Abstract
Phthalates with potential adverse health effects are being replaced by other phthalates or phthalate alternatives. Little is known about temporal trends of phthalate exposure in pregnant women in the U.S. We quantified 16 metabolites of 8 phthalates and di(isononyl) cyclohexane-1,2-dicarboxylate (DINCH) in 656 urine samples collected from 192 California pregnant women in 2007-2013 during their 2nd and 3rd trimesters of pregnancy who participated in the MARBLES (Markers of Autism Risk in Babies – Learning Early Signs) study. We used multiple regression to estimate least square geometric means of phthalate biomarker concentrations and annual percent changes over the study period. Biomarker concentrations of diethyl phthalate (DEP) and three phthalates with known toxicity and adverse health effects (i.e., butyl benzyl phthalate [BBzP], dibutyl phthalate [DBP], di(2-ethylhexyl) phthalate [DEHP]) decreased while those of di-isobutyl phthalate [DiBP], di-isononyl phthalate [DiNP], and di-n-octyl phthalate [DOP] increased in California pregnant women during our study period. To understand broad social forces that may influence temporal trends and geographic variations in phthalate exposure across countries, we compared our phthalate biomarker concentrations with those of other populations. We observed a factor of 2 differences in exposure across countries for some phthalate biomarkers and between pregnant and non-pregnant women for DEP.
Graphical Abstract
1. Introduction
Phthalates are synthetic chemicals widely used in personal care products (e.g., cosmetics, perfumes, lotions), consumer products (e.g., electronics, toys, food packaging, food containers), indoor residential materials (e.g., polyvinyl chloride (PVC) flooring, shower curtains), and medical devices.1-3 High molecular weight (HMW) phthalates such as di(2-ethylhexyl) phthalate (DEHP) and butyl benzyl phthalate (BBzP) are primarily used in PVC flooring, food packaging, and food containers, whereas low molecular weight (LMW) phthalates such as diethyl phthalate (DEP) and dibutyl phthalate (DBP) are primarily used in personal care products.2 Because of widespread use of products and materials containing phthalates, biomarkers of exposure to these chemicals, namely phthalate metabolites in urine, are widely detected in most of the U.S. general population.4
Prenatal exposure to phthalates is of increasing health concern. Phthalates may cross the placental barrier as some of their biomarkers are detected in cord blood.5 Moreover, in laboratory animal studies, DEHP, DBP, BBzP, di-isononyl phthalate (DiNP), di-isodecyl phthalate (DiDP), and di-n-octyl phthalate (DOP) can produce reproductive and developmental toxicity.6, 7 In addition, DEHP,8 DBP,9, 10 and BBzP11 have neurotoxic and neurobehavioral toxicity. In humans, higher prenatal metabolite concentrations of DEHP were associated with increased risk of attention-deficit/hyperactivity disorder (ADHD)12 and other neurobehavioral problems.13-16 Higher prenatal metabolite concentrations of BBzP and DEHP were associated with increased scores of autistic traits among children at 3 to 4 years of age.17 Furthermore, having PVC flooring (a source of certain phthalates) in a parent’s bedroom during pregnancy and child’s first year was associated with increased risk of autism spectrum disorder (ASD).18 These findings support that higher prenatal exposure to these phthalates may result in adverse health effects in pregnant women and their offspring.
With increasing concern over toxicity and potential adverse health effects associated with exposure to six phthalates (i.e., DEHP, DBP, BBzP, DiNP, DiDP, DOP) during pregnancy and early childhood, both the European Union (EU)19-21 and the U.S. federal government22 enacted similar regulations for those phthalates in the mid- to late 2000’s. The U.S. Consumer Product Safety Commission (CPSC) also made recommendations to either ban, impose an interim ban, or allow the continued use of phthalates and phthalate substitutes in children’s toys, childcare products, and in products used by women of childbearing age.23 Since these regulations were enacted or recommendations were made by the CPSC, DEHP appeared to have been replaced with other chemicals such as DiNP, DiDP, di-2-propylheptyl phthalate (DPHP), di(isononyl) cyclohexane-1,2-dicarboxylate (DINCH), dioctyl terephthalate (DOTP), and di(2-ethylhexyl) terephthalate (DEHTP);1, 24-30 DBP appeared to have been replaced with di-isobutyl phthalate (DiBP) until regulations restricted uses of DiBP.31-34 Phthalate content in other products is not subject to legislative oversight in the United States,35 but DEP use appeared to have been reduced in cosmetics after consumer advocates campaigned for the removal of phthalates from personal care products.36 Thus, as part of an effort to understand whether these regulations, recommendations, and campaigns resulted in reductions in pregnant women’s phthalate exposure, temporal trends of pregnant women’s phthalate exposure were examined in Sweden,37 Mexico City,38 and Puerto Rico.39 However, little is known about temporal trends of phthalate exposure in pregnant women in the U.S.
Phthalate exposure is multifactorial. Thus, this current study did not delve into sources or factors of phthalate exposure but explored broader social forces that may account for temporal trends in pregnant women’s phthalate exposure and explain differences in those trends across countries. We focused on pregnant women because they may have different exposure profiles for some phthalates from non-pregnant women, potentially due to changes in their daily routines or lifestyle after being pregnant. Studies showed that exposures in pregnant women were lower than those in non-pregnant women for some phthalates that are commonly used in fragrance or hair spray.40-42 Sources and patterns of pregnant women’s phthalate exposure may differ across countries and regions, partially because of differences in socioeconomic status or regulatory status.43-45 Thus, comparing pregnant women’s phthalate exposure across countries may help identify differences in exposure patterns over time and help understand broad social forces that influence temporal trends and geographic variations in phthalate exposure.
For the present study, we quantified metabolite concentrations of 8 phthalates (i.e., DEP, DiBP, DBP, BBzP, DEHP, DOP, DiNP, DiDP) and DINCH to examine temporal trends of biomarker concentrations among 192 California pregnant women during 2007-2013. We compared our measured biomarker concentrations with those of three pregnancy cohorts in Sweden,37 Mexico City38 and Puerto Rico,39 as well as those of pregnant and non-pregnant women in the U.S. National Health and Nutrition Examination Survey (NHANES). As the comparison demonstrated different patterns in temporal trends, we discussed implications in terms of broad social forces that may have played a role in temporal trends of phthalate exposure in the United States.
2. Methods
2.1. Study population
To examine temporal trends of phthalate exposure in California pregnant women, this study included women participating in MARBLES (Markers of Autism Risk in Babies – Learning Early Signs). MARBLES is a cohort study that began in 2006 and enrolls women who are pregnant with a child who has a first degree relative with ASD and thereby is at elevated risk (~20%) for developing ASD.46 MARBLES families are primarily recruited by beginning with lists of children receiving services for autism through the California Department of Developmental Services, as well as from other studies, by self- or provider referrals, and various clinics. Eligibility is then determined based on the mother’s pregnancy status or pregnancy planning. Details of study design, recruitment, eligibility, sample size, exposure data, and developmental diagnosis are available elsewhere.47
For the present study, we selected 192 mothers who provided first morning voids (FMVs) and/or 24-hour urine samples during pregnancy collected from January 2007 to December 2013. Five samples collected in January 2014 were also included in a batch of 2013. Among 192 mothers, 11 mothers participated in the study for two different pregnancies and one mother participated for three different pregnancies over four years. All urine samples included in the present study were collected from a total of 205 unique pregnancies.
This study was approved by the institutional review boards for the State of California and the University of California Davis (UC Davis). The analysis of blinded specimens by the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subjects research. Participants provided written informed consent before collection of any data.
2.2. Urine sample collection
The details of urine sample collection from MARBLES mothers and methods for pooling multiple urine samples are available in our previous studies.40, 48 Briefly, each woman in the MARBLES study collected three FMVs (taken one week apart) and one 24-hour urine sample in each trimester and placed samples collected prior to the day of her visit in their home refrigerator or freezer. Samples were returned to the laboratory at UC Davis, thawed, aliquoted, and then stored at −80 °C at the UC Davis biorepository, ensuring long-term stability of phthalate metabolites.49 Although almost all of the mothers included in the current study provided urine samples during the 2nd and 3rd trimesters, only 72 mothers provided urine samples during the 1st trimester and most of them provided a single urine sample. Thus, a total of 1,053 urine samples collected during the 2nd and 3rd trimesters were used in the current study. To reduce analytical costs, for mothers who provided three or more urine specimens within a trimester, we selected the first FMV as an individual sample and pooled all remaining samples for that trimester. After pooling, 656 samples from 205 pregnancies remained for analysis. The type and number of urine samples collected and analyzed in this study are summarized in the Supporting Information (see Figure S1).
2.3. Urinary metabolite quantification
We shipped the urine samples in 2 mL aliquots to CDC for chemical analysis. At CDC, we quantified the urinary concentrations for 14 metabolites of 8 phthalates and 2 metabolites of DINCH using online solid phase extraction coupled with high-performance liquid chromatography with isotope dilution-tandem mass spectrometry as described elsewhere.50 The metabolites were: monoethyl phthalate (MEP), monoisobutyl phthalate (MiBP), monohydroxyisobutyl phthalate (MHiBP), mono-n-butyl phthalate (MBP), monohydroxybutyl phthalate (MHBP), monobenzyl phthalate (MBzP), mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), mono-3-carboxypropyl phthalate (MCPP), mono-isononyl phthalate (MNP), mono-carboxyisooctyl phthalate (MCOP), mono-carboxyisononyl phthalate (MCNP), cyclohexane-1,2-dicarboxylic acid, and monohydroxy isononyl ester (MHiNCH), and cyclohexane-1,2-dicarboxylic acid, monocarboxy isooctyl ester (MCOCH).
In this study, depending on the analyte and quality control (QC) concentration, the average relative percent difference (RPD) of 59 repeated measures of these QCs was 2.6-8.5%. In addition, the laboratory analyzed 51 blind duplicates for quality assurance. Replicate analyses for individual pairs of duplicate samples showed good agreement: average RPD was 7% (range: 4%-15%, depending on the analyte). The limits of detection (LODs) varied between 0.2 and 1.2 nanograms per milliliter (ng/mL), depending on the analyte. For concentrations below the LOD, we used instrumental-reading concentrations without substituting values as there seems to be less bias with this approach than using an imputation method that assigns the same value (e.g., LOD/2) to all non-detectable concentrations.51, 52
2.5. Correction for urinary dilution
We measured specific gravity (SG) in each analyzed sample (either individual or pooled) using a digital handheld refractometer (Atago Co., Ltd., Tokyo, Japan) at UC Davis. Urinary phthalate metabolite concentrations were corrected for urinary dilution53, 54 using the following formula: CSG = C [(1.012 – 1)/(SG – 1)], where CSG is the SG-corrected metabolite concentration (in ng/mL), C is the measured metabolite concentration in urine (in ng/mL), 1.012 is the median SG of all analyzed samples, and SG is the specific gravity of each sample.55
2.6. Statistical analysis
We conducted all statistical analyses using R version 3.6.1. Based on a literature review, we selected a priori six candidate population characteristics that may influence phthalate exposure of our study population: race/ethnicity (non-Hispanic white, Hispanic, others), age at delivery (≤35 years, >35 years), education (less than college, bachelor, graduate or professional), pre-pregnancy body mass index (BMI) (underweight/normal, overweight, obese), homeownership (yes, no), and parity (1, >1).38, 39, 56 After confirming that our biomarker concentrations also differed for some phthalates by these population characteristics (refer to results in Supporting Information and Table S1), we included them as covariates in the regression model.
Because of the high correlations among the four DEHP metabolites measured (0.84-0.99 among four DEHP metabolites, Table S2), we computed the sum of DEHP metabolites (ΣDEHP = MEHP + MEHHP + MEOHP + MECPP) in all statistical analyses, instead of urinary concentrations of individual DEHP metabolites. For the same reason (r = 0.90 between MiBP and MHiBP; 0.84 between MBP and MHiBP), we also computed the sums of DBP and DiBP metabolites (ΣDBP = MBP + MHBP and ΣDiBP = MiBP + MHiBP, respectively) and used ΣDBP and ΣDiBP in regression analyses. We did not compute the molar sums of these three phthalates because there was no statistically significant difference between sums in ng/mL and molar sums. For MNP and two DINCH metabolites with less than 50% of detection frequency, we did not perform further statistical analyses.
For ΣDEHP, ΣDBP, ΣDiBP and 5 other metabolites, we tested significant changes in concentrations over time using multiple regression with adjustment for the selected covariates. We used ‘lsmeans’ function in R to estimate the least square mean (LSM), which is the mean of ln[C] for each sampling year. We used natural log-transformed phthalate biomarker concentrations as the outcome variable in the regression models to account for skewed distributions. Thus, we computed the least square geometric mean (LSGM) of phthalate biomarker concentrations for each sampling year as exp(LSM), with 95% confidence intervals (CIs) as exp(LSM ± 1.97·SELSM), where SELSM is the standard error of the LSM.36 To construct CIs, we used a critical value of 1.97 from the t distribution, based on the number of the samples and the number of the selected covariates. To examine the relative concentration changes over our study period, we computed average annual percent changes of phthalate biomarker concentrations using a formula [exp(β) − 1] × 100% with 95% CIs as [exp(β ± 1.97·SEβ) − 1], where β is the time-related regression coefficient and SEβ is the standard error of the time-related regression coefficient. To test for monotonic trends in biomarker concentrations over the sample collection period, we performed the Mann-Kendall test and computed the Kendall’s tau correlation coefficient (τ) between sampling dates and biomarker concentrations. An alpha of 0.05 was used as the criterion for statistical significance.
We computed geometric means (GMs) of MARBLES pregnant women as well as NHANES’s pregnant and non-pregnant women (20-50 years of age).4 For pregnant women in Sweden, we obtained LSGMs during 2007-2010 from the Swedish Environmental Longitudinal, Mother and child, Asthma and allergy (SELMA) cohort.37 For pregnant women in Mexico City, we used GMs during 2007-2010 provided from the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) cohort.38 For pregnant women in Puerto Rico, we used GMs during 2011-2017 from the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) cohort.39 Sampling time, sample size, a participants’ age range, and GMs or LSGMs of phthalate biomarker concentrations for each cohort are available in Table S3.
3. Results
3.1. Population characteristics
The average age of the participating women at delivery was 34.5 years old, ranging from 20.5 to 49.2 years old (Table 1). The women included in the study were 54% non-Hispanic white, 23% Hispanic, and 23% others (5% black, 14% Asian, and 3% multiracial). Approximately half of the women were normal or underweight (52%) and did not have a bachelor’s degree or a higher degree (49%). Summary statistics of other characteristics are available in Table 1.
Table 1.
Characteristics of study population (n =192 mothers from 205 unique pregnancies) included in the current study.
| Characteristics a | n | % |
|---|---|---|
| Race/ethnicity | ||
| White (non-Hispanic) | 110 | 54% |
| Hispanic | 47 | 23% |
| Other b | 48 | 23% |
| Pre-pregnancy BMI | ||
| Normal/ underweight | 106 | 52% |
| Overweight | 49 | 24% |
| Obese | 49 | 24% |
| Education | ||
| Less than college degree | 100 | 49% |
| Bachelor’s degree | 76 | 37% |
| Graduate or professional degree | 29 | 14% |
| Age at delivery | ||
| < 35 years | 110 | 54% |
| ≥ 35 years | 95 | 46% |
| Homeownership | ||
| Yes | 80 | 39% |
| No | 119 | 58% |
| Parity | ||
| 0 c | 2 | 1% |
| 1 | 84 | 41% |
| >1 | 113 | 55% |
| Number of urine samples collected during 2nd and 3rd trimesters | ||
| 1 – 3 | 56 | 27% |
| 4 – 6 | 75 | 37% |
| 7 – 12 | 74 | 36% |
Eleven mothers participated in the study for two different pregnancies and one mother participated for three different pregnancies over four years. The missing values for pre-pregnancy body mass index (BMI), homeownership, and parity are approximately 0.5%, 3%, and 3%, respectively.
Includes Black (23%), Asian (63%), and multiracial (15%).
One of them has an identical twin sister with an autistic child, and the other mother has multiple siblings with autism. These two mothers were included in the current study because they have high-risk ASD genetic factors.
3.2. Biomarker concentrations
Six metabolites were detected in all samples: MEP, MEHHP, MEOHP, MECPP, MCOP, and MCNP (Table 2). Except for MHBP (82%), MEHP (83%) and MNP (50%), the other five phthalate metabolites were detected in more than 90% of the samples. Two metabolites of DINCH were detected in less than 20% of the samples: 19.5% for MHiNCH and 5.3% for MCOCH. The highest median of SG-corrected metabolite concentrations was observed for MEP (23.7 ng/mL), followed by MECPP, MCOP, MBP, and MEHHP (18.4, 13.3, 12.6, and 11.5 ng/mL, respectively).
Table 2.
Distribution of uncorrected and specific gravity-corrected metabolite concentrations of phthalates and DINCH [ng/mL] in 656 urine samples collected from 205 pregnancies.
| Parent | Metabolite | LOD [ng/mL] |
% detect |
Uncorrected |
SG-corrected |
||||
|---|---|---|---|---|---|---|---|---|---|
| Percentiles |
Percentiles |
||||||||
| 5th | 50th | 95th | 5th | 50th | 95th | ||||
| DEP | MEP | 1.2 | 100 | 4.4 | 23.1 | 223.2 | 5.7 | 23.7 | 194.1 |
| DiBP | MiBP | 0.8 | 98 | 1.5 | 7.2 | 31.2 | 2.1 | 7.4 | 25.0 |
| MHiBP | 0.4 | 97 | 0.6 | 2.6 | 11.0 | 0.8 | 2.6 | 9.3 | |
| DBP | MBP a | 0.4 | 99 | 2.4 | 12.6 | 49.5 | 3.5 | 12.6 | 41.2 |
| MHBP | 0.4 | 82 | <LOD | 1.1 | 4.5 | <LOD | 1.1 | 3.7 | |
| BBzP | MBzP | 0.3 | 99 | 0.8 | 6.4 | 39.6 | 1.2 | 6.4 | 33.6 |
| DEHP | MEHP | 0.8 | 83 | <LOD | 2.6 | 26.1 | <LOD | 2.6 | 28.0 |
| MEHHP | 0.4 | 100 | 1.8 | 12.1 | 136.2 | 2.6 | 11.5 | 107.3 | |
| MEOHP | 0.2 | 100 | 1.5 | 9.3 | 88.5 | 2.2 | 9.3 | 80.2 | |
| MECPP | 0.4 | 100 | 4.0 | 19.2 | 158.4 | 5.6 | 18.4 | 162.9 | |
| DOP, others | MCPP b | 0.4 | 92 | <LOD | 1.8 | 14.9 | <LOD | 1.7 | 13.4 |
| DiNP | MNP | 0.9 | 50 | <LOD | 0.9 | 9.4 | <LOD | 0.9 | 8.3 |
| MCOP | 0.3 | 100 | 2.1 | 13.4 | 175.2 | 3.0 | 13.3 | 149.9 | |
| DiDP | MCNP c | 0.2 | 100 | 0.6 | 2.8 | 26.4 | 0.9 | 2.7 | 25.0 |
| DINCH | MHiNCH | 0.4 | 5.3 | <LOD | <LOD | 0.94 | <LOD | <LOD | 1.37 |
| MCOCH | 0.5 | 19.8 | <LOD | <LOD | 0.48 | <LOD | <LOD | 0.73 | |
MBP is also a minor metabolite of BBzP.
MCPP is also a minor metabolite of DBP and a non-specific metabolite of several high molecular weight phthalates, including DBP, DiNP, DiDP, and di-n-pentyl phthalate (DnPeP).
MCNP is also a minor metabolite of di-2-propylheptyl phthalate (DPHP).
Abbreviation: limit of detection (LOD), diethyl phthalate (DEP), di-isobutyl phthalate (DiBP), dibutyl phthalate (DBP), benzyl butyl phthalate (BBzP), di(2-ethylhexyl) phthalate (DEHP), di-n-octyl phthalate (DOP), di-isononyl phthalate (DiNP), di-isodecyl phthalate (DiDP), di(isononyl) cyclohexane-1,2-dicarboxylate (DINCH), monoethyl phthalate (MEP), monoisobutyl phthalate (MiBP), monohydroxyisobutyl phthalate (MHiBP), mono-n-butyl phthalate (MBP), monohydroxybutyl phthalate (MHBP), monobenzyl phthalate (MBzP), mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), mono-3-carboxypropyl phthalate (MCPP), mono-isononyl phthalate (MNP), mono-carboxyisooctyl phthalate (MCOP), mono-carboxyisononyl phthalate (MCNP), cyclohexane-1,2-dicarboxylic acid, and monohydroxy isononyl ester (MHiNCH), and cyclohexane-1,2-dicarboxylic acid, monocarboxy isooctyl ester (MCOCH).
3.3. Temporal trends of phthalate biomarker concentrations in California pregnant women
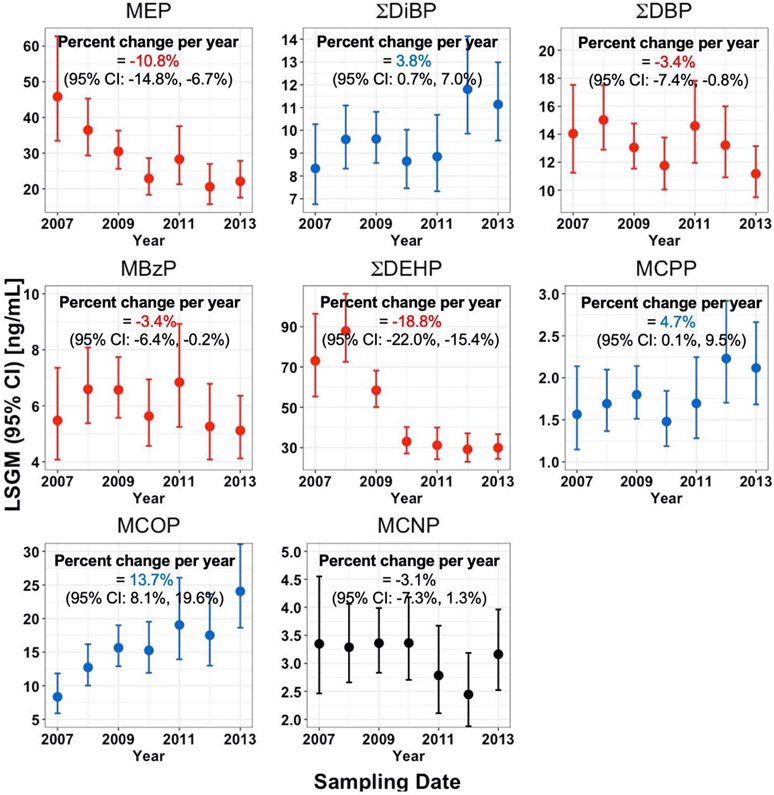
After adjusting for the selected covariates, the LSGMs of MEP, MBzP, ΣDBP, and ΣDEHP in California pregnant women decreased over the study period [percent change per year (95% CI): −10.8% (−14.8%, −6.7%); −3.4% (−7.4%, −0.8%); −3.4% (−6.4%, −0.2%); −18.8% (−22.0%, −15.4%), respectively] (Figure 1). In contrast, LSGMs of ΣDiBP, MCPP, and MCOP increased [percent change (95% CI): 3.8% (0.7%, 7.0%); 4.7% (0.1%, 9.5%); 13.7% (8.1%, 19.6%), respectively]. For MCNP, we did not observe a clear time trend (p-value = 0.16).
Figure 1.
Temporal trends and percent change per year with 95% confidence intervals (CI) in measured SG-corrected phthalate biomarker concentrations [ng/mL] in 656 urine samples collected from California pregnant women during 2007-2013. Biomarker concentrations were adjusted for SG, sampling year, race/ethnicity, pre-pregnancy body mass index (BMI), education, and age at delivery. Data points represent LSGMs (least square geometric means or adjusted geometric means) and error bars represent 95% CIs. Error bars in red and blue, respectively, represent decreasing and increasing trends that were statistically significant, while those in black represent no statistically significant trend. MCPP is a metabolite of DOP and other parent compounds, MCOP is a metabolite of DiNP, and MCNP is a metabolite of DiDP and DPHP.
Temporal trends of unadjusted concentrations from the Mann-Kendall trend test were similar to those of adjusted phthalate biomarker concentrations (i.e., LSGMs). Urinary concentrations of MEP (τ = −0.13, p-value <0.01), ΣDBP (τ = −0.06, p-value = 0.02), and ΣDEHP (τ = −0.27, p-value <0.01) decreased over the study period (see Figure S2). On the other hand, urinary concentrations of ΣDiBP (τ = 0.05, p-value = 0.03) and MCOP (τ = 0.14, p-value <0.01) increased. We did not observe any clear temporal trend among the other biomarkers.
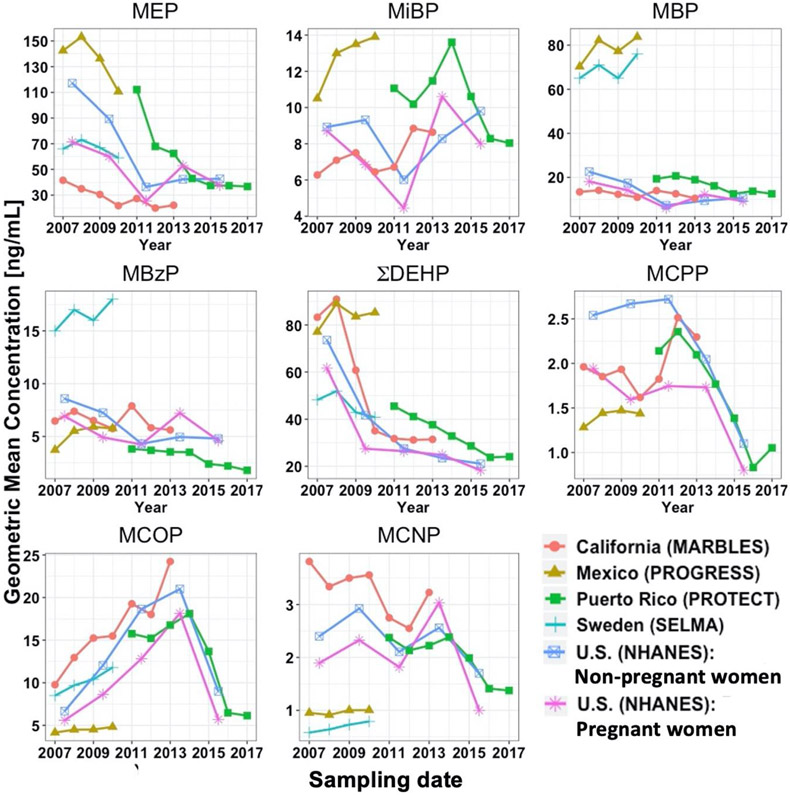
3.4. Temporal trends of phthalate biomarker concentrations in all studied populations
Overall, temporal trends in GM concentrations of California pregnant women’s phthalate biomarkers were comparable to those of both NHANES’s pregnant and non-pregnant women (Figure 2). For MBP, MBzP, ΣDEHP, MCPP, and MCOP, GM concentrations in California pregnant women varied within a factor of 2 of those in NHANES’s pregnant and non-pregnant women during most of the NHANES cycles. Compared to NHANES’s pregnant and non-pregnant women, GM concentrations in California pregnant women were lower for MEP and higher for ΣDEHP, MCPP, and MCOP during most of the NHANES cycles. GM concentrations in NHANES’s pregnant women were consistently lower than those in NHANES’s non-pregnant women for all biomarkers during most of the NHANES cycles.
Figure 2.
Geometric means (GMs) of biomarker concentrations [ng/mL] among different study cohorts from 2007 to 2017. All GMs were corrected for dilution either using specific gravity or creatinine. Only non-pregnant women aged 20 to 50 years were included in U.S. (NHANES): Non-pregnant women. The least square geometric means were used in the SELMA study and their model adjusted for urinary creatinine, age, body weight, smoking status, education and sampling season. For consistency across study cohorts, GMs for the sum of DEHP metabolites (ΣDEHP) were calculated by adding GMs [ng/mL] of individual DEHP metabolites. Phthalate metabolite concentrations for NHANES’s pregnant women were only available in a small sample size (range: 18 to 26 pregnant women), depending on the NHANES cycle, and hence comparisons with NHANES’s pregnant women should be interpreted cautiously. MCPP is a metabolite of DOP and other parent compounds, MCOP is a metabolite of DiNP, and MCNP is a metabolite of DiDP and DPHP.
For MBzP, GM concentrations in Swedish pregnant women were at least 2 times higher than those in pregnant women in other countries during the same study period. For MBP, GM concentrations in pregnant women in Sweden and Mexico City were 2 to 3 times higher than those in pregnant women in other countries during the same study period. For MCNP, GM concentrations in California pregnant women were at least 3 times higher than those in pregnant women in Sweden and Mexico City during the same study period. For MEP, MiBP, MBP, and ΣDEHP, GM concentrations tended to be higher in Puerto Rico pregnant women than those in California pregnant women and NHANES’s pregnant women during the same study period.
4. Discussion
In the mid- to late 2000’s, regulations were enacted for six phthalates (i.e., DEHP, DBP, BBzP, DiNP, DiDP, DOP) in the U.S. to reduce phthalate exposure during pregnancy and early childhood.22 However, little is known about temporal trends of phthalate exposure in pregnant women in the U.S. In this study, we used multiple urine samples collected from the MARBLES study to examine temporal trends of California pregnant women’s phthalate biomarker concentrations. We observed decreased GM concentrations for MEP, MBzP, ΣDBP, and ΣDEHP in California pregnant women over the study period, which appear to be responses to regulations or advocacy campaigns related to the parent compounds of these biomarkers in California. On the other hand, we observed increased GM concentrations for ΣDiBP, MCPP, and MCOP over the study period, which suggest that the parent compounds of these biomarkers may have been used as substitutes for DBP or DEHP in California. When comparing our phthalate biomarker concentrations with those in pregnant women in Sweden, Mexico City, Puerto Rico, and NHANES’s pregnant and non-pregnant women, California pregnant women had a few times lower or higher GM concentrations for some biomarkers during the same study period. We also observed that GM concentrations in NHANES’s pregnant women were consistently lower than those in NHANES’s non-pregnant women for all biomarkers during most of the NHANES cycles. Together, this current study highlights a need to examine various factors affecting pregnant women’s phthalate exposure.
Overall, the decreasing trends of MEP (a metabolite of DEP), MBzP (a metabolite of BBzP), ΣDBP (sum of individual DBP metabolites) and ΣDEHP (sum of individual DEHP metabolites) in California pregnant women were consistent with those in both NHANES’s pregnant and non-pregnant women. However, California pregnant women had consistently lower MEP concentrations than NHANES’s pregnant and non-pregnant women during the same study period. Because phthalates in cosmetics have been a target of advocacy campaigns,35 and California has been centered on the advocacy campaign,57, 58 California pregnant women may have used phthalates-free cosmetics more often than those in other states. During 2007-2010, Shu et al. observed a decreasing trend of DEHP metabolites only from Swedish pregnant women37 and Wu et al. observed a decreasing trend of a DEP metabolite only from pregnant women in Mexico City.38 Results of these two studies should be interpreted with caution because temporal trends were examined using samples collected during a short period of time. For example, the limited finding of Shu et al. might result from a relatively short sample collection period (i.e., 2.5 years) because Gyllenhammar et al. observed decreasing trends of metabolites of DEP, DBP, BBzP, and DEHP in Swedish non-pregnant women during 2009-2014.59 It is likely that because regulations of these three phthalates were not enacted in Mexico during their study period, Wu et al. did not observe decreasing trends of metabolites of BBzP, DBP and DEHP. While DEHP metabolite concentrations consistently decreased after 2009 among all included cohorts, only MCOP (a metabolite of DiNP) concentrations increased among the cohorts in the U.S. and Sweden during 2007-2014. It is likely that DiNP was one of the primary DEHP replacements in the U.S. and European countries during this period.60 DiBP is a known substitute of DBP until regulations restricted uses of DiBP.31-34 However, we observed no clear relationship in temporal trends between major DiBP and DBP metabolites among included study cohorts. DBP metabolites relatively consistently decreased over the study period, whereas DiBP metabolites fluctuated. The decreasing trends in biomarker concentrations of DEP, BBzP, ΣDBP and ΣDEHP in this study were also consistent with those in other U.S. studies36, 39 as well as outside the United States during a similar study period.27-29, 59, 61 Overall, the decreasing trends in biomarkers of DEP, BBzP, DBP and DEHP among studies in the U.S. and other European countries may result, at least in part, from the efforts of reducing exposure to phthalates via legislative activities or advocacy campaigns.36
Comparison of phthalate biomarker concentrations among pregnancy cohorts allowed us to observe that the magnitude and temporal trends of phthalate biomarker concentrations varied across countries during the same study period. For example, compared to California pregnant women, pregnant women in Sweden and Mexico City had higher MBP concentrations and lower MCNP (a major metabolite of DiDP and a minor metabolite of DPHP) concentrations. In addition, Swedish pregnant women had higher MBzP concentrations than California pregnant women. It is likely that regulatory status or sources of exposure to BBzP, DBP, and DiDP in Sweden and Mexico were different from those in the United States. Although Puerto Rico is a territory of the United States, we observed that concentrations of MEP, ΣDiBP, ΣDBP, and ΣDEHP were higher in pregnant women in Puerto Rico than those in California pregnant women and NHANES’s women. A median household income in Puerto Rico in 2017 was slightly above $20,000, while median household incomes in California and the entire United States in 2017 were above $70,000 and $60,000, respectively (www.census.gov). Thus, the significant difference in household incomes between Puerto Rico and other U.S. states may contribute to the different sources (e.g., type and number of personal care products containing DEP, DiBP, and DBP) and patterns (e.g., frequency of personal care product use or time spending indoors with vinyl flooring containing DEHP) of exposure to DEP, DiBP, DBP, and DEHP. Additionally, the marketing of products in Puerto Rico may differ from the mainland due to stringent restrictions on commercial shipments to Puerto Rico.
Strengths of this study include a relatively long sample collection period (i.e., 7 years), and comparison of phthalate biomarker concentrations with other pregnancy cohorts from various regions within and outside the United States, including pregnant and non-pregnant women in NHANES. We observed that samples collected during a relatively long collection period are needed to overcome the time lag between regulation changes and removal of regulated phthalates from products that women use. Comparison of phthalate biomarker concentrations over time across pregnancy cohorts allowed us to observe the potential effect of differences in regulations and sources on exposure to phthalates across countries or within the United States. In addition, from the comparison of phthalate biomarker concentrations within NHANES between pregnant women and non-pregnant women, lower GM concentrations were consistently observed in pregnant women for all biomarkers during most of the NHANES cycles. This may be additional evidence that phthalates are being transferred to fetus crossing the placental barrier or pregnant women have different metabolism of phthalates from non-pregnant women.5
Some limitations should be noted. First, apart from the fact that California is at the forefront of efforts to protect human health from chemical exposure,62 biomarker concentrations measured in the pregnant women of this study may not represent other populations or pregnancy cohorts in the United States. Compared to NHANES’s pregnant women and non-pregnant women, MARBLES pregnant women had two to three times lower MEP concentrations, respectively. Because the pregnant women in this study already had a child with ASD, some may have been aware of concerns about products with phthalates and hence may have reduced their use of cosmetics or personal care products that may contain endocrine disrupting chemicals such as phthalates.63 Second, given the primary focus of this paper on understanding time trends in phthalate metabolite concentrations and how they differ across populations worldwide, we did not comprehensively examine whether sources such as the use of products containing phthalates and diet affected our measured metabolite concentrations. Further studies are needed to investigate how diet and product use affected phthalate biomarker concentrations. Third, this study did not examine differences in phthalate biomarker concentrations across pregnancy cohorts with respect to population characteristics (e.g., race/ethnicity, BMI, education), pregnancy stage (e.g., 1st, 2nd or 3rd trimesters), type of samples (e.g., spot or first morning voids), or dilution correction method (e.g., specific gravity, creatinine). It has been reported that DEHP metabolism may be influenced by the pregnancy stage,64 but urine samples of four pregnancy cohorts included in this current study were collected at different trimesters of pregnancy. In addition, because biological elimination half-lives of phthalates are on the order of hours,65 phthalate metabolite concentrations in first morning voids may differ from those in other spot samples. Among four pregnancy cohorts included in this current study, one study used creatinine and the other three used SG to correct for urinary dilution. Although the correlation coefficient between creatinine and SG for all urine samples collected during pregnancy was 0.8966 and both creatinine- and SG-corrected phthalate biomarker concentrations were within a factor of 1.5 each other,55 some differences between creatinine- and SG-corrected phthalate biomarker concentrations would be expected. Thus, future research may need to ascertain which factor is the most important in explaining these differences in phthalates exposure between pregnant and non-pregnant women or among cohorts.
Future biomonitoring studies may need to include all phthalate replacements and other non-phthalate plasticizers with similar use or functions in consumer products or indoor residential materials. For example, because DEHP is being phased out, we suspect upward trends in exposure to DEHP replacements, including some that were not measured in this study such as DPHP, DOTP, DEHTP, acetyl tributyl citrate (ATBC), and bis(2-ethylhexyl) adipate (DEHA). Based on 2016 U.S. Environmental Protection Agency’s Chemical Data Reporting database (https://www.epa.gov/chemical-data-reporting), most of these replacements were produced in or imported to the United States in the amount of >1 million pounds (or 453.6 tonnes) per year in 2015. Among them, DiDP, DPHP, and DINCH metabolite concentrations increased in Swedish pregnant women during 2007-2010,37 and DEHTP metabolite concentrations increased in pregnant women in Puerto Rico during 2011-201739 and in Mexico City during 2007-2010.38 U.S. NHANES also showed an increasing trend of DINCH metabolite concentrations during 2011-2016.4, 67 Other than the eight phthalates and DINCH targeted in our study, three non-phthalate plasticizers that have replaced DEHP (i.e., DOTP, ATBC, and DEHA) were widely detected in California residential indoor dust.68 Because the median dust concentration of DOTP (35.7 μg/g of dust) was similar to that of DEHP (39.1 μg/g of dust), exposure to DOTP is also likely in California residents. Moreover, DEHP and parent compounds of non-phthalate plasticizers were widely detected in German surface water and DINCH and DPHP water concentrations increased from 2005/2006 to 2017 in all samples analyzed.69 Therefore, future biomonitoring studies may benefit from including biomarkers of additional DEHP replacements that are globally produced in high volumes.70
From this study, we observed that biomarker concentrations of DEP and three phthalates with known toxicity and adverse health effects (i.e., BBzP, DBP, DEHP) decreased while those of replacement phthalates (i.e., DiBP, DOP, DiNP) increased in California pregnant women during 2007-2013, which appear to reflect regulations or campaigns in California in the mid- to late 2000’s. Moreover, comparison across a broad set of cohorts enabled us to infer the potential effect of differences in broad social forces on phthalate exposure. Because there are various factors affecting pregnant women’s exposure to phthalates and phthalate alternatives, further studies are needed to comprehensively investigate those factors to provide insights in designing targeted intervention for susceptible populations. In the absence of transparency from manufacturers, characterizing temporal exposure trends of phthalates and phthalate alternatives will enable the prioritization of chemicals with an increasing trajectory over time so that further epidemiological and toxicological studies can be conducted to ensure the health and well-being of pregnant women and their offspring.
Supplementary Material
Acknowledgments
We would like to acknowledge the MARBLES participants for helping make this research possible and Deborah Bennett for coordination of MARBLES data and urine samples among UC Davis, UT Arlington, and CDC. We acknowledge Dr. Deborah Watkins at the University of Michigan, Ann Arbor and Dr. Haotian Wu at Columbia University who kindly provided annual GM concentrations of phthalate metabolites measured in PROTECT and PROGRESS studies, respectively. We would like to acknowledge and thank all members of the PROGRESS team for their tireless efforts in maintaining the cohort and the American British Cowdray Hospital for providing research facilities for the PROGRESS study. We also acknowledge the late Xiaoyun Ye, Manori Silva, Ella Samandar, Jim Preau, and Tao Jia for the urinary biomarker measurements for MARBLES, PROGRESS, and PROTECT studies.
Funding
This research was supported by grants from the National Institute of Environmental Health Sciences (R21-ES025551, R01-ES020392, R24-ES028533, R01-ES028802, P30-ES023513, UH3OD023342, UG3OD023342).
Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC (Centers for Disease Control and Prevention). Use of trade name is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.0c03857.
Characteristics of study populations (n =192 mothers) included in the current study from 205 unique pregnancies (Supplemental Table 1), geometric means of phthalate metabolite concentrations from various study cohorts (Supplemental Table 2), type and number of urine samples analyzed in this study (Supplemental Figure 1), Spearman’s correlation coefficients among 14 phthalate metabolite concentrations in 656 urine samples included in the current study (Supplemental Figure 2), and SG-corrected phthalate biomarker concentrations in log 10 scale during our study period and p-values from the Mann-Kendall trend test (Supplemental Figure 3).
Conflict of interest
All authors declare they have no actual or potential competing financial interests.
Ethics approval and consent to participate
The MARBLES study protocol and this study were approved by the institutional review boards for the State of California, the University of California Davis (UC Davis), and the University of Texas Arlington (UT Arlington). Participants provided written informed consent before collection of any data. The analysis of coded specimens at the CDC laboratory was determined by CDC not to constitute engagement in human subject research.
References
- 1.Dodson RE; Nishioka M; Standley LJ; Perovich LJ; Brody JG; Rudel RA, Endocrine Disruptors and Asthma-Associated Chemicals in Consumer Products. Environ. Health Perspect 2012, 120, (7), 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauser R; Calafat AM, Phthalates and human health. Occup. Environ. Med 2005, 62, (11), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heudorf U; Mersch-Sundermann V; Angerer E, Phthalates: Toxicology and exposure. Int. J. Hyg. Envir. Heal 2007, 210, (5), 623–634. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC), Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA, 2019; pp 1–235. [Google Scholar]
- 5.Kolatorova L; Vitku J; Vavrous A; Hampl R; Adamcova K; Simkova M; Parizek A; Starka L; Duskova M, Phthalate Metabolites in Maternal and Cord Plasma and Their Relations to Other Selected Endocrine Disruptors and Steroids. Physiol. Res 2018, 67, S473–S487. [DOI] [PubMed] [Google Scholar]
- 6.Lyche JL; Gutleb AC; Bergman A; Eriksen GS; Murk AJ; Ropstad E; Saunders M; Skaare JU, Reproductive and developmental toxicity of phthalates. J. Toxicol. Environ. Health. B Crit. Rev 2009, 12, (4), 225–49. [DOI] [PubMed] [Google Scholar]
- 7.Ambe K; Sakakibara Y; Sakabe A; Makino H; Ochibe T; Tohkin M, Comparison of the developmental/reproductive toxicity and hepatotoxicity of phthalate esters in rats using an open toxicity data source. J. Toxicol. Sci 2019, 44, (4), 245–255. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka T, Reproductive and neurobehavioural toxicity study of bis(2-ethylhexyl) phthalate (DEHP) administered to mice in the diet. Food Chem. Toxicol 2002, 40, (10), 1499–1506. [DOI] [PubMed] [Google Scholar]
- 9.Li X-J; Jiang L; Chen L; Chen H-S; Li X, Neurotoxicity of dibutyl phthalate in brain development following perinatal exposure: A study in rats. Environ. Toxicol. Pharmacol 2013, 36, (2), 392–402. [DOI] [PubMed] [Google Scholar]
- 10.Li Y; Zhuang M; Li T; Shi N, Neurobehavioral toxicity study of dibutyl phthalate on rats following in utero and lactational exposure. J. Appl. Toxicol 2009, 29, (7), 603–611. [DOI] [PubMed] [Google Scholar]
- 11.Betz AJ; Jayatilaka S; Joshi J; Ramanan S; Debartolo D; Pylypiw H; Franke E, Chronic exposure to benzyl butyl phthalate (BBP) alters social interaction and fear conditioning in male adult rats: Alterations in amygdalar MeCP2, ERK1/2 and ERα. Neuroendocrinology Letters 2013, 34, (5), 347–358. [PubMed] [Google Scholar]
- 12.Kim B-N; Cho S-C; Kim Y; Shin M-S; Yoo H-J; Kim J-W; Yang YH; Kim H-W; Bhang S-Y; Hong Y-C, Phthalates Exposure and Attention-Deficit/Hyperactivity Disorder in School-Age Children. Biol. Psychiatry 2009, 66, (10), 958–963. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y; Ha E-H; Kim E-J; Park H; Ha M; Kim J-H; Hong Y-C; Chang N; Kim B-N, Prenatal Exposure to Phthalates and Infant Development at 6 Months: Prospective Mothers and Children's Environmental Health (MOCEH) Study. Environ. Health Perspect 2011, 119, (10), 1495–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobrosly RW; Evans S; Miodovnik A; Barrett ES; Thurston SW; Calafat AM; Swan SH, Prenatal Phthalate Exposures and Neurobehavioral Development Scores in Boys and Girls at 6-10 Years of Age. Environ. Health Perspect 2014, 122, (5), 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tellez-Rojo MM; Cantoral A; Cantonwine DE; Schnaas L; Peterson K; Hu H; Meeker JD, Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci. Total Environ 2013, 461, 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.England-Mason G; Martin JW; MacDonald A; Kinniburgh D; Giesbrecht GF; Letourneau N; Dewey D, Similar names, different results: Consistency of the associations between prenatal exposure to phthalates and parent-ratings of behavior problems in preschool children. Environ. Int 2020, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oulhote Y; Lanphear B; Braun JM; Webster GM; Arbuckle TE; Etzel T; Forget-Dubois N; Seguin JR; Bouchard MF; MacFarlane A; Ouellet E; Fraser W; Muckle G, Gestational Exposures to Phthalates and Folic Acid, and Autistic Traits in Canadian Children. Environ. Health Perspect 2020, 128, (2), 27004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson M; Weiss B; Janson S; Sundell J; Bornehag C-G, Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6-8 years of age. Neurotoxicology 2009, 30, (5), 822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Union (EU). Commission Directive 2004/93/EC of 21 September 2004 Amending Council Directive 76/768/EEC for the Purpose of Adapting Its Annexes II and III to Technical Progress. Off J Eur Union L 300:25.September.2004, 13–41. [Google Scholar]
- 20.Eurpean Union (EU). Directive 2005/84/EC of the European Parliament and of the Council 14 December 2005 Amending for the 22nd time Council Directive 76/769/EEC on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Relating to Restrictions on the Marketing and Use of Certain Dangerous Substances and Preparations (Phthalates in Toys and Childcare Articles). Off J Eur Union L 344:27.December 2005, 40–43. [Google Scholar]
- 21.European Union (EU). Commission Directive 2007/19/EC of 30 March 2007 Amending Directive 2002/72/EC Relating to Plastic Materials and Articles Intended to Come into Contact with Food and Council Directive 85/572/EEC Laying Down the List of Simulants to Be Used for Testing Migration of Constituents of Plastic Materials and Articles Intended to Come into Contact with Foodstuffs. Off J Eur Union L 91:31.March 2007, 17–36. [Google Scholar]
- 22.Consumer Product Safety Improvement Act (CPSIA) of 2008. Public Law 110-314, 2008. Available: https://www.cpsc.gov/s3fs-public/pdfs/blk_media_cpsia.pdf [accessed: August 21, 2020]. [Google Scholar]
- 23.Lioy PJ; Hauser R; Gennings C; Koch HM; Mirkes PE; Schwetz BA; Kortenkamp A, Assessment of phthalates/phthalate alternatives in children's toys and childcare articles: Review of the report including conclusions and recommendation of the Chronic Hazard Advisory Panel of the Consumer Product Safety Commission. J Expo Sci Environ Epidemiol 2015, 25, (4), 343–353. [DOI] [PubMed] [Google Scholar]
- 24.A European Chemical Agency. Evaluation of New Scientific Evidence Concerning DINP and DIDP in Relation to Entry 52 of Annex XVII to Regulation (EC) No 1907/2006 (REACH); 2012. Available: https://echa.europa.eu/documents/10162/31b4067e-de40-4044-93e8-9c9ff1960715 [accessed: August 21, 2020]. [Google Scholar]
- 25.Silva MJ; Wong LY; Samandar E; Preau JL Jr.; Jia LT; Calafat AM, Exposure to di-2-ethylhexyl terephthalate in the U.S. general population from the 2015–2016 National Health and Nutrition Examination Survey. Environ. Int 2019, 123, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidtkunz C; Gries W; Weber T; Leng G; Kolossa-Gehring M, Internal exposure of young German adults to di(2-propylheptyl) phthalate (DPHP): Trends in 24-h urine samples from the German Environmental Specimen Bank 1999–2017. Int. J. Hyg. Environ. Health 2019, 222, (3), 419–424. [DOI] [PubMed] [Google Scholar]
- 27.Frederiksen H; Nielsen O; Koch HM; Skakkebaek NE; Juul A; Jorgensen N; Andersson AM, Changes in urinary excretion of phthalates, phthalate substitutes, bisphenols and other polychlorinated and phenolic substances in young Danish men; 2009-2017. Int. J. Hyg. Environ. Health 2020, 223, (1), 93–105. [DOI] [PubMed] [Google Scholar]
- 28.Koch HM; Ruther M; Schutze A; Conrad A; Palmke C; Apel P; Bruning T; Kolossa-Gehring M, Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. Int. J. Hyg. Environ. Health 2017, 220, (2), 130–141. [DOI] [PubMed] [Google Scholar]
- 29.Lessmann F; Kolossa-Gehring M; Apel P; Ruther M; Palmke C; Harth V; Bruning T; Koch HM, German Environmental Specimen Bank: 24-hour urine samples from 1999 to 2017 reveal rapid increase in exposure to the para-phthalate plasticizer di(2-ethylhexyl) terephthalate (DEHTP). Environ. Int 2019, 132. [DOI] [PubMed] [Google Scholar]
- 30.Schwedler G; Rucic E; Lange R; Conrad A; Koch HM; Palmke C; Bruning T; Schulz C; Schmied-Tobies MIH; Daniels A; Kolossa-Gehring M, Phthalate metabolites in urine of children and adolescents in Germany. Human biomonitoring results of the German Environmental Survey GerES V, 2014-2017. Int. J. Hyg. Envir. Heal 2020, 225. [DOI] [PubMed] [Google Scholar]
- 31.Borch J; Axelstad M; Vinggaard AM; Dalgaard M, Diisobutyl phthalate has comparable anti-androgenic effects to di-n-butyl phthalate in fetal rat testis. Toxicol. Lett 2006, 163, (3), 183–190. [DOI] [PubMed] [Google Scholar]
- 32.Koch HM; Christensen KLY; Harth V; Lorber M; Bruning T, Di-n-butyl phthalate (DnBP) and diisobutyl phthalate (DiBP) metabolism in a human volunteer after single oral doses. Arch. Toxicol 2012, 86, (12), 1829–1839. [DOI] [PubMed] [Google Scholar]
- 33.Consumer Product Safety Improvement Act (CPSIA) of 2008. Public Law 16 CRF part 1307, 2017. Available: https://www.govinfo.gov/content/pkg/FR-2017-10-27/pdf/2017-23267.pdf [accessed: August 21, 2020]. [Google Scholar]
- 34.European Union (EU). Regulation (EC) No 1907/2006 – Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Annex XIV (List of substances subject to authorisation). 2007. Available: https://echa.europa.eu/authorisation-list [accessed: August 21, 2020]. [Google Scholar]
- 35.Campaign for Safe Cosmetics. Market Shift: The Story of the Compact for Safe Cosmetics and the Growth in Demand for Safe Cosmetics. 2011. Available: http://www.safecosmetics.org/wp-content/uploads/2015/02/Market-Shift-report.pdf [accessed August 21, 2020]. [Google Scholar]
- 36.Zota AR; Calafat AM; Woodruff TJ, Temporal Trends in Phthalate Exposures: Findings from the National Health and Nutrition Examination Survey, 2001-2010. Environ. Health Perspect 2014, 122, (3), 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shu H; Jonsson BA; Gennings C; Svensson A; Nanberg E; Lindh CH; Knutz M; Takaro TK; Bornehag CG, Temporal trends of phthalate exposures during 2007-2010 in Swedish pregnant women. J. Expo. Sci. Environ. Epidemiol 2018, 28, (5), 437–447. [DOI] [PubMed] [Google Scholar]
- 38.Wu H; Kupsco AJ; Deierlein AL; Just AC; Calafat AM; Oken E; Braun JM; Mercado-Garcia A; Cantoral A; Tellez-Rojo MM; Wright RO; Baccarelli AA, Trends and Patterns of Phthalates and Phthalate Alternatives Exposure in Pregnant Women from Mexico City during 2007-2010. Environ. Sci. Technol 2020, 54, (3), 1740–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez-Carmona Y; Ashrap P; Calafat AM; Ye XY; Rosario Z; Bedrosian LD; Huerta-Montanez G; Velez-Vega CM; Alshawabkeh A; Cordero JF; Meeker JD; Watkins D, Determinants and characterization of exposure to phthalates, DEHTP and DINCH among pregnant women in the PROTECT birth cohort in Puerto Rico. J. Expo. Sci. Environ. Epidemiol 2020, 30, (1), 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin HM; Bennett DH; Barkoski J; Ye X; Calafat AM; Tancredi D; Hertz-Picciotto I, Variability of urinary concentrations of phthalate metabolites during pregnancy in first morning voids and pooled samples. Environ. Int 2019, 122, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodruff TJ; Zota AR; Schwartz JM, Environmental Chemicals in Pregnant Women in the United States: NHANES 2003-2004. Environ. Health Perspect 2011, 119, (6), 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houlihan J; Brody C; Schwan B Not Too Pretty. Phthalates, Beauty Products and the FDA; Environmental Working Group: Washington, DC, 2002. [Google Scholar]
- 43.Smolders R; Den Hond E; Koppen G; Govarts E; Willems H; Casteleyn L; Kolossa-Gehring M; Fiddicke U; Castano A; Koch HM; Angerer J; Esteban M; Sepai O; Exley K; Bloemen L; Horvat M; Knudsen LE; Joas A; Joas R; Biot P; Aerts D; Katsonouri A; Hadjipanayis A; Cerna M; Krskova A; Schwedler G; Seiwert M; Nielsen JKS; Rudnai P; Kozepesy S; Evans DS; Ryan MP; Gutleb AC; Fischer ME; Ligocka D; Jakubowski M; Reis MF; Namorado S; Lupsa IR; Gurzau AE; Halzlova K; Fabianova E; Mazej D; Snoj JT; Gomez S; Gonzalez S; Berglund M; Larsson K; Lehmann A; Crettaz P; Schoeters G, Interpreting biomarker data from the COPHES/DEMOCOPHES twin projects: Using external exposure data to understand biomarker differences among countries. Environ. Res 2015, 141, 86–95. [DOI] [PubMed] [Google Scholar]
- 44.Guo Y; Alomirah H; Cho HS; Minh TB; Mohd MA; Nakata H; Kannan K, Occurrence of Phthalate Metabolites in Human Urine from Several Asian Countries. Environ. Sci. Technol 2011, 45, (7), 3138–3144. [DOI] [PubMed] [Google Scholar]
- 45.Guo Y; Kannan K, Comparative Assessment of Human Exposure to Phthalate Esters from House Dust in China and the United States. Environ. Sci. Technol 2011, 45, (8), 3788–3794. [DOI] [PubMed] [Google Scholar]
- 46.Ozonoff S; Young GS; Carter A; Messinger D; Yirmiya N; Zwaigenbaum L; Bryson S; Carver LJ; Constantino JN; Dobkins K; Hutman T; Iverson JM; Landa R; Rogers SJ; Sigman M; Stone WL, Recurrence Risk for Autism Spectrum Disorders: A Baby Siblings Research Consortium Study. Pediatrics 2011, 128, (3), E488–E495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hertz-Picciotto I; Schmidt RJ; Walker CK; Bennett DH; Oliver M; Shedd-Wise KM; LaSalle JM; Giulivi C; Puschner B; Thomas J; Roa DL; Pessah IN; Van de Water J; Tancredi DJ; Ozonoff S, A Prospective Study of Environmental Exposures and Early Biomarkers in Autism Spectrum Disorder: Design, Protocols, and Preliminary Data from the MARBLES Study. Environ. Health Perspect 2018, 126, (11), 117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin HM; Schmidt RJ; Tancredi D; Barkoski J; Ozonoff S; Bennett DH; Hertz-Picciotto I, Prenatal exposure to phthalates and autism spectrum disorder in the MARBLES study. Environ. Health 2018, 17, (1), 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samandar E; Silva MJ; Reidy JA; Needham LL; Calafat AM, Temporal stability of eight phthalate metabolites and their glucuronide conjugates in human urine. Environ. Res 2009, 109, (5), 641–646. [DOI] [PubMed] [Google Scholar]
- 50.Silva MJ; Samandar E; Preau JL; Reidy JA; Needham LL; Calafat AM, Quantification of 22 phthalate metabolites in human urine. J. Chromatogr B 2007, 860, (1), 106–112. [DOI] [PubMed] [Google Scholar]
- 51.Richardson DB; Ciampi A, Effects of exposure measurement error when an exposure variable is constrained by a lower limit. Am. J. Epidemiol 2003, 157, (4), 355–363. [DOI] [PubMed] [Google Scholar]
- 52.Schisterman EF; Vexler A; Whitcomb BW; Liu AY, The limitations due to exposure detection limits for regression models. Am. J. Epidemiol 2006, 163, (4), 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adibi JJ; Whyatt RM; Williams PL; Calafat AM; Camann D; Herrick R; Nelson H; Bhat HK; Perera FA; Silva MJ; Hauser R, Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ. Health Perspect 2008, 116, (4), 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hauser R; Meeker JD; Park S; Silva MJ; Calafat AM, Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ. Health Perspect 2004, 112, (17), 1734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meeker JD; Hu H; Cantonwine DE; Lamadrid-Figueroa H; Calafat AM; Ettinger AS; Hernandez-Avila M; Loch-Caruso R; Tellez-Rojo MM, Urinary Phthalate Metabolites in Relation to Preterm Birth in Mexico City. Environ. Health Perspect 2009, 117, (10), 1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Philippat C; Bennett DH; Krakowiak P; Rose M; Hwang HM; Hertz-Picciotto I, Phthalate concentrations in house dust in relation to autism spectrum disorder and developmental delay in the CHildhood Autism Risks from Genetics and the Environment (CHARGE) study. Environ. Health 2015, 14, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zota AR; Singla V; Adamkiewicz G; Mitro SD; Dodson RE, Reducing chemical exposures at home: opportunities for action. J. Epidemiol. Community Health 2017, 71, (9), 937–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.California Safe Cosmetic Act (CSCA). Public Law 2005. Available: http://www.leginfo.ca.gov/pub/05-06/bill/sen/sb_0451-0500/sb_484_bill_20051007_chaptered.pdf [accessed: August 21, 2020]. [Google Scholar]
- 59.Gyllenhammar I; Glynn A; Jonsson BA; Lindh CH; Darnerud PO; Svensson K; Lignell S, Diverging temporal trends of human exposure to bisphenols and plastizisers, such as phthalates, caused by substitution of legacy EDCs? Environ. Res 2017, 153, 48–54. [DOI] [PubMed] [Google Scholar]
- 60.Wittassek M; Wiesmueller GA; Koch HM; Eckard R; Dobler L; Mueller J; Angerer J; Schlueter C, Internal phthalate exposure over the last two decades - A retrospective human biomonitoring study. Int. J. Hyg. Envir. Heal 2007, 210, (3-4), 319–333. [DOI] [PubMed] [Google Scholar]
- 61.Tranfo G; Caporossi L; Pigini D; Capanna S; Papaleo B; Paci E, Temporal Trends of Urinary Phthalate Concentrations in Two Populations: Effects of REACH Authorization after Five Years. Int. J. Environ. Res. Public Health 2018, 15, (9), 1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson MP; Schwarzman MR, Toward a New US Chemicals Policy: Rebuilding the Foundation to Advance New Science, Green Chemistry, and Environmental Health. Environ. Health Perspect 2009, 117, (8), 1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marie C; Cabut S; Vendittelli F; Sauvant-Rochat MP, Changes in Cosmetics Use during Pregnancy and Risk Perception by Women. Int. J. Environ. Res. Public Health 2016, 13, (4): 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao HZ; Li JF; Zhou YQ; Zhu L; Zheng YY; Xia W; Li YY; Xiang L; Chen W; Xu SQ; Cai ZW, Investigation on Metabolism of Di(2-Ethylhexyl) Phthalate in Different Trimesters of Pregnant Women. Environ. Sci. Technol 2018, 52, (21), 12851–12858. [DOI] [PubMed] [Google Scholar]
- 65.Hoppin JA; Brock JW; Davis BJ; Baird DD, Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ. Health Perspect 2002, 110, (5), 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MacPherson S; Arbuckle TE; Fisher M, Adjusting urinary chemical biomarkers for hydration status during pregnancy. J. Expo. Sci. Environ. Epidemiol 2018, 28, (5), 481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kasper-Sonnenberg M; Koch HM; Apel P; Ruther M; Palmke C; Bruning T; Kolossa-Gehring M, Time trend of exposure to the phthalate plasticizer substitute DINCH in Germany from 1999 to 2017: Biomonitoring data on young adults from the Environmental Specimen Bank (ESB). Int. J. Hyg. Environ. Health 2019, 222, (8), 1084–1092. [DOI] [PubMed] [Google Scholar]
- 68.Shin HM; Moschet C; Young TM; Bennett DH, Measured concentrations of consumer product chemicals in California house dust: Implications for sources, exposure, and toxicity potential. Indoor Air 2020, 30, (1), 60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagorka R; Koschorreck J, Trends for plasticizers in German freshwater environments - Evidence for the substitution of DEHP with emerging phthalate and non-phthalate alternatives. Environ. Pollut 2020, 262, 114237. [DOI] [PubMed] [Google Scholar]
- 70.Lioy PJ; Gennings C; Hauser R; Koch HM; Kortenkamp A, Changing Trends in Phthalate Exposures. Environ. Health Perspect 2014, 122, (10), A264–A264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.