPP (200 nM) and c-HDMAPP (2 nM, Innate Pharma, Marseille, France); IPP (10 μM), zoledronic acid monohydrate (Zometa, 1 or 5 μM), DAPI (1 μg/mL), and Probucol (10 μM, Sigma-Aldrich St. Louis, MO); 7-AAD viability staining solution (Sony Biotechnology).
Flow cytometry antibodies
Anti-CD3 mAb (BV510, clone UCHT1, BD Biosciences), anti-γ9TCR mAb (FITC, clone B3, BD Biosciences), anti-δ2TCR mAb (PE, clone REA771, Miltenyi Biotec), anti-γδTCR mAb (BV510, clone B1, Sony Biotechnology), PeCy5 or PE anti-CD107a (clone H4A3), APC-Cy7 anti-CD69 (clone FN50), PE anti-BTN3A1 (clone 232-5) and isotopic controls (BD Biosciences); anti-BTN2A1 (Biorbyt, Cambridge, United Kingdom); PeCy7 anti-IFNγ (clone B27) and isotype control (Sony Biotechnology).
Activating antibody
Anti-BTN3A activating antibody (clone 20.1, 10 μg/ml).
Blocking antibodies
Anti-BTN3A1 1 h at 10 μg/mL (103.2) and anti-BTN2A1 (7.48, ImCheck Therapeutics, Marseille, France); and anti-γδTCR 1 h at 0.5 mg/mL (clone B1, BioLegend).
Trogocytosis analysis and cytotoxic assay
Daudi cells were stained with the lipophilic green-emitting dye PKH67 (Sigma-Aldrich). Pure resting γδ T cells were stained with PKH67 or with cytoplasmic Cell Tracker™ Orange-CMTMR [5-(and-6)-(((4-chloromethyl) benzoyl) amino) tetramethylrhodamine), Molecular Probes, Oregon, USA] according to the manufacturer’s instructions. CMTMR+ γδ T cells were cocultured with PKH67+ cells (Daudi cells or autologous γδ T cells) at 37 °C in RPMI 1640 culture medium (Invitrogen, Cergy Pontoise, France) supplemented with 10% fetal calf serum (Hy1, Thermo Scientific, USA), 100 µg/ml streptomycin, and 100 IU/ml penicillin (Cambrex Biosciences, Rockland, ME, USA). The cocultures were performed in 96-well U-bottom culture plates at a cell ratio of 1:1 with a total of 200,000 cells per well. CD107a or IgG1 was added to the coculture, and brefeldin A (10 µg/ml) was added after 2 h of coculture. After 4 h of coculture, the cells were washed with 0.5 mM PBS/EDTA and then stained with 7-AAD and DAPI to identify dead cells. Trogocytosis was measured as the acquisition of PKH67 fluorescence by CMTMR+ γδ T cells, which was characterized by an increase in the mean fluorescence intensity of PKH67 by flow cytometry.
Flow cytometry analysis
Cells were labeled with the indicated antibodies or isotype controls at 5 µg/ml for 20 min at 4 °C and analyzed on an LSRII cytometer (BD Biosciences, Pont de Claix, France). Data were analyzed using BD FACSDiva software or FlowJo software. Intracellular staining was performed using BD Cytofix Cytoperm according to the manufacturer’s instructions (BD Biosciences).
Single-cell calcium video imaging
To assess the real-time activation of lymphocytes, measurements of the intracellular Ca2+ levels were performed with γδ T cells loaded with 2 μg Fluo-8 AM (Abcam) for 30 min at 37 °C in RPMI medium. γδ T cells were washed, resuspended in HBSS FCS 1% and seeded on Angiogenesis μ-Slides (Ibidi, Planegg/Martinsried, Germany) coated with poly-D-lysine for 5 min at 37 °C (10 µg/mL, Sigma-Aldrich) and two PBS washes.
γδ T cells were stimulated by BrHPP (200 nM), cHDMAPP (2 nM), IPP (15 µM), zoledronate (1 µM), or ionomycin (1 μM) as indicated. In some experiments, γδ T cells were previously treated with blocking antibodies for 2 h (anti-BTN3A1 10 μg/mL, anti-BTN2A1 10 μg/mL or anti-γδTCR 10 µg/mL), or with reagent (Probucol 10 μM overnight).
Measurements of intracellular Ca2+ responses were performed using a Zeiss LSM 880 FAST Airyscan confocal microscope equipped with a 63X Plan-Apochromat oil immersion objective with a 1.4 aperture and an incubation system (PeCon–Zeiss) to regulate the temperature (37 °C) and CO2 (5%).
To analyze calcium influx, the mean Fluo-8 AM ratio (mean Fluo-8 AM intensity/cell area) in isolated fresh γδ T cells over time was quantified and reported.
To study the reproducibility of calcium influx, these profiles were applied to several freshly isolated sets of γδ T cells, and the results were quantified. This quantification represents the quantitative difference between the maximum and minimum values (5% standard deviation) of the Fluo-8 AM intensities emitted by an isolated cell for each condition.
Immunofluorescence microscopy and colocalization analysis
Microscope slides were coated with poly-D-lysine (10 µg/mL, overnight, Sigma-Aldrich). Fresh purified γδ T cells were previously stained with anti-γ9TCR antibody (FITC, clone B3, BD Biosciences) and anti-BTN3A1 (BV421, clone 232-5, BD Biosciences) for 20 min at 4 °C. Next, freshly purified γδ T cells were untreated or treated with BrHPP (200 nM) for 3, 10, or 45 min or ionomycin (10 min at 10 µg/mL) under optimal culture conditions (37 °C and 5% CO2). Then, the cells were dropped on slides and fixed with 4% PFA for 10 min (room temperature). After a blocking incubation of 30 min (PBS 10% SVF, room temperature), γδ T cells were stained overnight at 4 °C with an anti-BTN2A1 (5 µg/mL, Biorbyt) primary antibody. After three washes, goat anti-rabbit (AF568) secondary antibody was added at 1 µg/mL for 1 h at room temperature. Finally, slides were mounted with Fluoromount-G mounting medium and analyzed on a Zeiss LSM 780 or 880 FAST Airyscan confocal microscope.
Quantitative colocalization analysis between BTN2A1, BTN3A1, and γ9TCR on the surface of freshly purified γδ T cells was achieved on the plot profile with the RGB profiler in ImageJ. For colocalization analysis, we determined Manders’ coefficient [27] on median optical sections using ImageJ and the JACoP plug-in.
Statistics
Data are expressed as the mean ± SEM. For comparison of two series of normally distributed variables, we used paired and one-tailed Student’s t tests with α = 0.05 for statistical significance. Statistical analysis was performed with Prism 7.0 software (GraphPad Inc.).
Results
PAg-activated Vγ9Vδ2 T cells are not sufficient for the activation of autologous resting Vγ9Vδ2 T cells
The goal of this study was to determine how purified resting Vγ9Vδ2 T cells can be activated by soluble PAgs such as BrHPP. Classically, target cells such as tumor cells activate Vγ9Vδ2 T cells through their butyrophilins, which are primarily activated by endogenous PAgs. However, even if the susceptibility of resting and activated Vγ9Vδ2 T cells to activation-induced cell death by TCR crosslinking is known [28], there is little research concerning the dialog between Vγ9Vδ2 T cells under PAg stimulation in the absence of an accessory cell. Thus, we wondered whether Vγ9Vδ2 T cells are also able to kill each other under PAg activation and whether the Vγ9Vδ2 killer needs PAg activation or if PAg sensing of the Vγ9Vδ2 target cell alone was sufficient.
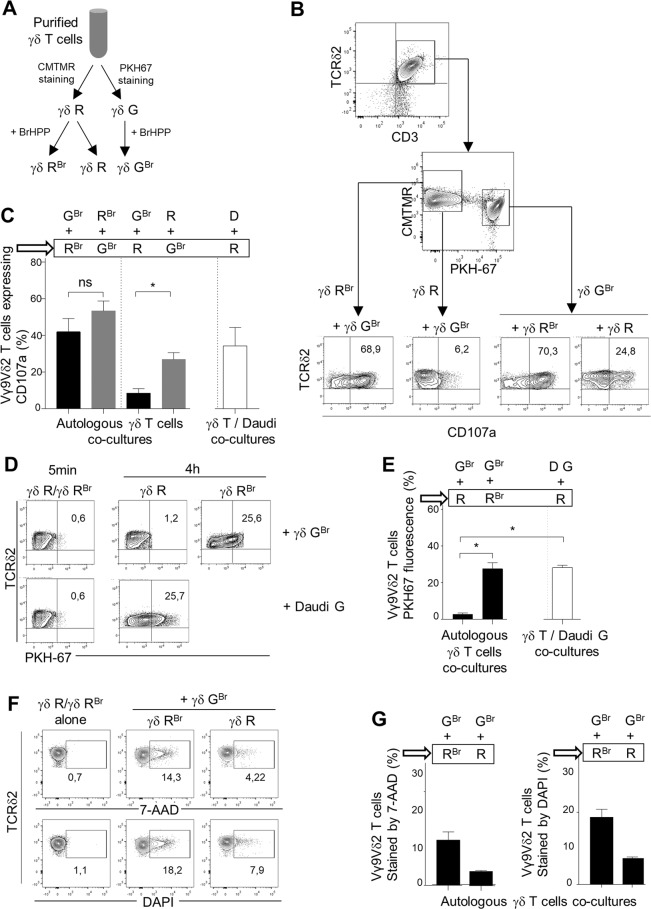
Purified resting γδ T cells from the same donor were divided into two groups. One group was stained for the intracellular marker CMTMR, and the other was stained for the lipophilic membrane marker PKH67 (Fig. 1A). We considered CMTMR+ Vγ9Vδ2 T cells (γδR) as effector cells and PKH67+ Vγ9Vδ2 T cells (γδG) as target cells. Daudi cells, from a cell line originating from a patient with Burkitt’s lymphoma, were also used as a target cell control. γδ T cells were prestimulated or not with BrHPP (γδ GBr, γδ R, γδ RBr). We next wanted to determine whether γδ T cells needed to be prestimulated with BrHPP to express CD107a. For that, we analyzed CD107a expression by γδ R or γδ RBr cocultured with γδ GBr by flow cytometry and showed that γδ RBr expressed CD107a after a coculture for 4 h but not γδ R. In the same cocultures, γδ GBr expressed CD107a in the two conditions: coculture with γδ R or with γδ RBr. The expression of CD107a by Vγ9Vδ2 T cells in contact with autologous Vγ9Vδ2 T cells requires BrHPP prestimulation. However, this prestimulation with PAg is not necessary for Vγ9Vδ2 T cells cultivated with Daudi cells, which are known to express endogenous PAgs [29] (Fig. 1B, C). CD107a is expressed following the establishment of the immunological synapse (IS), during which membrane patches are exchanged between the two partners involved in this IS, a phenomenon called trogocytosis [30]. Acquisition of membrane PKH67 fluorescence by the CMTMR+ group after 4 h of contact therefore reflects a stable IS, which is essential for the activation of lymphocytes [30]. We analyzed PKH67 fluorescence acquisition by γδ R or γδ RBr after 5 min or 4 h in coculture with γδ GBr. γδ RBr cells acquired PKH67 fluorescence after 4 h of contact with γδ GBr but not γδ R, although the same γδ R cells were able to acquire PKH67 fluorescence after contact with PKH67+ Daudi cells (Fig. 1D, E). Thus, autologous trogocytosis between two Vγ9Vδ2 T cells requires BrHPP prestimulation of the two partners.
Fig. 1.
Autologous killing of purified Vγ9Vδ2 T cells requires PAg activation. A Flow chart of the Vγ9Vδ2 T-cell preparation for coculture experiments (γδ R: CMTMR+ γδ T cells; γδ RBr: CMTMR+ γδ T cells pretreated with BrHPP; γδ GBr: PKH67+ γδ T cells pretreated with BrHPP). B, C Flow cytometry analysis of CD107a expression by either γδ RBr or γδ R cocultured with γδ GBr or by γδ GBr cocultured with γδ RBr or γδ R. B One representative experiment; C Twelve independent experiments included CD107a expression by γδR cells cocultured with Daudi cells (D)). D, E Flow cytometry of PKH67 fluorescence acquisition by γδ R or γδ RBr after coculture for 5 min or 4 h with γδ GBr or with PKH67+ Daudi cells (D G). (D One representative experiment (5 min of coculture gave the same result for γδ R or γδ RBr); E seven independent experiments). F, G Flow cytometry analysis of 7-AAD and DAPI staining of γδ RBr or γδ R cells cocultured with γδ GBr cells. (F One representative experiment. G Three independent experiments). Asterisk (*) indicates p < 0.05, Student’s paired t test; ns: not significant
To determine whether Vγ9Vδ2 T cells died following autologous trogocytosis, we quantified 7-AAD and DAPI staining of γδR or γδ RBr cocultured with γδ GBr. We showed that only γδ RBr cells were stained with 7-AAD and DAPI when cocultured with γδ GBr cells (Fig. 1F, G). Therefore, a Vγ9Vδ2 T cell needs to be stimulated by PAgs to kill another Vγ9Vδ2 T cell only if this cell is also activated by PAgs.
Vγ9Vδ2 T cells can self-activate through BrHPP in a TCR- and butyrophilin-dependent manner
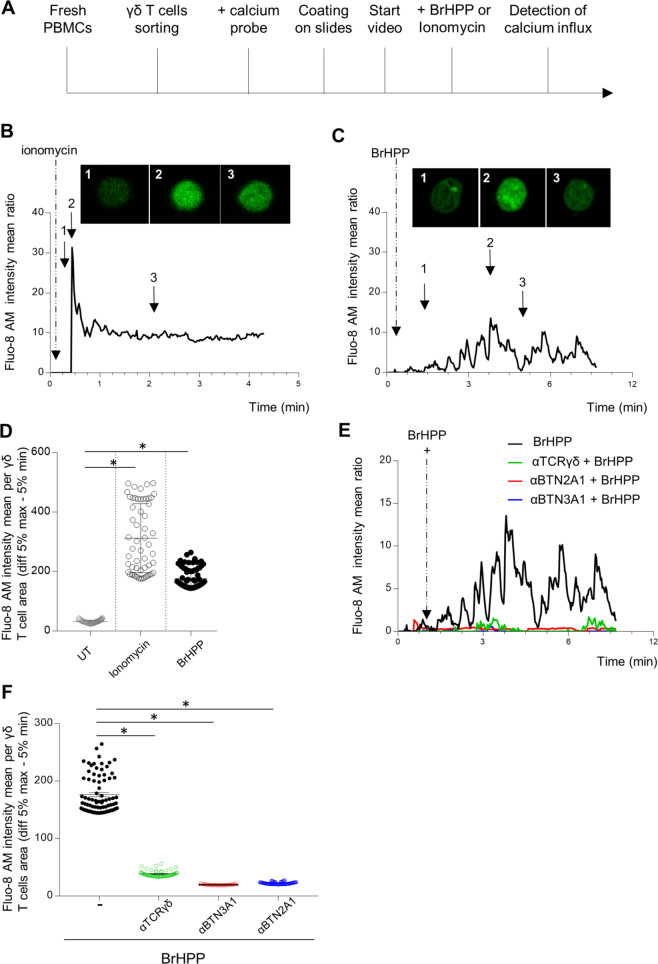
The above results led us to ask how purified γδ T lymphocytes can be activated by exogenous PAgs without any target cell and without cell contact. Thus, we monitored the calcium flux of individual Vγ9Vδ2 T cells by video microscopy under stimulation with exogenous PAgs (BrHPP, cHDMAP, or IPP) or with ionomycin, a calcium ionophore, as a positive control. Fresh γδ T cells sorted from blood samples of healthy donors were loaded with the calcium probe Fluo-8 AM-tagged and then coated on a microslide at a limited cell concentration to avoid cell contact. The stimulator was added with care to the well under the microscope 2 min after starting the video to detect the green fluorescence of the calcium flux (Fig. 2A). Stimulation with ionomycin led to a rapid increase in Fluo-8 AM fluorescence followed by stabilization (Fig. 2B). The Fluo-8 AM profile obtained with BrHPP stimulation was different, with several peaks of calcium flux in the same isolated Vγ9Vδ2 T cell (Fig. 2C, Supplementary Fig. 1 for the video). These profiles were reproduced for several isolated Vγ9Vδ2 T cells from different donors by measuring the ratio (Fluo-8 AM intensity mean/cell area) before and during stimulation with ionomycin or BrHPP (Fig. 2D). A significant increase in fluorescence in isolated γδ T cells was observed with ionomycin activation and BrHPP stimulation. This self-activation was shown with other PAgs, such as cHDMAP and IPP (Supplementary Fig. 2). The expression of IFN-γ, CD107a and CD69 measured by flow cytometry confirmed that Vγ9Vδ2 T cells can be activated by the exogenous PAgs BrHPP, cHDMAPP and IPP without a target or accessory cell. The same results were reproduced with anti-CD3/CD28 beads as a positive control (Supplementary Fig. 3). Interestingly, Vγ9Vδ2 T cells were not activated by an ABP such as zoledronate after incubation for 4 h, overnight or 4 days, whereas Vγ9Vδ2 T cells could be amplified in cultures of PBMCs as with BrHPP in the presence of IL2 and zoledronate (Supplementary Fig. 4).
Fig. 2.
Self-activation of resting purified Vγ9Vδ2 T cells by exogenous BrHPP is dependent on TCR, BTN3A1, and BTN2A1. A Sequence of actions for calcium flux detection by video in an individual Vγ9Vδ2 T cell. B–E Time lapse of the Fluo-8 AM intensity representing the calcium flux in one Vγ9Vδ2 T-cell stimulated by ionomycin (B) or exogenous BrHPP (C). Three images were extracted from the time lapses at three different time points of the stimulation. D Mean Fluo-8 AM intensity per γδ T cell area (difference 5% max–5% min). F Time lapse of the Fluo-8 AM intensity representing the calcium flux in one Vγ9Vδ2 T-cell stimulated by BrHPP in the presence or absence of blocking antibodies against γ9TCR, BTN3A1. or BTN2A1. Asterisk (*) indicates p < 0.05, Student’s paired t test; ns not significant
All these results show that Vγ9Vδ2 T cells can self-activate with exogenous PAgs without cell contact.
Next, we asked whether this PAg self-activation involved the same actors depicted for Vγ9Vδ2 T-cell activation in the context of contact with tumor cells. First, we checked that resting Vγ9Vδ2 T cells expressed BTN2A1 and BTN3A1 on their cell surface (Supplementary Fig. 5A) and that fresh Vγ9Vδ2 T cells could be activated by the BTN3A1 agonist (Supplementary Fig. 5B). Then, we analyzed the calcium flux in a single cell in the presence of blocking antibodies by video microscopy during BrHPP stimulation. Blocking antibodies against TCR and the two butyrophilins succeeded in decreasing calcium flux when added to cells stimulated by BrHPP (Fig. 2E, F and Supplementary Fig. 6 for the videos). Furthermore, the expression of IFN-γ, CD107a, and CD69 was measured by flow cytometry in purified resting Vγ9Vδ2 T cells stimulated with BrHPP in the presence or absence of blocking antibodies against TCR, BTN3A1, or BTN2A1. A clear decrease in the expression of the three markers was obtained for Vγ9Vδ2 T cells stimulated by BrHPP in the presence of each blocking antibody but not for Vγ9Vδ2 T cells stimulated with anti-CD3/CD28 beads (Supplementary Fig. 5C, D).
Thus, we showed that all the actors involved in the PAg-induced activation of Vγ9Vδ2 T cells in a cell contact context with tumor cells are also engaged in PAg self-activation.
Preexisting clustering of γ9TCR with BTN2A1 and BTN3A1 in resting Vγ9Vδ2 T cells
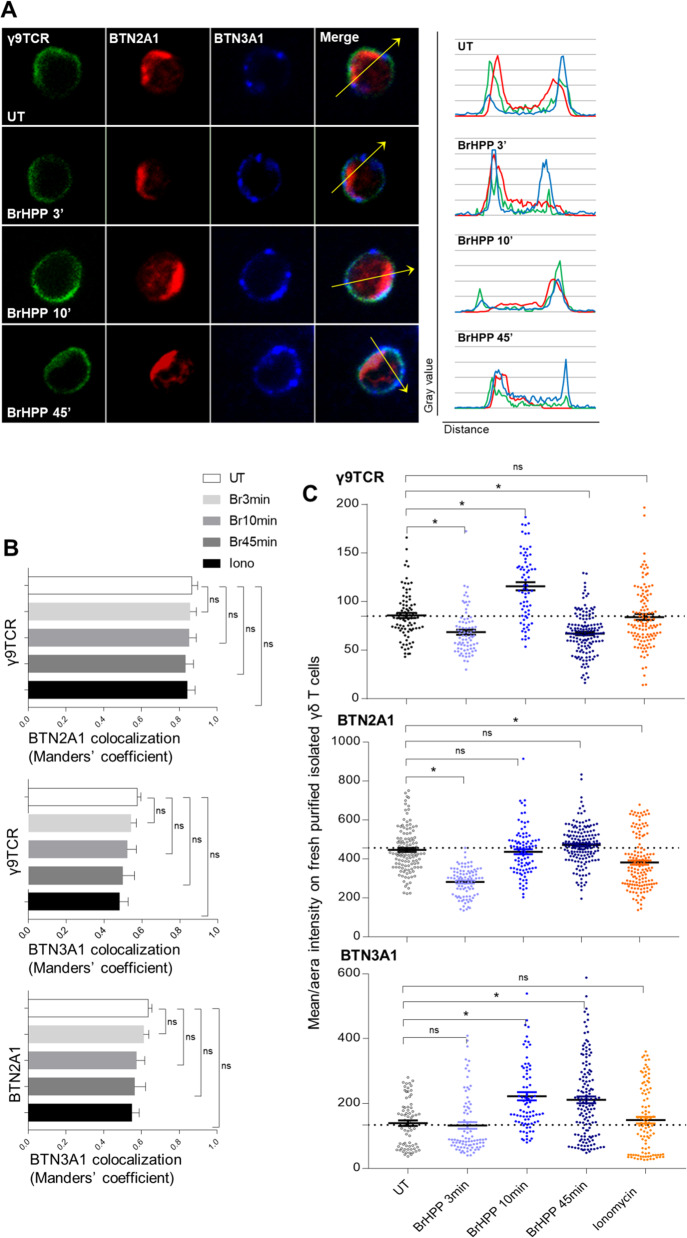
The next step was to understand the configuration of the involvement of γ9TCR, BTN2A1 and BTN3A1 at the surface of Vγ9Vδ2 T cells under BrHPP stimulation. To depict in detail Vγ9Vδ2 T-cell self-activation, we performed immunofluorescence staining of these three molecules on resting purified Vγ9Vδ2 T cells during activation with BrHPP (200 nM for 3, 10, or 45 min) or ionomycin (10 µM for 10 min) (Fig. 3A). As expected, we showed that γ9TCR expression (green fluorescence) was evenly distributed on the surface of fresh purified resting Vγ9Vδ2 T cells. γ9TCR, BTN2A1, and BTN3A1 were colocalized in several parts of the membrane before or after BrHPP stimulation, as shown in the profiles in the right panel of Fig. 3A. However, the colocalizations between the three structures were not equivalent. γ9TCR was more colocalized with BTN2A1 than with BTN3A1, with Manders’ coefficients of 0.86 vs. 0.56 (Fig. 3B). Then, we asked whether these colocalizations could be modulated upon BrHPP or ionomycin stimulation. The coefficient of colocalization of γ9TCR with BTN3A1 or BTN2A1 was not significantly modified, nor was that of BTN3A1/BTN2A1 colocalization (Fig. 3B).
Fig. 3.
Preexisting clusters of γ9TCR, BTN2A1 and BTN3A1 at the surface of resting Vγ9Vδ2 T cells and their modulation during BrHPP stimulation. A Immunofluorescence of γ9TCR, BTN2A1 and BTN3A1 on freshly purified isolated Vγ9Vδ2 T cells during BrHPP stimulation (representative images, γ9TCR: green fluorescence, BTN2A1: red fluorescence and BTN3A1: blue fluorescence and the merge) and colocalization profiles for each condition corresponding to the arrow). B Comparison of the colocalization of γ9TCR, BTN2A1 and BTN3A1 on freshly purified isolated Vγ9Vδ2 T cells during BrHPP or ionomycin stimulation quantified by Manders’ coefficient in ImageJ software. C Mean/area intensity of γ9TCR, BTN2A1, and BTN3A1 on freshly purified isolated Vγ9Vδ2 T cells during BrHPP or ionomycin stimulation. Asterisk (*) indicates p < 0.05, Student’s paired t test; ns not significant
To analyze the dynamics of these membrane proteins during stimulation with exogenous BrHPP, we quantified their fluorescence intensity according to the duration of the stimulation (Fig. 3C). The intensity of fluorescence was normalized to that of the untreated cells. A fluctuation of the fluorescence for γ9TCR was shown with a quick decrease after 3 min of stimulation with BrHPP and then an increase after 10 min to again decrease after a longer stimulation time (45 min). However, the fluorescence for γ9TCR observed after 10 min of stimulation with ionomycin was not modified compared to the unstimulated condition. Similar to γ9TCR fluorescence, BTN2A1 fluorescence decreased rapidly after 3 min of BrHPP stimulation to return to a similar intensity to the unstimulated control after 10 min. In contrast, the mean intensity of fluorescence for BTN3A1 increased but not in the first minutes, only from 10 min, and remained stable, while ionomycin stimulation did not change the fluorescence of BTN3A1.
Thus, all these results show that freshly purified Vγ9Vδ2 T cells transitorily modulate their early expression of BTN2A1 and γ9TCR and later BTN3A1 expression under BrHPP activation.
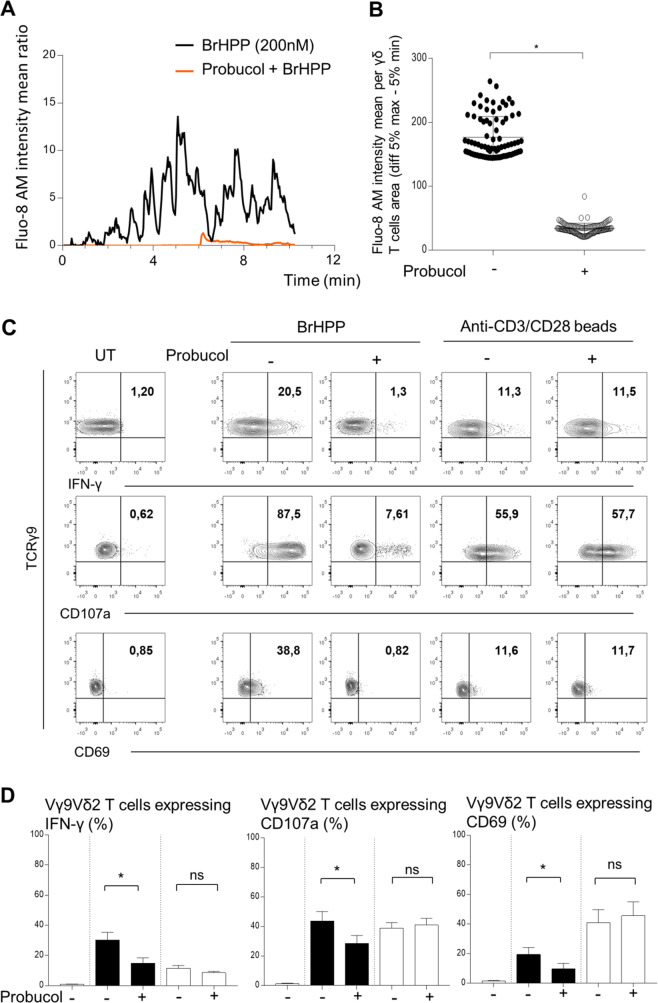
Finally, we wondered whether exogenous BrHPP needs to enter Vγ9Vδ2 T cells to activate them through the membrane cluster γ9TCR/BTN2A1/BTN3A1. As the Massimo Massaia group has shown that the ABCA1 transporter could be involved in IPP trafficking across the membrane [24], we investigated whether this transporter could be used for the transport of exogenous BrHPP inside Vγ9Vδ2 T cells. Using probucol, a specific inhibitor of ABCA1 that is not toxic to Vγ9Vδ2 T cells (Supplementary Fig. 7), we showed a total abrogation of calcium flux in Vγ9Vδ2 T cells activated by BrHPP in the presence of probucol (Fig. 4A), with a significant decrease in Fluo-8 AM intensity in Vγ9Vδ2 T cells treated with probucol before BrHPP stimulation (Fig. 4B). The involvement of ABCA1 in BrHPP stimulation was confirmed by a significant decrease in the expression of IFN-γ, CD107a and CD69 by purified resting Vγ9Vδ2 T cells stimulated by BrHPP, while stimulation with anti-CD3/CD28 beads was not affected (Fig. 4C (representative experiment) and Fig. 4D (pool of independent experiments)).
Fig. 4.
Inhibition of ABCA-1 by probucol impairs BrHPP self-activation of resting purified Vγ9Vδ2 T cells. A Time lapse of the Fluo-8 AM intensity representing the calcium flux in one Vγ9Vδ2 T-cell stimulated by BrHPP and previously treated with Probucol (10 µM, overnight). B Mean Fluo-8 AM intensity per γδ T-cell area (difference 5% max–5% min). C, D Flow cytometry analysis of the expression of CD69 and CD107a and IFN-γ production by fresh purified Vγ9Vδ2 T cells activated by BrHPP or anti-CD3/CD28 beads previously treated with Probucol (10 µM, overnight). (C A representative experiment; D Four independent experiments; black: Vγ9Vδ2 T cells activated by BrHPP; white: Vγ9Vδ2 T cells activated by anti-CD3/CD28 beads). Asterisk (*) indicates p < 0.05, Student’s paired t test; ns not significant
These results indicate that ABCA1 could be used by exogenous BrHPP to penetrate Vγ9Vδ2 T cells before self-activation.
Discussion
Recent years have seen renewed interest in Vγ9Vδ2 T-cell therapies and new strategies using Vγ9Vδ2 T cells (reviewed in [9]). Despite increased knowledge of many components involved in the Vγ9Vδ2 T-cell activation process, the mechanism is still elusive, and it must be known fully to understand the potential reasons for the failure of therapeutic strategies involving Vγ9Vδ2 T cells [31]. Among these components, butyrophilins, such as BTN3A1 and, more recently, BTN2A1, were shown to be key costimulatory molecules during Vγ9Vδ2 T-cell activation by PAg-sensing targets [18, 20, 21]. Several studies have demonstrated that BTN3A1 acts as a PAg sensor thanks to the interaction of its intracellular domain B30.2 with PAg [31–33] and to the cooperation of three BTN3 isoforms necessary for complete activation [19]. Although BTN3A1 was not shown to be directly linked to γ9TCR during PAg activation with a target cell, the interaction between BTN2A1 and TCR was shown to be essential in this process, as was the interaction between BTN2A1 and BTN3A1 [20, 21]. The interaction of BTN3A1 and γ9TCR is still an open question, as well as the possibility that BTN3A1 might serve as a chaperone molecule that brings another ligand to the cell surface, also not excluding the direct binding of BTN3A1 with γ9TCR [18].
All these discoveries were found in Vγ9Vδ2 T-cell activation in the presence of an accessory or a target cell, i.e., tumor cells naturally or pharmacologically overexpressing PAg or that were pretreated with exogenous PAg. Some early studies reported that Vγ9Vδ2 T cells could be activated directly by nonpeptidic mycobacterial ligands or by synthetic BrHPP, but there was no evidence of direct interaction of these PAgs and the TCR or other membrane molecules on Vγ9Vδ2 T cells [34, 35]. Based on these studies and others showing proliferation and production of IFN-γ by resting pure Vγ9Vδ2 T cells or δ2TCR T-cell clones treated with exogenous PAg and IL-2 [36–38], it seemed important to depict the mechanism of this direct activation.
In this study, we confirmed that Vγ9Vδ2 T cells could be directly activated by exogenous PAgs, i.e., BrHPP. We detected calcium flux in isolated pure resting cells very quickly after contact with exogenous PAg. The increase in intracellular calcium concentration was clearly shown to reflect TCR engagement and T-cell activation. Indeed, this calcium modulation is necessary for cytoskeletal remodeling during TCR signaling and the establishment of T-cell responses (reviewed in ref. [39]). Flow cytometry detection of the expression of CD107a, IFN-γ and CD69 and the fratricide of Vγ9Vδ2 T cells following BrHPP stimulation was consistent with self-activation. This phenomenon was also confirmed by the impossibility of Vγ9Vδ2 T cells to play the role of presenting cells for autologous resting Vγ9Vδ2 T cells. Furthermore, we showed that a Vγ9Vδ2 T cell needs to be stimulated by PAg to kill another Vγ9Vδ2 T cell after trogocytosis. CD107a expression and dead cells were not observed for Vγ9Vδ2 T cells not prestimulated by exogenous PAg in coculture with Vγ9Vδ2 T cells prestimulated by exogenous PAg (Fig. 1). A Vγ9Vδ2 T cell, therefore, cannot activate the TCR of another Vγ9Vδ2 T cell by its PAg-sensed butyrophilins.
Interestingly, we did not detect any increase in intracellular calcium concentration or expression of CD107a, IFN-γ and CD69 in Vγ9Vδ2 T cells treated with zoledronate for 4, 18, or 4 days. Aminobisphosphonate (ABP) treatments have been shown to activate Vγ9Vδ2 T cells but always in the context of contact between Vγ9Vδ2 T cells and accessory cells such as macrophages or dendritic cells in PBMCs or tumor cells. Indeed, accessory cells are generally pretreated with an ABP, such as zoledronate, to activate the production of endogenous PAg that can bind to the intracellular part of BTN [40–45]. The mechanism of action of zoledronate on Vγ9Vδ2 T cells remains unsolved: are Vγ9Vδ2 T cells unable to accumulate endogenous PAg, or are they unable to be activated by endogenous PAg?
PAg self-activation raised the question of the factors involved in this process. We showed here that γ9TCR, BTN3A1 and BTN2A1 were involved in BrHPP self-activation, as the application of blocking antibodies clearly decreased the calcium flux and expression of CD107a, IFN-γ, and CD69 on Vγ9Vδ2 T cells. Vγ9Vδ2 T-cell activation by anti-CD3/CD28 beads was, however, not impacted by blocking antibodies, therefore supporting the specific contribution of the three partners to BrHPP self-activation. Vγ9Vδ2 T cells are consequently able to respond to exogenous PAg through γ9TCR, BTN3A1 and BTN2A1, which are expressed at their surface. The modulation of the membrane expression of each of these membrane proteins also supports the PAg self-activation of Vγ9Vδ2 T cells. We have indeed shown that γ9TCR can be very quickly downregulated, similar to BTN2A1, while BTN3A1 was upregulated later. This is consistent with previous results showing the downregulation of the TCR following contact with PAg-sensing targets [46, 47] and the modulation of the expression of butyrophilins [19]. Modifications of the conformation of the butyrophilins in the presence of PAgs could also favor a weaker accessibility of antibodies to detect these proteins at the membrane surface [20, 21]. Moreover, very recent work has suggested that γ9TCR directly interacts with BTN2A1 at the target surface and not with BTN3A1, while the latter was shown to interact with BTN2A1 [20]. This could be accompanied by an upregulation in expression or a change in conformation later than for BTN2A1, as shown in Fig. 3C. The very rapid modulation of γ9TCR and BTN2A1 membrane expression in the first minutes after stimulation reflects the rapid modification of calcium flux observed under PAg stimulation. TCR engagement induces the modulation of calcium flux, which is mandatory for cytoskeletal remodeling, allowing calcium signaling as well as NKG2D costimulation, which leads to T-cell responses [39, 48]. Early modulation of γ9TCR and BTN2A1 expression and modulation of BTN3A1 are thus consistent with all of these observations.
Furthermore, the preexisting colocalization of γ9TCR, BTN3A1, and BTN2A1, which form several clusters at the surface of Vγ9Vδ2 T cells, was not modified in response to PAg treatment, which is consistent with the successive activation of isolated Vγ9Vδ2 T cells reflected by an irregular calcium flux profile compared to the uniform profile under ionomycin stimulation.
The sequence proposed by different studies, i.e., sensing of the intracellular domain of BTN3A1, conformational modification of BTN3A1, interaction with BTN2A1 and then interaction of BTN2A1 with γ9TCR [21, 44] could therefore be applied during PAg self-activation on a single γδ T cell. However, to date, nothing has been shown regarding the possible interaction of BrHPP and the B30.2 intracellular part of BTN3A1, as has been demonstrated for other PAgs, such as (E)-1-hydroxy-2-methyl-but-2-enyl 4-diphosphate (HMBPP) [32]. If BrHPP were able to interact with the B30.2 part of BTN3A1, the question is how this molecule can penetrate inside Vγ9Vδ2 T cells. ABCA1 was shown to physically associate with BTN3A1 on zoledronate-treated dendritic cells as a function of extracellular IPP release [24]. ABCA1, as another (ATP)-binding cassette (ABC) transporter, is a ubiquitous molecule expressed at the plasma membrane with bifunctional action, hydrolyzing ATP to ADP, and inorganic phosphate and exporting molecules such as cholesterol or calpains [49, 50]. ABCA1 can bind the extracellular domain of apolipoprotein A-I (apoA-I), which is required for the assembly of nascent high-density lipoprotein (HDL) mediating cholesterol transport [51]. Moreover, apoA-1 can bind F1-ATPase, which was shown to be an important molecule involved in Vγ9Vδ2 T-cell activation [52, 53]. These ABC transporters, which can also form complexes with other proteins to act as channels for export and import [49], can be inhibited by probucol. Indeed, several studies have shown that probucol was able to block cholesterol efflux, especially in smooth muscle cells, in the same way as ABCA1-KO of these cells [54, 55]. However, probucol was also shown to block ion channels, especially potassium channels, in cardiac cells [56, 57]. Using probucol, we showed in our study a clear decrease in Vγ9Vδ2 T-cell self-activation by exogenous BrHPP, with a total inhibition of calcium flux and CD107a and IFN-γ expression, while stimulation with anti-CD3/CD28 beads was not impaired by pretreatment with probucol. These results strongly suggested that exogenous BrHPP can penetrate inside Vγ9Vδ2 T cells through transporters inhibited by probucol. This can be extended to the BrHPP sensing of tumor cells thanks to the penetration of BrHPP through the same transporters, including ABCA1. Indeed, pretreatment with BrHPP in some lung cancer cell lines induced activation of Vγ9Vδ2 T cells [58].
Altogether, these results provide the first evidence that self-activation of pure resting Vγ9Vδ2 T cells from blood by exogenous PAg involves the γ9TCR, BTN3A1 and BTN2A1 organized in clusters at the plasma membrane. Some transporters associated with BTN3A1, such as ABCA1, could be considered critical for the entrance of PAgs into Vγ9Vδ2 T cells before activation of the B30.2 domains of BTN3A1.
Even though Vγ9Vδ2 T cells are now widely considered highly potent antitumor effectors, γδ T cell-based therapies with exogenous PAgs are lacking in efficacy. Self-activation with exogenous PAgs such as BrHPP leading to the autologous killing of Vγ9Vδ2 T cells could therefore contribute to this failure, in addition to their possible exhaustion and/or anergy.
Supplementary information
Video of fresh γδ T cells stimulated with BrHPP
Video of fresh γδ T cells stimulated with ionomycin
Video of fresh γδ T cells stimulated with cHDMAPP
Video of fresh γδ T cells stimulated with IPP
Video of fresh γδ T cells stimulated with zoledronate
Video of fresh γδ T cells stimulated with BrHPP after TCR blocking
Video of fresh γδ T cells stimulated with BrHPP after BTN3A1 blocking
Video of fresh γδ T cells stimulated with BrHPP after BTN2A1 blocking
Video of fresh γδ T cells stimulated with BrHPP after probucol treatment
Acknowledgements
This work was funded by INSERM, CNRS, the University Hospital of Bordeaux, and Toulouse III University. We acknowledge ImCheck for providing the 103.2 antibody and the 7.48 antibody. We are grateful to our healthcare professionals for their boundless efforts during the COVID-19 crisis.
Author contributions
C Laplagne performed the experiments. LL and FL participated in the microscopic experiments. JJF, CLaurent and SV participated in the discussion of the results. JF reviewed the English language. MP and CLaplagne designed the experiments and wrote the paper. MP supervised the study.
Competing interests
The authors declare no competing interests.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-021-00720-w.
References
- 1.Choudhary A, Davodeau F, Moreau A, Peyrat MA, Bonneville M, Jotereau F. Selective lysis of autologous tumor cells by recurrent gamma delta tumor-infiltrating lymphocytes from renal carcinoma. J Immunol. 1995;154:3932–40. [PubMed] [Google Scholar]
- 2.Kabelitz D, Wesch D, Pitters E, Zöller M. Characterization of tumor reactivity of human V gamma 9V delta 2 gamma delta T cells in vitro and in SCID mice in vivo. J Immunol. 2004;173:6767–76. doi: 10.4049/jimmunol.173.11.6767. [DOI] [PubMed] [Google Scholar]
- 3.Sicard H, Ingoure S, Luciani B, Serraz C, Fournié J-J, Bonneville M, et al. In vivo immunomanipulation of V gamma 9V delta 2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol. 2005;175:5471–80. doi: 10.4049/jimmunol.175.8.5471. [DOI] [PubMed] [Google Scholar]
- 4.Rei M, Pennington DJ, Silva-Santos B. The emerging Protumor role of γδ T lymphocytes: implications for cancer immunotherapy. Cancer Res. 2015;75:798–802. doi: 10.1158/0008-5472.CAN-14-3228. [DOI] [PubMed] [Google Scholar]
- 5.Donia M, Ellebaek E, Andersen MH, Straten PT, Svane IM. Analysis of Vδ1 T cells in clinical grade melanoma-infiltrating lymphocytes. OncoImmunology. 2012;1:1297–304. doi: 10.4161/onci.21659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Lin C, Li H, Li R, Wu Y, Liu H, et al. Tumor-infiltrating γδT cells predict prognosis and adjuvant chemotherapeutic benefit in patients with gastric cancer. OncoImmunology. 2017;6:e1353858. doi: 10.1080/2162402X.2017.1353858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived T cells of MICA and MICB. Proc Natl Acad Sci. 1999;96:6879–84. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corvaisier M, Moreau-Aubry A, Diez E, Bennouna J, Mosnier J-F, Scotet E, et al. Vγ9Vδ2 T cell response to colon carcinoma cells. J Immunol. 2005;175:5481–8. doi: 10.4049/jimmunol.175.8.5481. [DOI] [PubMed] [Google Scholar]
- 9.Sebestyen Z, Prinz I, Déchanet-Merville J, Silva-Santos B, Kuball J. Translating gammadelta (γδ) T cells and their receptors into cancer cell therapies. Nat Rev Drug Discov. 2020;19:169–84. doi: 10.1038/s41573-019-0038-z. [DOI] [PubMed] [Google Scholar]
- 10.Poupot M, Fournié J-J. Non-peptide antigens activating human Vγ9/Vδ2 T lymphocytes. Immunol Lett. 2004;95:129–38. doi: 10.1016/j.imlet.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Gober H-J, Kistowska M, Angman L, Jenö P, Mori L, De Libero G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hebbeler AM, Cairo C, Cummings JS, Pauza CD. Individual Vγ2-Jγ1.2+ T cells respond to both isopentenyl pyrophosphate and Daudi cell stimulation: generating tumor effectors with low molecular weight phosphoantigens. Cancer Immunol Immunother. 2007;56:819–29. doi: 10.1007/s00262-006-0235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoeres T, Holzmann E, Smetak M, Birkmann J, Wilhelm M. PD-1 signaling modulates interferon-γ production by Gamma Delta (γδ) T-cells in response to leukemia. Oncoimmunology. 2019;8:1550618. doi: 10.1080/2162402X.2018.1550618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossi C, Gravelle P, Decaup E, Bordenave J, Poupot M, Tosolini M, et al. Boosting γδ T cell-mediated antibody-dependent cellular cytotoxicity by PD-1 blockade in follicular lymphoma. Oncoimmunology. 2019;8:1554175. doi: 10.1080/2162402X.2018.1554175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ono K, Onishi Y, Kobayashi M, Hatta S, Nasu K, Watanabe S, et al. γδ T cell clonal proliferation early after PD-1 blockade. Ann Hematol. 2019;98:219–20. doi: 10.1007/s00277-018-3406-6. [DOI] [PubMed] [Google Scholar]
- 16.Chauvin C, Joalland N, Perroteau J, Jarry U, Lafrance L, Willem C, et al. NKG2D controls natural reactivity of Vγ9Vδ2 T lymphocytes against mesenchymal glioblastoma cells. Clin Cancer Res. 2019;25:7218–28. doi: 10.1158/1078-0432.CCR-19-0375. [DOI] [PubMed] [Google Scholar]
- 17.Chitadze G, Lettau M, Luecke S, Wang T, Janssen O, Fürst D, et al. NKG2D- and T-cell receptor-dependent lysis of malignant glioma cell lines by human γδ T cells: modulation by temozolomide and A disintegrin and metalloproteases 10 and 17 inhibitors. Oncoimmunology. 2016;5:e1093276. doi: 10.1080/2162402X.2015.1093276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harly C, Guillaume Y, Nedellec S, Peigné C-M, Mönkkönen H, Mönkkönen J, et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood. 2012;120:2269–79. doi: 10.1182/blood-2012-05-430470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vantourout P, Laing A, Woodward MJ, Zlatareva I, Apolonia L, Jones AW, et al. Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing γδ T cell biology. Proc Natl Acad Sci USA. 2018;115:1039–44. doi: 10.1073/pnas.1701237115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karunakaran MM, Willcox CR, Salim M, Paletta D, Fichtner AS, Noll A, et al. Butyrophilin-2A1 directly binds germline-encoded regions of the Vγ9Vδ2 TCR and is essential for phosphoantigen sensing. Immunity. 2020;52:487–98.e6. doi: 10.1016/j.immuni.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rigau M, Ostrouska S, Fulford TS, Johnson DN, Woods K, Ruan Z, et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science. 2020;367. 10.1126/science.aay5516. [DOI] [PubMed]
- 22.Sebestyen Z, Scheper W, Vyborova A, Gu S, Rychnavska Z, Schiffler M, et al. RhoB mediates phosphoantigen recognition by Vγ9Vδ2 T cell receptor. Cell Rep. 2016;15:1973–85. doi: 10.1016/j.celrep.2016.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhodes DA, Chen H-C, Price AJ, Keeble AH, Davey MS, James LC, et al. Activation of human γδ T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin. J Immunol. 2015;194:2390–8. doi: 10.4049/jimmunol.1401064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castella B, Kopecka J, Sciancalepore P, Mandili G, Foglietta M, Mitro N, et al. The ATP-binding cassette transporter A1 regulates phosphoantigen release and Vγ9Vδ2 T cell activation by dendritic cells. Nat Commun. 2017;8:15663. doi: 10.1038/ncomms15663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belmant C, Espinosa E, Halary F, Tang Y, Peyrat M-A, Sicard H, et al. A chemical basis for selective recognition of nonpeptide antigens by human δ T cells. FASEB J. 2000;14:1669–70. doi: 10.1096/fj.99-0909fje. [DOI] [PubMed] [Google Scholar]
- 26.Scotet E, Martinez LO, Grant E, Barbaras R, Jenö P, Guiraud M, et al. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Manders EM, Stap J, Brakenhoff GJ, van Driel R, Aten JA. Dynamics of three-dimensional replication patterns during the S-phase, analysed by double labelling of DNA and confocal microscopy. J Cell Sci. 1992;103:857–62. doi: 10.1242/jcs.103.3.857. [DOI] [PubMed] [Google Scholar]
- 28.Gan YH, Lui SS, Malkovsky M. Differential susceptibility of naïve and activated human gammadelta T cells to activation-induced cell death by T-cell receptor cross-linking. Mol Med Camb Mass. 2001;7:636–43. [PMC free article] [PubMed] [Google Scholar]
- 29.Fisch P, Malkovsky M, Kovats S, Sturm E, Braakman E, Klein BS, et al. Recognition by human V gamma 9/V delta 2 T cells of a GroEL homolog on Daudi Burkitt’s lymphoma cells. Science. 1990;250:1269–73. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- 30.Gertner J, Wiedemann A, Poupot M, Fournié J-J. Human gammadelta T lymphocytes strip and kill tumor cells simultaneously. Immunol Lett. 2007;110:42–53. doi: 10.1016/j.imlet.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Herrmann T, Fichtner AS, Karunakaran MM. An Update on the molecular basis of phosphoantigen recognition by Vγ9Vδ2 T cells. Cells. 2020;9. 10.3390/cells9061433. [DOI] [PMC free article] [PubMed]
- 32.Yang Y, Li L, Yuan L, Zhou X, Duan J, Xiao H, et al. A structural change in butyrophilin upon phosphoantigen binding underlies phosphoantigen-mediated Vγ9Vδ2 T cell activation. Immunity. 2019;50:1043–53.e5. doi: 10.1016/j.immuni.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Dustin ML, Scotet E, Olive D. An X-ray vision for phosphoantigen recognition. Immunity. 2019;50:1026–8. doi: 10.1016/j.immuni.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Lang F, Peyrat MA, Constant P, Davodeau F, David-Ameline J, Poquet Y, et al. Early activation of human V gamma 9V delta 2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J Immunol. 1995;154:5986–94. [PubMed] [Google Scholar]
- 35.Espinosa E, Belmant C, Pont F, Luciani B, Poupot R, Romagné F, et al. Chemical synthesis and biological activity of bromohydrin pyrophosphate, a potent stimulator of human gamma delta T cells. J Biol Chem. 2001;276:18337–44. doi: 10.1074/jbc.M100495200. [DOI] [PubMed] [Google Scholar]
- 36.Martinet L, Fleury-Cappellesso S, Gadelorge M, Dietrich G, Bourin P, Fournié J-J, et al. A regulatory cross-talk between Vgamma9Vdelta2 T lymphocytes and mesenchymal stem cells. Eur J Immunol. 2009;39:752–62. doi: 10.1002/eji.200838812. [DOI] [PubMed] [Google Scholar]
- 37.Martinet L, Poupot R, Mirshahi P, Rafii A, Fournié J-J, Mirshahi M, et al. Hospicells derived from ovarian cancer stroma inhibit T-cell immune responses. Int J Cancer. 2010;126:2143–52. doi: 10.1002/ijc.24881. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 39.Joseph N, Reicher B, Barda-Saad M. The calcium feedback loop and T cell activation: how cytoskeleton networks control intracellular calcium flux. Biochim Biophys Acta. 2014;1838:557–68. doi: 10.1016/j.bbamem.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Serrano R, Wesch D, Kabelitz D. Correction: Serrano, R.; Wesch, D.; Kabelitz, D. Activation of human γδ T cells: modulation by toll-like receptor 8 ligands and role of monocytes. Cells 2020, 9, 713. Cells. 2020;9. 10.3390/cells9091977.
- 41.Riganti C, Castella B, Massaia M. ABCA1, apoA-I, and BTN3A1: a legitimate ménage à Trois in dendritic cells. Front Immunol. 2018;9:1246. doi: 10.3389/fimmu.2018.01246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cabillic F, Toutirais O, Lavoué V, de La Pintière CT, Daniel P, Rioux-Leclerc N, et al. Aminobisphosphonate-pretreated dendritic cells trigger successful Vgamma9Vdelta2 T cell amplification for immunotherapy in advanced cancer patients. Cancer Immunol Immunother. 2010;59:1611–9. doi: 10.1007/s00262-010-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'asaro M, La Mendola C, Di Liberto D, Orlando V, Todaro M, Spina M, et al. V gamma 9V delta 2 T lymphocytes efficiently recognize and kill zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant chronic myelogenous leukemia cells. J Immunol. 2010;184:3260–8. doi: 10.4049/jimmunol.0903454. [DOI] [PubMed] [Google Scholar]
- 44.Sandstrom A, Peigné C-M, Léger A, Crooks JE, Konczak F, Gesnel M-C, et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity. 2014;40:490–500. doi: 10.1016/j.immuni.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen K, Li J, Puthenveetil R, Lin X, Poe MM, Hsiao CC, et al. The butyrophilin 3A1 intracellular domain undergoes a conformational change involving the juxtamembrane region. FASEB J. 2017;31:4697–706. doi: 10.1096/fj.201601370RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sireci G, Espinosa E, Di Sano C, Dieli F, Fournié JJ, Salerno A. Differential activation of human gammadelta cells by nonpeptide phosphoantigens. Eur J Immunol. 2001;31:1628–35. doi: 10.1002/1521-4141(200105)31:5<1628::AID-IMMU1628>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 47.Alcover A, Alarcón B. Internalization and intracellular fate of TCR-CD3 complexes. Crit Rev Immunol. 2000;20:325–46. doi: 10.1615/CritRevImmunol.v20.i4.20. [DOI] [PubMed] [Google Scholar]
- 48.Nedellec S, Sabourin C, Bonneville M, Scotet E. NKG2D costimulates human V gamma 9V delta 2 T cell antitumor cytotoxicity through protein kinase C theta-dependent modulation of early TCR-induced calcium and transduction signals. J Immunol. 2010;185:55–63. doi: 10.4049/jimmunol.1000373. [DOI] [PubMed] [Google Scholar]
- 49.Efferth T. Adenosine triphosphate-binding cassette transporter genes in ageing and age-related diseases. Ageing Res Rev. 2003;2:11–24. doi: 10.1016/S1568-1637(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 50.Perez J, Dansou B, Hervé R, Levi C, Tamouza H, Vandermeersch S, et al. Calpains released by T lymphocytes cleave TLR2 to control IL-17 expression. J Immunol. 2016;196:168–81. doi: 10.4049/jimmunol.1500749. [DOI] [PubMed] [Google Scholar]
- 51.Hafiane A, Genest JHDL. Atherosclerosis, and emerging therapies. Cholesterol. 2013;2013:891403. doi: 10.1155/2013/891403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scotet E, Martinez LO, Grant E, Barbaras R, Jenö P, Guiraud M, et al. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 53.Vantourout P, Mookerjee-Basu J, Rolland C, Pont F, Martin H, Davrinche C, et al. Specific requirements for Vgamma9Vdelta2 T cell stimulation by a natural adenylated phosphoantigen. J Immunol. 2009;183:3848–57. doi: 10.4049/jimmunol.0901085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Favari E, Zanotti I, Zimetti F, Ronda N, Bernini F, Rothblat GH. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler Thromb Vasc Biol. 2004;24:2345–50. doi: 10.1161/01.ATV.0000148706.15947.8a. [DOI] [PubMed] [Google Scholar]
- 55.Delvecchio CJ, Bilan P, Nair P, Capone JP. LXR-induced reverse cholesterol transport in human airway smooth muscle is mediated exclusively by ABCA1. Am J Physiol Lung Cell Mol Physiol. 2008;295:L949–57. doi: 10.1152/ajplung.90394.2008. [DOI] [PubMed] [Google Scholar]
- 56.Cubeddu LX. Drug-induced inhibition and trafficking disruption of ion channels: pathogenesis of QT abnormalities and drug-induced fatal arrhythmias. Curr Cardiol Rev. 2016;12:141–54. doi: 10.2174/1573403X12666160301120217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi Y-Q, Fan P, Zhang G-C, Zhang Y-H, Li M-Z, Wang F, et al. Probucol-induced hERG channel reduction can be rescued by matrine and oxymatrine in vitro. Curr Pharm Des. 2020;25:4606–12. doi: 10.2174/1381612825666191026170033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laplagne C, Meddour S, Figarol S, Michelas M, Calvayrac O, Favre G, et al. Vγ9Vδ2 T cells activation through phosphoantigens can be impaired by a RHOB rerouting in lung cancer. Front Immunol. 2020;11:1396. doi: 10.3389/fimmu.2020.01396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video of fresh γδ T cells stimulated with BrHPP
Video of fresh γδ T cells stimulated with ionomycin
Video of fresh γδ T cells stimulated with cHDMAPP
Video of fresh γδ T cells stimulated with IPP
Video of fresh γδ T cells stimulated with zoledronate
Video of fresh γδ T cells stimulated with BrHPP after TCR blocking
Video of fresh γδ T cells stimulated with BrHPP after BTN3A1 blocking
Video of fresh γδ T cells stimulated with BrHPP after BTN2A1 blocking
Video of fresh γδ T cells stimulated with BrHPP after probucol treatment