Abstract
Objective:
To estimate the effect of antenatal corticosteroid (ACS) administration on neonatal mortality and morbidity in preterm small-for-gestational age (SGA) infants through a systematic review and meta-analysis.
Data sources:
A predefined, systematic search was conducted through Ovid Medline, Embase, Scopus, Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials, World Health Organization International Clinical Trial Registry Portal, and ClinicalTrials.gov yielding 5,324 articles from 1970–2019.
Study eligibility criteria:
Eligible studies compared neonatal morbidity and/or mortality among SGA infants delivered preterm who received ACS to those who did not.
Study appraisal and synthesis methods:
The primary outcome was neonatal mortality. Secondary outcomes were respiratory distress syndrome (RDS), necrotizing enterocolitis (NEC), intraventricular hemorrhage and/or periventricular leukomalacia (IVH and/or PVL), bronchopulmonary dysplasia or chronic lung disease of prematurity (BPD or CLD), or neonatal sepsis. We assessed heterogeneity via Higgins I2 and Cochrane’s Q test, and calculated pooled odds ratios (OR) with 95% confidence intervals (CI) using random effects models.
Results:
Sixteen observational cohort and case-control studies published from 1995–2018 met selection criteria for the systematic review and included 8,989 preterm SGA infants. ACS administration was explicitly reported among 8,376 SGA infants; 4,631 (55.3%) received ACS and 3,741 (44.7%) did not. Thirteen studies including 6,387 preterm SGA infants were then included in the meta-analysis. Neonatal mortality was significantly lower among infants who received ACS compared to those who did not (12 studies: 12.8% vs. 15.1%, pooled odds ratio [OR] 0.63 [95% CI 0.46–0.86]), with significant heterogeneity between studies (I2=55.1%, p=0.011). There was no significant difference in RDS (12 studies: OR 0.89 [95% CI 0.69–1.15]), NEC (7 studies: OR 0.93 [95% CI 0.70–1.22]), IVH and/or PVL (10 studies: OR 0.82 [95% CI 0.56–1.20]), BPD or CLD (8 studies: OR 1.11 [95% CI 0.88–1.41]), or neonatal sepsis (6 studies: OR 1.13 [95% CI 0.86–1.49]).
Conclusions:
These data show that ACS reduces neonatal mortality in SGA infants delivered preterm, with no apparent effect on neonatal morbidity. This supports the use of ACS to reduce neonatal mortality in pregnancies with SGA infants at risk for preterm birth.
Keywords: small-for-gestational age, fetal growth restriction, antenatal corticosteroids, neonatal morbidity, neonatal mortality
Condensation
Antenatal corticosteroids reduce neonatal mortality in SGA infants delivered preterm, with no apparent effect on neonatal morbidity.
Introduction
Small-for-gestational age (SGA) is commonly defined as birthweight less than the tenth percentile. SGA infants can be either constitutionally small or pathologically growth-restricted antenatally.1–2 Clinically, it can be difficult to differentiate the etiology of FGR (fetal growth restriction). Approximately 3 to 7% of newborns are affected by pathologic FGR, a major risk factor for preterm birth, and the incidence of FGR increases with increasing prematurity.3–5 FGR in a preterm neonate specifically carries an increased risk of perinatal morbidity and mortality.5,6
Administration of antenatal corticosteroids (ACS) has become the standard of care in the setting of anticipated preterm delivery in order to prevent neonatal morbidity and mortality. ACS has been shown to reduce neonatal mortality by 31% in appropriate-for-gestational age (AGA) infants, with efficacy demonstrated specifically in reducing rates of respiratory distress syndrome, intraventricular hemorrhage and necrotizing enterocolitis, among other neonatal outcomes.7–9 However, large-scale prospective studies evaluating the effect of ACS on preterm birth outcomes have not made small-for-gestational age (SGA) infants a primary population of focus, with data for this population limited to mostly retrospective studies. Furthermore, clinical management related to ACS administration in pregnancies with SGA infants has wide variation largely guided by expert opinion without an evidence-based consensus.
Due to pathologic intrauterine stress, SGA infants may be exposed to higher levels of endogenous corticosteroids at baseline as a result of multiple mechanisms. These mechanisms include increased fetal adrenal cortisol production, compromised ability to remove corticosteroids through the blood brain barrier or placenta, and reduced ability to block the passage of maternal cortisol across the placenta.10–18 As SGA infants are already exposed to higher levels of endogenous steroids, the additional administration of exogenous ACS prior to impending preterm delivery may not offer additional benefit. In fact, exposure to single or repeated courses of corticosteroids in utero has been associated with reduced fetal growth; impaired cardiovascular and brain development; and impaired gas exchange and physiologic adaptive mechanisms in the growth-restricted neonate.10–18 Administration of exogenous ACS may ultimately alter the ability of an SGA infant to compensate for intrauterine stress caused by placental insufficiency.18 As a result, some researchers have postulated that administration of exogenous steroids may even be detrimental to SGA infants2.
Objective
Given limited and conflicting evidence guiding the use of ACS in SGA infants, the present study aims to summarize the totality of evidence on ACS administration in SGA infants at risk for preterm delivery. We performed a systematic literature review and meta-analysis to estimate the effect of ACS on neonatal mortality and morbidity in preterm SGA infants. We hypothesized that administration of ACS in preterm SGA infants would have limited benefit given adaptive physiologic mechanisms in SGA infants.
Methods
We used a predesigned methodology according to guidelines for Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) and Meta-analyses of Observational Studies in Epidemiology (MOOSE).19,20 The study protocol was registered with PROSPERO (#156264).
Information Sources and Search Strategy
A medical librarian searched published literature for records discussing ACS (i.e. betamethasone, dexamethasone, alternate drug names and suggested synonyms for dexamethasone and betamethasone), and preterm SGA infants. The librarian created search strategies using a combination of keywords and controlled vocabulary in Ovid Medline (1946- present), Embase.com (1947-present), Scopus (1823-present), Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), World Health Organization International Clinical Trial Registry Portal (WHO ICTRP), and Clinicaltrials.gov (1997-present). Animals were excluded using the OVID human filter recommended in Cochrane Handbook for Systematic Reviews of Interventions.21 The filter was translated to exclude animals in Embase and Scopus. All search strategies were completed initially in June 2019, and a total of 10,139 results were exported to EndNote. 5,204 records were deleted after using the deduplication processes described by Bramer et al.22 A total of 4,935 unique records remained in the project library. In addition to these, 35 records were identified in ClinicalTrials.gov, and 24 in World Health Organization International Clinical Trials Registry Portal (WHO ICTRP). A manual search of bibliographies of relevant articles was also performed.
The search was updated in all databases again in May 2020. A total of 10,151 search results were exported from the databases without any date limits and were added to the project Endnote project library (15086). A total of 9,824 duplicates were removed and deleted revealing 330 new citations. Due to the search and site no longer being available, the World Health Organization International Clinical Trials Registry Portal (WHO ICTRP) was not searched in May 2020. All references were exported to an excel workbook for review. Fully reproducible search strategies for each database can be found in the appendix.
Eligibility Criteria and Study Selection
Two investigators (SAB and KEB) independently screened abstracts and articles pertaining to ACS administration that reported on neonatal mortality and/or other perinatal outcomes that contribute to overall neonatal morbidity or mortality in SGA infants, and extracted data from each study. Study corresponding authors were contacted via email in attempt to obtain missing data for outcomes of interest. Discrepancies in coding required agreement between authors (SAB, KEB and MT) to be considered resolved.
Studies were included if they reported on SGA infants delivered preterm that received ACS, either betamethasone or dexamethasone, prior to delivery. Included studies reported on neonatal mortality and/or any of the following adverse perinatal outcomes: respiratory distress syndrome (RDS), bronchopulmonary dysplasia (BPD) or chronic lung disease of prematurity (CLD), necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH) and/or periventricular leukomalacia (PVL), or neonatal sepsis after delivery.
Studies were excluded if they were a review article; included non-human, animal fetuses; did not report on outcomes distinctly for SGA infants according to ACS administration; analyzed combined effect of surfactant and steroids on perinatal outcomes or compared steroids to an alternative intervention; reported on the effect of repeated or “rescue” doses of steroids; included duplicate data previously reported in another publication by the same author; or included multiple gestations. Additionally, studies were excluded from the meta-analysis if they did not report raw data for the included aforementioned neonatal outcomes.
Data Extraction
The primary outcome was neonatal mortality. Secondary outcomes of interest were RDS, BPD or CLD, IVH and/or PVL, NEC and neonatal sepsis, as defined in Supplementary Table 1. Long term childhood neurodevelopmental outcomes were also extracted when available.
For each study, when data were available, we extracted mean maternal age, maternal parity, mean gestational age at delivery, mean birth weight, number of infants delivered via Cesarean section, infant sex, number of infants who received surfactant, number of infants affected by chorioamnionitis, and use of surfactant or mechanical ventilation postnatally. Maternal risk factors and co-morbidities were also extracted, including gestational or pregestational diabetes mellitus and maternal hypertensive disorders (chronic hypertension, pregnancy-induced hypertension, pre-eclampsia, eclampsia or HELLP syndrome). Each of the aforementioned variables was stratified by the number of SGA infants who did or did not receive ACS.
Data Synthesis
Meta-analysis was performed using the metan add-on program in Stata (Stata 2015 Release 12, StataCorp, Texas, USA). Two-by-two contingency tables were created to compare the presence or absence of neonatal mortality or adverse neonatal outcome stratified by ACS administration. Although the majority of studies were cohort studies, we calculated pooled odds ratios (OR) as one case control study was included. Random effects models were used to account for clinical heterogeneity between studies even when statistical heterogeneity was not evident. To further account for heterogeneity related to varied time periods among included studies, we also performed a subgroup analysis for neonatal mortality among studies that evaluated patients up to the year 2010 analyzed separately from those that evaluated patients beyond the year 2010. Forest plots were created to visually assess both effect size and identify outliers.
We estimated heterogeneity across studies and tested its significance using the Higgins I2 statistic and Cochrane’s Q test. I2 of 50% was considered evidence of significant heterogeneity. Publication bias was evaluated visually using funnel plots and asymmetry was tested statistically using Egger’s test.
Assessment of Risk of Bias
Quality assessment to determine risk of bias of included studies was also performed using the Downs and Black assessment tool.23 The checklist is composed of 27 questions, with a total possible score of 28 for randomized and 25 for non-randomized studies. Downs and Black score ranges are given corresponding quality levels: excellent (26–28); good (20–25); fair (15–19); and poor (≤14). Only randomized studies can achieve a quality level of excellent according to the scoring methodology of the Downs and Black checklist. As all studies were observational and not randomized, the maximum quality level of included studies is “good.”
Results
Study Selection
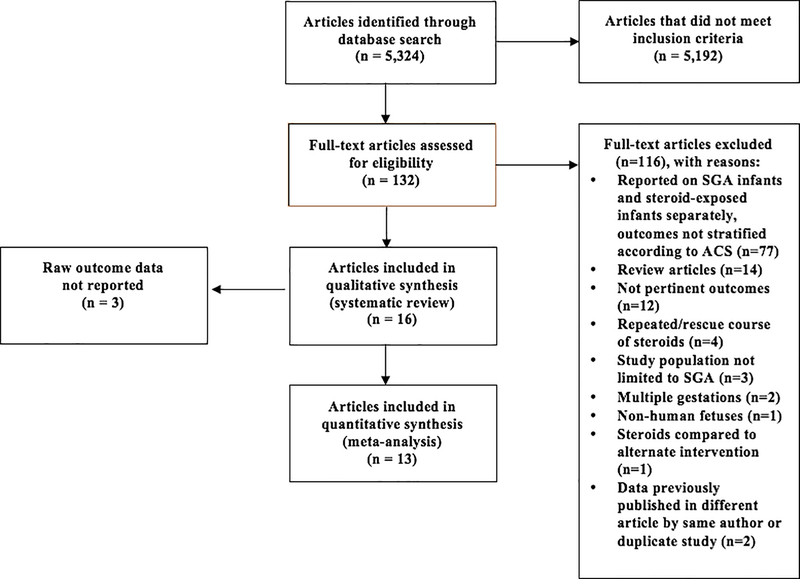
The search yielded 5,324 articles published from 1970–2019. Sixteen observational cohort and case-control studies published from 1995–2018 met inclusion and exclusion criteria and were selected for the systematic review. 24–39 In aggregate, the 16 studies included in the systematic review included 8,989 preterm SGA infants.
Study Characteristics
All studies were observational, with fourteen retrospective cohort studies, one prospective cohort study, and one case-control study included (Table 1). ACS administration was explicitly reported among 8,376 SGA infants; 4,631 (55.3%) received ACS and 3,741 (44.7%) did not. Nine studies reported on type of ACS administered, with betamethasone the most commonly used in 8 studies; two studies included infants who received either betamethasone or dexamethasone. Ten studies specified birth weight less than the tenth percentile in their definition for SGA. Additional maternal and neonatal characteristics in the included studies are detailed in Tables 2 and 3, respectively.
Table 1.
Baseline Study Characteristics
| Author | Publication year (study years) | Country | Study design | Gestational age at delivery (weeks) | Type of steroid | SGA defined | Number of SGA infants (n) | Number of SGA infants receiving ACS (n) | Number of SGA infants not receiving ACS (n) |
|---|---|---|---|---|---|---|---|---|---|
| Kim | 2018 (2009–2016) | Korea | Retrospective cohort | 29–34 | D | BW <10%ile | 82 | 45 | 37 |
| Collaborative Study Group for Respiratory Distress in Preterm Infants (CSGRDSPI) | 2017 (2013–2014) | China | Retrospective cohort | 24–34 | - | - | 602 | - | - |
| Melamed | 2016 (2010–2014) | Canada | Retrospective cohort | 24–33w6d | B or D | BW <10%ile | 918 | 698 | 220 |
| Hoellen | 2016 (2000–2011) | Germany | Retrospective cohort | 22w5d-29w6d | - | early onset FGR <32weeks and BW <10%ile | 92 | 58 | 23 |
| Riskin-Mashiah | 2015 (1995–2012) | Israel | Retrospective cohort | 24–31 | - | BW <10%ile | 1771 | 1246 | 525 |
| Griffin | 2015 (2005–2012) | United States | Retrospective cohort | 22–29w6d | - | BW < 1 %ile | 648 | 271 | 377 |
| Ishikawa | 2015 (2003–2007) | Japan | Retrospective cohort | 22–33w6d | B | BW <10%ile | 1929 | 719 | 1210 |
| Mitsiakos | 2013 (-) | Canada | Retrospective cohort | 24–31w6d | B | BW <10%ile | 149 | 87 | 62 |
| van Stralen | 2009 (2001–2005) | Netherlands | Retrospective cohort | <34 | B | BW <3%ile | 88 | 54 | 34 |
| Torrance | 2007 (1999–2003) | Netherlands | Retrospective cohort | <34 | B | Abnormal PI, UA or MCA dopplers | 165 | 146 | 19 |
| Foix-L’Helias | 2005 (1993–1996) | France | Retrospective cohort | 24–31 | - | FGR antenatally suspected and BW <10%ile | 151 | 96 | 55 |
| Schaap | 2001 (1984–1991) | Netherlands | Case-control | 26–31 | B | fundal height and biometry less than dates; placental dysfunction confirmed on histopathology | 124 | 62 | 62 |
| Bernstein | 1999 (1991–1996) | Canada/United States | Retrospective cohort | 25–30 | - | BW <10%ile | 1720 | 937 | 783 |
| Elimian | 1999 (1990–1997) | United States | Retrospective cohort | - | B | BW <10%ile | 220 | 63 | 157 |
| Ley | 1997 (1985–94) | Sweden | Retrospective cohort | 25–32 | - | Use of growth curve based on longitudinal EFW on US | 234 | 117 | 117 |
| Spinillo | 1995 (1988–1993) | Italy | Prospective cohort | <35 | B or D | BW<10% and AC or HC <10%ile on US | 96 | 32 | 64 |
SGA=small-for-gestational age, ACS=antenatal corticosteroids, B=betamethasone, D=dexamethasone, BW=birth weight, PI=pulsatility index, UA=umbilical artery, MCA=middle cerebral artery, FGR=fetal growth restriction, EFW=estimated fetal weight, US= ultrasound, AC=abdominal circumference, HC=head circumference
Blank cells represent missing data
Table 2.
Maternal Characteristicstab
| Author (publication year) | Number of SGA infants receiving ACS (n) | Number of SGA infants not receiving ACS (n) | Mean maternal age (years ± SD) | Nulliparity (n, %) | Maternal diabetes (n, %) | Maternal hypertensive disorder (n, %) | Chorioamnionitis† (n, %) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +ACS | −ACS | +ACS | −ACS | +ACS | −ACS | +ACS | −ACS | +ACS | −ACS | |||
| Kim (2018) | 45 | 37 | 34 | 33 | 29 (64.6) | 26 (70.3) | 2 (4.4) | 2 (5.4) | 25 (55.6) | 18 (48.6) | 0 (0.0) | 1 (2.7) |
| CSGRDSPI (2017) | - | - | - | - | - | - | - | - | - | - | - | - |
| Melamed (2016) | 698 | 220 | 32.4 ± 6.1 | 30.8 ± 5.3 | 395 (56.6) | 110 (50.0) | 71 (10.2) | 16 (7.3) | 489 (70) | 94 (44) | 0 (excluded) | 0 (excluded) |
| Hoellen (2016) | 58 | 23 | - | - | - | - | - | - | - | - | - | - |
| Riskin-Mashiah (2015) | 1246 | 525 | - | - | - | - | 64 (5.1) | 23 (4.4) | 753 (76.8) | 227 (23.2) | - | - |
| Griffin (2015) | 271 | 377 | - | - | - | - | - | - | - | - | - | - |
| Ishikawa (2015) | 719 | 1210 | 32.0 ± 4.9 | 32.1 ± 5.0 | 437 (60.8) | 746 (61.7) | 8 (1.1) | 25 (2.1) | 318 (44.2) | 605 (50.0) | clinical: 43 (6.0) histologic: 46 (9.1) | clinical: 75 (6.3) histologic: 46 (5.5) |
| Mitsiakos (2013) | 87 | 62 | 30.9 ± 6.3 | 29.5 ± 6.0 | - | - | - | - | 41 (47.1) | 35 (56.4) | 6 (6.9) | 2 (3.2) |
| van Stralen (2009) | 54 | 34 | 30.0 ± 5.9 | 32.8 ±5.5 | 22 (40.7) | 8 (23.5) | - | - | 46 (85.2) | 24 (70.5) | - | - |
| Torrance (2007) | 146 | 19 | - | - | - | - | - | - | 99 (67.8) | 7 (36.8) | - | - |
| Foix-L’Helias (2005) | 96 | 55 | - | - | - | - | - | - | - | - | - | - |
| Schaap (2001) | 62 | 62 | 29.0 ± 5.0 | 29.0 ± 5.0 | 37 (59.6) | 40 (64.5) | - | - | 37 (59.7) | 51 (82.3) | - | - |
| Bernstein (1999) | 937 | 783 | - | - | - | - | - | - | - | - | - | - |
| Elimian (1999) | 63 | 157 | - | - | - | - | - | - | - | - | clinical: 2 (3.2) histologic: 11 (17.5) | clinical: 6 (3.8) histologic: 34 (21.6) |
| Ley (1997) | 117 | 117 | - | - | - | - | - | - | - | - | - | - |
| Spinillo (1995) | 32 | 64 | - | - | - | - | - | - | - | - | - | - |
| Weighted-average | - | - | 32.0 | 31.7 | 58.3% | 59.5% | 5.4% | 3.3% | 59.1% | 48.9% | clinical: 5.6% histologic: 7.3% | clinical: 5.7% histologic: 5.9% |
SGA=small-for-gestational age, ACS=antenatal corticosteroids, SD=standard deviation, CSGRDSPI=Collaborative Study Group for Respiratory Distress in Preterm Infants, +ACS=infants received antenatal corticosteroids, −ACS=infants did not receive antenatal corticosteroids
Chorioamnionitis is clinically diagnosed unless otherwise specified
Blank cells represent missing data
Table 3.
Neonatal Characteristics
| Author (publication year) | Number of SGA infants receiving ACS (n) | Number of SGA infants not receiving ACS (n) | Mean gestational age (weeks ± SD) | Mean birthweight (g) | Cesarean delivery (n, %) | Male sex (n, %) | Post-natal surfactant (n, %) | Mechanical ventilation (n, %) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +ACS | −ACS | +ACS | −ACS | +ACS | −ACS | +ACS | −ACS | +ACS | −ACS | +ACS | −ACS | |||
| Kim (2018) | 45 | 37 | 32.7 (-) | 34.1 (-) | 1190 | 1450 | 40 (88.9) | 34 (91.9) | 24 (53.3) | 11 (29.7) | - | - | 23 (51.1) | 14 (37.8) |
| CSGRDSPI (2017) | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Melamed (2016) | 698 | 220 | 29.8 ± 2.6 | 30.7 ± 2.5 | 959 | 1061 | 636 (91.1) | 178 (80.9) | 369 (52.8) | 114 (51.8) | - | - | 312 (44.7%) | 106 (48%) |
| Hoellen (2016) | 58 | 23 | - | - | - | - | - | - | - | - | - | - | - | - |
| Riskin-Mashiah (2015) | 1246 | 525 | - | - | - | - | 1169 (72.8) | 436 (27.2) | 657 (69.7) | 286 (30.3) | - | - | - | - |
| Griffin (2015) | 271 | 377 | - | - | - | - | - | - | - | - | - | - | - | - |
| Ishikawa (2015) | 719 | 1210 | 29.1 ± 2.6 | 29.7 ± 2.7 | 886 | 959 | 661 (91.9) | 1058 (87.4) | 386 (53.8) | 615 (50.9) | - | - | - | - |
| Mitsiakos (2013) | 87 | 62 | 27.5 ± 2.5 | 27.8 ± 2.5 | 779 | 787 | 73 (83.9) | 53 (85.5) | 34 (39.1) | 615 (50.9) | - | - | - | - |
| van Stralen (2009) | 54 | 34 | 30.0 ± 1.7 | 30.4 ± 1.7 | - | - | 50 (92.6) | 33 (97.1) | 21 (38.9) | 15 (44.1) | 19 (35.2) | 13 (38.2) | 30 (55.6%) | 18 (52.9%) |
| Torrance (2007) | 146 | 19 | 30.3 ± 1.9 | 31.0 ± 1.6 | 899 | 903 | 13 (95.2) | 19 (100.0) | 79 (54.1) | 9 (47.4) | 45 (30.8) | 3 (15.8) | 66 (45.2%) | 11 (57.9%) |
| Foix-L’Helias (2005) | 96 | 55 | - | - | - | - | - | - | - | - | - | - | - | - |
| S chaap (2001) | 62 | 62 | 29.9 ± 1.3 | 30.3 ± 1.0 | 943 | 987 | 62 (100.0) | 62 (100.0) | 39 (62.9) | 39 (62.9) | 4 (6.5) | 9 (14.5) | 31 (50.0%) | 27 (43.5%) |
| Bernstein (1999) | 937 | 783 | - | - | - | - | - | - | - | - | - | - | - | - |
| Elimian (1999) | 63 | 157 | - | - | - | - | - | - | - | - | 17 (27.0) | 31 (19.7) | - | - |
| Ley (1997) | 117 | 117 | - | - | - | - | - | - | - | - | - | - | - | - |
| Spinillo (1995) | 32 | 64 | - | - | - | - | - | - | - | - | - | - | - | - |
| Weighted-average | - | - | 29.5 | 29.9 | 921 | 978 | 92.6% | 86.4% | 52.6% | 51.2% | 26.2% | 20.6% | 46.0% (mean 0.8 days) | 47.3% (mean 2.2 days) |
SGA=small-for-gestational age, ACS=antenatal corticosteroids, SD=standard deviation, CSGRDSPI=Collaborative Study Group for Respiratory Distress in Preterm Infants, +ACS=inJants received antenatal corticosteroids, −ACS=infants did not receive antenatal corticosteroids
Blank cells represent missing data
Table 4 contains weighted-averages for the primary and all secondary outcomes among SGA infants stratified by ACS administration. Fourteen studies reported on overall neonatal mortality, 14 studies reported on RDS, 8 studies reported on BPD or CLD, 7 studies reported on NEC and 6 studies reported on neonatal sepsis. Among 11 studies that reported on IVH and/or PVL, seven studies reported on IVH alone; 4 studies included grade 3 or 4 IVH and/or PVL as a combined outcome.26,32,37,38
Table 4.
Neonatal Outcomes
| Author (Publication Year) | Number of SGA infants receiving (n) | Number of SGA infants not ACS (n) | Neonatal mortality (n, %) | RDS (n, %) | BPD or CLD of Prematurity (n, %) | IVH and/or PVL (n, %) | NEC (n, %) | Neonatal sepsis (n, %) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +ACS | −ACS | +ACS | −ACS | +ACS | −ACS | +ACS | −ACS | +ACS | −ACS | +ACS | −ACS | |||
| Kim (2018) | 45 | 37 | 1 (2.2) | 1 (2.7) | 22 (48.9) | 11 (29.7) | - | - | 4 (8.7) | 1 (2.7) | 5 (11.1) | 2 (5.4) | 9 (20.0) | 3 (8.1) |
| CSGRDSPI (2017) | - | - | 13/77 (16.9) | 17/53 (32.1) | 37/77 (48.1) | 42/53 (79.2) | - | - | 27/248 (10.9) | 13/224 (5.8) | - | - | - | - |
| Melamed (2016) | 698 | 220 | 48 (6.9) | 27 (12.3) | 364 (52.1) | 98 (44.5) | 134 (19.2) | 30 (14.5) | 35 (5.0) | 20 (9.1) | 30 (4.3) | 7 (1.4) | - | - |
| Hoellen (2016) | 58 | 23 | 6 (10.3) | 10 (43.5) | - | - | - | - | - | - | - | - | - | - |
| Riskin- Mashiah (2015) | 1246 | 525 | 240 (19.3) | 169 (32.2) | 840/1236 (68.0) | 365/505 (72.3) | 225/1009 (22.3) | 68/363 (18.7) | IVH: 77/1180 (6.5) PVL: 41/992 (4.1) | IVH: 49/446 (11.0) PVL: 30/347 (8.6) | 101/1235 (8.2) | 55/505 (10.9) | - | - |
| GrifRn† (2015) | 271 | 377 | OR 0.16 (0.07–0.34) | - | - | - | - | - | - | - | - | - | - | |
| Ishikawa (2015) | 719 | 1210 | 56 (7.8) | 92 (7.6) | 341 (47.4) | 510 (42.1) | 194 (27) | 250 (21) | IVH: 54 (7.5) PVL: 11 (1.5) | IVH: 99 (8.2) PVL: 28 (2.3) | 13 (1.8) | 15 (1.2) | 51 (7.1) | 75 (6.2) |
| Mitsiakos (2013) | 87 | 62 | 19 (21.8) | 9 (14.5) | 45 (51.7) | 36 (58.1) | 16 (23.5) | 15 (28.3) | IVH: 3 (3.4) PVL: 1 (1.4) | IVH: 3 (4.8) PVL: 1 (1.8) | 10 (11.5) | 5 (8.0) | 29 (33.3) | 20 (32.3) |
| van Stralen (2009) | 54 | 34 | 5 (9.3) | 4 (11.8) | 22 (40.7) | 17 (50.0) | 6 (11.1) | 4 (11.8) | 4 (7.4) | 1 (2.9) | 3 (5.6) | 2 (5.9) | 33 (61.1) | 24 (70.6) |
| Torrance (2007) | 146 | 19 | 15 (10.3) | 3 (15.8) | 64 (43.8) | 8 (42.1) | 34 (23.3) | 6 (31.6) | - | - | - | - | - | - |
| Foix- L’Helias (2005) | 96 | 55 | 12 (12.5) | 12 (21.8) | 49 (51.0) | 35 (63.6) | 25 (26.4) | 21 (37.8) | - | - | - | - | - | - |
| Schaap (2001) | 62 | 62 | 9 (14.5) | 15 (24.2) | 23 (37.1) | 25 (40.3) | 16 (25.8) | 19 (30.6) | 8 (12.9) | 9 (14.5) | - | - | 12 (19.4) | 12 (19.4) |
| Bernstein† (1999) | 937 | 783 | - | - | OR 0.70 | - | - | - | - | - | - | - | - | |
| Elimian (1999) | 63 | 157 | 5 (7.9) | 11 (7.0) | 17 (26.9) | 38 (24.2) | - | - | 3 (4.8) | 8 (5.1) | 1 (1.6) | 3 (1.9) | 6 (9.5) | 9 (5.7) |
| Ley† (1997) | 117 | 117 | OR 0.53 (95% CI 0.21–1.32) | OR 1.20 (95% CI 0.62–2.34) | - | - | OR 0.72 (95% CI 0.28–1.84) | - | - | - | - | |||
| Spinillo (1995) | 32 | 64 | - | - | 10 (31.3) | 30 (46.9) | - | - | 2/28 (7.1) | 18/58 (31.0) | - | - | - | - |
| Weighted-Average | - | - | 12.8% | 15.1% | 55.3% | 49.0% | 22.6% | 20.4% | 6.8% | 8.8% | 5.6% | 4.0% | 13.6% | 9.2% |
SGA=small-for-gestational age, ACS=antenatal corticosteroids, RDS=respiratory distress syndrome, BPD=bronchopulmonary dysplasia, CLD=chronic lung disease, IVH=intraventricular hemorrhage, PVL=periventricular leukomalacia, NEC=necrotizing enterocolitis, CSGRDSPI=Collaborative Study Group for Respiratory Distress in Preterm Infants, +ACS=infants received antenatal corticosteroids, −ACS=infants did not receive antenatal corticosteroids, OR=odds ratios, CI=confidence interval
Raw data for neonatal outcomes according to ACS administration was not reported for 3 studies; only odds ratios with 95% confidence intervals were reported in these studies comparing SGA infants that received ACS to those that did not receive ACS
Blank cells represent missing data Blank cells represent missing data
Long-term neurodevelopmental outcomes were reported among three studies30,31,35 Two studies reported on severe global delay up to three years of age as determined by a development quotient (DQ) less than 70, or more than two standard deviations below the mean DQ of 100, as defined by the Kyoto Scale of Psychological Development test or the Griffiths test for mental developmental scales40,41 Among infants with long term follow up data, 16.8% (54/321) of infants that received ACS had severe global delay, while 13.5% (71/525) infants that did not receive ACS had severe global delay. Schaap et al. reported abnormal behavior in long-term follow-up at school age of surviving infants, with 43% (21/62) of children who received ACS and 45% (19/45) of children who did not receive ACS exhibiting abnormal behavior.34 However, this study did not report how it classified abnormal behavior.
Meta-analysis and Synthesis of Results
Three studies did not provide raw data for neonatal outcomes according to ACS administration and thus were unable to be included in the meta-analysis. Among these three studies, Griffin et al. reported odds ratios for neonatal mortality; Bernstein et al. reported odds ratios for RDS; and Ley et al. reported odds ratios for neonatal mortality, RDS and IVH and/or PVL (Table 4).29,36,38 The remaining thirteen studies reported raw data for neonatal outcomes among 6,387 preterm SGA infants and were quantitatively synthesized in the meta-analysis.24–28,30–35,37,39
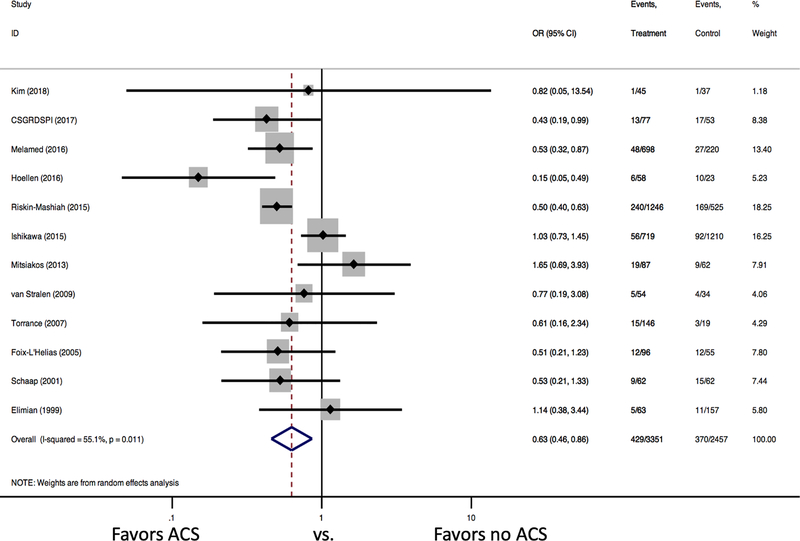
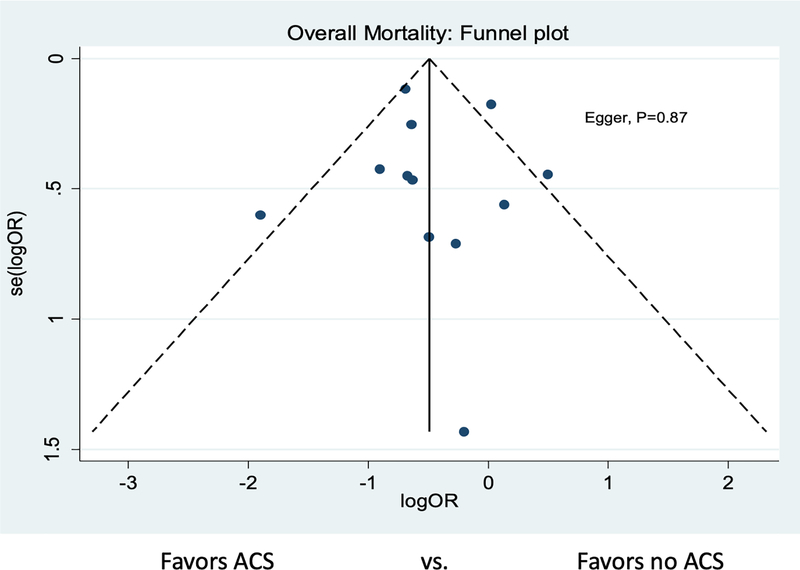
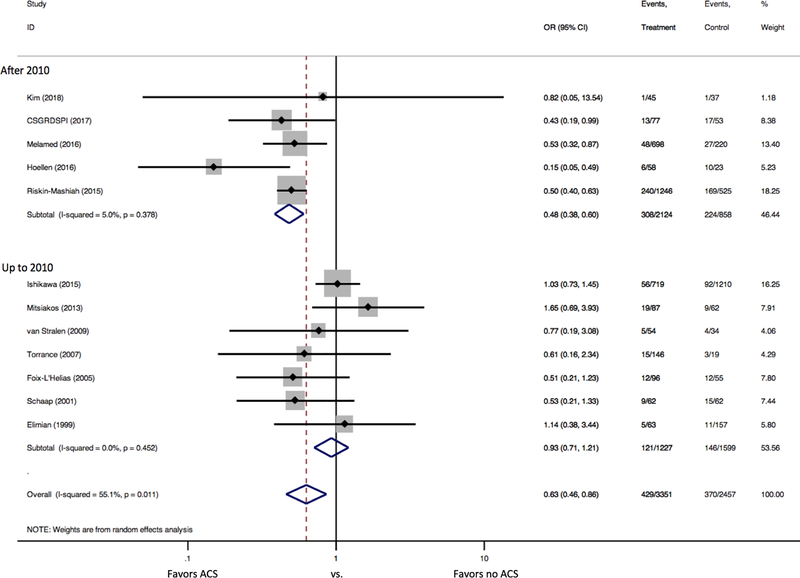
ACS administration was associated with a significant reduction in neonatal mortality (12 studies: 12.8% vs. 15.1%, OR 0.63 [95% confidence interval (CI) 0.46–0.86]). There was significant heterogeneity between studies (I2 =55.1% [p=0.011]) (Figure 2). There was no evidence of publication bias (Figure 3, Egger’s p=0.87). In the subgroup analysis by study year, no significant difference in mortality was detected among studies that followed patients up to 2010 (OR 0.93 [95% CI 0.71, 1.21], I2=0.0% [p=0.452], 7 studies), but a significant reduction in mortality was found among infants who received ACS among studies that followed patients after 2010 (OR 0.48 [95% CI 0.38, 0.60], I2=5.0% [p=0.378]; 5 studies, Figure 4).
Figure 2. Forest Plots for Neonatal Mortality.
Forest plot demonstrates a significant reduction in neonatal mortality for SGA infants that received ACS.
Figure 3. Funnel Plot for Publication Bias for Overall Mortality.
Funnel plot demonstrates symmetry for studies that reported overall mortality, suggesting a lack of publication bias.
Figure 4. Forest Plots for Neonatal Mortality by Study Year.
Forest plot demonstrates a significant reduction in neonatal mortality for SGA infants that received ACS among studies that followed patients after 2010, but no significant difference in mortality among studies that followed patients up to 2010.
Among the secondary outcomes, there was no significant difference in RDS (12 studies: OR 0.89 [95% CI 0.69–1.15], I=66.7% [p=0.001], Supplementary Figure 1), NEC (7 studies: OR 0.93 [95% CI 0.70–1.22], I2=0.0% [p=0.447], Supplementary Figure 2), or IVH and/or PVL (10 studies: OR 0.82 [95% CI 0.56–1.20], I2=53.1% [p=0.024], Supplementary Figure 3). Among the 3 studies that reported on individual values for IVH and PVL, only the values for IVH were included in the forest plot for IVH and/or PVL as IVH was more common in these studies.30–32 Significant heterogeneity was seen in studies reporting RDS and IVH and/or PVL, as reflected by the I2 statistic. There was no significant difference in risk of BPD or CLD (8 studies: OR 1.11 [95% CI 0.88–1.41], I2=40.2% [p=0.111], Supplementary Figure 4) and neonatal sepsis (6 studies: OR 1.13 [95% CI 0.86–1.49], I2=0.0% [p=0.583], Supplementary Figure 5).
Risk of Bias of Included Studies
In the quality assessment of included studies, the majority of studies were assessed to be “fair” quality, with two studies determined to be of “good” quality and two studies of “poor” quality (Table 5). Only two studies performed a power calculation and external validity was unable to be determined in most studies. While lack of randomization decreased the quality of all included studies, all studies achieved at least average (e.g. score of 3 or higher) internal validity in both the bias and confounding assessments by using appropriate statistical regression to adjust for potential confounders in the provided analyses.
Table 5.
Results of the Risk of Bias Assessment using the Downs and Black Assessment Tool
| Author (Publication Year) | Reporting (11)* | External validity (3)* | Bias (7)* | Confounding (6)* | Power (1)* | Total (28)* |
|---|---|---|---|---|---|---|
| Kim (2018) | 10 | 0 | 5 | 3 | 0 | 18 |
| CSGRDSPI (2017) | 5 | 0 | 5 | 2 | 0 | 12 |
| Melamed (2016) | 10 | 2 | 5 | 3 | 0 | 20 |
| Hoellen (2016) | 6 | 0 | 4 | 2 | 0 | 12 |
| Riskin- Mashiah (2015) | 9 | 2 | 5 | 3 | 0 | 19 |
| Griffin (2015) | 7 | 1 | 5 | 3 | 0 | 16 |
| Ishikawa (2015) | 10 | 2 | 5 | 3 | 0 | 20 |
| Mitsiakos (2013) | 10 | 0 | 5 | 3 | 0 | 18 |
| van Stralen (2009) | 10 | 0 | 5 | 3 | 0 | 18 |
| Torrance (2007) | 9 | 0 | 4 | 3 | 0 | 16 |
| Foix-L’Helias (2005) | 8 | 0 | 5 | 3 | 0 | 16 |
| Schaap (2001) | 10 | 0 | 5 | 2 | 1 | 18 |
| Bernstein (1999) | 8 | 1 | 4 | 3 | 0 | 16 |
| Elimian (1999) | 10 | 0 | 5 | 3 | 1 | 19 |
| Ley (1997) | 8 | 2 | 4 | 3 | 0 | 17 |
| Spinillo (1995) | 10 | 0 | 5 | 3 | 0 | 18 |
Maximum number can be scored in that criterion.
Comment
Main Findings and Comparison with with Existing Literature
We found that ACS reduces neonatal mortality in SGA infants delivered preterm, with no apparent effect on individual neonatal morbidities. Our results are similar to those of a 2016 systematic review and meta-analysis of 2,846 SGA infants in eight studies conducted up until 2010 that found that administration of ACS to growth-restricted preterm infants did not improve neonatal morbidity.42 However, in contrast to our findings, the 2016 meta-analysis was unable to detect a reduction in neonatal mortality with ACS. Our meta-analysis includes five studies with 2,982 SGA infants (46.7% of the study population included in the meta-analysis) followed after 2010, 2,124 (71.2%) of whom received ACS. Our meta-analysis provides a more current and comprehensive update to prior available data and supports ACS administration to SGA infants to reduce neonatal mortality.
Of note, studies in our analysis that followed patients beyond 2010 include data predominately from the 2000s to 2010s, whereas studies that followed patients up to 2010 included patient data also from the 1980s and 1990s. Multiple aspects of medical care and technology have evolved over the past few decades in an effort to reduce infant mortality with improved antenatal interventions, neonatal resuscitation, and other postnatal management among preterm infants. While our subgroup analysis seeks to account for these differences according to study period, it is plausible the reduction in mortality seen in studies that followed patients beyond 2010 could be attributed to other advancements in medical care for SGA infants delivered preterm, not solely due to ACS administration.
Strengths and Limitations
Our study offers several strengths. We included a large representative sample of 8,989 preterm SGA infants, most with birthweight less than the tenth percentile. We used a predefined protocol and comprehensive search strategy to limit selection bias. The SGA population as the specific target in our analysis represents a major strength of our study as SGA infants, albeit an important population of clinical interest, have been either excluded from prior large-scale trials evaluating ACS administration and neonatal outcomes or not specifically a population of focused analysis in these trials.
As with all meta-analyses, the limitations of the primary studies must be considered. Eleven of the thirteen included studies included in the meta-analysis were retrospective cohort studies, inherently limited in their study design compared to prospective or randomized controlled trials studies. Most studies did not distinguish etiology of SGA infants, whether constitutional versus pathologic, but the benefits and risks of ACS likely vary according to their physiology. As a result of variable definitions for SGA, we were unable to perform a subgroup analysis based on etiology of SGA or to evaluate for differences in the primary or secondary outcomes for more or less severely growth-restricted infants (for example, less than the fifth percentile versus less than the tenth percentile). Missing data for secondary outcomes, and variable ways in which data were reported or outcomes were defined, also limited data synthesis. Gestational age at delivery was highly variable and individual studies included neonates over a broad range of gestational ages, thus limiting our ability to perform subgroup analysis comparing outcomes among very early preterm (less than 28 or 32 weeks’ gestation, for example) versus preterm infants at more advanced gestational ages (32 to 34 weeks’ gestation). Similarly, heterogeneity in type of steroid used, betamethasone versus dexamethasone, limited subgroup analysis to determine which may be preferential in SGA infants. Few studies reported on what percentage of infants, if any, received a rescue course of ACS, nor did they report on the average time interval from ACS administration to infant delivery, specifically how close the timing of ACS administration was within the optimal window of 48 hours to within seven days of delivery. However, five of the sixteen included studies did exclude infants with suboptimal or partial ACS administration less than 24 hours before birth or greater than 7 days before delivery.
Future studies should further evaluate the effect of ACS administration on SGA infants in the late preterm period from 34 to 37 weeks and in non-singleton pregnancies, as data on ACS use in late preterm and multiple gestations is limited. In fact, the majority of studies excluded multiple gestations. More expansive investigation is also needed to further identify the effect of ACS on long term neurodevelopmental childhood outcomes in SGA infants, outcomes among constitutionally versus pathologically growth-restricted infants who receive ACS, and the benefit or harm of repeated or rescue doses of steroids in SGA infants delivered preterm.
Conclusions and Implications
Despite these limitations, our findings suggest ACS administration among preterm SGA infants could be beneficial in reducing neonatal mortality. Our study provides evidence-based support for the continued clinical use of ACS as the standard of care for reduction of neonatal mortality among infants at risk of preterm birth in the next seven days, including the SGA population, in accordance with current guidance set for by the American College of Obstetricians and Gynecologists.43 Although a large randomized-controlled trial (RCT) would provide a higher level of evidence and reduce the effect of bias and heterogeneity on study outcomes, an RCT is likely not feasible to evaluate the effect of ACS administration in SGA infants due to both ethical reasons and patient preference for an intervention that is likely to be beneficial. Our meta-analysis of thirteen observational studies provides the highest level of evidence currently available demonstrating benefit of ACS administration for reducing neonatal mortality in SGA infants at risk of preterm delivery.
Supplementary Material
Supplementary Figure 1. Forest Plot for RDS
Forest plot demonstrates no significant reduction in RDS with ACS administration to SGA infants.
Supplementary Figure 2. Forest Plot for NEC
Forest plot demonstrates no significant reduction in NEC with ACS administration to SGA infants.
Supplementary Figure 3. Forest Plot for IVH and/or PVL
Forest plot demonstrates no significant reduction in IVH and/or PVL with ACS administration to SGA infants.
Supplementary Figure 4. Forest Plot for BPD or CLD
Forest plot demonstrates no significant reduction in BPD or CLD with ACS administration to SGA infants.
Supplementary Figure 5. Forest Plot for Neonatal Sepsis
Forest plot demonstrates no significant reduction in neonatal sepsis with ACS administration to SGA infants.
Figure 1. Flow Chart of the Literature Review.
Flow chart demonstrates the literature search, including inclusion and exclusion of selected studies.
AJOG at a Glance.
A. Why was the study conducted?
Prior literature offers conflicting evidence guiding antenatal corticosteroid (ACS) administration in small-for-gestational age (SGA) infants given their increased endogenous steroid exposure due to pathologic intrauterine stress. The present study estimates the effect of ACS on neonatal mortality and morbidity in preterm SGA infants through a systematic literature review and meta-analysis.
B. What are the key findings?
ACS administration in preterm SGA infants significantly reduces neonatal mortality, with no apparent effect on neonatal morbidity.
C. What does the study add to what is already known?
The SGA population is one of clinical interest that has not been a population of focus in large-scale randomized trials evaluating ACS administration and neonatal outcomes. Our focused analysis on ACS administration in SGA infants provides the highest level of evidence currently available demonstrating benefit of ACS administration for reducing neonatal mortality in SGA infants delivered preterm.
Funding:
SB and KB have no funding sources to disclose. MJS has support from NICHD T32 (5 T32 HD055172-02), Washington University CTSA grant (UL1 TR000448), NIH/NICHD Women’s Reproductive Health Research Career Development Program at Washington University in St. Louis (5K12HD063086-05), and The March of Dimes Prematurity Research Center at Washington University in Saint Louis. MGT has support from NIH/NICHD grants (R01HD086007 and U01 HD077384) and supplemental funding from Acelity. The above funding sources had no role in the study design, collection/analysis/interpretation of data, or manuscript preparation.
Footnotes
Disclosure and Conflict of Interests: The authors report no financial or non-financial competing interests with the data in this manuscript, or other conflict of interest to disclose.
Clinical Trial Registration: Not applicable
Presentation This work was presented as a poster at the Society of Maternal Fetal Medicine 39th Annual Pregnancy Meeting, February 11–16, 2019, Las Vegas, NV.
Disclaimer: Not applicable
Ethics approval and consent to participate: Permission from the Washington University Institutional Review Board was not indicated for this study.
Consent for publication: All authors consent to publication of this data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alberry M, Soothill P. Management of fetal growth restriction. Arch Dis Child Fetal Neonatal Ed 2007;92:F62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ting JY, Kingdom JC, Shah PS. Antenatal glucocorticoids, magnesium sulfate, and mode of birth in preterm fetal small for gestational age. Am J Obstet Gynecol. 2018;218(2S):S818–S828. [DOI] [PubMed] [Google Scholar]
- 3.Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev. 2009;6(Suppl 3):332–336 [PubMed] [Google Scholar]
- 4.Morrison JL, Botting KJ, Soo PS, McGillick EV, Hiscock J, Zhang S, McMillen IC, Orgeig S Antenatal steroids and the IUGR fetus: Are exposure and physiological effects on the lung and cardiovascular system the same as in normally grown fetuses? J. Pregnancy 2012;2012:839656. doi: 10.1155/2012/839656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert WM, Danielsen B. Pregnancy outcomes associated with intrauterine growth restriction. Am J Obstet Gynecol. 2003;188(6):1596–9601. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe HM, Gross TL. increased risk to the growth retarded fetus. in: Gross TL, Sokol RJ, eds. intrauterine growth retardation: a practical approach. Chicago: Year Book Medical Publishers, 1989:111–24. [Google Scholar]
- 7.NIH. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273(5):413–8. [DOI] [PubMed] [Google Scholar]
- 8.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454. [DOI] [PubMed] [Google Scholar]
- 9.Atarod Z, Taghipour M, Roohanizadeh H, Fadavi S, Taghavipour M. Effects of single course and multicourse betamethasone prior to birth in the prognosis of the preterm neonates: A randomized, double-blind placebo-control clinical trial study. J Res Med Sci. 2014; 19(8):715–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Gould JB, Gluck L, Kulovich MV. The relationship between accelerated pulmonary maturity and accelerated neurological maturity in certain chronically stressed pregnancies. Am J Obstet Gynecol. 1977;127(2):181–6. [DOI] [PubMed] [Google Scholar]
- 11.Hawdon JM, Ward Platt MP. Metabolic adaptation in small for gestational age infants. Arch Dis Child 1993;68:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen E, Baerts W, van Bel F. Brain-sparing in intrauterine growth restriction: considerations for the neonatologist. Neonatology 2015;108: 269–76. [DOI] [PubMed] [Google Scholar]
- 13.Meyer K, Lubo Z. Fetal programming of cardiac function and disease. Reprod Sci 2007;14:209–16. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham FG, MacDonald PC, Leveno KJ, Gant NF, Gilstrap LC. Williams Obstetrics. London: Prentice Hall; 1993. [Google Scholar]
- 15.Economides DL, Nicolaides KH, Linton EA, Perry LA, Chard T. Plasma cortisol and adrenocorticotropin in appropriate and small for gestational age fetuses. Fetal Ther. 1988;3(3):158–64. [DOI] [PubMed] [Google Scholar]
- 16.McTernan CL, Draper N, Nicholson H, Chalder SM, Driver P, Hewison M, et al. Reduced placental 11betahydroxysteroid dehydrogenase type 2 mRNA levels in human pregnancies complicated by intrauterine growth restriction: an analysis of possible mechanisms. J Clin Endocrinol Metab. 2001;86(10):4979–83. [DOI] [PubMed] [Google Scholar]
- 17.Miller SL, Chai M, Loose J, Castillo Meléndez M, Walker DW, Jenkin G, et al. The effects of maternal betamethasone administration on the intrauterine growth restricted fetus. Endocrinology. 2007;148(3):1288–95. [DOI] [PubMed] [Google Scholar]
- 18.Hodges RJ, Wallace EM. Mending a growth-restricted fetal heart: should we use glucocorticoids? J Matern Fetal Neonatal Med. 2012;25(11):2149–2153. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, and the PRISMA Group. Preferred Reporting Items for Systematic reviews and Meta-Analyses: the PRISMA Statement. Ann Intern Med 2009;151:264–9. http://www.prisma-statement.org [DOI] [PubMed] [Google Scholar]
- 20.Stroup DF, Berline JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of Observational Studies in Epidemiology: A Proposal for Reporting. JAMA. 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- 22.Bramer WM, Giustini D, de Jonge GB, Holland L, & Bekhuis T De-duplication of database search results for systematic reviews in EndNote. JMLA 2016;104(3):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Downs SH and Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomized and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim WJ, Han YS, Ko HS, Park IY, Shin JC, Wie JH. Antenatal corticosteroids and outcomes of preterm small-for-gestational-age neonates in a single medical center. Obstet Gynecol Sci. 2018;61(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collaborative Study Group for Respiratory Distress Syndrome in Preterm Infants. Effect of antenatal corticosteroids therapy on the mortality and morbidity of small for gestational age infants born at 24–34 completed weeks: a retrospective multicenter study. Zhonghua Er Ke Za Zhi. 2017;55(8):613–618. [DOI] [PubMed] [Google Scholar]
- 26.Melamed N, Pittini A, Barrett J, Jyotsna S, Yoon EW, Lemyre B, Lee SK, Murphy KE, Shah PS. Antenatal corticosteroids and Outcomes of Small-for-Gestational-Age Neonates. Obstet Gynecol. 2016;128(5):1001–1008. [DOI] [PubMed] [Google Scholar]
- 27.Hoellen F, Beckmann AA, Banz-Jansen C, Weichert J, Rody A, Bohlmann MK. Management of Very Early-onset Fetal Growth Restriction: Results from 92 Consecutive Cases. In Vivo. 2016;30(2):123–132 [PubMed] [Google Scholar]
- 28.Riskin-Mashiah S, Riskin A, Bader D, Kugelman A, Boyko V, Lerner-Geva L, et al. Antenatal corticosteroid treatment in singleton, small-for-gestational-age infants born at 24–31 weeks’ gestation: a population-based study. BJOG. 2016; 2016;123(11):1779–86. [DOI] [PubMed] [Google Scholar]
- 29.Griffin U, Lee HC, Profit J, Tancedi DJ. The smallest of the small: short-term outcomes of profoundly growth-restricted and profoundly low birth weight preterm infants. J Perinat. 2015(35):503–510. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa H, Miyazaki K, Ikeda T, Murabayashi N, Hayashi K, Kai A, et al. The Effcts of Antenatal Corticosteroids on Short-and-Long-Term Outcomes in Small-for-Gestational- Age Infants. Int J Med Sci. 2015;12(4):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitsiakos G, Kovacs L, Papageorgiou A. Are antenatal steroids beneficial to severely growth restricted fetuses? J Matern Fetal Neonatal Med. 2016;26(15): 1496–1499. [DOI] [PubMed] [Google Scholar]
- 32.van Stralen G, van der Bos J, Lopriore E, te Pas AB, Bloemenkamp KWM, Walther FJ, et al. No short-term benefits of antenatal corticosteroid treatment in severely preterm growth restricted fetuses: A case-control study. Early Hum Dev. 2009; 85:253–257. [DOI] [PubMed] [Google Scholar]
- 33.Torrance HL, Mulder EJH, Brouwers HAA, van Bel F, Visser GHA. Respiratory outcome in preterm small for gestational age fetuses with or without abnormal umbilical artery Doppler and/or maternal hypertension. J Matern Fetal Neonatal Med. 2007;20(8):613–621. [DOI] [PubMed] [Google Scholar]
- 34.Foix-L’Helias L, Baud O, Lenden R, Kaminski M, Lacaze-Masmonteil T. Benefit of antenatal glucocorticoids according to the cause of very premature birth. Arch Dis Child Fetal Neonatal Ed 2005; 90:F46–F48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaap AH, Wolf H, Bruinse HW, Smolders-de Haas H, van Ertbruggen I, Treffers PE. Effects of Antenatal Corticosteroid Administration on Mortality and Long-term Morbidity in Early Preterm, Growth-Restricted Infants. Obstet Gynecol. 2001; 97(6):954–60. [DOI] [PubMed] [Google Scholar]
- 36.Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol. 2000;182:198–206. [DOI] [PubMed] [Google Scholar]
- 37.Elimian A, Verma U, Canterino J, Shah J, Visintainer P, Tejani N. Effectiveness of antenatal steroids in obstetric subgroups. Obstet Gynecol. 1999; 93(2):174–9. [DOI] [PubMed] [Google Scholar]
- 38.Ley D, Wide-Swensson D, Lindroth M, Svenningsen N, Marsal K. Respiratory distress syndrome in infants with impaired intrauterine growth. Acta Paediatr. 1997;86:1090–6. [DOI] [PubMed] [Google Scholar]
- 39.Spinillo A, Capuzzo E, Ometto A, Stronati M, Baltaro F, Iasci A. Value of antenatal corticosteroid therapy in preterm birth. Early Hum Dev. 1995; 42(1):37–47. [DOI] [PubMed] [Google Scholar]
- 40.Koyama T, Osada H, Tsujii H, et al. Utility of the Kyoto scale of psychological development in cognitive assessment of children with pervasive developmental disorders. Psychiatry Clin Neurosci. 2009; 63: 241–243. [DOI] [PubMed] [Google Scholar]
- 41.Huntley M The Griffiths mental developmental scales. Oxford: The Test Agency Ltd. 1996;114. [Google Scholar]
- 42.Amiya RM, Munde LB, Ota E, Swa T, Oladapo OT, Mori R. Antenatal Corticosteroids for reducing adverse maternal and child outcomes in special populations of women at risk for imminent preterm birth: a systematic review and meta-analysis. PLoS ONE. 2016; PLoS ONE 11(2): e0147604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ACOG. Committee Opinion No. 713 Summary: Antenatal Corticosteroid Therapy for Fetal Maturation. Obstet Gynecol. 2017; 130(2):e102–e109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Forest Plot for RDS
Forest plot demonstrates no significant reduction in RDS with ACS administration to SGA infants.
Supplementary Figure 2. Forest Plot for NEC
Forest plot demonstrates no significant reduction in NEC with ACS administration to SGA infants.
Supplementary Figure 3. Forest Plot for IVH and/or PVL
Forest plot demonstrates no significant reduction in IVH and/or PVL with ACS administration to SGA infants.
Supplementary Figure 4. Forest Plot for BPD or CLD
Forest plot demonstrates no significant reduction in BPD or CLD with ACS administration to SGA infants.
Supplementary Figure 5. Forest Plot for Neonatal Sepsis
Forest plot demonstrates no significant reduction in neonatal sepsis with ACS administration to SGA infants.