Abstract
Introduction
KEYNOTE-240 investigated the efficacy and safety of pembrolizumab plus best supportive care (BSC) in sorafenib-treated patients with advanced hepatocellular carcinoma (HCC). Results for the subgroup of patients from Asia are described.
Methods
Adults with advanced HCC previously treated with sorafenib were randomized 2:1 to pembrolizumab or placebo plus BSC. Here, the Asian subgroup comprised patients enrolled in Hong Kong, Japan, Korea, the Philippines, Taiwan, and Thailand. Primary endpoints were progression-free survival (PFS) per blinded central imaging review and overall survival (OS). Secondary endpoints included objective response rate (ORR) per blinded central imaging review, duration of response (DOR), and safety.
Results
The Asian subgroup included 157 patients. As of January 2, 2019, the median follow-up in this subgroup was 13.8 months for pembrolizumab and 8.3 months for placebo. The median PFS was 2.8 months for pembrolizumab (95% confidence interval [CI] 2.6–4.1) versus 1.4 months (95% CI 1.4–2.4) for placebo (hazard ratio [HR] 0.48; 95% CI 0.32–0.70). The median OS was 13.8 months (95% CI 10.1–16.9) for pembrolizumab versus 8.3 months (95% CI 6.3–11.8) for placebo (HR 0.55; 95% CI 0.37–0.80). ORR was 20.6% (95% CI 13.4–29.5) for pembrolizumab versus 2.0% (95% CI 0.1–10.6) for placebo (difference: 18.5%; 95% CI 8.3–27.6). The median DOR was 8.6 and 2.8 months for pembrolizumab and placebo, respectively. Any grade treatment-related adverse events (TRAEs) occurred in 63 patients (58.9%) receiving pembrolizumab and 24 patients (48.0%) receiving placebo; 14 (13.1%) and 2 (4.0%) patients experienced grade 3–5 TRAEs, respectively. No treatment-related deaths occurred.
Conclusion
Pembrolizumab demonstrated antitumor activity and was well tolerated in the Asian subgroup of KEYNOTE-240. A trend toward greater benefit with pembrolizumab in the Asian subgroup was observed compared with the overall cohort, supporting further evaluation of pembrolizumab treatment in this population.
Keywords: Pembrolizumab, Programmed death 1, Hepatocellular carcinoma
Introduction
Liver cancer is one of the top 10 most diagnosed malignancies worldwide and a leading cause of cancer-related mortality [1]. Hepatocellular carcinoma (HCC), the most common type of liver cancer, comprises more than three-fourths of all liver cancer cases [2]. The incidence of HCC is particularly high in Asia, accounting for approximately two-thirds of global cases [3]. Geographic variability between Asia and the West is largely due to differences in exposure to a variety of risk factors [3]. A common cause of HCC in Japan is hepatitis C virus (HCV), where mass vaccination against schistosomiasis with nonsterilized hypodermic needles in the 1950s resulted in high rates of infection [3]. In other Southeast Asian and East Asian countries, the primary risk factors are hepatitis B virus (HBV) infection, which has been estimated to account for 80% of all diagnosed cases, and exposure to aflatoxins, which can contaminate food supplies in humid areas [2, 3]. In the West (Europe and North America), the primary risk factors are HCV infection and metabolic syndrome [3]. In recent years, a shift in the epidemiology of HCC has occurred with rates decreasing in Asia due to implementation of HBV vaccination programs and improvement in farming policies and rates increasing in the West due to rising rates of metabolic syndrome and nonalcoholic fatty liver disease [3].
As a consequence of effective surveillance programs in Japan and Taiwan, >70% of patients in these countries are diagnosed with HCC in the early stages [4, 5, 6]. Treatment options for these patients include potentially curative therapies, such as liver resection, orthotopic liver transplantation, and ablation methods [7, 8, 9]. Most patients in other countries are diagnosed with intermediate or advanced disease, which cannot typically be cured [5]. Until recently, the only systemic therapy available for advanced HCC was the multikinase inhibitor sorafenib [7, 10]. Within the past several years, 6 additional agents have been introduced to the treatment paradigm, including lenvatinib and atezolizumab plus bevacizumab as an alternative to sorafenib in the first-line, and regorafenib, cabozantinib, ramucirumab, nivolumab (with or without ipilimumab), and pembrolizumab as subsequent-line therapies [7, 10, 11, 12, 13, 14, 15, 16, 17, 18]. However, the optimal therapeutic strategy for advanced HCC remains unclear. Due to the prevalence of HCC in the Asian region, identifying treatment options effective in these populations is of significant clinical importance.
Pembrolizumab is a programmed death-1 blocking antibody that initially showed activity in patients with HCC in the open-label, phase 2 KEYNOTE-224 study, which provided the precedent for approval in the USA, and for additional clinical research [17]. KEYNOTE-240 was a placebo-controlled phase 3 study that evaluated the efficacy and safety of pembrolizumab in combination with best supportive care (BSC) in patients who had progression after sorafenib treatment or intolerance to sorafenib treatment [19]. The results of the final analysis reported a median overall survival (OS) of 13.9 months for pembrolizumab compared with 10.6 months for placebo (hazard ratio [HR] 0.78; 95% confidence interval [CI] 0.61–1.00; p = 0.0238) and median progression-free survival (PFS) of 3.0 and 2.8 months, respectively (HR 0.72; 95% CI 0.57–0.90; p = 0.0022). These results did not reach the prespecified criteria for statistical significance but supported a favorable benefit-to-risk ratio for pembrolizumab in that population. Here, we report the outcomes of a post hoc subgroup analysis of patients in KEYNOTE-240 who were enrolled in Asia.
Methods
Study Design and Patients
KEYNOTE-240 (NCT02702401) was a double-blind, placebo-controlled, randomized phase 3 study [19]. The study protocol and all amendments were approved by the relevant ethics committee or institutional review board at each participating center, and the study was conducted in accordance with standards of Good Clinical Practice and the Declaration of Helsinki. All participants provided written informed consent prior to enrollment.
Detailed eligibility criteria for KEYNOTE-240 have been published previously [19]. In brief, patients were aged ≥18 years with a confirmed diagnosis of HCC; at least 1 measurable lesion per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1); documented radiographic progression after treatment with sorafenib or intolerance to sorafenib; Barcelona Clinic Liver Cancer (BCLC) stage C disease or stage B disease not amenable to or refractory to locoregional therapy and not amenable to curative treatment; a Child-Pugh class A liver score; Eastern Cooperative Oncology Group performance status of 0 or 1; and adequate organ function. Patients with treated or untreated HCV infection or controlled HBV infection were eligible (the latter having received antiviral therapy for at least 12 weeks with an HBV viral load <100 IU/mL before the first dose of study drug). In the current analysis, active hepatitis included patients with active HBV infection (defined as HBsAg-positive and/or -detectable HBV DNA) and active HCV infection (defined as detectable HCV RNA, which includes untreated patients and treatment failures).
Patients were randomly assigned 2:1 to receive pembrolizumab 200 mg or saline placebo by intravenous infusion once every 3 weeks. Treatment was continued until disease progression, unacceptable toxicity, withdrawal from the study, or the patient had received 35 doses of study treatment (approximately 2 years). BSC was provided to patients in both treatment arms at the discretion of the investigator per local treatment practices.
Treatment allocation was stratified by the geographic region (Asia without Japan vs. non-Asia with Japan), macrovascular invasion (yes vs. no), and alpha-fetoprotein (AFP) level (<200 vs. ≥ 200 ng/mL). Enrollment of patients from Asia was capped at approximately 30% of the total study population. In this analysis, the Asian subgroup comprised patients enrolled in Hong Kong, Japan, Korea, the Philippines, Taiwan, and Thailand.
Assessments and Endpoints
Disease progression and tumor response were assessed using computed tomography or magnetic resonance imaging. Imaging was first conducted 6 weeks after randomization, and subsequent imaging was performed every 6 weeks until disease progression, start of the new anticancer treatment, withdrawal of consent, or death, whichever occurred first. Response was assessed per RECIST 1.1 by blinded central imaging review. Patients who progressed or started a new anticancer therapy were followed up for survival every 12 weeks. Safety was monitored throughout the study and for 30 days following the end of treatment (90 days for serious adverse events [AEs] and immune-mediated AEs). AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
The primary efficacy endpoints were PFS per RECIST 1.1 by blinded central imaging review and OS. Secondary efficacy endpoints were time to progression (TTP), objective response rate (ORR), disease control rate (DCR), and duration of response (DOR) per RECIST 1.1 by blinded central imaging review. Safety and tolerability were also assessed as secondary endpoints.
Statistical Analysis
A detailed description of the statistical methods used in KEYNOTE-240 has been described previously [19]; the same methods were used in the analyses of the Asian subgroup. All analyses of the Asian subgroup were exploratory, and the nominal p values should be interpreted in this context. In brief, efficacy was analyzed in the intent-to-treat population, which included all patients randomly allocated to treatment. PFS, OS, and DOR were estimated using the nonparametric Kaplan-Meier method. Treatment differences in OS and PFS were tested using the stratified log-rank test, and a stratified Cox proportional hazards model with Efron's method of tie handling was used to estimate HRs between the treatment arms. The stratification factors (geographic region, macrovascular invasion, and AFP) with small strata collapsed as prespecified in the statistical analysis plan. Comparison of ORR and DCR between treatment arms was made using the stratified Miettinen and Nurminen method [20]. Safety was assessed in the as-treated population, which included all patients who were randomly allocated to treatment and received at least 1 dose of study drug. The data cutoff for the final analysis was January 2, 2019.
Results
Patients
A total of 413 patients were enrolled in KEYNOTE-240 between May 31, 2016 and November 23, 2017. Of these, 157 patients were enrolled in Asia and included in the Asian subgroup (pembrolizumab, n = 107; placebo, n = 50). Baseline demographics and disease characteristics are presented in Table 1. Baseline characteristics were generally similar between treatment arms in the Asian subgroup, although more patients in the pembrolizumab arm had AFP ≥200 ng/mL at baseline than in the placebo arm (51.4 vs. 40.0%, respectively) (Table 1). All 157 patients received ≥1 dose of study drug.
Table 1.
Baseline demographics and disease characteristics in the Asian subgroup and overall study population
| Characteristic | Asian subgroup |
Overall population [19] |
||
|---|---|---|---|---|
| pembrolizumab (n = 107) | placebo (n = 50) | pembrolizumab (n = 278) | placebo (n = 135) | |
| Age, median (range), years | 67 (35–85) | 65 (45–89) | 67 (18–91) | 65 (23–89) |
| Sex | ||||
| Male | 86 (80.4) | 41 (82.0) | 226 (81.3) | 112 (83.0) |
| Female | 21 (19.6) | 9 (18.0) | 52 (18.7) | 23 (17.0) |
| Region of enrolling site | ||||
| Asia without Japan | 67 (62.6) | 31 (62.0) | 67 (24.1) | 31 (23.0) |
| Japan | 40 (37.4) | 19 (38.0) | 40 (14.4) | 19 (14.1) |
| Rest of world | 0 | 0 | 171 (61.5) | 85 (62.9) |
| ECOG PS | ||||
| 0 | 59 (55.1) | 28 (56.0) | 162 (58.3) | 71 (52.6) |
| 1 | 48 (44.9) | 22 (44.0) | 116 (41.7) | 64 (47.4) |
| Child-Pugh class | ||||
| A | 107 (100.0) | 50 (100.0) | 277 (99.6) | 133 (98.5) |
| B | 0 | 0 | 1 (0.4) | 2 (1.5) |
| Overall BCLC stage | ||||
| B | 13 (12.1) | 8 (16.0) | 56 (20.1) | 29 (21.5) |
| C | 94 (87.9) | 42 (84.0) | 222 (79.9) | 106 (78.5) |
| HBV active positivea, c | 55 (51.4) | 25 (50.0) | 72 (25.9) | 29 (21.5) |
| HCV active positiveb, c | 18 (16.8) | 8 (16.0) | 43 (15.5) | 21 (15.6) |
| Discontinuation of previous sorafenib | ||||
| Intolerance | 7 (6.5) | 2 (4.0) | 36 (12.9) | 18 (13.3) |
| PD | 100 (93.5) | 48 (96.0) | 242 (87.1) | 117 (86.7) |
| Extrahepatic disease | 86 (80.4) | 37 (74.0) | 195 (70.1) | 93 (68.9) |
| Macrovascular invasion | 13 (12.1) | 6 (12.0) | 36 (12.9) | 16 (11.9) |
| AFP ≥200 ng/mL | 55 (51.4) | 20 (40.0) | 129 (46.4) | 58 (43.0) |
All data are n (%) unless stated otherwise. AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; BSC, best supportive care; ECOG PS, Eastern Cooperative Oncology Group performance status; HBV, hepatitis B virus; HCV, hepatitis C virus; PD, progressive disease; Q3W, every 3 weeks.
52 (48.6%) and 25 (50.0%) patients were negative for HBV in the pembrolizumab and placebo groups, respectively.
89 (83.2%) and 42 (84.0%) patients were negative for HCV in the pembrolizumab and placebo groups, respectively.
Patients with active hepatitis.
At the time of the data cutoff (January 2, 2019), 100 patients (93.5%) in the pembrolizumab arm and all patients (100%) in the placebo arm had discontinued treatment (see online suppl. Fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000515553). The most common reason for discontinuation was progressive disease, which occurred in 83 patients (77.6%) in the pembrolizumab arm and 45 patients (90%) in the placebo arm. Treatment was ongoing in 6 patients (5.6%), all of whom were in the pembrolizumab arm. The median duration of follow-up was 13.8 months (range, 1.0–27.0) in the pembrolizumab arm and 8.3 months (range, 1.6–25.3) in the placebo arm. The median duration of treatment was 3.4 months (range, 0.0–23.4) and 2.1 months (range, 0.0–16.4) in the pembrolizumab and placebo arms, respectively. Following disease progression, subsequent anticancer therapies were used by 50 patients (46.7%) in the pembrolizumab arm and 19 patients (38.0%) in the placebo arm. Although the overall proportion of patients receiving post-study anticancer therapies were lower in the placebo arm than in the pembrolizumab arm (likely because most placebo patients did not live long enough to start post-study anticancer therapy), examination of subsequent anticancer therapy use over time among those alive showed that the proportions of patients who started new anticancer therapy were similar (up to 5 months) or higher (up to 20 months) in the placebo arm than in the pembrolizumab arm (online suppl. Fig. 2).
Efficacy
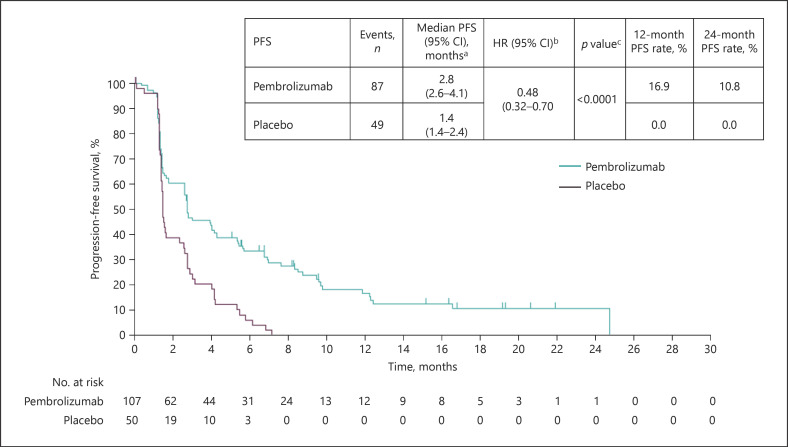
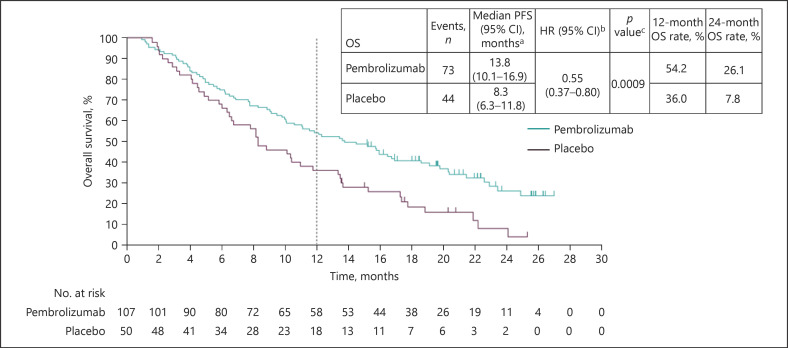
The median PFS was 2.8 months (95% CI 2.6–4.1) in the pembrolizumab arm and 1.4 months (95% CI 1.4–2.4) in the placebo arm (HR 0.48; 95% CI 0.32–0.70; p < 0.0001), as shown in Figure 1. Kaplan-Meier estimates of PFS rates at 12 months were 16.9% (95% CI 9.8–25.6) and 0.0% (95% CI not applicable [NA]–NA) for pembrolizumab and placebo arms, respectively. Kaplan-Meier estimates of PFS rates at 24 months were 10.8% (95% CI 5.1–19.1) and 0.0% (95% CI NA–NA) for pembrolizumab and placebo arms, respectively. The median TTP was 2.8 months (95% CI 2.6–4.3) in the pembrolizumab arm and 1.4 months (95% CI 1.4–2.4) in the placebo arm (HR 0.46; 95% CI 0.31–0.68; p< 0.0001). As of January 2, 2019, 72 patients (67.3%) in the pembrolizumab arm and 44 patients (88.0%) in the placebo arm had died. The median OS was 13.8 months (95% CI 10.1–16.9) in the pembrolizumab arm and 8.3 months (95% CI 6.3–11.8) in the placebo arm (HR 0.55; 95% CI 0.37–0.80; p = 0.0009), as shown in Figure 2. Kaplan-Meier estimates of OS rates at 12 months were 54.2% (95% CI 44.3–63.1) and 36.0% (95% CI 23.1–49.1) for the pembrolizumab and placebo arms, respectively. Kaplan-Meier estimates of OS rates at 24 months were 26.1% (95% CI 16.8–36.5) and 7.8% (95% CI 1.7–20.3) for the pembrolizumab and placebo arms, respectively.
Fig. 1.
Kaplan-Meier estimates of PFS per RECIST v1.1 by blinded central imaging review in the Asia subgroup (ITT population). CI, confidence interval; HR, hazard ratio; ITT, intent to treat; PFS, progression-free survival; RECIST v1.1, Response Evaluation Criteria in Solid Tumors, version 1.1.
Fig. 2.
Kaplan-Meier estimates of OS per RECIST 1.1 by blinded central imaging review in the Asia subgroup (ITT population). CI, confidence interval; HR, hazard ratio; ITT, intent to treat; OS, overall survival; RECIST v1.1, Response Evaluation Criteria in Solid Tumors, version 1.1.
ORR was 20.6% (95% CI 13.4–29.5) in the pembrolizumab arm and 2.0% (95% CI 0.1–10.6) in the placebo arm (treatment difference: 18.5%; 95% CI 8.3–27.6; p = 0.00135) (Table 2). DCR was 59.8% (95% CI 49.9–69.2) in the pembrolizumab arm and 40.0% (95% CI 26.4–54.8) in the placebo arm (treatment difference: 20.1%; 95% CI 3.1–35.7; p = 0.01014). The median time to response was 1.6 months (range, 1.2–9.8) in the pembrolizumab arm and 1.4 months for the 1 patient who responded in the placebo arm. The median DOR was 8.6 months (range, 3.8 + to 23.5) and 2.8 months for the pembrolizumab and placebo arms, respectively. The Kaplan-Meier estimate of patients with a DOR of ≥12 months was 7 (40.6%) patients in the pembrolizumab arm. The 1 patient in the placebo arm did not have an extended response duration.
Table 2.
Best overall response per RECIST 1.1 by blinded central imaging review in the Asian subgroup
| Response | Pembrolizumab (n = 107) |
Placebo (n = 50) |
Difference in rate vs. placebo |
|||
|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | % (95% CI)a | p valueb | |
| ORR | 22 | 20.6 (13.4–29.5) | 1 | 2.0 (0.1–10.6) | 18.5 (8.3–27.6) | 0.0014 |
| DCR | 64 | 59.8 (49.9–69.2) | 20 | 40.0 (26.4–54.8) | 20.1 (3.1–35.7) | 0.0101 |
AFP, alpha-fetoprotein; CI, confidence interval; DCR, disease control rate; ORR, objective response rate. a Based on the Miettinen and Nurminen method stratified by geographic region (Asia without Japan vs. non-Asia with Japan), macrovascular invasion (yes vs. no) and AFP level (<200 vs. ≥200 ng/mL) with small strata collapsed as prespecified in the statistical analysis plan. b One-sided p value for testing. H0: difference in % = 0 versus H1: difference in % >0.
Safety
AEs of any cause occurred in 103 patients (96.3%) in the pembrolizumab arm and 45 patients (90.0%) in the placebo arm (Table 3). Grade 3–5 AEs were reported in 51 patients (47.7%) and 19 patients (38.0%) in the pembrolizumab and placebo arms, respectively. AEs leading to treatment discontinuation occurred in 9 patients (8.4%) in the pembrolizumab arm and 2 patients (4.0%) in the placebo arm. Serious AEs were reported by 31 patients (29.0%) and 9 patients (18.0%) in the pembrolizumab and placebo arms, respectively.
Table 3.
Safety summary
| AE, n (%) | Pembrolizumab (n = 107) | Placebo (n = 50) |
|---|---|---|
| Any grade AE | 103 (96.3) | 45 (90.0) |
| Grades 3–5 | 51 (47.7) | 19 (38.0) |
| Serious AE | 31 (29.0) | 9 (18.0) |
| Led to discontinuation | 9 (8.4) | 2 (4.0) |
| TRAE | 63 (58.9) | 24 (48.0) |
| Grades 3–5 | 14 (13.1) | 2 (4.0) |
| Serious AE | 11 (10.3) | 0 (0.0) |
| Led to death | 0 (0.0) | 0 (0.0) |
| Led to discontinuation | 3 (2.8) | 0 (0.0) |
AE, adverse event; TRAE, treatment-related AE.
Treatment-related AEs (TRAEs) occurred in 63 patients (58.9%) in the pembrolizumab arm and 24 patients (48.0%) in the placebo arm. Grade 3–5 TRAEs occurred in 14 patients (13.1%) and 2 patients (4.0%) in the pembrolizumab and placebo arms, respectively. TRAEs leading to discontinuation occurred in 3 patients (2.8%) in the pembrolizumab arm and no patients in the placebo arm. One patient in the pembrolizumab arm died due to an AE; this was not considered related to treatment. Immune-mediated AEs occurred in 24 patients (22.4%) in the pembrolizumab arm and 2 patients (4.0%) in the placebo arm. These events were grades 3–5 in 7 (6.5%) patients in the pembrolizumab arm. No patient in the placebo arm had a grade 3–5 event. Immune-mediated hepatitis events on the basis of investigator assessment and sponsor assessment were infrequent in the pembrolizumab arm (1 [0.9%] and 2 [1.9%], respectively), and no immune-mediated hepatitis events by either assessment occurred in the placebo arm. No patients experienced HBV or HCV flares.
Discussion
The results from this subgroup analysis of patients in KEYNOTE-240 enrolled in Asia were generally consistent with those of the overall cohort [19] but with a trend toward improved efficacy among patients receiving pembrolizumab in the Asian subgroup. It is notable that pembrolizumab reduced the risk of death by 45% in the Asian subgroup compared with 22% in the overall cohort [19]. In the overall cohort, the smaller-than-expected risk of death reduction was attributed primarily to the higher-than-expected OS in the placebo group, which was thought to in part reflect the effect on survival of post-study treatment with newly approved agents, such as regorafenib and nivolumab [19]. The OS in the placebo arm of the Asian subgroup analysis was 8.3 months, which was closer to the expected survival time for this patient group, rather than 10.6 months reported in the overall cohort [19]. The trend toward improved outcomes in the Asian cohort compared with the overall cohort may have been the result of a combination of factors, including the availability of post-study anticancer therapy and inherent geographical differences in survival among patients with HCC. The OS HR in the overall population of the KEYNOTE-240 study may have been diluted by the post-study anticancer therapy because sensitivity analyses that adjusted for the use of such medication resulted in a lower HR [19]. In addition, shorter survival times in Asian patients receiving placebo versus non-Asian patients receiving placebo were expected given evidence from published studies [21, 22]. In patients with advanced HCC receiving sorafenib, the median OS in the placebo arm of a study conducted in Europe, North America, South America, and Australasia was 7.9 months [21], whereas the median OS in the placebo arm of a study conducted in China, South Korea, and Taiwan was 4.2 months [22]. It is possible that with shorter survival time in Asian patients, a smaller fraction of placebo patients had an opportunity to start new anticancer therapies compared with patients outside of Asia, which led to less dilution of the treatment effect by post-study anticancer therapy. Furthermore, published reports examining immune checkpoint inhibitors in patients with HCC have shown a trend toward greater survival in patients with a viral hepatitis etiology of HCC [18, 19].
The Asian cohort was balanced between the pembrolizumab and placebo arms but it did differ from the overall cohort in some important disease characteristics, including a higher proportion of the Asian cohort having an overall BCLC stage C, active HBV, and extrahepatic disease (Table 1). The median PFS was similar between the Asian subgroup and the overall cohort, with an improved PFS observed with pembrolizumab compared with placebo in the former. The plateau in the Kaplan-Meier curve for PFS between 13 and 25 months for the Asian subgroup was also indicative of a long-term benefit for some patients. The treatment difference in ORR was also larger in the Asian subgroup than in the overall cohort (18.5 vs. 13.8%). The median DOR, however, was shorter in the Asian subgroup than in the overall cohort. This may be a consequence of the Asian subgroup having more advanced disease, as is reflected in the higher proportion of patients in this population having BCLC stage C and extrahepatic disease. Safety and tolerability in the Asian subgroup were similar to that observed in the overall cohort, except that the discontinuation rate due to AEs was less than half that reported in the overall cohort. Immune-mediated AEs occurred in a generally similar proportion of patients in the Asian subgroup (pembrolizumab, 22.4%; placebo, 4.0%) and overall cohort (pembrolizumab, 18.0%; placebo, 8.2%), although the proportion of patients with AEs in the placebo arm of the Asian cohort was lower than that in the overall cohort [19]. Immune-mediated hepatitis events were infrequent and occurred in a similar proportion of patients in the Asian subgroup and overall cohort. The safety results were also consistent with the known profile of pembrolizumab.
Similar results to those observed in this analysis have been reported for nivolumab. In a post hoc analysis of the phase 1/2 CheckMate 040 study, the efficacy and safety of nivolumab in Asian patients was found to be comparable to the intent-to-treat population [23]. The differences in demographics were also similar to the Asian subgroup in this analysis, with a higher proportion of Asian patients having HBV-related HCC and more patients having advanced disease. Of note, the phase 3 study KEYNOTE-394 is underway and is evaluating pembrolizumab plus BSC compared with placebo plus BSC in previously treated Asian patients with advanced HCC (ClinicalTrials.gov identifier NCT03062358). By design, ∼80% of patients in KEYNOTE-394 are from mainland China, and this study differs from the Asian subgroup in KEYNOTE-240. This study will further elucidate the treatment effects observed in this subanalysis as well as the overall efficacy and safety of pembrolizumab in combination with BSC in patients with advanced HCC who are of Asian descent.
Trials investigating other second-line agents for HCC have also reported regional variation in outcome. The phase 3 RESORCE study, which investigated regorafenib in patients with HCC who had progressed on sorafenib, showed a trend toward improved OS, PFS, and TTP in Asian patients [13]. In the phase 3 REACH-2 study, which investigated ramucirumab after sorafenib in patients with advanced HCC who had increased AFP concentrations, the OS HRs were 0.65 for the Japanese cohort; 0.76 for the cohort that included patients from the Americas, Europe, Israel, and Australia; and 0.83 for the Asian cohort (without Japan) [15]. The results of the phase 3 CELESTIAL trial, which investigated cabozantinib in patients with previously treated advanced HCC, showed that patients from the Asian region gained no benefit from treatment with cabozantinib compared with the overall population (HR for death 1.01 vs. 0.76, respectively) [14]. These results support the importance of evaluating regional differences in response to treatment in future clinical trials.
There are several factors that should be considered when interpreting the results of this subgroup analysis. First, the selection of a subgroup results in a smaller study population for analysis, and smaller sample sizes may limit interpretation. Second, the population of patients included in the KEYNOTE-240 study were selected to have well-preserved liver function. Therefore, these patients may not reflect the broader population of patients who are treated in the real-world setting. The patients in the Asian subgroup were also more likely to have active HBV, BCLC stage C, and extrahepatic disease. The relatively higher rate of active HBV reflects the underlying epidemiology of HCC in Southeast Asian and East Asian countries, whereas HCV is more commonly the cause in Japan and the West [3].
Conclusion
The results of this analysis show that pembrolizumab had antitumor activity and a manageable safety profile in Asian patients with previously treated advanced HCC in the KEYNOTE-240 study. Furthermore, the results observed in the current analysis support a trend toward superior outcomes with pembrolizumab in the Asian subgroup than were reported for the overall cohort. Additional analysis validating the findings observed herein will elucidate the role of pembrolizumab in improving outcomes specifically in Asian patients. Notwithstanding, these results support the growing body of evidence that there may be regional differences in the treatment response for HCC, supporting a favorable benefit-to-risk ratio for use of second-line pembrolizumab in Asian patients with previously treated advanced HCC.
Statement of Ethics
The study protocol and all amendments were approved by the relevant ethics committee or institutional review board at each participating center, and the study was conducted in accordance with standards of Good Clinical Practice and the Declaration of Helsinki. All participants provided written informed consent prior to enrollment. Trial Registration: Clinicaltrials.gov: NCT02702401.
Conflict of Interest Statement
M. Kudo is the editor-in-chief of Liver Cancer. M. Kudo reports grant fees from Eisai, EA Pharma, Gilead Sciences, Taiho, Sumitomo Dainippon Pharma, Takeda, Otsuka, and AbbVie and personal fees from Eisai, Bayer, Bristol Myers Squibb, Eli Lilly, EA Pharma, Ono, and Roche. A.-L. Cheng is an associate editor of Liver Cancer. A.-L. Cheng reports consulting fees from AstraZeneca, Bristol Myers Squibb, Merck Serono, Exelixis, Nucleix, Ipsen Innovation, Bayer Healthcare, BeiGene, CSR Pharma Group, F. Hoffmann-La Roche, and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.; consulting and speakers bureau fees from Eisai, Novartis, and Ono Pharmaceutical; consulting and travel support fees from Roche/Genentech and IQVIA; and travel support and speakers bureau fees from Bayer Yakuhin. S. Ogasawara reports grants and personal fees from Bayer, Eisai, and Eli Lilly and personal fees from AstraZeneca and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.. M. Kurosaki reports lecture fees and advisory consulting fees from Eli Lilly, Bayer, and Eisai and lecture fees from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.. T. Yamashita is an editorial board member of Liver Cancer. K.-H. Lee reports other for advisory board from Bayer, Ono Pharmaceutical, Roche, Eisai, and AstraZeneca. E. Chen and A.B. Siegel are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.. H.Y. Lim, Y. Chao, T. Yau, N. Morimoto, K. Ohkawa, and B.-Y. Ryoo report no conflicts of interest.
Funding Sources
This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Author Contributions
M. Kudo contributed substantially to the acquisition of data and interpretation of the results; H.Y. Lim contributed substantially to the conception, design, or planning of the study, acquisition of data, analysis of the data, interpretation of the results, and drafting the manuscript; A-L. Cheng contributed substantially to the conception, design, or planning of the study, acquisition of data, and interpretation of the results; Y. Chao contributed substantially to the acquisition of data and interpretation of the results; T. Yau contributed substantially to the acquisition of data, analysis of the data, and interpretation of the results; S. Ogasawara contributed substantially to the acquisition of data; M. Kurosaki contributed substantially to acquisition of data; N. Morimoto contributed substantially to the acquisition of data; K. Ohkawa contributed substantially to the acquisition of data and interpretation of the results; T. Yamashita contributed substantially to the interpretation of the results; K-H. Lee contributed substantially to the acquisition of data and interpretation of the results; A.B. Siegel contributed substantially to the conception, design, or planning of the study, acquisition of data, analysis of the data, interpretation of the results, and drafting the manuscript; E. Chen contributed substantially to analysis of the data and interpretation of the results; B-Y. Ryoo contributed substantially to the conception, design, or planning of the study, acquisition of data, analysis of the data, interpretation of the results, and drafting the manuscript. All authors contributed substantially to critically reviewing or revising the manuscript for important intellectual content, provided approval of the final version of the manuscript to be submitted and published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Availability of Data and Material
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the USA and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Supplementary Material
Supplementary data
Acknowledgements
We thank the patients and their families and caregivers for participating in this trial, and all investigators and site personnel. We also thank Ariadna Holynskyj, MD (formerly of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA), for her contributions in the development of this article. Medical writing and editorial assistance were provided by Jemimah Walker of ApotheCom (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68((6)):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut Liver. 2016;10((3)):332–9. doi: 10.5009/gnl15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayaraman T, Lee YY, Chan WK, Mahadeva S. Epidemiological differences of common liver conditions between Asia and the West. JGH Open. 2020;4((3)):332–9. doi: 10.1002/jgh3.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudo M, Izumi N, Kubo S, Kokudo N, Sakamoto M, Shiina S, et al. Report of the 20th Nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res. 2020;50((1)):15–46. doi: 10.1111/hepr.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16((10)):589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35((9)):2155–66. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29((Suppl 4)):iv238–55. doi: 10.1093/annonc/mdy308. [DOI] [PubMed] [Google Scholar]
- 8.EASL Clinical Practice Guidelines Management of hepatocellular carcinoma. J Hepatol. 2018;69((1)):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68((2)):723–50. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network . Hepatobiliary cancers. Version 4.2020. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2020. NCCN clinical practice guidelines in oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391((10126)):1163–73. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 12.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382((20)):1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389((10064)):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 14.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379((1)):54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20((2)):282–96. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 16.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389((10088)):2492–502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19((7)):940–52. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 18.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkmate 040 randomized clinical trial. JAMA Oncol. 2020;6((11)):e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38((3)):193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 20.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4((2)):213–26. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
- 21.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359((4)):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 22.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10((1)):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 23.Yau T, Hsu C, Kim T-Y, Choo S-P, Kang Y-K, Hou M-M, et al. Nivolumab in advanced hepatocellular carcinoma: sorafenib-experienced Asian cohort analysis. J Hepatol. 2019;71((3)):543–52. doi: 10.1016/j.jhep.2019.05.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the USA and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.