Abstract
Spanking remains common around the world, despite evidence linking corporal punishment to detrimental child outcomes. This study tested whether children (Mage = 11.60) who were spanked (N = 40) exhibited altered neural function in response to stimuli that suggest the presence of an environmental threat compared to children who were not spanked (N = 107). Children who were spanked exhibited greater activation in multiple regions of the medial and lateral prefrontal cortex (PFC), including dorsal anterior cingulate cortex, dorsomedial PFC, bilateral frontal pole, and left middle frontal gyrus in response to fearful relative to neutral faces compared to children who were not spanked. These findings suggest that spanking may alter neural responses to environmental threats in a manner similar to more severe forms of maltreatment.
Corporal punishment—defined as the use of physical force to cause a child to experience pain or discomfort, however light—(Convention on the Rights of the Child, 2006), such as spanking, is a socially normative and legal punishment method in over 130 countries (Global Initiative to End Corporal Punishment of Children, 2021). In the United States, approximately half of parents reported spanking their children in the past year and one-third used spanking in the past week (Finkelhor, Turner, Wormuth, Vanderminden, & Hamby, 2019; Ryan, Kalil, Ziol-Guest, & Padilla, 2016). Despite the high prevalence and widespread social approval of spanking, developmental theories have long posited that spanking is associated with deleterious child outcomes (see Gershoff, 2002). Indeed, meta-analyses show consistent associations between spanking and internalizing and externalizing problems, poor cognitive development, and other maladaptive outcomes throughout the life span (Gershoff, 2002; Gershoff & Grogan-Kaylor, 2016).
More recently, some have argued that spanking may influence brain development in a similar manner as more extreme forms of maltreatment (e.g., Gershoff, 2016). The dimensional model of adversity (McLaughlin, Sheridan, & Lambert, 2014; Sheridan & McLaughlin, 2014), argues that exposure to experiences involving harm or threat of harm to the child, such as sexual abuse, physical or psychological maltreatment, witnessing domestic violence, and exposure to community violence will have similar influences on emotional and neural development that will scale in relation to the severity of the threat experienced. These developmental consequences are posited to be at least somewhat distinct from those associated with adverse experiences involving deprivation, such as neglect and lack of cognitive and social stimulation. Spanking and other forms of corporal punishment are threatening experiences that cause fear, pain, and threat of harm to the child (Gershoff, 2002). Consequently, spanking may influence neurodevelopmental processes in similar ways as more severe forms of maltreatment.
The dimensional model predicts that exposure to threatening experiences alters social and emotional processing in ways that facilitate the rapid identification of environmental threats, including heightened responses to negative emotional cues in the amygdala and other regions of the salience network (McLaughlin et al., 2014; Sheridan & McLaughlin, 2014), including the anterior insula and dorsal anterior cingulate cortex, which are involved in processing emotional and personal salience through the perception and regulation of internal bodily responses (Menon, 2011). Existing evidence from studies of harsh parenting and child abuse is consistent with this idea. Children exposed to physical and sexual abuse, domestic violence, or harsh parenting exhibit heightened neural responses to threatening or negative stimuli in the amygdala and broader salience network (Gard et al., 2017; Hein & Monk, 2017; McCrory et al., 2011, 2013; McLaughlin, Peverill, Gold, Alves, & Sheridan, 2015; McLaughlin, Weissman, & Bitr an, 2019; Pozzi et al., 2020). The dimensional model predicts that the magnitude of these neural changes varies as a function of the severity of the threat involved. Consistent with this prediction, greater severity of violence exposure has been associated with greater amygdala reactivity to threat cues (Ganzel, Kim, Gilmore, Tottenham, & Temple, 2013; Mclaughlin et al., 2015). Therefore, it is plausible that spanking may similarly contribute to heightened salience network responses to threat. We are unaware of prior research examining this possibility.
Little is known about the neural consequences of spanking. One study found that children who were spanked exhibit heightened cortisol reactivity to stressors such as repeated separation from the mother and the presence of a stranger, suggesting maladaptive changes in the stress response system (Bugental, Martorell, & Barraza, 2003). Adults who were exposed to harsh corporal punishment (e.g., hit with objects) during childhood exhibited structural brain differences, including less gray matter volume in the prefrontal cortex (PFC), than adults who did not experience harsh corporal punishment (Tomoda et al., 2009). However, little is currently known about functional neural correlates of forms of corporal punishment that are more socially normative in some countries, such as spanking.
The purpose of this study was to examine the association between spanking and neural responses to fearful faces, an indicator of the presence of threat in the environment (Tottenham, Phuong, Flannery, Gabard-Durnam, & Goff, 2013), using an emotional face task (Tottenham et al., 2009). Building on the dimensional model of adversity (Sheridan & McLaughlin, 2014) and prior work on neural correlates of child abuse (McCrory et al., 2011; McLaughlin et al., 2019), we expected that children who were spanked would exhibit greater neural activation to fearful than neutral faces in the amygdala and other nodes of the salience network, including the anterior insula and dorsal anterior cingulate, compared to children who were never spanked. As an exploratory aim, we also evaluated whether children who were spanked exhibited a profile of neural response to stimuli that suggests the presence of an environmental threat (i.e., fearful faces) that was similar to children who experienced severe physical and sexual abuse.
Method
Participants
The analytic sample of this study comprised 147 children (75 girls, Mage = 11.60) who were part of an ongoing longitudinal study and participated in a functional MRI (fMRI) assessment. A total of 97 (66%) participants identified as White, 19 (13%) as Black, 13 (9%) as Latinx, 13 (9%) as Asian, and 5 (3%) as another race or ethnicity. The families in the larger study (n = 302; Lengua et al., 2015) were recruited from a hospital birth register, day cares, preschools, clinics, and charitable agencies at the age of 36 months and were followed across multiple assessments prior to the current neuroimaging assessment. Families were recruited to achieve equal representation across income levels, and sampled families were required to be proficient in English and to understand the assessment procedures. Families with children diagnosed with a developmental disability were excluded from the sample (for more details on the larger study, see Lengua et al., 2015). Children were assessed at four time points between the ages of 3 and 5 (T1: 36–40 months, T2: 45–49 months, T3: 54–58 months, T4: 63–67 months). This report focuses on a fifth wave of data collection carried out on a subset of these participants (n = 227) when children were 10–12 years old. Each child participated in three laboratory sessions, which included assessments of corporal punishment and maltreatment. A subgroup of the sample (n = 183) also participated in a neuroimaging assessment. Of these participants, 10 participants were excluded from analyses due to poor fMRI data quality (see fMRI DATA Acquisition and Preprocessing) and 26 participants experienced physical or sexual abuse and were therefore excluded from analyses evaluating associations with spanking (n = 147).
All instances of child maltreatment were reported to the proper authorities, and facilitated clinical referrals were provided for families whose children exhibited clinically meaningful levels of psychopathology. All research procedures were approved by the Institutional Review Board of the University of Washington.
Measures
Spanking
Exposure to spanking at any point in the child’s life was assessed based on an item of the Violence Exposure Scale for Children-Revised (Raviv et al., 2001). Participants were first shown a cartoon picture of a character being spanked on the buttocks with an open hand and were told “A person spanks Chris.” They were then asked, “How many times has a person spanked you?” with response options of “never,” “one time,” “a few times,” or “lots of times.” Children who responded “a few times” or “lots of times,” who were not also exposed to severe physical or sexual abuse (described in the following section), were classified as spanked. In the sample, 40 (22 female) children were spanked and 107 (53 female) were neither spanked nor physically or sexually abused in their lifetime (see in the following section). These two groups of children did not differ in gender, race or ethnicity, or in age or birthweight (Table 1). The income-to-needs ratio of spanked children was significantly lower than that of children who were never spanked (d = −.76, t = .31, p < .05).
Table 1.
Sample Characteristics
| Spanking (n = 40) | Control (n = 107) | Physical and sexual abuse (n = 26) | ||||
|---|---|---|---|---|---|---|
| % | n | % | n | % | N | |
| Female | 55 | 22 | 49 | 53 | 65 | 17 |
| Racial/ethnic minority | 28 | 11 | 36 | 39 | 38 | 10 |
| M | SD | M | SD | M | SD | |
| Age | 11.68 | 0.87 | 11.57 | 0.49 | 11.69 | 0.55 |
| Income-to-needs | 3.12* | 1.77 | 3.89 | 1.65 | 3.21 | 2.15 |
| Birthweight | 6.17 | 1.53 | 5.9 | 1.6 | 6.87* | 2.63 |
Mean is significantly different from the mean in the control group (p < .05).
Severe Physical and Sexual Abuse
A multi-informant, multimethod approach used frequently in prior work (e.g., Jenness et al., 2020; Weissman et al., 2019) was used to assess exposure to physical or sexual abuse. Children were classified as experiencing physical or sexual abuse if abuse was endorsed by the child on the Childhood Experiences of Care and Abuse (CECA) interview (Bifulco, Brown, & Harris, 1994), UCLA PTSD Reaction Index (PTSD-RI) trauma screen (Steinberg et al., 2013), or above the validated threshold on the self-report Childhood Trauma Questionnaire (CTQ; Bernstein, Ahluvalia, Pogge, & Handelsman, 1997) or reported by the parent on the Juvenile Victimization Questionnaire (Finkelhor, Hamby, Ormrod, & Turner, 2005), or PTSD-RI. Sample items from these measures include: “has someone forced you to have sex when you didn’t want to” (CECA); “I got hit or beaten so badly that it was noticed by someone like a teacher, neighbor, or doctor” (CTQ); “Being hit, punched, or kicked very hard at home” (PTSD-RI, child-report); “Not including spanking on your child’s bottom, at any time in your child’s life, did a grown-up in your child’s life hit, beat, kick, or physically hurt your child in any way?” (PTSD-RI, parent-report). A total of 26 children (17 female) experienced physical or sexual abuse in childhood.
Covariates
We controlled for children’s sex and age in all analysis, as well as the income-to-needs ratio given that children who were spanked were also more likely to come from households with a lower income-to-needs ratio. Income-to-needs ratio was calculated by dividing parent-reported yearly family income by the federal poverty line for a family of a given size as indicated by the U.S. Census Bureau. Low income-to-needs ratio is associated with increased risk of exposure to many forms of adversity, including both deprivation and threat (e.g., Bradley, Corwyn, McAdoo, & García Coll, 2001), and has been used as a proxy for experiences of deprivation in studies that also examine experiences of threat (e.g., Lambert, King, Monahan, & McLaughlin, 2017; Sheridan, Peverill, & McLaughlin, 2017).
Emotional Face Task
The emotional face task is a computerized task that participants complete while lying in an MRI scanner looking at a computer screen where actors’ faces are displayed one at a time. The task was conducted in two “runs”. Each run was made up of nine 18-s blocks; three blocks showed neutral faces, three blocks showed fearful faces, and three blocks showed scrambled faces (see Figure 1). Blocks were displayed in a pseudorandom order that ensured that no block type was displayed twice in a row. During each block, 36 faces of different actors expressing the same emotion were displayed for 300 ms each, with a space of 200 ms following each face. The procedure was based on findings of a prior face processing task (Somerville, Kim, Johnstone, Alexander, & Whalen, 2004). Once during each block, participants were prompted to indicate by an index or middle finger button press, whether the last face they saw was male or female to assess whether they were paying attention. Otherwise, participants were only asked to keep their eyes open and view the faces. Three participants performed worse than chance on this attention check and were therefore excluded from analyses.
Figure 1.
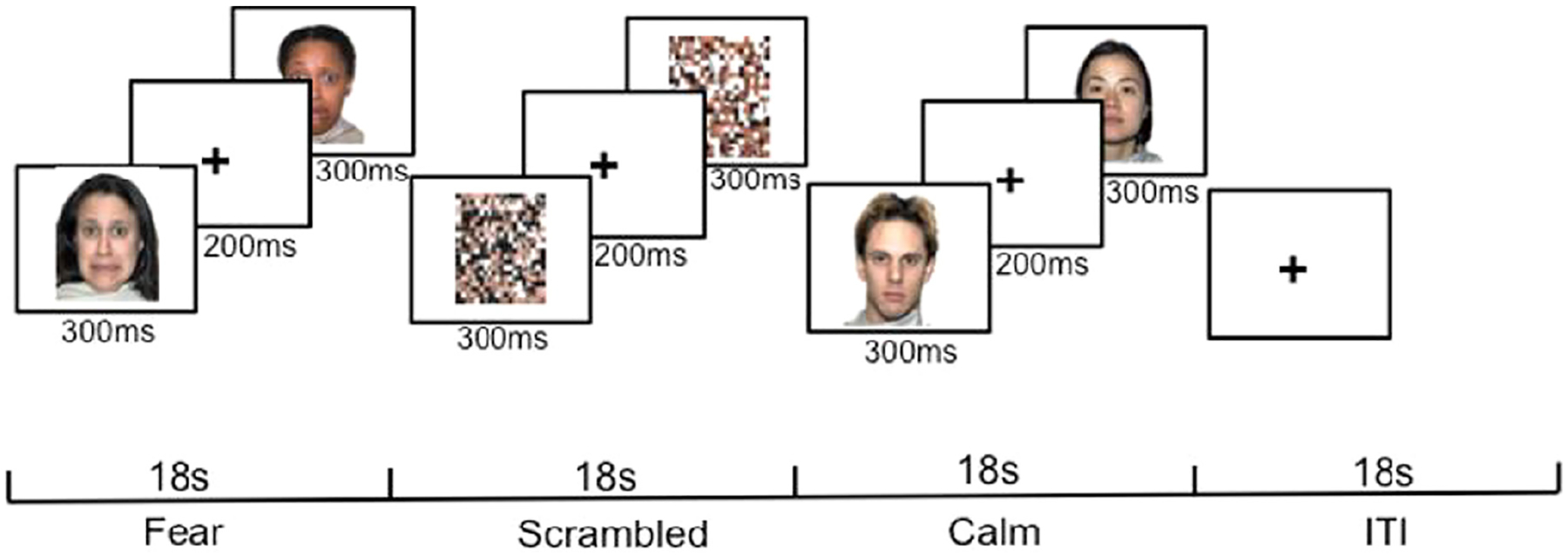
The emotional face task.
Faces were drawn from the NimStim stimulus set (Tottenham et al., 2009). The “calm” faces from this data set were used as neutral expressions, as these expressions are potentially less emotionally evocative than neutral faces, which are perceived as negatively valenced (Tottenham et al., 2009). The scrambled faces consisted of the images of neutral faces with the pixels scrambled so as to resemble random static.
fMRI Data Acquisition and Preprocessing
Before undergoing scanning, participants were trained to minimize head movements in a mock scanner. They watched a movie with a head-mounted motion tracker that stopped playing if a movement of over 2 mm occurred. When participants were able to watch the movie without 2 mm head movement for 1 min, the training was considered successful. This method has been shown to significantly reduce head motion once children are in the scanner (Raschle et al., 2012). In the scanner, we used an inflatable head-stabilizing pillow to further restrict movement.
Scanning and preprocessing of neuroimaging data were conducted using standard methods. Scanning was performed on a 3T Phillips Achieva scanner at the University of Washington Integrated Brain Imaging Center using a 32-channel head coil. T1-weighted MPRAGE volumes were acquired (repetition time = 2,530 ms, TE = 3.5 ms, flip angle = 7°, FOV = 256 × 256, 176 slices, in-plane voxel size = 1 mm3) for co-registration with fMRI data. Blood oxygenation level-dependent (BOLD) signal during functional runs was acquired using a gradient-echo T2*-weighted echo planar imaging sequence. Thirty-seven 3-mm-thick slices were acquired sequentially and parallel to the AC-PC line (TR = 2 s, TE = 25 ms, flip angle = 79°, Inter-slice gap = 0.6 mm, FOV = 224 × 224 × 132.6, matrix size = 76 × 74). Prior to each scan, four images were acquired and discarded to allow longitudinal magnetization to reach equilibrium.
Preprocessing and statistical analysis of fMRI data were performed in a pipeline using Gnu Make, a software development tool designed for building executables from source files that can be used to create neuroimaging workflows that rely on multiple software packages. The following preprocessing steps were applied: (a) motion correction followed by slice-time correction in FSL; (b) skull-stripping using FSL’s bet tool; (c) despiking using AFNI’s 3dDespike tool; and (d) smoothing with a 6-mm full-width half-max kernel using SUSAN in FSL. Outlier volumes in which framewise displacement exceeded 1 mm, the derivative of variance in BOLD signal across the brain (DVARS) exceeded the upper fence (above 75th percentile + 1.5 × inter-quartile range), or signal intensity was more than 3 SD from the mean were regressed out of person-level models. Six rigid-body motion regressors and the time-series extracted from white matter and ventricles were included in person-level models to reduce noise associated with motion and physiological fluctuations. Person- and group-level models were estimated in FSL. Following estimation of person-level models, the resulting contrast images were normalized into standard space, and anatomical co-registration of the functional data with each participant’s T1-weighted image was performed using Advanced Normalization Tools (ANTs) software (Avants et al., 2011).
Data were visually inspected for the presence of major artifacts or abnormalities in the structural and functional images by two trained researchers. Following person-level analyses, four participants were excluded from group-level analyses because of substantial signal dropout in the ventromedial PFC, indicating distortion of data in relevant brain regions for this analysis. One was excluded because of an incidental finding indicating a major structural abnormality, and one participant’s data were unusable due to a data storage error. Data were also excluded for four additional participants, two because of excessive motion, one because of a data acquisition error, and one because the scan was interrupted after the first run.
fMRI Analysis
FMRI data processing was performed using FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (Woolrich et al., 2001, 2004). For each participant, a model of the BOLD signal corresponding to neural activation across each face block was constructed for each stimulus type. These models were then regressed on the BOLD timeseries in each voxel of the brain. Model regressors were created by convolving a boxcar function of phase duration with the standard double-gamma hemodynamic response function for each phase of the task (fearful, neutral, and scrambled faces). A general linear model was constructed for each participant based on the models for each stimulus type, the six motion regressors, and the signals from white matter and ventricles.
To investigate the study hypotheses, we first conducted whole-brain analyses comparing neural activity for children who were spanked versus never exposed to violence for the contrast of fearful versus neutral faces. Higher level analysis was carried out using FLAME1 in FSL. While we did have a priori hypotheses that we would see greater activation in the anterior insula and dorsal anterior cingulate cortex among spanked children, this type of whole-brain analytic approach is exploratory in nature. We used a standard approach for identifying significant clusters of neural activation after correcting for multiple comparisons. Specifically, cluster thresholding was determined using AFNI’s 3dClust-Sim program (Cox, Chen, Glen, Reynolds, & Taylor, 2017), which generates Monte Carlo simulations to determine appropriate cluster sizes to correct for multiple comparisons, and AFNI’s 3dFWHMx program, which accounts for the number of voxels and the intrinsic spatial autocorrelation in the data residuals, addressing prior work indicating that failure to account for this autocorrelation in cluster correction can inflate type 1 error (Cox et al., 2017; Eklund, Nichols, & Knutsson, 2016). Based on output from these programs, a voxel-wise threshold of t = 2.33 (p < .01) with a minimum cluster size of 897 voxels was used, to set the corrected familywise error rate at 0.05. Sex, age, and income-to-needs ratio were included as covariates.
Because a minimum cluster size limits the ability to detect smaller clusters, particularly in subcortical regions, and given substantial evidence for differences in amygdala response to threat cues in children exposed to violence (McLaughlin et al., 2019), we also conducted a region of interest (ROI) analysis (i.e., a confirmatory analysis) in the amygdala. Activation to fearful versus neutral faces, transformed into z-scores and averaged across every voxel in the right and left amygdala, was extracted for each participant. Bilateral amygdala ROIs were constructed in FSL based on the Harvard Oxford subcortical probabilistic structural atlas, thresholded at 20% probability and warped back into each subjects’ native space. The mean of the z-scores of every voxel within the bilateral amygdala ROI were then extracted for the fear versus neutral contrast for each participant. Differences in amygdala response as a function of spanking were examined using linear regression, controlling for age, sex, and income-to-needs, using R version 3.5.1 (R Core Team, 2016). Sensitivity analyses were also conducted controlling for the frequency of witnessing violence (see Appendix S1). Although not the primary focus of this investigation, we also compared neural activation in the whole brain and amygdala in the children who were spanked to those who were physically or sexually abused, controlling for age, sex, and income-to-needs.
Results
Task-Related Neural Activation
On average, across the entire sample, fearful faces elicited greater activation than neutral faces in many regions throughout the brain, including the ventral visual stream, superior temporal sulcus, amygdala, and hippocampus, as well as widespread activation throughout PFC, including frontal pole and multiple regions in the dorsal and ventral lateral and medial PFC (Figure 2).
Figure 2.
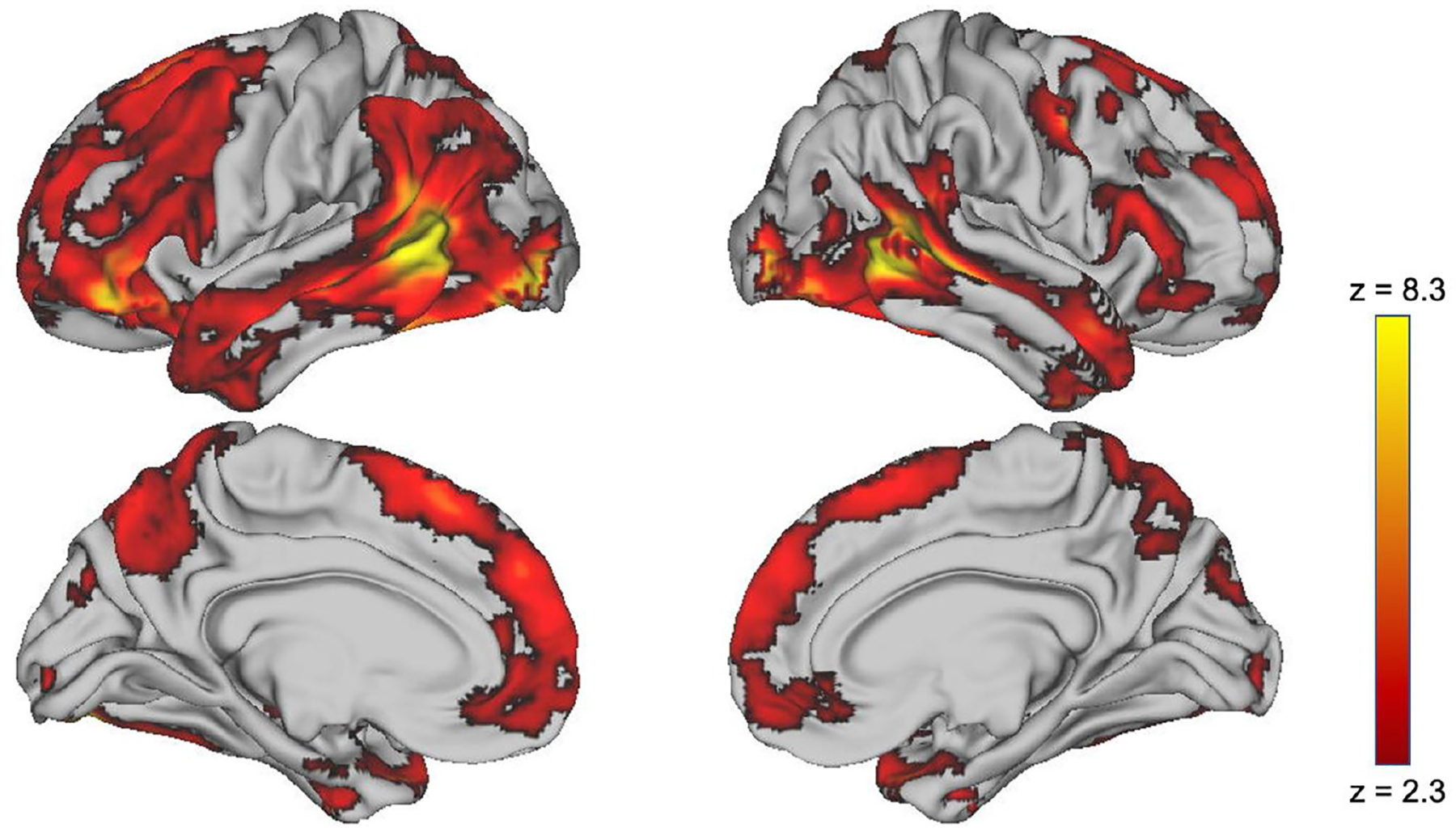
Brain regions with significantly greater activation, on average, to fearful compared to neutral faces.1
Note. The difference between activation to fearful versus neutral faces was greatest in regions depicted in yellow and lower, but still statistically significant, in regions depicted in red.
Spanking and Neural Response to Fearful Faces
Children who were spanked demonstrated greater activation in multiple regions of PFC to fearful relative to neutral faces than children who were never spanked (Table 2; Figure 3). These included a large cluster in the left middle frontal gyrus (MFG) and a second large cluster in the bilateral dorsomedial prefrontal cortex (dmPFC), encompassing bilateral dorsal anterior cingulate cortex (dACC), and the bilateral frontal pole. There were no regions of the brain where activation to fearful relative to neutral faces differed between children who were abused and children who were spanked.
Table 2.
Differences in Neural Reactivity to Fearful Versus Neutral Faces Between Spanked and Never-Spanked Children
| Voxels | Peak (x, y, z) | Region | BA | Peak voxel z-score |
|---|---|---|---|---|
| Fear > neutral, spanked > control | ||||
| 1,261 | −28, 6, 38 | Middle frontal gyrus | 6 | 4.20 |
| −36, 10, 44 | Middle frontal gyrus | 6 | 3.50 | |
| 1,095 | −2, 60, 34 | Frontal pole | 9 | 4.03 |
| −4, 46, 26 | Paracingulate gyrus | 9 | 3.55 | |
Note. Voxels = number of 2 mm3 voxels in the cluster; Peak (x, y, z) = MNI coordinates for the voxels with the highest coefficients within each cluster as well as subcluster local maxima; Region = label of the brain region at the location of Peak based on the Harvard Oxford Statistical Atlas; BA = Brodmann’s area.
Figure 3.
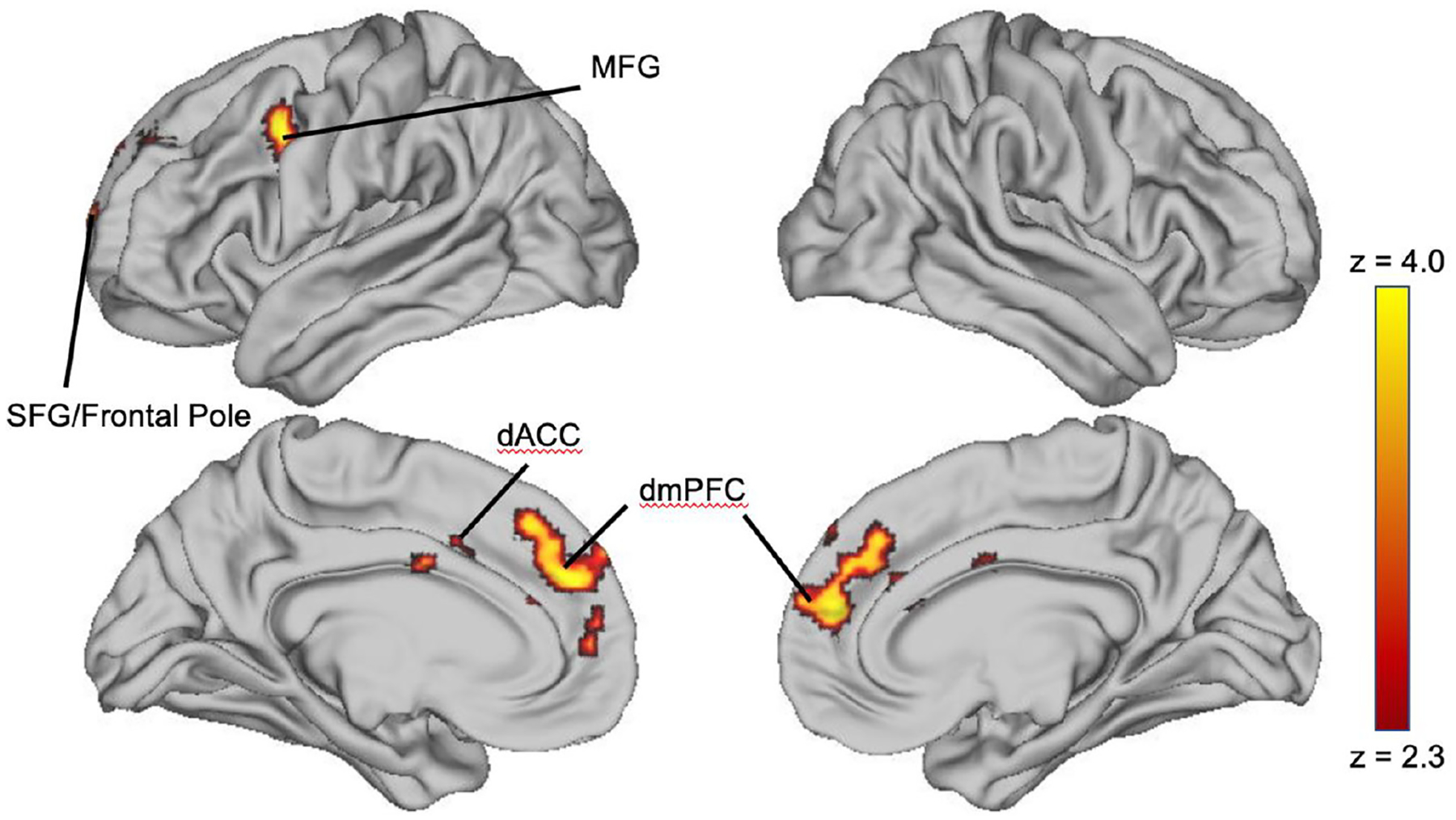
Differences in neural reactivity to fearful versus neutral faces between spanked and never spanked children.
Note. Regions where spanked children exhibit significantly greater activation to fearful versus neutral faces than never spanked children. Regions where the magnitude of increased activation to fearful versus neutral faces in spanked children was the largest are depicted in yellow, and smaller but still statistically significant are depicted in red. dACC = dorsal anterior cingulate cortex; MFG = middle frontal gyrus; SFG = superior frontal gyrus; dmPFC = dorsal medial prefrontal cortex.
We used the scrambled face condition to decompose these associations, to evaluate whether they were driven more by neural responses to fearful or neutral faces. Examining the association of spanking with activation in the left MFG to fearful versus scrambled faces (β = .136, p = .108) and neutral versus scrambled faces (β = −.322, p < .001) revealed that the association between spanking and activation to fearful versus neutral faces was driven primarily by lower activation to neutral faces in this region. Examining the association of spanking with activation in the dmPFC cluster to fearful versus scrambled faces (β = .144, p = .087) and neutral versus scrambled faces (β = −.176, p < .037) revealed that the association between spanking and activation to fearful versus neutral faces was driven by both lower activation to neutral faces and greater activation to fearful faces.
Amygdala activation to fearful veruss neutral faces did not differ significantly between children who were spanked and children who were never spanked nor exposed to more severe abuse (β = .02; SE = .15; p = .88). Amygdala activation to fearful versus neutral faces also did not differ significantly between children who were spanked and children who were exposed to more severe abuse β = .08; SE = .24; p = .73).
Discussion
Spanking remains common in the United States and worldwide (Cuartas et al., 2019; Finkelhor et al., 2019; Ryan et al., 2016). Children who are spanked tend to exhibit higher levels of cognitive, behavioral, and emotional problems than their never-spanked peers (Gershoff & Grogan-Kaylor, 2016). Despite these widespread developmental differences, we are unaware of prior work examining differences in neurodevelopment as a function spanking. In this study, we examined whether children who were spanked exhibited altered neural responses to stimuli that suggest the presence of an environmental threat (i.e., fearful faces; Tottenham et al., 2013) relative to children were never spanked nor exposed to physical or sexual abuse.
Our findings reveal that spanking was associated with greater activation to fearful versus neutral faces in multiple regions of the PFC. First, we observed elevated responses to fearful relative to neutral faces in the dACC, a key node in the salience network, among spanked relative to never-spanked children. Altered neural responses to emotional stimuli in the salience network, have been consistently reported in prior research on children exposed to abuse and domestic violence (McLaughlin et al., 2019). Our results suggest that spanking may influence children’s neural response to emotional cues in a way that is qualitatively similar to more severe violence.
In addition, we observed increased activation in the left MFG to fearful versus neutral faces among children who were spanked. This effect was driven primarily by lower activation to neutral faces in children who were spanked relative to those who were never spanked. The MFG is frequently engaged during effortful attempts to regulate emotional responses, such as when using cognitive reappraisal (Goldin, McRae, Ramel, & Gross, 2008; Kanske, Heissler, Schönfelder, Bongers, & Wessa, 2010; Silvers, Weber, Wager, & Ochsner, 2014). Children who have been exposed to violence recruit regions of the dorsolateral PFC more during cognitive reappraisal of negative emotion than those who have never encountered violence, particularly during adolescence (Jenness et al., 2020; McLaughlin et al., 2015). Neutral faces are ambiguous stimuli, and are often interpreted as negative by children (Tottenham et al., 2009). While no explicit instructions were given to regulate emotions in this task, it is plausible that children who were spanked were less likely to engage these types of effortful regulation strategies in response to the ambiguous neutral faces than their never-spanked peers.
We also observed heightened activation among children who were spanked in a wide swath of the dorsomedial PFC and bilateral frontal pole. These regions are part of the default mode network, which is involved in a wide range of social-cognitive processes including autobiographical memory as well as mentalizing, theory of mind, and other aspects of social information processing more broadly (Buckner, Andrews-Hanna, & Schacter, 2008; Buckner & DiNicola, 2019). Fearful faces are a signal of potential danger in the environment. Therefore, this pattern could reflect that spanked children devote greater attentional resources to processing the mental state of others expressing fear, perhaps in the service of understanding the source of that fear, due to greater vigilance to potential threats in the environment. Such a pattern is consistent with evidence that exposure to violence is associated with enhanced perceptual sensitivity and attention to threat cues relative to neutral cues (McCoy, Roy, & Raver, 2016; Pollak, Cicchetti, Hornung, & Reed, 2000; Pollak & Sinha, 2002; Pollak & Tolley-Schell, 2003), which may contribute to greater vigilance toward possible threats in the immediate environment. This heightened vigilance may be adaptive in the short term, as it increases the salience of threatening emotional information in ways that may allow children exposed to violence to more readily identify potential threats and mobilize defensive responses in order to avoid harm (McCoy et al., 2016; McLaughlin et al., 2014). However, these responses are likely to be maladaptive in the long-term, as they may promote elevated emotional reactivity, difficulties with emotion regulation, hostile attribution biases, and increased risk for psychopathology (Dodge, 1993; Heleniak, Jenness, Van der Stoep, McCauley, & McLaughlin, 2016; McLaughlin & Lambert, 2017; Weissman et al., 2019).
Increased activation to fearful faces in the same areas of the mPFC have been observed previously in adolescents exposed to physical abuse (Hart et al., 2018) and adolescent girls with a history of violent victimization (Cisler, Steele, Smitherman, Lenow, & Kilts, 2013). Furthermore, the brain regions where activation to fearful compared to neutral faces was higher among children who had been spanked relative to children who had not been spanked also overlapped considerably with the regions where reductions in regional gray matter volume have previously been observed in young adults exposed to harsh corporal punishment (Tomoda et al., 2009). Reductions in gray matter volume in the same regions of the mPFC have also been observed in children exposed to abuse (Edmiston et al., 2011; Hanson et al., 2010) and community violence (Butler et al., 2018). These results suggest that spanking may influence children’s neural response to emotional cues in the same way as more severe forms of violence and in the same brain regions where brain structure is altered following more severe corporal punishment and other forms of violence exposure. In other words, the neurodevelopmental consequences of corporal punishment as compared to abuse may be a difference more of degree than type, as predicted by the dimensional model of adversity (McLaughlin et al., 2014). Indeed, we observed no differences here between children who were spanked from those who were more severely abused. However, these findings should be interpreted with caution given the relatively small number of children who experienced abuse in our sample. These results are broadly consistent with observational studies linking spanking with externalizing and internalizing behavior problems in a qualitatively similar manner as more severe physical abuse (Gershoff, 2002; Gershoff & Grogan-Kaylor, 2016).
Surprisingly, spanking was not associated with heightened reactivity in the amygdala or anterior insula. Increased reactivity of the amygdala and insula to emotional cues is often observed in studies of violence exposure or other forms of childhood maltreatment (McCrory et al., 2011; McLaughlin et al., 2015). Although the anterior insula, is a key node of the salience network and is frequently co-activated with dorsal ACC, including in this study, significant differences in insula activation were not observed. These null findings may be a product of task design. This study’s paradigm did not constrain attention given that prior evidence indicates that attentional constraints caused by task demands produce lower amygdala activation (Costafreda, Brammer, David, & Fu, 2008), and faces were displayed for only 300 ms each. A prior study found that adults exposed to childhood adversity had greater amygdala reactivity to fearful and angry faces when attention was constrained, but lower amygdala reactivity when it was not (Taylor, Eisenberger, Saxbe, Lehman, & Lieberman, 2006). Furthermore, this study included fearful, but not angry, faces. Although fearful face expressions indicate the presence of a potential threat in the environment, as reflected in another person’s fear or distress, the fearful expression itself is not threatening in the way an angry expression is. Alternatively, this finding may suggest that differences in salience network responses to threat cues following corporal punishment are more constrained than following more severe forms of violence. These are important questions to evaluate in future studies.
Limitations
This study has several strengths but also some limitations. First, while we controlled for children’s age, gender, and income-to-needs ratio in the analysis, we could not rule out all potential confounders, so it is not possible to draw causal conclusions. Second, a limitation that is common to the corporal punishment literature (Gershoff & Grogan-Kaylor, 2016), is that it was not possible to measure the severity of spanking or identify the person who spanked the child, which are factors that may relate to different developmental outcomes. Third, we assessed spanking using child report, which could involve under-reporting. However, it is important to note that classifying children who were spanked as nonspanked would have biased our results toward the null hypothesis. Finally, more research is warranted to understand whether neural mechanisms can explain the association between spanking and cognitive and behavioral problems that have been associated with spanking in prior studies.
Conclusion
This study complements previous research linking harsh forms of corporal punishment to atypical structural brain development (Tomoda et al., 2009), and reveals that spanking is linked to atypical brain functioning in regions known to be influenced by more severe forms of physical and sexual abuse. Growing evidence suggests that spanking is associated with deleterious cognitive and behavioral outcomes and changes in the neural processing of threatening emotional stimuli in children. The United States and other countries around the world should discourage the use of corporal punishment through public education and legal prohibition, following the Convention on the Rights of the Child, the United Nations Sustainable Development Goals, and the robust scientific evidence on the harmful consequences of corporal punishment.
Supplementary Material
Appendix S1. Sensitivity analysis.
Acknowledgments
This research was supported by the National Institute of Mental Health, under Grant R01-MH106482.
Contributor Information
Margaret A. Sheridan, University of North Carolina, Chapel Hill
Liliana Lengua, University of Washington.
Katie A. McLaughlin, Harvard University
References
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, & Gee JC (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage, 54(3), 2033–2044. 10.1016/j.neuroimage.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, & Handelsman L (1997). Validity of the childhood trauma questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 340–348. 10.1097/00004583-199703000-00012 [DOI] [PubMed] [Google Scholar]
- Bifulco A, Brown GW, & Harris TO (1994). Childhood Experience of Care and Abuse (CECA): A Retrospective Interview Measure. Journal of Child Psychology and Psychiatry, 35, 1419–1435. 10.1111/j.1469-7610.1994.tb01284.x [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF, McAdoo HP, & García Coll C (2001). The home environments of children in the United States part I: Variations by age, ethnicity, and poverty status. Child Development, 72, 1844–1867. 10.1111/1467-8624.t01-1-00382 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: Anatomy, function, and relevance to disease. In Kingstone A, & Miller MB (Eds.), Annals of the New York Academy of Sciences: Vol. 1124. The year in cognitive neuroscience 2008 (pp. 1–38). Oxford, UK: Blackwell. [DOI] [PubMed] [Google Scholar]
- Buckner RL, & DiNicola LM (2019). The brain’s default network: Updated anatomy, physiology and evolving insights. Nature Reviews Neuroscience, 20, 593–608. 10.1038/s41583-019-0212-7 [DOI] [PubMed] [Google Scholar]
- Bugental DB, Martorell GA, & Barraza V (2003). The hormonal costs of subtle forms of infant maltreatment. Hormones and Behavior, 43, 237–244. 10.1016/S0018-506X(02)00008-9 [DOI] [PubMed] [Google Scholar]
- Butler O, Yang X-F, Laube C, Kühn S, & Immordino-Yang MH (2018). Community violence exposure correlates with smaller gray matter volume and lower IQ in urban adolescents. Human Brain Mapping, 39(5), 2088–2097. 10.1002/hbm.23988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Steele JS, Smitherman S, Lenow JK, & Kilts CD (2013). Neural processing correlates of assaultive violence exposure and PTSD symptoms during implicit threat processing: A network-level analysis among adolescent girls. Psychiatry Research: Neuroimaging, 214, 238–246. 10.1016/j.pscychresns.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convention on the Rights of the Child. (2006) General comment No. 8: The right of the child to protection from corporal punishment and other cruel or degrading forms of punishment. CRC/C/GC/8. Geneva, Switzerland: UN OHCHR—Committee on the Rights of the Child. [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, & Fu CHY (2008). Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 10.1016/j.brainresrev.2007.10.012 [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, & Taylor PA (2017). fMRI clustering and false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 114, E3370–E3371. 10.1073/pnas.1614961114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuartas J, McCoy DC, Rey-Guerra C, Britto P, Beatriz E, & Salhi C (2019). Early childhood exposure to non-violent discipline and physical and psychological aggression in low- and middle-income countries: National, regional, and global prevalence estimates. Child Abuse & Neglect, 92, 93–105. 10.1016/j.chiabu.2019.03.021 [DOI] [PubMed] [Google Scholar]
- Dodge KA (1993). Social-cognitive mechanisms in the development of conduct disorder and depression. Annual Review of Psychology, 44, 559–584. 10.1146/annurev.ps.44.020193.003015 [DOI] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, & Blumberg HP (2011). Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Archives of Pediatrics & Adolescent Medicine, 165, 1069–1077. 10.1001/archpediatrics.2011.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113, 7900–7905. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelhor D, Hamby SL, Ormrod R, & Turner H (2005). The Juvenile Victimization Questionnaire: Reliability, validity, and national norms. Child Abuse and Neglect. 10.1016/j.chiabu.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Finkelhor D, Turner H, Wormuth BK, Vanderminden J, & Hamby S (2019). Corporal punishment: Current rates from a national survey. Journal of Child and Family Studies, 28, 1991–1997. 10.1007/s10826-019-01426-4 [DOI] [Google Scholar]
- Ganzel BL, Kim P, Gilmore H, Tottenham N, & Temple E (2013). Stress and the healthy adolescent brain: Evidence for the neural embedding of life events. Development and Psychopathology, 25(4Pt.1), 879–889. 10.1017/S0954579413000242 [DOI] [PubMed] [Google Scholar]
- Gard AM, Waller R, Shaw DS, Forbes EE, Hariri AR, & Hyde LW (2017). The long reach of early adversity: Parenting, stress, and neural pathways to antisocial behavior in adulthood. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2, 582–590. 10.1016/j.bpsc.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoff ET (2002). Corporal punishment by parents and associated child behaviors and experiences: A meta-analytic and theoretical review. Psychological Bulletin, 128, 539–579. 10.1037/0033-2909.128.4.539 [DOI] [PubMed] [Google Scholar]
- Gershoff ET (2016). Should parents’ physical punishment of children be considered a source of toxic stress that affects brain development? Family Relations, 65, 151–162. 10.1111/fare.12177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoff ET, & Grogan-Kaylor A (2016). Spanking and child outcomes: Old controversies and new meta-analyses. Journal of Family Psychology, 30, 453–469. 10.1037/fam0000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Initiative to End Corporal Punishment of Children. (2021). States which have prohibited all corporal punishment of children. Retrieved from http://www.endcorporalpunishment.org/progress/prohibiting-states/
- Goldin PR, McRae K, Ramel W, & Gross JJ (2008). The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry, 63, 577–586. 10.1016/j.biopsych.2007.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, & Pollak SD (2010). Early stress is associated with alterations in the orbito-frontal cortex: A tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience, 30, 7466–7472. 10.1523/JNEUROSCI.0859-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Lim L, Mehta MA, Simmons A, Mirza KAH, & Rubia K (2018). Altered fear processing in adolescents with a history of severe childhood maltreatment: An fMRI study. Psychological Medicine, 48, 1092–1101. 10.1017/S0033291716003585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein TC, & Monk CS (2017). Research Review: Neural response to threat in children, adolescents, and adults after child maltreatment—A quantitative meta-analysis. Journal of Child Psychology and Psychiatry and Allied Disciplines, 58, 222–230. 10.1111/jcpp.12651 [DOI] [PubMed] [Google Scholar]
- Heleniak C, Jenness J, Van der Stoep A, McCauley E, & McLaughlin KA (2016). Childhood maltreatment exposure and disruptions in emotion regulation: A transdiagnostic pathway to adolescent internalizing and externalizing psychopathology. Cognitive Therapy and Research, 40, 394–415. 10.1007/s10608-015-9735-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness JL, Peverill M, Miller AB, Heleniak C, Robertson MM, Sambrook KA, … McLaughlin KA (2020). Alterations in neural circuits underlying emotion regulation following child maltreatment: A mechanism underlying trauma-related psychopathology. Psychological Medicine, 1–10. 10.1017/S0033291720000641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Bongers A, & Wessa M (2010). How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex, 21, 1379–1388. 10.1093/cercor/bhq216 [DOI] [PubMed] [Google Scholar]
- Lambert HK, King KM, Monahan KC, & McLaughlin KA (2017). Differential associations of threat and deprivation with emotion regulation and cognitive control in adolescence. Development and Psychopathology, 29, 929–940. 10.1017/S0954579416000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua LJ, Moran L, Zalewski M, Ruberry EJ, Kiff CJ, & Thompson S (2015). Relations of growth in effortful control to family income, cumulative risk, and adjustment in preschool-age children. Journal of Abnormal Child Psychology, 43, 705–720. 10.1007/s10802-014-9941-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy DC, Roy A, & Raver C (2016). Neighborhood crime as a predictor of individual differences in emotional processing and regulation. Developmental Science, 19, 164–174. 10.1111/desc.12287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Kelly PA, Bird G, Sebastian CL, Mechelli A, … Viding E (2013). Amygdala activation in maltreated children during preattentive emotional processing. The British Journal of Psychiatry, 202, 269–276. 10.1192/bjp.bp.112.116624 [DOI] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, & Viding E (2011). Heightened neural reactivity to threat in child victims of family violence. Current Biology. 10.1016/j.cub.2011.10.015 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, & Lambert HK (2017). Child trauma exposure and psychopathology: Mechanisms of risk and resilience. Current Opinion in Psychology, 14, 29–34. 10.1016/j.copsyc.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, & Sheridan MA (2015). Child maltreatment and neural systems underlying emotion regulation. Journal of the American Academy of Child and Adolescent Psychiatry, 54, 753–762. 10.1016/j.jaac.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014). Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews, 47, 578–591. 10.1016/j.neubiorev.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Weissman D, & Bitr an D (2019). Childhood adversity and neural development: A systematic review. Annual Review of Developmental Psychology, 1, 277–312. 10.1146/annurev-devpsych-121318-084950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15, 483–506. 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Hornung K, & Reed A (2000). Recognizing emotion in faces: Developmental effects of child abuse and neglect. Developmental Psychology, 36, 679–688. 10.1037/0012-1649.36.5.679 [DOI] [PubMed] [Google Scholar]
- Pollak SD, & Sinha P (2002). Effects of early experience on children’s recognition of facial displays of emotion. Developmental Psychology, 38, 784–791. 10.1037/0012-1649.38.5.784 [DOI] [PubMed] [Google Scholar]
- Pollak SD, & Tolley-Schell SA (2003). Selective attention to facial emotion in physically abused children. Journal of Abnormal Psychology, 112, 323–338. 10.1037/0021-843X.112.3.323 [DOI] [PubMed] [Google Scholar]
- Pozzi E, Simmons JG, Bousman CA, Vijayakumar N, Bray KO, Dandash O, … Whittle SL (2020). The influence of maternal parenting style on the neural correlates of emotion processing in children. Journal of the American Academy of Child & Adolescent Psychiatry, 59, 274–282. 10.1016/j.jaac.2019.01.018 [DOI] [PubMed] [Google Scholar]
- Raschle N, Zuk J, Ortiz-Mantilla S, Sliva DD, Franceschi A, Grant PE, … Gaab N (2012). Pediatric neuroimaging in early childhood and infancy: Challenges and practical guidelines. Annals of the New York Academy of Sciences, 1252, 43–50. 10.1111/j.1749-6632.2012.06457.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviv A, Erel O, Fox NA, Leavitt LA, Raviv A, Dar I, … Greenbaum CW (2001). Individual measurement of exposure to everyday violence among elementary schoolchildren across various settings. Journal of Community Psychology, 29, 117–140. [DOI] [Google Scholar]
- R Core Team (2016). R: A Language and Environment for Statistical Computing. Vienna, Austria. Retrieved from: https://www.R-project.org/. [Google Scholar]
- Ryan R, Kalil A, Ziol-Guest K, & Padilla C (2016). Socioeconomic gaps in parent’s discipline strategies from 1998 to 2011. Pediatrics, 138. 10.1542/peds.2016-0720 [DOI] [PubMed] [Google Scholar]
- Sheridan MA, & McLaughlin KA (2014). Dimensions of early experience and neural development: Deprivation and threat. Trends in Cognitive Sciences, 18, 580–585. 10.1016/j.tics.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Peverill M, Finn AS, & McLaughlin KA (2017). Dimensions of childhood adversity have distinct associations with neural systems underlying executive functioning. Development and Psychopathology, 29, 1777–1794. 10.1017/S0954579417001390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Weber J, Wager TD, & Ochsner KN (2014). Bad and worse: Neural systems underlying reappraisal of high- and low-intensity negative emotions. Social Cognitive and Affective Neuroscience, 10, 172–179. 10.1093/scan/nsu043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, & Whalen PJ (2004). Human amygdala responses during presentation of happy and neutral faces: Correlations with state anxiety. Biological Psychiatry. 10.1016/j.biopsych.2004.01.007 [DOI] [PubMed] [Google Scholar]
- Steinberg AM, Brymer MJ, Kim S, Briggs EC, Ippen CG, Ostrowski SA, … Pynoos RS (2013). Psychometric properties of the UCLA PTSD reaction index: Part I. Journal of Traumatic Stress. 10.1002/jts.21780 [DOI] [PubMed] [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, & Lieberman MD (2006). Neural responses to emotional stimuli are associated with childhood family stress. Biological Psychiatry, 60, 296–301. 10.1016/J.BIOPSYCH.2005.09.027 [DOI] [PubMed] [Google Scholar]
- Tomoda A, Suzuki H, Rabi K, Sheu Y-S, Polcari A, & Teicher MH (2009). Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. NeuroImage, 47, T66–T71. 10.1016/j.neuroimage.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Phuong J, Flannery J, Gabard-Durnam L, & Goff B (2013). A negativity bias for ambiguous facial-expression valence during childhood: Converging evidence from behavior and facial corrugator muscle responses. Emotion, 13, 92–103. 10.1037/a0029431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, … Nelson C (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168, 242–249. 10.1016/J.PSYCHRES.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DG, Bitran D, Miller AB, Schaefer JD, Sheridan MA, & McLaughlin KA (2019). Difficulties with emotion regulation as a transdiagnostic mechanism linking child maltreatment with the emergence of psychopathology. Development and Psychopathology, 31, 899–915. 10.1017/S0954579419000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, & Smith SM (2004). Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage, 21(4), 1732–1747. 10.1016/j.neuroimage.2003.12.023 [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, & Smith SM (2001). Temporal Autocorrelation in Univariate Linear Modeling of FMRI Data. NeuroImage, 14(6), 1370–1386. 10.1006/nimg.2001.0931 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Sensitivity analysis.


