Abstract
Background
Coronary artery calcification (CAC) is an independent risk factor of major adverse cardiovascular events; however, the impact of CAC on in-hospital death and adverse clinical outcomes in patients with coronavirus disease 2019 (COVID-19) remains unclear.
Objective
To explore the association between CAC and in-hospital mortality and adverse events in patients with COVID-19.
Methods
This multicenter retrospective cohort study enrolled 2067 laboratory-confirmed COVID-19 patients with definitive clinical outcomes (death or discharge) admitted from 22 tertiary hospitals in China between January 3, 2020 and April 2, 2020. Demographic, clinical, laboratory results, chest CT findings, and CAC on admission were collected. The primary outcome was in-hospital death and the secondary outcome was composed of in-hospital death, admission to intensive care unit (ICU), and requiring mechanical ventilation. Multivariable Cox regression analysis and Kaplan–Meier plots were used to explore the association between CAC and in-hospital death and adverse clinical outcomes.
Results
The mean age was 50 years (SD,16) and 1097 (53.1%) were male. A total of 177 patients showed high CAC level, and compared with patients with low CAC, these patients were older (mean age: 49 vs. 69 years, P < 0.001) and more likely to be male (52.0% vs. 65.0%, P = 0.001). Comorbidities, including cardiovascular disease (CVD) ([33.3%, 59/177] vs. [4.7%, 89/1890], P < 0.001), presented more often among patients with high CAC, compared with patients with low CAC. As for laboratory results, patients with high CAC had higher rates of increased D-dimer, LDH, as well as CK-MB (all P < 0.05). The mean CT severity score in high CAC group was also higher than low CAC group (12.6 vs. 11.1, P = 0.005). In multivariable Cox regression model, patients with high CAC were at a higher risk of in-hospital death (hazard ratio [HR], 1.731; 95% CI 1.010–2.971, P = 0.046) and adverse clinical outcomes (HR, 1.611; 95% CL 1.087–2.387, P = 0.018).
Conclusion
High CAC is a risk factor associated with in-hospital death and adverse clinical outcomes in patients with confirmed COVID-19, which highlights the importance of calcium load testing for hospitalized COVID-19 patients and calls for attention to patients with high CAC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42058-021-00072-4.
Keywords: Coronavirus disease 2019, Coronary artery calcification, Cardiovascular disease, Mortality
Introduction
Since December 2019, coronavirus disease-19 (COVID-19) has ravaged more than 200 countries and regions with an increasing number of cases every day around the world. Although the overall case fatality rate of COVID-19 (7%) seems to be lower than that of severe acute respiratory syndrome coronavirus (SARS) (10%) and Middle East respiratory syndrome (MERS) (37%) [1–4], COVID-19 has caused far more deaths than SARS and MERS combined. As of June 29, 2020, a total of 10,021,401 patients have been diagnosed with COVID-19, including 499,913 deaths [1].
Although patients with confirmed COVID-19 mainly present with respiratory symptoms, lots of patients were reported to have developed serious cardiac complications [4, 5], which were reported to be associated with adverse outcomes. In addition, cardiovascular comorbidities are common in patients with COVID-19 who are at higher risk of morbidity and mortality [6–7]. The detection of coronary artery calcification (CAC) is considered to play an indelible role in primary prevention of coronary artery disease (CAD) [8], as the existence, scope, and progress of CAC has been recognized as a strong predictor for cardiovascular event and all-cause mortality in general population [9, 10]. However, despite being a well-described imaging biomarker of the burden of atherosclerosis, to date, the association between CAC and the risk of in-hospital mortality as well as adverse outcomes in patients with confirmed COVID-19 remains unclear.
Therefore, this study aimed to analyze the association between CAC and in-hospital mortality and adverse events in patients with COVID-19. We retrospectively analyzed data from a total of 2067 patients with laboratory-confirmed COVID-19 with definitive clinical outcomes from 22 tertiary hospitals in China to investigate the impact of the severity degree of CAC on in-hospital mortality and adverse events in COVID-19 patients to further optimize patients’ clinical management.
Materials and methods
Study participants
This multicenter retrospective cohort study was performed in 22 medical centers across 11 provinces and municipalities in China (Figure S1). Consecutive patients admitted from January 3, 2020 to April 2, 2020 were included. Inclusion criteria were: (a) infection of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was confirmed by reverse transcription–polymerase chain reaction (RT–PCR) assay for nasal and pharyngeal swab specimens or high-throughput sequencing; (b) thin-section chest CT scan was performed on admission; (c) definitive prognosis information was available (death or discharge). The exclusion criteria were: (a) unavailable CT images, and (b) previous history of percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). This study was approved by the institutional review boards of Jinling Hospital (2020NZKY-005–02). Written informed consent was waived owing to retrospective data. This study has been registered with the Chinese Clinical Trial Registry (the registration number: ChiCTR2000030863).
Data collection
All data of patients on admission were extracted from electronic medical record systems by primary investigators of each center. The following demographic and clinical characteristics were collected: age, sex, preexisting comorbidity (cardiovascular disease [CVD], diabetes, hypertension, chronic obstructive pulmonary disease [COPD], chronic liver disease, chronic kidney disease, malignancy). Laboratory values included lymphocyte, D-dimer, lactate dehydrogenase (LDH), creatine kinase myocardial band (CK-MB), cardiac troponin, and procalcitonin. Data were sent to a computerized database and cross-checked by two physicians (S.L. and P.P.X.) in core lab in Jinling Hospital, Medical School of Nanjing University. Incomplete data were reconfirmed and clarified by primary investigators of each center.
Chest CT acquisition and image analysis
CT scanners and scanning protocols included in this study are presented in Supplemental materials. All chest CT images were reviewed by four cardiothoracic radiologists (Z.Y.S., L.Q., F. X., and X.L.Z. with 18, 6, 5, and 5 years of experiences, respectively) in core lab in Jinling Hospital, Medical School of Nanjing University. They independently evaluated initial CT images without being informed of patient’s clinical or laboratory results. Any disagreement between reviewers was resolved through consultation with another senior physician (G.M.L., with 32 years experiences in chest imaging). The following CT manifestations were analyzed and recorded: pure ground-glass opacity (GGO), pure consolidation, GGO with consolidation, interstitial lung disease (ILD), crazy-paving pattern, pleural effusion, and pericardial effusion. We proposed a CT severity score based on lung segment to evaluate the severity of COVID-19 pneumonia [11]. If both lungs are involved, the highest score is 20.
CAC evaluation
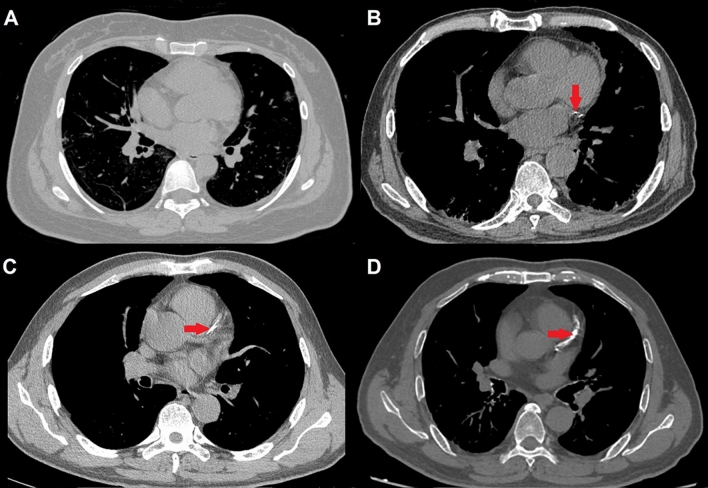
In this study, we adopted a simple visual CAC scoring method throughout the entire coronary circulation in noncontrast noncardiac chest CT scans recommended by 2016 SCCT/STR guidelines [12]. In the CAC scoring adopted, the calcification degree was recorded according to the severity of calcification: 0: no calcification; 1: only isolated spot of CAC within a segment; 2: the degree of calcification is between 1 and 3 points; 3: continuous CAC within a segment [12]. Some representative images are shown in Fig. 1. We merged points 0 and 1 into low CAC group, while points 2 and 3 were combined into high CAC group. CAC was evaluated independently by two cardiothoracic radiologists (J.Z. and S.L. both with 12 years of experiences in cardiovascular radiology) in core lab of Jinling Hospital, Medical School of Nanjing University. Any disagreement was resolved by consensus reading.
Fig. 1.
Representative CAC evaluation of patients with COVID-19, A Point 0: no coronary calcification is observed on chest CT image; B Point 1: only isolated spot of CAC is seen within a segment; C Point 2: the degree of calcification is between points 1 and 3; D Point 3: continuous CAC is observed within a segment. Points 0 and 1 are classified as low CAC groups, while points 2 and 3 are classified as high CAC groups
Outcomes
The primary outcome was COVID-19-associated in-hospital death, and the secondary composite outcome included in-hospital death, admission to ICU, and requiring mechanical ventilation. The composite outcome was adopted, because ICU admission, mechanical ventilation, as well as death were serious outcomes of COVID-19 and had been used to evaluate the severity of other serious infectious diseases in the previous studies [13, 14]. Discharge criteria included: afebrile for at least 3 days, both lungs showed significant improvement on chest CT, respiratory symptoms were alleviated in clinical practice, and repeated negative RT–PCR results ≥ 24 h interval [15].
Statistical analysis
Kolmogorov–Smirnov test was used to assess the normality of quantitative data. Mean with standard deviation (SD) and median with interquartile range (IQR) were used to describe continuous variables where necessary, while frequency with percentage was used to describe categorical variables. Student’s t tests and Wilcoxon’s rank sum tests were used for comparing the differences between continuous variables, while Pearson’s Chi-square tests and Fisher’s exact tests were used for comparing categorical variables. Univariable Cox proportional hazard regression model was performed for identifying potential risk factors for death and adverse outcomes. Those variables with P < 0.05 in univariable analysis were selected into multivariable Cox regression model by stepwise regression method. Hazard ratio (HR) and 95% confidence interval (CI) were calculated and missing data in multivariable analysis for categorical variables were coded as an unknown class to regress. The proportionality of hazard assumption was evaluated by Schoenfeld residuals. Kaplan–Meier plots and log-rank test were used to compare the cumulative event rate of death and adverse outcomes between groups. A P value < 0.05 was considered as statistical significance. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, North Carolina, USA).
Results
Patient characteristics
A total of 2067 patients with mean age of 50 years (SD 16), including 1097 (53.1%) males and 970 (46.9%) females, were enrolled in this study. 222 patients were excluded because of unavailable CT images (n = 200), previous percutaneous coronary intervention (n = 21), and coronary artery bypass grafting (n = 1). The study flowchart is shown in Figure S2.
Table 1 shows the clinical characteristics, laboratory results, and chest CT findings of patients with COVID-19 in different CAC groups. The median interval between admission and discharge/death was 19 [14–25] days. A total of 1989 (96.2%, 1989/2067) patients recovered and discharged, while 78 patients (3.8%, 78/2067) did not survive. It was noteworthy that during hospitalization, 165 patients (8%, 165/2067) developed adverse outcomes. In addition to 78 (3.8%, 78/2067) deceased, 128 patients (6.2%, 128/2067) were transmitted to ICU, and 96 patients (4.6%, 96/2067) received mechanical ventilation.
Table 1.
Clinical characteristics, laboratory results, and chest CT findings of patients with COVID-19 in different CAC groups
| Variables | All patients (n = 2067) | Low CAC (n = 1890) | High CAC (n = 177) | P value |
|---|---|---|---|---|
| Age, year ± SD | 50.3 ± 15.6 | 48.5 ± 14.8 | 68.7 ± 12.4 | < 0.001 |
| Age > 60 years—no. (%) | 578 (28.0) | 442 (23.4) | 136 (76.8) | < 0.001 |
| Sex (male)—no. (%) | 1097 (53.1) | 982 (52.0) | 115 (65.0) | 0.001 |
| Comorbidity—no. (%) | ||||
| CVD | 148 (7.2) | 89 (4.7) | 59 (33.3) | < 0.001 |
| Diabetes | 216 (10.5) | 161 (8.5) | 55 (31.1) | < 0.001 |
| Hypertension | 414 (20.0) | 320 (16.9) | 94 (53.1) | < 0.001 |
| COPD | 39 (1.9) | 28 (1.5) | 11 (6.2) | 0.0003 |
| Chronic liver disease | 77 (3.8) | 72 (3.8) | 5 (2.8) | 0.678 |
| Chronic kidney disease | 21 (1.0) | 17 (0.9) | 4 (2.3) | 0.099 |
| Malignancy | 39 (1.9) | 30 (1.6) | 9 (5.1) | 0.005 |
| Adverse outcomes—no. (%) | 165 (8.0) | 132 (6.9) | 45 (27.3) | < 0.001 |
| Death | 78 (3.8) | 52 (2.8) | 27 (15.3) | < 0.001 |
| ICU admission | 128 (6.2) | 91 (4.8) | 37 (20.9) | < 0.001 |
| Mechanical ventilation | 96 (4.6) | 73 (3.9) | 23 (13.0) | < 0.001 |
| The interval between admission and discharge/death (IQR) | 19 (14–25) | 18 (13–24) | 20 (16–27) | < 0.001 |
| Laboratory results—no. (%) | ||||
| Lymphopenia | 819 (39.6) | 748 (39.6) | 69 (39.0) | 0.288 |
| Missing | 413 (20.0) | 393 (20.8) | 20 (11.3) | |
| D-dimer (increased) | 405 (19.6) | 331 (17.5) | 74 (41.8) | < 0.001 |
| Missing | 989 (47.8) | 931 (49.3) | 58 (32.8) | |
| LDH (increased) | 516 (25.0) | 450 (23.8) | 66 (37.3) | 0.007 |
| Missing | 805 (39.0) | 754 (39.9) | 51 (28.8) | |
| CK-MB (increased) | 63 (3.0) | 51 (2.7) | 12 (6.8) | 0.026 |
| Missing | 924 (44.7) | 860 (45.5) | 64 (36.2) | |
| Cardiac troponin (increased) | 358 (17.3) | 309 (16.3) | 49 (27.7) | 0.206 |
| Missing | 1623 (78.5) | 309 (16.3) | 49 (27.7) | |
| Procalcitonin (increased) | 206 (10.0) | 184 (9.7) | 22 (12.4) | 0.980 |
| Missing | 1023 (49.9) | 957 (50.6) | 66 (37.3) | |
| Chest CT finding | ||||
| Signs—no. (%) | ||||
| GGO | 123 (6.0) | 113 (6.0) | 10 (6.0) | 0.860 |
| Consolidation | 56 (2.7) | 48 (2.5) | 8 (4.5) | 0.140 |
| GGO + consolidation | 1769 (85.6) | 1612 (85.3) | 157 (88.7) | 0.263 |
| ILD | 1675 (81.0) | 1515 (80.2) | 160 (90.4) | 0.001 |
| Crazy paving pattern | 1291 (62.5) | 1179 (62.4) | 112 (63.3) | 0.814 |
| Pleural effusion | 125 (6.0) | 98 (5.2) | 27 (15.3) | < 0.001 |
| Pericardial effusion | 56 (2.7) | 43 (2.3) | 13 (7.3) | 0.001 |
| CT severity score (SD) | 11.2 ± 7.0 | 11.1 ± 7.0 | 12.6 ± 6.9 | 0.005 |
Data are given as mean (SD), n (%) or median (IQR). The normal range refers to the criteria of each hospital. Increased means over the upper limit of the normal range and decreased means below the lower limit of the normal range
COVID-19 Coronavirus Disease-19, CAC coronary artery calcification, SD standard deviation, IQR interquartile range, CVD cardiovascular disease, COPD chronic obstructive pulmonary disease, ICU intensive care unit, LDH lactate dehydrogenase, CK-MB creatine kinase myocardial band, GGO ground-glass opacity, ILD interstitial lung disease
Compared with patients in low CAC group (91.4%, 1890/2067), patients with high CAC (8.6%, 177/2067) were older (mean age: 68.7 vs. 48.5, P < 0.001) and more likely to be male ([65.0%, 115/177] vs. [52.0%, 982/1890], P = 0.001). More comorbidities, including CVD ([33.3%, 59/177] vs. [4.7%, 89/1890], P < 0.001), diabetes ([31.1%, 55/177] vs. [8.5%, 161/1890], P < 0.001), hypertension ([53.1%, 94/177] vs. [16.9%, 320/1890], P < 0.001), COPD ([6.2%, 11/177] vs. [1.5%, 28/1890], P = 0.0003), and malignancy ([5.1%,9 /177] vs. [1.6%, 30/1890], P = 0.005), were found in patients with high CAC than patients with low CAC. As for laboratory results, patients with high CAC had higher rates of increased D-dimer, LDH, as well as CK-MB (all P < 0.05). In terms of signs of CT, high CAC group showed more ILD ([90.4%, 160/177] vs. [80.2%, 1515/1890], P = 0.001), pleural effusion ([15.3%, 27/177], vs. [5.2%, 98/1890] P < 0.001), as well as pericardial effusion ([7.3%, 13/177] vs. [2.3%, 43/1890], P = 0.001) than low CAC group. The mean CT severity score in high CAC group was also higher than low CAC group (12.6 vs. 11.1, P = 0.005). Comparisons between discharge and death, stable, and adverse outcomes groups are shown in Supplemental Table 1.
Relations between clinical characteristics, laboratory results, CT Imaging features, and death and adverse outcomes on cox regression analysis
In univariable analysis, older age (> 60 years), sex (male), high CAC, comorbidity (CVD, hypertension, diabetes, COPD, chronic kidney disease, malignancy), lymphopenia, higher level of D-dimer, CK-MB, LDH, procalcitonin, higher CT severity score, the presence of pleural effusion, and pericardial effusion were shown to be associated with in-hospital death and adverse outcomes (all P < 0.05) (Table 2, Supplemental Table 2).
Table 2.
Univariable and multivariable cox regression results on the risk factors associated with mortality and adverse outcomes in patients with COVID-19
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P values | HR | 95% CI | P values | |
| Mortality | ||||||
| Age > 60 years | 5.859 | 3.314–9.769 | < 0.001 | 2.892 | 1.668–5.014 | < 0.001 |
| Sex (Male) | 1.999 | 1.235–3.235 | 0.005 | 1.979 | 1.217–3.217 | 0.006 |
| Higher CAC | 4.511 | 2.811–7.242 | < 0.001 | 1.731 | 1.010–2.971 | 0.046 |
| CVD | 6.621 | 4.175–10.499 | < 0.001 | 3.184 | 1.901–5.334 | < 0.001 |
| Lymphopenia | 5.500 | 2.818–10.733 | < 0.001 | 4.559 | 2.333–8.911 | < 0.001 |
| CT severity score | 1.084 | 1.043–1.126 | < 0.001 | 1.069 | 1.029–1.111 | < 0.001 |
| Adverse outcomes | ||||||
| Age > 60 years | 5.263 | 3.807–7.276 | < 0.001 | 2.097 | 1.455–3.022 | < 0.001 |
| CVD | 5.553 | 3.959–7.787 | < 0.001 | 1.952 | 1.324–2.878 | 0.001 |
| Diabetes | 2.755 | 1.924–3.946 | < 0.001 | 1.517 | 1.036–2.222 | 0.032 |
| COPD | 10.231 | 6.464–16.192 | < 0.001 | 3.592 | 2.177–5.927 | < 0.001 |
| High CAC | 4.224 | 2.998–5.951 | < 0.001 | 1.611 | 1.087–2.387 | 0.018 |
| Lymphopenia | 2.698 | 1.898–3.835 | < 0.001 | 2.010 | 1.405–2.874 | 0.0001 |
| Pleural effusion | 5.517 | 3.874–7.856 | < 0.001 | 2.121 | 1.499–3.213 | < 0 .001 |
| Pericardial effusion | 5.400 | 3.347–8.713 | < 0.001 | 2.194 | 1.283–3.505 | 0.003 |
| CT severity score | 1.124 | 1.093–1.156 | < 0.001 | 1.080 | 1.050–1.111 | < 0 .001 |
Adverse outcomes group was composed of in-hospital death, admission to ICU and requiring mechanical ventilation
COVID-19 Coronavirus Disease-19, ICU intensive care unit, HR hazard ratio, CI confidence interval, CVD cardiovascular disease, CAC coronary artery calcium, COPD chronic obstructive pulmonary disease
In multivariate Cox regression model, older age (> 60 years) (HR, 2.892; 95% CI 1.668–5.014, P < 0.001), sex (male) (HR,1.979; 95% CI 1.217–3.217, P = 0.006), CVD (HR, 3.184; 95% CI 1.901–5.334, P < 0.001), higher CAC (HR, 1.731; 95% CL 1.010–2.971, P = 0.046), lymphopenia (HR, 4.559; 95% CI 2.333–8.911, P < 0.001), and higher CT severity score (HR, 1.070; 95% CI 1.030–1.111, P < 0.001) were associated with higher risk of in-hospital death. Risk factors associated with adverse outcomes included older age (> 60 years) (HR, 2.097; 95% CI 1.455–3.022, P < 0.001), CVD (HR, 1.952; 95% CI 1.324–2.878, P = 0.001), diabetes (HR, 1.517; 95% CI 1.036–2.222, P = 0.032), COPD (HR, 3.592; 95% CI 2.177–5.927, P < 0.001), higher CAC (HR, 1.611; 95% CI 1.087–2.387 P = 0.018), lymphopenia (HR, 2.010; 95% CI 1.405–2.874, P = 0.0001), pleural effusion (HR, 2.121; 95% CI 1.499–3.213, P < 0.001), pericardial effusion (HR, 2.194; 95% CI 1.283–3.505, P = 0.003), and higher CT severity score (HR, 1.080; 95% CI 1.050–1.111, P < 0.001).
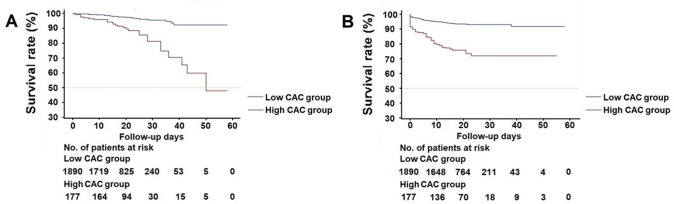
As shown in Fig. 2, Kaplan–Meier curves with log-rank test were generated to test the ability of CAC to distinguish cumulative event rates of mortality and adverse outcomes groups. Apparently, patients with higher CAC significantly improved the performance of predicting in-hospital death (log-rank: X2 = 46.264, P < 0.0001) as well as adverse outcomes (log-rank: X2 = 81.248, P < 0.0001). The outcome of lower CAC group without comorbidities was better than the high CAC group without comorbidities (Supplemental Table 3).
Fig. 2.
Kaplan–Meier survival curve for mortality and adverse outcomes during hospitalization between low and high CAC groups. A Patients with high CAC had a higher rate of mortality in log-rank test (log-rank: X2 = 46.264, P < 0.0001). B Patients with high CAC had a higher rate of adverse outcomes in log-rank test (log-rank: X2 = 81.248, P < 0.0001). CAC coronary artery calcium, No number
Discussion
CAC has been widely known to be associated with cardiovascular events. Recent evidences showed that elevated CAC was significantly associated with non-CVD, such as cancer, chronic kidney disease, COPD, and even hip fractures [16, 17]. Some researchers pointed out that elevated CAC may be a marker of an individual's overall health status [17]. In this study, we stratified COVID-19 patients with definitive prognosis information according to the degree of CAC severity and proved for that higher CAC was associated with higher risk of in-hospital death and adverse outcomes in COVID-19 patients. This highlights the important of special attention of CAC load to make the proper risk stratification and management.
Chest CT is used to detect COVID-19 pneumonia [18–19], which can easily and quickly detect and quantify CAC [12]. Previous studies have demonstrated that good agreement was noted between the visual assessment in non-gated chest CT examinations and Agatston score categories [13, 20]. Chiles et al. evaluated 1575 low-dose non-gated chest CT scans from the National Lung Screening Trial using a simple visual assessment of CAC compared with Agatston scoring and demonstrated that the simple visual way could be feasible for risk assessment of outcome [13]. In this study, we adopted visual assessment from the conventional chest CT examinations to quantify CAC burden. We found the correlation between CAC and adverse outcomes including in-hospital death and adverse outcomes in patients with confirmed COVID-19. Many studies have confirmed that CVD was the main comorbidity and risk factor associated with fatal outcome in patients with COVID-19 [5–7, 16, 21]. A nationwide study in China showed [21] that CVD was an independent risk factor associated with fatal outcome. Our study also supported that high CAC was a strong risk factor for the fatal outcome. Virmani et al. [22] summarized that 2–7% of acute coronary events resulted from the formation of coronary thrombosis caused by calcified nodules. Protruding calcified nodules may cause discontinuities in endothelial lining and underlying collagen matrix, eventually leading to acute luminal thrombosis [23]. Inflammation can also cause endothelial dysfunction and increase the blood procoagulant activity, which may contribute to the formation of occlusive thrombosis [7]. Patients with sepsis showed an increased inflammatory response in coronary plaque and adventitia compared with patients who died from non-infectious disease [24]. Additionally, as age increases, CAC is more common and severe in males [25]. Our study also showed that older age and males were independent predictors of death. Although the association between SARS-CoV-2 and human atherosclerotic plaques has not been investigated, based on above-mentioned evidences, it is reasonable to speculate that the superimposed effect of COVID-19 infection and CAC may exacerbate the disease in patients with COVID-19.
Similar with previous studies [11, 15, 21], our results also showed that older age, CVD, lymphopenia, and higher CT severity score were independent predictors of death and adverse outcomes in patients with COVID-19, while diabetes, COPD, incidence of pleural effusion, or pericardial effusion increased the risk odds of adverse outcomes, but were not associated with death. We speculated that although diabetes and COPD aggravated the symptoms in patients with COVID-19, the lethality might be less than cardiovascular disease. Of course, it cannot be ruled out that the lack of awareness and related tests might lead to inadequate diagnosis of these two comorbidities [21, 26]. On the other hand, when patients presented with pleural effusion or pericardial effusion on CT images, physicians should pay great importance to it and intervene as soon as possible to avoid adverse outcomes.
This study still has some limitations. First, as a large retrospective cohort study, it was inevitable that some data might be missing, and CT data of 200 patients were excluded because of unavailable CT images. The laboratory values although we double checked all data with principal investigators of each enter. To solve this problem, in multivariable Cox regression analysis, missing categorical variable data were encoded as an unknown class for statistics. Second, we did not take account of the impact of treatment measures on the prognosis. However, clinical interventions on COVID-19 patients are still limited to symptomatic treatment, without specialized treatment available to date. Third, this study only included PCR-positive hospitalized patients, we did not include PCR-negative hospitalized patients and out-patients with COVID-19. Further studies are needed to clarify the role of these factors.
Conclusions
This large retrospective prognostic study from China shows that high CAC is an independent risk factor associated with in-hospital death and adverse outcomes in patients with COVID-19, which highlights the importance of calcium load testing for hospitalized COVID-19 patients and calls for paying special attention to patients with high CAC to make the proper risk stratification and timely management.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- COVID-19
Coronavirus disease-19
- CFR
Case fatality rate
- SARS
Severe acute respiratory syndrome
- MERS
Middle East respiratory syndrome
- ACS
Acute coronary syndrome
- CAC
Coronary artery calcium
- CHD
Coronary heart disease
- ACM
All-cause mortality
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus-2
- RT–PCR
Reverse transcription–polymerase chain reaction
- PCI
Percutaneous coronary intervention
- CABG
Coronary artery bypass grafting
- CVD
Cardiovascular disease
- COPD
Chronic obstructive pulmonary disease
- LDH
Lactate dehydrogenase
- CK-MB
Creatine kinase myocardial band
- ICU
Intensive-care unit
- GGO
Ground-glass opacity
- ILD
Interstitial lung disease
- SD
Standard deviation
- IQR
Interquartile range
- HR
Hazard ratio
- CI
Confidence interval
Funding
None.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declaration
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethics approval
This study was approved by the institutional review boards of Jinling Hospital (2020NZKY-005-02).
Consent to participate
Written informed consent was waived.
Consent for publication
All authors read and approved the final manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Song Luo, Xiao Ming Qiu, Xian Jun Zeng, and Dong You Zhang have equally contributed to this work.
Contributor Information
Long Jiang Zhang, Email: kevinzhlj@163.com.
Yu Xiu Liu, Email: liu_yuxiu@163.com.
Guang Ming Lu, Email: cjr.luguangming@vip.163.com.
References
- 1.[Internet] WHO. Coronavirus disease 2019 (COVID-19) situation report -161. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200525-covid-19-sitrep-126.pdf?sfvrsn=887dbd66_2. Published at June 29, 2020.
- 2.[Internet] WHO. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Dec 31, 2003. https://www.who.int/csr/sars/country/table2004_04_21/en/. Accessed 16 May 2020.
- 3.[Internet] WHO. Middle East respiratory syndrome coronavirus (MERS-CoV). November, 2019. http://www.who.int/emergencies/mers-cov/en/. Accessed 16 May 2020.
- 4.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan. China JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong TY, Redwood S, Prendergast B, et al. Coronaviruses and the cardiovascular system acute and long-term implications. Eur Heart J. 2020;41:1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung MK, Zidar DA, Bristow MR, et al. COVID-19 and cardiovascular disease: from bench to bedside. Circ Res. 2021;128:1214–1236. doi: 10.1161/CIRCRESAHA.121.317997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keelan PC, Bielak LF, Ashai K, et al. Long-term prognostic value of coronary calcification detected by electron-beam computed tomography in patients undergoing coronary angiography. Circulation. 2001;104:412–417. doi: 10.1161/hc2901.093112. [DOI] [PubMed] [Google Scholar]
- 9.Shaw LJ, Giambrone AE, Blaha MJ, et al. Long-term prognosis after coronary artery calcification testing in asymptomatic patients: a cohort study. Ann Intern Med. 2015;163:14–21. doi: 10.7326/M14-0612. [DOI] [PubMed] [Google Scholar]
- 10.Shao L, Yan AT, Lebovic G, et al. Prognostic value of visually detected coronary artery calcification on unenhanced non-gated thoracic computed tomography for prediction of non-fatal myocardial infarction and all-cause mortality. J Cardiovasc Comput Tomogr. 2017;11:196–202. doi: 10.1016/j.jcct.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Xu PP, Tian RH, Luo S, et al. Risk factors for adverse clinical outcomes with COVID-19 in China: a multicenter, retrospective, observational study. Theranostics. 2020;10:6372–6383. doi: 10.7150/thno.46833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hecht HS, Cronin P, Blaha MJ, et al. 2016 SCCTSTR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans A report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Cardiovasc Comput Tomogr. 2017;11:74–84. doi: 10.1016/j.jcct.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Chiles C, Duan F, Gladish GW, et al. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276:82–90. doi: 10.1148/radiol.15142062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handy CE, Desai CS, Dardari ZA, et al. The association of coronary artery calcium with noncardiovascular disease: the multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. 2016;9:568–576. doi: 10.1016/j.jcmg.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi H, HX, Jiang N,, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geoffrey DR, Christopher JR, Linda BH, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner society. Radiology. 2020;296(1):172–180. doi: 10.1148/radiol.2020201365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie X, Zhao Y, de Bock GH, et al. Validation and prognosis of coronary artery calcium scoring in nontriggered thoracic computed tomography: Systematic review and meta-analysis. Circ Cardiovasc Imaging. 2013;6:514–521. doi: 10.1161/CIRCIMAGING.113.000092. [DOI] [PubMed] [Google Scholar]
- 21.Chen RH, Liang WH, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virmani R, Kolodgie FD, Burke AP, et al. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.ATV.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 23.Whelton SP, Al Rifai M, Dardari Z, et al. Coronary artery calcium and the competing long-term risk of cardiovascular vs. cancer mortality: the CAC Consortium. Eur Heart J Cardiovasc Imaging. 2019;20:389–395. doi: 10.1093/ehjci/jey176. [DOI] [PubMed] [Google Scholar]
- 24.Haidari M, Wyde PR, Litovsky S, et al. Influenza virus directly infects, inflames, and resides in the arteries of atherosclerotic and normal mice. Atherosclerosis. 2010;208:90–96. doi: 10.1016/j.atherosclerosis.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 25.Lessmann N, de Jong PA, Celeng C, et al. Sex differences in coronary artery and thoracic aorta calcification and their association with cardiovascular mortality in heavy smokers. JACC Cardiovasc Imaging. 2019;12:1808–1817. doi: 10.1016/j.jcmg.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6:421–430. doi: 10.1016/S2213-2600(18)30103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.