Abstract
Background: Comorbid disease is a risk factor for severe coronavirus disease 2019 (COVID-19) infection. However, initial rates of chronic obstructive pulmonary disease (COPD) in case series were low and severity of COVID-19 in COPD patients was variable.
Methods: We performed a retrospective study of patients admitted with COVID-19 and evaluated outcomes in those with and without COPD and/or emphysema. Patients were identified as having COPD if they had a diagnosis in the medical record and a history of airflow-obstruction on spirometry, or a history of tobacco use and prescribed long-acting bronchodilator(s). Computed tomography scans were evaluated by radiologists. Propensity matching was performed for age, body mass index (BMI), and serologic data correlated with severity of COVID-19 disease (D-dimer, C-reactive protein, ferritin, fibrinogen, absolute lymphocyte count, lymphocyte percentage, and lactate dehydrogenase).
Results: Of 577 patients admitted with COVID-19, 103 had a diagnosis of COPD and/or emphysema. The COPD/emphysema cohort was older (67 versus 58, p<0.0001) than the other cohort and had a lower BMI. Among unmatched cohorts those with COPD/emphysema had higher rates of intensive care unit (ICU) admission (35% versus 24.9%, p=0.036) and maximal respiratory support requirements, with more frequent invasive mechanical ventilation (21.4% versus 11.8%), but no significant difference in mortality. After propensity-matching there was no difference in ICU admission, maximal respiratory support requirements, or mortality. Univariate and multivariate regression analyses yielded similar results.
Discussion: Our propensity-matched retrospective cohort study suggests that patients hospitalized with COVID-19 who have COPD and/or emphysema may not have worse outcomes than those without these comorbid conditions.
Keywords: copd, emphysema, chronic obstructive pulmonary disease, comorbidities, COVID-19, outcomes, coronavirus 2019
Background
Coronavirus disease 2019 (COVID-19) presents with a wide range of severity and has caused a worldwide pandemic.1,2 Initial reports from China described a disease where 81% of individuals who test positive are asymptomatic, have mild symptoms, or a mild pneumonia, while 19% have disease severe enough to warrant hospitalization, and 5% overall progressing to critical illness and requiring intensive care.3 Predictors for disease severity, intensive care unit admission, use of mechanical ventilation, or death reported initially out of China included age over 65 years and presence of comorbid disease.4 Comorbid disease included cardiovascular disease, diabetes, hypertension, chronic obstructive pulmonary disease (COPD), and immunosuppression.
Severity of outcomes in patients with COPD hospitalized with COVID-19 in reported data varies. An early report out of China of 1099 patients with COVID-19 had 137 current smokers, 12 former smokers, and only 12 patients (1.1%) with COPD.4 A study of 191 hospitalized patients with COVID-19 from China only reported 6 patients with COPD, with 4 dying (7% of total deaths) and 2 surviving to hospital discharge and only 11 patients of the 191 were smokers.5 A report of 90 patients infected reported only 1 with COPD, with more common comorbidities including coronary artery disease, diabetes, and hypertension.6 A series of 1591 patients admitted to the intensive care unit (ICU) in Italy with COVID-19 reported 4% of patients with COPD, while other comorbidities such as hypertension were more prevalent.7 In the United States, a case series describing 21 patients admitted to ICUs in the Seattle area with COVID-19 included 5 smokers and only 1 person with COPD.8 Two cohorts from New York City had the prevalence of COPD between 5.1%–5.4%.9,10
Chronic lung disease was only present in 2.5% of patients in a cohort study of patients assessing risk of progression to acute respiratory distress syndrome (ARDS) or death in China11 and emphysema was present in <2 % in multiple studies assessing computed tomography (CT) imaging features of COVID-19 disease.12,13 A meta-analysis of 7 studies with a total of 1592 patients with COVID-19 found only 1 study where COPD was a significant predictor of severe COVID-19 disease. When pooling the data of all 7 studies, however, COPD was associated with severe COVID-19 disease with an odds ratio of 5.69 (confidence interval, [CI] 2.49 to13.00).14 Another analysis of 1590 patients with COVID-19 found that a diagnosis of COPD carried an odds ratio of 2.68 (CI 1.42 to5.04) for ICU admission, mechanical ventilation, or death.15 COPD was predominantly reported as a comorbidity in these studies either by patient report on hospital admission or via extraction of an existing diagnosis in the medical record.5,16,17
Herein, in this single-center retrospective study we seek to assess the impact of a diagnosis of COPD and/or the presence of emphysema on CT on the severity of presentation and short-term outcomes in patients with COVID-19.
Methods
Patient Population
We performed a retrospective chart review of adult patients 18 years or older admitted to Temple University Hospital in Philadelphia, Pennsylvania with COVID-19 from March 18, 2020 to May 04, 2020. Data was collected through manual data extraction via the electronic medical record with the use of quality evaluations by the investigators.
Diagnosis of Coronavirus 2019 Infection
The diagnosis of patients with COVID-19 pneumonia was based on symptoms, presence of infiltrates on chest X-ray or high-resolution CT scan with a positive reverse transcription polymerase chain reaction (RT-PCR) nasopharyngeal swab for COVID-19. High resolution CT scans were performed for clinical utility and graded and interpreted by a board-certified radiologist.
Patients with negative RT-PCR for COVID-19 test were excluded. The study was approved by the Temple University Institutional Review Board (Protocol number 26854).
Definition of COPD/Emphysema
Patients were divided into 2 cohorts for analysis - patients with a history of COPD and/or emphysema on the CT of the chest and those with neither. All patients included in the COPD/emphysema cohort had either available spirometry showing irreversible airflow obstruction or a history of cigarette smoking with outpatient use of an inhaled bronchodilator, and/or findings of emphysema on CT as interpreted by a board-certified radiologist.
Clinical Variables
Clinical variables collected included age, sex, race, body mass index (BMI), comorbidities, and smoking status. Laboratory markers suggestive of severity of COVID-19 collected included absolute lymphocyte count (ALC), such as C-reactive protein (CRP), lactate dehydrogenase (LDH), interleukin-6 (IL-6), D-dimer, and ferritin. Also analyzed were troponin and brain natriuretic peptide. Outcomes analyzed included maximal ventilatory support required (oxygen supplementation via nasal cannula, high-flow nasal cannula oxygen therapy [HFNT], noninvasive positive-pressure ventilation [NIPPV], or invasive mechanical ventilation), need for intensive care, total hospital length-of-stay, and mortality.
Propensity Scores Matching
To identify comparable groups with and without COPD/emphysema, we used propensity scores matching to derive the 2 matched groups for data analysis. A nearest neighbor 1:1 variable ratio, parallel, balanced propensity scores matching method was used after generating the propensity scores from the variables age, BMI, d-dimer, CRP, ferritin, fibrinogen, absolute lymphocyte count, percentage of lymphocytes, and LDH. Given the relatively small sample size and to confirm that bias was not introduced through propensity matching, univariate and multivariate regression analyses were also performed for the outcomes of mortality, invasive mechanical ventilation, intensive care unit admission, and hospital length-of-stay.
Primary Objective
The primary objective of this study was to determine if patients admitted with a clinical history of COPD and/or radiographic diagnosis of emphysema have worse outcomes associated with COVID-19 pneumonia as compared to patients without COPD/emphysema.
Statistical Analysis
Data were expressed as mean ± standard deviation or median (interquartile range [IQR]) where applicable for continuous variables and frequencies with percentages for categorical variables. Continuous variables were compared between the 2 groups using 2-sample t-test and categorical variables with the use of the Pearson chi-square test. The non-normally distributed laboratory variables were compared between the 2 groups using a 2-sample Wilcoxon rank-sum test. Survival data was assessed by Kaplan-Meier curve and compared by log-rank test. A p-value <0.05 was considered statistically significant. Univariate and multivariate regression analysis was performed for 4 primary outcomes–mortality, invasive mechanical ventilation, intensive care unit admission, and hospital length of stay–in order to confirm the findings of the propensity-matching analysis. For each outcome, COPD/emphysema was analyzed as well as several variables that were deemed clinically relevant and had significant differences on initial analysis with 2-sample t-test, Pearson chi-square test, or 2-sample Wilcoxon rank-sum test. All the data were analyzed using SAS 9.4 (SAS Institute, Cary, North Carolina) and Stata 14.0 (StataCorp. LP, College Station, Texas).
Results
A total of 577 patients with nasopharyngeal RT-PCR positive for COVID-19, admitted to the hospital between March 18-May 4, 2020, were included in the analysis. Of these patients, 103 had either a historical diagnosis of COPD and/or radiographic evidence of significant emphysema.
Patients with COPD/Emphysema
Fifty-seven of the 103 patients in the COPD/emphysema cohort had a clinical diagnosis of COPD with 46 of the 103 patients having significant radiographic evidence of emphysema. Sixteen patients had pulmonary function testing available via our electronic medical records, with a median forced-expiratory volume in 1 second (FEV1) of 1.5 liters (69% of predicted). Eleven patients had evidence of chronic hypercapnia on arterial blood gas and 10 patients were on supplemental oxygen at home.
Fifty-three patients (51.5%) were using either a long-acting muscarinic agent, long-acting beta2-agonist, or inhaled corticosteroid as an outpatient. Forty-nine of these patients (47.6%) were on combination therapy. Sixty-one patients (59.2%) were initiated on a long-acting muscarinic, long-acting beta2- agonist, or inhaled corticosteroid delivered via nebulized or hand-held metered dose inhaler while hospitalized. Ninety-seven patients (94.1%) received systemic corticosteroids while hospitalized for COVID-19. The 7 patients who did not receive steroids had either no pulmonary symptoms, a relative contraindication such as sepsis from bacterial infection or active diverticulitis or left the hospital against medical advice before treatment was initiated.
All patients received CT scans for clinical care purposes and all imaging was interpreted and reviewed by a board-certified radiologist. CTs showed evidence of emphysema in 46 patients. The degree of emphysema was quantified via CT densitometry with a cutoff criterion of –950 Hounsfield units. Of these patients, the average percentage of parenchymal emphysema was 5.6% (+5.11%) in the left lung and 5.6% (+5.3%) in the right lung.
Patient Demographics
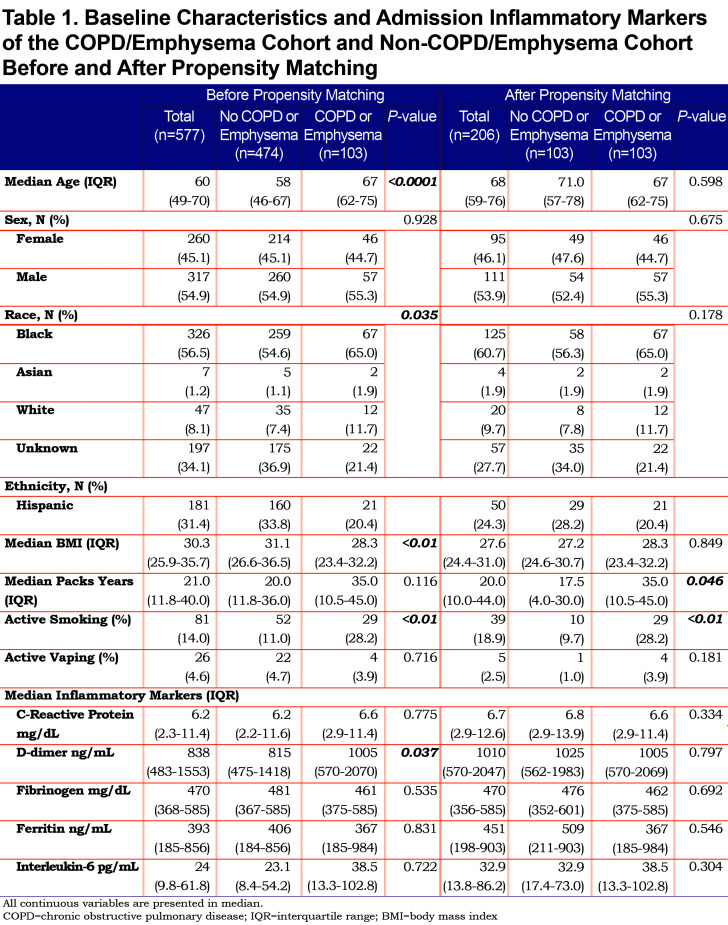
The COPD/emphysema cohort had a median age of 67 years (62–75), which was significantly older than the non-COPD cohort with a median age of 58 years (46–67) (p=<0.0001). Gender was statistically similar between the 2 cohorts. The COPD/emphysema cohort had a lower median BMI (28.3) compared to the non-COPD cohort (31.1) (p=<0.0001). Smoking history determined through intake history showed the COPD/emphysema cohort had higher median pack years (35, IQR 10.5–45) compared to the non-COPD cohort (20, 11.8–36), but this was not a statistically significant finding. More patients in the COPD/emphysema cohort (28.2%) were still actively smoking. Complete demographics are shown in Table 1.
Laboratory Findings
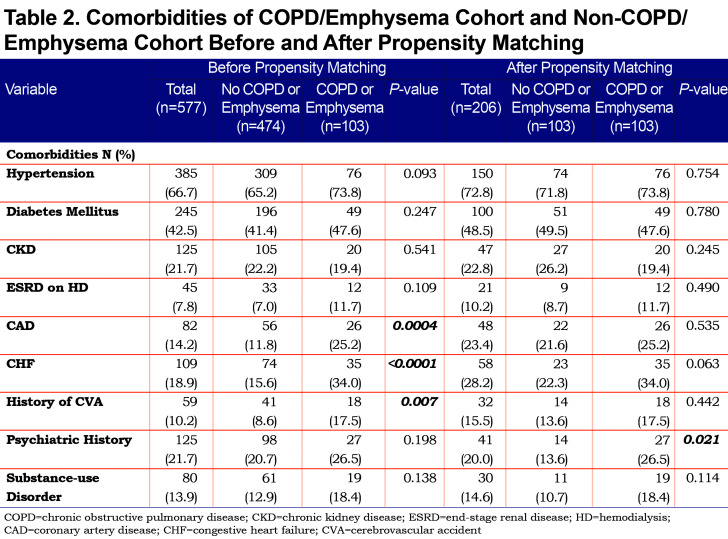
On admission, we used inflammatory markers as a surrogate to represent the severity of a COVID-19 associated cytokine storm. The COPD cohort had higher IL-6 levels compared to the non-COPD cohort, but the difference was not statistically significant. However, the COPD cohort had a significantly higher median D-Dimer (1005 ng/mL) compared to the non-COPD cohort. As expected, the COPD cohort had more comorbidities than the non-COPD cohort (represented in Table 2), with the difference in coronary artery disease (25.2% versus 11.8%), congestive heart failure (34% versus 15.6%) and history of stroke (17.5% versus 8.6%) being statistically significant.
Clinical Outcomes
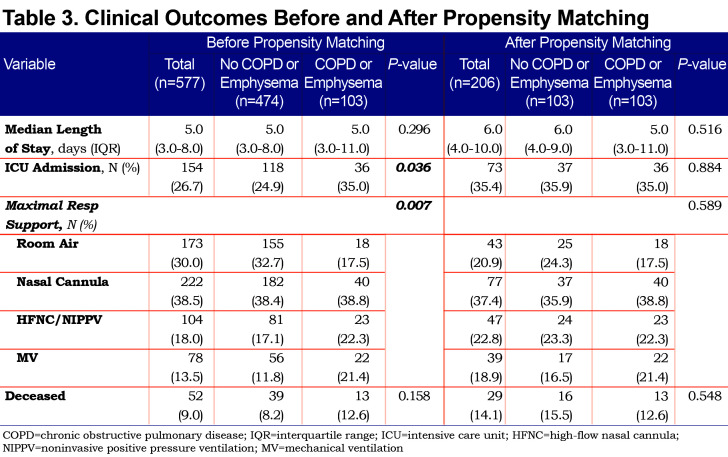
Outcomes between the 2 cohorts are shown in Table 3. The COPD/emphysema cohort had significantly more ICU admissions (35% versus 24.9%, p=0.036) and needed more advanced respiratory support, including oxygen via HFNT, NIPPV (22.3% versus 17.1%) and mechanical ventilation (21.4% versus 11.8%) compared to the non-COPD cohort. Mortality was not significantly different between the 2 cohorts.
Propensity Matching
We applied propensity matching for potential confounders of age, BMI, and severity of COVID-19 on presentation. After propensity matching, the 103 patients of the COPD cohort were compared to 103 patients in the non-COPD cohort. Full demographic, comorbidities, and laboratory data are shown in Table 1.
After propensity matching, there was no statistically significant difference observed between the COPD and the non-COPD cohort in age, BMI, or any of the selected inflammatory markers. Median pack years and active smoking status were statistically higher in the COPD cohort. Of note, the statistical difference seen prior in coronary artery disease, congestive heart failure, and stroke history was not observed in the cohorts after propensity matching (Table 2).
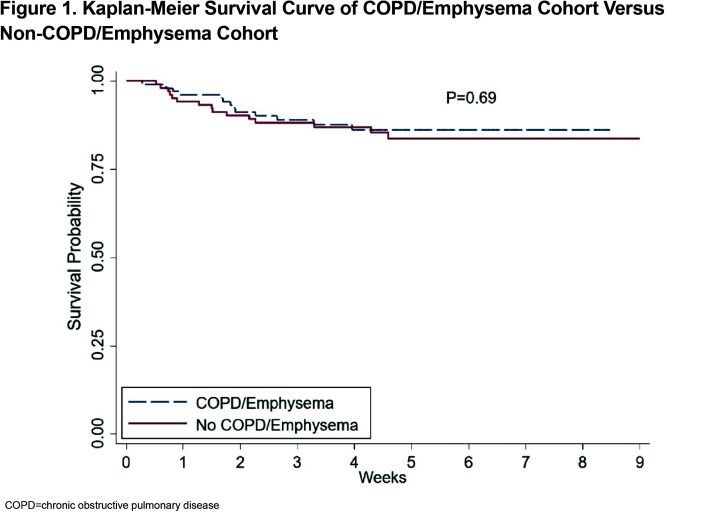
Outcomes of the matched cohorts are shown in Table 3. There was no statistically significant difference between ICU admissions and level of respiratory failure after propensity matching between the COPD and non-COPD cohort. Survival probability was assessed via Kaplan-Meier and there was no difference in survival between the COPD and non-COPD cohort over the first 80 days after initial admission for COVID-19. (Figure 1)
Univariate and Multivariate Regression Analysis
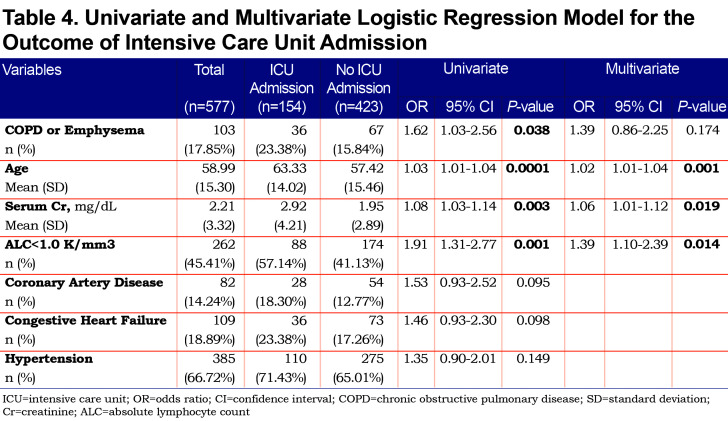
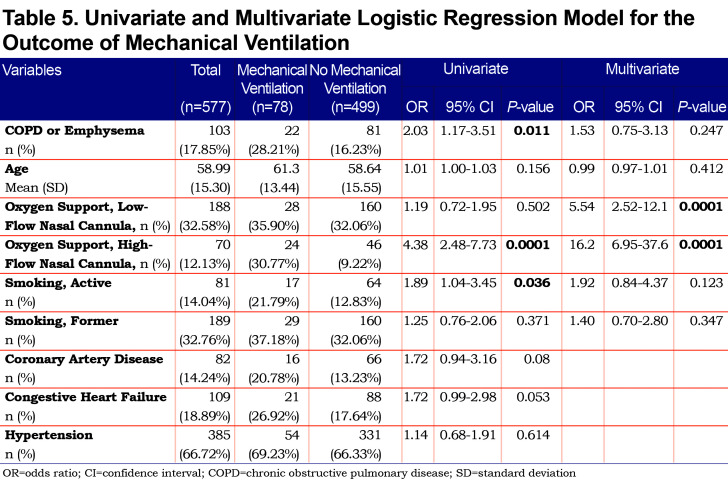
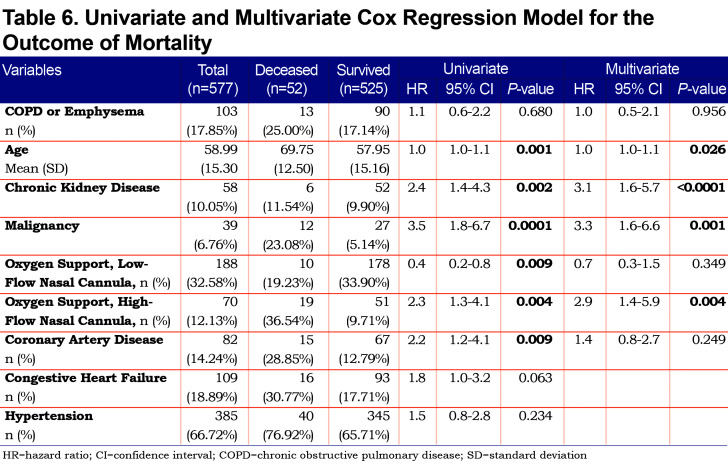
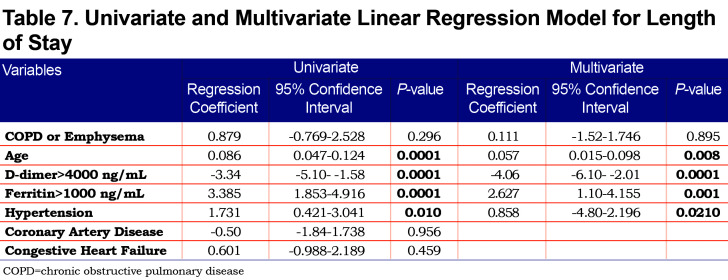
On univariate analysis a diagnosis of COPD emphysema was associated with a higher rate of ICU admission with an odds ratio of 1.62 (1.03–2.56, p=0.038) and with a higher risk of invasive mechanical ventilation with an odds ratio of 2.03 (1.17–3.51, p=0.011). There was no significant difference for mortality with an odds ratio of 1.01 (0.6–2.2, p=0.68) or hospital length-of-stay (p=0.296). When multivariate regression analysis with variables significant on univariate analysis was performed, a diagnosis of COPD/emphysema was not associated with significant differences in any of the primary outcomes. This analysis is shown in Tables Table 4, Table 5, Table 6, Table 7.
Discussion
This retrospective observational study of patients hospitalized with COVID-19 found no major differences in the outcomes between patients with and without COPD and/or emphysema after propensity matching for age, BMI, and markers associated with COVID-19 disease (ALC, ferritin, D-Dimer, LDH, fibrinogen, and CRP). In the unmatched cohorts we do not report a higher rate of mortality or hospital length of stay in patients with COPD and/or emphysema but did find that they more frequently were admitted to the ICU and required higher levels of maximal respiratory support. Univariate and multivariate regression analyses yielded similar results.
Patients with COPD and/or emphysema were significantly older, had a lower BMI, had higher D-Dimer values, and higher rates of cardiovascular comorbidity (coronary artery disease, congestive heart failure, and stroke). Of note, the higher D-dimer level may have been due to the older age in the COPD/emphysema group. Univariate and multivariate regression analysis did confirm that age, but not COPD/emphysema, was correlated with an elevated D-dimer level.
The cohort of patients had a relatively high prevalence of COPD/emphysema, likely due to high rates of cigarette smoking in the area around the study hospital and the fact that the hospital is a referral center for patients with COPD. While active smokers had a higher rate of mechanical ventilation on univariate analysis, neither active nor former smokers did after multivariate analysis (Table 5). A significant number of patients were on controller-inhaler therapy as an outpatient, some on inhaled corticosteroids. Debate over potential impairment of antiviral responses in those on inhaled corticosteroids with COVID-19 exists, however, a recent meta-analysis and a systematic review have not shown a difference in mortality or rates of severe disease.18,19 We chose to propensity-match for age because older patients with COVID-19 have been shown to have worse outcomes and higher mortality.8,15,16,20 BMI was also matched both because obesity has been associated with worse COVID-19 outcomes21,22 and because both obesity and low BMI are associated with worse outcomes in patients with COPD.23,24 COVID-19 has been associated with lymphopenia as well as elevated inflammatory markers. These markers of elevation can correlate with COVID-19 disease severity so we chose to include it in our propensity matching.5,10,25-26-27 We did not propensity match for a history of coronary artery disease, congestive heart failure, or history of prior stroke because cardiovascular disease is a known, associated comorbidity in patients with COPD,28,29 but it is worth noting that when controlling for age, our propensity-matched groups did not have significant differences with regards to these comorbidities, nor with others such as hypertension, diabetes mellitus, or chronic kidney disease. Unsurprisingly, active smoking was higher in the COPD/Emphysema cohort both before and after propensity matching.
Comorbid disease has been associated with worse outcomes in patients with COVID-19. Specifically, comorbid hypertension, diabetes, heart disease, and kidney disease have been correlated with increased risk of hospitalization and death.7,9,15,30,31 In a meta-analysis, although only 1 of 7 studies had shown a significant association, pooled data showed COPD to be associated with severe COVID-19 disease. The value of our propensity matching methodology is to control for age, BMI, and known laboratory markers associated with COVID-19 disease in comparing patients with and without COPD/emphysema.
The similar outcomes for patients with and without COPD/emphysema at our large academic center suggest that patients with COPD may not necessarily be at higher risk for worse outcomes when hospitalized for COVID-19. Similar findings have recently been reported on multivariate analysis for the outcomes of ICU admission and death.32 We report data from the earlier months of the pandemic in the United States when practice varied on utilization of invasive mechanical ventilation rather than using NIPPV or HFNT out of a concern for aerosolization of virus particles and increased risk of disease transmission to health care workers.33-34-35-36 Invasive mechanical ventilation has been associated with higher mortality in COVID-19 patients,7,37,38 and evidence is accumulating that aerosolization from the respiratory tract on HFNT and NIPPV is less concerning.39,40 Data reported by our institution in a separate study reported relatively low use of invasive mechanical ventilation and favorable outcomes associated with the use of HFNT.41 In addition, many of our patients received glucocorticoids and bronchodilators either in the form of inhalers or nebulized therapy regardless when indicated. The avoidance of invasive mechanical ventilation, use of glucocorticoids, and use of bronchodilators all are factors that may explain why our propensity-matched group of patients with COPD and/or emphysema did not have worse outcomes.
Our study has several limitations, including the following examples. It is a single-center, retrospective study. COPD is a heterogenous disease with various phenotypes and this study is limited in not evaluating these differences such as disease severity and exacerbation history. Our control group had a high rate of active smoking, although the COPD/emphysema group had significantly higher rates before and after matching. We chose to group patients with a diagnosis of COPD along with patients with emphysema on CT imaging as these are related conditions and it allowed a greater number of patients to be compared. We attempted to objectively characterize COPD/emphysema based on a history of smoking, prior prescription of a long-acting bronchodilator and by having all CT scans evaluated by a radiologist to determine presence of emphysema. It is established that COPD is underdiagnosed in the population in general and that may lead to missing some patients affected with early, asymptomatic, or undiagnosed disease which we attempt to mitigate with our radiologic inclusion criteria.42-43-44
In conclusion, our findings suggest that although patients with COPD and/or emphysema hospitalized with COVID-19 were found to have higher maximal respiratory requirements and more frequent ICU admission prior to propensity matching, they do not necessarily have worse outcomes including mortality. Further investigation into outcomes of patients with these common diseases in the setting of the ongoing COVID-19 pandemic is warranted.
Abbreviations
Abbreviations: coronavirus-disease-2019, COVID-19; chronic obstructive pulmonary disease, COPD; body-mass index, BMI; high-flow nasal therapy, HFNT; noninvasive positive pressure ventilation, NIPPV; interquartile range, IQR; intensive care unit, ICU; acute respiratory distress syndrome, ARDS; computed tomography, CT; confidence interval, CI; reverse transcription polymerase chain reaction, RT-PCR; C-reactive protein, CRP; lactate dehydrogenase, LDH; interleukin-6, IL-6; forced expiratory volume in 1 second, FEV1; coronary artery disease, CAD; congestive heart failure, CHF; chronic kidney disease, CKD; end-stage renal disease, ESRD; hemodialysis, HD; mechanical ventilation, MV; absolute lymphocyte count, ALC; hazard ratio, HZ; Thoracic Medicine and Surgery department, TMS
Funding Statement
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1.World Health Organization (WHO). Novel coronavirus-China. WHO website. Published 2020. Accessed November 2020. http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ [Google Scholar]
- 2.Center for Systems Science and Engineering at Johns Hopkins University (JHU). COVID-19 Dashboard. JHU website. Published 2020. Accessed November 2020. https://coronavirus.jhu.edu/map.html [Google Scholar]
- 3.Wu Z,McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi:https://doi.org/10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 4.Guan W,Ni Z,Hu Y,et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720. doi:https://doi.org/10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F,Yu T,Du R,et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:https://doi.org/10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X,Yu C,Qu J,et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47(5):1275-1280. doi:https://doi.org/10.1007/s00259-020-04735-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G,Zangrillo A,Zanella A,et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16). doi:https://doi.org/10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatraju PK,Ghassemieh BJ,Nichols M,et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382(21):2012-2022. doi:https://doi.org/10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson S,Hirsch JS,Narasimhan M,et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:https://doi.org/10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goyal P,Choi JJ,Pinheiro LC,et al. Clinical characteristics of Covid-19 in New York City. New Engl J Med. 2020;382(24):2372-2374. doi:https://doi.org/10.1056/NEJMc2010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C,Chen X,Cai Y,et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934-943. doi:https://doi.org/10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernheim A,Mei X,Huang M,et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295(3):685-691. doi:https://doi.org/10.1148/radiol.2020200463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung M,Bernheim A,Mei X,et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 2020;295(1):202-207. doi:https://doi.org/10.1148/radiol.2020200230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lippi G,Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir Med. 2020:105941. doi:https://doi.org/10.1016/j.rmed.2020.105941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan W-J,Liang W-H,Zhao Y,et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi:https://doi.org/10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D,Hu B,Hu C,et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi:https://doi.org/10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung JM,Niikura M,Yang CWT,Sin DD. COVID-19 and COPD. Eur Respir J. 2020;56(2):2002108. doi:https://doi.org/10.1183/13993003.02108-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kow CS,Hasan SS. Preadmission use of inhaled corticosteroids and risk of fatal or severe COVID-19: a meta-analysis. J Asthma. 2021 Feb 8:1-4. doi:https://doi.org/10.1080/02770903.2021.1878531 [DOI] [PubMed] [Google Scholar]
- 19.Halpin DMG,Singh D,Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020;55(5):2001009. doi:https://doi.org/10.1183/13993003.01009-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michelozzi P,de'Donato F,Scortichini M,et al. Mortality impacts of the coronavirus disease (COVID-19) outbreak by sex and age: rapid mortality surveillance system, Italy, 1 February to 18 April 2020. Euro Surveill. 2020;25(19):2000620. doi:https://doi.org/10.2807/1560-7917.ES.2020.25.19.2000620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalligeros M,Shehadeh F,Mylona EK,et al. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity (Silver Spring). 2020;28(7):1200-1204. doi:https://doi.org/10.1002/oby.22859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malik VS,Ravindra K,Attri SV,Bhadada SK,Singh M.Higher body mass index is an important risk factor in COVID-19 patients: a systematic review and meta-analysis. Environ Sci Pollut Res Int. 2020;27:42115-42123. doi:https://doi.org/10.1007/s11356-020-10132-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert AA,Putcha N,Drummond MB,et al. Obesity is associated with increased morbidity in moderate to severe COPD. Chest. 2017;151(1):68-77. doi:https://doi.org/10.1016/j.chest.2016.08.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celli BR,Cote CG,Marin JM,et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005-1012. doi:https://doi.org/10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 25.Huang C,Wang Y,Li X,et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi:https://doi.org/10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Q,Meng M,Kumar R,et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int J Infect Dis. 2020;96:131-135. doi:https://doi.org/10.1016/j.ijid.2020.04.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou C,Huang Z,Hu Y,et al. Predictive factors of severe coronavirus disease 2019 in previously healthy young adults: a single-center, retrospective study. Respir Res. 2020;21:157. doi:https://doi.org/10.1186/s12931-020-01412-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatila WM,Thomashow BM,Minai OA,Criner GJ,Make BJ. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):549-555. doi:https://doi.org/10.1513/pats.200709-148ET [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnes PJ,Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165-1185. doi:https://doi.org/10.1183/09031936.00128008 [DOI] [PubMed] [Google Scholar]
- 30.Flythe JE,Assimon MM,Tugman MJ,et al. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis. 2020;77(2):190-203. doi:https://doi.org/10.1053/j.ajkd.2020.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Q,Zhou Y,Wang X,et al. Effect of hypertension on outcomes of adult inpatients with COVID-19 in Wuhan, China: a propensity score-matching analysis. Respir Res. 2020;21:172. doi:https://doi.org/10.1186/s12931-020-01435-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calmes D,Graff S,Maes N,et al. Asthma and COPD are not risk factors for ICU stay and death in case of SARS-CoV2 infection. J Allergy Clin Immunol Pract. 2021;9(1):160-169. doi:https://doi.org/10.1016/j.jaip.2020.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alhazzani W,Møller MH,Arabi YM,et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. doi:https://doi.org/10.1097/CCM.0000000000004363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu C. Correspondence. Brit J Surg. 2019;106(7):949. doi:https://doi.org/10.1002/bjs.11197 [DOI] [PubMed] [Google Scholar]
- 35.Ñamendys-Silva SA. Respiratory support for patients with COVID-19 infection. Lancet Respir Med. 2020;8(4):e18. doi:https://doi.org/10.1016/S2213-2600(20)30110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kluge S,Janssens U,Welte T,Weber-Carstens S,Marx G,Karagiannidis C.German recommendations for critically ill patients with COVID 19. Med Klin Intensivmed Notfmed. 2020;115(Suppl 3):S111-S114. doi:https://doi.org/10.1007/s00063-020-00689-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X,Yu Y,Xu J,et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. doi:https://doi.org/10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tobin MJ,Laghi F,Jubran A.Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care. 2020;10(1):78. doi:https://doi.org/10.1186/s13613-020-00692-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaeckle NT,Lee J,Park Y,Kreykes G,Evans MD,Jr CJH.Aerosol generation from the respiratory tract with various modes of oxygen delivery. Am J Resp Crit Care. 2020;202(8):1115-1124. doi:https://doi.org/10.1164/rccm.202006-2309OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J,Fink JB,Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur Respir J. 2020;55(5):2000892. doi:https://doi.org/10.1183/13993003.00892-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel M,Gangemi A,Marron R,et al. Retrospective analysis of high flow nasal therapy in COVID-19-related moderate-to-severe hypoxaemic respiratory failure. BMJ Open Respir Res. 2020;7(1):e000650. doi:https://doi.org/10.1136/bmjresp-2020-000650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labonté LE,Tan WC,Li PZ,et al. Undiagnosed chronic obstructive pulmonary disease contributes to the burden of health care use. Data from the CanCOLD study. Am J Resp Crit Care. 2016;194(3):285-298. doi:https://doi.org/10.1164/rccm.201509-1795OC [DOI] [PubMed] [Google Scholar]
- 43.Martinez CH,Mannino DM,Jaimes FA,et al. Undiagnosed obstructive lung disease in the United States. Associated factors and long-term mortality. Ann Am Thorac Soc. 2015;12(12):1788-1795. doi:https://doi.org/10.1513/AnnalsATS.201506-388OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gershon AS,Thiruchelvam D,Chapman KR,et al. Health services burden of undiagnosed and overdiagnosed COPD. Chest. 2018;153(6):1336-1346. doi:https://doi.org/10.1016/j.chest.2018.01.038 [DOI] [PubMed] [Google Scholar]