Abstract
Context
Immune checkpoint inhibitors (ICIs) have gained a revolutionary role in management of many advanced malignancies. However, immune-related endocrine events (irEEs), have been associated with their use. irEEs have nonspecific clinical presentations and variable timelines, making their early diagnosis challenging.
Objective
To identify risk factors, timelines, and prognosis associated with irEEs development.
Design and Setting
Retrospective observational study within the Cleveland Clinic center.
Patients
Metastatic cancer adult patients who received ICIs were included.
Methods
570 charts were reviewed to obtain information on demographics, ICIs used, endocrine toxicities, cancer response to treatment with ICI, and overall survival.
Main Outcome Measures
Incidence of irEEs, time to irEEs development and overall survival of patients who develop irEEs.
Results
The final cohort included 551 patients. The median time for the diagnosis of irEEs was 9 weeks. Melanoma was associated with the highest risk for irEEs (31.3%). Ipilimumab appeared to have the highest percentage of irEEs (29.4%), including the highest risk of pituitary insufficiency (11.7%), the most severe (Grade 4 in 60%) and irreversible (100%) forms of irEEs. Forty-five percent of patients with irEEs had adequate cancer response to ICI compared to 28.3% of patients without irEEs (P = 0.002). Patients with irEEs had significantly better survival compared to patients without irEEs (P < 0.001).
Conclusions
In the adult population with metastatic cancer receiving treatment with ICI, irEEs development may predict tumor response to immunotherapy and a favorable prognosis. Ipilimumab use, combination ICI therapy, and melanoma are associated with a higher incidence of irEEs.
Keywords: immune-checkpoint inhibitors, immune-related adverse events, endocrine toxicities, cancer
The treatment paradigm for many advanced malignancies has changed with the advent of immune checkpoint inhibitors (ICIs). Over the last 10 years, ICIs have rapidly gained an important role in treating various types of solid cancers. This now includes small cell and nonsmall cell lung cancers (NSCLCs), triple-negative breast cancer, gastric carcinoma, urothelial cancers, ovarian cancer, cervical cancer, renal cell carcinoma, hepatocellular carcinoma, skin squamous cell carcinoma, Merkel cell carcinoma, head and neck squamous cell carcinomas, and malignant melanoma. In addition, ICIs were approved for use in some hematologic malignancies including Hodgkin’s lymphoma and primary mediastinal B-cell lymphoma. As more ICI agents and new immune targets (checkpoints such as B- and T-lymphocyte attenuator; costimulatory molecules such as CD137) are being identified, the potential for immunotherapy in cancer treatment is thriving [1,2].
Immune checkpoint proteins on immune cells are involved in immune-inhibitory pathways, which play a vital role in immune system balance [3]. ICIs enhance antitumor immunity by blocking negative regulators (checkpoints) of T cell function involved in both immune and tumor cells [4]. These agents target programmed death-1 receptor (PD-1; nivolumab, pembrolizumab, cemiplimab), programmed death-ligand 1 (PD-L1; atezolizumab, avelumab, durvalumab), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4; ipilimumab, tremelimumab).
Despite their promising therapeutic effects, various severe immune-related adverse events (irAEs) have been reported and questioned the safety of ICIs [4]. irAEs may affect almost any organ system and have broad clinical presentations. The most common toxicities due to ICIs include dermatological, endocrine, respiratory, gastrointestinal, and rheumatological adverse effects [5,6]. Endocrinopathies are frequently encountered during treatment with ICIs [7-10]. Immune-related endocrine events (irEEs) have heterogenous clinical manifestations such as hypothyroidism, thyroiditis, hypophysitis, adrenal insufficiency, diabetes mellitus, and hypopituitarism [7,9,11-13]. irEEs may be irreversible and life-threatening if not diagnosed and treated early [7-9]. irEEs presentation may be subclinical or nonspecific, making their early diagnosis challenging [8,14]. The study aimed to identify risk factors associated with the development of irEEs, timelines for irEEs and to evaluate the prognosis of patients who develop irEEs. This included evaluating the reversibility and severity of irEEs, cancer response to treatment with ICIs, and overall survival of patients who develop irEEs.
Materials and Methods
This is a retrospective observational study from several sites within the Cleveland Clinic health system. After institutional review board approval, a chart review was done for patients diagnosed with malignancy who received ICIs within a 3-year period from August 31, 2014 to August 31, 2017. Inclusion criteria included male and female adult patients (aged ≥ 18 years old), with a confirmed diagnosis of cancer, who received treatment with ICIs for cancer management. ICIs included in the study were anti–PD-1 (nivolumab and pembrolizumab), anti–PD-L1 (atezolizumab, durvalumab, avelumab) and anti–CTLA-4 (ipilimumab).
Exclusion criteria were
Thyroid dysfunction with history of IV contrast exposure within 3 months prior to diagnosis of thyroid dysfunction.
Thyroid dysfunction in patients treated with tyrosine kinase inhibitors such as sunitinib, sorafenib, imatinib; amiodarone; and lithium.
Patients who had acute illness at the time of checking thyroid function labs.
Patients with suppressed thyroid-stimulating hormone (TSH) levels who received steroids within 48 h prior to thyroid function testing.
Patients with primary adrenal insufficiency who had adrenal metastasis.
Patients with any pituitary hormone deficiencies with hypothalamic or pituitary metastasis.
Patients with isolated secondary adrenal insufficiency on long-term systemic steroids or extensive topical or inhaled steroids.
Patients with isolated hypogonadotropic hypogonadism who is on chronic opioids.
If the endocrinopathy cannot be clearly attributed to ICI use for any other possible reason.
Patients who had baseline endocrinopathies of clear etiology were still included, but, of course, their endocrinopathies were not attributed to ICIs. Since the number of patients who had preexisting endocrinopathies was too small in our cohort, we did not specifically study the association between having a baseline endocrinopathy and developing a different type of ICI-related endocrine toxicity.
The Cleveland Clinic pharmacy database provided the authors with a full list of all adult patients who were treated with ICIs within a 3-year period from August 31, 2014 to August 31, 2017. Charts were reviewed on the fully integrated EPIC electronic medical records to screen patients’ eligibility based on the previously discussed inclusion criteria. A total of 570 eligible patients were identified, and their charts were reviewed for previously described exclusion criteria and for many other variables including demographics and baseline characteristics, type of cancer treated, immunotherapy regimens, dates of ICIs initiation, information about cancer response to ICIs, the reason for stopping ICIs if stopped, development of irEEs, type of irEEs, date of irEEs development, severity grade of irEEs, type of treatment required for irEEs, reversibility of irEEs as indicated by the duration of treatment required for irEEs, date of last follow-up, and date of death if the patient died.
The authors designed a data collection sheet by utilizing a secure Red Cap research software to include all of the previously described variables. Authors had secure access to the Red Cap data collection sheet. All relevant data obtained from the electronic medical records was entered into the Red Cap data collection sheets, where each patient has a separate sheet.
Baseline characteristics, type of metastatic cancer treated, the response of cancer to treatment with ICIs, and overall survival were compared between patients who developed irEEs vs those who did not develop irEEs. Development and incidence of irEEs, the timeline of irEEs development, type of irEEs, development of symptoms with irEEs, severity of irEEs, and reversibility of irEEs were compared by ICI regimen.
How Patient’s Symptoms Were Assessed
Based on chart review, by the time of starting ICI treatment, patients were routinely educated about possible side effects and about the symptoms to watch for during treatment with an ICI. They were also instructed to self-report any new symptoms or side effects. Based on documentation, visits with the treating oncologists have also documented the review of systems, including the endocrine system. As this is an observational retrospective study, pertinent positive and negative endocrine symptoms documented varied based on the treating clinician. Patients who reported symptoms were tested case by case for relevant endocrine toxicity based on their symptoms.
How Patients Were Tested for Endocrine Toxicity
Based on chart review, we observed that almost all patients regardless of symptoms were screened with a TSH level every 4 to 6 weeks. Unfortunately, not all patients had a free thyroxine (T4) check. All patients had a basic metabolic panel at baseline and about 4 to 6 weeks later to assess for hyponatremia and hyperkalemia. Patients in whom symptoms or electrolytes suggested possible adrenal insufficiency were screened with am serum cortisol and subsequently with an adrenocorticotropin (ACTH) stimulation test when indicated. No routine testing or brain imaging were done to assess for hypophysitis. Patients who were diagnosed with 1 or more of the pituitary hormone deficiencies or patients who reported symptoms such as new onset headaches or visual symptoms were further evaluated for hypophysitis. All patients, regardless of symptoms, were routinely tested with basic metabolic panel (including serum glucose). No routine testing with hemoglobin A1c was done.
Definitions
Primary hypothyroidism was defined as free T4 level below the lower limit of normal accompanied by TSH level above the upper limit of normal as reported by Cleveland Clinic laboratory.
Secondary hypothyroidism was defined as low free T4 level accompanied by low or inappropriately normal TSH level. Patients in this group may include isolated secondary hypothyroidism or secondary hypothyroidism combined with other pituitary hormone deficiencies and/or hypophysitis.
Primary hyperthyroidism was defined as elevated free T4 or 3,3′,5-triiodo-L-thyronine (T3) levels accompanied by TSH level below the lower limit of normal plus evidence of high uptake thyrotoxicosis, positive antibodies (eg, thyroid-stimulating immunoglobulin antibodies) or persistence of thyrotoxicosis for more than 3 months.
Secondary hyperthyroidism was defined as free T4 or T3 level above the upper limit of normal accompanied by elevated or inappropriately normal TSH level.
Thyroiditis was defined as a thyrotoxicosis phase followed by a spontaneous hypothyroidism phase or thyrotoxicosis accompanied by evidence of low uptake of thyrotoxicosis plus/minus elevated thyroglobulin levels. Since this was a retrospective study and most patients were not managed by endocrinologists, unfortunately no thyroid uptake tests, thyroid imaging, thyroid antibodies, or thyroglobulin levels were checked.
Primary adrenal insufficiency was defined as 8 Am serum cortisol less than 3 mcg/dL or serum cortisol that fails to increase above 18 mcg/dL at 30 or 60 min intervals after ACTH stimulation test using 250 mcg of subcutaneous cosyntropin injection associated with elevated serum ACTH level.
Secondary adrenal insufficiency was defined as 8 Am serum cortisol less than 3 mcg/dL or serum cortisol that fails to increase above 18 mcg/dL at 30 or 60 min intervals after ACTH stimulation test using 250 mcg of subcutaneous cosyntropin injection associated with low or inappropriately normal serum ACTH level. Patients in this group may include isolated secondary adrenal insufficiency or secondary adrenal insufficiency combined with other pituitary deficiencies and/or hypophysitis.
Hypophysitis was defined by evidence of pituitary inflammation based on imaging studies and oncologist documentation.
Number of total endocrine events was defined as more than 1 endocrine hormone deficiency that was related to the same event, which was counted as 1 endocrine event. For example, a patient with secondary hypothyroidism who also had hypophysitis at the same time was included in both corresponding groups, but the related events were counted as a 1 single endocrine event. On the other hand, 2 independent endocrine events (eg, thyroiditis and hypophysitis) it was counted as 2 endocrine events.
iRECIST guidelines are used by oncologists to define disease progression, partial response, complete response, and stable disease. This includes the following:
Immune-related complete response defined as complete resolution of all measurable and nonmeasurable lesions, with no new lesions, on 2 occasions at least 4 weeks apart.
Immune-related partial response defined as a decrease in the total tumor burden of 50% or more compared with baseline, on 2 occasions at least 4 weeks apart. This category allows for the inclusion of progression of some lesions or the appearance of new lesions as long as the total tumor burden meets the response criterion.
Immune-related progressive disease was defined as an increase in the total tumor burden of 25% or more relative to the minimum recorded tumor burden. This must be confirmed by a second, consecutive assessment no fewer than 4 weeks after the initial documentation of an increase in tumor.
Immune-related stable disease was defined as not meeting the criteria for either a partial or complete response or for progressive disease.
As this is a retrospective study, we relied on the treating oncologist’s opinion and documentation to define disease progression, partial response, complete response, and stable disease. To simplify our documentation and analysis, we classified patients based on their response into 2 main categories: (1) patients who had disease progression, referred to as “patients with no response to treatment” and (2) patients who did not meet criteria for disease progression (including patients who had a partial response, complete response, or stable disease), referred to as “patients with favorable response.” Some patients did not have sufficient documentation on their response status, and we referred to this group as “patients with unknown response.”
irEEs was defined as new evidence of endocrine dysfunction after starting ICIs with no alternative explanation or as clearly documented by oncologists or endocrinologist to be caused by ICIs.
The severity of endocrine toxicity was defined by the grading system of specific endocrine toxicities as defined by the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 5; CTCAE) [15]. Although CTCAE grading of endocrinopathies has limitations, we used it because in real life endocrinopathies related to ICIs are mostly managed by oncologists who used this grading at the time of this study.
Grade 1: asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated
Grade 2: moderate; minimal, local or noninvasive intervention indicated; limiting age-appropriate instrumental activities of daily living
Grade 3: severe or medically significant but not immediately life-threatening; hospitalization or prolongation of existing hospitalization indicated; limiting self-care activities of daily living
Grade 4: life-threatening consequences; urgent intervention indicated
Grade 5: death
Statistical Methods
Categorical variables were described using frequencies and percentages. Comparisons involving ordered data used Kruskal-Wallis tests, while nonordered comparisons used Pearson’s chi-square or Fisher’s exact tests. Normally distributed continuous variables were described using means and SDs, and comparisons were evaluated using t-tests. Continuous variables with nonnormal distributions were described using medians and quartiles, and comparisons were evaluated using Kruskal-Wallis tests. Ad hoc comparisons were performed when analyses comparing more than 2 groups were significant. Analyses were performed using SAS® Software (version 9.4; Cary, NC, USA). Statistical significance was set at P < 0.05.
Overall survival was calculated from the date of starting ICIs until the date of last follow-up in alive patients and until the date of death in patients who died. Overall survival was analyzed using the Kaplan-Meier curve.
Results
Patient Characteristics
Out of 570 eligible patients, in 19 patients, the etiology of irEEs was unclear; hence, they were excluded from the study based on previously discussed exclusion criteria. The final cohort included for analysis were 551 patients. The median age was 67.4 years. The majority of patients were white (n = 493, 90%) and male (n = 359, 65.2%), and the most common underlying malignancy was NSCLC (n = 246, 44.6%), followed by melanoma (n = 96, 17.4%), renal cell carcinoma (n = 68, 12.3%), and bladder cancer (n = 47, 8.5%). Multiple other tumor types were also included in the study. Out of the 551 patients, 276 patients received nivolumab monotherapy; 117 patients, pembrolizumab monotherapy; 54 patients, atezolizumab monotherapy; and 17 patients, ipilimumab monotherapy. Sixty-three patients were on 2 or more agents with ipilimumab, and 24 patients were on 2 or more agents without ipilimumab. The baseline characteristics are outlined in Table 1.
Table 1.
Endocrine toxicity and baseline characteristics
| Overall (N = 551) | No endocrine toxicity (N = 453) | Endocrine toxicity (N = 98) | |||||
|---|---|---|---|---|---|---|---|
| Factor | N | Statistics | N | Statistics | N | Statistics | P-value |
| Age | 551 | 67.4 ± 13.3 | 453 | 67.8 ± 13.5 | 98 | 65.7 ± 12.0 | 0.16a |
| Gender | 551 | 453 | 98 | 0.37c | |||
| Male | 359 (65.2) | 299 (66.0) | 60 (61.2) | ||||
| Female | 192 (34.8) | 154 (34.0) | 38 (38.8) | ||||
| BMI | 551 | 26.5 ± 6.1 | 453 | 26.4 ± 5.9 | 98 | 27.4 ± 6.9 | 0.15b |
| Race | 547 | 450 | 97 | 0.086c | |||
| White | 493 (90.1) | 402 (89.3) | 91 (93.8) | ||||
| Black | 31 (5.7) | 30 (6.7) | 1 (1.03) | ||||
| Other | 23 (4.2) | 18 (4.0) | 5 (5.2) | ||||
| Cancer type treated | |||||||
| Breast | 551 | 8 (1.5) | 453 | 6 (1.3) | 98 | 2 (2.0) | 0.64d |
| Prostate | 551 | 0(0.00) | 453 | 0(0.00) | 98 | 0(0.00) | N/A |
| Bladder | 551 | 47 (8.5) | 453 | 37 (8.2) | 98 | 10 (10.2) | 0.51c |
| Renal cell carcinoma | 551 | 68 (12.3) | 453 | 54 (11.9) | 98 | 14 (14.3) | 0.52c |
| Colon | 551 | 2 (0.36) | 453 | 2 (0.44) | 98 | 0 (0.00) | 0.99d |
| Pancreatic | 551 | 0(0.00) | 453 | 0(0.00) | 98 | 0(0.00) | N/A |
| Esophageal | 551 | 2 (0.36) | 453 | 2 (0.44) | 98 | 0 (0.00) | 0.99d |
| Gastric | 551 | 0(0.00) | 453 | 0(0.00) | 98 | 0(0.00) | N/A |
| Brain | 551 | 4 (0.73) | 453 | 4 (0.88) | 98 | 0 (0.00) | 0.99d |
| Melanoma | 551 | 96 (17.4) | 453 | 66 (14.6) | 98 | 30 (30.6) | <0.001 c |
| Small cell lung cancer | 551 | 18 (3.3) | 453 | 17 (3.8) | 98 | 1 (1.02) | 0.22d |
| Nonsmall cell lung cancer | 551 | 246 (44.6) | 453 | 212 (46.8) | 98 | 34 (34.7) | 0.029 c |
| Sarcoma | 551 | 0(0.00) | 453 | 0(0.00) | 98 | 0(0.00) | N/A |
| Thyroid cancer | 551 | 0(0.00) | 453 | 0(0.00) | 98 | 0(0.00) | N/A |
| Testicular | 551 | 1 (0.18) | 453 | 1 (0.22) | 98 | 0 (0.00) | 0.99d |
| Others (detail) | 551 | 27 (4.9) | 453 | 24 (5.3) | 98 | 3 (3.1) | 0.45d |
| Lymphoma | 551 | 18 (3.3) | 453 | 16 (3.5) | 98 | 2 (2.0) | 0.75d |
| Leukemia | 551 | 1 (0.18) | 453 | 0 (0.00) | 98 | 1 (1.02) | 0.18d |
| Ovarian | 551 | 9 (1.6) | 453 | 8 (1.8) | 98 | 1 (1.02) | 0.99d |
| Oropharyngeal carcinoma | 551 | 10 (1.8) | 453 | 9 (2.0) | 98 | 1 (1.02) | 0.99d |
Statistics presented as mean ± SD or N (%).
Abbreviations: BMI, body mass index; N/A, not applicable.
a t-test.
bSatterthwaite t-test.
cPearson’s chi-square test.
dFisher’s exact test.
The Pattern of Endocrine Toxicities Due to ICIs
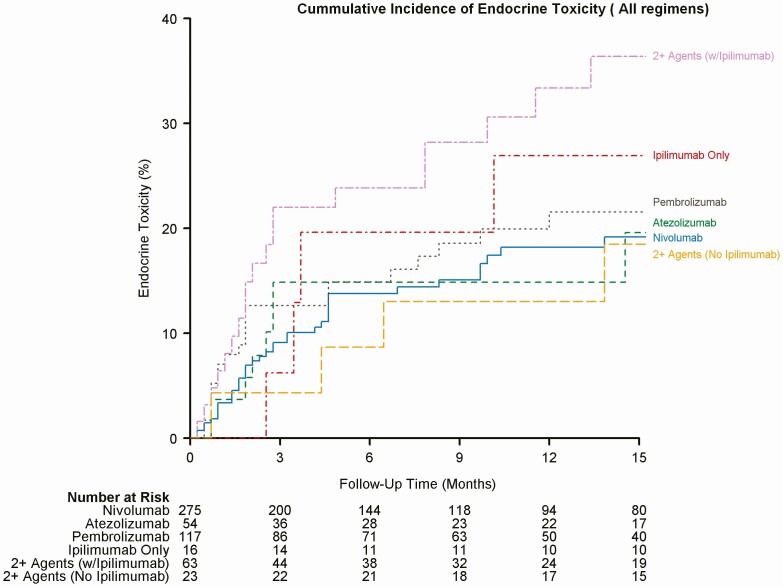
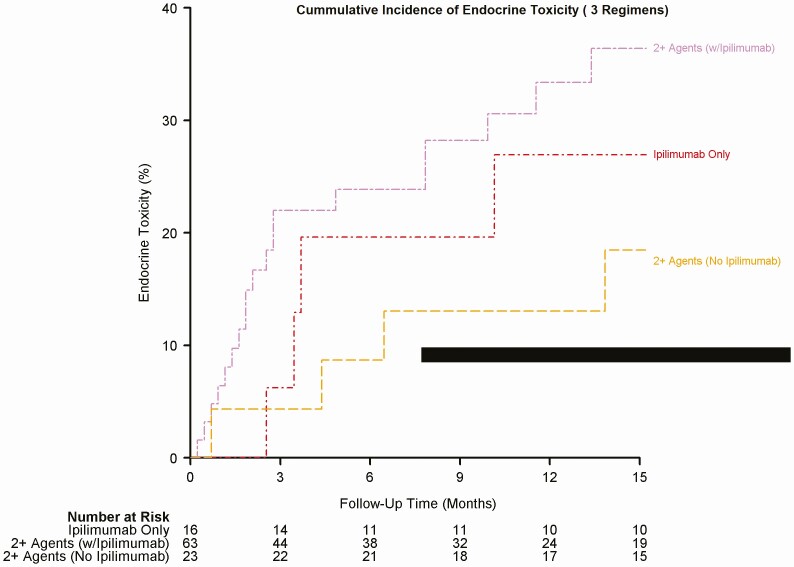
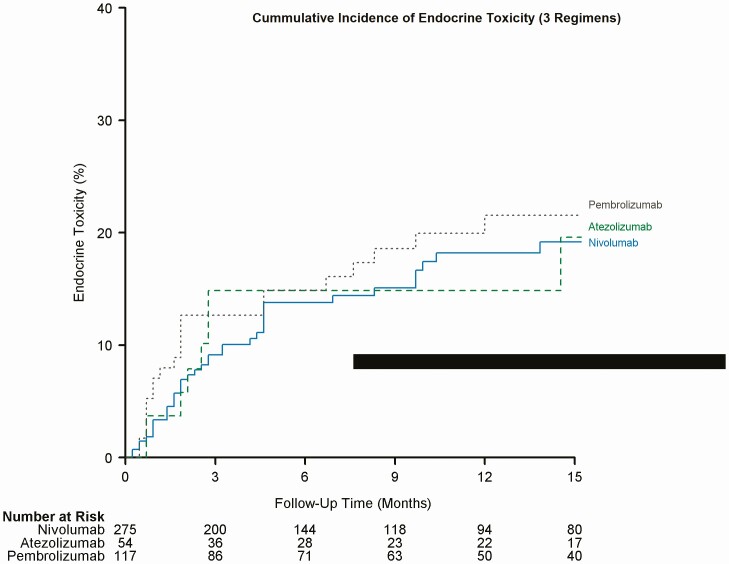
We documented endocrine-related adverse effects due to ICIs in 98 (17.7%) patients. The overall median timeline for the development of irEEs regardless of regimen was about 9 weeks (range 1.0-63.0). The median timeline for irEEs observed with nivolumab was 10 weeks, with atezolizumab was 10 weeks, pembrolizumab was 8 weeks, ipilimumab was 15.5 weeks, 2+ agents including ipilimumab was 9 weeks, and finally for 2+ agents not including ipilimumab was 23.5 weeks. Figures 1 to 3 depict a plot of the timeline for endocrine toxicity events by ICIs regimen. Table 2 summarizes the median timelines for endocrine toxicity development based on the ICI regimen.
Figure 1.
Cumulative incidence of endocrine toxicity (all regimens).
Figure 3.
Cumulative incidence of endocrine toxicity (3 regimens).
Table 2.
Timeline of endocrine toxicity by regimen
| Immune checkpoint inhibitors regimen | N (observed) | N | 25th percentile | Median (weeks) |
75th percentile |
|---|---|---|---|---|---|
| Nivolumab | 276 | 39 | 6.0 | 10.0 | 20 |
| Atezolizumab | 54 | 8 | 5.5 | 10.0 | 12 |
| Pembrolizumab | 117 | 21 | 3.0 | 8.0 | 20 |
| Ipilimumab only | 17 | 4 | 13.0 | 15.5 | 30 |
| 2+ agents, including ipilimumab | 63 | 19 | 5.0 | 9.0 | 34 |
| 2+ agents, not including ipilimumab | 24 | 4 | 11.0 | 23.5 | 44 |
Out of all irEEs, 56 (57.1%) patients were symptomatic, and 42 (42.9%) were asymptomatic. Most symptoms were nonspecific including fatigue, nausea, vomiting, and weight changes, which are also common symptoms among many cancer patients. However, most symptomatic patients reported some improvement in these symptoms after treating their endocrinopathy. Of note, the percentage of symptoms development may vary based on the subtype of endocrine toxicity as shown in Table 3. Among all cancer types, melanoma was associated with the highest risk of irEEs; In our cohort, 30 out of 96 melanoma patients had irEEs (31.3%), whereas only 68 out of 455 patients with other, nonmelanoma cancer types had irEEs (14.9%; P < 0.001). In addition, patients with irEEs were mostly treated for NSCLC (n = 34, 34.7%; P = 0.029) followed by melanoma (30.6%; P < 0.001) as shown in Table 1.
Table 3.
Percentage of patients who reported symptoms with different endocrine toxicity subtypes
| Endocrine toxicity subtype | Overall number | Symptomatic patients, n (%) |
|---|---|---|
| Primary hypothyroidism | 45 | 25 (55) |
| Thyroiditis | 29 | 17 (58.6) |
| Primary adrenal insufficiency | 1 | 1 (100) |
| Hypophysitis | 9 | 7 (77.7) |
| Secondary hypothyroidism | 11 | 9 (81.8) |
| Secondary adrenal insufficiency | 14 | 13 (92.8) |
| Central hypogonadism | 5 | 5 (100) |
| New onset insulin-dependent DM | 2 | 1 (50) |
Abbreviation: DM, diabetes mellitus
Among patients with endocrine toxicities, most of them had Grade 1 (n = 33, 33.7%) and Grade 2 (n = 33, 33.7%) toxicity; Grade 3 toxicity were documented in 21 (21.4%) patients. Eleven patients (11.2%) had life-threatening (Grade 4) toxicities; interestingly, Grade 4 toxicity was most commonly observed in patients who received ipilimumab monotherapy or combination regimen (Table 4). irEEs were fairly well tolerated by most of the patients in this study; only 9 patients were documented to have stopped ICIs due to endocrine toxicity. The most common reasons for stopping ICIs were disease progression (n = 212, 54.4%), followed by nonendocrine toxicity (n = 77, 19.7%), completion of treatment (n = 37, 9.5%), and transitioning to hospice (n = 31, 7.9%). Table 5 summarizes the reasons for stopping ICIs based on the type of ICIs.
Table 4.
Severity of endocrine toxicity by regimen
| Factor | Overall (N = 98) | Nivolumab (n = 41) | Atezolizumab (n = 8) | Pembrolizumab (n = 21) | Ipilimumab only (n = 5) | 2+ agents, including ipilimumab (n = 19) | 2+ agents, not including ipilimumab (n = 4) | P-value |
|---|---|---|---|---|---|---|---|---|
| Severity of endocrine toxicity | 0.041 a | |||||||
| Mild (Grade 1) | 33 (33.7) | 18 (43.9) | 3 (37.5) | 5 (23.8) | 0 (0.00) | 6 (31.6) | 1 (25.0) | |
| Moderate (Grade 2) | 33 (33.7) | 15 (36.5) | 2 (25.0) | 8 (38.1) | 1 (20.0) | 5 (26.3) | 2 (50.0) | |
| Severe (Grade 3) | 21 (21.4) | 6 (14.6) | 2 (25.0) | 6 (28.6) | 1 (20.0) | 5 (26.3) | 1 (25.0) | |
| Life-threatening (Grade 4) | 11 (11.2) | 2 (4.9) | 1 (12.5) | 2 (9.5) | 3 (60.0) | 3 (15.8) | 0 (0.00) |
Statistics are presented as n (%).
a Kruskal-Wallis test.
Table 5.
Reasons for stopping ICI agent by regimen
| Factor | Overall (N = 551) | Nivolumab (n = 276) | Atezolizumab (n = 54) | Pembrolizumab (n = 117) | Ipilimumab only (n = 17) | 2+ agents, including ipilimumab (n = 63) | 2+ agents, not including ipilimumab (n = 24) |
|---|---|---|---|---|---|---|---|
| Agent stopped, yes | 390 (70.8) | 187 (67.8) | 35 (64.8) | 81 (69.2) | 13 (76.5) | 51 (81.0) | 23 (95.8) |
| Reason for stopping agent | |||||||
| Endocrine toxicity | 9 (2.3) | 2 (1.06) | 0 (0.00) | 0 (0.00) | 4 (30.8) | 3 (5.9) | 0 (0.00) |
| Nonendocrine toxicity | 77 (19.7) | 29 (15.4) | 5 (14.3) | 14 (17.1) | 3 (23.1) | 20 (39.2) | 6 (28.6) |
| Completion of treatment | 37 (9.5) | 18 (9.6) | 0 (0.00) | 9 (11.0) | 3 (23.1) | 4 (7.8) | 3 (14.3) |
| Other | 24 (6.2) | 13 (6.9) | 4 (11.4) | 4 (4.9) | 0 (0.00) | 3 (5.9) | 0 (0.00) |
| Disease progression/treatment failure | 212 (54.4) | 114 (60.6) | 22 (62.9) | 42 (51.2) | 3 (23.1) | 19 (37.3) | 12 (57.1) |
| Hospice | 31 (7.9) | 12 (6.4) | 4 (11.4) | 13 (15.9) | 0 (0.00) | 2 (3.9) | 0 (0.00) |
In this study, the thyroid gland was the most commonly involved endocrine organ, which manifested as primary hypothyroidism (n = 45, 45.9%), thyroiditis (n = 29, 29.6%), secondary hypothyroidism (n = 11, 11.2%), thyrotoxicosis of undetermined mechanism (n = 6, 5.1%) (The 6 patients with thyrotoxicosis of undetermined mechanism did not have definitive evidence of primary hyperthyroidism or biochemical results to support a diagnosis of thyroiditis or subsequent development of hypothyroidism. Because destructive thyroiditis is the most common etiology of thyrotoxicosis after ICI therapy, we presume that these 6 patients were most likely in the early thyrotoxic phase of thyroiditis at the time the biochemical test results documented in their electronic medical records). Nivolumab (n = 25, 60.9%), and pembrolizumab (n = 8, 38%), were most commonly associated with primary hypothyroidism as monotherapy.
Primary hypothyroidism was the most common irEEs with the overall incidence of 8.2% (45.9% of all endocrine toxicities; P < 0.031) and was associated with all ICIs except for ipilimumab monotherapy.
Secondary adrenal insufficiency was found in 14% of all irEEs (P < 0.001). It was associated with all ICIs therapy except ipilimumab and atezolizumab groups.
About 9% of all irEEs had hypophysitis (P < 0.001). It was frequently observed in patients on ipilimumab monotherapy and those who were on a combination ICI regimen including ipilimumab.
Other rare but significant irEEs in this study include central hypogonadism (n = 5, 5%; P = 0.016), new onset insulin-dependent diabetes mellitus (n = 2, 2%; P = 0.99), hypoprolactinemia (n = 2, 2%; P = 0.12), growth hormone deficiency (n = 2, 2%; P = 0.22), and central diabetes insipidus (n = 1, 1%; P = 0.38).
Overall, ipilimumab was associated with the highest incidence for irEEs whether as ipilimumab monotherapy (29.4%) or ipilimumab combination therapy (30.2%). A complete description of various endocrine toxicities based on the type of ICIs is summarized in Table 6.
Table 6.
Endocrine toxicity subtypes by regimen
| Factor | Overall (N = 98) | Nivolumab (N = 41) | Atezolizumab (N = 8) | Pembrolizumab (N = 21) | Ipilimumab only (N = 5) | 2+ agents, including Ipilimumab (N = 19) | 2+ agents, not including Ipilimumab (N = 4) | P-value |
|---|---|---|---|---|---|---|---|---|
| Endocrine toxicity | 98 (17.8) | 41 (14.8) | 8 (14.8) | 21 (17.9) | 5 (29.4) | 19 (30.2) | 4 (16.7) | 0.058a |
| Primary hypothyroidism | 45 (45.9) | 25 (60.9) | 3 (37.5) | 8 (38.1) | 0 (0.00) | 6 (31.6) | 3 (75.0) | 0.031 b |
| Thyrotoxicosis, undetermined mechanism | 6 (5.1) | 2 (4.9) | 1 (12.5) | 1 (4.8) | 1 (20.0) | 0 (0.00) | 1 (25.0) | 0.20b |
| Primary adrenal insufficiency | 1 (1.02) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (5.3) | 0 (0.00) | 0.38b |
| Hypophysitis | 9 (9.2) | 0 (0.00) | 0 (0.00) | 1 (4.8) | 2 (40.0) | 6 (31.6) | 0 (0.00) | <0.001 b |
| New onset DM/hyperglycemia | 2 (2.0) | 1 (2.4) | 0 (0.00) | 1 (4.8) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0.99b |
| Other | 1 (1.02) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (5.3) | 0 (0.00) | 0.38b |
| Secondary adrenal insufficiency | 14 (14.3) | 2 (4.9) | 0 (0.00) | 3 (14.3) | 3 (60.0) | 6 (31.6) | 0 (0.00) | 0.005 b |
| Secondary hypothyroidism | 11 (11.2) | 1 (2.4) | 0 (0.00) | 2 (9.5) | 3 (60.0) | 5 (26.3) | 0 (0.00) | 0.003 b |
| Thyroiditis (first hyperthyroid and then hypothyroid) | 29 (29.6) | 11 (26.8) | 4 (50.0) | 9 (42.8) | 1 (20.0) | 4 (21.0) | 0 (0.00) | 0.15b |
| Central hypogonadism | 5 (5.1) | 0 (0.00) | 0 (0.00) | 1 (4.8) | 2 (40.0) | 2 (10.5) | 0 (0.00) | 0.016 b |
| Hypoprolactinemia | 2 (2.0) | 0 (0.00) | 0 (0.00) | 1 (4.8) | 1 (20.0) | 0 (0.00) | 0 (0.00) | 0.12b |
| GH deficiency | 2 (2.0) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (10.5) | 0 (0.00) | 0.22b |
| Central DI | 1 (1.02) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (5.3) | 0 (0.00) | 0.38b |
Abbreviations: DI, diabetes insipidus; DM, diabetes mellitus; GH, growth hormone.
aPearson’s chi-square test.
bFisher’s exact test.
Cancer Response to ICIs and Endocrine Toxicity
Patients who developed irEEs demonstrated better tumor response to ICIs as compared to those who did not develop endocrine toxicity. Forty-one out of the 91 patients with endocrine toxicity (45%) vs 102 out of the 361 patients without endocrine toxicity (28%) demonstrated a favorable response of the primary tumor to ICIs (P = 0.002).
Overall Survival and irEEs
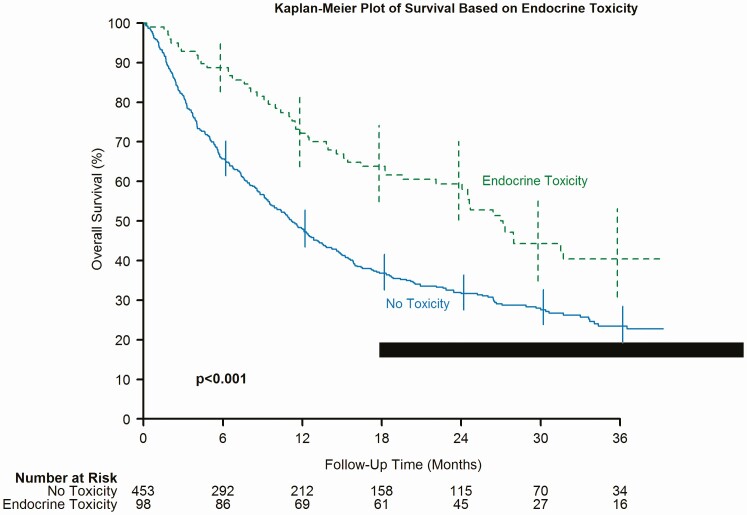
Notably, our cohort with endocrine toxicity demonstrated a significantly better overall survival as compared to the cohort without endocrine toxicity [hazard ratio (HR) 1.86, 95% CI (1.39, 2.49); P < 0.001] (Fig. 4). In addition, those treated with nivolumab (P < 0.001) and those treated with 2 + agents including ipilimumab (P = 0.037) had significantly better survival if they had endocrine toxicity. Table 7 shows the multivariable model of endocrine toxicity and survival adjusting for age, gender, race, body mass index (BMI), and cancer type (restricted to prevalent cancer types that differed). After adjustment for these factors, those without endocrinology remain at higher risk for mortality [HR 1.77, 95% CI (1.31, 2.38); P < 0.001]. In addition, subgroup analysis was done after including response to immunotherapy in the multivariable model. Patients with undocumented response to treatment were excluded from analysis in Table 7; thus, including this variable drops the sample size by 20%. Regardless, the association between endocrine toxicity and survival remains significant [HR 1.57, 95% CI (1.15, 2.15); P = 0.004].
Figure 4.
Kaplan-Meier plot of survival based on endocrine toxicity.
Table 7.
Multivariable model for endocrine toxicity and overall survival
| Variable | Cox multivariable hazard ratio (95% CI) | Cox multivariable Wald P-value (n = 547) |
|---|---|---|
| Endocrine toxicity | ||
| No | 1.77 (1.31, 2.38) | <0.001 |
| Yes | — | |
| Gender | . | |
| Male | — | . |
| Female | 1.09 (0.87, 1.35) | 0.46 |
| Race | . | |
| White | — | . |
| Black | 0.70 (0.43, 1.11) | 0.13 |
| Other | 1.21 (0.74, 1.98) | 0.45 |
| Cancer type treated | . | |
| Melanoma | ||
| Unchecked | — | . |
| Checked | 0.52 (0.37, 0.72) | <0.001 |
| Nonsmall cell lung cancer | . | |
| Unchecked | — | . |
| Checked | 1.11 (0.89, 1.39) | 0.35 |
| Age | 1.007 (0.999, 1.015) | 0.085 |
| BMI | 0.975 (0.957, 0.993) | 0.006 |
Abbreviation: BMI, body mass index
Figure 2.
Cumulative incidence of endocrine toxicity (3 regimens).
Reversibility of Endocrine Toxicity After Stopping Immunotherapy
Among patients who had to stop their immunotherapy regimen for any cause and had developed immune-related endocrine toxicity that required treatment, 82% of patients required long-term treatment for their endocrine disorder. Table 8 summarizes this finding.
Table 8.
Duration of treatment required for endocrine toxicity in patients who had to stop ICI
| Total (N = 56) | |
|---|---|
| Duration of treatment | n (%) |
| Limited short course | 1 (1.8) |
| Limited long course | 9 (16.1) |
| Life long | 46 (82.1) |
Discussion
Immune checkpoint inhibitors have demonstrated promising outcomes and have revolutionized cancer treatment. ICIs are associated with a broad spectrum of adverse effects and are clinically different from traditional chemotherapies in cancer patients [7]. Stimulation of T cell response plays a vital role in the autoimmune processes. Increased tendency for autoimmunity can result from ICI therapy as it promotes T-cell response to tumor cell antigen [16]. The exact mechanism of irAEs is yet to be elucidated; however, recently published data suggests autoantibody production with ICIs may correlate with irAEs [17]. irAEs are frequently encountered complications after ICI use [18]. The overall incidence of irEEs is approximately 10% based on systemic review and meta-analysis of randomized clinical trials [12,18]. The spectrum of irEEs includes thyroid dysfunction, hypopituitarism caused by hypophysitis, primary adrenal insufficiency, and insulin-dependent diabetes mellites [9,11-13]. If not promptly recognized, irEEs can be life-threatening, which may raise a concern about the safety of ICI use. However, data about the outcome of patients who develop IrEEs are limited. In addition, no well-established risk factors exist to predict irEEs development [4,12].
Role of Symptoms in Diagnosing irEEs
We found that 42.9% of patients with irEEs were asymptomatic. Even patients who were symptomatic, most symptoms were nonspecific including fatigue, nausea, vomiting, and weight changes, which are also very common symptoms among most cancer patients. This highlights the importance of routine screening for early diagnosis and treatment of endocrine toxicity after ICI therapy.
Table 3 shows percentage of symptomatic patients in each endocrine toxicity subgroup.
Effects of Combination Therapy vs Monotherapy on IREEs
Researchers had reported a higher incidence of thyroid disorders when combination therapy was used [10,16,19]. In our study, we also observed that combination therapy was associated with a higher risk of thyroid disorders (up to 16.6%) as compared to monotherapy as low as (5.8%).
Effects of Anti–CTLA-4 vs Other ICIs on IREEs
Hypophysitis is the inflammation of the pituitary gland, which often leads to irreversible hypopituitarism and can potentially lead to an adrenal crisis if left untreated [8,12,20]. Previous data suggest that the incidence of hypophysitis is much higher in patients receiving treatment with anti-CTLA agents as compared to patients treated with other groups of ICIs [8]. Our study also showed a higher risk of hypophysitis with ipilimumab use, whether as a monotherapy (11.7%) or in combination with other agents (9.5%), as compared to other ICI regimens without ipilimumab (as low as 0.8% with pembrolizumab monotherapy; P < 0.001). Ipilimumab use was also associated with the most severe (Grade 4 in 60%) and irreversible (100 %) forms of irEEs compared to other ICIs.
Effects of Primary Cancer Type on the Development of IREEs
In 1 study of patients treated for metastatic melanoma with nivolumab plus ipilimumab, treatment-related AEs were reported in 96.8% of patients and were Grades 3 and 4 in 58.5% of patients [21]. Patients treated for melanoma were significantly more likely to develop endocrine toxicity (31.3%) compared to those treated for any other cancer type (14.9%; P < 0.001). This association may be partly explained by the fact that patients with melanoma were more likely to receive combination ICI regimens, including ipilimumab.
Timeline and Incidence of Endocrine Toxicity
The onset of endocrine toxicity has been extremely variable in the literature, ranging from 3 weeks after starting treatment up to 10 months following therapy [19]. Our study shows that different regimens of ICIs have different timelines for different types of endocrine toxicity. Table 2 demonstrate time to endocrine toxicity development by regimen. In this study, the median timeline for irEEs, in general, was around 9 weeks. Figures 1 to 3 depict the cumulative incidence of irEEs by regimen.
Prognosis of Patients Who Develop IREEs
Our findings suggest that most of the immune-related endocrine toxicities, around 82%, remain irreversible and require long-term treatment even after stopping ICI therapy (Table 8).
Previous data have linked endocrine and other irAEs to better patient outcomes [19,22]. In 1 retrospective study, out of 154 patients with metastatic melanoma treated with ipilimumab, 17 patients developed hypophysitis. Tahir et al observed that hypophysitis was associated with better survival [17]. Another study included a total of 73 patients with NSCLC, melanoma, and Hodgkin lymphoma treated with nivolumab. Seventeen patients developed thyroiditis. Their results suggest that patients with NSCLC and nivolumab-induced thyroiditis might have better survival [22]. Another recently published retrospective study that evaluated 186 patients with advanced melanoma treated with single anti–PD-1 agent demonstrated better overall survival among patients who developed irAEs (endocrine or nonendocrine toxicities in general) [23]. Also, 1 study that evaluated 576 melanoma patients treated with nivolumab suggested irAEs were associated with a higher objective response rate but had no progression-free survival benefit [24]. Based on the previous studies, it is unclear if this association is specific to certain types of irAEs, specific cancer type, specific ICI agents, or specific race. However, other studies had conflicting results demonstrating no association between overall survival and development of irAEs. For example, a retrospective study evaluated 298 metastatic melanoma patients managed with ipilimumab. The study concluded that overall survival was not affected by the occurrence of irAEs [25]. Our study supports the presence of significant association between better survival and development of irEEs. Furthermore, in our study, even after adjusting for age, gender, race, BMI, and cancer type, those with irEEs show better overall survival. This may suggest that this association is probably not specific to a certain cancer type or certain ICI agent. Patients who developed any endocrine toxicity were more likely to respond to immunotherapy (45% favorable response) compared to those who did not develop endocrine toxicity (28% favorable response; P < 0.001). This better cancer response also translated to better overall survival. Figure 4, a Kaplan-Meier graph, demonstrates that those with endocrine toxicity tend to have significantly better survival compared to those without endocrine toxicity (P < 0.001). We hypothesize that the development of IrEEs is an indicator of adequate activation of the immune response, not only strong enough to damage self-tissue and cause autoimmune adverse effects but also strong enough to reach the threshold needed to kill cancer cells and induce partial or complete remission or maintain stable disease.
Thyroid Hormone and Cancer Biology
The association of improved survival in patients with solid tumors has been previously reported and extensively reviewed. An initial report from a Harvard study showed significant response with improvement in renal cell and melanoma patients who were treated with immunotherapy interleukin-2 and lymphokine-activated killer cells therapy [26]. A Phase 2 study to induce medical hypothyroidism in recurrent high-grade cerebral glioma showed significant prolongation of survival in patients whose free F4 levels were reduced by >40% from baseline [27].
The discovery of alfavbeta3 integrin expression of a thyroid hormone receptors on cell membranes of malignant cells and associated vascular endothelium has led to the elucidation of divergence between T4 and T3 actions on malignant cells [28,29]. F4, acting nongenomically, blocks mitogenesis of tumor cells and vascular endothelium. F4 was shown to be 10 to 100 times more potent than liothyronine at inducing mitogenesis [30]. The alfavbeta3 integrin comprises 2 thyroid hormone binding sites, S1 and S2, which activate the pathways responsible for the activation of multiple pro-oncogenic genes [31]. T3 binds to S1, and T4 binds to S2, however, with less affinity as compared to T3. Blocking of this receptor (alfavbeta3) by the analog tetraiodothyroacetic acid almost entirely bocks this effect and induces apoptosis and tumor regression [32]. T3, on the other hand, acting via S1 and S2 receptors, blocks metabolic functions. This divergence of action between T4 and T3 has been exploited and utilized in the treatment of advanced cancer patients who had been taking exogenous levothyroxine and switched to liothyronine supplementation. Significant gross tumor regression was subsequently observed as well as prolongation of survival beyond expectation [28].
Spontaneous or medically induced hypothyroidism may beneficially alter the clinical behavior of several cancers including breast cancer [33], glioblastoma [27], head-and-neck tumors [34], and renal cell carcinoma [26,35]. Induction of the clinical state of euthyroid hypothyroxinemia—in which euthyroidism is maintained by administered T3 in the absence of host levothyroxine—has also been shown to slow the course of end-stage carcinomas of various origins [28]. Levothyroxine in physiological concentrations in vitro has been shown to cause the proliferation of a wide variety of human cancer cells [30,36], whereas T3 in physiological concentrations does not appear to promote cancer cell proliferation in vitro [37]. Reports that T3 may stimulate tumor cell proliferation in vitro have relied upon high concentrations of the hormone [38,39]. Clinically, circulating T3 may be quite low in cancer patients subject to the nonthyroidal illness syndrome [28]. A possible confounding factor in tumor response in hypothyroid individuals supplemented with levothyroxine is that PD-1/PD-L1 may be upregulated by T4, and so replacement with this hormone in patients undergoing immunotherapy may negate the otherwise beneficial antioncogenic effects of T4 depletion [40]. It is important to note that a diagnosis of hypothyroidism, which is a clinical diagnosis, does not equate to pure hypothyroxinemia, which experimentally is an optimal thyroid function status to obtain tumor regression as free T4 is the ligand for the thyroid hormone receptor on the cell membrane expressed alfavbeta3 integrin [29].
Where Do Our Findings Stand Among Previous Studies?
Our results highlight the importance of routine screening for irEEs and suggest possible risk factors and expected timelines for endocrine toxicities by regimen that may guide more efficient future testing protocols for high-risk patients. In addition, different studies showed conflicting results for the association between survival and irAEs [23-25]. Although some studies have suggested better outcomes in patients who develop irAEs, it is unclear if this association is specific to certain types of irAEs, specific cancer type, or specific ICI agents. Our study not only supports this association but also suggests that even after adjusting for age, gender, race, BMI, and cancer type, those without endocrine toxicity remain at higher risk for mortality. Recently published data frequently has emphasized the serious nature of endocrine toxicity [5-7,21]. Furthermore, current guidelines recommend holding ICIs in moderate to severe cases of endocrine toxicity [5]. On the other hand, we emphasize that endocrine toxicity development is associated with favorable outcomes. Our findings also suggest that most endocrine toxicities that require treatment are irreversible even after stopping ICI therapy. These findings may indicate that holding ICIs for nonlife-threatening, treatable, moderate to severe endocrine toxicity may not be useful to reverse endocrine toxicity and is probably harmful from a cancer management standpoint as shown in recently published studies [41]. An exception would be life-threatening endocrine toxicities that cannot be managed by hormone replacement alone (eg, hypophysitis with intracranial mass effect). In summary, our findings suggest that the risks of holding ICI for nonlife-threatening, treatable endocrine toxicity may outweigh the benefits. However, this needs further prospective evaluation and long-term follow-up.
Our study is limited by its retrospective design. In addition, most of our patients are of the white race, so our observations may not necessarily be generalizable to other races. Also, CTCAE was used by treating oncologists for grading endocrinopathies. This may occasionally overestimate the severity of some toxicities. Because our study was retrospective, we were unable to assess the severity of endocrine toxicity in a different way. In addition, where we have a significant overall number of endocrine toxicities, we have limited number of endocrine toxicity cases per individual treatment group and per individual specific endocrine toxicity subtype. This makes it difficult to judge if the association of specific ICI agents with specific subtype of endocrine toxicity is real. For example, the association of nivolumab and pembrolizumab with hypothyroidism may be because these drugs were used more commonly. Also, a proportion of our primary hypothyroidism cohort may have had actually destructive thyroiditis with an undocumented or missed thyrotoxic phase. Finally, longer duration follow-up is needed for a better understanding of the long-term outcomes of patients with endocrine toxicities.
Conclusions
In white adults with metastatic cancer receiving treatment with ICIs, irEEs development may predict better cancer response to immunotherapy and better overall survival. Stopping ICIs will not reverse endocrine toxicity most of the time. Thus, the development of nonlife-threatening irEEs may be a reason to continue treatment with ICIs, if tolerated, rather than discontinue. Many patients with irEEs are either asymptomatic or have nonspecific symptoms, indicating the importance of routine surveillance to diagnose and treat irEEs early on. The pattern of endocrine toxicity is highly variable based on regimen. The overall median time for irEEs development is around 9 weeks from the start date. Ipilimumab use, combination ICI therapy, and melanoma as the cancer type are associated with a higher incidence of irEEs. Further prospective studies are needed to confirm our findings regarding the impact on overall survival.
Additional Information
Disclosures: The authors declare that they have no relevant or financial interests that relate to the research described in this paper.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in references.
References
- 1. Fourcade J, Sun Z, Pagliano O, et al. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72(4):887-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gough MJ, Crittenden MR, Sarff M, et al. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J Immunother. 2010;33(8):798-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Azoury SC, Straughan DM, Shukla V. Immune checkpoint inhibitors for cancer therapy: clinical efficacy and safety. Curr Cancer Drug Targets. 2015;15(6):452-462. [DOI] [PubMed] [Google Scholar]
- 5. Brahmer JR, Lacchetti C, Schneider BJ, et al. ; National Comprehensive Cancer Network . Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Puzanov I, Diab A, Abdallah K, et al. ; Society for Immunotherapy of Cancer Toxicity Management Working Group . Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elia G, Ferrari SM, Galdiero MR, et al. New insight in endocrine-related adverse events associated to immune checkpoint blockade. Best Pract Res Clin Endocrinol Metab. 2020:34(1):101370. [DOI] [PubMed] [Google Scholar]
- 8. Girotra M, Hansen A, Farooki A, et al. ; Investigational Drug Steering Committee (IDSC) Immunotherapy Task Force Collaboration . The current understanding of the endocrine effects from immune checkpoint inhibitors and recommendations for management. JNCI Cancer Spectr. 2018;2(3):pky021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res. 2019;51(3):145-156. [DOI] [PubMed] [Google Scholar]
- 10. Zhai Y, Ye X, Hu F, et al. Endocrine toxicity of immune checkpoint inhibitors: a real-world study leveraging US Food and Drug Administration adverse events reporting system. J Immunother Cancer. 2019;7(1):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98(4):1361-1375. [DOI] [PubMed] [Google Scholar]
- 12. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):173-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. 2019;40(1):17-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events. Version 5. Published November 27, 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf
- 16. Joshi MN, Whitelaw BC, Palomar MT, Wu Y, Carroll PV. Immune checkpoint inhibitor-related hypophysitis and endocrine dysfunction: clinical review. Clin Endocrinol (Oxf). 2016;85(3):331-339. [DOI] [PubMed] [Google Scholar]
- 17. Tahir SA, Gao J, Miura Y, et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proc Natl Acad Sci U S A. 2019;116(44):22246-22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thapa B, Roopkumar J, Kim AS, et al. Incidence and clinical pattern of immune related adverse effects (irAE) due to immune checkpoint inhibitors (ICI). JCO. 2019;37(15 Suppl):e14151. [Google Scholar]
- 19. Faje AT, Sullivan R, Lawrence D, et al. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014;99(11):4078-4085. [DOI] [PubMed] [Google Scholar]
- 20. Wei KZ, Baxter M, Casasola R. Hypophysitis induced by immune checkpoint inhibitors in a Scottish melanoma population. Melanoma Manag. 2019;6(1):MMT13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Callahan MK, Kluger H, Postow MA, et al. Nivolumab plus ipilimumab in patients with advanced melanoma: updated survival, response, and safety data in a phase I dose-escalation study. J Clin Oncol. 2018;36(4):391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480-1492. [DOI] [PubMed] [Google Scholar]
- 23. Suo A, Chan Y, Beaulieu C, et al. Anti-PD1-induced immune-related adverse events and survival outcomes in advanced melanoma. Oncologist. 2020;25(5):438-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785-792. [DOI] [PubMed] [Google Scholar]
- 25. Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Atkins MB, Mier JW, Parkinson DR, Gould JA, Berkman EM, Kaplan MM. Hypothyroidism after treatment with interleukin-2 and lymphokine-activated killer cells. N Engl J Med. 1988;318(24):1557-1563. [DOI] [PubMed] [Google Scholar]
- 27. Hercbergs AA, Goyal LK, Suh JH, et al. Propylthiouracil-induced chemical hypothyroidism with high-dose tamoxifen prolongs survival in recurrent high grade glioma: a phase I/II study. Anticancer Res. 2003;23(1B):617-626. [PubMed] [Google Scholar]
- 28. Hercbergs A, Johnson RE, Ashur-Fabian O, Garfield DH, Davis PJ. Medically induced euthyroid hypothyroxinemia may extend survival in compassionate need cancer patients: an observational study. Oncologist. 2015;20(1):72-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hercbergs A. Clinical implications and impact of discovery of the thyroid hormone receptor on integrin αvβ3: a review. Front Endocrinol (Lausanne). 2019;10:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davis FB, Tang HY, Shih A, et al. Acting via a cell surface receptor, thyroid hormone is a growth factor for glioma cells. Cancer Res. 2006;66(14):7270-7275. [DOI] [PubMed] [Google Scholar]
- 31. Davis PJ, Glinsky GV, Lin HY, et al. Cancer cell gene expression modulated from plasma membrane integrin αvβ3 by thyroid hormone and nanoparticulate tetrac. Front Endocrinol (Lausanne). 2015;6:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glinskii AB, Glinsky GV, Lin H-Y, et al. Modification of survival pathway gene expression in human breast cancer cells by tetraiodothyroacetic acid (tetrac). Cell Cycle. 2009;8(21):3562-3570. [DOI] [PubMed] [Google Scholar]
- 33. Cristofanilli M, Yamamura Y, Kau SW, et al. Thyroid hormone and breast carcinoma. Primary hypothyroidism is associated with a reduced incidence of primary breast carcinoma. Cancer. 2005;103(6):1122-1128. [DOI] [PubMed] [Google Scholar]
- 34. Smith GL, Smith BD, Garden AS, et al. Hypothyroidism in older patients with head and neck cancer after treatment with radiation: a population-based study. Head Neck. 2009;31(8):1031-1038. [DOI] [PubMed] [Google Scholar]
- 35. Rosenberg AG, Dexeus F, Swanson DA, von Eschenbach AC. Relationship of thyroid disease to renal cell carcinoma: an epidemiologic study. Urology. 1990;35(6):492-498. [DOI] [PubMed] [Google Scholar]
- 36. Yalcin M, Bharali DJ, Lansing L, et al. Tetraidothyroacetic acid (tetrac) and tetrac nanoparticles inhibit growth of human renal cell carcinoma xenografts. Anticancer Res. 2009;29(10):3825-3831. [PubMed] [Google Scholar]
- 37. Davis PJ, Davis FB, Mousa SA, Luidens MK, Lin HY. Membrane receptor for thyroid hormone: physiologic and pharmacologic implications. Annu Rev Pharmacol Toxicol. 2011;51:99-115. [DOI] [PubMed] [Google Scholar]
- 38. Davis PJ, Tang HY, Hercbergs A, Lin HY, Keating KA, Mousa SA. Bioactivity of thyroid hormone analogs at cancer cells. Front Endocrinol (Lausanne). 2018;9:739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin HY, Landersdorfer CB, London D, et al. Pharmacodynamic modeling of anti-cancer activity of tetraiodothyroacetic acid in a perfused cell culture system. PloS Comput Biol. 2011;7(2):e1001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin HY, Chin YT, Nana AW, et al. Actions of l-thyroxine and Nano-diamino-tetrac (Nanotetrac) on PD-L1 in cancer cells. Steroids. 2016;114:59-67. [DOI] [PubMed] [Google Scholar]
- 41. Naqash AR, Ricciuti B, Owen DH, et al. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol Immunother. 2020;69(7):1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in references.