Significance
This study investigates the cause and effect relationship between the timing of food intake and blood pressure (BP) circadian rhythm in diabetic db/db mice. The results show that nondipping BP is associated with the loss of food intake rhythm and imposing food intake rhythms by time-restricted feeding (TRF, food available only during active nighttime) protects BP dipping in db/db mice. Mechanistically, TRF suppressed the sympathetic activity during the resting daytime, thus protecting BP dipping in db/db mice. Our study reveals a critical role of the daily timing of food intake in the prevention and treatment of nondipping BP in diabetic mice. The utility of this approach to the treatment of hypertension in patients with diabetes remains to be determined.
Keywords: time-restricted feeding, blood pressure circadian rhythm, diabetes, nondipping blood pressure, sympathetic nervous system
Abstract
The quantity and quality of food intake have been considered crucial for peoples' wellness. Only recently has it become appreciated that the timing of food intake is also critical. Nondipping blood pressure (BP) is prevalent in diabetic patients and is associated with increased cardiovascular events. However, the causes and mechanisms of nondipping BP in diabetes are not fully understood. Here, we report that food intake and BP were arrhythmic in diabetic db/db mice fed a normal chow diet ad libitum. Imposing a food intake diurnal rhythm by time-restricted feeding (TRF; food was only available for 8 h during the active phase) prevented db/db mice from developing nondipping BP and effectively restored the already disrupted BP circadian rhythm in db/db mice. Interestingly, increasing the time of food availability from 8 h to 12 h during the active dark phase in db/db mice prompted isocaloric feeding and still provided robust protection of the BP circadian rhythm in db/db mice. In contrast, neither 8-h nor 12-h TRF affected BP dipping in wild-type mice. Mechanistically, we demonstrate that TRF protects the BP circadian rhythm in db/db mice via suppressing the sympathetic activity during the light phase when they are inactive and fasting. Collectively, these data reveal a potentially pivotal role of the timing of food intake in the prevention and treatment of nondipping BP in diabetes.
As the incidence of type 2 diabetes mellitus continues to rise, diabetes has become one of the most prevalent and costly chronic diseases worldwide (1). About 75% of type 2 diabetic patients have hypertension (2), which worsens diabetic vascular complications and significantly contributes to their morbidity and mortality (3). With the increased use of ambulatory blood pressure (BP) monitoring, accumulating evidence indicates that not only the level but also the circadian rhythm of BP is critical for cardiovascular health (4). BP exhibits a circadian rhythm, with a rise during the early morning and a higher level maintained throughout the day, followed by a decrease of about 10 to 20% at night (dip). Nondipping BP, defined as a less than 10% nocturnal decline in BP (5), is highly prevalent in patients with type 2 diabetes (6). The prevalence of nondipping BP varies among patient populations but is as high as 73% in type 2 diabetic patients (7). Nondipping BP, independent of hypertension, is associated with left ventricular hypertrophy, increased proteinuria, secondary forms of hypertension, increased insulin resistance, and increased fibrinogen level (8). Moreover, a recent large meta-analysis of 17,312 hypertensive individuals with 4 to 8 y follow-up revealed that nondippers, relative to dippers, have a significant 27% higher risk for total cardiovascular events (9). Despite the risk associated with nondipping BP in diabetic patients, the mechanisms underlying this problem are largely unknown. As a result, there is currently no effective medication for the prevention and treatment of nondipping BP in diabetes.
It is well known that excessive food intake is a major risk factor for the onset of obesity and type 2 diabetes (10). Research over the past decade has demonstrated that the quantity of food intake (how much we eat) and/or the quality of food intake (what we eat) has a profound impact on obesity and diabetes (10). Only recently has it become appreciated that the timing of food intake (when we eat) is also critical for diet-induced metabolic diseases (11). Observational human studies revealed that late-night eating is higher in obese and diabetic patients compared to healthy individuals (12) and is associated with the development of metabolic disorders (13). In line with these human studies, animal work further demonstrated a causal relationship between the timing of food intake and the development of obesity and metabolic diseases. Of interest, when mice are fed an obesogenic diet ad libitum, they develop obesity and metabolic disorders (14, 15). In contrast, when mice are fed the same diet only during the active dark phase (time-restricted feeding [TRF]), they do not develop obesity and metabolic disorders (14, 15). Together, these studies suggest that the timing of food intake has a profound impact on metabolic health. However, the relationship between the timing of food intake and BP circadian rhythm is unknown, and whether TRF can serve as a therapeutic strategy against nondipping BP in diabetes is also unknown.
The current study seeks to investigate the cause-and-effect relationship between the timing of food intake and the BP circadian rhythm in diabetic db/db mice. Our results show that the loss of the food intake diurnal rhythm coincides with nondipping BP in db/db mice, and imposing a food intake diurnal rhythm by TRF during the active dark phase effectively protects the BP circadian rhythm in db/db mice. Mechanistically, we demonstrated that TRF protects the BP circadian rhythm via suppressing the sympathetic nervous system (SNS) activity during the fasting inactive light phase in db/db mice. Our results reveal a potentially pivotal role of the timing of food intake in BP circadian rhythm in diabetes.
Results
Loss of Food Intake Diurnal Rhythm Coincides with Nondipping BP in db/db Mice.
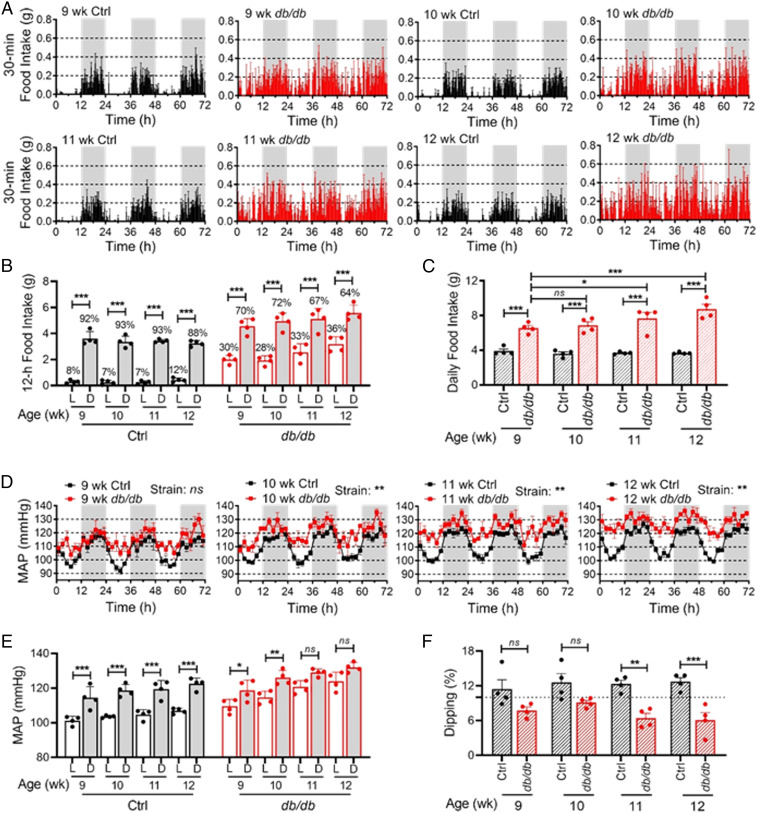
We and others have reported that the food intake diurnal rhythm (16) and BP circadian rhythm (17–19) are altered in db/db mice. To investigate whether the altered food intake diurnal rhythm is involved in nondipping BP in diabetes, we simultaneously monitored food intake and BP circadian rhythms by BioDAQ and telemetry in the same db/db mice and age-matched nondiabetic control (db/+) mice at 9, 10, 11, and 12 wk of age. BioDAQ is a computer-automated instrument that can be programmed to control the gates to food chambers at specified times and to monitor the amount and pattern of food intake continuously. The daily profiles of food intake demonstrated that the db/db mice consumed food throughout the day compared with the control mice that consumed food mostly during the dark phase throughout 9 to 12 wk of age (Fig. 1A). As such, the difference in food intake between the light and dark phase was significantly dampened in the db/db mice in an age-dependent manner (Fig. 1B). As expected, the db/db mice consumed more food than the control mice (Fig. 1C). Consequently, the db/db mice developed obesity and hyperglycemia (SI Appendix, Fig. S1 A and B).
Fig. 1.
Time course of the food intake diurnal rhythm and BP circadian rhythm in db/db and control mice. Food intake and BP were recorded by BioDAQ and telemetry in the same 9-, 10-, 11-, and 12-wk-old diabetic db/db mice and age-matched nondiabetic control mice (db/+). Four mice are in each group. (A) Daily profiles of food intake in 30-min intervals over 72 h (h) during the light (L) and dark (D) phases, shown in white and gray, respectively. (B) The 12-h food intake during the light and dark phases. Percent of daily food intakes are indicated above each bar. (C) Daily food intake. (D) Daily profiles of MAP over 72 h in 2-h intervals during the light and dark phases. (E) The 12-h average MAP during the light and dark phases. (F) MAP dipping was calculated as percentage of MAP decrease during the light phase compared to the dark phase. The dashed line indicates a 10% dipping. The data were analyzed by two-way ANOVA (C, D, and F) and three-way ANOVA (B and E) with multiple comparisons test and were expressed as the mean ± SE (SEM). *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
The circadian rhythm in mean arterial pressure (MAP) was monitored by telemetry in these conscious free-moving db/db and control mice at the same time when their food intake was monitored. Consistent with our previous reports (17, 18), a robust MAP circadian rhythm was found in the control mice but gradually diminished in the db/db mice in an age-dependent manner (Fig. 1D). Interestingly, the diminishment of BP circadian rhythm in the db/db mice primarily resulted from the larger increase of MAP during the light phase than during the dark phase (Fig. 1D), which coincided with the increased food intake during the light phase in the db/db mice (Fig. 1 A and B). In the db/db mice, the daytime elevation of MAP was evident as early as 9 wk of age before the nighttime elevation of MAP occurred (Fig. 1D). As a result, the normal diurnal MAP variation, as seen in control mice, was blunted in the db/db mice in an age-dependent manner (Fig. 1E). Moreover, the db/db mice developed nondipping BP by 9 wk of age (Fig. 1F).
Imposing a Food Intake Diurnal Rhythm by TRF Prevents db/db Mice from the Development of Nondipping BP.
The concomitant attenuation of the food intake diurnal rhythm and BP circadian rhythm indicates that the attenuation in the food intake diurnal rhythm may cause nondipping BP in db/db mice. To test this possibility, we investigated whether imposing a food intake diurnal rhythm by TRF prevents db/db mice from the development of nondipping BP. The 6-wk-old db/db and control mice were fed a chow diet ad libitum feeding (ALF), or 8-h TRF, during the active dark phase. Food intake and BP were measured by indirect calorimetry and telemetry, respectively, after 10 to 12 wk of ALF or TRF when mice were 16 to 18 wk of age.
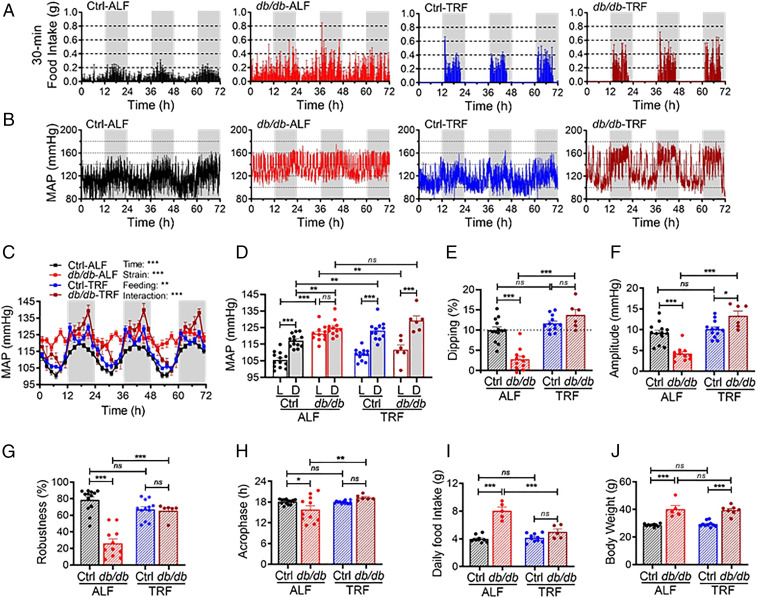
The daily food intake profile showed that the db/db mice under ALF exhibited modest diurnal rhythm, but TRF, as expected, imposed a robust food intake rhythm in the db/db mice (Fig. 2A). Representative telemetry recordings (Fig. 2B) and daily food intake profile (Fig. 2A) illustrate a significant temporal correlation between MAP and food intake in the db/db and control mice under ALF or TRF. The control mice under ALF exhibited a MAP circadian rhythm, characterized by a higher MAP during the dark phase than during the light phase (Fig. 2 B–D), which coincided with their food intake diurnal rhythm (Fig. 2A). In contrast, the db/db mice under ALF exhibited an impaired MAP circadian rhythm (Fig. 2 B and C), abolished diurnal MAP variation (Fig. 2D), and nondipping MAP (Fig. 2E), which correlated with their diminished food intake diurnal rhythm (Fig. 2A). Importantly, imposing a food intake diurnal rhythm by TRF prevented the db/db mice from the development of impaired MAP circadian rhythm, abolished diurnal MAP variation, and nondipping MAP, with little effect on these parameters in the control mice (Fig. 2 B–E). Notably, TRF selectively dwindled the MAP during the light phase while the db/db mice were fasting, whereas it had little effect on the MAP during the dark phase when the db/db mice were feeding (Fig. 2 B–D). These data suggest that TRF protects db/db mice from the development of nondipping BP by inducing fasting during the light phase.
Fig. 2.
Imposing a food intake circadian rhythm by TRF prevents db/db mice from the development of BP nondipping. Both 6-week-old db/db and control (db/+) mice were fed ALF or 8-h TRF. Food intake was measured by indirect calorimetry 12 wk after TRF or ALF when mice were 18 wk of age. BP was monitored by telemetry 10 wk after TRF or ALF when mice were 16 wk of age. (A) Daily profiles of food intake in 30-min intervals over 72 h during the light and dark phases, shown in white and gray, respectively. Ctrl-ALF (n = 10), db/db-ALF (n = 5), Ctrl-TRF (n = 10), and db/db-TRF (n = 5). (B) Representative continuous MAP recordings by telemetry in 10-s intervals over 72 h during the light and dark phases. (C) Daily profiles of the MAP in 2-h intervals over 72 h during the light and dark phases in Ctrl-ALF (n = 13), db/db-ALF (n = 11), Ctrl-TRF (n = 12), and db/db-TRF (n = 6) mice. (D) The 12-h average MAP during the light (L) and dark (D) phase. (E) MAP dipping (percentage of MAP decrease during the light phase compared to the dark phase). The dashed line indicates a 10% dipping. (F−H) Amplitude (F), robustness (G), and acrophase (H) of the MAP circadian rhythm. (I and J) Daily food intake (I) and body weight (J) were measured 10 to 12 wk after ALF or TRF. The data were analyzed by three-way ANOVA (C and D) and two-way ANOVA (E−J) with multiple comparisons test and were expressed as the mean ± SE (SEM). *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
The effect of the TRF on the BP circadian rhythm was further analyzed by cosinor analysis (20). We calculated the amplitude (the half value between the peak and trough of a rhythm), robustness (the strength and stability of a rhythm), and acrophase (the time at which the peak of rhythm occurs) of the MAP circadian rhythm in the same groups of the db/db and control mice under ALF or TRF aforementioned. During ALF, the amplitude, robustness, and acrophase of MAP circadian rhythm were all significantly lower in the db/db mice compared to those in the control mice (Fig. 2 F–H). In contrast, during TRF, the amplitude, robustness, and acrophase of MAP circadian rhythm were significantly preserved in the db/db mice at the levels comparable to control mice (Fig. 2 F–H). Importantly, similar effects of TRF on systolic BP (SBP) and diastolic BP (DBP) circadian rhythms were also found in the same groups of db/db and control mice after 10 wk of ALF or TRF (SI Appendix, Fig. S2 A–L).
We also determined daily food intake, body weight, body composition, blood glucose, and diurnal rhythm in metabolism in db/db and control mice after 7 to 12 wk of ALF or TRF. As shown in Fig. 2I, TRF did not affect the daily food intake in control mice but significantly reduced daily food intake in db/db mice. Although db/db-TRF mice consumed less food than db/db-ALF mice, they had similar body weights (Fig. 2J) and body compositions (SI Appendix, Fig. S3A). Blood glucose was measured at zeitgeber time (ZT)13 (corresponding to the time after 16-h fasting) and ZT21 (corresponding to the time after 8-h feeding) in db/db and control mice under ALF or TRF. Whereas TRF did not affect blood glucose at either ZT13 or ZT21 in the control mice, it selectively decreased blood glucose at ZT13 but not ZT21 in the db/db mice (SI Appendix, Fig. S3 B and C). The diurnal rhythm in energy expenditure (EE) and respiratory exchange ratio (RER) was measured by indirect calorimetry in db/db and control mice under ALF or TRF. TRF did not affect the EE diurnal rhythm but markedly enhanced the RER diurnal rhythm in the control mice (SI Appendix, Fig. S3 D and F). In contrast, TRF protected the db/db mice from the loss of diurnal rhythms in both EE and RER (SI Appendix, Fig. S3 E and G).
TRF Restores BP Dipping in the db/db Mice that Already Have Nondipping BP.
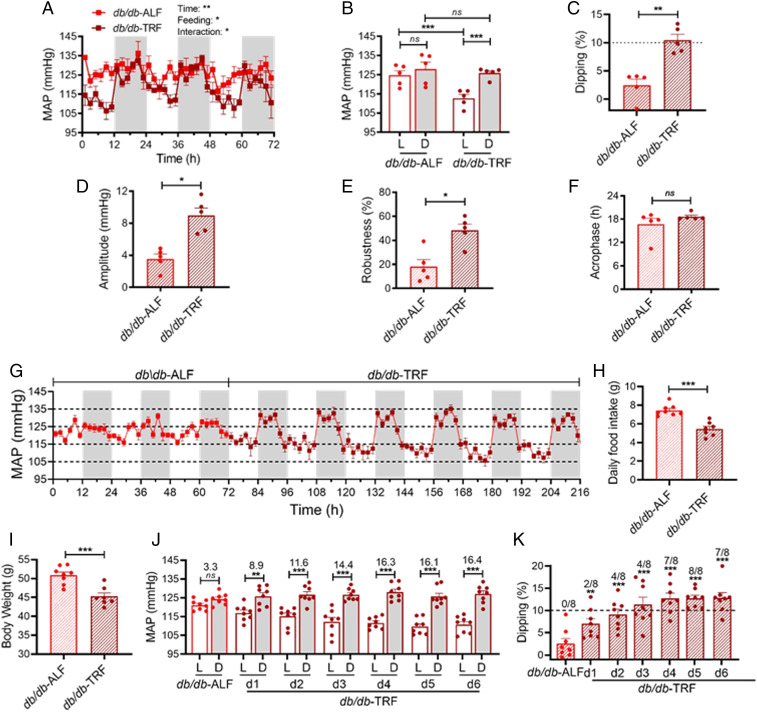
To investigate whether TRF can restore BP dipping in nondipping db/db mice, which is highly relevant to diabetic patients, we investigated the effect of TRF on BP circadian rhythm in 15-wk-old db/db mice that already exhibit nondipping BP (17). BP was recorded by telemetry at baseline with ALF and again after 9 d of TRF. As expected, the db/db mice under ALF lost the MAP circadian rhythm (Fig. 3A), diurnal MAP variation (Fig. 3B), and MAP dipping (Fig. 3C). Surprisingly, only 9 d of switching ALF to TRF sufficiently restored the MAP circadian rhythm, diurnal MAP variation, and MAP dipping in db/db mice (Fig. 3 A–C). Similar to the effect of TRF in the prevention studies (Fig. 2), TRF also selectively dropped the MAP during the light phase but not the dark phase (Fig. 3 A and B), indicating that TRF restores the BP circadian rhythm through fasting during the light phase in db/db mice. Interestingly, the amplitude and robustness but not the acrophase of MAP circadian rhythm were significantly restored in db/db-TRF mice relative to that in db/db-ALF mice (Fig. 3 D–F). Moreover, similar effects on the SBP and DBP circadian rhythms were also found in the same groups of db/db mice after 9 d of TRF (SI Appendix, Fig. S4 A–L).
Fig. 3.
TRF restores BP dipping in nondipping db/db mice. BP was monitored by telemetry. In A–F, MAP was first recorded for 3 d in the 15-week-old db/db mice (n = 5) under ALF and then another 3 d after 9 d of 8-h TRF. (A) Daily profiles of the MAP in 2-h intervals over 72 h during the light and dark phases, shown in white and gray, respectively. (B) The 12-h average MAP during the light (L) and dark (D) phase. (C) MAP dipping. The dashed line indicates 10% dipping. (D–F) Amplitude (D), robustness (E), and acrophase (F) of MAP circadian rhythm. In G–K, MAP was continuously recorded in the 16-week-old db/db mice (n = 8) under ALF for 3 d and then under TRF for 6 d. (G) Daily profiles of the MAP in 2-h intervals over 216 h during the light and dark phases, shown in white and gray, respectively. (H and I) Daily food intake (H) and body weight (I) were measured 8 and 9 d after ALF or TRF, respectively. (J) The 12-h average MAP during the light (L) and dark (D) phase. The difference in MAP between the light and dark phases are indicated above the bars. (K) MAP dipping. The dashed line indicates 10% dipping. The number of mice with ≥ 10% dipping are indicated above each bar. The data were analyzed by two-way ANOVA with multiple comparisons test (A and B), t test (C–F; H and I), and one-way ANOVA (K), and were expressed as the mean ± standard error (SEM). *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
To define how rapidly TRF can rescue nondipping BP in db/db mice, BP was continuously monitored by telemetry in an independent group of 16-wk-old db/db mice under ALF for 3 d and then TRF for 6 d. A time-dependent protective effect of TRF on BP circadian rhythm (Fig. 3G), diurnal MAP variation (Fig. 3J), and MAP dipping (Fig. 3K) was observed in db/db-TRF mice compared to db/db-ALF mice. A moderate but significant effect on these parameters could be detected as early as 1 d after TRF (Fig. 3 G, J, and K). Importantly, sustained and robust effects were observed 4 d after TRF (Fig. 3 G, J, and K). Daily food intake and body weight were measured 8 to 9 d after ALF or TRF. Both food intake and body weight were significantly reduced in db/db-TRF mice compared to db/db-ALF mice (Fig. 3 H and I).
Increasing TRF from 8 h to 12 h Promotes Isocaloric Feeding and Similarly Rescues BP Dipping in Nondipping db/db Mice.
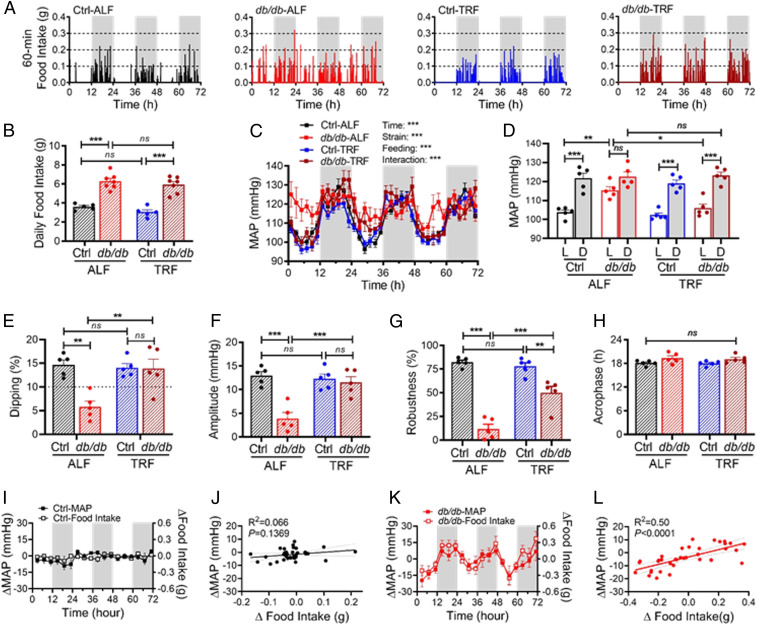
To determine whether TRF without reducing food intake can also rescue nondipping BP in db/db mice, we increased the time of food availability from 8 h to 12 h during the active dark phase. We simultaneously monitored the food intake and BP by BioDAQ and telemetry in the same 14-wk-old db/db and control mice for 3 d under ALF and then another 3 d after 4 d of 12-h TRF. Representative food intake recordings illustrated that 12-h TRF imposed a robust food intake diurnal rhythm on the db/db mice with little effect on the control mice (Fig. 4A). Quantitative analysis of the profile of food intake showed that 12-h TRF did not affect the number of feeding bouts, duration of feeding bouts, and percent of time spent in feeding bouts in the db/db and control mice (SI Appendix, Fig. S5 A–C). Unlike 8-h TRF that reduces daily food intake and body weight in db/db mice (Fig. 3 H and I), 12-h TRF had no significant effect on daily food intake and body weight in db/db mice (Fig. 4B and SI Appendix, Fig. S5D). These results suggest that the db/db mice under 12-h TRF during the active phase were under isocaloric feeding. Nevertheless, similar to 8-h TRF (Fig. 3), 12-h TRF effectively restored the MAP circadian rhythm in the db/db mice, including diurnal MAP variation, MAP dipping, amplitude, and robustness (Fig. 4 C–H).
Fig. 4.
Increasing TRF from 8 h to 12 h promotes isocaloric feeding and rescues BP dipping in nondipping db/db mice. Food intake and BP were simultaneously recorded by BioDAQ and telemetry in the same 14-wk-old db/db and control (db/+) mice for 3 d under ALF and then another 3 d after 4 d of 12-h TRF. Five mice are in each group. (A) Representative BioDAQ recordings of daily profiles of the food intake in 1-min intervals over 72 h during the light and dark phases, shown in white and gray, respectively. (B) Total daily food intake. (C) Daily profiles of the MAP in 2-h intervals over 72 h during the light and dark phase. (D) The 12-h average MAP during the light (L) and dark (D) phase. (E) MAP dipping. The dashed line indicates a 10% dipping. (F−H) Amplitude (F), robustness (G), and acrophase (H) of the MAP circadian rhythm. (I and K) Daily profiles of the changes in MAP (ΔMAP, left y-axis) and food intake (Δfood intake, right y-axis) in 4-h intervals in the control mice (I) and db/db mice (K). (J and L) There is a correlation between ΔMAP and Δfood intake in db/db mice (L) but not in control mice (J). The data were analyzed by two-way ANOVA (B and E−H) and three-way ANOVA (C and D) with multiple comparisons test and simple linear regression (J and L) and were expressed as the mean ± SE (SEM). *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
Compared with the db/db and control mice under ALF, the db/db but not control mice under 12-h TRF exhibited a selective reduction in food intake and MAP during the light phase than the dark phase (Fig. 4 A, C, and D). To investigate whether the reduced consumption of food temporally correlates with the deceased MAP during the light phase, we took advantage of our food intake and BP data that were simultaneously collected from the same db/db and control mice. We calculated the change in food intake (Δfood intake) and the change in MAP (ΔMAP) by subtracting the food intake or MAP at corresponding times over 72 h in db/db-ALF and Ctrl-ALF mice from db/db-TRF and Ctrl-TRF mice, respectively. In the control mice, TRF induced minimal changes in food intake and MAP (Fig. 4I), and there was no correlation between Δfood intake and ΔMAP (Fig. 4J). In contrast, in the db/db mice, TRF simultaneously induced substantial changes in food intake and MAP (Fig. 4K), and there was a significant correlation between Δfood intake and ΔMAP (Fig. 4L).
The SNS Mediates the TRF Protection of BP Circadian Rhythm in db/db Mice.
To explore the mechanism by which TRF protects db/db mice from nondipping BP, we investigated the potential roles of the sympathetic nervous system (SNS) as it responds to food intake, exhibits a daily oscillation, and plays a predominant role in the BP circadian rhythm (21, 22). Multiple approaches were taken to investigate this potentially important mechanism.
First, we investigated whether TRF affects the heart rate (HR) circadian rhythm in db/db mice, as HR is regulated by the balance of sympathetic and parasympathetic tone (23). HR circadian rhythm was measured by telemetry in 16-wk-old db/db and control mice that had been fed ALF or TRF for 10 wk. HR circadian rhythm was moderately altered in the db/db-ALF mice relative to the Ctrl-ALF mice (SI Appendix, Fig. S6 A and B). TRF significantly decreased the HR during the light phase without affecting the HR during the dark phase and thereby effectively protected the HR circadian rhythm, diurnal HR variation, HR dipping, amplitude, robustness, and acrophase in the db/db mice (SI Appendix, Fig. S6 A–F). Similar benefits of TRF on HR circadian rhythm were also observed in 15-wk-old db/db mice, whose HR circadian rhythm had already been disrupted (SI Appendix, Fig. S6 G–L).
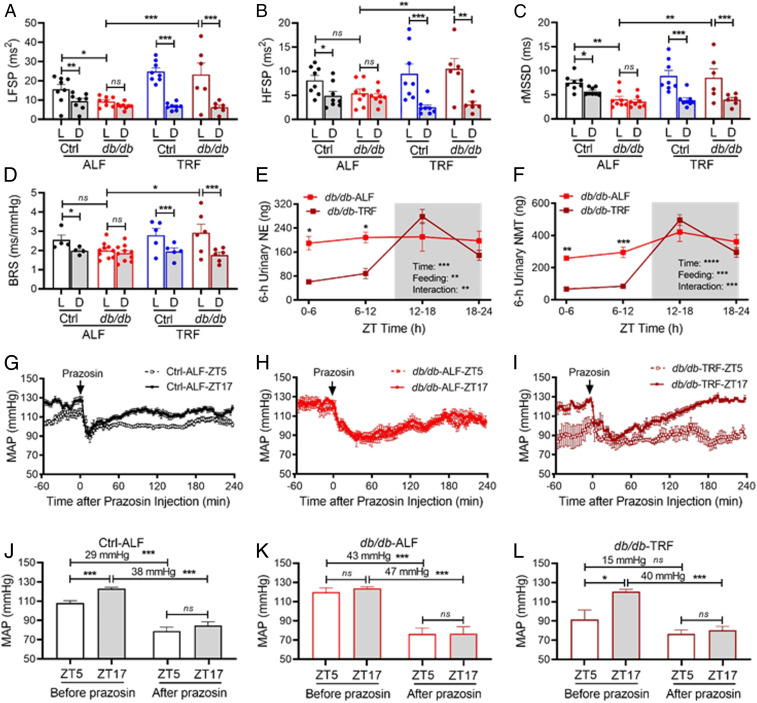
Second, we investigated whether TRF affects diurnal variation in HR variability (HRV) in db/db mice, as the HRV is considered as an index of the sympathetic and parasympathetic activity (24, 25). The HRV is defined as a physiological variation in the time intervals between heartbeats and can be analyzed by the frequency domain and time domain techniques, including the low-frequency spectral power (LFSP), high-frequency spectral power (HFSP), and root mean square of successive RR interval differences (rMSSD) (24, 25). The effect of TRF on diurnal variations of HRV was determined in 16-wk-old db/db and control mice that had been fed ALF for or TRF 10 wk. LFSP, HFSP, and rMSSD were all significantly higher during the light phase than during the dark phase in Ctrl-ALF mice (Fig. 5 A–C and SI Appendix, Fig. S7 A, C, and E). In contrast, these diurnal variations were abolished in db/db-ALF mice (Fig. 5 A–C and SI Appendix, Fig. S7 B, D, and F). Interestingly, TRF significantly increased LFSP, HFSP, and rMSSD during the light phase with little effect during the dark phase, thus preventing db/db mice from the loss of the diurnal variations in LFSP, HFSP, and rMSSD (Fig. 5 A–C and SI Appendix, Fig. S7 A–F). Similar beneficial effects of TRF on diurnal variation in HRV were also observed in 15-wk-old db/db mice that already lost their HRV diurnal variations (SI Appendix, Fig. S8 A–F).
Fig. 5.
The SNS mediates the TRF protection of BP circadian rhythm in db/db mice. (A−D) The 12-h average LFSP (A), HFSP (B), average rMSSD (C), and spontaneous BRS (D) during the light (L) and dark (D) phase in 16-wk-old db/db and control (db/+) mice that had been fed ALF or 8-h TRF for 10 wk. (E and F) Levels of NE (E) and its metabolite NMT (F) in 6-h urine samples collected at ZT0-6, ZT6-12, ZT12-18, and ZT18-24 from 15-wk-old db/db mice that had been fed ALF or 8-h TRF for 8 wk (n = 4 to 5). ZT, zeitgeber time (ZT0 = lights on and ZT12 = lights off). Levels of NE and NMT are expressed as total contents, calculated by concentration x urine volumes. The gray box indicates the dark phase. (G−I) The 3-min average MAP response to prazosin, an α1 adrenergic receptor antagonist (1 mg/kg; i.p.), at ZT5 and ZT17 in 18- to 21-wk-old Ctrl-ALF (G, n = 12), db/db-ALF (H, n = 9), and db/db-TRF (I, n = 4) mice after 14 d of TRF or ALF. (J−L) The 1-h average MAP before prazosin injection (baseline) and the lowest MAP after prazosin injection at ZT5 and ZT17 in the Ctrl-ALF (J; n = 12), db/db-ALF (K; n = 9), and db/db-TRF (L; n = 4) mice. The data were analyzed by three-way ANOVA (A−D) and two-way ANOVA (E−L) with multiple comparisons test and were expressed as the mean ± SE (SEM). *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
Third, we investigated whether TRF affects diurnal variation in spontaneous baroreflex sensitivity (BRS) in db/db mice, as BRS has been widely used as a cardiac autonomic index (26). The effect of TRF on BRS was determined by sequence techniques (27) in 16-wk-old db/db and control mice that had been fed ALF or TRF for 10 wk. In accordance with our previous report (18, 28), BRS was higher during the light phase than during the dark phase in Ctrl-ALF mice (Fig. 5D and SI Appendix, Fig. S9A). In contrast, this BRS diurnal variation was lost in db/db-ALF mice (Fig. 5D and SI Appendix, Fig. S9B). TRF significantly increased the BRS during the light phase, while having little effect on the BRS during the dark phase, thus protecting db/db mice from the loss of the BRS diurnal variation (Fig. 5D and SI Appendix, Fig. S9B). TRF had similar beneficial effects in 15-wk-old db/db mice that already lost their diurnal variations in BRS (SI Appendix, Fig. S9 C and D).
Fourth, to more directly investigate whether the SNS is involved in the TRF protection of BP circadian rhythm in diabetes, we measured diurnal variations of urinary norepinephrine (NE) excretion in 15- to 16-wk-old db/db and control mice under ALF or TRF. The db/db mouse urine was collected in the 6-h interval during ZT0-6, ZT6-12, ZT12-18, and ZT18-24, whereas the control mouse urine was collected in the 12-h interval during ZT0-12 and ZT12-24 due to the difficulty to collect sufficient volumes of urine from the control mice. In Ctrl-ALF mice, urinary NE excretion exhibited a diurnal variation with a higher level during the dark phase than during the light phase (SI Appendix, Fig. S10A). In contrast, in db/db-ALF mice, the diurnal NE variations were abolished (Fig. 5E). Interestingly, TRF did not significantly affect urinary NE excretion during the light and dark phases in the control mice (SI Appendix, Fig. S10A) but potently protected the db/db mice from the loss of diurnal variation of NE (Fig. 5E). We also measured 6-h urinary excretion of normetanephrine (NMT), a metabolite of NE, in the same urine samples that we measured NE in db/db mice under ALF or TRF. TRF significantly protected the db/db mice from the loss of diurnal variation of NMT (Fig. 5F). Similar to its effects on BP, HR, HRV, and BRS, TRF also selectively decreased high urinary NE and NMT levels during the light phase (ZT0-6 and ZT6-12) but not the dark phase (ZT12-18 and ZT18-24) in the db/db mice (Fig. 5 E and F). These results suggest that TRF may selectively inhibit sympathetic activity during the light phase, thus protecting db/db mice from nondipping BP. To verify that the TRF-induced NE decrease during the light phase in urine samples reflects circulating NE levels in the db/db mice, we measured the plasma NE levels at ZT6 during the light phase in the db/db mice under ALF or TRF. The results confirmed that plasma NE levels were indeed lower during the light phase in db/db-TRF mice than in db/db-ALF mice (SI Appendix, Fig. S10B).
Finally, to explore a potential cause-and-effect relationship between the sympathetic vascular tone and BP diurnal variation, we determined instant MAP responses to prazosin at ZT5 during the light phase and ZT17 during the dark phase in 18- to 21-wk-old db/db and control mice after 14 d of TRF or ALF. Prazosin is a potent α1 adrenergic receptor antagonist that explicitly blocks the effect of the SNS on smooth muscle contraction, thus lowing BP. The MAP in 1 h before prazosin injection (i.p., 1 mg/kg body weight) and 2 h after prazosin injection were recorded by telemetry, respectively, in Ctrl-ALF, db/db-ALF, and db/db-TRF mice (Fig. 5 G–I). The decrease in MAP between before and after prazosin injection reflected the sympathetic vascular tone at ZT5 or ZT17. In Ctrl-ALF mice, the MAP was higher at ZT17 than ZT5 before prazosin injection, and prazosin reduced MAP to the same value at both times (Fig. 5 G and J). As a result, there was a larger MAP decreasing effect of prazosin at ZT17 than ZT5 (Fig. 5 G and J). This data indicates a higher sympathetic vascular tone during the dark phase than the light phase in the control mice under ALF. In contrast, in db/db-ALF mice, the MAP at ZT5 was increased, and the MAP at ZT5 and ZT17 was similar before and after prazosin injection so that prazosin had a similar MAP decreasing effect at both times (Fig. 5 H and K). This data indicates that the sympathetic vascular tone was increased during the light phase in db/db mice under ALF, which leads to the loss of the diurnal variation in sympathetic vascular tone. In db/db-TRF mice, the MAP at ZT5 was diminished, and as a result, the diurnal variation in MAP was restored (Fig. 5 I and L). Since the MAP at ZT5 and ZT17 was similar after prazosin injection, there was a larger MAP decreasing effect of prazosin at ZT17 than ZT5 (Fig. 5 I and L). This data indicates that TRF suppresses the sympathetic vascular tone during the light phase and thereby restored the diurnal variation in MAP in db/db mice under TRF.
In addition to the SNS, we also explored other mechanisms that may play a role in the TRF protection of BP dipping in db/db mice, including vascular reactivity, adrenal gland hormones, renin-angiotensin-aldosterone system (RAAS), locomotor activity, and clock genes. Vascular reactivity is defined as the responsiveness of a blood vessel to a specific stimulus. We first determined α1a and α1d adrenergic receptor (Adra1a and Adra1d) mRNA (messenger ribonucleic acid) daily oscillations in the mesenteric arteries of 21-wk-old db/db and control mice that had been fed ALF or TRF for 4 wk. TRF did not affect Adra1a and Adra1d mRNA daily oscillations in db/db or control mice (SI Appendix, Fig. S11 A and B). To more directly explore the role of vascular reactivity in the TRF protection of BP dipping, we determined the instant pressor responses to an intravenous bolus injection of phenylephrine (PE) or angiotensin II (Ang II) at ZT5 and ZT17 in db/db mice after 9 d of TRF. If TRF protects BP dipping via vascular reactivity, we expected that TRF would selectively inhibit vascular reactivity at ZT5 during the light phase. In contrast to our expectation, there was no significant difference in the pressor responses to PE or Ang II between ZT5 and ZT17 in db/db-TRF mice (SI Appendix, Fig. S11 C and D), indicating that vascular reactivity is unlikely involved in the TRF protection of BP dipping.
Adrenal gland hormones, including epinephrine (EPI), aldosterone (ALDO), and corticosterone (CORT), are regulated by the SNS and are well recognized for their pivotal roles in BP homeostasis (29). To explore the role of EPI, ALDO, and CORT in the TRF protection of BP dipping, we determined the time-of-day variations of EPI, ALDO, and CORT in the db/db mice and EPI diurnal rhythm in the control mice under ALF and TRF in the same urine samples that we used to measure NE. In contrast to NE (Fig. 5E), EPI, ALDO, and CORT possessed diurnal variation in db/db-ALF mice (SI Appendix, Fig. S12 A–C). TRF did not affect the urinary EPI excretion during the light phase (ZT0 through 12) in the db/db (SI Appendix, Fig. S12A) or control mice (SI Appendix, Fig. S12D). However, TRF increased urinary EPI excretion during the dark phase (ZT12 through 18) in the db/db mice (SI Appendix, Fig. S12A), thus enhancing its diurnal variation. In contrast, TRF suppressed ALDO and CORT in the db/db mice during the light phase (ZT6 through 12), thus improving their diurnal variations (SI Appendix, Fig. S12 B and C).
The RAAS is primarily comprised of angiotensinogen (Agt), renin (Ren1), angiotensin-converting enzyme (Ace), Ace2, and angiotensin II receptor 1a (Agtr1a). Accumulating evidence suggests that the RAAS activation contributes to BP nondipping via the SNS in diabetes (19, 30). To explore whether the RAAS is involved in the TRF protection of BP dipping in db/db mice, we determined the daily oscillation of Agt, Ren1, Ace, Ace2, and Agtr1a mRNAs in the liver or kidney from 21-wk-old db/db and control mice that had been fed ALF or TRF for 4 wk. TRF did not significantly affect the daily oscillation of Agt, Ren1, Ace, Ace2, and Agtr1a mRNAs in the db/db mice (SI Appendix, Fig. S13 A–F).
Locomotor activity and BP are synchronized by the clock genes in the suprachiasmatic nucleus (SCN) and exhibit similar circadian rhythms under physiological conditions (28). To determine whether TRF protects BP circadian rhythm through locomotor activity, we measured locomotor activity by telemetry in the same db/db and control mice under ALF or TRF in which we measured BP (Fig. 2). Consistent with our previous report (17), db/db-ALF mice showed little locomotor activity circadian rhythm compared to Ctrl-ALF mice (SI Appendix, Fig. S14 A and B). In contrast to its potent protection on the BP circadian rhythm in the db/db mice (Fig. 2), TRF had minimal effect on locomotor activity circadian rhythm in the db/db mice (SI Appendix, Fig. S14 A–F). In 15-wk-old db/db mice that already had lost their locomotor activity circadian rhythm, TRF induced a brief increase in locomotor activity at the onset of the dark phase but failed to restore the locomotor activity circadian rhythm (SI Appendix, Fig. S14 G–L).
BP circadian rhythms are controlled at the molecular level by interacting transcriptional–translational feedback loops composed of a set of clock genes, which are expressed in cells throughout the body (31, 32). To investigate whether the clock genes participate in the TRF protection of BP circadian rhythm, we determined the daily transcript oscillations of a panel of clock genes, including Bmal1 (aryl hydrocarbon receptor nuclear translocator-like [Arntl]), Clock, Per 1 (period 1), Per2 (period 2), Cry 1 (cryptochrome 1), Cry2 (cryptochrome 2), Rev-erbα (nuclear receptor subfamily 1, group D, member 1 [Nr1d1]), and Rorc (RAR-related orphan receptor gamma), in the liver, kidney, heart, adrenal gland, and mesenteric arteries from db/db and control mice. We chose the liver because clock genes in the liver are extensively studied and are well documented for their robust and prompt responses to feeding (33). We chose the kidney, heart, adrenal gland, and mesentery arteries because these tissues are critical for BP homeostasis and circadian rhythm (34). We did not choose the SCN, the master circadian pacemaker, because previous studies have shown that the SCN does not respond to TRF (33).
The daily mRNA oscillation of Bmal1, Clock, Per1, Per2, Cry1, Cry2, Rev-erbα, and Rorc were determined by real-time PCR in the liver, kidney, heart, adrenal gland, and mesentery arteries at ZT5, ZT11, ZT17, and ZT23 in 21-wk-old db/db and control mice that had been fed ALF or TRF for 4 wk. Consistent with our previous report (35), the daily mRNA oscillation of Bmal1, Clock, Per1, Per2, Cry1, Cry2, Rev-erbα, and Rorc was significant altered in the db/db mice compared to Ctrl-ALF mice (SI Appendix, Figs. S15–S22). Importantly, TRF was capable of restoring the daily mRNA oscillation of Bmal1, Clock, Rev-erbα, Per1, Per2, Cry1, Cry2, and Rorc in the db/db mice in a time-, gene-, and tissue-specific manner (SI Appendix, Figs. S15–S22). Among all clock genes examined, Bmal1 is the only one whose single-gene deletion results in immediate and complete loss of circadian rhythmicity (36), including loss of the BP circadian rhythm (32, 37). Consistent with its potential role in the TRF protection of BP circadian rhythm in db/db mice, Bmal1 was most significantly altered in db/db-ALF mice relative to Ctrl-ALF mice and consistently rescued by TRF in all tissues examined in db/db-TRF mice (SI Appendix, Figs. S15–S22). In Ctrl-ALF mice, Bmal1 mRNA oscillated with the peak at ZT23 and trough at ZT11 in all tissues examined (SI Appendix, Fig. S15). In contrast, in db/db-ALF mice, Bmal1 mRNA oscillated with the peak at ZT17 and trough at ZT5 in all tissues examined except for the mesentery arteries in which Bmal1 daily oscillation was suppressed (SI Appendix, Fig. S15). While TRF did not affect daily Bmal1 oscillation in the control mice, TRF rescued Bmal1 mRNA daily oscillation in the db/db mice to a pattern similar to that in Ctrl-ALF mice (SI Appendix, Fig. S15).
Discussion
The major findings of the current study include 1) loss of the food intake diurnal rhythm coincides with nondipping BP in diabetic db/db mice, 2) imposing a food intake diurnal rhythm by TRF prevents db/db mice from the development of nondipping BP, 3) TRF restores BP dipping in db/db mice that already have nondipping BP, 4) increasing TRF from 8 h to 12 h promotes isocaloric feeding and similarly rescues nondipping BP in db/db mice, and 5) mechanistically, TRF suppresses the sympathetic nervous system activity during the inactive light phase via fasting, thereby protecting the BP circadian rhythm in db/db mice.
What is the significance of our findings? There is a growing body of literature demonstrating that the timing of food intake is critical for diet-induced metabolic disorders (11–15). However, only sparse information about the effect of the timing of food intake on BP circadian rhythm is presently available. It has been shown that changing the time when pellet food was presented to rabbits or dogs immediately shifted the peak of the BP circadian rhythm (38, 39). It has also been shown that rats receiving feeding for 1 h only during the light phase rather than the dark phase exhibited a suppressed BP circadian rhythm (40). Recently, Zhang et al. reported that reverse feeding (food is only available during the 12-h lights-on period) for 6 d inverted the diurnal rhythm of BP in C57BL/6J mice (41). These studies suggest that the timing of food intake can influence BP circadian rhythm in nondiabetic healthy animals. However, it is unknown whether nondipping BP, which is a serious risk factor for adverse cardiovascular consequences, is causally associated with altered daily timing of food intake.
The current study demonstrated that the loss of food intake diurnal rhythm coincides with nondipping BP in diabetic db/db mice (Figs. 1 and 2). More importantly, the current study demonstrated that imposing a food intake diurnal rhythm by TRF not only prevented db/db mice from the development of nondipping BP (Fig. 2 and SI Appendix, Fig. S2) but also effectively restored BP dipping in db/db mice that already have nondipping BP (Figs. 3 and 4 and SI Appendix, Fig. S4). The results from the current study suggest that the loss of food intake diurnal rhythm contributes to the etiology and development of nondipping BP in diabetes, and the lifestyle changes (i.e., limiting eating to the right time of day) may effectively improve nondipping BP in diabetes.
The TRF used in the current study differs from intermittent fasting or periodic fasting that lately has become very popular in humans (42). TRF, intermittent fasting, and periodic fasting all impose a cycle consisting of fasting periods alternating with nonfasting periods and effectively protect against obesity, diabetes, cancers, heart disease, asthma, rheumatoid arthritis, neurodegeneration, and hypertension (11, 42). However, the TRF used in the current study imposes the fasting and feeding cycle within one 24-h day, and importantly, the feeding phase aligns with the dark phase when most of the food is normally consumed in nocturnally active rodents. In contrast, intermittent fasting imposes fasting for 1 d every other day or 2 d a week, and periodic fasting imposes fasting 3 d or longer every 2 or more weeks, both of which have 20 to 40% of caloric restriction (42). Given that BP exhibits a circadian rhythm, it is tempting to speculate that TRF, but not intermittent fasting or periodic fasting, can effectively protect against nondipping BP in diabetes. It is also worth pointing out that TRF often induces caloric restriction, which may contribute to, at least in part, the TRF protection of BP circadian rhythm in diabetes.
The TRF used in the current study also differs from the bedtime ingestion of anti-hypertension medications, which has been shown to be more effective than traditionally recommended morning ingestion of anti-hypertension medicines (43). Compared with morning ingestion of anti-hypertension medications, bedtime ingestion of anti-hypertension medications not only improves BP control and nondipping BP but also significantly reduces the risk of new-onset type 2 diabetes and cardiovascular events (43, 44). However, it is unlikely that bedtime ingestion of anti-hypertension medications is effective for the treatment of metabolic disorders that commonly coexist with hypertension in type 2 diabetic patients. It is also unclear whether bedtime ingestion of anti-hypertension medications is effective in the prevention of the development of metabolic disorders and nondipping BP in people with obesity and prediabetes. In contrast, the TRF used in the current study not only effectively restores the BP circadian rhythm in db/db mice that have already developed nondipping BP (Figs. 3 and 4) but also prevents the development of nondipping BP and metabolic disorder in db/db mice (Fig. 2 and SI Appendix, Fig. S3). Recent small but rigorously controlled proof-of-concept clinical trials reported that early 4-h or self-selected 10-h TRF effectively lowered SBP and DBP in men with prediabetes or metabolic syndrome (45, 46). Although these clinical trials did not monitor BP circadian rhythm, it suggests the feasibility of TRF in the prevention of nondipping BP in humans. However, large clinical trials are needed to investigate further the efficacy of this nonpharmacological, chrono-nutritional, and low-cost approach for restoration and maintenance of a healthy BP rhythm in diabetic patients.
How does TRF protect BP circadian rhythm in diabetes? One of the intriguing findings from the current study is that imposing food intake diurnal rhythm by TRF selectively lowered BP during the light or fasting phase but not the dark or nonfasting phase in db/db mice (Figs. 2 B–D, 3 A and B, and 4 C and D). While the underlying mechanism remains elusive, previous studies have shown that nondipping BP was associated with increased sympathetic vascular tone in db/db mice (17, 19), and fasting was capable of potently suppressing sympathetic activity in nondiabetic rats (22, 47). These studies suggest that TRF may selectively decrease sympathetic vascular tone during the light phase via fasting in db/db mice and thereby protect their BP circadian rhythm. Several lines of evidence from the current study support this potential mechanism. First, TRF selectively decreased HR during the light phase but not the dark phase in db/db mice (SI Appendix, Fig. S6). Second, TRF selectively increased the HRV (LFSP, HFSP, and rMSSD) during the light phase but not the dark phase in db/db mice (Fig. 5 A–C and SI Appendix, Figs. S7 and S8). Third, TRF selectively increased the BRS during the light phase but not the dark phase in db/db mice (Fig. 5D and SI Appendix, Fig. S9). Since the HR, HRV, and BRS reflect the balance of sympathetic and parasympathetic activity (24, 25), these findings suggest that TRF protects BP circadian rhythm in db/db mice by inhibiting the sympathetic activity or activating parasympathetic vascular tone during the light phase via fasting in db/db mice. Fourth, perhaps the most important, TRF selectively decreased sympathetic neurotransmitter NE and its metabolite NMT during the light phase but not the dark phase in db/db mice (Fig. 5 E and F and SI Appendix, Fig. S10B). Finally, the selective effect of TRF on BP during the light phase was sensitive to α1 adrenergic receptor antagonist prazosin in db/db mice (Fig. 5 G–L). Collectively, these data suggest that TRF inhibits sympathetic vascular tone during the light phase via fasting, thus protecting the BP circadian rhythm in db/db mice.
In addition to the SNS, accumulating evidence suggests that many additional mechanisms may also mediate the TRF protection of BP circadian rhythm in db/db mice. These potential mechanisms include but are not limited to vascular reactivity, adrenal gland hormones, RAAS, locomotor activity, sleep–wake cycle, gut microbiota, and clock genes. While the results from the current study do not support the role of vascular reactivity and locomotor activity in the TRF protection of BP circadian rhythm in db/db mice (SI Appendix, Figs. S11 and S14), the following mechanisms need to be investigated further. First, although we did not find that TRF affects the daily mRNA oscillation of Agt, Ren1, Ace1, Ace2, and Agtr1a in the liver or kidney in db/db mice (SI Appendix, Fig. S13), the role of the RAAS in the TRF protection of BP circadian rhythm in diabetes cannot be excluded. It has been shown that chronic treatment of db/db mice with the Ang II antagonist losartan or the ACE inhibitor enalapril blocked the increase in MAP and improved autonomic regulation (19, 30). It also has been shown that feeding time has a marked influence on the chronobiology of renin cascade, urinary electrolytes, and BP (39). Consistent with these findings, the current study demonstrated that TRF selectively diminished urine aldosterone during the light phase but not the dark phase in db/db mice (SI Appendix, Fig. S12B). Second, we have shown in other studies that TRF can restore a normal daily sleep–wake cycle in db/db mice (48). Since the role of the sleep–wake cycle in BP circadian rhythm is well recognized (49), TRF may improve the sleep–wake cycle and thereby protect the BP circadian rhythm in db/db mice. Third, several recent studies indicated that gut microbiota is involved in BP control and hypertension (50). Moreover, the gut microbiome is highly dynamic and exhibits daily cyclical fluctuations in composition, which can be affected by TRF and links to the host circadian clock and metabolism (51).
At the molecular level, how does TRF suppress sympathetic activity via fasting in diabetes? Over the last half-century, accumulated evidence has demonstrated that fasting can reduce catecholamine turnover, an index of sympathetic activation, in the rat heart and brain (22, 52). While multiple mechanisms may be involved, it has been shown that fasting significantly inhibits the activity of tyrosine hydroxylase (TH), the rate-limiting enzyme in NE synthesis, in the rat brain (53). The current study demonstrates that TRF restores the daily mRNA oscillations of a number of clock genes, including the Bmal1, Clock, and Rev-erbα, in multiple tissues in the db/db mice in a time-, gene-, and tissue-specific manner (SI Appendix, Figs. S15–S22). Interestingly, Rev-erbα, Clock, and Bmal1 have been shown to negatively regulate TH mRNA and protein expression in the mouse brain (54–56). Given the predominant role of the SNS in the BP circadian rhythm (21, 22) and the finding that adrenal TH was up-regulated in db/db mice (57), it is tempting to speculate that TRF may protect db/db mice from nondipping BP by acting through TH, SNS, and clock genes during the light phase when they are fasting. While further studies are required to test this potential mechanism, it should be pointed out that the role of clock genes in the cardiovascular system is complicated. We and others reported that global or selective deletion of Bmal1 from smooth muscle cells compromised the BP circadian rhythm and decreased BP under physiological conditions (28, 32, 37). In contrast, we and others also found that selective deletion of the Bmal1 from smooth muscle cells or myeloid cells protects mice from aldosterone and high salt– or Ang II and hyperlipidemia–induced aortic aneurysms (58, 59). Recently, it has been shown that reverse feeding inverted the diurnal rhythm of BP independent of Bmal1 (41). Hence, whether clock genes are involved in the TRF-induced and TH-mediated sympathoadrenal function in diabetes remains to be established.
There are some limitations in the current studies that need to be addressed in future studies. First, the molecular mechanisms by which TRF modulates the SNS via Bmal1 remain to be investigated. Second, whether the TRF used in the current study is also effective in the protection of BP circadian rhythm in females as well as males and other diabetic and nondipping BP animal models remains to be elucidated. Third, whether the TRF used in the current study also effectively protects target organ damage associated with nondipping BP in diabetic patients remains to be explored.
In summary, the current study demonstrated that TRF not only prevents db/db mice from nondipping BP but restores BP dipping in nondipping db/db mice. Mechanistically, we unveiled that imposing fasting during the light phase by TRF inhibits sympathetic activity, thus protecting BP circadian rhythm in db/db mice. The current study suggests that people with prediabetes or patients with diabetes can improve their BP circadian rhythm by lifestyle changes (i.e., eating only at the right time), which can potentially improve their health outcomes.
Materials and Methods
Detailed materials and methods are provided in SI Appendix.
Mice and Methods.
Diabetic db/db and control (db/+) mice were fed a chow diet with free (ALF) or 8- or 12-h access (TRF) to food under a 12:12 light: dark cycle. Food intake was measured by a BioDAQ system or metabolic chamber. BP, HR, and locomotor activity were recorded by telemetry. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Kentucky.
Supplementary Material
Acknowledgments
We thank Dr. Wendy Katz for assistance with the indirect calorimetry measurements. We thank Dr. Harald M. Stauss for his help with the baroreflex sensitivity analysis. This work is supported by National Heart, Lung, and Blood Institute (HL106843, HL141103, and HL142973 to M.C.G. and Z.G.), Biomedical Laboratory Research and Development, Veterans Affairs Office of Research and Development (I01BX002141 to Z.G.), and Institutional Development Award from National Institute of General Medical Sciences of NIH (P30GM127211).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2015873118/-/DCSupplemental.
Data Availability
All study data are included in the article and SI Appendix.
References
- 1.Cho N. H., et al., IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 138, 271–281 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Geiss L. S., Rolka D. B., Engelgau M. M., Elevated blood pressure among U.S. adults with diabetes, 1988-1994. Am. J. Prev. Med. 22, 42–48 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Baena-Díez J. M.et al.; FRESCO Investigators , Risk of cause-specific death in individuals with diabetes: A competing risks analysis. Diabetes Care 39, 1987–1995 (2016). [DOI] [PubMed] [Google Scholar]
- 4.O’Brien E., Parati G., Stergiou G., Ambulatory blood pressure measurement: What is the international consensus? Hypertension 62, 988–994 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Kario K., Nocturnal hypertension: New technology and evidence. Hypertension 71, 997–1009 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Cuspidi C., Vaccarella A., Leonetti G., Sala C., Ambulatory blood pressure and diabetes: Targeting nondipping. Curr. Diabetes Rev. 6, 111–115 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Pistrosch F., Reissmann E., Wildbrett J., Koehler C., Hanefeld M., Relationship between diurnal blood pressure variation and diurnal blood glucose levels in type 2 diabetic patients. Am. J. Hypertens. 20, 541–545 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Mahabala C., Kamath P., Bhaskaran U., Pai N. D., Pai A. U., Antihypertensive therapy: Nocturnal dippers and nondippers. Do we treat them differently? Vasc. Health Risk Manag. 9, 125–133 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salles G. F.et al.; ABC-H Investigators , Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: The ambulatory blood pressure collaboration in patients with hypertension (ABC-H) meta-analysis. Hypertension 67, 693–700 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Sami W., Ansari T., Butt N. S., Hamid M. R. A., Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. (Qassim) 11, 65–71 (2017). [PMC free article] [PubMed] [Google Scholar]
- 11.Panda S., Circadian physiology of metabolism. Science 354, 1008–1015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morse S. A., Ciechanowski P. S., Katon W. J., Hirsch I. B., Isn’t this just bedtime snacking? The potential adverse effects of night-eating symptoms on treatment adherence and outcomes in patients with diabetes. Diabetes Care 29, 1800–1804 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Berg C., et al., Eating patterns and portion size associated with obesity in a Swedish population. Appetite 52, 21–26 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Hatori M., et al., Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15, 848–860 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaix A., Zarrinpar A., Miu P., Panda S., Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 20, 991–1005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grosbellet E., et al., Leptin modulates the daily rhythmicity of blood glucose. Chronobiol. Int. 32, 637–649 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Su W., et al., Hypertension and disrupted blood pressure circadian rhythm in type 2 diabetic db/db mice. Am. J. Physiol. Heart Circ. Physiol. 295, H1634–H1641 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou T., Su W., Guo Z., Gong M. C., A novel diabetic mouse model for real-time monitoring of clock gene oscillation and blood pressure circadian rhythm. J. Biol. Rhythms 34, 51–68 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goncalves A. C., et al., Diabetic hypertensive leptin receptor-deficient db/db mice develop cardioregulatory autonomic dysfunction. Hypertension 53, 387–392 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Cornelissen G., Cosinor-based rhythmometry. Theor. Biol. Med. Model. 11, 16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panza J. A., Epstein S. E., Quyyumi A. A., Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N. Engl. J. Med. 325, 986–990 (1991). [DOI] [PubMed] [Google Scholar]
- 22.Young J. B., Landsberg L., Suppression of sympathetic nervous system during fasting. Science 196, 1473–1475 (1977). [DOI] [PubMed] [Google Scholar]
- 23.Gordan R., Gwathmey J. K., Xie L. H., Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 7, 204–214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baudrie V., Laude D., Elghozi J. L., Optimal frequency ranges for extracting information on cardiovascular autonomic control from the blood pressure and pulse interval spectrograms in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R904–R912 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Thireau J., Zhang B. L., Poisson D., Babuty D., Heart rate variability in mice: A theoretical and practical guide. Exp. Physiol. 93, 83–94 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Kardos A., et al., Determinants of spontaneous baroreflex sensitivity in a healthy working population. Hypertension 37, 911–916 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Di Rienzo M., et al., Baroreflex effectiveness index: An additional measure of baroreflex control of heart rate in daily life. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R744–R751 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Xie Z., et al., Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J. Clin. Invest. 125, 324–336 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leliavski A., Dumbell R., Ott V., Oster H., Adrenal clocks and the role of adrenal hormones in the regulation of circadian physiology. J. Biol. Rhythms 30, 20–34 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Senador D., Kanakamedala K., Irigoyen M. C., Morris M., Elased K. M., Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp. Physiol. 94, 648–658 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green C. B., Takahashi J. S., Bass J., The meter of metabolism. Cell 134, 728–742 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtis A. M., et al., Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc. Natl. Acad. Sci. U.S.A. 104, 3450–3455 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damiola F., et al., Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14, 2950–2961 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudic R. D., Fulton D. J., Pressed for time: The circadian clock and hypertension. J Appl Physiol (1985) 107, 1328–1338 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su W., et al., Altered clock gene expression and vascular smooth muscle diurnal contractile variations in type 2 diabetic db/db mice. Am. J. Physiol. Heart Circ. Physiol. 302, H621–H633 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bunger M. K., et al., Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis 41, 122–132 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Yang G., et al., Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci. Transl. Med. 8, 324ra16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Buuse M., Malpas S. C., 24-hour recordings of blood pressure, heart rate and behavioural activity in rabbits by radio-telemetry: Effects of feeding and hypertension. Physiol. Behav. 62, 83–89 (1997). [DOI] [PubMed] [Google Scholar]
- 39.Mochel J. P., et al., Influence of feeding schedules on the chronobiology of renin activity, urinary electrolytes and blood pressure in dogs. Chronobiol. Int. 31, 715–730 (2014). [DOI] [PubMed] [Google Scholar]
- 40.van den Buuse M., Circadian rhythms of blood pressure and heart rate in conscious rats: Effects of light cycle shift and timed feeding. Physiol. Behav. 68, 9–15 (1999). [DOI] [PubMed] [Google Scholar]
- 41.Zhang D., et al., Timing of food intake drives the circadian rhythm of blood pressure. Function (Oxf) 2, zqaa034 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longo V. D., Mattson M. P., Fasting: Molecular mechanisms and clinical applications. Cell Metab. 19, 181–192 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hermida R. C., Ayala D. E., Mojón A., Fernández J. R., Influence of circadian time of hypertension treatment on cardiovascular risk: Results of the MAPEC study. Chronobiol. Int. 27, 1629–1651 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Hermida R. C., Ayala D. E., Mojón A., Fernández J. R., Bedtime ingestion of hypertension medications reduces the risk of new-onset type 2 diabetes: A randomised controlled trial. Diabetologia 59, 255–265 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Sutton E. F., et al., Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 27, 1212–1221.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilkinson M. J., et al., Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 31, 92–104.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mager D. E., et al., Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 20, 631–637 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Hou T., et al., Active time-restricted feeding improved sleep-wake cycle in db/db mice. Front. Neurosci. 13, 969 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smolensky M. H., Hermida R. C., Castriotta R. J., Portaluppi F., Role of sleep-wake cycle on blood pressure circadian rhythms and hypertension. Sleep Med. 8, 668–680 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Raizada M. K.et al., Report of the national heart, lung, and blood Institute working group on the role of microbiota in blood pressure regulation: Current status and future directions. Hypertension 70, 479–485 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zarrinpar A., Chaix A., Yooseph S., Panda S., Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 20, 1006–1017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schweiger U., Warnhoff M., Pirke K. M., Brain tyrosine availability and the depression of central nervous norepinephrine turnover in acute and chronic starvation in adult male rats. Brain Res. 335, 207–212 (1985). [DOI] [PubMed] [Google Scholar]
- 53.Philipp E., Pirke K. M., Effect of starvation on hypothalamic tyrosine hydroxylase activity in adult male rats. Brain Res. 413, 53–59 (1987). [DOI] [PubMed] [Google Scholar]
- 54.Chung S., et al., Impact of circadian nuclear receptor REV-ERBα on midbrain dopamine production and mood regulation. Cell 157, 858–868 (2014). [DOI] [PubMed] [Google Scholar]
- 55.McClung C. A., et al., Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc. Natl. Acad. Sci. U.S.A. 102, 9377–9381 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo D., et al., Tyrosine hydroxylase down-regulation after loss of Abelson helper integration site 1 (AHI1) promotes depression via the circadian clock pathway in mice. J. Biol. Chem. 293, 5090–5101 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carson K. A., Hanker J. S., Kirshner N., The adrenal medulla of the diabetic mouse (C57BL/KsJ, db/db): Biochemical and morphological changes. Comp. Biochem. Physiol. A Comp. Physiol. 72, 279–285 (1982). [DOI] [PubMed] [Google Scholar]
- 58.Lutshumba J., et al., Deletion of BMAL1 in smooth muscle cells protects mice from abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 38, 1063–1075 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang G., et al., Bmal1 deletion in myeloid cells attenuates atherosclerotic lesion development and restrains abdominal aortic aneurysm formation in hyperlipidemic mice. Arterioscler. Thromb. Vasc. Biol. 40, 1523–1532 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.