Significance
The canonical NF-κB pathway mediates controlled nuclear activation of RelA factors, which induce proinflammatory genes. Uncontrolled RelA activity, however, fuels aberrant intestinal inflammation. What triggers pathological RelA activity in the colitogenic gut remains unclear. The noncanonical NF-κB module typically directs immune organogenesis involving Nfkb2 gene products. We find that this otherwise harmless noncanonical signaling amplifies canonical RelA activity in the inflamed colon in inflammatory bowel disease patients and in colitogenic mice, aggravating gut pathologies. Our work further suggests that noncanonical signaling supplements copious amounts of RelA dimers, whose activation by canonical signaling exacerbates gut inflammation. In sum, we reveal a mechanism regulating disease-associated inflammation and present the noncanonical Nfkb2 pathway as an attractive therapeutic option in inflammatory diseases.
Keywords: NF-κB, noncanonical, Nfkb2, inflammatory bowel disease, intestinal inflammation
Abstract
Aberrant inflammation, such as that associated with inflammatory bowel disease (IBD), is fueled by the inordinate activity of RelA/NF-κB factors. As such, the canonical NF-κB module mediates controlled nuclear activation of RelA dimers from the latent cytoplasmic complexes. What provokes pathological RelA activity in the colitogenic gut remains unclear. The noncanonical NF-κB pathway typically promotes immune organogenesis involving Nfkb2 gene products. Because NF-κB pathways are intertwined, we asked whether noncanonical signaling aggravated inflammatory RelA activity. Our investigation revealed frequent engagement of the noncanonical pathway in human IBD. In a mouse model of experimental colitis, we established that Nfkb2-mediated regulations escalated the RelA-driven proinflammatory gene response in intestinal epithelial cells, exacerbating the infiltration of inflammatory cells and colon pathologies. Our mechanistic studies clarified that cell-autonomous Nfkb2 signaling supplemented latent NF-κB dimers, leading to a hyperactive canonical RelA response in the inflamed colon. In sum, the regulation of latent NF-κB dimers appears to link noncanonical Nfkb2 signaling to RelA-driven inflammatory pathologies and may provide for therapeutic targets.
The disruption of the intestinal barrier exposes tissue-resident cells, including intestinal epithelial cells (IECs), to luminal microbial contents setting off inflammation. Calibrated expressions of proinflammatory genes by tissue-resident cells limit local infections by orchestrating the recruitment and activation of effector immune cells, such as neutrophils, macrophages, and helper T cells, leading to the restoration of intestinal homeostasis. However, excessive inflammation provokes unabated activity of these effector cells, causing tissue damage in mice subjected to experimental colitis and contributing to the pathogenesis of human inflammatory bowel disease (IBD), including ulcerative colitis (1–3).
Microbial substances signal through the canonical NF-κB module for activating the RelA:p50 transcription factor (4). In unstimulated cells, preexisting RelA dimers are sequestered in the latent cytoplasmic complexes by the inhibitory IκB proteins, the major isoform being IκBα. Canonical NF-κB signaling directs NEMO:IKK2- (alternately known as NEMO:IKKβ) mediated phosphorylation of IκBs, which are then degraded by the ubiquitin–proteasome system. Signal-induced degradation of IκBs liberates the bound RelA dimers into the nucleus, where they activate genes encoding proinflammatory cytokines and chemokines. Negative regulators of canonical signaling, including IκBα and A20, normally ensure a controlled RelA activity in physiological settings. In contrast, inordinate RelA response by tissue-resident cells culminates into nonresolving pathological intestinal inflammation (5, 6). Indeed, the severity of disease correlated with the extent of RelA activation in human IBD (7), whereas lessening canonical signaling using antisense oligo or peptide inhibitors mitigated experimental colitis in mice (8, 9). What intensifies nuclear RelA activity in the colitogenic gut remains unclear.
The noncanonical NF-κB pathway mediates the nuclear accumulation of the RelB:p52 heterodimer (10). A select set of TNF receptor superfamily members, including lymphotoxin-β receptor (LTβR), induces noncanonical signaling, which stimulates NIK-dependent phosphorylation of the NF-κB precursor p100 encoded by Nfkb2. Subsequent proteasomal processing of p100 generates the mature p52 subunit, which, in association with RelB, translocates to the nucleus and induces the expression of immune organogenic genes.
Interestingly, genome-wide association studies identified LTBR and NFKB2 as candidate genes linked to the susceptibility loci for human IBD (11). On the other hand, an IEC-intrinsic deficiency of LTβR or NIK exacerbated chemically induced colitis in knockout mice (12, 13). It was suggested that LTβR protects against intestinal injury by driving the production of IL-23, which induces IL-22–mediated tissue repair. Dendritic cells (DCs) are the prime producers of IL-23 in the intestinal niche. Surprisingly, NIK’s depletion in DCs ameliorated colitis in mice (14). More so, biochemical studies indicated that genes encoding IL-23 subunits p19 and p40 are generic NF-κB targets and are not activated solely by RelB:p52 (15). Of note, LTβR and NIK also have functions beyond noncanonical NF-κB signaling (16). It is less well understood if the noncanonical Nfkb2 signaling per se contributes to the inflammatory pathologies observed in human IBD or in colitogenic mice. While global Nfkb2−/− mice were partially resistant to experimental colitis (17), the molecular mechanism and relevant cell types that link Nfkb2 functions to gut inflammation remain obscure.
The canonical and noncanonical NF-κB pathways are intertwined at multiple levels (18). Proteasomal processing of p105, another NF-κB precursor encoded by Nfkb1, produces the mature p50 subunit that forms RelA:p50. While p105 processing is mostly constitutive, noncanonical signaling stimulates further p50 production involving a mechanism requiring the C-terminal destruction box of p100 (19). Second, the noncanonical signaling gene Nfkb2 represents a RelA target (20). Third, p100, in association with p105, forms high-molecular-weight complexes, which also sequester RelA in the cytoplasm (21, 22). Unlike canonical signal-responsive latent NF-κB complexes where preexisting RelA dimers are sequestered by IκBs, these high-molecular-weight complexes consist of monomeric RelA species bound to the individual NF-κB precursors. Finally, p52 interacts with RelA producing a minor RelA:p52 heterodimer (23). Akin to RelA:p50, RelA:p52 is sequestered by IκBs, activated upon canonical signaling, and mediates proinflammatory gene expressions. This intertwining enables cross-talks between canonical and noncanonical NF-κB pathways (23–25). We asked if the noncanonical pathway modulated RelA-driven inflammation in the colitogenic gut, leveraging this interlinked NF-κB system.
Here, we present experimental evidence of frequent activation of the noncanonical Nfkb2 pathway in IBD patients. In a mouse model, we ascertained that an IEC-intrinsic Nfkb2 function exacerbated RelA-driven inflammation in the colitogenic gut. Our mechanistic analyses explained that tonic noncanonical Nfkb2 signaling supplemented latent RelA:p50 and RelA:p52 dimers by inducing simultaneous processing of both p105 and p100. The Nfkb2-dependent regulation of latent RelA dimers provoked a hyperactive canonical response in epithelial cells during the initiation of experimental colitis, aggravating intestinal inflammation. Finally, we established a tight association between heightened RelA activity and elevated processing of both the NF-κB precursors in human IBD. We argue that the regulation of latent NF-κB dimers by the noncanonical Nfkb2 pathway provides for a therapeutic target in RelA-driven inflammatory pathologies.
Results
Engagement of the Noncanonical NF-κB Pathway in Human IBD.
To understand the molecular basis for the inordinate RelA activity associated with pathological intestinal inflammation, we biochemically analyzed colonic epithelial biopsies from 30 IBD patients suffering from ulcerative colitis (Methods and SI Appendix, Fig. S1 A–D). As controls, we utilized biopsies from 10 otherwise IBD-free individuals ailing from hemorrhoids. We first measured the NF-κB DNA-binding activity in nuclear extracts derived from these tissues by electrophoretic mobility shift assay (EMSA; Fig. 1A and SI Appendix, Fig. S1A). Corroborating previous studies (26), our investigation broadly revealed a heightened nuclear NF-κB (NF-κBn) activity in IBD patients as compared to the control cohort. Our shift-ablation assay suggested that this activity consisted mostly of RelA:p50 with a modest contribution of RelA:p52 (Fig. 1B). Albeit at low levels, we also detected RelB complexes in some subjects, for example, patient #P29 (Fig. 1B). Focusing on nuclear RelA (nRelA) activity in RelA-EMSA, we further ascertained that the heightened NF-κBn activity in IBD patients was mainly attributed to RelA (Fig. 1C and SI Appendix, Fig. S1A). Quantitative immunoblot analyses of whole-cell extracts derived from colon biopsies revealed that the level of total RelA protein was not discernably different between control and IBD patients (Fig. 1D and SI Appendix, Fig. S1B). Therefore, our results suggested that the heightened nRelA activity observed in IBD patients was not due to elevated RelA expressions. We further noticed a reciprocal twofold decrease in the median abundance of IκBα in IBD patients compared to controls indicating that ongoing canonical signaling in the inflamed gut caused nuclear RelA activation (Fig. 1E and SI Appendix, Fig. S1B). Notably, IBD patients exhibited substantial variations in nRelA (Fig. 1C). We cataloged IBD patients with nRelA levels equal to or greater than 1.5-fold of the median value in the control cohort as nRelAhigh; otherwise, they were designated as nRelAlow. The median nRelA value for the nRelAhigh subgroup comprising of 23 patients was 8.9, whereas the nRelAlow subgroup had a median of 4.6. Interestingly, IκBα levels were almost equivalently reduced in nRelAhigh and nRelAlow patient subgroups (Fig. 1F). These studies disclose a disconnect between the intensity of canonical NF-κB signaling and the amplitude of nuclear RelA activity in IBD.
Fig. 1.
Heightened RelA activity correlates with elevated noncanonical NF-κB signaling in human IBD. (A and C) Nuclear extracts obtained using colon biopsies derived from 10 non-IBD control patients and 30 IBD patients were examined by EMSA (SI Appendix, Fig. S1). Signals corresponding to total (NF-κBn) (A) and RelA (nRelA) (C) NF-κB DNA-binding activities were quantified and graphed as a dot plot. The superimposed box plot indicates the median of the data along with first and third quartiles. (B) The composition of nuclear NF-κB complexes present in colonic tissues was determined by shift-ablation assay. Antibodies against the indicated NF-κB subunits were used for ablating the respective DNA-binding complexes in EMSA. A representative data has been shown. The arrow and arrowhead indicate RelA- and RelB-containing complexes, respectively. (D, E, and G) Cell extracts obtained using colonic tissues from control individuals, and IBD patients were examined by immunoblot analyses for the presence of total RelA protein (total RelAp), IκBα, and p52 as well as p100 (SI Appendix, Fig. S1). Signals corresponding to total RelA (D) or IκBα (E) in controls and IBD patients were quantified and graphed. Similarly, the abundance of p52 in relation to p100 was determined in these sets (G, F, and H) IBD patient subgroups with high or low nRelA activities in colonic tissues were compared for the abundance of IκBα (F) or the relative abundance of p52 to p100 (H). nRelAlow and nRelAhigh patients have been color coded in other figure panels. In all panels, the statistical significance was determined by Welch’s t test on unpaired samples.
Next, we examined the plausible involvement of the noncanonical pathway in IBD. We measured the abundance of p52 in relation to p100 in colonic extracts as a surrogate of noncanonical signaling. Remarkably, up to 53% of IBD patients displayed elevated p100 processing as determined from a 1.5-fold or more increase in the p52:p100 ratio compared to the median p52:p100 value in the control cohort (Fig. 1G and SI Appendix, Fig. S1B). Overall, IBD patients exhibited a more than twofold increase in the median p52:p100 value. Interestingly, nRelAhigh patients revealed significantly elevated p100 processing to p52 compared to nRelAlow patients (Fig. 1H). Our cohort consisted of both steroid-naïve and steroid-treated individuals; however, steroid seemingly did not impact either the disease severity or NF-κB signaling at the time of biopsy acquisition (SI Appendix, Fig. S1C). We conclude that the noncanonical NF-κB pathway is frequently engaged in human IBD and that the strength of impinging noncanonical signaling correlates with the amplitude of nuclear RelA activity induced by the canonical NF-κB module in IBD patients.
The Noncanonical Nfkb2 Pathway Amplifies Epithelial RelA NF-κB Response in the Colitogenic Murine Gut.
Because our investigation involving colonic epithelial biopsies implicated elevated noncanonical signaling in the heightened RelA activity observed in human IBD, we asked if the noncanonical Nfkb2 pathway truly modulated epithelial RelA activation in the colitogenic gut. To address this, we treated mice with the colitogenic agent dextran sulfate sodium (DSS), collected IECs from these mice at various times postonset of DSS treatment (SI Appendix, Fig. S2A), and subjected those cells to biochemical analyses. As such, DSS damages the mucosal layer triggering epithelial RelA activation via the canonical NF-κB module (8, 27). We found that wild-type (WT) mice elicited a strong NF-κBn activity at 12 h postonset of DSS treatment that persisted, albeit at a reduced level, even at 48 h (Fig. 2A). As observed with IBD biopsies, this activity consisted of mostly RelA:p50 and, to some extent, RelA:p52 (Fig. 2B). Our immunoblot analyses further revealed substantial basal processing of p100 into p52 in IECs derived from untreated WT mice; as suggested earlier (28), DSS treatment augmented this noncanonical Nfkb2 signaling in the gut (Fig. 2C). In response to DSS, Nfkb2−/− mice produced a rather weakened, but not completely abrogated, NF-κBn activity at 12 h that further declined to a near-basal level by 48 h (Fig. 2A). Basal NF-κBn activity in untreated mice was comparable between these genotypes. Notably, LTBR was linked to IBD susceptibility loci in humans (11), and our prior study revealed a role of LTβR in activating the noncanonical Nfkb2 pathway in the IECs of mice (23). Accordingly, intraperitoneal administration of an antagonistic LTβR-Ig fusion protein in mice diminished basal processing of p100 into p52 in IECs (SI Appendix, Fig. S2B). When subsequently challenged with DSS, mice administered with LTβR-Ig indeed produced a threefold-lessened NF-κBn activity, indicating a role of tonic noncanonical Nfkb2 signaling in shaping signal-induced canonical RelA response in IECs (Fig. 2D).
Fig. 2.
LTβR-Nfkb2 signaling strengthens RelA NF-κB responses elicited by IECs in DSS-treated mice. (A) WTand Nfkb2−/− mice were administered with 2.5% DSS. IECs were collected at the indicated times from the onset of DSS treatment and analyzed for NF-κBn activities. (Bottom) NF-κBn signals were quantified and presented in the bar graphs below the respective lanes. (B) Shift-ablation assay characterizing the composition of NF-κBn complexes induced in IECs of DSS-treated WT mice at 12 h. Antibodies against the indicated NF-κB subunits were used for ablating the respective DNA-binding complexes in EMSA. (C) IECs collected from WT mice treated with 2.5% DSS were subjected to immunoblot analyses. Quantified signals corresponding to p52:p100 has been indicated. (D) WT mice were intraperitoneally injected with control-Ig or LTβR-Ig, which blocks LTβR signaling, 24 h prior to the onset of DSS treatment. Subsequently, IEC were isolated from mice treated with DSS for 24 h and analyzed for NF-κBn by EMSA. (E) GSEA comparing IECs derived from WT and Nfkb2−/− mice, either left untreated or treated with DSS for 48 h (n = 3 each), for RelA-driven gene expressions. Briefly, RNA-seq analyses were performed and a list 8,199 genes with expressions in different datasets were prepared. The difference in the fold changes (FC) in the mRNA levels upon DSS treatment between WT and Nfkb2−/− mice was calculated for these genes, and genes were ranked in descending order of the FC difference values (Bottom). The relative enrichment of RelA-target genes was determined by GSEA (Top) ES denotes enrichment score. Each of the horizontal dashed lines represents a RelA-target gene. (F) Enrichment analyses revealing the top 10 most enriched GO for biological processes terms among genes induced at least four folds in IECs upon DSS treatment of WT mice; corresponding enrichment scores have been plotted. The enrichment scores of these GO terms were further determined for genes induced in Nfkb2−/− mice and presented in the overlaid bar gram. GO identifications have been indicated in the respective bars. (G) RT-qPCR revealing the abundance of indicated mRNAs in IECs derived from WT and Nfkb2−/− mice administered with DSS for 2 or 5 d. mRNA FC values were calculated in relation to corresponding untreated mice. Quantified data represent means ± SEM. Two-tailed Student’s t test was performed. ***P < 0.001; **P < 0.01; *P < 0.05.
Proinflammatory gene activation by NF-κB factors in IECs represents a key event in the initiation of colitis (1, 29). We performed RNA sequencing (RNA-seq) analyses to address if the Nfkb2 pathway tuned the transcriptional response of IECs in the colitogenic gut (Methods). A comparison of DSS-induced fold changes in the messenger RNA (mRNA) levels between WT and Nfkb2−/− mice revealed that Nfkb2 deficiency led to both diminished inductions as well as a hyperactivation of genes in IECs at 48 h postonset of treatment (Fig. 2E and SI Appendix, Fig. S2C). Interestingly, a gene set enrichment analysis (GSEA) of our transcriptomic data identified significant enrichment of RelA-targets among genes, whose DSS-responsive expressions in IECs were augmented by Nfkb2 (Fig. 2E). It was also suggested that LTβR-stimulated noncanonical signaling promotes RelB-dependent expressions of goblet cell–specific genes in Listeria monocytogenes–infected mice (30, 31). In our experimental colitis studies, WT and Nfkb2−/− mice exhibited an equivalent enrichment of goblet cell–specific genes among transcripts induced in IECs upon DSS treatment (SI Appendix, Fig. S2D). Moreover, we examined the enrichment of Gene Ontology (GO) for biological process terms among genes induced in IECs upon DSS treatment of mice. In the expected line, the top 10 most enriched GO terms in WT mice were related to broadly inflammatory and innate immune processes (Fig. 2F). Importantly, these GO terms were consistently less enriched in global Nfkb2−/− mice (Fig. 2F), suggesting that global Nfkb2 deficiency lessened specifically RelA-driven inflammatory gene expressions in IECs during the course of colitis.
Our RT-qPCR analyses further demonstrated that the abundance of mRNAs encoding proinflammatory cytokines and chemokines was increased in IECs upon DSS treatment of WT mice (Fig. 2F). While the levels of Ccl20 and Cxcl2 mRNAs were elevated within 2 d of the treatment, TNF, IL-1β, Ccl5, and Ccl2 mRNAs gradually accumulated over a period of 5 d. Importantly, Nfkb2 deficiency restrained DSS-induced expressions of these RelA-target proinflammatory genes in IECs. The abundances of c-FOS mRNA, whose expression involves NF-κB–independent mechanisms, or mRNA-encoding TGF-β, which typically limits intestinal inflammation, were not considerably different between WT and Nfkb2−/− mice (Fig. 2G). Together, our results indicate that by amplifying epithelial RelA activity in the colitogenic gut, the tonic noncanonical Nfkb2 pathway aggravates RelA-driven inflammatory gene expressions in mice.
A Stromal Nfkb2 Function Exacerbates Experimental Colitis in Mice.
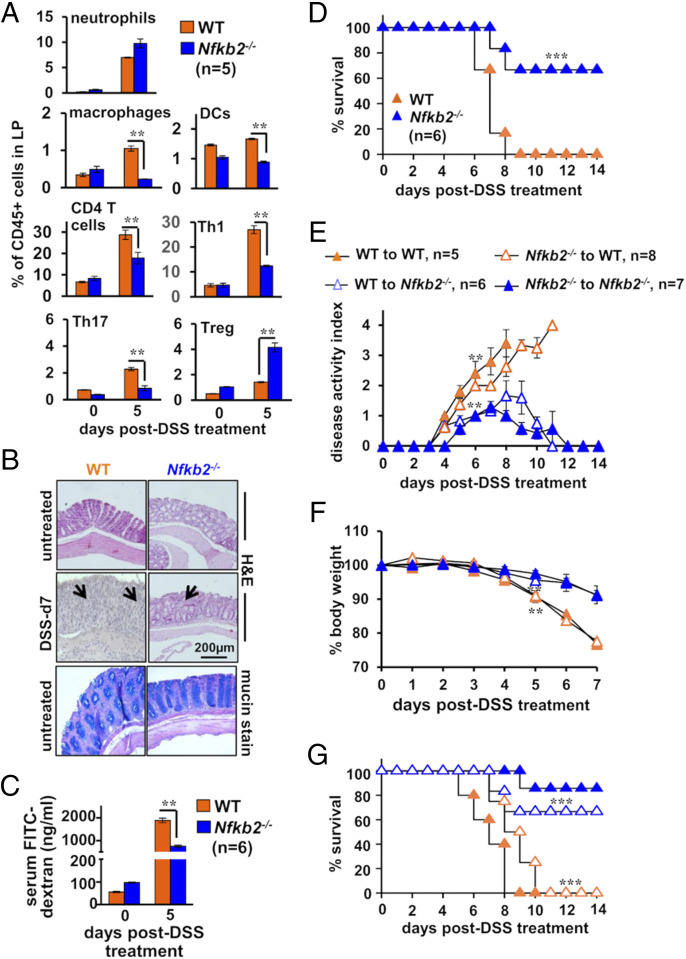
In a recent study, Lyons et al. quantitatively assessed the contribution of IECs in experimental colitis (32). Their investigation suggested that proinflammatory gene expressions by particularly undifferentiated IECs play a pivotal role in the infiltration of inflammatory immune cells in the colitogenic gut, aggravating tissue injuries. Following those lines, we inquired if limiting RelA-driven gene response in IECs altered the course of DSS-induced ulcerating colitis in Nfkb2−/− mice. First, we collected cells from the colonic lamina propria of mice subjected to DSS treatment at day 5 and determined the composition of effector immune cell subsets by flow cytometry (SI Appendix, Fig. S3). In comparison to WT mice, Nfkb2−/− mice exhibited less marked accumulation of inflammatory cells in the gut upon DSS treatment (Fig. 3A). DSS-treated Nfkb2−/− mice displayed a fivefold reduced frequency of macrophages—identified as CD11b+F4/80+ cells, a close to twofold decrease in the abundance of DCs—broadly considered as CD11c+F4/80− cells and a 2.5-fold reduced frequency of CD4+ T cells. Further investigation revealed a substantially lower colonic abundance of IFN-γ+ Th1 cells and IL17A+ Th17 cells in DSS-treated Nfkb2−/− mice. However, DSS caused a more profound accumulation of suppressive CD4+CD25+FoxP3+ Treg cells in Nfkb2−/− mice, presumably owing to their increased stability in the context of timid gut inflammation. The frequency of neutrophils—identified as Gr1+SiglecF− cells—was not discernibly different between WT and Nfkb2−/− mice subjected to DSS treatment. Untreated WT and Nfkb2−/− mice presented almost equivalent frequencies of these immune cells in the gut (Fig. 3A).
Fig. 3.
Nfkb2 deficiency in the stromal compartment ameliorates chemically induced colitis in mice. (A) Bar plot revealing relative frequencies of the indicated immune cells among CD45.2+ cells present in the lamina propria (LP) of WT and Nfkb2−/− mice. Mice were either left untreated or administered with 2.5% DSS. The composition of LP cells was examined by flow cytometry. (B) Representative image showing H&E-stained colon sections derived from untreated or DSS-treated mice of the indicated genotypes (Top). Colon sections from untreated mice were additionally stained using Alcian Blue (Bottom). The data represent n = 4; four fields per section and a total of five sections from each set were examined. Panels show 20× magnification. (C) Bar chart revealing the concentration of FITC-dextran in serum of untreated or DSS-treated WT and Nfkb2−/− mice. FITC-dextran was gavaged 6 h prior to serum collection. (D) WT and Nfkb2−/− mice were administered with 2.5% DSS for 7 d and monitored for survival for 14 d. (E–G) Reciprocal bone marrow chimera generated using WT and Nfkb2−/− mice were subjected to 2.5% DSS treatment and then evaluated for the disease activity (E), body weight changes (F), and mortality (G). The statistical significance was determined by comparing WT to Nfkb2−/− chimeras with WT to WT chimeras or Nfkb2−/− to WT chimeras with Nfkb2−/− to Nfkb2−/− mice. Quantified data represent means ± SEM. For D and G, the statistical significance was determined using log-rank (Mantel–Cox) test. Otherwise, two-tailed Student’s t test was performed. ***P < 0.001; **P < 0.01.
We then compared WT and Nfkb2−/− mice for DSS-inflicted pathologies. Our histological analyses revealed that colons from untreated WT and Nfkb2−/− mice were largely indistinguishable with respect to epithelial architecture and mucin expressions (Fig. 3B). More so, DSS treatment for 36 h caused almost equivalent IEC apoptosis, indicating early epithelial injury of the similar extent in these genotypes (SI Appendix, Fig. S4A). At day 7, however, WT mice displayed an extensive disruption of the epithelial barrier and widespread infiltration of leukocytes in the submucosa, while Nfkb2−/− mice exhibited less pervasive intestinal damage (Fig. 3B). We also evaluated the intestinal barrier permeability by scoring serum concentrations of fluorescein isothiocyanate (FITC)-dextran gavaged orally to DSS-treated mice. Consistent with histological studies, DSS treatment caused only a modest increase in the intestinal permeability in Nfkb2−/− mice at day 5 (Fig. 3C). WT mice subjected to acute DSS treatment also exhibited profound disease activity in these later days that was accompanied by a significant shortening of the colon and a close to 15% loss in the body weight at day 5 (SI Appendix, Fig. S4 B–D). As reported (17), these colitis phenotypes were less pronounced in Nfkb2−/− mice (SI Appendix, Fig. S4 B–D). Indeed, the entire WT cohort succumbed to DSS-induced colitis by day 8 postonset of DSS treatment, while two-thirds of Nfkb2−/− mice survived the course of colitis (Fig. 3D).
Nfkb2 signaling in the hematopoietic compartment curtails the generation of Treg cells (33, 34), while our global Nfkb2−/− mice displayed an increased frequency of Tregs in the colitogenic gut. Therefore, we further examined reciprocal bone marrow chimeras generated using WT and Nfkb2−/− mice for dissecting the hematopoietic and the stromal Nfkb2 functions in experimental colitis. Regardless of WT or Nfkb2−/− hematopoietic cells, WT recipients were sensitive to DSS-induced colitis, exhibiting heightened disease activity, substantial body weight loss, and mortality (Fig. 3 E–G). On the other hand, Nfkb2−/− mice showed resilience, even in the presence of WT hematopoietic cells. We conclude that a stromal Nfkb2 function exacerbates experimental colitis by directing inflammatory infiltrates to the gut and that Nfkb2 signaling in the hematopoietic compartment is less consequential for DSS-inflicted intestinal pathologies.
An IEC-Intrinsic Role of Nfkb2 Aggravates Experimental Colitis in Mice.
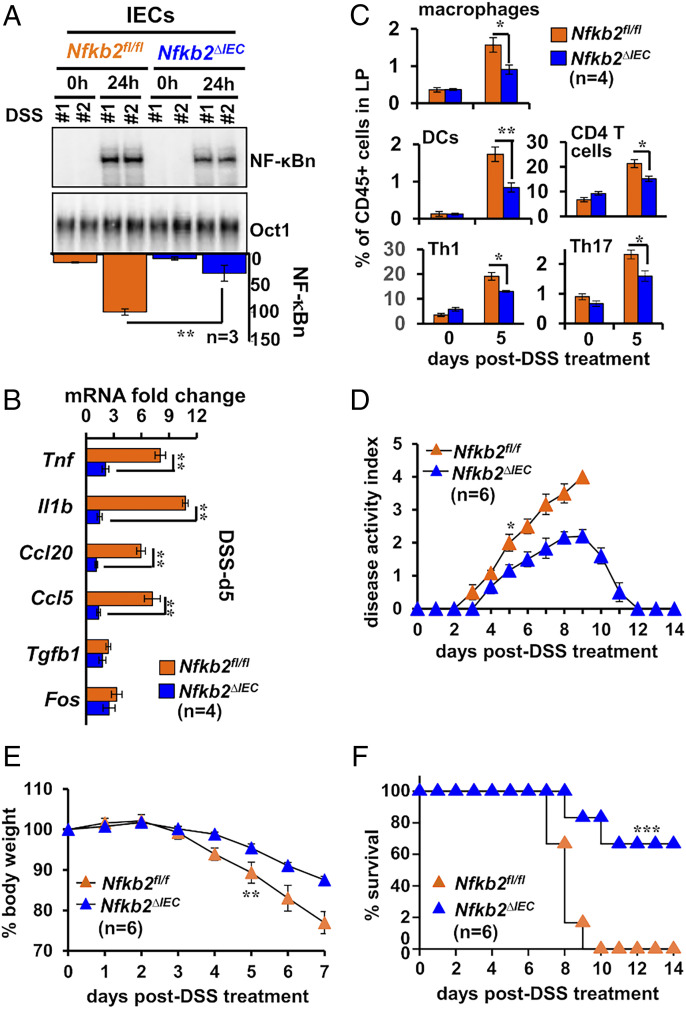
Next, we asked if Nfkb2 signaling in IECs was sufficient for aggravating intestinal inflammation. To test this, we generated Nfkb2ΔIEC mice, which specifically lacked Nfkb2 expressions in IECs (SI Appendix, Fig. S4E). As compared to control Nfkb2fl/fl mice possessing otherwise functional floxed Nfkb2 alleles, Nfkb2ΔIEC mice elicited a relatively moderate NF-κBn activity in IECs upon DSS treatment (Fig. 4A). We consistently noticed a subdued expression of RelA-target proinflammatory genes in the IECs of Nfkb2ΔIEC mice at day 5 of the DSS regime (Fig. 4B). However, NF-κB–independent expressions of mRNAs encoding c-FOS or TGF-β were not substantially altered. Our flow cytometry analyses further revealed a reduced accumulation of inflammatory immune cells, including macrophages, Th1, and Th17 cells, in the colonic lamina propria of DSS-treated Nfkb2ΔIEC mice (Fig. 4C). Finally, a dysfunctional Nfkb2 pathway in IECs alleviated experimental colitis—DSS treatment led to less severe disease activity and only a marginal loss of the body weight in Nfkb2ΔIEC mice (Fig. 4 D and E). In contrast to DSS-inflicted mortality in Nfkb2fl/fl mice, most of the Nfkb2ΔIEC mice survived acute DSS treatment (Fig. 4F). Our results substantiate that an IEC-intrinsic Nfkb2 pathway exacerbates experimental colitis by amplifying RelA-driven inflammatory gene responses in IECs. In other words, we establish the functional significance of Nfkb2-mediated modulation of epithelial canonical signaling in aberrant intestinal inflammation.
Fig. 4.
Deficiency of Nfkb2 in IECs restrains RelA-driven inflammation in the colitogenic gut. (A and B) IECs isolated from DSS-treated control Nfkb2fl/fl or Nfkb2∆IEC mice were examined for NF-κBn by EMSA (A) or the expression of indicated mRNAs by RT-qPCR (B and C) Bar plot revealing relative frequencies of the indicated immune cells in the lamina propria of Nfkb2fl/fl or Nfkb2∆IEC mice subjected to DSS treatment. (D–F) Nfkb2∆IEC and Nfkb2fl/fl mice were subjected to DSS treatment and evaluated for the disease activity (D), body weight changes (E), and mortality (F). Log-rank (Mantel–Cox) test was used in F. Two-tailed Student’s t test was performed in other instances. Quantified data represent means ± SEM ***P < 0.001; **P < 0.01; *P < 0.05.
Noncanonical Nfkb2 Signaling Amplifies Canonical NF-κB Responses by Supplementing Latent RelA Dimers.
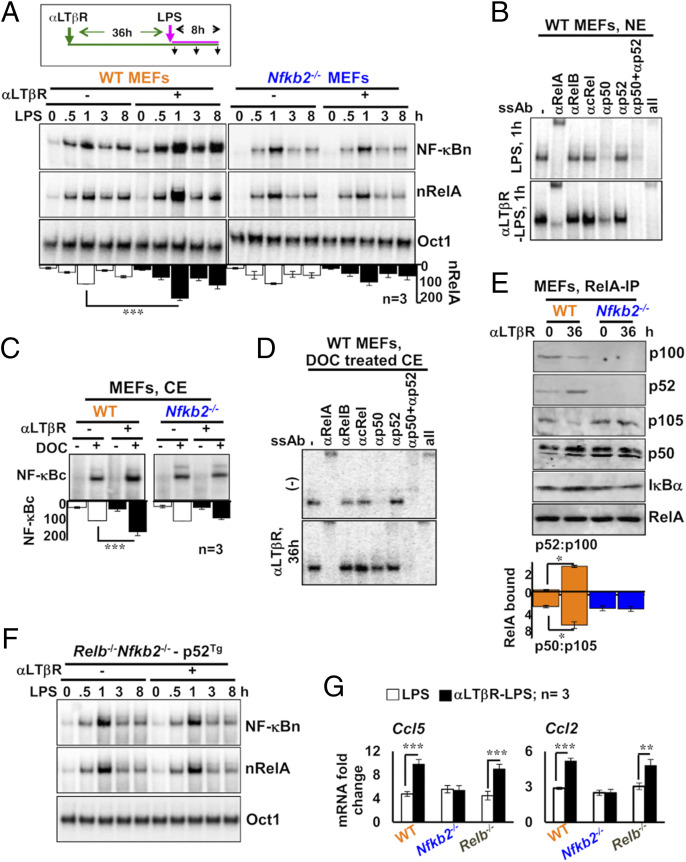
We sought to examine the mechanism linking noncanonical signaling to the proinflammatory RelA activity. In their anatomic niche, IECs receive tonic signals through LTβR from innate lymphoid cells expressing the cognate lymphotoxin ligand (35). To recapitulate this chronic LTβR signaling ex vivo, we first stimulated mouse embryonic fibroblasts (MEFs) for 36 h using 0.1 μg/mL of an agonistic anti-LTβR antibody (αLTβR), which activates NIK-dependent noncanonical signaling (23). We then activated the canonical pathway in these lymphotoxin-conditioned cells by treating them with microbial-derived lipopolysaccharides (LPS) in the continuing presence of αLTβR. Our EMSA analyses revealed that chronic LTβR signaling alone induced a minor NF-κBn activity in WT MEFs (Fig. 5A). In lymphotoxin-naïve cells, LPS triggered a moderate, RelA-containing NF-κBn activity that peaked at 1 h and constituted a weakened late phase. Lymphotoxin conditioning considerably strengthened both the early nRelA peak as well as the late activity induced by LPS. LPS primarily activated RelA:p50 in lymphotoxin-naïve cells (Fig. 5B). Lymphotoxin conditioning not only potentiated RelA:p50 activation but also led to a substantial nuclear accumulation of RelA:p52 in response to LPS. As such, Nfkb2−/− MEFs lack RelA:p52 heterodimers. Importantly, chronic LTβR signaling was ineffective in enhancing even the LPS-induced RelA:p50 activity in Nfkb2−/− cells (Fig. 5A). Moreover, lymphotoxin conditioning aptly strengthened LPS-induced RelA activity in Relb−/− MEFs but not in NIK-deficient cells (SI Appendix, Fig. S5A), whereas pretreatment of WT MEFs with recombinant TWEAK, which engages noncanonical Nfkb2 signaling through Fn14, enhanced RelA response to LPS (SI Appendix, Fig. S5 B and C). These studies establish that LTβR-stimulated noncanonical Nfkb2 signaling reinforces canonical RelA activity involving a NIK-dependent, but RelB-independent, cell-autonomous mechanism. Our data also imply that multiple noncanonical pathway-activating cytokines are capable of engaging in cross-pathway signaling.
Fig. 5.
Nfkb2-dependent accumulation of latent RelA dimers in LTβR-stimulated MEFs augments canonical NF-κB responses. (A) EMSA revealing NF-κBn induced in WT and Nfkb2−/− MEFs in a stimulation time course (Top). Cells were treated with LPS alone or stimulated using agonistic αLTβR antibody for 36 h and then treated with LPS in the continuing presence of αLTβR. Ablating RelB and cRel DNA-binding activity using anti-RelB and anti-cRel antibodies, nRelA complexes were examined (Middle). Oct1 DNA binding (Bottom) served as a control. The data represent three experimental replicates. (B) The composition of NF-κBn complexes induced in naïve or LTβR-conditioned MEFs subjected to LPS treatment for 1 h was characterized by shift-ablation assay. (C) EMSA revealing the abundance of latent NF-κB dimers in the cytoplasm (NF-κBc) of WT and Nfkb2−/− MEFs subjected to αLTβR stimulation for 36 h. Cytoplasmic extracts were treated with DOC before being subjected to EMSA for unmasking latent NF-κB DNA-binding activities. The data represent three experimental replicates. (D) The composition of NF-κBc complexes accumulated in the cytoplasm of MEFs subjected to the indicated treatment regimes was determined by shift-ablation assay. (E) Immunoblot of RelA coimmunoprecipitates obtained using whole-cell extracts derived from αLTβR-treated MEFs. Band intensities were quantified from three independent experiments. Accordingly, the relative abundance of p52 to p100 (p52:p100) and p50 to p105 (p50:p105) in the RelA coimmunoprecipitate was determined and plotted (see below the gel picture). (F) p100-deficient Relb−/−Nfkb2−/− cells stably expressing p52 from a retroviral transgene were subjected to cell stimulation as described in A. Subsequently, NF-κBn or nRelA activities were captured in EMSA. Data represent two biological replicates. (G) qRT-PCR analyses revealing proinflammatory gene expressions induced by LPS in naïve or LTβR-conditioned MEFs of the indicated genotypes. Quantified data represent means ± SEM. Two-tailed Student’s t test was performed. ***P < 0.001; **P < 0.01; *P < 0.05.
Canonical signaling entails NEMO-IKK–mediated activation of preexisting RelA dimers from the latent cytoplasmic complexes. It was suggested that NIK might directly regulate the NEMO-IKK activity induced via the canonical pathway (36). We found that the low dose of αLTβR used in our experiments, although promoted efficient processing of p100 into p52, did not impact LPS-induced NEMO-IKK activity (SI Appendix, Fig. S5 D and E). We then argued that increased availability of latent RelA dimers in the cytoplasm led to a hyperactive LPS response in lymphotoxin-conditioned cells. The treatment of cytoplasmic extracts with the detergent deoxycholate (DOC) dissociates RelA dimers from IκBs, unmasking the DNA-binding activity of the preexisting RelA dimers present in the latent complexes (37). We scored latent NF-κB complexes by analyzing DOC-treated extracts in EMSA. Lymphotoxin conditioning significantly augmented the abundance of latent NF-κB dimers in WT, but not Nfkb2−/−, MEFs (Fig. 5C and SI Appendix, Fig. S5F). Our shift-ablation assay revealed that the latent NF-κB activity in lymphotoxin-naïve cells consisted of RelA:p50 (Fig. 5D). Lymphotoxin conditioning elevated the level of latent RelA:p50 complexes and also accumulated latent RelA:p52 dimer in WT MEFs.
As also reported previously (19), LTβR signaling stimulated simultaneous processing of both p105 to p50 and p100 to p52 in our experiments (SI Appendix, Fig. S5D); although the extent was less remarkable in MEF extracts, signal-responsive p105 processing required the presence of p100 (SI Appendix, Fig. S5D). Our quantitative immunoblot analyses involving RelA coimmunoprecipitates rather clearly revealed that αLTβR treatment of WT MEFs led to a significantly increased association of RelA with p50 and p52 at the expense of RelA binding to the respective p105 and p100 precursors (Fig. 5E). We also noticed an augmented level of IκBα in the RelA immunoprecipitates derived from LTβR-stimulated WT cells (Fig. 5E). Notably, RelA binding to p50 was insensitive to LTβR signaling in Nfkb2−/− MEFs (Fig. 5E). Our analyses suggest that the noncanonical Nfkb2 pathway targets high-molecular-weight NF-κB precursor complexes to supplement RelA:p50 and RelA:p52 heterodimers, which IκBα then sequesters in the latent NF-κB complexes. Consistent with the requirement of the C-terminal destruction box of p100 in imparting lymphotoxin responsiveness to the precursor complexes (19), expression of the mature p52 subunit in p100-deficient Relb−/−Nfkb2−/− cells was inadequate in generating the lymphotoxin-conditioning effect on canonical RelA activity (Fig. 5F and SI Appendix, Fig. S5G). We conclude that full-length p100 is important for linking the noncanonical pathway to canonical signaling.
Notably, RelA:p50 and RelA:p52 was shown to exhibit overlap in relation to proinflammatory gene expressions (23, 38). Accordingly, lymphotoxin conditioning amplified LPS-induced expressions of generic RelA-target chemokine genes encoding Ccl5 and Ccl2 in WT, but not Nfkb2−/−, cells (Fig. 5G). LTβR signaling also stimulates nuclear RelB activity. Lymphotoxin-mediated enhancement of LPS-induced gene expressions in Relb−/− MEFs asserted that the observed gene effects were independent of RelB. We infer that tonic LTβR-Nfkb2 signaling increases the abundance of latent RelA heterodimers, leading to a hyperactive canonical NF-κB response, which amplifies TLR-induced expressions of RelA-target proinflammatory genes.
Noncanonical Nfkb2 Signaling in the Intestinal Niche Instructs the Homeostasis of Latent RelA Dimers in IECs.
Next, we asked if the Nfkb2 pathway modulated latent NF-κB complexes also in the intestinal niche. Quantitative immunoblot analyses of extracts from IECs revealed a muted processing of p105 to p50 in Nfkb2−/− compared to WT mice (Fig. 6A). The reduced cellular abundance of p50 in Nfkb2−/− mice led to decreased RelA binding to p50 in RelA coimmunoprecipitates (Fig. 6B). On the other hand, the treatment of WT mice with LTβR-Ig prevented p100 processing to p52 and also diminished p50 production from p105. LTβR-Ig treatment of WT mice significantly lessened the association of RelA with both p50 and p52 (Fig. 6B). The latent NF-κB activity in IECs consisted of mostly RelA:p50, and a moderate amount of RelA:p52, in WT mice (Fig. 6 C and D). Global (Fig. 6C) or IEC-specific (Fig. 6E) Nfkb2 deficiency or LTβR-Ig treatment (Fig. 6F) substantially reduced the abundance of latent RelA dimers in IECs. Together, tonic LTβR-Nfkb2 signaling determines the homeostasis of latent RelA dimers in IECs by promoting simultaneous production of RelA-interacting partners p50 and p52 from their respective precursors.
Fig. 6.
LTβR-Nfkb2 signaling regulates the abundance of latent RelA dimers in IECs. (A and B) Immunoblot of whole-cell extracts (A) or RelA coimmunoprecipitates obtained using whole-cell extracts (B) derived from IECs. IECs were obtained from WT or Nfkb2−/− mice or WT mice administered with either control-Ig or LTβR-Ig 24 h prior to tissue collection. Signals were quantified from three independent experiments. Accordingly, the relative level of p52 to p100 and p50 to p105 (A) or the abundance of p52 and p50 present in the RelA coimmunoprecipitate (B) were determined and plotted (see below the respective gel pictures). (C, E, and F) EMSA revealing the abundance of latent NF-κBc dimers present in the cytoplasm of IECs derived from the indicated mice. Cytoplasmic extracts were treated with DOC for unmasking latent NF-κB DNA-binding activity. Data represent (C) five or (E and F) three biological replicates. (D) The composition of latent NF-κBc dimers present in IECs derived from WT mice was determined by shift-ablation assay. Quantified data represent means ± SEM. Two-tailed Student’s t test was performed. ***P < 0.001; *P < 0.05.
Increased Availability of p50 and p52 Connects Elevated Noncanonical NF-κB Signaling to Heightened nRelA Activity in Human IBD.
Because human IBD was associated with increased p100 processing to p52, we asked if IBD patients also exhibited increased p50 production from p105. Immunoblot analyses of extracts derived from colonic epithelial biopsies identified a total of 23 IBD patients with heightened p105 processing as assessed from a 1.5-fold or more increase in the p50:p105 ratio compared to the median value in the control cohort (Fig. 7A and SI Appendix, Fig. S1D). There was an overall 1.7-fold increase in the median p50:p105 value in IBD patients. In an upset plot, we could further capture that the fraction of patients displaying elevated ratios of both p50:p105 and p52:p100 was substantially higher than those with augmented ratios of either p50:p105 or p52:p100 (Fig. 7B). A high odds ratio for a simultaneous increase in the p50:p105 and p52:p100 values indicated interdependent processing of p105 and p100 in IBD. Finally, odds ratio measurements established that heightened nRelA activity in IBD was closely linked with increased processing of both the NF-κB precursors (Fig. 7C). These studies ascribe increased availability of the RelA dimerization partners p50 and p52, generated through interdependent processing of NF-κB precursors, to the heightened RelA activity in human IBD.
Fig. 7.
A link between elevated processing of p100 as well as p105 and heightened nRelA activity in human IBD. (A) The abundance of p50 in relation to p105 in colon biopsies from controls and IBD patients was quantified involving immunoblot analyses (SI Appendix, Fig. S1D) and presented as a dot plot. The statistical significance was determined by Welch’s t test. (B) Upset plot showing the fraction of IBD patients with elevated processing of both p100 and p105. Patients with a p52:p100 ratio above 1.5-fold of the median of the control cohort were termed as “p52:p100 High”; they were otherwise considered as “p52:p100 Low.” Similarly, patients were cataloged as either p50:p105 High or p50:p105 Low. The number of patients in each category has been indicated. The interdependence of p100 and p105 processing was determined from the odds ratio of a patient to have high p50:p105 ratio if p52:p100 ratio was elevated (90% CI, 1.44 to 69.41). (C) Upset plot revealing the fraction of IBD patients with heightened nRelA activity as well as increased processing of both the NF-κB precursors p100 and p105. The interdependence was determined from the odds ratio (90% CI, 0.83 to 18.24). (D) A mechanistic model explaining the proposed role of the noncanonical Nfkb2 pathway in amplifying canonical RelA activity that causes aberrant inflammation in the colitogenic gut. In this cartoon, brown, magenta, and green lines represent constitutive, canonical signal-responsive, and noncanonical signal-responsive cellular processes, respectively. The weight of the lines indicates the strength of the respective biochemical reactions.
Taken together, we put forward a mechanistic model explaining aberrant intestinal inflammation (Fig. 7D). In this model, noncanonical Nfkb2 signaling in IECs supplemented latent RelA heterodimers aggravating canonical NF-κB response in the colitogenic gut. We suggest that latent RelA dimer homeostasis connects the noncanonical NF-κB pathway to RelA-driven inflammatory pathologies.
Discussion
Despite the well-established role of RelA in fueling aberrant intestinal inflammation, the molecular mechanism underlying inordinate RelA activation in the colitogenic gut remains unclear. As such, the canonical NF-κB pathway drives controlled nuclear activity of RelA. Our investigation revealed not only the triggering of canonical signaling but also the frequent engagement of the noncanonical NF-κB pathway in human IBD. In a mouse model, we ascertained that tonic, noncanonical Nfkb2 signaling amplified epithelial RelA activity induced during the initiation of experimental colitis. This Nfkb2-mediated regulation escalated RelA-driven inflammatory gene response in IECs, exacerbating the infiltration of inflammatory cells and colon pathologies. Our analyses involving cultured cells confirmed that LTβR-stimulated, NIK-dependent noncanonical Nfkb2 signaling intensified canonical RelA response to TLRs in a cell-autonomous mechanism. These studies closely linked the Nfkb2-RelA signaling axis to aggravated inflammation in the colitogenic mouse gut. Although multifactorial human IBD cannot be fully recapitulated in animal models, our finding suggests that impinging noncanonical signaling aggravates inflammatory canonical RelA activity in the colitogenic gut of IBD patients. Unlike basally elevated noncanonical signaling in the mouse colon, however, p100 processing was rather timid in IBD-free human subjects. While this difference warrants further examination, future studies ought to determine if the onset of human IBD is indeed preceded by strengthening Nfkb2 signaling or if engagement of the noncanonical pathway coincides with the disease progression.
What provides signals to the Nfkb2-RelA axis in the colitogenic gut remains unclear. In our experiments, the impairment of LTβR signaling using LTβR-Ig substantially diminished RelA activation in IECs of DSS-treated mice. It was suggested that LIGHT, rather than the lymphotoxinα1β2 heterotrimer, likely engage intestinal LTβR (39). Importantly, human IBD was associated with an augmented colonic abundance of LIGHT (40). However, Nfkb2 signaling induced by TWEAK also amplified the canonical RelA response in our ex vivo studies. More so, it was reported that the TWEAK-Fn14 pathway aggravates human IBD (41). Together, these observations support the notion that the nonredundant functioning of multiple noncanonical pathway-activating cytokines promotes inflammatory RelA activity during the course of colitis. Further investigating the dynamic engagement of immune cells bearing triggers for noncanonical Nfkb2 signaling may offer important insights.
Previous mouse studies revealed a rather complex role of the canonical pathway in the colon (42, 43). Genetic deficiency of the inhibitory IκBα in IECs caused spontaneous intestinal inflammation (29). However, a complete lack of canonical signaling in IEC-specific knockouts of RelA or IKK2 sensitized those mice to experimental colitis despite reduced inflammatory gene activation (44, 45). In addition to immune genes, RelA also activates the expression of antiapoptotic factors. It was found that increased apoptosis of IECs in the absence of functional canonical signaling overwhelms mucosal healing. Targeting pathway components using antisense oligo or peptide inhibitors achieved partial inhibition of canonical signaling in mice subjected to colitogenic insults (8, 9). Interestingly, such incomplete pathway inhibition effectively alleviated gut inflammation while circumventing cellular apoptosis in the intestinal niche. An absence of Nfkb2 diminished, but not completely abrogated, canonical RelA signaling in IECs in our study. Accordingly, Nfkb2 deficiency restrained intestinal inflammation without exacerbating cell death in the colitogenic gut.
Investigating the colitogenic function of the noncanonical pathway in gene knockout mice yielded confounding outcomes. Increased intestinal inflammation observed in Nlrp12−/− mice was attributed to the elevated level of NIK and p52 (28). We have also earlier reported that noncanonical Nfkb2 signaling reinforces protective inflammatory responses against gut pathogens (23). Our current study revealed that Nfkb2-mediated amplification of canonical RelA activity in IECs conferred vulnerability in mice enduring chemically induced, pervasive colon damage. While our finding pertaining to the colitis-resilient Nfkb2−/− phenotype was consistent with those published by Burkitt et al. (17), we further established a nonredundant role of LTβR in triggering the pathological Nfkb2-RelA axis in IECs. In contrast, previous studies involving LTβR-Ig–administered WT mice or cell type–specific knockouts revealed both disease-promoting as well as protective roles of LTβR in experimental colitis (46–49). In particular, it was initially suggested that IEC-specific deletion of LTβR exacerbates DSS-induced colitis (13). A more recent investigation by Riffelmacher et al. (49) clarified that LTβR signaling, if anything, plays a moderately procolitogenic role in IECs. However, LTβR was also shown to promote the differentiation of gut-protective goblet cells independent of canonical NF-κB signaling (30). Similarly, IEC-specific ablation of NIK sensitized mice to DSS, owing to a presumably NF-κB–independent role of NIK in maintaining M cells, which produced gut-protective IL-17A and IgA (12). We reconcile that LTβR and NIK possess pleiotropic functions in the gut. In this proposition, LTβR, or other noncanonical signal transducers, and NIK exacerbate colitis involving noncanonical Nfkb2 signaling-mediated amplification of inflammatory RelA activity in IECs. LTβR and NIK also counteract colitis, albeit involving RelA-independent mechanisms in IECs or another cell type. In support of our model, when overexpressed in IECs, NIK indeed up-regulated the colonic expression of proinflammatory cytokines in mice (12).
The amplitude of canonical RelA response depends on both the magnitude of signal-induced NEMO-IKK activity as well as the abundance of IκB-bound latent dimers, which are acted upon by NEMO-IKK. While NEMO-IKK activation has been extensively studied in the context of inflammation, molecular processes regulating the generation of latent NF-κB dimers remain less explored. It was demonstrated that by preferentially stabilizing RelA homodimers in resting cells, IκBβ ensured their availability for activation via the canonical pathway (50). Our study revealed that noncanonical signaling tuned the homeostasis of latent RelA heterodimers. Scheidereit’s group earlier reported that LTβR stimulates simultaneous processing of p100 and p105 and that LTβR-responsive p105 processing to p50 requires p100 (19). We found that the noncanonical pathway accumulated both RelA:p50 and RelA:p52 involving this interdependent processing mechanism, which required full-length p100. The sequestration of these RelA dimers by IκBs in deoxycholate-sensitive latent complexes provided for heightened canonical response in lymphotoxin-conditioned cells. The absence of Nfkb2 diminished LTβR-responsive, but not constitutive, RelA:p50 generation. We propose that the previously described high-molecular-weight complexes comprising RelA, p105, and p100 (22) produced latent RelA dimers in response to noncanonical pathway-activating stimuli and that a lack of p100 prevented proteolytic machinery from recruiting to these complexes. Nonetheless, our data suggested that interdependent processing of p105 and p100 modulated intestinal inflammation in colitogenic mice and in human patients. In other words, coordinated generation of p50 and p52 by noncanonical Nfkb2 signaling amplified proinflammatory canonical RelA responses in the colitogenic gut. Despite impinging noncanonical signaling in the gut, however, we could detect only a minor nuclear RelB DNA-binding activity in IECs from DSS-treated mice. Importantly, the expression of goblet cell–specific genes, which were proposed to be RelB targets (30), was largely unaffected in colitogenic Nfkb2−/− mice. While underscoring the necessity for further investigating RelB regulations in the gut, our results assured that the observed resilience of Nfkb2−/− mice to colitogenic insults was not due to altered RelB functions.
We present evidence that the convergence of seemingly harmless tonic signals with inflammatory signals may provoke pathological RelA activity. Within the intestinal niche, epithelial cells receive noncanonical NF-κB signals, which inflate the repertoire of latent RelA dimers; mucosal damage provides for the inflammatory signal via the canonical NF-κB pathway that then fuels aberrant RelA activity in the gut. Direct therapeutic targeting of the canonical pathway precipitates undesired side effects because of the engagement of canonical signaling in a myriad of physiological processes. In this context, the noncanonical pathway offers an attractive therapeutic option. However, we argue that the therapeutic targeting of noncanonical pathway-activating receptors or kinase may lead to adverse outcomes because of their pleiotropic functions, and insulating high-molecular-weight NF-κB precursor complexes from noncanonical signaling may alleviate RelA-driven inflammatory pathologies, including those associated with IBD. In sum, our study emphasizes that signal-crossregulatory mechanisms may provide therapeutic opportunities in human ailments.
Methods
Patients and Collection of Colon Biopsies.
Patients older than 18 y were registered at the All India Institute of Medical Sciences, New Delhi, India, and diagnosed based on European Crohn’s and Colitis Organization guidelines. We examined IBD patients suffering from ulcerative colitis. Colonic epithelial biopsies were derived from the inflamed region of the rectum. As controls, we utilized samples from IBD-free individuals suffering from hemorrhoids. Experimental procedures were approved by the human ethics committees of the All India Institute for Medical Sciences (protocol no. IHEC-667/07–12-2018) and the National Institute of Immunology (protocol no. IHEC#106/18), and informed consent was obtained from patients.
Animal Use.
All mouse strains were housed at the National Institute of Immunology and used adhering to the institutional guidelines (approval no. IAEC 400/15). Nfkb2fl/fl mice (stock no. #028720, C57BL/6) were from the Jackson Laboratory and Villin-Cre mice were a generous gift from F. Greten, Georg-Speyer-Haus Institute of Tumor Biology and Experimental Therapy, Frankfurt am Main, Germany. Villin-Cre mice were crossed with Nfkb2fl/fl mice to generate Nfkb2∆IEC mice, which lacked Nfkb2 functions in IECs. The Nfkb2∆IEC and control Nfkb2fl/fl mice used in our experiments were in the mixed background.
Induction and Assessment of Colitis in Mice.
As described (3), 7- to 9-wk-old male mice of the indicated genotypes were administered with 2.5% of DSS in drinking water for 7 d. Subsequently, mortality, body weight, and disease activity were assessed for 14 d from the onset of treatment. The protocol details for assessment of colitis, colon length and histological studies is provided in the SI Appendix. For specific experiments, mice were killed at the indicated days postonset of DSS treatment. A total of 200 µg antagonistic LTβR-Ig fusion protein was injected via intraperitoneal route into mice as described (23), and IECs were collected after another 24 h. As a control, MOPC21 antibody was used.
Generation of Bone Marrow Chimeras.
Bone marrow chimeras were generated and analysed as previously described (23).
Isolation of IECs from Mouse Colons.
Colons were surgically removed from the mice, and IECs were isolated as described (23).
Isolation of Lamina Propria Cells from Mouse Colons.
Colons were longitudinally cut open and then cut into small pieces. These small pieces were rocked on a shaker platform submerged in Hanks’ Balanced Salt Solution (HBSS) containing 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM DTT, and 10% fetal bovine serum (FBS). Subsequently, pieces were washed gently and then subjected to enzymatic digestion for 60 min in HBSS containing 0.5 mg/mL collagenase IV (Worthington), 0.05 mg/mL deoxyribonuclease I (Worthington), and 10% FBS. The supernatant was filtered through a nylon cell strainer with 70-μm pore size (Corning). Cells from the filtrate were collected, stained with fluorochrome-conjugated antibodies, and analyzed by flow cytometry. The details for flow cytometric analysis is provided in the SI Appendix.
Gene Expression Studies.
Protocol details for qRT-PCR analysis are provided in the SI Appendix. Total RNA isolated from IECs was also subjected to the RNA-seq analysis at the Immunogenomics core facility of the Singapore Immunology Network. If the cumulative read count of a transcript estimated from a total of 12 experimental sets was less than 500, the corresponding gene was excluded from analyses. Ensemble identifications lacking assigned gene names were excluded. Accordingly, we arrive onto a list of 8,199 genes from 46,517 entries. The average read counts of these genes in various experimental sets were determined. Next, fold changes in the mRNA levels upon DSS treatment were determined using the DESeq2 package from Bioconductor (52). The difference in the fold-change values between WT and Nfkb2−/− mice was calculated for 8,199 genes, and genes were ranked in descending order of this fold-change difference. This ranked gene list was subjected to the GSEA involving fgsea package using a previously described list of RelA-target genes (23, 53). A previously published goblet cell–specific gene signature was also used for GSEA (31). The enrichment scores of GO terms were determined by Fisher’s exact test with an elimination algorithm utilizing R package topGO (54). All genes with 50 or more read counts in the RNA-seq data were selected as backgrounds for the respective WT or Nfkb2−/− genotypes.
Ex Vivo Cell Stimulations.
WT, Nfkb2−/−, or Relb−/− MEFs, obtained from day 14.5 embryos, were treated with 0.1 μg/mL αLTβR or 1 μg/mL LPS for the indicated times. Alternately, MEFs were stimulated with αLTβR for 36 h and then were additionally treated with LPS in the continuing presence of αLTβR. Adhering to our previously published protocol (23), we stably expressed p52 in immortalized Relb−/−Nfkb2−/− MEFs from a retroviral transgene, and the resultant cell line was further subjected to the combinatorial stimulation regime. For a select set of experiments, MEFs were treated with 100 ng/mL recombinant mouse TWEAK for 24 h and then subjected to LPS treatment.
Biochemical Studies.
The methods for preparing nuclear, cytoplasmic, and whole-cell extracts from MEFs and mouse IECs have been described elsewhere (23). Adapting from a previously published protocol (26), we optimized a nucleocytoplasmic fractionation method for colonic epithelial biopsies derived from human subjects. Detailed protocol for preparation of nuclear and cytoplasmic extract from colon biopsies is provided in the SI Appendix. The detailed protocols for NEMO-IKK kinase assay, EMSA and shift-ablation assay, immunoblot, and immunoprecipitation studies have been described earlier (23). For assessing latent NF-κB dimers, 2 μg cytoplasmic extract was treated with 0.8% DOC for 30 min and then analyzed in EMSA (55). For immunoblot analyses of RelA coimmunoprecipitates, a TrueBlot secondary antibody was used. We used a Cy5-conjugated secondary antibody for immunoblotting colon biopsies. Gel images were acquired using a Typhoon 9400 Variable Mode Imager, and signal intensities were quantified in ImageQuant 5.2.
Statistical Analysis.
Error bars are shown as SEM of four to eight mice in animal studies and as SEM of three to five replicates in biochemical experiments. Quantified data are means ± SEM. Unless otherwise mentioned, paired two-tailed Student’s t test was used for calculating statistical significance in datasets involving mouse or derived cells. Human data were subjected to Welch’s unpaired t test.
Supplementary Material
Acknowledgments
We sincerely thank Biogen, Inc. for providing the αLTβR antibody and LTβR-Ig fusion protein and Prof. F. Greten for the generous gift of Villin-Cre mice. We thank V. Kumar for technical help, B. Lee for bioinformatics help with RNA-seq analyses, and Dr. P. Nagarajan for the help with animal husbandry. We deeply appreciate Prof. G. Ghosh, University of California, San Diego and Dr. R. Gokhale for critical comments. The research in the Principal Investigator’s laboratory was funded by the Department of Biotechnology, Government of India [BT/PR36631/BRB/10/1862/2020] and National Institute of Immunology Core. S.K.B. thanks core funding from the Singapore Immunology Network Agency for Science, Technology and Research (A*STAR). M.C. and A.D. thank DBT and the Department of Science and Technology Innovation in Science Pursuit for Inspired Research for research fellowships, respectively.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. S.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2024828118/-/DCSupplemental.
Data Availability
The raw read counts of the RNA-seq dataset generated and analyzed in the current study are available on National Center for Biotechnology Information Gene Expression Omnibus (accession no. GSE148577).
References
- 1.Friedrich M., Pohin M., Powrie F., Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 50, 992–1006 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Kotas M. E., Medzhitov R., Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiesler P., Fuss I. J., Strober W., Experimental models of inflammatory bowel diseases. Cell. Mol. Gastroenterol. Hepatol. 1, 154–170 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell S., Vargas J., Hoffmann A., Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 8, 227–241 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu T., Zhang L., Joo D., Sun S.-C., NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2, 17023 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karrasch T., Kim J.-S., Muhlbauer M., Magness S. T., Jobin C., Gnotobiotic IL-10-/-;NF-κ B(EGFP) mice reveal the critical role of TLR/NF-κ B signaling in commensal bacteria-induced colitis. J. Immunol. 178, 6522–6532 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Han Y. M., et al., NF-kappa B activation correlates with disease phenotype in Crohn’s disease. PLoS One 12, e0182071 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibata W., et al., Cutting edge: The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks inflammatory injury in murine colitis. J. Immunol. 179, 2681–2685 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Neurath M. F., Pettersson S., Meyer zum Büschenfelde K.-H., Strober W., Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-κ B abrogates established experimental colitis in mice. Nat. Med. 2, 998–1004 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Sun S.-C., The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 17, 545–558 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J. Z.et al.; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium , Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 47, 979–986 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramakrishnan S. K., et al., Intestinal non-canonical NFκB signaling shapes the local and systemic immune response. Nat. Commun. 10, 660 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macho-Fernandez E., et al., Lymphotoxin beta receptor signaling limits mucosal damage through driving IL-23 production by epithelial cells. Mucosal Immunol. 8, 403–413 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jie Z., et al., NIK signaling axis regulates dendritic cell function in intestinal immunity and homeostasis. Nat. Immunol. 19, 1224–1235 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mise-Omata S., et al., A proximal kappaB site in the IL-23 p19 promoter is responsible for RelA- and c-Rel-dependent transcription. J. Immunol. 179, 6596–6603 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Boutaffala L., et al., NIK promotes tissue destruction independently of the alternative NF-κB pathway through TNFR1/RIP1-induced apoptosis. Cell Death Differ. 22, 2020–2033 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkitt M. D., et al., NF-κB1, NF-κB2 and c-Rel differentially regulate susceptibility to colitis-associated adenoma development in C57BL/6 mice. J. Pathol. 236, 326–336 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shih V. F.-S. S., Tsui R., Caldwell A., Hoffmann A., A single NFκB system for both canonical and non-canonical signaling. Cell Res. 21, 86–102 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yılmaz Z. B., et al., Quantitative dissection and modeling of the NF-κB p100-p105 module reveals interdependent precursor proteolysis. Cell Rep. 9, 1756–1769 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Basak S., Shih V. F.-S., Hoffmann A., Generation and activation of multiple dimeric transcription factors within the NF-kappaB signaling system. Mol. Cell. Biol. 28, 3139–3150 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao Z., et al., p100/IκBδ sequesters and inhibits NF-κB through kappaBsome formation. Proc. Natl. Acad. Sci. U.S.A. 111, 15946–15951 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savinova O. V., Hoffmann A., Ghosh G., The Nfkb1 and Nfkb2 proteins p105 and p100 function as the core of high-molecular-weight heterogeneous complexes. Mol. Cell 34, 591–602 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banoth B., et al., Stimulus-selective crosstalk via the NF-κB signaling system reinforces innate immune response to alleviate gut infection. eLife 4, 1–56 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almaden J. V., et al., A pathway switch directs BAFF signaling to distinct NFκB transcription factors in maturing and proliferating B cells. Cell Rep. 9, 2098–2111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee T., et al., A TNF-p100 pathway subverts noncanonical NF-κB signaling in inflamed secondary lymphoid organs. EMBO J. 36, 3501–3516 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andresen L., et al., Activation of nuclear factor kappaB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut 54, 503–509 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi Y., et al., Roxithromycin inhibits nuclear factor kappaB signaling and endoplasmic reticulum stress in intestinal epithelial cells and ameliorates experimental colitis in mice. Exp. Biol. Med. (Maywood) 240, 1664–1671 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen I. C., et al., NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-κB signaling. Immunity 36, 742–754 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikuda N., et al., Deficiency in IκBα in the intestinal epithelium leads to spontaneous inflammation and mediates apoptosis in the gut. J. Pathol. 251, 160–174 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Pian Y., et al., Type 3 innate lymphoid cells direct goblet cell differentiation via the LT-LTβR pathway during Listeria infection. J. Immunol. 205, 853–863 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Haber A. L., et al., A single-cell survey of the small intestinal epithelium. Nature 551, 333–339 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyons J., et al., The colonic epithelium plays an active role in promoting colitis by shaping the tissue cytokine profile. PLoS Biol. 16, e2002417 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grinberg-Bleyer Y., et al., The alternative NF-κB pathway in regulatory T cell homeostasis and suppressive function. J. Immunol. 200, 2362–2371 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhar A., et al., Role of NF-kappaB2-p100 in regulatory T cell homeostasis and activation. Sci. Rep. 9, 13867 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Upadhyay V., Fu Y.-X., Lymphotoxin signalling in immune homeostasis and the control of microorganisms. Nat. Rev. Immunol. 13, 270–279 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zarnegar B., Yamazaki S., He J. Q., Cheng G., Control of canonical NF-kappaB activation through the NIK-IKK complex pathway. Proc. Natl. Acad. Sci. U.S.A. 105, 3503–3508 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baeuerle P. A., Baltimore D., IκB: A specific inhibitor of the NF-κB transcription factor. Science 242, 540–546 (1988). [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann A., Leung T. H., Baltimore D., Genetic analysis of NF-kappaB/Rel transcription factors defines functional specificities. EMBO J. 22, 5530–5539 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krause P., et al., The tumor necrosis factor family member TNFSF14 (LIGHT) is required for resolution of intestinal inflammation in mice. Gastroenterology 146, 1752–62.e4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohavy O., Zhou J., Ware C. F., Targan S. R., LIGHT is constitutively expressed on T and NK cells in the human gut and can be induced by CD2-mediated signaling. J. Immunol. 174, 646–653 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Dohi T., Burkly L. C., The TWEAK/Fn14 pathway as an aggravating and perpetuating factor in inflammatory diseases: Focus on inflammatory bowel diseases. J. Leukoc. Biol. 92, 265–279 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Wullaert A., Bonnet M. C., Pasparakis M., NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 21, 146–158 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaidi D., Wine E., Regulation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κβ) in inflammatory bowel diseases. Front Pediatr. 6, 317 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinbrecher K. A., Harmel-Laws E., Sitcheran R., Baldwin A. S., Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J. Immunol. 180, 2588–2599 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Eckmann L., et al., Opposing functions of IKKbeta during acute and chronic intestinal inflammation. Proc. Natl. Acad. Sci. U.S.A. 105, 15058–15063 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jungbeck M., et al., Blocking lymphotoxin beta receptor signalling exacerbates acute DSS-induced intestinal inflammation–opposite functions for surface lymphotoxin expressed by T and B lymphocytes. Mol. Immunol. 45, 34–41 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Stopfer P., et al., Blocking lymphotoxin-β receptor activation diminishes inflammation via reduced mucosal addressin cell adhesion molecule-1 (MAdCAM-1) expression and leucocyte margination in chronic DSS-induced colitis. Clin. Exp. Immunol. 136, 21–29 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wimmer N., et al., Lymphotoxin-beta receptor activation on macrophages ameliorates acute DSS-induced intestinal inflammation in a TRIM30α-dependent manner. Mol. Immunol. 51, 128–135 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Riffelmacher T., et al., Metabolic activation and colitis pathogenesis is prevented by lymphotoxin β receptor expression in neutrophils. Mucosal Immunol. 14, 679–690 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsui R., et al., IκBβ enhances the generation of the low-affinity NFκB/RelA homodimer. Nat. Commun. 6, 7068 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franco N. H., Correia-Neves M., Olsson I. A. S., How “humane” is your endpoint? Refining the science-driven approach for termination of animal studies of chronic infection. PLoS Pathog. 8, e1002399 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sergushichev A. A., An algorithm for fast preranked gene set enrichment analysis using cumulative statistic calculation. bioRxiv [Preprint] (2016). 10.1101/060012 (Accessed 24 February 2021). [DOI]
- 54.Alexa A., Rahnenführer J., Lengauer T., Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22, 1600–1607 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Basak S., et al., A fourth IkappaB protein within the NF-kappaB signaling module. Cell 128, 369–381 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw read counts of the RNA-seq dataset generated and analyzed in the current study are available on National Center for Biotechnology Information Gene Expression Omnibus (accession no. GSE148577).