Abstract
Background
The secretion of organic solutes by the proximal tubules is an essential intrinsic kidney function. The degree to which secretory solute clearance corresponds with the glomerular filtration rate (GFR) and potential metabolic implications of net secretory clearance are largely unknown.
Methods
We evaluated 1240 participants with chronic kidney disease (CKD) from the multicenter Chronic Renal Insufficiency Cohort (CRIC) Study. We used targeted mass-spectrometry to quantify candidate secretory solutes in paired 24-h urine and plasma samples. CRIC study personnel measured GFR using 125I-iothalamate clearance (iGFR). We used correlation and linear regression to determine cross-sectional associations of secretory clearances with iGFR and common metabolic complications of CKD.
Results
Correlations between iGFR and secretory solute clearances ranged from = +0.30 for hippurate to = +0.58 for kynurenic acid. Lower net clearances of most secretory solutes were associated with higher serum concentrations of parathyroid hormone (PTH), triglycerides and uric acid. Each 50% lower kynurenic acid clearance was associated with a 21% higher serum PTH concentration [95% confidence interval (CI) 15–26%] and a 10% higher serum triglyceride concentration (95% CI 5–16%) after adjustment for iGFR, albuminuria and other potential confounders. Secretory solute clearances were not associated with statistically or clinically meaningful differences in serum calcium, phosphate, hemoglobin or bicarbonate concentrations.
Conclusions
Tubular secretory clearances are modestly correlated with measured GFR among adult patients with CKD. Lower net secretory clearances are associated with selected metabolic complications independent of GFR and albuminuria, suggesting potential clinical and biological relevance.
Keywords: CKD complications, glomerular filtration rate, proximal tubular secretion, secretory solutes clearances
INTRODUCTION
The estimated glomerular filtration rate (GFR) and urinary albumin excretion are used to determine the severity of chronic kidney disease (CKD) and to monitor its progression [1]. However, the kidneys perform many other important functions, including reabsorption, synthesis and secretion, that are not commonly measured. Consequently, little is known about variability in these functions and their potential metabolic consequences.
The secretion of retained solutes and medications by the proximal tubules is an essential intrinsic kidney function [2]. Organic anion and cation transporters located on the basolateral surface of the proximal tubules uptake solutes from the vasculature, including protein-bound substances that cannot be filtered [3–5]. On the luminal surface, solutes are secreted into the urine by an energy-dependent process mediated by transporters that include members of the ATP-binding cassette transporter family [6]. Despite the recognized importance of proximal tubular secretory clearance, this kidney function is rarely measured due to a lack of validated assays and uncertainty regarding clinical interpretation.
We previously reported individual-level differences in tubular secretory solute clearance for a given level of estimated GFR among persons with CKD [7]. These data were limited by assessment of estimated, rather than directly measured GFR, evaluation of a small group of endogenous secretory solutes and recruitment from a single study. In this study, we compare 24-h kidney clearances of 11 endogenous secretory solutes with gold-standard measurements of GFR, determined by 125I-iothalamate clearance, in a national cohort study of CKD. We then delineate associations of net tubular secretory clearances with common metabolic complications of CKD independent of GFR and albuminuria.
MATERIALS AND METHODS
Data source and study population
This study is complementary to one of our previously published works, which assessed associations of kidney clearances of secretory solutes with incident CKD progression and all-cause mortality in the Chronic Renal Insufficiency Cohort (CRIC) study [8]. The CRIC study is a multicenter, prospective study designed to investigate risk factors for CKD progression and cardiovascular complications among adults with mild to moderate CKD [9, 10]. The CRIC study excluded screened participants with known polycystic kidney disease, active immunosuppression for glomerulonephritis, prior kidney transplantation, multiple myeloma, HIV infection and advanced heart failure [10]. The institutional review board at each CRIC site approved the study protocol. All participants provided written informed consent.
We focused this ancillary study on the weighted sub-sample of CRIC study participants who completed 125I-iothalamate clearance studies at baseline (n = 1432). Participants were excluded from iothalamate testing if they had recently undergone thallium stress imaging, were unable to void, required self-catheterization or had a known iodine allergy. We further excluded 192 CRIC study participants whose plasma or 24-h urine samples were not available, leaving a final analytic sample of 1240.
Measurements of secretory solute clearance
We previously reported our methods for the measurement of the kidney clearances of secretory solutes [8]. Briefly, we selected candidate secretory solutes from the published literature based on known affinity for organic anion or cation transporters, increased blood concentrations in transporter knockout models, a high degree of protein binding and/or reported kidney clearances that are higher than that of creatinine or GFR [11–13] (Table 1). We then quantified these solutes in plasma and urine using a targeted liquid chromatography-tandem mass spectrometry assay with labeled internal standards and single-point calibrators. Subsequent protein binding studies performed in our laboratory demonstrated three solutes, i.e. isovalerylglycine, tiglylglycine and xanthosine, had lower protein binding compared with results from previous studies (Table 1). Nonetheless, as these solutes had high kidney clearances relative to GFR, indicating tubular secretion as a primary kidney elimination route, we kept these solutes in our analyses.
Table 1.
Associations of secretory solute clearances with iGFR
| Solutes | Protein binding in healthy controls (%)a | Protein binding in CKD (%)a | Kidney clearance, mL/min/1.73 m2 b | Ratio of clearance to iGFRc | Correlation with iGFR (ρ)d |
|---|---|---|---|---|---|
| Iothalamate | – | – | 47 (34–61) | – | – |
| Kynurenic acid | 97 ±1 | 96 ±2 | 82 (58–119) | 1.7 | 0.58 |
| p-cresol sulfate | 97 ±1 | 96 ±2 | 8 (5–13) | 0.2 | 0.53 |
| Indoxyl sulfate | 97 ±1 | 93 ±2 | 30 (20–45) | 0.6 | 0.57 |
| Cinnamoylglycine | 91 ±12 | 95 ±3 | 52 (30–91) | 1.1 | 0.36 |
| Pyridoxic acid | 83 ±4 | 87 ±1 | 399 (255–604) | 8.5 | 0.54 |
| Dimethyluric acid | 72 ±4 | 68 ±7 | 427 (245–752) | 9.1 | 0.34 |
| Hippurate | 68 ±4 | 51 ±13 | 435 (255–711) | 9.3 | 0.30 |
| Trimethyluric acid | 59 ±33 | 80 ±11 | 257 (139–489) | 5.5 | 0.35 |
| Tiglylglycine | 33 ±20 | 24 ±15 | 164 (105–258) | 3.5 | 0.55 |
| Xanthosine | 11 ± 14 | 15 ±13 | 72 (43–109) | 1.5 | 0.44 |
| Isovalerylglycine | 6 ±13 | 4 ±7 | 206 (131–313) | 4.4 | 0.48 |
Mean and SD protein binding percentage in 14 healthy persons with normal kidney function (estimated GFR ≥ 90 mL/min/1.73 m2 and no albuminuria) and 14 patients with advanced CKD from the Seattle Kidney Study (estimated GFR <15 mL/min/1.73 m2 not receiving dialysis). Plasma was filtered using a centrifugal filter (Amicon Ultra, 3kD MWCO) at 11 200g for 30 min at room temperature. The concentration of solutes in the filtrate was then determined using the same method as for plasma and compared with the concentration of solutes in unfiltered plasma.
Median (interquartile range).
Ratio of medians.
Correlation coefficient between secretory solutes clearances and iGFR (all log-transformed); all P < 0.001.
For this study, plasma samples were precipitated in organic solvent followed by solid phase extraction (Phree phospholipid removal plate) [8]. Urine samples underwent two different solid-phase extractions (HLB or MCX µElution plates, Waters) to increase the capture of target solutes. Dried extracts were reconstituted in 80 µL of 5% acetonitrile/0.2% formic acid in H2O and filtered through a large-pore filter plate (Millipore, MSBVN1210) to remove particulates before introduction into a triple quadrupole tandem mass spectrometer (Sciex 6500). Data were normalized to labeled internal standards consisting of purified compounds that were added to each well. We used a single-point calibration approach to calibrate solutes concentrations data with five replicates of calibrators on each study plate (pooled human serum and urine). We have previously used quantitative nuclear magnetic resonance to quantify absolute concentrations of secretory solutes in the calibrators by standard addition of purified compounds. Inter- and intra-assay coefficients of variation (COV) for individual solutes in plasma and urine ranged from 3.4% to 14.7% (Supplementary data, Table S1).
We calculated the kidney clearance of each secretory solute as:
In this equation, UX represents the urine concentration of the secretory solute, V represents the corresponding urine volume in mL per minute and PX represents the plasma concentration of the solute. For direct comparisons with iothalamate measurements of GFR (iGFR), we standardized secretory solute clearances to 1.73 m2.
Measurement of covariates
At the baseline CRIC study visit, participants self-reported their information on sociodemographic characteristics and medical history, including cardiovascular conditions and lifestyle behaviors. Current medications were ascertained using the inventory method [9, 10]. iGFR was measured using a standardized protocol after a low protein (<10 g) meal [14, 15]. In brief, following a water load and saturated potassium iodine solution, study personnel administered a subcutaneous injection of 125I-iothalamate and collected timed urine and serum samples during four subsequent collection periods. The first collection period was dropped from analyses and iGFR was calculated as the weighted average of iothalamate clearance during collection periods 2–4, corrected for body surface area. The median COV for iGFR measurements was 9.7% [14, 15].
Serum creatinine (enzyme-based assay), albumin (dye-binding assay), calcium, phosphorus and plasma glucose were measured on the Hitachi Vitros 950 AT. Twenty-four-hour urine albumin was measured on Siemens Immulite [14, 16]. Three seated blood pressure measurements were obtained, and the mean of the second and third readings was used for analysis [17, 18]. Total parathyroid hormone (PTH) was measured by the Scantibodies immunoradiometric assay [16, 19]. Alkaline phosphatase (ALP) was measured by enzyme-linked immunosorbent assay [19]. Triglyceride and high-density lipoprotein (HDL) were measured by spectrophotometry and low-density lipoprotein (LDL) by β quantification after separation by ultracentrifugation [20]. Serum uric acid was measured using the uricase/peroxidase methods [21]. High-sensitivity C-reactive protein (CRP) was measured using particle-enhanced immunonephelometry [22]. Hemoglobin was measured at each CRIC clinical center [23]. Serum bicarbonate was measured using Beckman Coulter DxC [15]. Participants were considered as having diabetes mellitus at baseline based on a fasting glucose concentration ≥126 mg/dL, a nonfasting glucose ≥200 mg/dL or the use of antidiabetic medication [22]. Physical activity was calculated from the Typical Week Physical Activity Survey as the total metabolic equivalent task (MET) score, which is derived from the number of hours per week spent in each of 27 activities, weighted by each activity’s MET value [24]. Study participants self-reported their cause of CKD at baseline as categories of hypertension, diabetes, glomerulonephritis and obstruction.
Statistical analyses
We summarized correlations between secretory clearances and iGFR using scatter plots and Pearson’s correlation. To facilitate the presentation of baseline characteristics, we computed a summary secretion score by standardizing each log-transformed secretory clearance to a 0–100 scale and then taking the average of these values:
In this equation, ln(secretory clearance) represents secretory clearance after natural log-transformation, min(ln(secretory clearance)) represents the minimum value of log-transformed secretory clearance and range(ln(secretory clearance)) represents the difference between the maximum and minimum values.
We used linear regression to estimate cross-sectional associations of secretory solute clearances with metabolic complications of CKD. We log-transformed secretory solute clearances and metabolic markers to obtain better model fit and produce comparable results among models. Base models were adjusted for log-transformed iothalamate GFR, log-transformed 24-h urinary albumin excretion, age, race, sex, attained education level, current smoking status, body mass index (BMI) and history of diabetes mellitus. Models of mineral metabolism markers were further adjusted for the use of active vitamin D, phosphate binders, and calciferols and serum concentrations of calcium (not in the calcium model) and phosphate (not in the phosphate model). Models of dyslipidemia, uric acid, bicarbonate, hemoglobin and CRP were further adjusted for waist circumference, physical activity levels, hemoglobin A1c and the use of statins, nonstatin lipid-lowering medications, thiazide diuretics and allopurinol. To assess potential confounding by CKD etiologies, we further adjusted for self-reported causes of CKD in a sensitivity analysis. We used the Hommel method to correct for multiple comparisons [25]. We defined the number of comparisons in each table/figure as either the number of exposures or the number of characteristics or outcomes, whichever was larger. For example, the number of comparisons for Table 2 was 21 and the number of comparisons in Figures 2 and 3 was 11. A two-sided corrected P-value of 0.05 was used to define statistical significance. Data analyses were performed using Stata/IC version 14.2 for Windows (StataCorp. 2015; Stata Statistical Software: Release 14; StataCorp LP, College Station, TX, USA) and RStudio version 3.4.3 (R Development Core Team 2017, Vienna, Austria).
Table 2.
Baseline participant characteristics by quartiles of the summary secretion scorea
| Characteristics | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-value |
|---|---|---|---|---|---|
| iGFR, mL/min/1.73 m2 | 33 ± 15 | 43 ± 15 | 51 ± 14 | 68 ± 21 | <0.001* |
| Sociodemographic characteristics | |||||
| Age, years | 57 ± 12 | 57 ± 11 | 56 ± 12 | 54 ± 12 | 0.002* |
| Female | 144 (47) | 127 (41) | 127 (41) | 143 (46) | 0.38 |
| Black | 141 (46) | 104 (34) | 100 (32) | 107 (35) | 0.001* |
| Hispanic | 55 (18) | 58 (19) | 46 (15) | 23 (7) | <0.001* |
| Education categoriesb | <0.001* | ||||
| Less than high school | 80 (26) | 54 (17) | 52 (17) | 25 (8) | |
| High school graduate | 70 (23) | 64 (21) | 57 (18) | 40 (13) | |
| Some college | 83 (27) | 96 (31) | 73 (23) | 89 (29) | |
| College graduate or higher | 76 (25) | 96 (31) | 129 (41) | 154 (50) | |
| Health characteristics | |||||
| Current smoker | 42 (14) | 33 (11) | 31(10) | 35 (11) | 0.19 |
| History of diabetes | 163 (53) | 165 (53) | 150 (48) | 122 (39) | 0.02 |
| History of cardiovascular disease | 107 (35) | 94 (30) | 78 (25) | 47 (15) | <0.001* |
| History of heart failure | 37 (12) | 26 (8) | 20 (6) | 3 (1) | <0.001* |
| BMI, kg/m2 | 32 ± 7 | 32 ± 7 | 31 ± 7 | 30 ± 7 | <0.001* |
| Systolic blood pressure, mmHg | 134 ± 23 | 129 ± 22 | 128 ± 21 | 124 ± 19 | <0.001* |
| Total MET score | 197 ± 163 | 196 ± 129 | 224 ± 139 | 223 ± 140 | 0.004* |
| Lab measurements | |||||
| Hemoglobin A1cc | 6.1 (5.5, 7.4) | 6.2 (5.6, 7.2) | 6.1 (5.5, 7.3) | 5.8 (5.3, 6.9) | 0.04 |
| 24-h urine albumin excretionc | 0.26 (0.03, 1.14) | 0.12 (0.02, 0.92) | 0.07 (0.01, 0.57) | 0.02 (0.01, 0.21) | <0.001* |
| Nephrotic range proteinuria >3 g | 31 (10) | 31 (10) | 22 (7) | 12 (4) | 0.005* |
| Medications | |||||
| Insulin | 80 (26) | 83 (27) | 82 (27) | 54 (18) | 0.10 |
| Statin | 177 (57) | 173 (56) | 177 (57) | 139 (45) | 0.006* |
| Loop diuretic | 158 (51) | 121 (39) | 96 (31) | 59 (19) | <0.001* |
| Thiazide diuretic | 68 (22) | 90 (29) | 100 (32) | 87 (28) | 0.12 |
| ACEi/ARB | 204 (66) | 222 (72) | 242 (78) | 174 (56) | 0.17 |
ACEi, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker.
For continuous variable: mean ± SD, P-values were from linear regression with participant characteristics as the dependent variable and the summary secretion score as the independent variable. For binary variables: n (%), P-values were from logistic regression with participant characteristics as the dependent variable and the summary secretion score as the independent variable.
P-value from the Chi-squared test.
Median (interquartile range), P-values were from linear regression with log-transformed participant characteristics as the dependent variable and the summary secretion score as the independent variable.
*Denotes statistical significance after correction for multiple comparisons using the Hommel method.
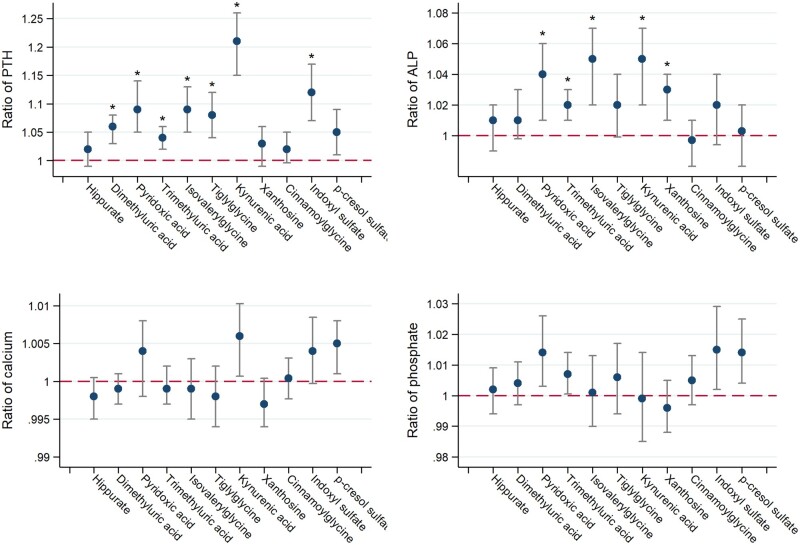
FIGURE 2.
Adjusted associations of secretory solute clearances with markers of mineral metabolism. Model adjusted for log-transformed i GFR, log-transformed 24-h urinary albumin excretion, age, race, sex, attained education, current smoking status, BMI, diabetes mellitus, serum calcium (not in the calcium model), serum phosphate (not in the phosphate model), and the use of active vitamin D, phosphate binders and calciferols. Ratio expressed per 50% lower clearance of each individual solute (log-transformed). Conversion factors for units: calcium in mg/dL to mmol/L, ×0.2495; phosphate in mg/dL to mmol/L, ×0.3229. Asterisks denote statistical significance after correction for multiple comparisons using the Hommel method.
RESULTS
Associations of secretory solute clearances with measured GFR
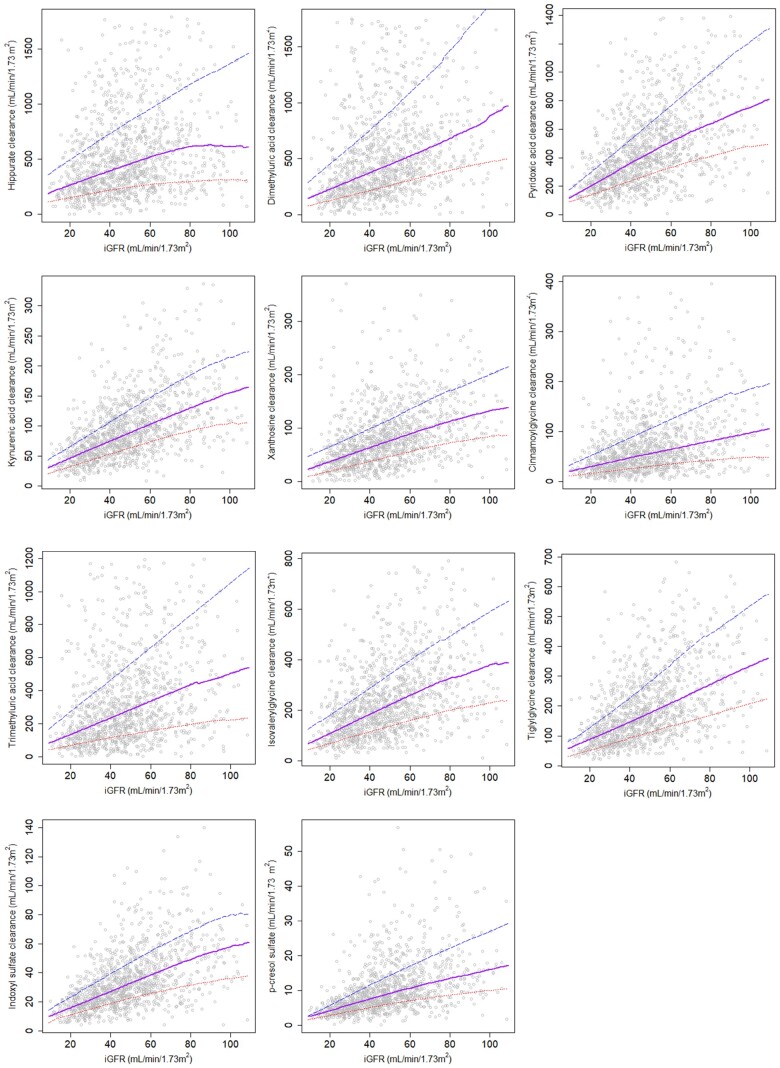
Among the 1240 CRIC participants in this ancillary study, the mean age was 56 years; 44% were women; 37% were Black and 15% reported Hispanic race. The median iGFR was 47 mL/min/1.73 m2 (interquartile range: 34–61 mL/min/1.73 m2). The kidney clearances of 9 of the 11 candidate secretory solutes were higher than measured GFR (Table 1). The highest clearance was observed for hippurate (median 435 mL/min/1.73 m2) and the lowest clearance was observed for p-cresol sulfate (median 8 mL/min/1.73 m2). Considerable interindividual variation in the clearance of each secretory solute was observed across the measured range of GFR (Figure 1). Secretory solutes clearances moderately correlated with iGFR, with a range between +0.30 for hippurate and +0.58 for kynurenic acid (Table 1). Correlations were slightly higher between secretory solute clearances and creatinine clearance obtained from the same urine sample (Supplementary data, Table S2). Correlations among the individual solute clearances are shown in Supplementary data, Table S2.
FIGURE 1.
Associations between iGFR and clearances of secretory solutes. Extreme values omitted for presentation. The lower dotted line represents the 20th percentile; the middle solid line represents the 50th percentile; the upper dashed line represents the 80th percentile.
Associations of secretory solute clearances with baseline characteristics
Participants with a higher summary secretion score were more likely to be non-black and non-Hispanic race, and have a higher iGFR, a higher attained education, a lower prevalence of diabetes, cardiovascular disease, heart failure, and lower systolic blood pressure and 24-h urine albumin excretion (Table 2). Associations for individual secretory solute clearances are presented in Supplementary data, Table S3. Among 726 participants who self-reported their cause of CKD, those with glomerulonephritis tended to have higher kidney clearances of secretory solutes (Table 3). For example, the kidney clearance of kynurenic acid, a highly protein-bound secretory solute, was 91 mL/min/1.73 m2 among patients with glomerulonephritis and 74 and 78 mL/min/1.73 m2 in patients with hypertension and diabetes, respectively.
Table 3.
Kidney clearances of secretory solutes by self-reported etiologies of chronic kidney diseasea
| Solute clearance, mL/min/1.73 m2 |
||||||
|---|---|---|---|---|---|---|
| Clearance | All participants with self-reported cause of CKD (n = 726) | Hypertension (n = 342) | Diabetes (n = 281) | Glomerular disease (n = 45) | Obstructive uropathy (n = 58) | P-valueb |
| Hippurate | 413 (243–683) | 351 (230–643) | 466 (279–778) | 499 (316–721) | 438 (229–677) | 0.02 |
| Dimethyluric acid | 406 (226–710) | 377 (215–669) | 412 (241–740) | 400 (233–799) | 460 (250–436) | 0.39 |
| Pyridoxic acid | 363 (229–541) | 341 (217–478) | 373 (257–555) | 375 (221–600) | 406 (247–617) | 0.02 |
| Trimethyluric acid | 234 (128–434) | 222 (121–392) | 243 (130–432) | 288 (149–576) | 218 (152–575) | 0.29 |
| Isovalerylglycine | 187 (112–278) | 172 (105–261) | 195 (120–272) | 274 (145–400) | 185 (115–298) | <0.001* |
| Tiglylglycine | 151 (94–226) | 142 (90–205) | 151 (97–236) | 211 (136–377) | 183 (88–244) | <0.001* |
| Kynurenic acid | 77 (54–107) | 74 (49–103) | 78 (58–111) | 91 (63–143) | 80 (51–117) | 0.002* |
| Xanthosine | 67 (39–100) | 61 (37–93) | 73 (40–111) | 72 (41–105) | 71 (49–100) | 0.06 |
| Cinnamoylglycine | 48 (28–84) | 45 (28–78) | 52 (29–86) | 62 (37–92) | 50 (23–83) | 0.13 |
| Indoxyl sulfate | 27 (18–40) | 25 (17–37) | 28 (21–43) | 26 (19–41) | 31 (20–42) | 0.01 |
| p-cresol sulfate | 8 (5–11) | 7 (5–11) | 8 (6–11) | 8 (5–14) | 8 (5–13) | 0.34 |
Secretory solutes clearance shown in mL/min/1.73 m2 as median (interquartile range).
P-value from analysis of variance on log-transformed secretory solutes clearances.
Statistical significance after correction for multiple comparisons using the Hommel method.
Association of secretory solute clearances with markers of mineral metabolism
After controlling for iGFR, urinary albumin excretion, demographics, smoking, diabetes, BMI, serum calcium and phosphate concentrations, and the use of active vitamin D, phosphate binders and calciferols, lower clearances of most secretory solutes were associated with higher serum concentrations of PTH (Figure 2). Associations reached statistical significance for seven solutes after correction for multiple comparisons. The strongest association was observed for kynurenic acid clearance. Each 50% lower clearance of this solute was associated with an estimated 21% higher serum PTH concentration [95% confidence interval (CI) 15–26% higher, P < 0.001] after full adjustment. Lower clearances of five secretory solutes were associated with higher serum concentrations of ALP after controlling for covariates. No clear relationships were observed between net secretory solute clearances and the serum calcium or phosphate concentration and the upper bounds of the 95% CIs for these associations exclude clinically or scientifically meaningful effects.
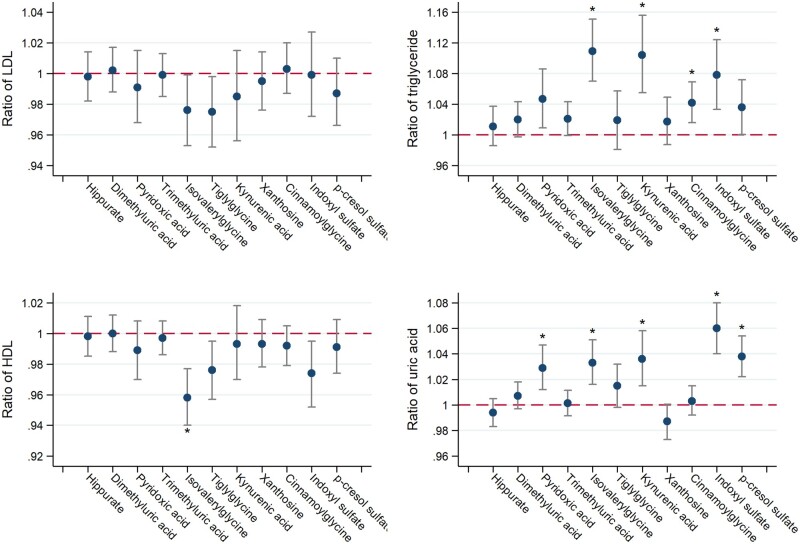
Association of secretory solute clearances with markers of dyslipidemia and uric acid
Lower clearances of isovalerylglycine, kynurenic acid, cinnamoylglycine and indoxyl sulfate were associated with higher serum triglyceride concentrations after full adjustment and correction for multiple comparisons (Figure 3). Similarly, lower clearances of five secretory solutes were associated with higher serum uric acid concentrations. In contrast, associations were generally null or inconsistent for serum concentrations of LDL-cholesterol and HDL-cholesterol.
FIGURE 3.
Adjusted associations of secretory solute clearances with markers of lipid metabolism and uric acid. Model adjusted for log-transformed iGFR, log-transformed 24-h urinary albumin, age, race, sex, attained education, current smoking status, BMI, diabetes mellitus, waist circumference, physical activity levels, hemoglobin A1C, statins, non-statin lipid-lowering medications, thiazide diuretics and allopurinol. Ratio expressed per 50% lower clearance of each individual solute (log-transformed). Conversion factors for units: LDL in mg/dL to mmol/L, ×0.02586; triglyceride in mg/dL to mmol/L, ×0.01129; HDL in mg/dL to mmol/L, ×0.02586; uric acid in mg/dL to μmol/L, ×59.48. Asterisks denote statistical significance after correction for multiple comparisons using the Hommel method.
Association of secretory solute clearances with other metabolic complications
Lower secretory clearances of four solutes were associated with higher serum CRP concentrations; however, associations remained significant for only isovalerylglycine clearance after correction for multiple comparisons (Supplementary data, Figure S1). No associations were observed between secretory solute clearances and serum bicarbonate or hemoglobin concentrations. Additional adjustment for self-reported causes of CKD only negligibly impacted the observed associations between the kidney clearances of secretory solutes and serum concentrations of PTH, triglycerides, uric acid and CRP (Supplementary data, Table S4).
DISCUSSION
In a representative cohort study of patients with CKD, we found modest correlations between the kidney clearances of secretory solutes and iothalamate clearance measurements of GFR. Yet, considerable variability in secretory clearances was observed for a given level of GFR. Lower net clearances of many secretory solutes were associated with higher serum concentrations of PTH, triglycerides and uric acid after adjustment for iGFR and potential confounding characteristics. Taken together, these findings suggest that tubular secretory clearance may indicate biologically and clinically relevant information about kidney function in addition to established glomerular measures of GFR and albuminuria.
The proximal tubules are responsible for eliminating a wide range of small molecules, including drugs, endobiotics, nutrients and uremic solutes and toxins [26, 27]. Many secretory transporters are members of the solute carrier (SLC) superfamily, which includes organic anion transporters (OAT) and organic cation transporters. The OAT, OAT1 and OAT3, primarily expressed on the basolateral membrane of proximal tubular cells, mediate uptake of organic anions from the circulation via exchange with dicarboxylates, most notably alpha-ketoglutarate. OAT1 and OAT3 have been recognized as primary basolateral transporters of nine of the secretory solutes in this study [28–30]. Apical transporters most relevant to this study are multidrug resistance protein 4 (MRP4) and breast cancer resistance protein (BCRP), both of which are members of the ATP-binding cassette transporters (ABC) superfamily. ATP hydrolysis is the driving force of the extrusion of solutes by these apical transporters [26]. MRP4 and BCRP both mediate the transport of indoxyl sulfate and kynurenic acid, while BCRP is likely also responsible for the extrusion of p-cresol sulfate [31–33]. Hippuric acid has been shown to inhibit the active transport of MRP4 and BCRP at clinically relevant concentrations; however, whether it is a substrate for these transporters is not known [34].
Prior studies have demonstrated lower protein binding of indoxyl sulfate and p-cresol sulfate among patients receiving chronic dialysis compared with healthy controls [35, 36]. However, conflicting results have been observed in patients with nondialysis requiring CKD. Poesen et al. have found progressively lower protein binding of p-cresol sulfate with lower GFR [37]; however, two other studies have reported similar protein binding of indoxyl sulfate comparing nondialysis CKD patients with healthy individuals [38, 39]. In our study, we did find lower protein binding of indoxyl sulfate, p-cresol sulfate and hippurate among patients with nondialysis requiring CKD patients with healthy controls. However, the amount of difference was less than that reported by Poesen et al. possibly due to differences in laboratory methods and small sample sizes in our protein binding studies.
The kidney clearances of most secretory solutes exceeded measured values of GFR, highlighting tubular secretion as an important kidney mechanism of elimination. These findings from a contemporary CKD cohort study underscore the potential for efficient extraction of specific solutes by the proximal tubules. In contrast to glomerular filtration, which is typically limited to 20–25% of renal plasma flow, tubular secretion can remove >80% of a substance from the circulation in a single pass through the kidneys [40]. However, the kidney clearances of some solutes, specifically hippurate, dimethyluric acid and pyridoxic acid, were more than eight times higher than GFR, exceeding normal physiological parameters. These ratios were nearly identical when computed using creatinine clearance from the same 24-h urine sample. An important possible explanation for these high clearance values is diurnal variation. The clearance of a substance, when calculated as (UX) * V/(PX), may be overestimated if PX obtained on a single occasion is lower than the true average plasma value over the urine collection period. For example, an unrealistically high hippurate clearance may be obtained if the morning plasma hippurate concentration is lower than the daily average, which may occur if plasma hippurate levels increase later in day due to the intake of certain foods. Alternatively, we cannot rule out the possibility of intra-tubular synthesis or metabolism of these solutes, though we have found no literature to support this assumption. It is also possible that some participants with CKD have a relatively low filtration fraction, resulting in a high secretory clearance relative to GFR. Low filtration fractions have been reported in specific kidney diseases; however, this characteristic has not been measured in large cohort studies of patients with CKD. We also observed relatively low kidney clearances of some solutes, specifically indoxyl sulfate and p-cresol sulfate, as previously reported [7]. It is likely that differences in individual secretory solute clearances reflect variation in affinities for tubular transporters, binding sites on serum albumin and rates of movement through the interstitial space. The slow, yet consistent kidney elimination of indoxyl sulfate and p-cresol sulfate, which are highly protein bound and suspected to contribute to uremic toxicity, represents an important homeostatic kidney function [41, 42].
Recent reports have raised doubts about the validity of GFR as a unique marker of uremia [43–45]. These studies demonstrate that among patients with Stages 2–5 CKD, estimated GFR is poorly associated with circulating concentrations of low molecular weight uremic solutes, such as hippuric acid, indoxyl sulfate and p-cresol sulfate, as well as concentrations of middle molecular weight uremic solutes, suggesting that factors other than GFR play a larger role in affecting uremic solute concentrations. Our results support this conclusion by showing that measured GFR only modestly correlated with kidney clearances of secretory solutes, which have a direct impact on their concentration.
Lower net secretory solute clearances were associated with higher serum PTH concentrations after adjustment for iGFR and other potential confounding characteristics. This finding may reflect the metabolism of PTH by the proximal tubules, where its receptors are most abundant. In animal models of ureteral obstruction, which halts glomerular filtration, the kidney elimination of PTH is only partially reduced. In human studies utilizing direct renal artery and vein sampling, the single-pass kidney elimination of PTH ranges from 34% to 47%, which is considerably higher than that of a filtered substance such as creatinine [46–48].
We also observed consistent associations between lower net secretory clearances and higher serum triglyceride levels after adjustment for iGFR and other characteristics. The retention of uremic solutes, many of which are secreted by the proximal tubules, promotes the accumulation of apolipoprotein C-III, an inhibitor of lipoprotein lipase (LPL), leading to subsequent inhibition of the hydrolysis of triglycerides into free fatty acids. Some studies have also suggested that PTH, which was associated with lower secretory clearances in this study, can downregulate the synthesis of LPL leading to accumulation of triglycerides [49–52].
Uric acid is filtered, reabsorbed and secreted. Secretion occurs predominantly in the S2 segment of the proximal tubules via basolateral OAT1/3 and apical ATP-Binding Cassette transporter G2 (ABCG2) [53]. In population-based studies, genetic variation in ABCG2 is associated with higher circulating uric acid levels [54, 55]. Lower clearances of endogenous secretory solutes may parallel that of uric acid to explain the observed associations of these clearances with serum uric acid concentrations.
High sensitivity CRP is a sensitive marker of systemic inflammation. The retention of many uremic solutes, e.g. hippuric acid, indoxyl sulfate and p-cresol sulfate, is postulated to contribute to systemic inflammation, which could explain the observed association between lower net secretory clearances and higher levels of CRP [56–62]. Alternatively, it is possible that chronic inflammation promotes local atherosclerosis, fibrosis and scarring of capillaries and interstitium around proximal tubules, leading to lowered secretory capacity [63–66]. It is unlikely that the observed association is due to decreased renal clearance of high sensitivity of CRP, as in both healthy subjects and patients with kidney disease, urine levels of CRP are negligibly low [67, 68].
Secretory solute clearances were not associated with markers of anemia or acidosis. Acid–base homeostasis involves the concerted actions of multiple tubular segments, including ammonia synthesis and bicarbonate reabsorption in the proximal tubules, recycling in the loop of Henle and distal acidification. The complexity of acid–base balance and the relative insensitivity of serum bicarbonate levels to these processes may explain the lack of association with secretory clearances. Similarly, erythropoietin is primarily synthesized by peritubular interstitial cells and hemoglobin concentrations may be influenced by many characteristics other than erythropoietin production, potentially explaining the lack of association with secretory clearances.
One strength of this study is the comparison of secretory solute clearances with direct measurements of GFR in a large, broadly representative CKD population. Other strengths include the use of targeted mass spectrometry assays to quantify specific secretory solutes with high accuracy and precision and research protocol-driven 24-h urine collections. Several limitations of the study warrant discussion. First, the variation in secretory solute clearances relative to iGFR may be inflated due to imperfect measurement. Specifically, misreporting of 24-h urine collection times, diurnal variation in circulating concentrations of secretory solutes and measurement errors in laboratory procedures may have contributed to variability in the estimates of secretory clearance. Moreover, iGFR is subject to some degree of error, as demonstrated by the median 9.7% coefficient of variation in the CRIC study [14, 15]. Third, CKD etiologies in CRIC were determined based on self-report, suggesting value from further studies in other cohorts that include dedicated kidney histology data. Finally, the cross-sectional study design cannot inform the temporality of the observed associations between secretory solute clearances and metabolic complications of CKD.
In conclusion, we report individual-level differences in the kidney clearances of endogenous secretory solutes relative to measured GFR in a large, diverse cohort of adults with CKD. Lower net secretory clearances were associated with higher serum concentrations of PTH, triglycerides and uric acid independent of iGFR, albuminuria and other potential confounding characteristics. Our findings suggest that proximal tubular secretory clearance may provide additional insight into the assessment of kidney function and metabolic complications of CKD.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
CRIC Study Investigators: Lawrence J. Appel, MD, MPH; Harold I. Feldman, MD, MSCE; Alan S. Go, MD; Jiang He, MD, PhD; James P. Lash, MD; Panduranga S. Rao, MD; Mahboob Rahman, MD; Raymond R. Townsend, MD.
FUNDING
This work was supported by National Institutes of Health (NIH) R01 DK107931. Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963 and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the NIH and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSAUL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/The National Center for Research Resources (NCRR) UCSF-CT SI UL1 RR-024131.
AUTHORS’ CONTRIBUTIONS
B.R.K., H.I.F., A.S.G. and J.P.L. designed the study; Y.C., K.W., R.K. and B.R.K. conducted literature search; A.N.H, J.O.B. and B.R.K. collected data; Y.C., L.R.Z., R.K., A.N.H., J.O.B. and B.R.K. analyzed data; all authors interpreted data; Y.C. and B.R.K. drafted the manuscript; all authors revised and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared. Results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Stevens PE, Levin A.. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825–830 [DOI] [PubMed] [Google Scholar]
- 2. Rose BD, Post TW.. Clinical Physiology of Acid-base and Electrolyte Disorders. London: McGraw-Hill, 2001 [Google Scholar]
- 3. Vallon V, Rieg T, Ahn SY. et al. Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. Am J Physiol Renal Physiol 2008; 294: F867–F873 [DOI] [PubMed] [Google Scholar]
- 4. Koepsell H, Lips K, Volk C.. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 2007; 24: 1227–1251 [DOI] [PubMed] [Google Scholar]
- 5. Motohashi H, Inui K.. Organic cation transporter OCTs (SLC22) and MATEs (SLC47) in the human kidney. AAPS J 2013; 15: 581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yacovino LL, Aleksunes LM.. Endocrine and metabolic regulation of renal drug transporters. J Biochem Mol Toxicol 2012; 26: 407–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suchy-Dicey AM, Laha T, Hoofnagle A. et al. Tubular secretion in CKD. J Am Soc Nephrol 2016; 27: 2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Y, Zelnick LR, Wang K. et al. Kidney clearance of secretory solutes is associated with progression of CKD: the CRIC study. J Am Soc Nephrol 2020; 31: 817–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feldman HI, Appel LJ, Chertow GM. et al. ; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. The chronic renal insufficiency cohort (CRIC) study: design and methods. J Am Soc Nephrol 2003; 14: S148–S153 [DOI] [PubMed] [Google Scholar]
- 10. Lash JP, Go AS, Appel LJ. et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 2009; 4: 1302–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bush KT, Wu W, Lun C. et al. The drug transporter OAT3 (SLC22A8) and endogenous metabolite communication via the gut-liver-kidney axis. J Biol Chem 2017; 292: 15789–15803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rhee EP, Clish CB, Ghorbani A. et al. A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol 2013; 24: 1330–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sirich TL, Aronov PA, Plummer NS. et al. Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int 2013; 84: 585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anderson AH, Yang W, Hsu CY. et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 2012; 60: 250–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsu CY, Propert K, Xie D. et al. Measured GFR does not outperform estimated GFR in predicting CKD-related complications. J Am Soc Nephrol 2011; 22: 1931–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fisher H, Hsu CY, Vittinghoff E. et al. Comparison of associations of urine protein-creatinine ratio versus albumin-creatinine ratio with complications of CKD: a cross-sectional analysis. Am J Kidney Dis 2013; 62: 1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Radulescu V, Goyfman M, Mohler ER III. et al. Prevalence and correlates of mitral annular calcification in adults with chronic kidney disease: results from CRIC study. Atherosclerosis 2015; 242: 117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson AH, Yang W, Townsend RR. et al. Time-updated systolic blood pressure and the progression of chronic kidney disease: a cohort study. Ann Intern Med 2015; 162: 258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scialla JJ, Leonard MB, Townsend RR. et al. Correlates of osteoprotegerin and association with aortic pulse wave velocity in patients with chronic kidney disease. Clin J Am Soc Nephrol 2011; 6: 2612–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rahman M, Yang W, Akkina S. et al. Relation of serum lipids and lipoproteins with progression of CKD: the CRIC study. Clin J Am Soc Nephrol 2014; 9: 1190–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Srivastava A, Kaze AD, McMullan CJ. et al. Uric acid and the risks of kidney failure and death in individuals with CKD. Am J Kidney Dis 2018; 71: 362–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bundy JD, Chen J, Yang W. et al. Risk factors for progression of coronary artery calcification in patients with chronic kidney disease: the CRIC study. Atherosclerosis 2018; 271: 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pfeffer MA, Burdmann EA, Chen CY. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]
- 24. Jepson C, Hsu JY, Fischer MJ. et al. Incident type 2 diabetes among individuals with CKD: findings from the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis 2018; 73: 72–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hommel G. A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika 1988; 75: 383–386 [Google Scholar]
- 26. Nigam SK, Wu W, Bush KT. et al. Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol 2015; 10: 2039–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lepist EI, Ray AS.. Beyond drug-drug interactions: effects of transporter inhibition on endobiotics, nutrients and toxins. Expert Opin Drug Metab Toxicol 2017; 13: 1075–1087 [DOI] [PubMed] [Google Scholar]
- 28. Sugawara M, Mochizuki T, Takekuma Y. et al. Structure–affinity relationship in the interactions of human organic anion transporter 1 with caffeine, theophylline, theobromine and their metabolites. Biochim Biophys Acta 2005; 1714: 85–92 [DOI] [PubMed] [Google Scholar]
- 29. Masereeuw R, Mutsaers HA, Toyohara T. et al. The kidney and uremic toxin removal: glomerulus or tubule? Semin Nephrol 2014; 34: 191–208 [DOI] [PubMed] [Google Scholar]
- 30. Nigam SK, Bush KT, Martovetsky G. et al. The organic anion transporter (OAT) family: a systems biology perspective. Physiol Rev 2015; 95: 83–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takada T, Yamamoto T, Matsuo H. et al. Identification of ABCG2 as an exporter of uremic toxin indoxyl sulfate in mice and as a crucial factor influencing CKD progression. Sci Rep 2018; 8: 11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mutsaers HA, Caetano-Pinto P, Seegers AE. et al. Proximal tubular efflux transporters involved in renal excretion of p-cresyl sulfate and p-cresyl glucuronide: implications for chronic kidney disease pathophysiology. Toxicol In Vitro 2015; 29: 1868–1877 [DOI] [PubMed] [Google Scholar]
- 33. Dankers AC, Mutsaers HA, Dijkman HB. et al. Hyperuricemia influences tryptophan metabolism via inhibition of multidrug resistance protein 4 (MRP4) and breast cancer resistance protein (BCRP). Biochim Biophys Acta 2013; 1832: 1715–1722 [DOI] [PubMed] [Google Scholar]
- 34. Mutsaers HA, Van Den Heuvel LP, Ringens LH. et al. Uremic toxins inhibit transport by breast cancer resistance protein and multidrug resistance protein 4 at clinically relevant concentrations. PLoS One 2011; 6: e18438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Viaene L, Annaert P, de Loor H. et al. Albumin is the main plasma binding protein for indoxyl sulfate and p‐cresyl sulfate. Biopharm Drug Dispos 2013; 34: 165–175 [DOI] [PubMed] [Google Scholar]
- 36. Deltombe O, de Loor H, Glorieux G. et al. Exploring binding characteristics and the related competition of different protein-bound uremic toxins. Biochimie 2017; 139: 20–26 [DOI] [PubMed] [Google Scholar]
- 37. Poesen R, Evenepoel P, de Loor H. et al. Metabolism, protein binding, and renal clearance of microbiota–derived p-cresol in patients with CKD. Clin J Am Soc Nephrol 2016; 11: 1136–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rossi M, Campbell K, Johnson D. et al. Uraemic toxins and cardiovascular disease across the chronic kidney disease spectrum: an observational study. Nutr Metab Cardiovasc Dis 2014; 24: 1035–1042 [DOI] [PubMed] [Google Scholar]
- 39. Rossi M, Johnson DW, Morrison M. et al. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial. Clin J Am Soc Nephrol 2016; 11: 223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith HW, Goldring W, Chasis H.. The measurement of the tubular excretory mass, effective blood flow and filtration rate in the normal human kidney. J Clin Invest 1938; 17: 263–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fujii H, Goto S, Fukagawa M.. Role of uremic toxins for kidney, cardiovascular, and bone dysfunction. Toxins (Basel) 2018; 10: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vanholder R, Argiles A, Baurmeister U. et al. Uremic toxicity: present state of the art. Int J Artif Organs 2001; 24: 695–725 [PubMed] [Google Scholar]
- 43. Vanholder R, Eloot S, Schepers E. et al. An obituary for GFR as the main marker for kidney function? Semin Dial 2012; 25: 9–14 [DOI] [PubMed] [Google Scholar]
- 44. Eloot S, Schepers E, Barreto DV. et al. Estimated glomerular filtration rate is a poor predictor of concentration for a broad range of uremic toxins. Clin J Am Soc Nephrol 2011; 6: 1266–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Neirynck N, Eloot S, Glorieux G. et al. Estimated glomerular filtration rate is a poor predictor of the concentration of middle molecular weight uremic solutes in chronic kidney disease. PLoS One 2012; 7: e44201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Van Ballegooijen AJ, Rhee EP, Elmariah S. et al. Renal clearance of mineral metabolism biomarkers. J Am Soc Nephrol 2015; 27: 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oldham SB, Finck EJ, Singer FR.. Parathyroid hormone clearance in man. Metabolism 1978; 27: 993–1001 [DOI] [PubMed] [Google Scholar]
- 48. Corvilain J, Manderlier T, Struyven J. et al. Metabolism of human PTH by the kidney and the liver. Horm Metab Res 1977; 9: 239–242 [DOI] [PubMed] [Google Scholar]
- 49. Kwan BC, Kronenberg F, Beddhu S. et al. Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol 2007; 18: 1246–1261 [DOI] [PubMed] [Google Scholar]
- 50. Stegmayr B, Olivecrona T, Olivecrona G.. Lipoprotein lipase disturbances induced by uremia and hemodialysis. Semin Dial 2009; 22: 442–444 [DOI] [PubMed] [Google Scholar]
- 51. Akmal M, Kasim SE, Soliman AR. et al. Excess parathyroid hormone adversely affects lipid metabolism in chronic renal failure. Kidney Int 1990; 37: 854–858 [DOI] [PubMed] [Google Scholar]
- 52. Akmal M, Perkins S, Kasim SE. et al. Verapamil prevents chronic renal failure-induced abnormalities in lipid metabolism. Am J Kidney Dis 1993; 22: 158–163 [DOI] [PubMed] [Google Scholar]
- 53. Eraly SA, Vallon V, Rieg T. et al. Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol Genomics 2008; 33: 180–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Woodward OM, Köttgen A, Coresh J. et al. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci USA 2009; 106: 10338–10342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dehghan A, Köttgen A, Yang Q. et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet 2008; 372: 1953–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lowenstein J, Grantham JJ.. The rebirth of interest in renal tubular function. Am J Physiol Renal Physiol 2016; 310: F1351–F1355 [DOI] [PubMed] [Google Scholar]
- 57. Rossi M, Campbell KL, Johnson DW. et al. Protein-bound uremic toxins, inflammation and oxidative stress: a cross-sectional study in stage 3–4 chronic kidney disease. Arch Med Res 2014; 45: 309–317 [DOI] [PubMed] [Google Scholar]
- 58. Vanholder R, Baurmeister U, Brunet P. et al. A bench to bedside view of uremic toxins. J Am Soc Nephrol 2008; 19: 863–870 [DOI] [PubMed] [Google Scholar]
- 59. Glorieux G, Cohen G, Jankowski J. et al. PROGRESS IN UREMIC TOXIN RESEARCH: platelet/leukocyte activation, inflammation, and uremia. Semin Dial 2009; 22: 423–427 [DOI] [PubMed] [Google Scholar]
- 60. Ito S, Yoshida M.. Protein-bound uremic toxins: new culprits of cardiovascular events in chronic kidney disease patients. Toxins (Basel) 2014; 6: 665–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Borges NA, Barros AF, Nakao LS. et al. Protein-bound uremic toxins from gut microbiota and inflammatory markers in chronic kidney disease. J Renal Nutr 2016; 26: 396–400 [DOI] [PubMed] [Google Scholar]
- 62. Lau WL, Kalantar-Zadeh K, Vaziri ND.. The gut as a source of inflammation in chronic kidney disease. Nephron 2015; 130: 92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stuveling EM, Hillege HL, Bakker SJ. et al. C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int 2003; 63: 654–661 [DOI] [PubMed] [Google Scholar]
- 64. Rockey DC, Bell PD, Hill JA.. Fibrosis—a common pathway to organ injury and failure. N Engl J Med 2015; 372: 1138–1149 [DOI] [PubMed] [Google Scholar]
- 65. Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999; 340: 115–126 [DOI] [PubMed] [Google Scholar]
- 66. Hashimoto H, Kitagawa K, Hougaku H. et al. C-reactive protein is an independent predictor of the rate of increase in early carotid atherosclerosis. Circulation 2001; 104: 63–67 [DOI] [PubMed] [Google Scholar]
- 67. Westhuyzen J, Healy H.. Biology and relevance of C-reactive protein in cardiovascular and renal disease. Ann Clin Lab Sci 2000; 30: 133–143 [PubMed] [Google Scholar]
- 68. Laiho K, Tiitinen S, Teppo AM. et al. Serum C-reactive protein is rarely lost into urine in patients with secondary amyloidosis and proteinuria. Clin Rheumatol 1998; 17: 234–235 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.