Abstract
The inclusion of women in preclinical pain studies has become more commonplace in the last decade as the National Institutes of Health (NIH) released its “Sex as a Biological Variable” mandate. Presumably, basic researchers have not had a comprehensive understanding about neuroimmune interactions in half of the population and how hormones play a role in this. To date, we have learned that sex hormones contribute to sexual differentiation of the nervous system and sex differences in behavior throughout the lifespan; however, the cycling of sex hormones does not always explain these differences. Here, we highlight recent advances in our understanding of sex differences and how hormones and immune interactions influence sensory neuron activity to contribute to physiology and pain. Neuroimmune mechanisms may be mediated by different cell types in each sex, as the actions of immune cells are sexually dimorphic. Unfortunately, the majority of studies assessing neuronal contributions to immune function have been limited to males, so it is unclear if the mechanisms are similar in females. Finally, pathways that control cellular metabolism, like nuclear receptors, have been shown to play a regulatory role both in pain and inflammation. Overall, communication between the neuroimmune and endocrine systems modulate pain signaling in a sex-dependent manner, but more research is needed to reveal nuances of these mechanisms.
Keywords: sensory neuron, hormone, pain, neuroimmune, bidirectional, neuroendocrine, sex differences
In 2015, the National Institutes of Health (NIH) mandate “Sex as a Biological Variable” required applicants to consider biological sex as an experimental variable in NIH research with animals and cells (1). This mandate has opened up a realm of possibility in the pain field and now NIH-submitted research projects include both sexes. Moreover, several prominent pain and neuroscience journals are requiring full reporting of the sexes of participants and the number of each (2-4). Previously, preclinical pain studies used only male animals despite the greater prevalence of chronic pain in women (5-7). Consequentially, current pain treatments fail to offer alleviation in more than two-thirds of patients (2, 8, 9). In people without chronic pain pathology, few sex differences have been reported in pain perception (10); it stands to reason that the lack of efficacy of current chronic pain treatments may be in part due to inherent differences in pain signaling between males and females. Because preclinical studies of chronic pain has traditionally used only male animals, the recent inclusion of female animals has uncovered evidence of sex differences in neuroimmune mechanisms contributing to pain. There is still much to understand about these differences and the contributing mechanisms (Table 1). Additionally, it is important to acknowledge that in humans the affective components of pain may contribute to differences in self-reported incidences of pain among the sexes, particularly in cases where mechanistic evidence may not directly correlate with reported sex-dependent bias of pain (11, 12). One example of this phenomenon was found in postoperative pain, where men have higher amounts of proinflammatory biomarkers, but report less pain than women (13). However, women consistently report increased pain following multiple forms of postoperative pain, musculoskeletal pain, and pain in response to disease states (8, 14-16).
Table 1.
Summary of sex differences across various neuro-, immune-, and endocrine-based targets
| Target | Context | Sex differences | References |
|---|---|---|---|
| TRPV1 | Lung infection | Both sexes used; sex differences not assessed | (151-156) |
| Allergic airway inflammation | Sex used not stated | (154) | |
| Gut infection | Not assessed; only males | (22, 157) | |
| Colitis | Not assessed; only males | (24-26) | |
| TLR4 | LPS activation | No differences | (30, 31, 158-160) |
| Inflammatory pain | Cell-type differences: immune cell driven in males but not females | (161, 162) | |
| Macrophages | Pain signaling | Larger role in males | (161, 162, 176, 177) |
| Inflammation | Proinflammatory in males; anti-inflammatory in females and modulated by E2 | (40, 49, 55, 136, 172-175) | |
| Microglia | Pain signaling | Larger role in males | (57-60, 67, 68, 178) |
| Inflammation | More proliferative and proinflammatory in males; anti-inflammatory and greater phagocytosis in females | (51, 66) | |
| T cells | Pain signaling | Inconsistent across models: no differences, male biased, and female biased | (54, 73-76) |
| Inflammation | Females have stronger responses to infection and function is modulated by E2 | (40, 43, 45, 71, 72) | |
| Testosterone | Analgesia | Similar effects in both sexes | (77-86) |
| E2 | Pain signaling | ERs are more abundant in trigeminal sensory neurons in females, and fluctuations in E2 may be responsible for migraine attacks | (36, 87-94) |
| Inflammation | Decreases in E2 following menopause, increases proinflammatory cytokines | (91, 92) | |
| Prolactin | Pain signaling | Female-biased roles, little to no effects in males | (65, 95-101) |
| AMPK | Inflammation | Activation is anti-inflammatory in male macrophages | (111, 112, 186) |
| Pain signaling | Activation promotes pain relief in male but not female mice; reduces effects of transition from acute to chronic pain | (116, 192-195) | |
| LKB1 | Pain signaling | Increased female reproductive tract innervation in endometriosis | (189) |
| Innervation | Potentially responsive to variations in sex hormones in females | (113-115) | |
| LXR | Inflammation | Not assessed; only males | (117, 123) |
| Pain signaling | Not assessed; only males | (119, 123) | |
| PPARα | Inflammation | Protective/anti-inflammatory in males | (183, 198) |
| Pain signaling | Analgesic in males or no differences | (183, 198, 202, 203) | |
| PPARγ | Inflammation | Anti-inflammatory in macrophages, promoted by E2 | (205-207) |
| Pain signaling | Analgesic in females only (PNI) or both sexes (CIPN) | (183, 201, 204) |
Abbreviations: AMPK, adenosine 5′-monophosphate–activated protein kinase; CIPN, chemotherapy-induced neuropathy; E2, estradiol; ER, estrogen receptor; LKB1, liver kinase B1; LPS, lipopolysaccharide; LXR, liver X receptor; PNI, peripheral nerve injury; PPAR, peroxisome proliferator-activated receptor; TLR4, toll-like receptor 4; TRPV1, TRP channel, vanilloid subtype.
Historically, we recognize sensory neurons as detectors of distinct environmental stimuli; they send out signals for the integration and control of effector organs. The ability of an organism to detect and respond to stimuli is paramount for survival, and sensory neurons function as the body’s mechanism by which to understand the external and internal environment (124, 125). Cutaneous sensory neurons have nerve endings that reside in the skin and are responsible for responding to external stimuli, such as heat and touch (125, 126). Visceral sensory neurons innervate internal organs for detection of inflammation, damage, and other changes in the internal environment (127-130). Activation of these sensory neurons is protective: they teach the organism what in the environment is safe or dangerous.
Nociceptors are a class of sensory neuron that detect and respond to noxious stimuli, including noxious thermal, chemical, and mechanical stimuli from the environment (131, 132). These signals are ultimately received via higher-order neurons that allow for the perception of pain (133, 134). Nociceptor activation also occurs endogenously via molecules from immune cells (cytokines, chemokines, and prostaglandins) (34, 131, 135). During acute tissue inflammation, such as infection or injury, resident tissue immune cells including neutrophils and macrophages secrete proinflammatory cytokines to recruit additional immune cells to the site (136). Cytokines released from immune cells directly activate nociceptors via receptors on the terminals of sensory neurons, which induces intracellular signaling cascades that results in increased ion channel expression and increases nociceptor excitability (137, 138). Direct activation and this signaling cascade both result in pain signaling as sensory neurons become more sensitive to their environment and increase their activation state (131). While the majority of what we know about the peripheral nervous system (PNS) development comes from studies in male animals, we now understand that there are some time and structural differences between the sexes and sensory neurons do more than respond to specific modalities, but they can directly detect foreign stimuli (139, 140) (Fig. 1).
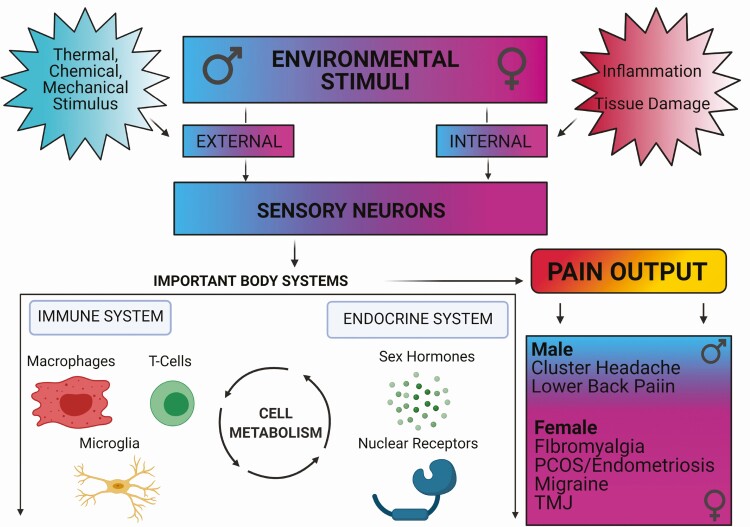
Figure 1.
Interactions between sensory neurons, immune system, and endocrine system to produce pain in a sex-biased manner. External and internal noxious stimuli are detected by sensory neurons, which communicate to the immune and endocrine systems to modulate pain outcomes. Interactions between these systems are further affected by changes in cellular metabolism. Chronic pain disorders are more commonly diagnosed in women. Furthermore, women are disproportionately affected by chronic pain disorders, such as fibromyalgia and migraine, suggesting female-biased mechanisms in pain signaling and communication between the nervous, immune, and endocrine systems.
Little is known about sex differences in the PNS and the role of sex hormones in modulating these differences. Two recent studies have shown that sensory neurons from males and females have different gene expression patterns at baseline and following nerve injury (141, 142). Estradiol (E2) and estrogen receptors (ERs), the former of which is more predominant in female mice and rats (143-145), may play a role in sex differences observed in neuron survival and synaptic transmission in the periphery (145-150). Studies have shown the role of E2 signaling in reduced synapse formation and inhibition of apoptosis during development and in response to immune challenge (146, 149, 150).
Sensory Neurons Directly Detect Distinct Environmental Stimuli to Control Effector Organs
More recent studies have revealed that, in addition to detecting inflammation and damage in the body, sensory neurons can also be directly activated by environmental stimuli and affect the organs they innervate. Direct activation of sensory neurons by bacteria via pathogen-associated molecular patterns (PAMPs) or tissue damage via damage-associated molecular patterns (DAMPS) not only leads to pain or changes in pain tolerance, but also affects the production of immune signals and influences immune cell populations (19, 151, 152). Unfortunately, most studies have either used only male mice or used both sexes without direct comparisons between males and females. In studies using both sexes that report no differences, experiments are often not appropriately powered to be able to detect potential effects. Because of this, it is currently unclear whether there are sex differences in communication between sensory neurons, the immune system, and peripheral organs.
Transient Receptor Potential Expressing Sensory Neurons
Sensory neurons that express transient receptor potential (TRP) channels, cation channels involved in neuronal activation, play a major role in mediating host defense against bacterial infection, including mediation of sepsis induced-mortality, immune infiltration into the lungs, and pain (151, 153, 154). While this has been shown in male rodents, these results have not been recapitulated in females to our knowledge. TRP channel, vanilloid subtype (TRPV1) in particular is an ion receptor expressed on nociceptors that detects noxious heat, chemicals, and both proinflammatory and anti-inflammatory cytokines (17, 18, 155). During lung infection, ablation of sensory neurons that express TRPV1 increases the infiltration of immune cells (ie, neutrophils) into the lung and improves survival rates following bacterial lung infection (156); however, TRPV1-expressing sensory neurons are not the only nociceptors that mediate response to infection and inflammation. A similar but distinct population of nociceptors that express a voltage-gated sodium channel (Nav1.8) highly expressed on peripheral sensory neurons also plays a similar role in bacterial clearance and immune response to TRPV1-expressing sensory neurons (20, 156). TRPV1 and Nav1.8 neurons are also involved in the inflammatory response during allergic airway inflammation, in which ablation of these neurons reduces lung inflammation (154). Given that ablation of nociceptors has differing effects based on the source of lung inflammation, the role of sensory neurons in mediating the response to inflammation is variable and warrants further study across different models of pain and inflammation (Fig. 2). Furthermore, potential sex differences in the role of sensory neurons in lung inflammation has not been assessed, even in the few studies that have included females.
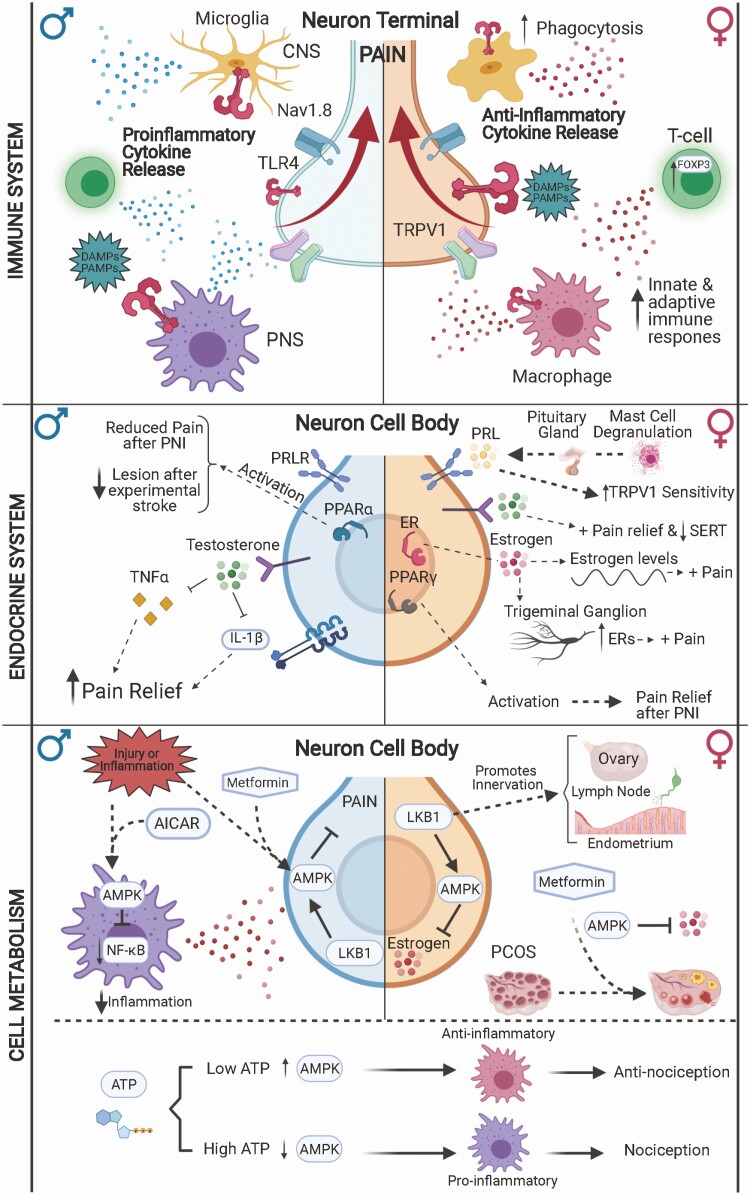
Figure 2.
Sex-biased mechanisms in pain signaling involving the immune system, endocrine system, and cellular metabolism. Neuroimmune signaling in chronic pain potentially uses alternate pathways and cell types in males and females. In males (left), immune cells are thought to be prominent drivers of pain signaling, for example, through toll-like receptor 4 (TLR4) signaling. After infection, injury, or inflammation, TLR4 on immune cells (microglia in the central nervous system [CNS] and macrophages in the periphery) detects damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) to promote proinflammatory cytokine release and subsequent pain signaling in sensory neurons. In females, sensory neurons directly drive pain signaling (such as through direct TLR4 activation), whereas immune cells tend to have a more anti-inflammatory phenotype. However, neuroimmune interactions may be modulated by sex hormone signaling and cell metabolism. Fluctuations in estrogen levels may contribute to increased pain sensitivity in females, despite promotion of protective immune responses. Testosterone, on the other hand, promotes pain relief by inhibiting proinflammatory cytokines like interleukin-1β (IL-1β) and tumor necrosis factor α (TNFα). Prolactin (PRL) promotes pain signaling by sensitizing sensory neurons in females, but not males. Neuroimmune and endocrine interactions can also occur by modulation of cellular metabolism. Metformin, an activator of adenosine 5′-monophosphate–activated protein kinase (AMPK), decreases the proinflammatory actions of macrophages in males. In females, AMPK activation contributes to decreasing estrogen levels and can be used to treat polycystic ovary syndrome (PCOS). AMPK activity in immune cells contributes to anti-inflammatory phenotypes and subsequent pain relief.
Similar to studies in the lungs, sensory neurons that innervate the gastrointestinal tract have been shown to modulate immune responses, inflammation, and pain in the gut (21, 22, 128). Similar to studies of the lung, a majority of studies on the gut and experimentally induced colitis have been performed in male rodents. Ablation of Nav1.8 and TRPV1-expressing nociceptors increases the infiltration of bacteria into the gut following infection and increases disease severity, indicating a protective role of nociceptors in host defense in the gut (22, 157). Sensory neurons are also involved in regulating gut inflammation in diseases like colitis, in which uncontrolled inflammation in the gastrointestinal tract leads to pain, sickness, diarrhea, and weight loss (23). Mice that lack TRPV1 have reduced inflammation and less sickness in experimental models of colitis (24); Furthermore, TRPV1 expression is increased during colitis and is thought to mediate visceral pain caused by the disease (25, 26). Sex differences in colitis and gut inflammation have not been reported, whether because of the use of only one sex or because no direct comparisons between males and females were assessed.
Toll-like Receptors
Toll-like receptors (TLRs) are a mechanism by which immune cells and sensory neurons detect PAMPs and DAMPs (27-29). TLR4 is a pattern recognition receptor expressed on sensory neurons and immune cells and is heavily involved in the innate immune response to bacterial infection (30). TLR4 gene expression at baseline is not different between sexes; however, cell surface TLR4 expression is known to decrease after activation as the receptor is internalized to activate intracellular signaling cascades (31, 158, 159). TLR4 signaling mechanisms were traditionally studied in the context of innate immune activation (30, 160). However, it was recognized that TLR4 on sensory neurons mediates both immune and neuronal communication and is an important molecule in pain development (see Fig. 2). TLR4 signaling on sensory neurons that express Nav1.8 stimulates the release of calcitonin gene-related peptide after treatment with lipopolysaccharide, a component of gram-negative bacteria that has high affinity for TLR4, but these studies have not been performed in females (75). TLR4 signaling on different cell types has been investigated as a mechanism for sex differences in pain signaling pathways. In inflammatory pain models, TLR4 signaling on different cell types mediates pain responses in males and females; TLR4 signaling in immune cells drives acute and chronic pain responses in males, whereas TLR4 signaling in nociceptors drives pain responses in females (161, 162).
Sex differences
Historically, sex differences were not commonly assessed because of the limited amount of female-driven studies in preclinical literature. Although women comprise the majority of chronic pain patients, preclinical studies historically used only male animals, presumably because of the perceived inconvenience of the estrous cycle and hormonal fluctuation in females (2). This belief was unfounded, as analyses of trait and behavioral variability between males and females has shown that females are not inherently more variable than males (163, 164). In 2016, the NIH implemented the policy “Sex as a Biological Variable,” in which biological sex must be considered as a variable in NIH-funded research (1). Prior to this, more than 75% of published articles in the journal Pain, a prominent source of literature on studies of pain mechanisms, were performed exclusively in males (2). With the inclusion of females, sexual dimorphic studies reveal the importance in understanding differences in pain processing in females (35).
Sex differences are traditionally defined as the biological differences that emerge because of differential gene expression from the sex chromosomes. Major drivers of phenotypic sex differences are gonadal hormones, androgens and estrogens, which drive the expression of sexual dimorphisms such as reproductive function, metabolism, and immune function. During nervous system development, exposure to testosterone leads to the development of male-specific characteristics, whereas its absence leads to the development of female-specific characteristics (145, 148). Furthermore, the actions of E2 in the nervous system are protective, contributing to the suppression of apoptosis and inflammation during development and synaptic pruning (146, 147, 150). Across human development, sex-specific hormones have long been implicated in several pain conditions such as back, temporomandibular disorder, and migraine pain, all of which are more likely to increase in females with increasing pubertal development (124). Although there was evidence that suggested women suffered more commonly from chronic pain conditions (5, 7, 8) and that hormones were likely to play a role in the dichotomous nature of these disorders (36-38), little preclinical evidence existed until recently (39, 165-168).
Sex Differences in the Immune System
Sex differences in the immune system are more consistent than in the nervous system. In fact, the literature is contentious as to whether the sex differences observed during nervous system development lead to overt changes in behavior and physiology at all (148, 169, 170). It seems more likely that changes that happen during development are more relevant to sex differences during adulthood than cyclic changes of sex hormones during adulthood, by evidence of inconsistent outcomes. Across many species, it has been recognized that there are distinct differences in immune responses between sexes. The immune system has sex-dependent actions, as evidenced by male vs female predominance across immune disorders (40-44, 136). In general, males are more prone to cancers, sepsis during infection, and increased macrophage production of proinflammatory cytokines (40, 45). Females, on the other hand, are more prone to autoimmune disorders and immune dysregulation and have stronger T-cell responses to infection and vaccination (41, 42, 44). Sex differences in immune system function are likely due to a combination of X-linked genes and sex hormone signaling, which work in concert to create the dimorphisms seen in the immune system between males and females (41, 43). In the following sections, we will review sex differences across different subtypes of immune cells that are commonly studied in the pain field.
Monocytes and Macrophages
Monocytes or macrophages are immune cells that circulate throughout the body and act as the primary responders of the innate immune system in the periphery (32, 46). As antigen-presenting cells, they phagocytose and then present antigens to T cells in the lymph nodes, which then initiate the adaptive immune response discussed later (33). Macrophages can detect bacteria (PAMPs) and tissue damage (DAMPs) via TLR signaling (47). In inflammation, they skew their activation toward promoting or resolving inflammation (48) and release cytokines to recruit immune cells to the site of inflammation and phagocytose foreign debris (171) (see Fig. 2).
Multiple studies have shown that macrophage function is sexually dimorphic and can be further modulated by the actions of sex hormones, most notably estrogens (40, 55, 172-175). Estrogens promote protective immune responses. During an immune challenge, E2 action on macrophages induces increased expression of TLRs, which allows for a more robust response (49, 136). Furthermore, estrogens typically promote the production of anti-inflammatory cytokines and inhibit proinflammatory cytokines; studies report that removal of endogenous estrogens via ovariectomy results in dampened pro-inflammatory responses to immune challenge (55, 173).
Macrophages are thought to play a larger role in pain signaling in males compared to females, indicating sex-specific mechanisms by which macrophages and nociceptors interact (161, 162, 176). Following peripheral nerve injury (PNI), male and female mice develop pain sensitivity similarly. Interestingly, male and female macrophages have different phenotypes within the dorsal root ganglia (DRG) after PNI, indicating that male macrophages are proinflammatory and females are anti-inflammatory (177); however, it has also been reported that macrophage-nociceptor interactions are required for development of neuropathic pain after PNI both in males and females (50). Most likely, the mechanisms by which macrophages and nociceptors interact during pain signaling are different in males and females, but sex-specific mechanisms in neuroimmune interactions continue to be elucidated.
Microglia
Microglia are the resident innate immune cells of the central nervous system (CNS). Differential function between microglia of males and females drives sex differentiation within the brain as well as sex dimorphisms in pain and inflammation (51, 56). Quiescent microglia constantly survey the CNS for inflammation and damage with processes that extend from the cell body. When neuroinflammation or damage occurs, microglia remove debris, dead cells, and foreign antigens (52). These reactive microglia retract their processes and become ameboid shaped and more proinflammatory to release cytokines. Reactive microglia may also increase their phagocytosis activity, which aids in the response to inflammation and damage in the CNS (53).
Within the CNS, spinal microglia interact with nerve terminals of sensory neurons to influence neurotransmitter release, synaptic plasticity, and signaling between the PNS and CNS (62). When tissue damage occurs, the damaged cells release adenosine 5′-triphosphate (ATP). Surveying microglia detect ATP via purinergic receptors (P2X and P2Y) and become activated to respond to the tissue damage and inflammation (63). Activated microglia release cytokines that increase the activity of excitatory neurons and decreases the activity of inhibitory neurons in pain pathways, contributing to the development of chronic pain (57,64,65). The mechanisms of microglial-neuron interactions are heavily influenced by studies in males with much less literature on the communication between microglia and sensory neurons in females.
Based on the current literature, microglial activation during neuropathic pain states seems to be a male-biased mechanism (58, 59). At baseline, adult male and female microglia have differing morphological and functional characteristics (51). Male microglia typically have larger cell bodies and are more proliferative. When activated, male microglia are more involved in pain signaling compared to female microglia, as inhibition of microglial signaling in the spinal cord is protective in males but not females (60, 178). Interestingly, female microglia are more phagocytotic than males, indicating a more anti-inflammatory role of microglia in females (66); however, it has also been shown that microglial activation in the periaqueductal gray reduces the effectiveness of morphine-based pain relief in females but not males (67). Microglial activation has also been shown to contribute to pain induced by bone cancer in female rats (68), and microgliosis within the spine is similar in males and females following nerve injury (57, 178). The majority of studies looking at the role of microglia in pain signaling mechanisms have been in male rodents; as such there is much less that is understood about the role of microglial activation in pain signaling for females.
T cells
T cells are heavily involved in orchestrating the adaptive immune response to antigens (69). When antigen-presenting cells find and present antigens to immature T cells, they then mature and differentiate to control the immune response by releasing cytokines and recruiting other immune cells (69, 70). T-cell function is heavily modified by X-linked genes and sex hormone signaling (43). Transcription factors, such as forkhead box protein 3, that help to regulate T-cell functions, are often X linked and have higher expression in females (40, 45, 71). Additionally, E2 signaling on T cells promotes proinflammatory cytokine production and T-cell proliferation (72).
Several studies have shown the role of T cells in sexually dimorphic action in pain states (73). Recently, a study of recombination activating gene 1/2 (RAG1 and RAG2) knockout mice, which are immunodeficient and lack mature T and B cells, (74) revealed that T cells mediate certain forms of pain in male mice. CD8+ T-cell reconstitution in RAG1- and RAG2-knockout female and male mice has been observed to resolve tactile allodynia following chemotherapy-induced pain (54, 76). Interestingly, a study within the last year using males and females found that CD4+ T cells foster microglial maturation within the brain (80), indicating that interactions between microglia and T cells may be an important aspect of immune system development and function in both sexes. Together these data suggest that macrophage/microglia and T-cell activation likely contribute to different forms of pain both in males and females, as opposed to completely different signaling systems that are required for development of all chronic pain.
Sex Differences in Neuroendocrinology
Testosterone
Differences in hormone signaling and production are important factor in the prevalence of chronic pain conditions in women. The predominantly male hormone testosterone has long been thought to serve a protective role in inflammatory and chronic pain conditions. Testosterone treatment in men decreases the presence of the proinflammatory cytokines tumor necrosis factor α (TNFα), interleukin (IL)-1β, and IL-6 (77, 81, 82). Additionally, testosterone not only contributes to antinociception in males, but has similar effects in females. For example, increased testosterone contributes to decreased neck and shoulder pain in women (78), and testosterone treatment reduces rheumatoid arthritis–induced pain in both sexes (79). These effects have been previously observed in female rats, in which treatment with testosterone abates nociceptive responses to formalin (83). More recently, testosterone was found to reduce hyperalgesia in female mice in a model of muscle pain via reducing the serotonin transporter (39). Interestingly, the protective effects of testosterone are also applicable to female-specific pain conditions, as localized vulvodynia can be treated with a combination of topical E2 and testosterone (84). The increased amount of testosterone in males may additionally help explain the increased prevalence of chronic pain conditions in women (85, 86).
Estradiol
As previously stated, the literature establishes the role of testosterone as being widely protective against pain and inflammation. However, the role of E2 remains unclear. E2 was shown to increase facial receptive fields of trigeminal ganglia (TG) neurons in rats as well as decrease the thresholds for these fields, making the argument that E2 may contribute to female-specific pain states mediated by the TGs, such as migraine (87). Within the last year, ERs were found to be more abundant in female than male rats in the TG (88). These data, combined with data showing ER agonists lead to blood vessel dilation in the dura (88), have led to speculation that E2 may contribute to predominantly female pain disorders such as migraine and temporomandibular joint syndrome. In contrast, many data have been published that suggest estrogen plays a protective role against inflammation and pain disorders. Estrogen has been shown to attenuate lymphocyte extravasation following exposure to TNFα, as well as reduce the trafficking of immune cells in response to inflammation (89). Moreover, the estrogen steroid hormone 17β-E2 serves a protective role at the blood-brain barrier, helping to maintain tight junctions between endothelial cells (88, 90). Decreases in E2 following menopause correlate with increases in the proinflammatory cytokines IL-6, IL-1β, and TNFα (91). Ultimately, E2 levels may be responsible for these conflicting reports, as low levels of estrogen have been shown to increase inflammatory mediators, but estrogen replacement can lead to a reduction in these inflammatory cytokines. In agreement with this statement, certain migraine attacks are evoked by estrogen withdrawal (92); however, the use of estrogen-containing contraceptives has been noted to induce migraine attacks (36, 93, 94). These data indicate that fluctuations in E2 levels may aid in the development of pain states in women, whereas stability of E2 levels may serve a protective role against nociceptive signaling (see Fig. 2).
Prolactin
Another example of sex hormone signaling differentiation is the increased evidence for a role of prolactin (PRL) in chronic pain. PRL has recently been linked to a female-specific role in multiple pain conditions (61, 95-101). Previously, it was known that women who develop increases in PRL from microprolactinomas—small, benign, PRL-secreting tumors—develop migraine in correlation with increasing PRL levels (103). Previous work has demonstrated that different stressors increase plasma levels of PRL (102, 179): Stress is thought to directly contribute to female-biased pain disorders such as migraine and chronic back pain (180). Additionally, mast cell degranulation, shown to happen during stress, has been shown to increase PRL in plasma (181). This may offer insight into stress-induced pain in women (182). Furthermore, the recent increased prevalence of females in preclinical research has revealed a significant and clear role for stress in chronic and inflammatory pain models. Female mice that have the PRL receptor (PRLR) deleted from Nav1.8-expressing neurons show reduced behavioral responses in models of chronic pain (99). Additionally, PRL leads to sensitization of TRPV1, TRPA1, and TRPM8 within the DRG neurons of female, but not male, mice (96). These findings agree with an additional study that demonstrated only female mice develop cold hyperalgesia following plantar incision (61). Whereas both males and females in this study experienced heat-induced hyperalgesia, only female PRL and PRLR-knockout mice exhibited reduced responses (61). These data suggest that PRL and PRLR strongly contribute to pain in females, with little to no effect on pain in males.
When considering the role of hormones in pain conditions, it is necessary to discuss potential differences in the role of T cells in female-specific inflammation. Data have shown Complete Freund’s Adjuvant, which has long been used to induce inflammatory pain (87), requires the presence of microglia signaling to cause allodynia in males, but not in females. However, females that are treated with testosterone also required microglial signaling to develop allodynia; this is also true of females that were T- and B-cell deficient (183).
While the complexity of hormonal regulation of pain requires much more in-depth exploration, the increased prevalence of females in preclinical studies continues to develop and expand our current understanding.
Recent Advances/Reorganization of Thought
Recent advances in our knowledge of bidirectional communication between sensory neurons and immune cells reveal immunomodulatory mechanisms, and these interactions are heavily influenced by cellular metabolism (137, 153, 154, 156). The metabolic activity of macrophages and microglia is a determinant of their polarization toward proinflammatory or anti-inflammatory activity, as anti-inflammatory functions are more energy intensive (109, 184, 185). The metabolic kinase adenosine 5′-monophosphate–activated protein kinase (AMPK) acts as a sensor for when cells are low on energy, specifically when cells are low on ATP, and helps to maintain cellular energy homeostasis (110). AMPK also plays an important role in immune cell polarization and pain signaling. When macrophages lack AMPK, they are unable to perform anti-inflammatory functions in response to an immune challenge, resulting in chronic inflammation and pain (111). Additionally, AMPK signaling promotes anti-inflammatory signaling pathways in macrophages (112). In male mice, activation of AMPK has been shown to promote pain relief after induction of inflammatory pain by reducing activity of nuclear factor κB (NF-κB) in macrophages, thus reducing their proinflammatory activity (186). Liver kinase B1 (LKB1) is an upstream activator of AMPK via phosphorylation (104, 187). Removal of LKB1 from macrophages, which results in reduced AMPK activity, increases proinflammatory signaling pathways in macrophages in response to lipopolysaccharide (105). These results show that LKB1 and AMPK activity have anti-inflammatory effects on macrophage function and provide an important link between immune cell metabolism and inflammation.
LKB1 and AMPK also play important roles in modulating neuron functions. In developing neurons, LKB1 signaling is an essential component of axon development and neuron polarization (106, 107). When LKB1 activity is blocked, cultured neurons develop shorter axons with fewer branches (108). Similar findings have been shown in vivo, where cortical neurons that lack LKB1 have reduced axon projections and less branching (188). To our knowledge, these studies have not been performed in the periphery, so it is unclear whether LKB1 activity regulates axon development and subsequently innervation of peripheral organs. There is, however, evidence that modulation of innervation of the female reproductive tract is a component of pain experienced by patients with endometriosis, as histological analysis of peritoneal lesions collected during surgery for diagnosis and treatment of endometriosis has shown that patients with endometriosis have increased sensory neuron innervation compared to those without endometriosis (189). There is also evidence that innervation may be sex hormone dependent and is affected by circulating sex hormones throughout the reproductive cycle both in humans and rodents (113-115). Interestingly, AMPK activity may play an important role in E2 production in endometrial tissues: Treatment with metformin significantly suppresses local E2 production (190). Additionally, a recent study has shown that female mice with peripheral sensory neurons lacking LKB1 have enhanced fertility, with increased follicular turnover and subsequently larger litters than wild-type mice (191). Based on these data, it is possible that cellular metabolism of sensory neurons may be an important aspect of sensory neuron interactions with peripheral organs, including the regulation of hormone production, immune function, and metabolism.
The effects of AMPK activity on pain signaling has been studied recently thanks to evidence that metformin, a drug commonly described for diabetes and other metabolic disorders, has been shown to treat neuropathic pain in males, but not females (192). Interestingly, metformin treatment may also reduce short-term pain sensitization in both sexes, a mechanism used to investigate the transition from acute to chronic pain (116, 193, 194). This transition entails large-scale transcriptional and translational activity within neurons to increase nociceptor excitability. This transition requires a vast amount of protein synthesis; thus, the modulation of neuronal metabolism may be an important aspect of sensory neuron response to prevent this transition (116, 195).
In addition to hormones, other ligands that act on nuclear receptors have sex-dependent activity. Agonism of nuclear receptors leads to a cell changing its overall phenotype. In addition to sex-specific androgens and estrogens, several other nuclear receptor types have been identified, including glucocorticoids, mineralocorticoids, vitamin D3, and retinoic acid. Interestingly, many nuclear receptors have been shown to have some effect on pain or inflammation via the modulation of cellular metabolism. As discussed earlier in relation to AMPK, cellular metabolism shifts toward protein production are typically pronociceptive and proinflammatory, whereas shifts toward lipid metabolism are typically antinociceptive and anti-inflammatory (109, 116, 185, 193). Here we will focus on the metabolic nuclear receptors peroxisome proliferator-activated receptors (PPAR) α and γ, as well as the liver X receptor (LXR), which have been shown to have both pain-relieving efficacy and anti-inflammatory properties across numerous models.
To exert their activities on lipid synthesis, LXRs form dimers with retinoid X receptors (122). Although there are 2 isoforms of LXR, LXRα and LXRβ, they highly colocalize, and while it is possible to distinguish between the 2 isoforms, existing tools are not specific enough to appropriately accomplish this (118). Thus, potential sex differences relating to this receptor are masked. Part of LXR efficacy has been linked to its role in relieving metabolic stress in DRG neurons, both delaying endoplasmic reticulum stress and delaying the onset of neuropathy (119). While it is possible that neuronal LXR activity is sufficient to relieve metabolic stress associated with a chronic pain phenotype, LXRs also show prolific anti-inflammatory properties (123). Interestingly, its anti-inflammatory qualities have been directly linked to its regulation of cholesterol and has also been shown to decrease immune cell recruitment after infection (117). When LXR is knocked out of mice, they show increased inflammation and a subsequent increase in inflammatory pain (123). Furthermore, taken with existing data showing a reduction of metabolic stress in DRG neurons, it is not clear if this reduction of inflammatory pain is directly due to reduced inflammation, especially when inflammation and pain do not consistently correlate (19, 120). These studies either used only male animals or did not report the sex of the animals used; as such, additional work is necessary to elucidate the role of LXRs in females.
Contrary to LXR, PPAR isoforms are typically distinctly located in different cell types, allowing for more specific targeting (121). Although 3 isoforms (α, β/δ, and γ) of PPAR have been identified, PPARα and PPARγ have been most widely studied in pain and inflammation. However, the 2 receptors play opposing roles. Whereas PPARα activation results in the use of fatty acids (196), PPARγ regulates storage and production of lipids, including the induction of adipose tissue (197). These actions not only can change the phenotype of a specific cell, such as causing the cell to shift to a more glycolytic profile, but and also shift the nutrients for other tissues can use. Thus, the metabolic properties of a single cell can affect whole-body metabolism, including contributing to antinociceptive processes in cells that were not directly activated.
Agonism of either PPAR has been linked to a sex-specific antinociceptive phenotype. After PNI, female mice exhibited antinociception after PPARγ activation and male mice after PPARα activation, an effect thought to be dependent on sex hormones (183). This is consistent with literature showing interactions between sex hormones, specifically estrogens, and PPAR activity (174, 198-200).
Similarly, following PPARα agonism, males but not females experienced reduced lesions after experimental stroke, further suggesting a sexually dimorphic role for this receptor, although neither sex hormones nor PPARγ agonism were examined in this study (198). However, PPARγ agonism has been shown both to reduce chemotherapy-induced neuropathy and restore mitochondrial function in males and females (201), suggesting interactions with sex hormones are not entirely responsible for the downstream effects of PPARγ. This is particularly pertinent in other literature that explicitly does not report sex differences in nociception after PPARα (202, 203), reports pain relief in males after PPARγ activation (204), or reports no sex differences after PPARγ activation (201). These conflicting reports suggest more needs to be done to investigate the pain-relieving capabilities of PPAR agonism, and if sex hormones contribute to these properties.
In addition to these antinociceptive qualities, PPARα and -γ both have been linked to anti-inflammatory properties. In macrophages, PPARα and -γ have been linked not only to the control of fatty acid metabolism, consistent with their roles in other tissues, but also to contributing to the macrophage polarization state (205). PPARα activation in M1 cells negatively interacts with the NF-κB pathway, leading to overall reduced inflammation (205). Despite its opposing metabolic actions to PPARα, PPARγ also interferes with NF-κB activity, causing similar anti-inflammatory effects (205). Furthermore, PPARγ assists in M2 differentiation, including colocalizing with ERα on anti-inflammatory macrophages (206, 207). Estrogens alone (17β-E2 treatment or ovariectomy of female mice) have been linked to macrophage polarization (49, 172-174), although the interactions both of estrogens and PPARγ in macrophage polarization are not fully understood. Thus, similarly to LXR, additional work needs to be done to distinguish the anti-inflammatory and antinociceptive qualities of PPARs, including how their metabolic functions influence each of these processes.
Overall, anti-inflammatory qualities of nuclear receptor activation mask neuromodulatory effects, especially as studies relating to the anti-inflammatory capabilities of nuclear receptors are typically conducted either in vitro or in a nonspecific whole-body model (eg, treating an animal with a PPAR agonist, taking its macrophages, and testing the skewing). Existing studies also fail to take into account neuroimmune interactions, whereby an immune cell can release cytokines or chemokines that then interact with neurons, especially as nuclear receptor agonists have been shown to play roles in a variety of cell types, suggesting the possibility of cell-to-cell communication being responsible for their effects. Furthermore, although the metabolic properties of nuclear receptor agonism are well known, it is not yet known how these properties can cause antinociception via their activities on neurons. It is also not yet clear whether males and females are affected differently by different nuclear receptor agonism or if different cells are responsible for antinociception in different sexes. Thus, more data are needed to investigate a cell-specific role of nuclear receptor agonism in antinociception, including how their anti-inflammatory properties can modulate antinociceptive processes.
Limitations, Caveats, and Gaps in Research
There are always limitations in translating preclinical research to clinical settings. Pain is an inherently subjective experience and as such can be influenced by factors including sex and gender, physician and experimenter bias, and experimental conditions (2, 5, 8, 208). In animal studies, measurement of pain occurs through behavioral measurements, which are inherently different from self-reported pain used in the clinic (209); however, self-report has been shown to be a reliable indicator of quality of life and function in chronic pain populations (210, 211).
A majority of the studies discussed in this review used male rodents only, unless otherwise specified. The basis of understanding for neuroimmune mechanisms is therefore a male-specific understanding, and whether sex differences exist in these pathways (TRPV1, TLR4, etc) continues to be explored. The focus on sex differences in future publications should aid in expanding knowledge of where male- and female-specific or biased mechanisms exist and, importantly, will help in understanding which mechanisms are not different between the sexes. Increased understanding of where these differences lie should improve the overall efficacy of therapeutics for chronic pain.
Acknowledgments
We thank present and past laboratory members for their contributions and support. Figures were created with BioRender.com.
Financial Support: This work was supported by the National Institutes of Health (NIH grant No. K22NS096030 to M.D.B.), an American Pain Society Future Leaders grant (to M.D.B.), a Rita Allen Foundation Award in Pain (to M.D.B.), and The University of Texas System Rising STARS program grant (to M.D.B.).
Glossary
Abbreviations
- AMPK
adenosine 5′-monophosphate–activated protein kinase
- ATP
adenosine 5′-triphosphate
- CNS
central nervous system
- DAMPS
damage-associated molecular patterns
- DRG
dorsal root ganglia
- E2
estradiol
- ER
estrogen receptor
- IL
interleukin
- LKB1
liver kinase B1
- LXR
liver X receptor
- NF-κB
nuclear factor κB
- NIH
National Institutes of Health
- PAMPs
pathogen-associated molecular patterns
- PGC-1a
PPARγ coactivating protein 1a
- PNI
peripheral nerve injury
- PNS
peripheral nervous system
- PPAR
peroxisome proliferator-activated receptor
- PRL
prolactin
- PRLR
prolactin receptor
- RAG1/2
recombination activating gene 1/2
- SABV
sex as a biological variable
- TG
trigeminal ganglia
- TLR
toll-like receptor
- TNFα
tumor necrosis factor α
- TRP
transient receptor potential
- TRPV1
TRP channel, vanilloid subtype
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. National Institutes of Health. Consideration of Sex as a Biological Variable in NIH-funded Research. National Institutes of Health; 2015:Notice Number: NOT-OD-15-102. https://grants.nih.gov/grants/guide/notice-files/not-od-15-102.html [Google Scholar]
- 2. Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci. 2020;21(7):353-365. [DOI] [PubMed] [Google Scholar]
- 3. Bhargava A, Arnold AP, Bangasser DA, et al. Considering sex as a biological variable in basic and clinical studies: an Endocrine Society scientific statement. Endocr Rev. 2021;42(3):219-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cavanaugh C, Abu Hussein Y. Do journals instruct authors to address sex and gender in psychological science? Res Integr Peer Rev. 2020;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL III. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Umeda M, Kim Y. Gender differences in the prevalence of chronic pain and leisure time physical activity among US adults: a NHANES study. Int J Environ Res Public Health. 2019;16(6):988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greenspan JD, Craft RM, LeResche L, et al. ; Consensus Working Group of the Sex, Gender, and Pain SIG of the IASP . Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26-S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35(3):565-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Otto A, Emery K, Côté JN. Sex differences in perceptual responses to experimental pain before and after an experimental fatiguing arm task. Biol Sex Differ. 2019;10(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt RF, Gebhart GF. Affective component (aspect, dimension) of pain. In: Gebhart GF, Schmidt RF, eds. Encyclopedia of Pain. Springer Berlin Heidelberg; 2013:77. [Google Scholar]
- 12. Talbot K, Madden VJ, Jones SL, Moseley GL. The sensory and affective components of pain: are they differentially modifiable dimensions or inseparable aspects of a unitary experience? A systematic review. Br J Anaesth. 2019;123(2):e263-e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Solheim N, Östlund S, Gordh T, Rosseland LA. Women report higher pain intensity at a lower level of inflammation after knee surgery compared with men. Pain Rep. 2017;2(3):e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ochroch EA, Gottschalk A, Troxel AB, Farrar JT. Women suffer more short and long-term pain than men after major thoracotomy. Clin J Pain. 2006;22(5):491-498. [DOI] [PubMed] [Google Scholar]
- 15. Rosseland LA, Stubhaug A. Gender is a confounding factor in pain trials: women report more pain than men after arthroscopic surgery. Pain. 2004;112(3):248-253. [DOI] [PubMed] [Google Scholar]
- 16. Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859-866. [DOI] [PubMed] [Google Scholar]
- 17. Bujak JK, Kosmala D, Szopa IM, Majchrzak K, Bednarczyk P. Inflammation, cancer and immunity-implication of TRPV1 channel. Front Oncol. 2019;9:1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boonen B, Alpizar YA, Meseguer VM, Talavera K. TRP channels as sensors of bacterial endotoxins. Toxins (Basel). 2018;10(8):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiu IM, Heesters BA, Ghasemlou N, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501(7465):52-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han C, Huang J, Waxman SG. Sodium channel Nav1.8: Emerging links to human disease. Neurology. 2016;86(5):473-483. [DOI] [PubMed] [Google Scholar]
- 21. Lai NY, Mills K, Chiu IM. Sensory neuron regulation of gastrointestinal inflammation and bacterial host defence. J Intern Med. 2017;282(1):5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lai NY, Musser MA, Pinho-Ribeiro FA, et al. Gut-innervating nociceptor neurons regulate Peyer’s patch microfold cells and SFB levels to mediate Salmonella host defense. Cell. 2020;180(1):33-49.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6(1):74. [DOI] [PubMed] [Google Scholar]
- 24. Utsumi D, Matsumoto K, Tsukahara T, Amagase K, Tominaga M, Kato S. Transient receptor potential vanilloid 1 and transient receptor potential ankyrin 1 contribute to the progression of colonic inflammation in dextran sulfate sodium-induced colitis in mice: links to calcitonin gene-related peptide and substance P. J Pharmacol Sci. 2018;136(3):121-132. [DOI] [PubMed] [Google Scholar]
- 25. De Schepper HU, De Winter BY, Van Nassauw L, et al. TRPV1 receptors on unmyelinated C-fibres mediate colitis-induced sensitization of pelvic afferent nerve fibres in rats. J Physiol. 2008;586(21):5247-5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lapointe TK, Basso L, Iftinca MC, et al. TRPV1 sensitization mediates postinflammatory visceral pain following acute colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309(2):G87-G99. [DOI] [PubMed] [Google Scholar]
- 27. Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res. 2011;90(6):759-764. [DOI] [PubMed] [Google Scholar]
- 28. Xu Y, Tao X, Shen B, et al. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature. 2000;408(6808):111-115. [DOI] [PubMed] [Google Scholar]
- 29. Acosta C, Davies A. Bacterial lipopolysaccharide regulates nociceptin expression in sensory neurons. J Neurosci Res. 2008;86(5):1077-1086. [DOI] [PubMed] [Google Scholar]
- 30. Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388(4):621-625. [DOI] [PubMed] [Google Scholar]
- 31. Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145-151. [DOI] [PubMed] [Google Scholar]
- 32. Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643-675. [DOI] [PubMed] [Google Scholar]
- 33. Gaudino SJ, Kumar P. Cross-talk between antigen presenting cells and T cells impacts intestinal homeostasis, bacterial infections, and tumorigenesis. Front Immunol. 2019;10:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peirs C, Seal RP. Neural circuits for pain: recent advances and current views. Science. 2016;354(6312):578-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arnegard ME, Whitten LA, Hunter C, Clayton JA. Sex as a biological variable: a 5-year progress report and call to action. J Womens Health (Larchmt). 2020;29(6):858-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Allais G, Chiarle G, Sinigaglia S, Airola G, Schiapparelli P, Benedetto C. Estrogen, migraine, and vascular risk. Neurol Sci. 2018;39(Suppl 1):11-20. [DOI] [PubMed] [Google Scholar]
- 37. Schertzinger M, Wesson-Sides K, Parkitny L, Younger J. Daily fluctuations of progesterone and testosterone are associated with fibromyalgia pain severity. J Pain. 2018;19(4):410-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Delaruelle Z, Ivanova TA, Khan S, et al. ; European Headache Federation School of Advanced Studies (EHF-SAS) . Male and female sex hormones in primary headaches. J Headache Pain. 2018;19(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lesnak JB, Inoue S, Lima L, Rasmussen L, Sluka KA. Testosterone protects against the development of widespread muscle pain in mice. Pain. 2020;161(12):2898-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jaillon S, Berthenet K, Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol. 2019;56(3):308-321. [DOI] [PubMed] [Google Scholar]
- 41. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626-638. [DOI] [PubMed] [Google Scholar]
- 42. Shepherd R, Cheung AS, Pang K, Saffery R, Novakovic B. Sexual dimorphism in innate immunity: the role of sex hormones and epigenetics. Front Immunol. 2020;11:604000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taneja V. Sex hormones determine immune response. Front Immunol. 2018;9:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vázquez-Martínez ER, García-Gómez E, Camacho-Arroyo I, González-Pedrajo B. Sexual dimorphism in bacterial infections. Biol Sex Differ. 2018;9(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010;10(8):594-604. [DOI] [PubMed] [Google Scholar]
- 46. Watanabe S, Alexander M, Misharin AV, Budinger GRS. The role of macrophages in the resolution of inflammation. J Clin Invest. 2019;129(7):2619-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li Q, Cherayil BJ. Role of Toll-like receptor 4 in macrophage activation and tolerance during Salmonella enterica serovar Typhimurium infection. Infect Immun. 2003;71(9):4873-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rettew JA, Huet YM, Marriott I. Estrogens augment cell surface TLR4 expression on murine macrophages and regulate sepsis susceptibility in vivo. Endocrinology. 2009;150(8):3877-3884. [DOI] [PubMed] [Google Scholar]
- 50. Yu X, Liu H, Hamel KA, et al. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun. 2020;11(1):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guneykaya D, Ivanov A, Hernandez DP, et al. Transcriptional and translational differences of microglia from male and female brains. Cell Rep. 2018;24(10):2773-2783.e6. [DOI] [PubMed] [Google Scholar]
- 52. Schetters STT, Gomez-Nicola D, Garcia-Vallejo JJ, Van Kooyk Y. Neuroinflammation: microglia and T cells get ready to tango. Front Immunol. 2017;8:1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Walker FR, Beynon SB, Jones KA, et al. Dynamic structural remodelling of microglia in health and disease: a review of the models, the signals and the mechanisms. Brain Behav Immun. 2014;37:1-14. [DOI] [PubMed] [Google Scholar]
- 54. Krukowski K, Eijkelkamp N, Laumet G, et al. CD8+ T cells and endogenous IL-10 are required for resolution of chemotherapy-induced neuropathic pain. J Neurosci. 2016;36(43):11074-11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521-574. [DOI] [PubMed] [Google Scholar]
- 56. Lenz KM, McCarthy MM. A starring role for microglia in brain sex differences. Neuroscientist. 2015;21(3):306-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tsuda M. Microglia-mediated regulation of neuropathic pain: molecular and cellular mechanisms. Biol Pharm Bull. 2019;42(12):1959-1968. [DOI] [PubMed] [Google Scholar]
- 58. Chen G, Luo X, Qadri MY, Berta T, Ji RR. Sex-dependent glial signaling in pathological pain: distinct roles of spinal microglia and astrocytes. Neurosci Bull. 2018;34(1):98-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron. 2018;100(6):1292-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Paige C, Maruthy GB, Mejia G, Dussor G, Price T. Spinal inhibition of P2XR or p38 signaling disrupts hyperalgesic priming in male, but not female, mice. Neuroscience. 2018;385:133-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Patil MJ, Green DP, Henry MA, Akopian AN. Sex-dependent roles of prolactin and prolactin receptor in postoperative pain and hyperalgesia in mice. Neuroscience. 2013;253:132-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Di Filippo M, Sarchielli P, Picconi B, Calabresi P. Neuroinflammation and synaptic plasticity: theoretical basis for a novel, immune-centred, therapeutic approach to neurological disorders. Trends Pharmacol Sci. 2008;29(8):402-412. [DOI] [PubMed] [Google Scholar]
- 63. Calovi S, Mut-Arbona P, Sperlágh B. Microglia and the purinergic signaling system. Neuroscience. 2019;405:137-147. [DOI] [PubMed] [Google Scholar]
- 64. Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther. 2006;109(1-2):210-226. [DOI] [PubMed] [Google Scholar]
- 65. Tsuda M, Tozaki-Saitoh H, Inoue K. Purinergic system, microglia and neuropathic pain. Curr Opin Pharmacol. 2012;12(1):74-79. [DOI] [PubMed] [Google Scholar]
- 66. Yanguas-Casás N, Crespo-Castrillo A, de Ceballos ML, et al. Sex differences in the phagocytic and migratory activity of microglia and their impairment by palmitic acid. Glia. 2018;66(3):522-537. [DOI] [PubMed] [Google Scholar]
- 67. Doyle HH, Eidson LN, Sinkiewicz DM, Murphy AZ. Sex differences in microglia activity within the periaqueductal gray of the rat: a potential mechanism driving the dimorphic effects of morphine. J Neurosci. 2017;37(12):3202-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yang Y, Li H, Li TT, et al. Delayed activation of spinal microglia contributes to the maintenance of bone cancer pain in female Wistar rats via P2X7 receptor and IL-18. J Neurosci. 2015;35(20):7950-7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout life. Immunity. 2018;48(2):202-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pennock ND, White JT, Cross EW, Cheney EE, Tamburini BA, Kedl RM. T cell responses: naive to memory and everything in between. Adv Physiol Educ. 2013;37(4):273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lu L, Barbi J, Pan F. The regulation of immune tolerance by FOXP3. Nat Rev Immunol. 2017;17(11):703-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mohammad I, Starskaia I, Nagy T, et al. Estrogen receptor α contributes to T cell–mediated autoimmune inflammation by promoting T cell activation and proliferation. Sci Signal. 2018;11(526):1-12. [DOI] [PubMed] [Google Scholar]
- 73. Rosen SF, Ham B, Haichin M, et al. Increased pain sensitivity and decreased opioid analgesia in T-cell-deficient mice and implications for sex differences. Pain. 2019;160(2):358-366. [DOI] [PubMed] [Google Scholar]
- 74. Ménoret S, Fontanière S, Jantz D, et al. Generation of Rag1-knockout immunodeficient rats and mice using engineered meganucleases. FASEB J. 2013;27(2):703-711. [DOI] [PubMed] [Google Scholar]
- 75. Jia L, Lee S, Tierney JA, Elmquist JK, Burton MD, Gautron L. TLR4 signaling selectively and directly promotes CGRP release from vagal afferents in the mouse. eNeuro. 2021;8(1):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Laumet G, Edralin JD, Dantzer R, Heijnen CJ, Kavelaars A. Cisplatin educates CD8+ T cells to prevent and resolve chemotherapy-induced peripheral neuropathy in mice. Pain. 2019;160(6):1459-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Maggio M, Basaria S, Ble A, et al. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab. 2006;91(1):345-347. [DOI] [PubMed] [Google Scholar]
- 78. Kaergaard A, Hansen AM, Rasmussen K, Andersen JH. Association between plasma testosterone and work-related neck and shoulder disorders among female workers. Scand J Work Environ Health. 2000;26(4):292-298. [DOI] [PubMed] [Google Scholar]
- 79. Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994;78(6):1360-1367. [DOI] [PubMed] [Google Scholar]
- 80. Pasciuto E, Burton OT, Roca CP, et al. Microglia require CD4 T cells to complete the fetal-to-adult transition. Cell. 2020;182(3):625-640.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89(7):3313-3318. [DOI] [PubMed] [Google Scholar]
- 82. Norata GD, Tibolla G, Seccomandi PM, Poletti A, Catapano AL. Dihydrotestosterone decreases tumor necrosis factor-α and lipopolysaccharide-induced inflammatory response in human endothelial cells. J Clin Endocrinol Metab. 2006;91(2):546-554. [DOI] [PubMed] [Google Scholar]
- 83. Aloisi AM, Ceccarelli I, Fiorenzani P, De Padova AM, Massafra C. Testosterone affects formalin-induced responses differently in male and female rats. Neurosci Lett. 2004;361(1-3):262-264. [DOI] [PubMed] [Google Scholar]
- 84. Burrows LJ, Goldstein AT. The treatment of vestibulodynia with topical estradiol and testosterone. Sex Med. 2013;1(1):30-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Smith BH, Elliott AM, Chambers WA, Smith WC, Hannaford PC, Penny K. The impact of chronic pain in the community. Fam Pract. 2001;18(3):292-299. [DOI] [PubMed] [Google Scholar]
- 86. Breslau N, Rasmussen BK. The impact of migraine: epidemiology, risk factors, and co-morbidities. Neurology. 2001;56(6 Suppl 1):S4-12. [DOI] [PubMed] [Google Scholar]
- 87. Duarte DB, Vasko MR, Fehrenbacher JC. Models of inflammation: carrageenan air pouch. Curr Protoc Pharmacol. 2016;72:5.6.1-5.6.9. [DOI] [PubMed] [Google Scholar]
- 88. Warfvinge K, Krause DN, Maddahi A, Edvinsson JCA, Edvinsson L, Haanes KA. Estrogen receptors α, β and GPER in the CNS and trigeminal system—molecular and functional aspects. J Headache Pain. 2020;21(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Maggioli E, McArthur S, Mauro C, et al. Estrogen protects the blood-brain barrier from inflammation-induced disruption and increased lymphocyte trafficking. Brain Behav Immun. 2016;51:212-222. [DOI] [PubMed] [Google Scholar]
- 90. Kuruca SE, Karadenizli S, Akgun-Dar K, Kapucu A, Kaptan Z, Uzum G. The effects of 17β-estradiol on blood brain barrier integrity in the absence of the estrogen receptor alpha; an in-vitro model. Acta Histochem. 2017;119(6):638-647. [DOI] [PubMed] [Google Scholar]
- 91. Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23(1):90-119. [DOI] [PubMed] [Google Scholar]
- 92. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211. [DOI] [PubMed] [Google Scholar]
- 93. Granella F, Sances G, Zanferrari C, Costa A, Martignoni E, Manzoni GC. Migraine without aura and reproductive life events: a clinical epidemiological study in 1300 women. Headache. 1993;33(7):385-389. [DOI] [PubMed] [Google Scholar]
- 94. Massiou H, MacGregor EA. Evolution and treatment of migraine with oral contraceptives. Cephalalgia. 2000;20(3):170-174. [DOI] [PubMed] [Google Scholar]
- 95. Scotland PE, Patil M, Belugin S, et al. Endogenous prolactin generated during peripheral inflammation contributes to thermal hyperalgesia. Eur J Neurosci. 2011;34(5):745-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Patil MJ, Ruparel SB, Henry MA, Akopian AN. Prolactin regulates TRPV1, TRPA1, and TRPM8 in sensory neurons in a sex-dependent manner: contribution of prolactin receptor to inflammatory pain. Am J Physiol Endocrinol Metab. 2013;305(9):E1154-E1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Diogenes A, Patwardhan AM, Jeske NA, et al. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci. 2006;26(31):8126-8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Patil M, Hovhannisyan AH, Wangzhou A, et al. Prolactin receptor expression in mouse dorsal root ganglia neuronal subtypes is sex-dependent. J Neuroendocrinol. 2019;31(8):e12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Patil M, Belugin S, Mecklenburg J, et al. Prolactin regulates pain responses via a female-selective nociceptor-specific mechanism. iScience. 2019;20:449-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Paige C, Barba-Escobedo PA, Mecklenburg J, et al. Neuroendocrine mechanisms governing sex differences in hyperalgesic priming involve prolactin receptor sensory neuron signaling. J Neurosci. 2020;40(37):7080-7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Avona A, Mason BN, Burgos-Vega C, et al. Meningeal CGRP-prolactin interaction evokes female-specific migraine behavior. Ann Neurol. 2021;89(6):1129-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kehoe L, Janik J, Callahan P. Effects of immobilization stress on tuberoinfundibular dopaminergic (TIDA) neuronal activity and prolactin levels in lactating and non-lactating female rats. Life Sci. 1992;50(1):55-63. [DOI] [PubMed] [Google Scholar]
- 103. Cavestro C, Rosatello A, Marino MP, Micca G, Asteggiano G. High prolactin levels as a worsening factor for migraine. J Headache Pain. 2006;7(2):83-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9(8):563-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Liu Z, Zhang W, Zhang M, Zhu H, Moriasi C, Zou MH. Liver kinase B1 suppresses lipopolysaccharide-induced nuclear factor κB (NF-κB) activation in macrophages. J Biol Chem. 2015;290(4):2312-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Barnes AP, Lilley BN, Pan YA, et al. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129(3):549-563. [DOI] [PubMed] [Google Scholar]
- 107. Shelly M, Poo MM. Role of LKB1-SAD/MARK pathway in neuronal polarization. Dev Neurobiol. 2011;71(6):508-527. [DOI] [PubMed] [Google Scholar]
- 108. Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129(3):565-577. [DOI] [PubMed] [Google Scholar]
- 109. Thapa B, Lee K. Metabolic influence on macrophage polarization and pathogenesis. BMB Rep. 2019;52(6):360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mounier R, Théret M, Arnold L, et al. AMPKα1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013;18(2):251-264. [DOI] [PubMed] [Google Scholar]
- 112. Zhu YP, Brown JR, Sag D, Zhang L, Suttles J. Adenosine 5′-monophosphate-activated protein kinase regulates IL-10–mediated anti-inflammatory signaling pathways in macrophages. J Immunol. 2015;194(2):584-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bajaj P, Arendt-Nielsen L, Bajaj P, Madsen H. Sensory changes during the ovulatory phase of the menstrual cycle in healthy women. Eur J Pain. 2001;5(2):135-144. [DOI] [PubMed] [Google Scholar]
- 114. Brauer MM, Smith PG. Estrogen and female reproductive tract innervation: cellular and molecular mechanisms of autonomic neuroplasticity. Auton Neurosci. 2015;187:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zoubina EV, Smith PG. Sympathetic hyperinnervation of the uterus in the estrogen receptor α knock-out mouse. Neuroscience. 2001;103(1):237-244. [DOI] [PubMed] [Google Scholar]
- 116. Ferrari LF, Araldi D, Levine JD. Distinct terminal and cell body mechanisms in the nociceptor mediate hyperalgesic priming. J Neurosci. 2015;35(15):6107-6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Thomas DG, Doran AC, Fotakis P, et al. LXR suppresses inflammatory gene expression and neutrophil migration through cis-repression and cholesterol efflux. Cell Rep. 2018;25(13):3774-3785.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Schulman IG. Liver X receptors link lipid metabolism and inflammation. FEBS Lett. 2017;591(19):2978-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gavini CK, Bookout AL, Bonomo R, Gautron L, Lee S, Mansuy-Aubert V. Liver X receptors protect dorsal root ganglia from obesity-induced endoplasmic reticulum stress and mechanical allodynia. Cell Rep. 2018;25(2):271-277.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Moy JK, Kuhn JL, Szabo-Pardi TA, Pradhan G, Price TJ. eIF4E phosphorylation regulates ongoing pain, independently of inflammation, and hyperalgesic priming in the mouse CFA model. Neurobiol Pain. 2018;4:45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2(4):236-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yoshikawa T, Shimano H, Amemiya-Kudo M, et al. Identification of liver X receptor-retinoid X receptor as an activator of the sterol regulatory element-binding protein 1c gene promoter. Mol Cell Biol. 2001;21(9):2991-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bao X, Cai Y, Wang Y, et al. Liver X receptor β is involved in formalin-induced spontaneous pain. Mol Neurobiol. 2017;54(2):1467-1481. [DOI] [PubMed] [Google Scholar]
- 124. Sneddon LU. Comparative physiology of nociception and pain. Physiology (Bethesda). 2018;33(1):63-73. [DOI] [PubMed] [Google Scholar]
- 125. Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013;79(4):618-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. McGlone F, Reilly D. The cutaneous sensory system. Neurosci Biobehav Rev. 2010;34(2):148-159. [DOI] [PubMed] [Google Scholar]
- 127. Bielefeldt K, Christianson JA, Davis BM. Basic and clinical aspects of visceral sensation: transmission in the CNS. Neurogastroenterol Motil. 2005;17(4):488-499. [DOI] [PubMed] [Google Scholar]
- 128. Holzer P. Efferent-like roles of afferent neurons in the gut: blood flow regulation and tissue protection. Auton Neurosci. 2006;125(1-2):70-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Christianson JA, Bielefeldt K, Altier C, et al. Development, plasticity and modulation of visceral afferents. Brain Res Rev. 2009;60(1):171-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Robinson DR, Gebhart GF. Inside information: the unique features of visceral sensation. Mol Interv. 2008;8(5):242-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Rostock C, Schrenk-Siemens K, Pohle J, Siemens J. Human vs. mouse nociceptors—similarities and differences. Neuroscience. 2018;387:13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R. General pathways of pain sensation and the major neurotransmitters involved in pain regulation. Int J Mol Sci. 2018;19(8):2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11(12):823-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Chen O, Donnelly CR, Ji RR. Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr Opin Neurobiol. 2020;62:17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011;118(22):5918-5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Pinho-Ribeiro FA, Verri WA Jr, Chiu IM. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. 2017;38(1):5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Baral P, Udit S, Chiu IM. Pain and immunity: implications for host defence. Nat Rev Immunol. 2019;19(7):433-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lim JY, Choi SI, Choi G, Hwang SW. Atypical sensors for direct and rapid neuronal detection of bacterial pathogens. Mol Brain. 2016;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Marmigère F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci. 2007;8(2):114-127. [DOI] [PubMed] [Google Scholar]
- 141. Stephens KE, Zhou W, Ji Z, et al. Sex differences in gene regulation in the dorsal root ganglion after nerve injury. BMC Genomics. 2019;20(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Mecklenburg J, Zou Y, Wangzhou A, et al. Transcriptomic sex differences in sensory neuronal populations of mice. Sci Rep. 2020;10(1):15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nilsson ME, Vandenput L, Tivesten Å, et al. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology. 2015;156(7):2492-2502. [DOI] [PubMed] [Google Scholar]
- 144. Saito T, Ciobotaru A, Bopassa JC, Toro L, Stefani E, Eghbali M. Estrogen contributes to gender differences in mouse ventricular repolarization. Circ Res. 2009;105(4):343-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88(1):91-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Barbati C, Pierdominici M, Gambardella L, et al. Cell surface estrogen receptor alpha is upregulated during subchronic metabolic stress and inhibits neuronal cell degeneration. PloS One. 2012;7(7):e42339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kajta M, Beyer C. Cellular strategies of estrogen-mediated neuroprotection during brain development. Endocrine. 2003;21(1):3-9. [DOI] [PubMed] [Google Scholar]
- 148. McCarthy MM. Sex differences in the developing brain as a source of inherent risk. Dialogues Clin Neurosci. 2016;18(4):361-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. McEwen B, Akama K, Alves S, et al. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc Natl Acad Sci U S A. 2001;98(13):7093-7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Patrone C, Andersson S, Korhonen L, Lindholm D. Estrogen receptor-dependent regulation of sensory neuron survival in developing dorsal root ganglion. Proc Natl Acad Sci U S A. 1999;96(19):10905-10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Blake KJ, Baral P, Voisin T, et al. Staphylococcus aureus produces pain through pore-forming toxins and neuronal TRPV1 that is silenced by QX-314. Nat Commun. 2018;9(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15(8):1063-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Cohen JA, Edwards TN, Liu AW, et al. Cutaneous TRPV1+ neurons trigger protective innate type 17 anticipatory immunity. Cell. 2019;178(4):919-932.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Talbot S, Abdulnour RE, Burkett PR, et al. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron. 2015;87(2):341-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Tsuji F, Aono H. Role of transient receptor potential vanilloid 1 in inflammation and autoimmune diseases. Pharmaceuticals (Basel). 2012;5(8):837-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Baral P, Umans BD, Li L, et al. Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia. Nat Med. 2018;24(4):417-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Ramirez V, Swain S, Murray K, Reardon C. Neural immune communication in the control of host-bacterial pathogen interactions in the gastrointestinal tract. Infect Immun. 2020;88(9):e00928-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Nhu QM, Cuesta N, Vogel SN. Transcriptional regulation of lipopolysaccharide (LPS)-induced Toll-like receptor (TLR) expression in murine macrophages: role of interferon regulatory factors 1 (IRF-1) and 2 (IRF-2). J Endotoxin Res. 2006;12(5):285-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Nomura F, Akashi S, Sakao Y, et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164(7):3476-3479. [DOI] [PubMed] [Google Scholar]
- 160. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Burton M, Szabo-Pardi T, Garner K, Tierney J, Price T. Uncovering cell-specific mechanisms in sex differences in TLR4-dependent pain. J Pain. 2019;20(4 Suppl):S1. [Google Scholar]
- 162. Rudjito R, Agalave NM, Farinotti AB, et al. Sex- and cell-dependent contribution of peripheral high mobility group box 1 and TLR4 in arthritis-induced pain. Pain. 2021;162(2):459-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1-5. [DOI] [PubMed] [Google Scholar]
- 164. Becker JB, Prendergast BJ, Liang JW. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol Sex Differ. 2016;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Xu S, Cheng Y, Keast JR, Osborne PB. 17β-estradiol activates estrogen receptor β-signalling and inhibits transient receptor potential vanilloid receptor 1 activation by capsaicin in adult rat nociceptor neurons. Endocrinology. 2008;149(11):5540-5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Guo D, Liu X, Zeng C, et al. Estrogen receptor β activation ameliorates DSS-induced chronic colitis by inhibiting inflammation and promoting Treg differentiation. Int Immunopharmacol. 2019;77:105971. [DOI] [PubMed] [Google Scholar]
- 167. Rosen S, Ham B, Mogil JS. Sex differences in neuroimmunity and pain. J Neurosci Res. 2017;95(1-2):500-508. [DOI] [PubMed] [Google Scholar]
- 168. Hernandez-Leon A, De la Luz-Cuellar YE, Granados-Soto V, González-Trujano ME, Fernández-Guasti A. Sex differences and estradiol involvement in hyperalgesia and allodynia in an experimental model of fibromyalgia. Horm Behav. 2018;97:39-46. [DOI] [PubMed] [Google Scholar]
- 169. De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145(3):1063-1068. [DOI] [PubMed] [Google Scholar]
- 170. Kaczkurkin AN, Raznahan A, Satterthwaite TD. Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology. 2019;44(1):71-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Bolego C, Cignarella A, Staels B, Chinetti-Gbaguidi G. Macrophage function and polarization in cardiovascular disease: a role of estrogen signaling? Arterioscler Thromb Vasc Biol. 2013;33(6):1127-1134. [DOI] [PubMed] [Google Scholar]
- 173. Calippe B, Douin-Echinard V, Delpy L, et al. 17β-Estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor α signaling in macrophages in vivo. J Immunol. 2010;185(2):1169-1176. [DOI] [PubMed] [Google Scholar]
- 174. Dou C, Ding N, Zhao C, et al. Estrogen deficiency–mediated M2 macrophage osteoclastogenesis contributes to M1/M2 ratio alteration in ovariectomized osteoporotic mice. J Bone Miner Res. 2018;33(5):899-908. [DOI] [PubMed] [Google Scholar]
- 175. Lambert KC, Curran EM, Judy BM, Milligan GN, Lubahn DB, Estes DM. Estrogen receptor α (ERα) deficiency in macrophages results in increased stimulation of CD4+ T cells while 17β-estradiol acts through ERα to increase IL-4 and GATA-3 expression in CD4+ T cells independent of antigen presentation. J Immunol. 2005;175(9):5716-5723. [DOI] [PubMed] [Google Scholar]
- 176. Agalave NM, Rudjito R, Farinotti AB, et al. Sex-dependent role of microglia in disulfide high mobility group box 1 protein-mediated mechanical hypersensitivity. Pain. 2021;162(2):446-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Szabo-Pardi TA, Syed UM, Castillo ZW, Burton MD. Use of integrated optical clearing and 2-photon imaging to investigate sex differences in neuroimmune interactions after peripheral nerve injury. Front Cell Dev Biol. 2021;9:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Taves S, Berta T, Liu DL, et al. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: sex-dependent microglial signaling in the spinal cord. Brain Behav Immun. 2016;55:70-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Terkel J, Blake CA, Sawyer CH. Serum prolactin levels in lactating rats after suckling or exposure to ether. Endocrinology. 1972;91(1):49-53. [DOI] [PubMed] [Google Scholar]
- 180. Abdallah CG, Geha P. Chronic pain and chronic stress: two sides of the same coin? Chronic Stress (Thousand Oaks). 2017;1:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Baldwin AL. Mast cell activation by stress. Methods Mol Biol. 2006;315:349-360. [DOI] [PubMed] [Google Scholar]
- 182. Donoso AO, Zárate MB. Release of prolactin and luteinizing hormone by histamine agonists in ovariectomized, steroid-treated rats under ether anesthesia. Exp Brain Res. 1983;52(2):277-280. [DOI] [PubMed] [Google Scholar]
- 183. Sorge RE, Mapplebeck JC, Rosen S, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18(8):1081-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Freemerman AJ, Johnson AR, Sacks GN, et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. 2014;289(11):7884-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, Castegna A. The metabolic signature of macrophage responses. Front Immunol. 2019;10:1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Xiang HC, Lin LX, Hu XF, et al. AMPK activation attenuates inflammatory pain through inhibiting NF-κB activation and IL-1β expression. J Neuroinflammation. 2019;16(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137-163. [DOI] [PubMed] [Google Scholar]
- 188. Courchet J, Lewis TL Jr, Lee S, et al. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell. 2013;153(7):1510-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Arnold J, Barcena de Arellano ML, Rüster C, et al. Imbalance between sympathetic and sensory innervation in peritoneal endometriosis. Brain Behav Immun. 2012;26(1):132-141. [DOI] [PubMed] [Google Scholar]
- 190. Xu JN, Zeng C, Zhou Y, Peng C, Zhou YF, Xue Q. Metformin inhibits StAR Expression in human endometriotic stromal cells via AMPK-mediated disruption of CREB-CRTC2 complex formation. J Clin Endocrinol Metab. 2014;99(8):2795-2803. [DOI] [PubMed] [Google Scholar]
- 191. Lenert ME, Burton MD. Sensory neuron metabolism mediates changes in ovarian function via LKB1. J Endocr Soc. 2020;4(Suppl 1):A665. [Google Scholar]
- 192. Inyang KE, Szabo-Pardi T, Wentworth E, et al. The antidiabetic drug metformin prevents and reverses neuropathic pain and spinal cord microglial activation in male but not female mice. Pharmacol Res. 2019;139:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Burton MD, Tillu DV, Mazhar K, et al. Pharmacological activation of AMPK inhibits incision-evoked mechanical hypersensitivity and the development of hyperalgesic priming in mice. Neuroscience. 2017;359:119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Inyang KE, McDougal TA, Ramirez ED, et al. Alleviation of paclitaxel-induced mechanical hypersensitivity and hyperalgesic priming with AMPK activators in male and female mice. Neurobiol Pain. 2019;6:100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Ferrari LF, Bogen O, Levine JD. Nociceptor subpopulations involved in hyperalgesic priming. Neuroscience. 2010;165(3):896-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kersten S. Integrated physiology and systems biology of PPARα. Mol Metab. 2014;3(4):354-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Ahmadian M, Suh JM, Hah N, et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19(5):557-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Dotson AL, Wang J, Chen Y, et al. Sex differences and the role of PPAR alpha in experimental stroke. Metab Brain Dis. 2016;31(3):539-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Schreiber SN, Emter R, Hock MB, et al. The estrogen-related receptor α (ERRα) functions in PPARγ coactivator 1α (PGC-1α)-induced mitochondrial biogenesis. Proc Natl Acad Sci U S A. 2004;101(17):6472-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Dupont J, Chabrolle C, Ramé C, Tosca L, Coyral-Castel S. Role of the peroxisome proliferator-activated receptors, adenosine monophosphate-activated kinase, and adiponectin in the ovary. PPAR Res. 2008;2008:176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Duggett NA, Griffiths LA, Flatters SJL. Paclitaxel-induced painful neuropathy is associated with changes in mitochondrial bioenergetics, glycolysis, and an energy deficit in dorsal root ganglia neurons. Pain. 2017;158(8):1499-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Caillaud M, Patel NH, Toma W, et al. A fenofibrate diet prevents paclitaxel-induced peripheral neuropathy in mice. Cancers (Basel). 2020;13(1):1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Caillaud M, Patel NH, White A, et al. Targeting peroxisome proliferator-activated receptor-α (PPAR-α) to reduce paclitaxel-induced peripheral neuropathy. Brain Behav Immun. 2021;93:172-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Zhou YQ, Liu DQ, Chen SP, et al. PPARγ activation mitigates mechanical allodynia in paclitaxel-induced neuropathic pain via induction of Nrf2/HO-1 signaling pathway. Biomed Pharmacother. 2020;129:110356. [DOI] [PubMed] [Google Scholar]
- 205. Rigamonti E, Chinetti-Gbaguidi G, Staels B. Regulation of macrophage functions by PPAR-α, PPAR-γ, and LXRs in mice and men. Arterioscler Thromb Vasc Biol. 2008;28(6):1050-1059. [DOI] [PubMed] [Google Scholar]
- 206. Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Bouhlel MA, Derudas B, Rigamonti E, et al. PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6(2):137-143. [DOI] [PubMed] [Google Scholar]
- 208. Edwards RR, Fillingim RB. Self-reported pain sensitivity: lack of correlation with pain threshold and tolerance. Eur J Pain. 2007;11(5):594-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10(4):283-294. [DOI] [PubMed] [Google Scholar]
- 210. Dailey DL, Vance CGT, Rakel BA, et al. Transcutaneous electrical nerve stimulation reduces movement‐evoked pain and fatigue: a randomized, controlled trial. Arthritis Rheumatol. 2020;72(5):824-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Noehren B, Dailey DL, Rakel BA, et al. Effect of transcutaneous electrical nerve stimulation on pain, function, and quality of life in fibromyalgia: a double-blind randomized clinical trial. Phys Ther. 2015;95(1):129-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.