Abstract
Glioblastoma cells release extracellular vesicles (EVs), sometimes referred to as microvesicles and exosomes, to transfer immune modulating molecules to immune cells, resulting in an immune privileged microenvironment. Here we discuss the potential EV-mediated mechanisms underlying glioma immune modulation, as well as the technical difficulties in studying these interactions.
Glioblastomas are the most common and lethal intracranial primary malignancies in adults. They are heterogeneous tumors with tumor cells and nonmalignant stromal cells [1]. The stromal population consists of resident brain glial cells, including oligodendrocytes, astrocytes, ependymal cells, and microglia; and infiltrating immune cells, such as myeloid-derived monocytes/macrophages and lymphocytes [1]. Together, the stromal and malignant cells form a microenvironment that in general enables the tumor cells to proliferate and infiltrate [1]. Within this microenvironment, cells communicate through secretion of cytokines and other (soluble) proteins, direct cell–cell contact through gap junctions or nanotubes, and extracellular vesicles (EVs) [1]. EVs is the collective term for nanosized and microsized (~50–10 000 nm) membrane-enclosed vesicles that are released by all cell types [2]. As different cellular pathways can result in the release of EVs, different terminology (e.g., exosomes, microvesicles, ectosomes) has been used for the potential subpopulations of EVs (Figure 1) [2,3]. However, since clear markers for these subpopulations are lacking, current consensus is to use the umbrella term ‘EVs’ [3]. EVs have a similar membrane topology as their cells of origin, and thus (mutant) extracellular domains of transmembrane proteins can be present on the surface of EVs. Simultaneously, donor cell cytosolic components, such as (mutant) proteins, m(i)RNA, and DNA molecules, are contained as cargo inside EVs and can be transferred from donor to recipient cells. This transfer of receptor and/or cargo molecules can induce intracellular signaling in EV recipient cells [4]. During the past 50 years these concepts have been gradually laid bare, starting with the identification of vesicle-like structures around mammalian cells, to the functional intercellular transfer of mRNAs in 2007 [2]. In different types of tumors, including gliomas, EVs transfer oncogenic messages between malignant cells that enhance their migratory capacities and proliferation, and dampen immunological responses [2]. This forum article first focuses on the role of glioma-derived EVs in the establishment of an immune privileged microenvironment, and then discusses technical challenges and future prospects for this field of research.
Figure 1. Extracellular Vesicles as a Mode of Intercellular Communication in Glioma Immunity.
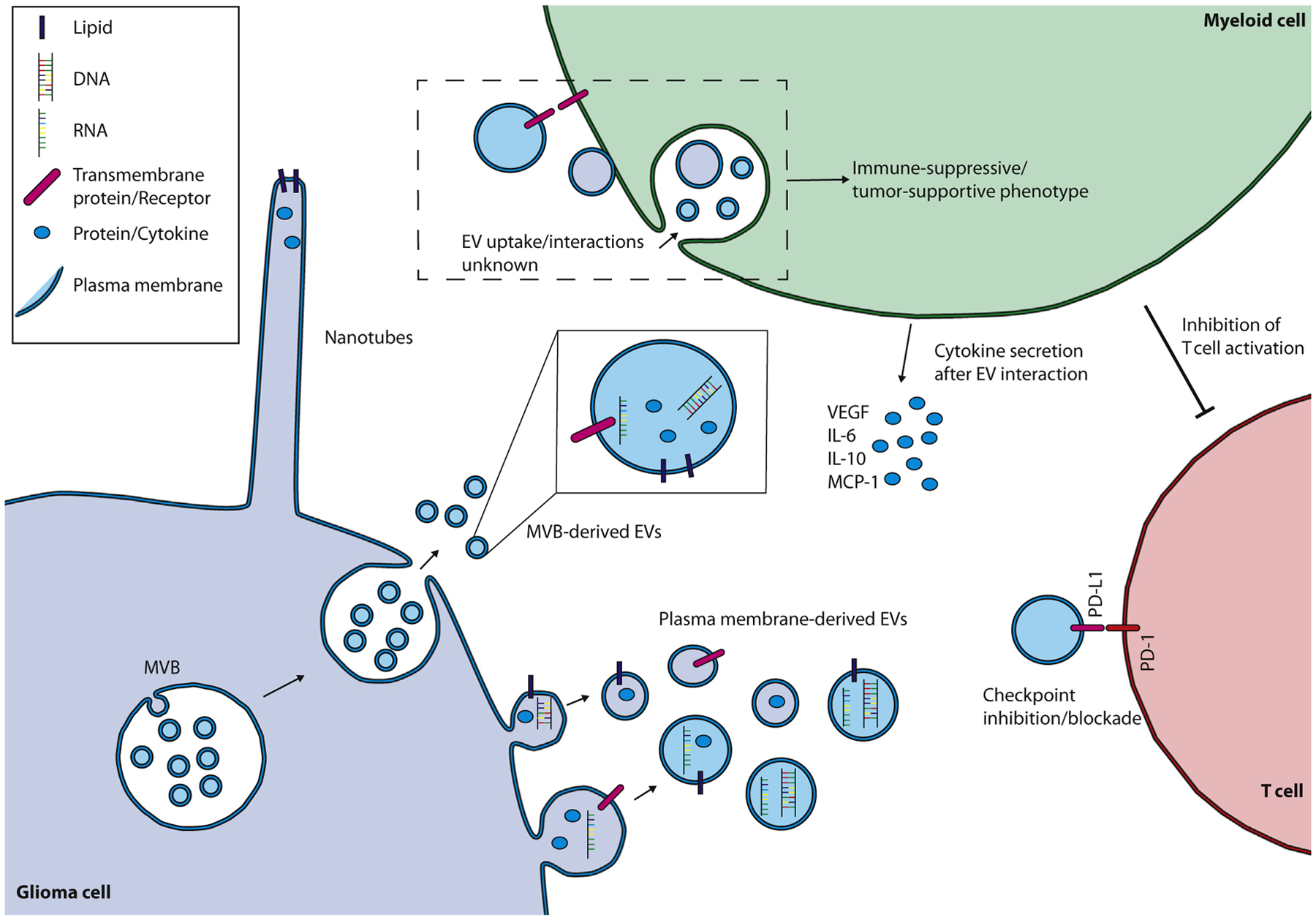
Extracellular vesicles (EVs) can be formed by both the budding of the plasma membrane or through the fusion of a multivesicular body (MVB) with the plasma membrane. Cell–cell contact and the subsequent exchange of cellular components through nanotubes is an alternative method of (local) intercellular communication. EV uptake by a myeloid-derived innate immune cell can change its phenotype into an immune-suppressive, tumor-supportive effector cell, inhibiting T cell activation and supporting tumor growth by secretion of specific cytokines. Direct interaction between glioma EV surface programmed death-ligand 1 (PD-L1) and programmed cell death-1 (PD-1) expressed on T cells is an alternative direct method for glioma EVs to suppress the T cell response. Abbreviations: IL-6, interleukin 6; IL-10, interleukin 10; MCP-1, monocyte chemoattractant protein-1; VEGF, vascular endothelial growth factor.
EVs and Glioma Immunity
One of the first indications that brain tumor-derived EVs could influence the (systemic) immune response was the identification of transforming growth factor (TGF)-β1 in EVs isolated from serum of high-grade glioma patients [5]. As TGF-β1 could not be detected in EVs from healthy controls, this finding suggested loading of TGF-β1 into circulating tumor EVs. EVs derived from high-grade gliomas also contained mutant epidermal growth factor receptor (EGFR); (EGFRvIII). This indicates that at least some of the EVs in the serum from glioma patients are derived from the tumor. TGF-β1 has pleiotropic effects, including stimulation and activation of T cells and monocytes, but in malignancies the effect is mainly immune suppressive [5]. To achieve immune suppression, EV-associated TGF-β1 has to interact with innate and adaptive immune cells. This interaction of glioma EVs with immune cells was identified in subsequent studies. First, proteomic profiling of EVs isolated from glioma cell lines and glioma stem cell-like cultures identified selective enrichment of proteins involved in recruitment of leukocytes and their focal adhesion [6]. These pathways are required for proliferation, movement, and phagocytosis by monocytic leukocytes, and provide indirect evidence of interaction of glioma EVs with immune cells. Evidence for direct interaction, however, came from culture experiments where glioma EVs were added to peripheral blood mononuclear cells (PBMCs) or purified monocytes. Compared with EVs from nonmalignant cells, addition of glioma EVs resulted in increased survival of PBMCs and purified monocytes, as well as their secretion of multiple cytokines, including interleukin 6 (IL-6), IL-10, monocyte chemoattractant protein-1 (MCP-1), and vascular endothelial growth factor (VEGF); [6]. These soluble secreted cytokines have different roles in the tumor microenvironment as IL-6 and IL-10 can both support and reduce tumor growth, MCP-1 attracts myeloid-derived monocytes, and VEGF induces angiogenesis, vital for continued tumor growth [1]. A separate study investigating cytokine release by microglia (brain resident innate immune cells) reported increased levels of cytokines after incubation of microglia with glioma EVs [7]. These studies revealed the potential for direct interaction between glioma EVs and innate immune cells; however, since the spatiotemporal distribution and concentration of EVs in an in vivo glioma are unknown, it is unclear to what extent these in vitro results represent the true EV/innate immune cell interaction. This challenge was elegantly highlighted in a study that showed different and even opposite (decreased versus increased) levels of cytokine production when two different EV concentrations were added to PBMC cultures [8]. However, as the studies discussed previously used different donor cells and employed different EV isolation techniques, direct comparisons between studies may not be possible.
In glioblastoma the adaptive T cell response is dependent on the activation state and the composition of different types of T cells [1]. Similar to cells of the innate immune system, glioma EVs can influence T cells both indirectly, through intermediate myeloid-derived innate immune cells, or directly (Figure 1). Factors associated with T-helper (Th)2 immunity (generally assumed to be a tumor-supportive T cell response) found in EVs in the peripheral blood of glioblastoma patients, led to the hypothesis that glioma-derived EVs can suppress the T cell-mediated adaptive immune response [9]. Specifically, the presence of immunoglobulins IgG2 and IgG4 on patient-derived EVs, together with elevated levels of CD14/CD163-positive monocytes, as well as high levels of colonystimulating factor 2 (CSF2), CSF3, IL-2, IL-4, and IL-13, were considered an indication of Th2 immunity. In addition, it was shown that monocytes after incubation with glioma EVs suppress T cell activation [10]. Although the exact mechanism for the suppression of T cell activation by monocytes after incubation with glioma EV is unknown, it was suggested that glioma EVs induced upregulation of pathways controlled by arginase-1, increased IL-10 secretion, and decreased human leukocyte antigen-DR isotope (HLA-DR) expression [10]. Contrarily to glioma EV induced effects requiring monocytes as intermediates, a direct effect of glioma EVs on T cells has recently been described [11]. In this study, binding of programmed death-ligand 1 (PD-L1) present on the surface of glioblastoma-derived EVs to the programmed cell death-1 (PD-1) receptor on T cells resulted in inhibition of T cell function, a phenotype that was reversed with the addition of anti-PD-1 receptor blockers. PD-L1/PD-1 inhibition of T cells mediated by glioma EVs does not require intermediate monocytes, as another study failed to detect monocytic PD-L1 expression after incubation with glioma EVs [12].
Together, these results describe capacities for glioma EVs to interfere with the adaptive immune response, however, similar to the findings in innate immune cells, all evidence supporting EV-mediated T cell immune suppression is based on in vitro testing, and lacks direct evidence from in vivo experiments.
Concluding Remarks and Future Perspectives
As highlighted earlier, challenges in identifying the role of EVs in glioma immunity result from the paucity of results from in vivo models and the inability to compare different studies, as virtually every publication uses a different EV isolation technique, yielding varying EV purity, concentration, and subpopulation composition (Box 1). To address the lack of standardization, the EV research community has generated a ‘Minimal Information for Studies of Extracellular Vesicles (MISEV)’ guideline that includes strong recommendations and reporting requirements to improve reproducibility and transferability of published results [3].
Box 1. Guidelines for Studying Extracellular Vesicles.
The interest and number of publications relating to EVs has significantly grown in recent years. However, variability in experimental methods currently impacts progression in this field. A number of factors are responsible for this variability. First, cell culture conditions, including methods to harvest EVs, can heavily impact composition and purity of EVs. For example, the presence of fetal calf/bovine serum in culture can introduce contamination with bovine-derived EVs. Additionally, selection of different centrifugation steps can result in isolation of specific EV subpopulations selected based on size and density. Different storage methods of EVs can affect their function and integrity. Another major obstacle is the lack of standardized methods to quantify EVs and robust markers for EV subtypes. An effort to standardize EV research has been made under the guidelines of ‘Minimal Information for Studies of EVs (MISEV)’, in which a number of recommendations are listed to guide and structure EV characterization, separation, isolation, and quantification to improve the reproducibility of EV research [3].
Since the immune response in the glioma microenvironment involves malignant and immune cells, including cells from both the innate and adaptive immune systems, ultimately the effect of EVs needs to be studied in vivo. Although different models have been developed to address this situation, setting up proper conditions and controls remains an issue. For example, researchers attempted to investigate the effect of EVs in vivo by injecting isolated EVs into (tumor bearing) mice (reviewed in [2]). Since the endogenous concentration and spatiotemporal distribution of EVs are unknowns, these attempts can only partially mimic the interactions between EVs and immune cells. Other in vivo strategies have also been developed. For example, optical reporters can be introduced into tumor cells generating EVs in vivo, thus avoiding the injection of EVs. One approach is the introduction of tetraspanin-based pH-sensitive CD63 protein reporters. These reporters are fluorescent only after fusion of the multivesicular body with the plasma membrane, and thus generate fluorescent glioma EVs [13]. Alternatively, palmitoylated-GFP/tdTomato reporters expressed in glioma cells label all cellular membranes, including all EVs released from those cells [14]. In addition, a CRE–lox-based system was used to show that CRE is functionally transferred by EVs from tumor to innate immune cells, resulting in activation of reporters that can be used to track EV uptake [15]. These reporters help to visualize the interaction of glioma EVs with immune cells in vivo and represent an important development for in vivo validation of EV effects observed in vitro. Although promising, these models still do not allow for non-EV effects, such as secreted cytokines that may dominate the glioma–immune interaction, making EVs a bystander rather than an instigator. To control for this, a model that allows for the selective and complete knockout of EV release by glioma cells in vivo would be invaluable in this research. However, since interference in many of the intracellular pathways involved in EV release affects the vitality of the cell, this may not be feasible [2].
Overall, current yet circumstantial evidence describes a role for glioma-derived EVs in the establishment of an immune privileged tumor microenvironment. This framework of evidence now needs to be built upon using novel reproducible in vivo models.
References
- 1.Broekman ML et al. (2018) Multidimensional communication in the microenvirons of glioblastoma. Nat. Rev. Neurol 14, 482–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maas SLN et al. (2017) Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 27, 172–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Théry C et al. (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Nedawi K et al. (2008) Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol 10, 619–624 [DOI] [PubMed] [Google Scholar]
- 5.Graner MW et al. (2009) Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 23, 1541–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vrij J et al. (2015) Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. Int. J. Cancer 137, 1630–1642 [DOI] [PubMed] [Google Scholar]
- 7.van der Vos KE et al. (2016) Directly visualized glioblastoma-derived extracellular vesicles transfer RNA to microglia/macrophages in the brain. Neuro-Oncology 18, 58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellwinkel JE et al. (2016) Glioma-derived extracellular vesicles selectively suppress immune responses. Neuro-Oncology 18, 497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harshyne LA et al. (2016) Serum exosomes and cytokines promote a T-helper cell type 2 environment in the peripheral blood of glioblastoma patients. Neuro-Oncology 18, 206–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domenis R et al. (2017) Systemic T cells immunosuppression of glioma stem cell-derived exosomes is mediated by monocytic myeloid-derived suppressor cells. PLoS ONE 12, e0169932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricklefs FL et al. (2018) Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv 4, eaar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iorgulescu JB et al. (2016) The limited capacity of malignant glioma-derived exosomes to suppress peripheral immune effectors. J. Neuroimmunol 290, 103–108 [DOI] [PubMed] [Google Scholar]
- 13.Verweij FJ et al. (2018) Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J. Cell Biol 217, 1129–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai CP et al. (2015) Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat. Commun 6, 7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridder K et al. (2015) Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology 4, e1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]


