Abstract
Introduction
Real-world management of patients with hepatocellular carcinoma (HCC) is crucially challenging in the current rapidly evolving clinical environment which includes the need for respecting patient preferences and autonomy. In this context, regional/national treatment guidelines nuanced to local demographics have increasing importance in guiding disease management. We report here real-world data on clinical outcomes in HCC from a validation of the Consensus Guidelines for HCC at the National Cancer Centre Singapore (NCCS).
Method
We evaluated the NCCS guidelines using prospectively collected real-world data, comparing the efficacy of treatment received using overall survival (OS) and progression-free survival (PFS). Treatment outcomes were also independently evaluated against 2 external sets of guidelines, the Barcelona Clinic Liver Cancer (BCLC) and Hong Kong Liver Cancer (HKLC).
Results
Overall treatment compliance to the NCCS guidelines was 79.2%. Superior median OS was observed in patients receiving treatment compliant with NCCS guidelines for early (nonestimable vs. 23.5 months p < 0.0001), locally advanced (28.1 vs. 22.2 months p = 0.0216) and locally advanced with macrovascular invasion (10.3 vs. 3.3 months p = 0.0013) but not for metastatic HCC (8.1 vs. 6.8 months p = 0.6300), but PFS was similar. Better clinical outcomes were seen in BCLC C patients who received treatment compliant with NCCS guidelines than in patients with treatment only allowed by BCLC guidelines (median OS 14.2 vs. 7.4 months p = 0.0002; median PFS 6.1 vs. 4.0 months p = 0.0286). Clinical outcomes were, however, similar for patients across all HKLC stages receiving NCCS-recommended treatment regardless of whether their treatment was allowed by HKLC.
Conclusion
The high overall compliance rate and satisfactory clinical outcomes of patients managed according to the NCCS guidelines confirm its validity. This validation using real-world data considers patient and treating clinician preferences, thus providing a realistic analysis of the usefulness of the NCCS guidelines when applied in the clinics.
Keywords: Real-world data, Hepatocellular carcinoma, Practice guidelines, Liver cancer
Introduction
Hepatocellular carcinoma (HCC) is a highly malignant cancer. It is the sixth most common cancer worldwide, and the fourth most common cause of cancer death [1]. Untreated, its general prognosis is poor with a median survival of 9 months [2]. More than 80% of HCC cases in the world occur in sub-Saharan Africa and Eastern Asia where viral hepatitis is endemic [3]. In Singapore, HCC is the fourth most common cancer in men and the third most common cause of cancer deaths. Globally, a number of guidelines have been proposed for the management of HCC, none of which have been universally adopted. The more widely cited guidelines are from the West, in which Asian patient demographics are underrepresented [4].
Asian management guidelines on HCC include those from the Japan Society of Hepatology [5], the Korean Liver Cancer Study Group [6], and the Asia Pacific Association for the Study of the Liver [7]. Notably, the Hong Kong Liver Cancer (HKLC) guidelines have been shown to outperform the Barcelona Clinic Liver Cancer (BCLC) guidelines in the management of Asian patients with HCC in several institutions [8]. Each of these guidelines caters to patients of different demographics, with more consideration for local disease aetiology and availability of medical resources.
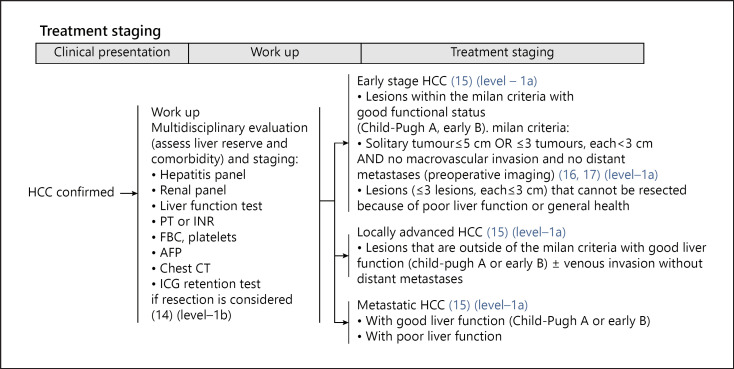
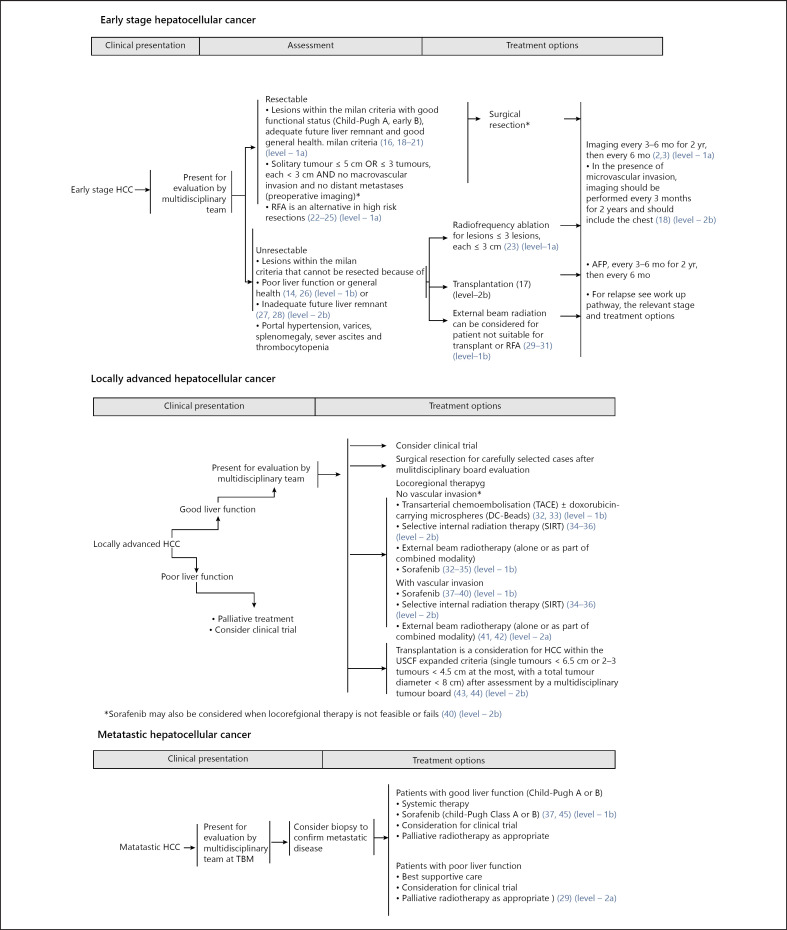
Singapore is a multiracial and multicultural country of 5.6 million in the centre of Southeast Asia. The Comprehensive Liver Cancer Clinic (CLCC) at the National Cancer Centre Singapore (NCCS) provides multidisciplinary care to HCC patients in the country. Singapore has a mixed public-private healthcare delivery system where patients co-share healthcare funding with state-sanctioned insurance and have significant autonomy [9]. The NCCS consensus guidelines [10] (shown in Fig. 1, 2) was developed in June 2014 by a group of 24 clinical specialists from multiple disciplines based on extensive review of regional and global guidelines, and current evidence in HCC treatment modalities. Consistent with many other Asian guidelines, the NCCS consensus guidelines stages patients based on tumour burden, liver function and functional status before recommending multiple treatment options. Specific provisions in the NCCS guidelines allow a multidisciplinary tumour board (MDTB) flexibility in recommending individualized treatment for patients. Autonomy and the treatment preferences of both patient and the treating clinician are consistently respected. The MDTB may also recommend treatment outside of the consensus guidelines if such treatment were deemed more suitable because of the unique circumstances of the patient. Thus, whilst the majority of patients with HCC managed at the NCCS received treatment consistent with the guidelines, a significant minority received treatment outside of the guidelines.
Fig. 1.
HCC staging system based on the NCCS consensus guidelines (retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4906434/). NCCS, National Cancer Centre Singapore; HCC, hepatocellular carcinoma.
Fig. 2.
NCCS consensus guidelines (retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4906434/). NCCS, National Cancer Centre Singapore.
Here, we report a prospective evaluation of the NCCS consensus guidelines for HCC, reviewing its prognostic accuracy, efficacy of treatments recommended, and overall compliance to recommendation in patients treated for HCC at the NCCS. Efficacy of treatment received was assessed by patients' clinical outcomes, specifically overall survival (OS), and progression-free survival (PFS). Other factors influencing treatment decision and outcomes, such as MDTB recommendations and individual patient and attending clinician preferences were also reviewed.
Outcomes of patients who received treatment consistent with the NCCS guidelines were further validated against 2 external staging system based on treatment compliance to the external set of guidelines, namely the BCLC guidelines and HKLC guidelines. Clinical outcomes from both sets of guidelines across the different cancer stages were then compared.
Materials and Methods
Study Design
This study was conducted at the NCCS, a tertiary care academic medical centre in Singapore and was approved by the Institutional Review Board (CIRB Ref: 2017-2000).
Study Population
Patients with HCC treated at the multidisciplinary CLCC at NCCS from the period of June 1, 2014, to December 31, 2018, and who met the study inclusion criteria were included in this study.
Inclusion criterion included (1) patients with HCC diagnosed on biopsy or imaging based on the American Association for the Study of Liver Diseases (AASLD) Guidelines 2011 (shown in Fig. 1) and (2) who received treatment for HCC from the multidisciplinary team at CLCC from June 1, 2014, to December 30, 2018.
The following patients were excluded from this study: patients who did not receive further treatment at NCCS after initial consultation and/or diagnosis (e.g., patients seeking second opinions) and patients with other concurrent malignancy, except for adequately treated basal cell or squamous cell skin cancer, in situ cervical cancer, or other cancers for which the patient has been disease-free for at least 5 years.
Definitions
Date of diagnosis was defined as the date of definitive diagnosis of HCC at NCCS by multiphasic imaging according to AASLD criteria, or biopsy when imaging was not conclusive. Date of progression/recurrence was defined as the date of imaging showing progressive disease according to the Response Evaluation Criteria in Solid Tumours (RECIST) criteria.
“NCCS guidelines compliant” was defined as patients receiving treatment for HCC that were consistent with the NCCS guidelines for a given HCC stage. “NCCS guidelines non-compliant” was defined as patients receiving treatment outside of those recommended by the NCCS guidelines.
Data Collection
Data were collected prospectively using the Sunrise Clinical Manager® (Allscripts Health Solutions INC, Chicago, IL, USA) electronic patient medical records at NCCS. Date of data censorship was July 18, 2019. Baseline characteristics for patients namely: gender, race, age, hepatitis status, Child-Pugh (CP) score, albumin-bilirubin (ALBI) score, and Eastern Cooperative Oncology Group (ECOG) performance status were entered into an electronic clinical record form (CRF). Data for date of diagnosis, first treatment administered, date of last follow-up, date of demise, date of progression, MDTB discussion status, MDTB discussion and assessment outcomes, including treatment recommendations and reasons for noncompliance to recommendations were also entered into the clinical record form. ALBI grade was created based on grade 1: ALBI ≤−2.60, grade 2: −2.60 < ALBI ≤−1.39, and grade 3: >−1.39.
The patients' HCC was staged according to the NCCS guidelines and the BCLC and HKLC guidelines and recorded. HCC stage was determined based on the first CT or MRI reviewed during the first consultation at the CLCC. The NCCS guidelines classify patients with HCC into 1 of 3 clinical stages based on tumour burden (shown in Fig. 2).
Patients were first grouped by their HCC stage according to the NCCS guidelines, then further subdivided into “NCCS guidelines compliant” and “NCCS guidelines non-compliant” based on their treatment compliance to guidelines. Treatment compliance to recommendations in the NCCS guidelines was evaluated based on the first treatment administered and recorded using the Sunrise Clinical Manager®.
Statistical Analysis
The primary objective of this study was to compare OS and PFS between patients' NCCS treatment compliant status − “guidelines compliant” and “guidelines non-compliant” within each HCC stage. The primary outcomes of this study were OS and PFS. Patient's demographics and clinical characteristics were summarized with respect to NCCS guidelines compliance status within each HCC stage. Continuous and categorical characteristics were summarized as mean (standard deviation) or median (minimum and maximum), whichever appropriate, and frequency (proportion), respectively. OS was calculated from the date of diagnosis to the date of demise or the date the patient was last seen before the date of data censorship. PFS was calculated from the date of diagnosis to time of disease progression or until death from any cause, whichever was earlier. Kaplan-Meier plots were used to investigate differences in both OS and PFS for patients with different NCCS guidelines compliance status. Differences were tested using Wilcoxon test. Median OS and PFS with 2-sided 95% confidence interval (95% CI) were also estimated from Kaplan-Meier plot for OS and PFS. Univariate Cox proportional hazard (CPH) regression model was used to find prognostic association between OS and ALBI grade and Child-Pugh. Association from CPH was expressed as hazard ratio (HR) with 95% CI. All tests were 2 sided, and p value <0.05 was considered as statistically significant. SAS software version 9.4 (SAS Institute, Cary, NC, USA) was used for analysis.
Results
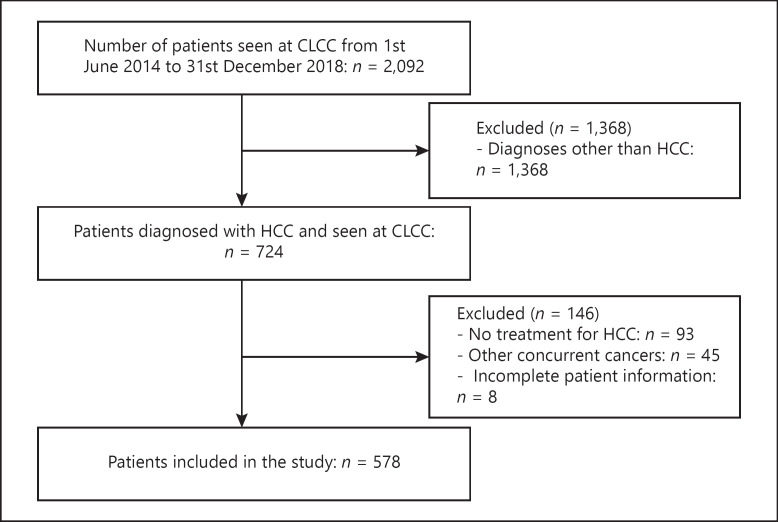
From June 1, 2014, to December 31, 2018, 2092 unique patients attended the multidisciplinary CLCC at NCCS. 724 (34.6%) of these patients had a primary diagnosis of HCC. After initial diagnosis and assessment, 93 (12.8%) patients did not receive further treatment at NCCS and were, therefore, excluded from the study. Also excluded from the analysis were 45 (6.2%) patients who had other concurrent cancers, and 8 (1.1%) patients with incomplete information. The remaining 578 patients met the study inclusion criteria and were included in the study (shown in Fig. 3).
Fig. 3.
Study CONSORT diagram.
In the cohort, 129 (22.3%) patients had Early HCC, 266 (46.0%) patients had locally advanced HCC (which included patients with macrovascular invasion), and 183 (31.7%) patients had Metastatic HCC as defined by the NCCS staging system (shown in Table 1). Within the locally advanced group, 127 (21.9%) patients had macrovascular invasion, and 139 (24.0%) patients were without macrovascular invasion.
Table 1.
Baseline characteristic based on NCCS compliance status stratified by HCC stages
| Baseline characteristic for early HCC patients | Early stage HCC |
Locally advanced HCC |
Metastatic HCC |
||||||
|---|---|---|---|---|---|---|---|---|---|
| noncompliant to NCCS guidelines N = 13 (10.1%) | compliant to NCCS guidelines N = 116 (89.9%) | p value | noncompliant to NCCS guidelines N = 57 (21.4%) | compliant to NCCS guidelines N = 209 (78.6%) | p value | noncompliant to NCCS guidelines N = 50 (27.3%) | compliant to NCCS guidelines N = 133 (72.7%) | p value | |
| Race, n (%) | |||||||||
| Chinese | 8 (61.54) | 65 (56.03) | 50 (87.72) | 122 (58.37) | 42 (84.00) | 90 (67.67) | |||
| Indian Malay |
1 (7.69) 2 (15.38) |
4 (03.45) 7 (06.03) |
0.2074 | 0 (0.0) 2 (3.51) |
8 (3.83) 15 (7.18) |
0.1385 | 1 (2.00) 1 (2.00) |
3 (2.26) 5 (3.76) |
0.1385 |
| Others | 2 (15.38) | 40 (34.48) | 5 (8.77) | 64 (30.62) | 6 (12.00) | 35 (26.32) | |||
| Gender, n (%) | |||||||||
| Male | 10 (76.92) | 31 (26.72) | 1.0000 | 50 (87.72) | 171 (81.82) | 0.3271 | 42 (84.00) | 117 (87.97) | 0.4699 |
| Female | 3 (23.08) | 85 (73.28) | 7 (12.28) | 38 (18.18) | 8 (16.00) | 16 (12.03) | |||
| Age (years) | |||||||||
| Mean (SD) | 68.9 (10.78) | 64.2 (10.30) | 0.1516 | 66.8 (12.55) | 64.8 (9.98) | 0.2670 | 66.6 (11.18) | 62.7 (10.30) | 0.0369 |
| Median (IQR) | 69.0 (12.0) | 64.0 (12.5) | 68.0 (18.0) | 65.0 (12.0) | 68.5 (17.0) | 64.0 (13.0) | |||
| Hepatitis B, n (%) | |||||||||
| Yes | 3 (23.08) | 58 (50.00) | 0.0822 | 25 (43.86) | 101 (48.33) | 0.6538 | 29 (58.00) | 77 (57.89) | 1.0000 |
| No | 10 (76.92) | 58 (50.00) | 32 (56.14) | 108 (51.67) | 21 (42.00) | 56 (42.11) | |||
| Hepatitis C, n (%) | |||||||||
| Yes | 5 (38.46) | 19 (16.38) | 0.0660 | 3 (5.26) | 28 (13.40) | 0.1055 | 5 (10.00) | 14 (10.53) | 1.0000 |
| No | 8 (61.54) | 97 (83.62) | 54 (94.74) | 181 (86.60) | 45 (90.00) | 119 (89.47) | |||
| Child-Pugh Score, n (%) | |||||||||
| A | 10 (76.92) | 110 (94.83) | 33 (57.89) | 167 (79.90) | 32 (64.00) | 102 (76.69) | |||
| B | 3 (23.08) | 5 (4.31) | 0.0475 | 24 (42.11) | 39 (18.66) | 0.0012 | 17 (34.00) | 25 (18.80) | 0.0835 |
| C | 0 (0.0) | 1 (0.86) | 0 (0.0) | 3 (1.44) | 1 (2.00) | 6 (4.51) | |||
| ECOG Scores, n (%) | |||||||||
| 0–2 | 13 (100.0) | 116 (100.0) | 53 (92.98) | 200 (95.69) | 0.4855 | 49 (98.00) | 126 (94.74) | 0.4497 | |
| 3–4 | − | − | − | 4 (7.02) | 9 (4.31) | 1 (2.00) | 7 (5.26) | ||
p values are based on Fisher's exact test for categorical variables and 2 sample t test for continuous variables. EOCG, Eastern Cooperative Oncology Group; SD, standard deviation; HCC, hepatocellular carcinoma.
Patient Demographics and Baseline Characteristics
The demographics and baseline characteristics of the 578 patients are described in Table 1. Within each HCC stage, baseline characteristics were compared between patients receiving guidelines compliant treatment and patients receiving guidelines noncompliant treatment. Amongst patients with early HCC and locally advanced HCC, those with guidelines noncompliant treatment had a larger proportion of patients with Child-Pugh B (p = 0.0475 and p = 0.0012, respectively) compared to patients with guidelines compliant treatment. In the metastatic group, patients with guidelines noncompliant treatment had a higher median age compared to patients with guidelines compliant treatment (p = 0.0369) (shown in Table 1).
Compliance with Recommendations of the National Cancer Centre Singapore Consensus Guidelines for Hepatocellular Carcinoma
Overall treatment compliance rate to the NCCS consensus guidelines for patients seen from the period of June 1, 2014, to December 31, 2018, was 79.2%. Treatment compliance rates for the different stages were 89.9% for early HCC, 78.6% for locally advanced HCC and 72.7% for metastatic HCC. The reasons for noncompliance were reviewed for the 120 (20.8%) patients (shown in Table 2). HCC stage was found to influence compliance rates (p = 0.0019), with compliance rates decreasing with more advanced HCC stage.
Table 2.
Reasons for treatment noncompliance to NCCS consensus guidelines recommendations
| HCC stage | Frequency, n = 120 | Reason for treatment noncompliance to NCCS consensus guidelines recommendations |
|---|---|---|
| Early Stage HCC | 13 | − 5, patient or family's decision − 3, MDTB recommended treatment outside of guidelines − 5, individual physician decision |
| Locally advanced-stage HCC | 57 | − 23, patient or family's decision − 28, individual physician's decision − 3, MDTB recommended treatment outside of guidelines − 1, death before treatment initiation − 1, rapid disease progression before treatment − 1, poor tolerance of therapy |
| Metastatic stage HCC | 50 | − 31, patient or family's decision − 1, MDTB recommended treatment outside of guidelines − 13, individual physician's decision − 3, rapid disease progression before treatment − 2, poor tolerance of therapy |
MDTB, multidisciplinary tumour board; HCC, hepatocellular carcinoma; NCCS, National Cancer Centre Singapore.
Overall Survival and Progression-Free Survival
Early Hepatocellular Carcinoma
The median OS was not reached after a median follow-up of 25.2 (min: 0.2 and max: 60.5) months. Median OS for patients with guidelines compliant treatment was not reached but was 23.5 months for patients with guidelines noncompliant treatment (p = < 0.0001) (shown in Table 3). Median PFS for all patients with early HCC was 53.4 months but was not reached for patients with guidelines compliant treatment. Median PFS was 10.6 months for patients with guidelines noncompliant treatment (p < 0.0001).
Table 3.
Comparison of median OS and PFS between NCCS compliant groups within each HCC stage
| HCC stage | Status | Median OS (95% CI) | p value | Median PFS (95% CI) | p value |
|---|---|---|---|---|---|
| Early stage HCC | Noncompliant | 23.52 (6.70, ne) | 10.64 (3.35, 51.78) | ||
| Compliant | Median OS not reached | <0.0001 | Median PFS not reached | <0.0001 | |
| Overall | Median OS not reached | 53.39 (28.88, ne) | |||
| Locally advanced-stage HCC | |||||
| No vascular invasion | Noncompliant | 22.21 (7.00, ne) | 23.13 (5.98, ne) | ||
| Compliant | 28.09 (19.65, 40.94) | 0.0216 | 9.43 (6.83, 11.70) | 0.2052 | |
| Overall | 25.66 (19.61, 38.60) | 10.32 (7.96, 11.99) | |||
| Vascular invasion | Noncompliant | 3.29 (2.43, 9.20) | 5.06 (2.27, ne) | ||
| Compliant | 10.25 (6.74, 14.16) | 0.0013 | 6.18 (4.34, 8.48) | 0.4806 | |
| Overall | 8.41 (5.45, 11.83) | 6.05 (4.34, 8.48) | |||
| Metastatic stage HCC | Noncompliant | 6.77 (4.34, 13.50) | 5.36 (3.52, 8.74) | ||
| Compliant | 8.05 (7.10, 10.45) | 0.6300 | 3.98 (3.32, 5.59) | 0.1390 | |
| Overall | 8.02 (6.64, 10.15) | 4.57 (3.48, 5.88) |
ne, not estimable; CI, confidence interval; HCC, hepatocellular carcinoma.
Locally Advanced Hepatocellular Carcinoma
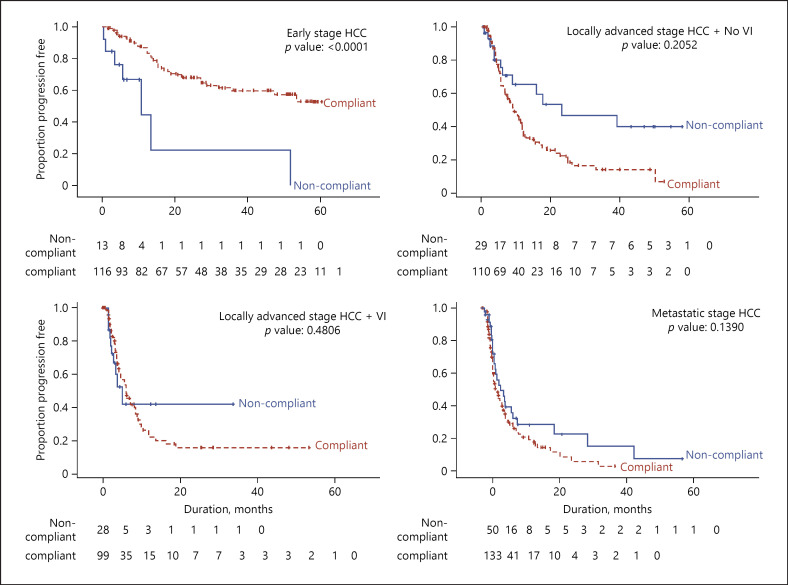
The median OS for locally advanced patients without vascular invasion was 25.7 months. OS for patients with guidelines compliant treatment was 28.1 versus 22.2 months for patients with guidelines noncompliant treatment (p = 0.0216) (shown in Table 3). Median PFS was 10.3 months, and there was no significant difference observed between these 2 groups (p = 0.2052).
The median OS for locally advanced patients with vascular invasion was 8.4 months. Patients with guidelines compliant treatment had a median OS of 10.3 months. Patients with guidelines noncompliant treatment had a median OS of 3.3 months (p = 0.0013) (shown in Table 3). Median PFS was 6.1 months and was not significantly different between the 2 groups (p = 0.4806).
Metastatic Hepatocellular Carcinoma
The median OS for patients with metastatic disease was 8.0 months. Patients with guidelines compliant treatment had a median OS of 8.1 months compared to 6.8 months for patients with guidelines noncompliant treatment (p = 0.6300) (shown in Table 3). Median PFS was 4.6 monthsand was not significantly different between the 2 groups (p = 0.1390).
Prognostic Values of ALBI and Child-Pugh
Univariate CPH showed that higher ALBI grade and Child-Pugh score significantly associated with poorer survival. ALBI grade 2 (HR [95% CI]: 2.58 [1.96, 3.40]) or 3 (HR [95% CI]: 5.62 [3.77, 8.39]) had poorer survival compared to grade 1. Similarly, higher Child-Pugh score B (HR [95% CI]: 3.94 [3.01, 5.15]) or C (HR [95% CI]: 5.08 [2.60, 9.93]) had poorer survival compared to Child-Pugh A (shown in Table 4). Integrated time dependent area under the curve was 0.47 (CP) and 0.48 (ALBI).
Table 4.
Prognostic values of Child-Pugh (CP) and Albumin-Bilirubin (ALBI) score
| Variables | Unadjusted HR (95% CI) | p value |
|---|---|---|
| CP Stage (ref = A) | <0.0001+ | |
| B | 3.94 (3.01, 5.15) | <0.0001 |
| C | 5.08 (2.60, 9.93) | <0.0001 |
| ALBI grade (ref = 1) | <0.0001+ | |
| 2 | 2.58 (1.96, 3.40) | <0.0001 |
| 3 | 5.62 (3.77, 8.39) | <0.0001 |
CI, confidence interval.
type −3 or overall p value.
Performance Assessment of the National Cancer Centre Singapore Consensus Guidelines
The efficacy of the NCCS consensus guidelines was further evaluated by comparison with 2 other external guidelines, namely the BCLC and the HKLC guidelines. Analysis was conducted on patients with NCCS compliant treatment, who were then restaged using the external staging system and stratified according to their treatment compliance. OS and PFS were then compared between compliant and noncompliant patients. The same analytical method was used for analysis with the BCLC and HKLC guidelines.
Comparison with the Barcelona Clinic Liver Cancer Guidelines
To provide external validation of the NCCS treatment guidelines, further analysis was conducted on patients with guidelines compliant treatment. Patients with compliant treatment were grouped according to their BCLC stages and treatment compliance to BCLC guidelines (shown in Table 5). The efficacy of NCCS-recommended treatment and specifically new treatment modalities recommended by the NCCS guidelines was evaluated by comparing the OS of patients who received only NCCS-recommended treatments (BCLC noncompliant treatment) with those who received treatments recommended by both guidelines (BCLC compliant) (shown in Table 5). The type of treatment these patients received at NCCS CLCC was tabulated across different BCLC stages, contrasting the different treatment modalities recommended by the 2 guidelines (shown in Table 6). In BCLC stage A and 0, median survival was not reached in both BCLC noncompliant and compliant group (p = 0.8106), median PFS was 24.9 months in BCLC noncompliant patients, and median PFS was not reached in the BCLC compliant group (p = 0.5282) (shown in Table 5).
Table 5.
Clinical outcomes for patients with treatment compliant to NCCS guidelines stratified by the BCLC system
| BCLC stage | Compliance status | Median OS (95% CI) | p value | Median PFS (95% CI) | p value |
|---|---|---|---|---|---|
| Stage 0/A, N = 135 | Non-BCLC compliant BCLC compliant |
Median OS not reached Median OS not reached |
0.8106 | 24.87 (14.95, ne) Median PFS not reached |
0.5282 |
| Stage B, N = 60 | Non-BCLC compliant BCLC compliant |
25.10 (14.36, 61.31) 14.60 (11.57, ne) |
0.7216 | 7.20 (4.80, 11.63) 7.03 (3.09, 10.84) |
0.9054 |
| Stage C, N = 239 | Non-BCLC compliant BCLC compliant |
14.16 (10.25, 16.66) 7.39 (5.09, 9.03) |
0.0002 | 6.05 (4.67, 8.48) 3.98 (3.22, 5.06) |
0.0286 |
| Stage D, N = 24 | Non-BCLC compliant BCLC compliant |
3.45 (1.54, 16.79) 2.40 (0.36, 9.07) |
0.3000 | 8.89 (2.00, ne) Median PFS did not reach |
0.3681 |
OS and PFS refers to OS and PFS. ne, not estimable; OS, overall survival; PFS, progression-free survival; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; NCCS, National Cancer Centre Singapore. p values are based on Gehan-Breslow-Wilcoxon test.
Table 6.
First treatment for patients compliant to NCCS guidelines staged by BCLC classification
| BCLC stage | First treatment at Singhealth Institutions |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Resection, N = 82 | RFA, N = 46 | SIRT, N = 89 | Radiotherapy, N = 4 | TACE, N = 14 | Sorafenib, N = 76 | Other systemic therapy, N = 43 | Clinical trials, N = 78 | Combination therapy, N = 4 | Best supportive care, N = 22 | |
| 0/A (N = 135) | 71 | 44 | 17 | 1 | 1 | 1 | ||||
| B (N = 60) | 7 | 25 | 10 | 8 | 10 | |||||
| C (N = 239) | 4 | 1 | 47 | 3 | 3 | 68 | 42 | 67 | 4 (Y90 + systemic) | |
| D (N = 24) | 1 | 1 | 22 | |||||||
Italicized: number of patients undergoing BCLC compliant treatments. BCLC, Barcelona Clinic Liver Cancer; SIRT, selective internal radiation therapy; NCCS, National Cancer Centre Singapore.
In BCLC stage B, median OS in BCLC noncompliant group (25.1 months) appeared to trend toward superior outcomes compared to BCLC compliant group (14.6 months) (p = 0.7216) but was not significant. Median PFS in BCLC noncompliant group was 7.2 months versus 7.0 months in BCLC compliant group (p = 0.9054) (shown in Table 5).
In BCLC stage C, median OS was 14.2 months in BCLC noncompliant group, which was significantly higher compared to 7.4 months in BCLC compliant group (p = 0.0002). Median PFS in the noncompliant group was 6.1 months compared BCLC compliant group at 4.0 months (p = 0.0286) (shown in Table 5).
In BCLC stage D, median OS for BCLC noncompliant group was 3.5 months, whilst BCLC compliant group had a median OS of 2.4 months (p = 0.3000). Median PFS was not reached in BCLC compliant group and median PFS for BCLC noncompliant group was 8.9 months (p = 0.3681) (shown in Table 5).
Comparison with the Hong Kong Liver Cancer Staging System
The comparison was done in the same manner as the comparison with the BCLC guidelines. All NCCS compliant patients were grouped according to their HKLC stage and compliance to HKLC guidelines accessed. OS and PFS data were then obtained and compared between compliant and noncompliant patients stratified by stage.
In HKLC stage 1, median OS was 61.3 months in all patients as all patients compliant to NCCS were also compliant to HKLC. Median PFS was nonestimable in all patients (shown in Table 7).
Table 7.
Clinical outcomes for patients with treatment compliant to NCCS guidelines stratified by the HKLC system
| HKLC stage | Status | Median OS (95% CI) | p value | Median PFS (95% CI) | p value |
|---|---|---|---|---|---|
| Stage 1 (N = 123) | Compliant (N = 123) | 61.31 (ne, ne) | − | ne | − |
| Stage 2 (N = 61) | Noncompliant (N = 10) Compliant (N = 51) |
36.67 (10.19, 38.60) 40.94 (25.43, ne) |
0.3289 | 10.32 (5.49, 22.67) 9.89 (6.57, 16.69) |
0.8336 |
| Stage 3 (N = 100) | Noncompliant (N = 17) | 9.36 (4.86, 23.23) | 0.0844 | 9.07 (3.71, 21.52) | 0.5271 |
| Compliant (N = 83) | 22.83 (13.11, ne) | 7.43 (4.44, 10.02) | |||
| Stage 4 (N = 138) | Noncompliant (N = 4) | 21.88 (11.83, ne) | 0.1232 | 7.13 (3.61, 7.20) | 0.2931 |
| Compliant (N = 134) | 8.57 (7.40, 11.17) | 4.14 (3.39, 5.59) | |||
| Stage 5 (N = 36) | Noncompliant (N = 14) Compliant (N = 22) |
4.67 (1.64, 5.52) 2.66 (1.48, 4.50) |
0.2285 | 12.29 (1.58, 12.29) ne |
0.3298 |
ne, not estimable; HKLC, Hong Kong Liver Cancer; CI, confidence interval; NCCS, National Cancer Centre Singapore.
In HKLC stage 2, there was no difference in OS (36.7 vs. 40.9 months, p = 0.3289) and PFS (10.3 vs. 9.9 months, p = 0.8336) between HKLC noncompliant patients and compliant patients, respectively (shown in Table 7).
In HKLC stage 3, there was no difference in OS (9.4 vs. 22.8 months, p = 0.0844) and PFS (9.1 and 7.4 months, p = 0.5271) between HKLC noncompliant patients and compliant patients, respectively (shown in Table 7). In HKLC stage 4, there was no difference in OS (21.9 vs. 8.6 months, p = 0.1232) and PFS (7.1 vs. 4.1 months, p = 0.2931) between HKLC noncompliant and compliant patients, respectively (shown in Table 7). In HKLC stage 5, there was no difference in OS (4.7 vs. 2.7 months, p = 0.2285) and PFS (12.3 months vs. nonestimable, p = 0.3298) between HKLC noncompliant and compliant patients, respectively (shown in Table 7). The number of patients who have stage migrated in each stage stratified by treatment received are annotated (shown in Table 8). Reasons for stage migration are summarized in Table 9.
Table 8.
First treatment for patients compliant to NCCS guidelines staged by HKLC guidelines
| HKLC stage | First treatment at Singhealth Institutions |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| resection, N = 82 | RFA, N = 46 | SIRT, N = 89 | radiotherapy, N = 4 | TACE, N = 14 | sorafenib, N = 76 | other systemic therapy, N = 43 | clinical trials, N = 78 | combination therapy, N = 4 | best supportive care, N = 22 | |
| 1 (N = 123) | 72 | 45 | 2 (TSM) | 1 (TSM) | 3 (TSM) | |||||
| 2 (N = 61) | 9 |
25 (TSM) 5 |
8 (TSM) 2 |
4 (TSM) 1 |
5 (TSM) 2 |
|||||
| 3 (N = 100) | 1 (TSM) | 53 | 2 (TSM) | 3 |
11 (TSM) 3 |
5 (TSM) 2 |
8 (TSM) 12 |
|||
| 4 (N = 138) | 3 | 1 | 53 | 30 | 47 | 4 (systemic + y90) | ||||
| 5 (N = 36) | 1 | 1 | 1 | 4 | 6 | 1 | 22 | |||
Italicized: number of patients undergoing HKLC compliant treatments. TSM, treatment stage migration; HKLC, Hong Kong Liver Cancer; SIRT, selective internal radiation therapy; NCCS, National Cancer Centre Singapore.
Table 9.
Reasons for treatment stage migration
| HCC stage | Frequency, n = 75 | Reason for treatment stage migration |
|---|---|---|
| 1 | 6 | − 2, poor ICG − 1, other comorbidities − 3, progressive disease on resection |
| 2 | 42 | − 24, poor ICG/functional liver remnant − 6, other comorbidities − 10, progressive disease on previous treatment − 2, unresectable tumour |
| 3 | 27 | − 14, rapidly progressive disease/worsening liver function/failed MAA − 3, other comorbidities − 10, progressive disease on previous treatment |
HCC, hepatocellular carcinoma.
Discussion
Underpinning the numerous guidelines for HCC staging and management is the wide geographic variance in tumour biology and resource availability. A universal guideline for HCC management does not currently appear feasible or effective. Real-world practice decisions are largely guided by local preferences and local availability of specialist opinions and treatment options.
This is the first validation of the NCCS consensus guidelines since its implementation in 2014, reviewing the effectiveness of the current practice guidelines in staging, prognosticating, and recommending treatment for the diverse patient population treated at the NCCS CLCC.
The NCCS consensus guidelines accurately stratified patients into 3 distinct stages of disease: early, locally advanced, and metastatic; the naming system used directly correlates the stage with disease burden. The veracity of the NCCS guidelines is confirmed by the worsening OS and PFS observed with increasing disease stage and/or vascular involvement and this offer good prognostic applications.
Treatment compliance to consensus guidelines was seen in 79.2% of patients treated at the NCCS CLCC. Of note, HCC stage was found to influence compliance rates (p = 0.0019), with compliance rates decreasing with more advanced HCC stages, at 89.9, 78.6, and 72.7%, respectively (shown in Table 1). In more advanced-stage HCC, treatment recommendations become increasing complex and are associated with less favourable risk-benefit ratios. Thus, in advanced-stage HCC, factors like patients' personal preference and individual physician's recommendations become increasingly weighted.
Similarly, Child-Pugh (CP) score was also found to influence compliance rates in the early and locally advanced group (p = 0.0475, 0.0012, respectively) but not in the metastatic group (p = 0.0835). A higher compliance rate was observed amongst all CP A patients as patients with better basal liver function would be amendable to more treatment options. Compliance rate amongst CP A patients was 91.7% (early), 83.5% (locally advanced), and 76.1% (metastatic). Notably, 93.0% of all early HCC patients were CP A, which could be a product of regular follow-up and intervention for basal liver disease in primary and secondary centres leading to early detection and referral to our tertiary centre. Subsequent studies involving the follow-up of CP A patients could be beneficial in ascertaining the effectiveness of the guidelines in preserving hepatic function in HCC patients and prognosticating guidelines compliant patients to further improve outcomes.
Multidisciplinary Tumour Board Recommendations and Patient and Treating Physician Autonomy
Patients who attend the CLCC are routinely assessed by the MDTB where at least 4 clinical disciplines are represented (from surgical oncology/Hepato-pancreato-biliary surgery, medical oncology, nuclear medicine, radiation oncology, interventional radiology, and diagnostic radiology), and treatment options and recommendations are discussed. Patients with HCC are a highly complex patient group, with multiple risk factors and comorbidities. At the CLCC, provisions in the guidelines specifically allow the MDTB the option of consensus-based recommendation of individualized treatment regime for each patient independent of the NCCS guidelines. During the study period, the MDTB made recommendations outside of the CLCC treatment guidelines in 3 patients with early stage HCC, in 3 patients with locally advanced HCC and in 1 patient with metastatic HCC. Patient and treating physician preferences become increasingly weighted as the disease becomes more advanced. Patient autonomy in treatment decisions was exercised in 5 patients with early stage HCC, in 23 patients with locally advanced HCC, and in 31 patients with metastatic HCC. Treating physician autonomy in treatment decisions was exercised in 5 patients with early stage HCC, in 28 patients with locally advanced HCC, and in 13 patients with advanced HCC (shown in Table 2). OS and PFS in patients who received treatment compliant/noncompliant to guidelines patients with early stage and locally advanced HCC had better OS when they received guidelines compliant treatment. Similarly, patients with early stage HCC had better PFS when they received guidelines compliant treatment.
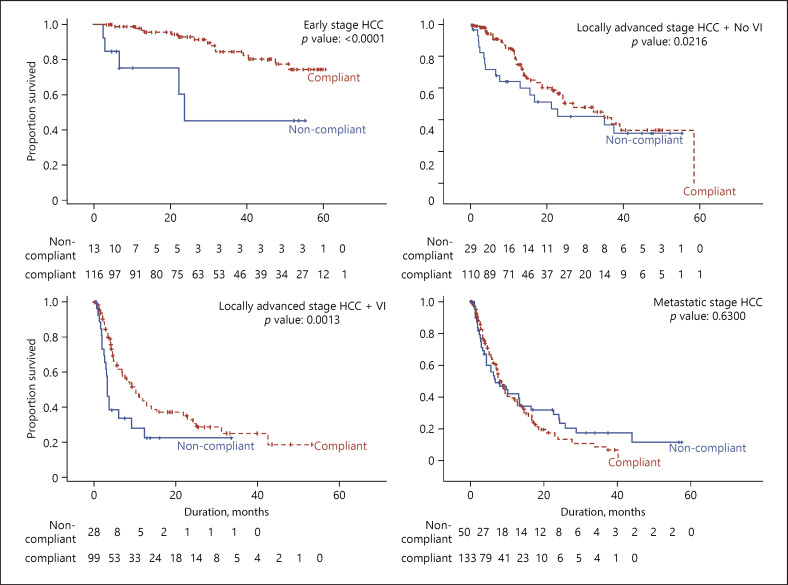
In patients with early stage HCC who received guidelines compliant treatment, median OS and progression-free survival were not reached in this study after a median follow-up of 25.2 months (p = <0.0001) (shown in Fig. 4, 5). The majority of patients (89.9%) in this group received either surgical resection or RFA. Consistent with many Asian countries, Singapore faces shortages in donor organs and continues to use liver resection and RFA aggressively with similar satisfactory survival outcomes seen in other Asian countries [11]. OS and PFS were significantly better in patients who received guidelines compliant treatment versus those who did not (p < 0.0001 for OS and p < 0.0001 for PFS).
Fig. 4.
KM plot comparing OS between NCCS treatment compliant groups within each HCC stage. OS, overall survival; KM, Kaplan-Meier; HCC, hepatocellular carcinoma; VI, vascular invasion.
Fig. 5.
KM curves comparing PFS between NCCS treatment compliant groups within each HCC stage. NCCS, National Cancer Centre Singapore; PFS, progression-free survival; KM, Kaplan-Meier; HCC, hepatocellular carcinoma; VI, vascular invasion.
In patients with locally advanced HCC, median OS was significantly better in patients with treatment compliant to the NCCS guidelines than in patients who received guidelines noncompliant treatments (shown in Fig. 4). PFS was not significantly different between the 2 groups (shown in Fig. 5). That significantly better OS was seen although there was no difference in PFS may be because many patients at the CLCC receive multiple sequential treatment modalities as the disease progresses in their treatment journey. Patients are closely followed up for early detection of disease progression at which point additional therapy modalities would be offered. With frequent follow-up, multiple modalities of therapy and multiple treatment points, improved OS can be achieved even in patients with locally advanced HCC with macrovascular invasion.
Median OS and median PFS in metastatic HCC patients showed no significant difference in patients treated within or outside of guidelines (shown in Fig. 4, 5). This was not unexpected since therapy for patients with extrahepatic metastatic HCC remains largely unsatisfactory; with first-line therapy sorafenib prolonging survival by only 2.8 months in clinical trial [12]. Coupled with widely varying tumour sensitivity to sorafenib, only 30% of patients can expect to derive survival benefits from the drug [13]. Many centres, such as our own have applied a multidisciplinary approach to treat these patients with advanced disease. Whilst such multidisciplinary approach has been reported to yield superior survival outcomes, especially for patients with more advanced disease [14], we have not been able to demonstrate this in our cohort. For metastatic HCC patients compliant to NCCS consensus guidelines, median OS was 8.1 months, which is comparable with other Asian cohorts [15].
Performance Assessment Outcomes of the National Cancer Centre Singapore Consensus Guidelines
Takeaways from the Barcelona Clinic Liver Cancer Guidelines
Similar to other Asian guidelines like the HKLC, which has been internally and externally validated to outperform the BCLC, the NCCS consensus guidelines stages a patient first based on their tumour burden, with consideration for liver function (CP score) and functional status during treatment selection. This is unlike the BCLC guidelines which consider a patient's functional status, liver function and tumour burden simultaneously during staging. Therefore, some patients categorized in the NCCS system as having early or locally advanced HCC would be categorized as advanced HCC in the BCLC system because of poorer ECOG status or liver function although the tumour burden might be low. Such patients will receive more aggressive therapies (shown in Table 6) as recommended in the NCCS consensus guidelines.
By comparing the clinical outcomes of patients who received treatment compliant to both NCCS and BCLC guidelines with clinical outcomes of patients who received treatment compliant to only the NCCS guidelines, the efficacy of the additional treatment modalities recommended by the NCCS guidelines can be assessed as both these groups were treated at the same tertiary centre.
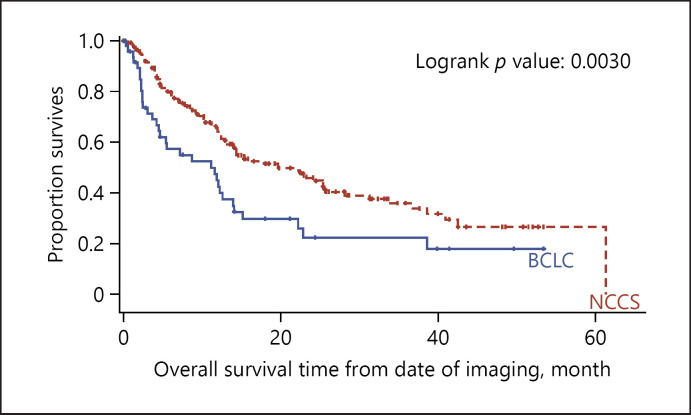
From the perspective of the BCLC guidelines, patients in BCLC C who received treatment compliant to NCCS guidelines but outside of the BCLC guidelines have a significantly better OS (14.2 vs. 7.4 months, p = 0.0002) and PFS (6.1 vs. 4.0 months, p = 0.0286) compared to patients who received treatment that is only compliant to be BCLC guidelines. This was not seen in patients with BCLC stage 0, A, B or D, where patients who received treatment compliant to NCCS guidelines and BCLC guidelines have similar OS and PFS compared to patients who received treatment that is only compliant to the NCCS guidelines.
From the perspective of the NCCS guidelines, patients with locally advanced HCC treated according to the NCCS guidelines had significant better OS than those with treatment compliant to both BCLC and NCCS guidelines (p = 0.0030) (shown in Fig. 6). The BCLC treatment guidelines recommend only sorafenib for patients with vascular invasion whereas the NCCS consensus guidelines allow for more aggressive treatment after MDTB discussion such as resection or selective internal radiation therapy (SIRT) in addition to sorafenib. The median survival of 14.2 months in BCLC C patients in our cohort outperforms cohorts from other institutions primarily using SIRT for BCLC C patients [16]. Favourable treatment outcomes could be result of multimodal therapy and multidisciplinary management facilitating optimal treatment selection.
Fig. 6.
Performance assessment of the NCCS consensus guidelines (locally advanced HCC). NCCS, National Cancer Centre Singapore; HCC, hepatocellular carcinoma.
Takeaways from the Hong Kong Liver Cancer Staging System
The HKLC staging system has been internally and externally validated and shown to outperform the BCLC in the management of HCC in Asian cohorts. The geographical proximity between the 2 countries allows the comparison of guidelines management of HCC of similar aetiologies (mainly hepatitis B) [17].
From the perspective of HKLC guidelines, all stage 1 patients are compliant with both guidelines, with median OS 61.3 months. PFS was nonestimable in our small cohort analysis. Overall, the overlap in treatment of early HCC is reflective of international consensus supporting resection in early HCC.
Both the NCCS and HKLC guidelines are less conservative in their approach to HCC management. However, the main difference in the 2 guidelines involves patients with tumours beyond the Milan criteria, and tumours with vascular involvement.
For patients beyond Milan criteria and no vascular involvement, the NCCS guidelines classify these patients as locally advanced without vascular invasion. NCCS guidelines recommend resection by MDT consensus, clinical trials, locoregional therapy, or systemic therapy (shown in Fig. 2). From the perspective of the HKLC, these patients are intermediate stage (Stage 2), and recommended treatment is resection. However, there was no difference in median OS (36.7 vs. 40.9 months, p = 0.3289) and PFS (10.3 vs. 9.9 months, p = 0.8336) between patients only compliant to NCCS guidelines or compliant to both guidelines, respectively.
The HKLC guidelines further subdivide vascular invasion into intrahepatic and extrahepatic. The NCCS guidelines do not make this distinction and patients with vascular invasion receive resection by MDT consensus, clinical trials, locoregional therapy, systemic therapy, or transplantation (shown in Fig. 2). Intravascular invasions fall into HKLC stage 2 or 3 depending on number and size of tumour. Treatment recommended under HKLC would be resection for stage 2, and resection or locoregional therapy for stage 3. However, again there was no difference in median OS (9.4 vs. 22.8 months, p = 0.0844) and PFS (9.1 and 7.4 months, p = 0.5271) between HKLC noncompliant patients and HKLC compliant in stages 3 and 2 (as discussed above). A significant number of patients treated at NCCS who were HKLC stages 3 received locoregional therapy in the form of SIRT.
Extrahepatic invasion in HKLC is stage 4 where the only recommended treatment is systemic therapy, whilst the NCCS guidelines recommend a wide range of treatment modalities (shown in Fig. 2). However, again there was no difference in median OS (21.9 vs. 8.6 months, p = 0.1232) and PFS (7.1 vs. 4.1 months, p = 0.2931) in HKLC noncompliant and HKLC compliant patients, respectively.
In our analysis cohort, none of the patients in stage 5 was amendable for transplant (12 MVI, 12 EHS, 4 PVT and EHS, and 8 beyond up-to-seven criteria). The median OS of 2.7 months in HKLC compliant patients and 4.7 months in HKLC noncompliant patients and is comparable to median OS of 1.6 months reported in the Hong Kong paper.
In terms of treatment recommendation, the NCCS guidelines cover a wider range of treatment options for each stage compared to the HKLC guidelines. The overlap in treatment recommendation across all stages and the lack of significant differences in median OS and PFS between patients compliant to both guidelines and patients only compliant to NCCS guidelines highlights the comparability in all NCCS-recommended treatment across all HKLC stages 1,2,3,4, and 5.
Treatment Stage Migration
The NCCS consensus guideline deviates from the traditional “stage hierarchy” approach [18] adopted by some western guidelines. Instead, by (1) incorporating alternative treatment modalities or (2) treatment stage migration, our guidelines delink disease stage and its specific treatment, providing flexibility based on expert consensus to improve patient outcomes.
In clinical practice, some patients will receive treatment recommended for later stage patients when first-line therapy recommended for that stage is not feasible, a concept known as treatment stage migration [19].
In this study, when assessed using the BCLC guidelines, 20 BCLC stage 0/A patients had treatment stage migration. Analysis of these 20 patients showed that they are not suitable for liver resection due to poor liver function (as measured by the indocyanine green retention test) or inadequate future liver remnant or both (13 patients) or were in poor clinical health and not fit for surgery (7 patients). Of these 20 patients, 1 received TACE and 17 received SIRT (shown in Table 6). Under the guidelines of the European Association for the Study of the Liver (EASL), these patients would be migrated to BCLC Stage B and receive TACE [20].
In the perspective of the NCCS guidelines, however, these patients are staged as locally advanced HCC without vascular invasion where recommended therapy for unresectable cases is SIRT, TACE, external beam radiotherapy, or sorafenib (shown in Fig. 2). The wide range of therapeutic alternatives under NCCS guidelines for the unresectable patient is a key distinguishing point from other HCC treatment guidelines. This approach has been associated with better survival outcomes as seen in BCLC stage C patients (shown in Table 6), as sequential treatment opportunities on progression is associated with survival benefits [21].
In our comparison with the HKLC guidelines, many patients across HKLC stages 1, 2, 3, and 4 have undergone the usual left-to-right treatment stage migration (shown in Table 8). The aggressive and selective nature of the HKLC guidelines is not unlike ours and treatment stage migration is the norm in real-world practice. Reasons for stage migration was summarized in Table 9, and all involved factors related to patients' physiological reserve. Consequently, comparison of survival outcomes between patients with and without stage migration poses a dilemma of comparability given the intrinsic differences in physiological reserve in the 2 groups. In some centres, select patient groups have benefited from treatment stage migration possibly driven by exposure to multiple lines of treatment [21].
Conclusion
In the context of the Singapore population, the NCCS guidelines for HCC successfully prognosticate patients and treatments compliant with the NCCS guidelines confer superior clinical outcomes compared with treatments not compliant with the NCCS guidelines. When validated against external set of guidelines namely the BCLC guidelines, in advanced HCC patients (BCLC C), treatment compliant with the NCCS guidelines conferred better clinical outcomes than treatment compliant only with the BCLC guidelines. Further comparison with the HKLC guidelines showed similar survival outcomes for patients compliant to both guidelines and patients compliant to only NCCS guidelines, highlighting the utility of the wide range of treatments recommended by the NCCS guidelines. In real-world clinical practice, considerable patient and treating physician autonomy will be exercised in treatment decisions in HCC especially when the disease is more advanced. Multidisciplinary management alongside treatment guidelines is shown to enhance patient selection and optimizes treatment outcomes for individual patient.
Statement of Ethics
This study was approved by the Institutional Review Board (CIRB Ref: 2017-2000) and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Exemption from patient consent was granted by IRB as the study only analysed anonymized data collected in the course of clinical service.
Conflict of Interest Statement
Pierce K.H. Chow declares the following conflicts of interest: Editorial Board Member of Liver Cancer, Consulting or Advisory Role: Sirtex Medical, Bayer, Roche, New B Innovation, MSD, BTG PLC, Eisai, Abbott, IQVIA, Genentech, L.E.K Consulting, Astra Zeneca, Guerbet, Ipsen, OncoSil Medical, and Bristol-Myers Squibb; Speakers' Bureau: Sirtex Medical; and Research Funding: Sirtex Medical (Inst), New B Innovation (Inst), and IQVIA (Inst).
Funding Sources
The authors have received no funding relevant to this study.
Author Contributions
Pierce K.H. Chow was responsible for the design of the study and to the analysis of data and the writing of the paper. Xin Hui Chew and Eshani N. Mathew prepared the manuscript and conducted the literature searches and literature selection. Rehena Sultana carried out the primary data analysis. Xin Hui Chew, Eshani N. Mathew, Fiona N.N. Moe, Jacelyn S.S. Chua, Reiko W.T. Ang, Aldwin D. Ong, Ashley W.Y. Ng, Marjorie T.Q. Hoang, Wanyi Kee, and Jaclyn H.M. Chan performed the data extraction. Xin Hui Chew, Rehena Sultana, David C.E. Ng, Richard H.G. Lo, Han Chong Toh, David W.M. Tai, Su Pin Choo, Brian K.P. Goh, Sean X. Yan, Kelvin S.H. Loke, Sue Ping Thang, Apoorva Gogna, Nanda Venkatanarasimha, Aaron K.T. Tong, Chow Wei Too, Choon Hua Thng, Wan Ying Chan, Wanyi Kee, Jaclyn H.M. Chan, Farah Irani, Sum Leong, Kiat Hon Lim, Michael L.C. Wang, and Pierce K.H. Chow contributed to the data interpretation and writing, reviewing, and revision of the manuscript, and all the authors gave their final approval for submission.
Acknowledgements
The authors acknowledge the nursing staff of the CLCC (Clinic D) at the NCCS for their kind assistance in the management of the patients.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68((6)):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Giannini EG, Farinati F, Ciccarese F, Pecorelli A, Rapaccini GL, Di Marco M, et al. Prognosis of untreated hepatocellular carcinoma. Hepatology. 2015 Jan;61((1)):184–90. doi: 10.1002/hep.27443. [DOI] [PubMed] [Google Scholar]
- 3.Mak LY, Cruz-Ramón V, Chinchilla-López P, Torres HA, LoConte NK, Rice JP, et al. Global epidemiology, prevention, and management of hepatocellular carcinoma. Am Soc Clin Oncol Educ Book. 2018 May 23;38:262–79. doi: 10.1200/EDBK_200939. [DOI] [PubMed] [Google Scholar]
- 4.Barman PM, Su GL. Limitations of the Barcelona Clinic Liver Cancer staging system with a focus on transarterial chemoembolization as a key modality for treatment of hepatocellular carcinoma. Clinical Liver Disease. 2016 Feb;7((2)):32–5. doi: 10.1002/cld.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the japan society of hepatology (JSH) 2010 updated version. Dig Dis. 2011;29((3)):339–64. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 6.Korean Liver Cancer Study, National Cancer Center Korean liver cancer study group-national cancer center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015 May–Jun;16((3)):465–522. doi: 10.3348/kjr.2015.16.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian pacific association for the study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010 Mar 18;4((2)):439–74. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong liver cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014 Jun;146((7)):1691–700.e3. doi: 10.1053/j.gastro.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 9.Lim J. Myth or magic: the Singapore healthcare system. Select Publishing; 2013. Myth or magic: the Singapore healthcare system; pp. p. 65–77. [Google Scholar]
- 10.Chow PK, Choo SP, Ng DC, Lo RH, Wang ML, Toh HC, et al. National cancer centre Singapore consensus guidelines for hepatocellular carcinoma. Liver Cancer. 2016 Apr;5((2)):97–106. doi: 10.1159/000367759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotewall CN, Cheung TT. Optimizing hepatectomy for hepatocellular carcinoma in Asia − patient selection and special considerations. Transl Gastroenterol Hepatol. 2018;3:75. doi: 10.21037/tgh.2018.09.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008 Jul 24;359((4)):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 13.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009 Jan;10((1)):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 14.Sinn DH, Choi GS, Park HC, Kim JM, Kim H, Song KD, et al. Multidisciplinary approach is associated with improved survival of hepatocellular carcinoma patients. PLoS One. 2019;14((1)):e0210730. doi: 10.1371/journal.pone.0210730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, Kondo Y, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011 Oct 1;117((19)):4475–83. doi: 10.1002/cncr.25960. [DOI] [PubMed] [Google Scholar]
- 16.Salem R, Gabr A, Riaz A, Mora R, Ali R, Abecassis M, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology. 2018 Oct;68((4)):1429–40. doi: 10.1002/hep.29691. [DOI] [PubMed] [Google Scholar]
- 17.Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut Liver. 2016 May 23;10((3)):332–9. doi: 10.5009/gnl15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vitale A, Trevisani F, Farinati F, Cillo U. Treatment of hepatocellular carcinoma in the precision medicine era: from treatment stage migration to therapeutic hierarchy. Hepatology. 2020 Feb 16;72((6)):2206–18. doi: 10.1002/hep.31187. [DOI] [PubMed] [Google Scholar]
- 19.Foerster F, Galle PR. Comparison of the current international guidelines on the management of HCC. JHEP Rep. 2019 Aug;1((2)):114–9. doi: 10.1016/j.jhepr.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Association for the Study of the Liver Electronic address eee, European association for the study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018 Jul;69((1)):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Yen C, Sharma R, Rimassa L, Arizumi T, Bettinger D, Choo HY, et al. Treatment stage migration maximizes survival outcomes in patients with hepatocellular carcinoma treated with sorafenib: an observational study. Liver Cancer. 2017 Nov;6((4)):313–24. doi: 10.1159/000480441. [DOI] [PMC free article] [PubMed] [Google Scholar]