Abstract
The present clinical case reports an increased zone of keratinized gingiva that was generated following surgical excision of the gingiva during periodontally accelerated osteogenic orthodontics. The present case consists of angle Class I with mal-aligned teeth and impacted #11. The patient was evaluated up to 2 years and 1 month (2.1) showing a stable increased zone of keratinized tissue. Possible causes for this event are discussed in this case report. Additional long-term clinical studies are necessary to support these results.
Keywords: Continuous stimulation of gingival growth, gingival growth, periodontally accelerated osteogenic orthodontics, wilckodontics
Clinical Relevance
Scientific rationale: A secondary effect of the PAOO has shown to optimize the band of keratinized tissue by a process we defined as “crawling” attachment. A case of PAOO for resolution of bilateral Angel class I with mal-aligned teeth and impacted #11 is presented. Principal Findings: The PAOO resulted in an increase in the band of keratinized tissue during treatment. During PAOO, the band of KT increased progressively and stabilized at the 2 years and 1 month (2.1 years) postoperative visit. Practical implications: In addition to the acceleration to tooth movement, a single intervention of PAOO seems to be an effective treatment that increases the band of KT. Increasing the band of keratinized tissues may facilitate homeostasis of the periodontal tissues if committed with periodontal diseases due to difficulty of conducting ideal oral hygiene (OH), especially in the presence of local factors that interfere with an ideal OH regimen.
Background
The zone of keratinized and attached gingiva may regenerate following surgical excision of the gingiva.[1] A clinical study showed that 1 month after the removal of the entire zone of keratinized and attached gingiva either by a “gingivectomy” or a “flap-excision” procedure, all “gingivectomy” units and approximately 64% of the “flap-excision” units demonstrated presence of a zone of keratinized gingiva. Previous reports on healing following mucogingival surgery in monkeys and dogs[2,3,4] supports this finding. Karring and collaborators have characterized the healing process of a surgical periodontal wound. A granulation tissue is formed, over which epithelial cells migrate from the surrounding tissues. The properties of the newly formed tissue will depend on the properties of the tissue that originated the granulation tissue. If the granulation tissue originated from the periodontal ligament and the adjacent connective tissue, the surface of the epithelium becomes keratinized.[3,5,6]
However, the progressive event that occurs with the keratinized tissue (KT) during the periodontally accelerated osteogenic orthodontics (PAOO) is still to be characterized histologically and further studied clinically.
The present clinical case is a report of a bilateral Angle class I with mal-aligned teeth and impacted #11 patient which had the band of KT evaluated 6-month postoperatively while being submitted to PAOO.
Procedure
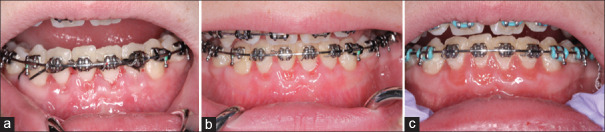
A 14.7-year-old Caucasian female presented for periodontal evaluation for PAOO procedure. The clinical evaluation revealed moderate gingivitis on an intact periodontium with probing depths ranging from 2 mm to 3 mm. The patient presented slight marginal erythematous tissues characterized by a thin biotype with scalloped gingival margins and a limited width of KT [Figure 1a and b]. The patient presented with bilateral angle Class I with mal-aligned teeth and impacted #11. The radiographic examination showed absence of vertical or horizontal bony defects. Cone beam computerized tomography (CBCT) scan revealed a thin mandibular labial bone [Figure 1c and d].
Figure 1.
Preoperative frontal (a) and lingual (b) views. Note presence of c d slight marginal erythematous tissues localized to the mandibular arch. Note the thin biotype with scalloped gingival margins and a limited width of keratinized tissue that ranges from 2 to 3 mm from #23 to 26. Preoperative cone-beam computerized tomography scan showing lingual view of the mandibular incisors (c), and a thin mandibular labial bone on the transaxial section for tooth #22 (d)
The patient was informed that the optimal treatment plan involved orthodontic mandibular and maxillary arch expansion using PAOO. The patient signed the consent forms and the procedure and PAOO were scheduled 2 weeks prior to initiation of orthodontic movement. PAOO consisted of intrasulcular incisions from #21 to 28. A full-thickness buccal flap was elevated, and 2–3 mm depth vertical corticotomy was conducted on the proximal bone with no. 2 round carbide bur extending to distal aspect of #21–28 under profuse irrigation with saline solution [Figure 2a]. Undermining incision was conducted at the base of the buccal flap. Cortico-cancellous bone chips (MinerOss®, Biohorizons, Birmingham, AL, USA) were mixed with bone mineral matrix (Equimatrix®, Osteohealth, Shirley, NY, USA), hydrated with saline, and placed on the buccal aspect of the alveolar ridge. The flap was re-positioned passively using continuous interrupted sling sutures with 4.0 silk thread [Figure 2b]. Digital pressure was applied with gauze for hemostasis. Postoperative instructions were given written and verbally to patient. The patient was escorted home.
Figure 2.
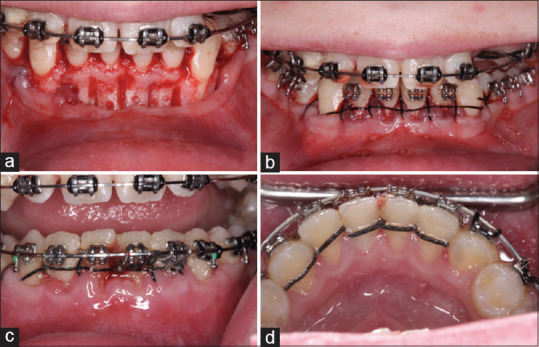
Transoperative view after full-thickness flap elevation form #23 to 26. (a) Note interdental linear perforations and single cortical perforations made from #23 to 26. (b) Continuous sling suture made with 4-0 silk thread. (c) Frontal and (d) lingual views of mandibular arch 1-week postoperatively. Note the band of keratinized tissue is approximately 2–3 mmm as measured in the preoperative frontal view
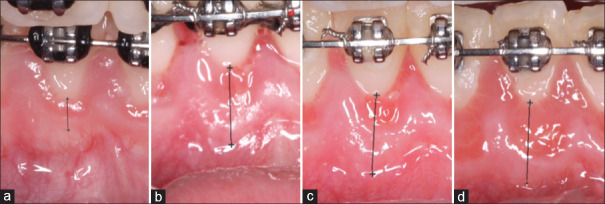
The healing process was uneventful during the PAOO. Postoperative [Figure 2c and d] evaluations of the mean band width of KT increase at baseline [Figure 1a], 2-week [Figure 3a], 4-week [Figure 3b], and 6-month [Figure 3c) postoperatively. All pictures were calibrated using the preestablished size of the bracket slot that is 0.022 inches (0.5588 mm) for further measurement using Photoshop-based image analysis (Adobe Photoshop CC, 2019 v20.0.6 software, Adobe Systems, San Jose, CA, USA). Next, calibrated measurement of the band width of the KT from #23 to 26, for baseline, 2-weeks, 4-week, and 6-month postoperatively to PAOO, the values were compiled on Table 1.
Figure 3.
Frontal view of the mandibular arch 2-week (a), 4-week (b), and 6-month (c) postoperatively. Note a progressive increase in the band of keratinized tissue from an additional 1–2 mm at 4-month to 3–4 mm at 6-month (c), and postoperatively
Table 1.
Width of keratinized tissue band during the evaluation time from baseline to 6-month postoperatively
| Band of KT/tooth in millimeters)/time period | #26 | #25 | #24 | #23 | Total | Mean value (mm) |
|---|---|---|---|---|---|---|
| Baseline | 1.25 | 1.31 | 1.51 | 1.43 | 5.50 | 1.37 |
| 2-weeks | 4.40 | 6.70 | 3.67 | 3.69 | 18.46 | 4.61 |
| 4-weeks | 5.71 | 7.19 | 5.54 | 3.99 | 22.43 | 5.60 |
| 6-months | 7.01 | 7.87 | 5.42 | 6.95 | 27.25 | 6.81 |
| Mean value/tooth | 4.65 | |||||
| Initial-final band of KT (mm) | 5.76 | 6.59 | 3.91 | 5.52 | 5.44 |
At baseline, the mean band width of KT for #23–26 was 1.37 mm. At the 6-month postoperative evaluation, it was 6.81 mm. That results in a mean value for crawling attachment for #23-26 of 5.44 mm in a 6-month period of PAOO. That is a rate of 0.90 mm/month. The highest value of crawling attachment obtained during the 6-month period of PAOO in this case report was for #25. At baseline, #25 showed a band width of KT of 1.31 mm. At 6-month, the band increased to 7.87 mm, which is a crawling attachment of 6.56 mm augmentation at a rate of 1.09 mm/month [Table 1].
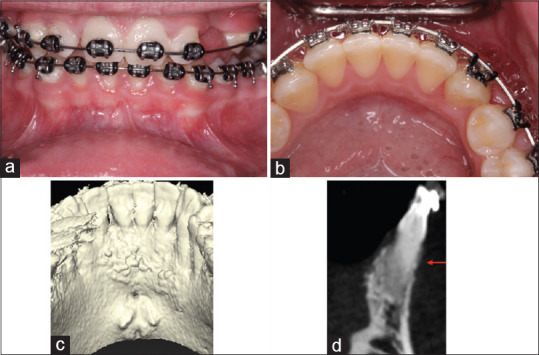
The “Crawling attachment” means reduction during the nonactive (from 6 months to 2.1 years) period of orthodontic treatment from #23 to 26 was 2.59 mm and is detailed in Table 2. The total value of the CA for teeth #23–26 was 10.39 mm at 2.1 years after PAOO [Figure 4]. The 2.1-year follow-up showed stability of the soft tissues in spite of the slight reduction of the band of KTs. At 2.1 years PO, the general band of KT was 10.4 mm wider when compared to baseline values, giving a mean increase for teeth #23–26 of 2.6 mm [Table 2 and Figure 5a]. At the 2.1-year follow-up, the periodontal tissues showed probing depths similar to the presurgical numbers. Comparison of the baseline [Figure 4] and the 2.1-year CBCT scans [Figure 5b] shows stability of the image and increased periodontal osseous tissues [Figure 5c].
Table 2.
Width of keratinized tissue band during the evaluation time from 6-month postoperatively to 2.1 years’ postoperatively
| Band of KT/tooth in millimeters)/time period | #26 | #25 | #24 | #23 | Total | Mean value (mm) |
|---|---|---|---|---|---|---|
| Baseline | 1.25 | 1.31 | 1.51 | 1.43 | 5.50 | 1.37 |
| 6-months | 7.01 | 7.87 | 5.42 | 6.95 | 27.25 | 6.81 |
| 2.1 years PO | 3.92 | 4.78 | 3.82 | 4.34 | 15.9 | 3.97 |
| Reduction from 6-month PO | 3.09 | 3.09 | 1.60 | 2.61 | 10.39 | 2.59 |
| Increase from baseline | 2.67 | 3.47 | 2.31 | 2.91 | 10.4 | 2.6 |
Figure 4.
Calibrated images showing measurement of the band of keratinized tissue for (a) baseline, (b) 2-week, (c) 4-week, and (d) 6-month postoperatively for tooth #25. The measurements made after calibration of the image were in millimeters: A = 1.31, B = 6.70, C = 7.19, D = 7.87. Note a progressive increase in the band of keratinized tissue
Figure 5.
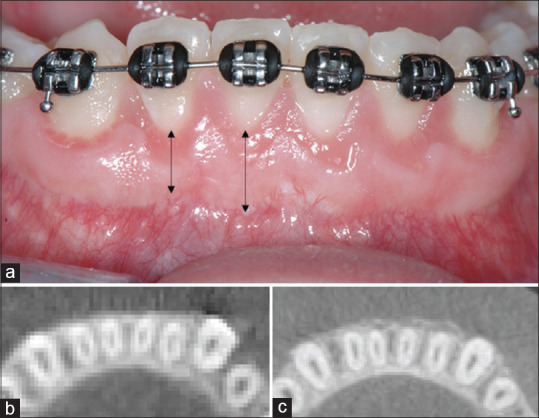
(a) Frontal view of the buccal aspect of the mandibular anterior teeth 2.1-year postoperatively to periodontally accelerated osteogenic orthodontics. Note the presence of stable band of keratinized tissue that ranged from 4 to 6 mm. (b) Preoperative and (c) 2.1 years' postperiodontally accelerated osteogenic orthodontics cone beam computerized tomography axial section. Note presence buccal bone increased on the mandibular anterior teeth (b), where prior to periodontally accelerated osteogenic orthodontics was absent (c)
Discussion
The authors have defined crawling attachment as an increase in clinical texture of the gingival tissues, which occurs during the active phase of PAOO. This band of KT may reduce during the inactive stage of orthodontic therapy; however, it stabilized within approximately 1.6 years of the inactive PAOO treatment phase.
During PAOO, the intra-sulcular incision exposes the superjacent tissues; which include the periodontal ligament, connective tissues, and epithelial attachment. Epithelialization from the peripheral gingival margins starts within 24 h and becomes complete after 1–5 weeks, depending on local traumatic factors.[7] The exposure of these tissues stimulates their regenerative potential.[8]
Gingival wound healing comprises a series of sequential cellular signaling processes that allow the closure of breaches in the masticatory mucosa.[9] During wound healing, tension derived from the ectodermal mesenchymal cells transmitted from the collagen fibers to the cells is also important for the modulation of gene expression, cell proliferation, and locomotion.[10]
Fibroblasts play an essential role in the angiogenic process during wound healing. They produce extracellular matrix molecules[11] and release vascular endothelial growth factor,[12] transforming growth factor-b,[13] and platelet-derived growth factor,[14] which are responsible for the regeneration of the tissues.
In addition, mechanical forces play an important role in the organization, growth, and function of tissues. Skin fibroblasts have shown to display varied mechanotransduction properties and biochemical reactions in response to applied mechanical stimulation, which contributes to the increased susceptibility to hypertrophic tissue formation at certain areas of the body characterized by higher skin and muscle tension. Mechanical stimulation is believed to play a major role in the pathogenesis of human hypertrophic tissue. Fibroblasts from scapular upper back skin, subjected to mechanical loads proliferated at a higher rate during in vitro culturing when compared to those that did not receive mechanical stimulation.[15]
Cell migration and mechanical stimulation have shown to play a role in regenerative processes. The suggested en masse fibroblast migration seems to have a degree of coordination in fibroblast behavior during wound healing in vivo.[16] Traction forces applied simultaneously by a group of migrating cells have shown to give rise to large-scale deformation of the matrix, as well as stress and mechanical property gradients across the matrix.[17] Mechanical Stretch have shown to upregulated cell proliferation and collagen synthesis of mesenchymal stem cells and ligament fibroblast in vitro.[18]
Clinically, mechanical stress has shown to stimulate gingival overgrowth (GO). Patients undergoing orthodontic treatment even while maintaining excellent oral hygiene, have shown GO.[19] Mechanical stresses have shown to increase fibroblast production of MMP-9, and to onset gingival growth. These same growth factors were shown at higher levels in the crevicular sulcus of patients undergoing orthodontic treatment and under a strict periodontal maintenance regimen.[20]
The present study showed an average band width of KT of 1.37 mm at baseline and a mean increase during active orthodontic treatment of 6.81 mm 6 months' postoperatively to the PAOO treatment. The mean increase in the width of KT during this time was 5.44 mm. The authors define this phenomenon as “crawling” attachment. The authors suggest that this phenomenon can be a result of mechanical stimulus of the orthodontic tooth movement after surgically stimulating the site by the periodontally accelerated procedure osteogenic orthodontic. This phenomenon does not only seem to stimulate bone regeneration, but also, keratinization of the soft tissues.
Keratinized gingiva height increases after alveolar corticotomy and bone augmentation was evaluated by Wilcko et al.[21] In this study, changes in the band of KT were registered after orthodontic treatment with alveolar corticotomy, with and without bone graft. An average of 1.5 years' postoperatively showed a 0.78 mm increase in the band of KT when compared to a reduction of 0.38 mm in the group that did not receive corticotomy and bone graft. The present case report concurs with the conclusions from Wilcko et al., which indicate that orthodontic therapy combined with alveolar decortication and augmentation bone grafting resulted in an increase in KT height.
The CeA was described as occurring after mucogingival procedures.[22] This CeA is a postoperative migration of the gingival marginal tissue in a coronal direction, which seems to be best observed on lower teeth with narrow recessions.[22] Clinical reports on creeping attachment after free gingival grafts varied from 0.12 to 3.5 mm during a period of 2 years.[22,23,24,25] Our report shows a continuous augmentation of 0.90 mm/month for the keratinized gingival tissues submitted to PAOO during the active phase. This could be partially explained by the CeA phenomenon described earlier by Matter and Cimasoni in 1976,[22] and further supported by clinical reports.[24,25] This phenomenon is in agreement with the findings of the present case report, which evaluated the crawling attachment in the anterior mandible region.
The authors believe that the explanation for the CeA phenomenon is bifold. In addition to the effect of mechanostimulation of fibroblasts as a stimulant for fibroblast proliferation and growth, as shown by Kuang et al.,[15] the origin of the granulation tissue will dictated the fate of the tissue in question, as shown by Karring et al. in 1971. If the granulation tissue originated from the periodontal ligament and the adjacent connective tissue, the surface of the epithelium becomes keratinized.[3]
Classic literature indicates the regenerative potential of the periodontal ligament and its adjacent tissues. The present case report shows that in addition to a periodontally accelerated orthodontic osteogenic procedure, a form of regeneration of the periodontal gingival tissues occurs during the orthodontic treatment. The authors propose a name change of the technique from PAOO to periodontally accelerated gingivo-alveolar regenerative orthodontics. In addition, the authors propose that the PAGARO occurs by means of a proposed nomenclature for the increase in the periodontal gingival tissues, denominated “crawling” attachment.
Conclusion
This case report indicates that PAOO may augment the band width of KT during the PAOO treatment at an average of 0.90 mm/month and that this band of KT is stable for 1.5 years after PAOO treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wennström J. Regeneration of gingiva following surgical excision. A clinical study. J Clin Periodontol. 1983;10:287–97. doi: 10.1111/j.1600-051x.1983.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 2.Smith RM. A study of the intertransplantation of gingiva. Oral Surg Oral Med Oral Pathol. 1970;29:169–77. doi: 10.1016/0030-4220(70)90076-9. [DOI] [PubMed] [Google Scholar]
- 3.Karring T, Ostergaard E, Löe H. Conservation of tissue specificity after heterotopic transplantation of gingiva and alveolar mucosa. J Periodontal Res. 1971;6:282–93. doi: 10.1111/j.1600-0765.1971.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 4.Wennström J, Lindhe J. Role of attached gingiva for maintenance of periodontal health. Healing following excisional and grafting procedures in dogs. J Clin Periodontol. 1983;10:206–21. doi: 10.1111/j.1600-051x.1983.tb02208.x. [DOI] [PubMed] [Google Scholar]
- 5.Karring T, Cumming BR, Oliver RC, Löe H. The origin of granulation tissue and its impact on postoperative results of mucogingival surgery. J Periodontol. 1975;46:577–85. doi: 10.1902/jop.1975.46.10.577. [DOI] [PubMed] [Google Scholar]
- 6.Karring T, Lang NP, Löe H. The role of gingival connective tissue in determining epithelial differentiation. J Periodontal Res. 1975;10:1–1. doi: 10.1111/j.1600-0765.1975.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 7.Amler MH. The time sequence of tissue regeneration in human extraction wounds. Oral Surg Oral Med Oral Pathol. 1969;27:309–18. doi: 10.1016/0030-4220(69)90357-0. [DOI] [PubMed] [Google Scholar]
- 8.Peterson LW, Artis D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–53. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 9.Smith PC, Cáceres M, Martínez C, Oyarzún A, Martínez J. Gingival wound healing: An essential response disturbed by aging? J Dent Res. 2015;94:395–402. doi: 10.1177/0022034514563750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CC. The requirement for fibroblasts in angiogenesis: Fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol Biol Cell. 2011;22:3791–800. doi: 10.1091/mbc.E11-05-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kellouche S, Mourah S, Bonnefoy A, Schoëvaert D, Podgorniak MP, Calvo F, et al. Platelets, thrombospondin-1 and human dermal fibroblasts cooperate for stimulation of endothelial cell tubulogenesis through VEGF and PAI-1 regulation. Exp Cell Res. 2007;313:486–99. doi: 10.1016/j.yexcr.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Paunescu V, Bojin FM, Tatu CA, Gavriliuc OI, Rosca A, Gruia AT, et al. Tumour-associated fibroblasts and mesenchymal stem cells: More similarities than differences. J Cell Mol Med. 2011;15:635–46. doi: 10.1111/j.1582-4934.2010.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antoniades HN, Galanopoulos T, Neville-Golden J, Kiritsy CP, Lynch SE. Injury induces in vivo expression of platelet-derived growth factor (PDGF) and PDGF receptor mRNAs in skin epithelial cells and PDGF mRNA in connective tissue fibroblasts. Proc Natl Acad Sci U S A. 1991;88:565–9. doi: 10.1073/pnas.88.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuang R, Wang Z, Xu Q, Liu S, Zhang W. Influence of mechanical stimulation on human dermal fibroblasts derived from different body sites. Int J Clin Exp Med. 2015;8:7641–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Rørth P. Collective cell migration. Annu Rev Cell Dev Biol. 2009;25:407–29. doi: 10.1146/annurev.cellbio.042308.113231. [DOI] [PubMed] [Google Scholar]
- 17.Pan Z, Ghosh K, Hung V, Macri LK, Einhorn J, Bhatnagar D, et al. Deformation gradients imprint the direction and speed of en masse fibroblast migration for fast healing. J Invest Dermatol. 2013;133:2471–9. doi: 10.1038/jid.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun L, Qu L, Zhu R, Li H, Xue Y, Liu X, et al. Effects of mechanical stretch on cell proliferation and matrix formation of mesenchymal stem cell and anterior cruciate ligament fibroblast. Stem Cells Int. 2016;2016:9842075. doi: 10.1155/2016/9842075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zachrisson S, Zachrisson BU. Gingival condition associated with orthodontic treatment. Angle Orthod. 1972;42:26–34. doi: 10.1043/0003-3219(1972)042<0026:GCAWOT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Surlin P, Rauten AM, Mogoantă L, Siloşi I, Oprea B, Pirici D. Correlations between the gingival crevicular fluid MMP8 levels and gingival overgrowth in patients with fixed orthodontic devices. Rom J Morphol Embryol. 2010;51:515–9. [PubMed] [Google Scholar]
- 21.Wilcko MT, Ferguson DJ, Makki L, Wilcko WM. Keratinized gingiva height increases after alveolar corticotomy and augmentation bone grafting. J Periodontol. 2015;86:1107–15. doi: 10.1902/jop.2015.150074. [DOI] [PubMed] [Google Scholar]
- 22.Matter J, Cimasoni G. Creeping attachment after free gingival grafts. J Periodontol. 1976;47:574–9. doi: 10.1902/jop.1976.47.10.574. [DOI] [PubMed] [Google Scholar]
- 23.Bernimoulin JP, Lüscher B, Mühlemann HR. Coronally repositioned periodontal flap. Clinical evaluation after one year. J Clin Periodontol. 1975;2:1–3. doi: 10.1111/j.1600-051x.1975.tb01721.x. [DOI] [PubMed] [Google Scholar]
- 24.Yukna RA, Tow HD, Carroll PB, Vernino AR, Bright RW. Evaluation of the use of freeze-dried skin allografts in the treatment of human mucogingival problems. J Periodontol. 1977;48:187–93. doi: 10.1902/jop.1977.48.4.187. [DOI] [PubMed] [Google Scholar]
- 25.Fagan F. Clinical comparison of the free soft tissue autograft and partial thickness apically positioned flap–preoperative gingival or mucosal margins. J Periodontol. 1975;46:586–95. doi: 10.1902/jop.1975.46.10.586. [DOI] [PubMed] [Google Scholar]