Abstract
Declining and variable levels of estrogens around the time of menopause are associated with a suite of metabolic, vascular, and neuroendocrine changes. The archetypal adverse effects of perimenopause are vasomotor symptoms, which include hot flashes and night sweats. Although vasomotor symptoms are routinely treated with hormone therapy, the risks associated with these treatments encourage us to seek alternative treatment avenues. Understanding the mechanisms underlying the effects of estrogens on temperature regulation is a first step toward identifying novel therapeutic targets. Here we outline findings in rodents that reveal neural and molecular targets of estrogens within brain regions that control distinct components of temperature homeostasis. These insights suggest that estrogens may alter the function of multiple specialized neural circuits to coordinate the suite of changes after menopause. Thus, defining the precise cells and neural circuits that mediate the effects of estrogens on temperature has promise to identify strategies that would selectively counteract hot flashes or other negative side effects without the health risks that accompany systemic hormone therapies.
Keywords: hypothalamus, hot flashes, menopause, estrogens, temperature, neural circuits
Hot flashes, characterized by episodic and transient increases in skin vasodilation, sweating, and shivering, are reported by 75% to 80% of menopausal cis women in the United States and occur over an average span of 7.4 years (1). Hot flashes are associated with periods of estrogen withdrawal due to oophorectomy or menopause and can be effectively prevented with estrogen therapy (2). Although hormone therapy is an effective treatment, the associated risks, including reproductive cancers (3, 4), have curbed the use of hormone therapy for the treatment of hot flashes. Alternative treatments include less effective lifestyle changes and pharmaceutical interventions (5-7) but additional options are in development. Increasing evidence in rodents indicates that estrogens modulate body temperature in females via the central nervous system (8, 9). Estrogen receptors are widely detected in the brain, especially in brain regions that maintain temperature and energy homeostasis (10, 11). Thus, we suggest that a full understanding of hot flashes and the identification of alternative treatments will require a dissection and mechanistic interrogation of the effects of estrogens on the neural circuits that maintain temperature homeostasis.
In this review we will outline current knowledge on the effects of estrogens on thermoregulation in female rodents. Estrogens appear to modulate multiple interrelated components of thermoregulation, including heat dissipation, heat production, and core body temperature (8, 12). The effects of estrogens on temperature appear to be mediated by distinct estrogen-sensitive neuron populations and circuitry (12). However, the effect of estrogens on these brain regions are not in isolation. Endogenous estrogens are widely circulated and can act at multiple estrogen-sensitive regions and tissues simultaneously. Systemic manipulations of estrogen levels may overlook the interactions between brain regions or tissues and the synergistic or compensatory actions of estrogens (13). Therefore, dissecting the effects of estrogens on individual cell populations or circuit connections will be necessary for a better understanding of the effects of estrogens on thermoregulation.
Estrogens and Estrogen Receptors
As sex hormones, estrogens are best known for their reproductive function in females. There are 3 major endogenous forms of estrogens: estrone, estradiol, and estriol, with 17β-estradiol (E2) being the major circulating estrogen in cycling females. The majority of E2 is produced in the ovaries, although local production of estrogens exists in many nonreproductive organs, including the brain (14, 15). The local synthesis of estrogens, independent of levels in the systemic circulation, enables site-specific or cell-type specific estrogen metabolism and function (14, 16). Biological effects of estrogens are mediated largely by 3 receptors, estrogen receptors alpha and beta (ERα and ERβ) (17, 18) and the G protein–coupled estrogen receptor (GPER1) (19). Estrogen receptor signaling can alter gene expression through nucleus-initiated transcriptional effects of nuclear receptors ERα and ERβ or through membrane-initiated signaling pathways downstream of ERα, ERβ, or GPER1 (see reviews (20-22)). The latter rapid nongenomic effects may contribute to synaptic transmission as a “neurotransmitter” (23), through effects on ion channels or other second messengers (20, 24). The heterogeneous expression of estrogen receptors in the hypothalamus has been nicely demonstrated at the transcript and protein levels (25-27). ERα immunoreactivity shows dominant expression in specific hypothalamic regions that are implicated in temperature and metabolic regulation, including the preoptic area (POA), ventromedial nucleus of the hypothalamus (VMH) and arcuate nucleus of the hypothalamus (ARC) (Table 1). Receptor-selective pharmacological (28) and genetic manipulations (29-31) suggest a primary role for ERα in mediating the metabolic effects of estrogens on the hypothalamus, although other receptor(s) are clearly involved in the effects of estrogens on temperature. For example, treating ovariectomized rats or mice with selective ligands for ERβ can mimic the effects of estradiol treatment on lowering tail skin temperature (Ttail) without affecting body or uterine weights (32, 33). Similarly, a Gq-coupled estrogen receptor agonist is able to decrease core body temperature similar to E2 in guinea pigs after ovariectomy (34). However, it is unclear where these effects manifest within neural circuitries in the brain. In this review, we primarily describe the thermoregulatory effects mediated by ERα unless otherwise indicated.
Table 1.
Candidate brain regions for estrogenic modulation of temperature in mice
| Brain region | Cell types or molecular targets | ERα expression* | ERβ expression* | Thermoregulatory roles |
|---|---|---|---|---|
| MPA | ERα-expressing neurons (47), GABAergic neurons, warm-responsive neurons | high | low | thermogenesis, heat dissipation (50) |
| MnPO | NK3R-expressing neurons (116), Glutamatergic neurons (130) | high (67, 68) low (50) |
low | cutaneous vasoconstriction (115), hot flashes (131) |
| VMH | ERα-expressing neurons (96), AMPK (53), Rprm-expressing neurons (96) | high | low | BAT thermogenesis (53, 96) |
| ARC | Kisspeptin or KNDy neurons (56, 72) | high | low | cutaneous vasoconstriction (56, 72) |
| DMH | Glutamatergic neurons | low | low | BAT thermogenesis (50) |
Abbreviations: AMPK, adenosine monophosphate–activated protein kinase; ARC, arcuate nucleus; BAT, brown adipose tissue; DMH, dorsomedial nucleus of the hypothalamus; ERα, estrogen receptor alpha; ERβ, estrogen receptor beta; GABAergic neurons, γ-aminobutyric acid–expressing neurons; KNDy, neurons co-expressing kisspeptin/neurokinin B/dynorphin; MnPO, median preoptic nucleus; MPA, medial preoptic area; NK3R, NK3 receptor; VMH, ventromedial nucleus of the hypothalamus.
* Based on immunoreactivity in mouse (26), unless otherwise noted
Effects of Estrogens on Thermoregulatory Neural Circuitry
The hypothalamus is a pivotal center for maintaining stable body temperature through the combination of feedforward and feedback systems. The feedforward pathway transmits sensory temperature information from the periphery to the POA via relays from the lateral parabrachial nucleus and spinal (or trigeminal) dorsal horn (10, 11). In addition, the POA contains temperature-sensitive neurons with the intrinsic ability to detect local brain temperature changes (35). This peripheral and central temperature information is integrated to direct various thermoregulatory responses. The feedback thermoregulatory network includes hypothalamic nuclei (eg, dorsomedial hypothalamus [DMH], VMH and ARC) and caudal brain regions (eg, raphe pallidus [RPa]) where distinct groups of premotor neurons independently regulate sympathetic activation of brown adipose tissue (BAT) thermogenesis and tail artery vasoconstriction (10, 36-38). Although the thermoregulatory network coordinates both heat dissipation and heat production, each thermoregulatory effector is driven separately via parallel but distinct efferent pathways (39). That is, certain neurons and circuits may be dedicated to one specific thermal effector. For example, synaptic connections from warm-responsive neurons in the POA to the DMH may regulate thermogenesis without affecting heat dissipation at the skin (40). In addition, 2 distinct populations of thermosensory neurons in the lateral parabrachial nucleus were found to innervate the POA and differentially affect vasodilation and BAT thermogenesis (41, 42). Consistent with specialization within these circuits, the POA is highly heterogeneous (27) and integrates multiple nonthermal inputs (43) that may also impact temperature homeostasis. For example, the POA mediates the febrile response to inflammation (44, 45), rhythmic temperature fluctuations along day-night periods or the ovarian cycle in response to circadian or sex hormones inputs (46, 47), adaptive thermogenesis in response to metabolic signals (43), the initiation of torpor in response to fasting (48-50), and water balance and osmolarity as a result of evaporative heat loss (51).
Due to the molecular and functional complexity of these thermoregulatory neurons, estrogens may modulate the thermoregulatory circuitry in ways that compensate or amplify their effects on individual neural populations. Studies indicate that estrogens can act at multiple thermosensory and thermoregulatory nodes to modulate body temperature (Table 1). This modulation by estrogens might be specific to one aspect of thermal responses, or mixed (Table 1). For example, warm-responsive neurons in the POA that suppress heat production and increase heat loss also express estrogen receptors and show changes in neuronal activity in response to E2 (47, 52). E2 in the VMH increases core body temperature and BAT thermogenesis in rats (53, 54). However, the effects of E2 on neuronal activity can differ, as E2 can have either inhibitory or excitatory effects on kisspeptin neurons in the ARC or anteroventral periventricular nucleus, respectively (55). Given that the thermoregulatory circuitry is sensitive to estrogens at multiple nodes (Table 1), the bidirectional effects of estrogens may lead to opposing or synergistic effects within the thermoregulatory circuitry. Indeed, there is evidence that E2 can suppress kisspeptin neurons in the ARC to reduce heat loss from the tail in rats (56) but also can activate warm-sensitive neurons in the medial POA (MPA) to increase tail heat loss in mice (47, 50). These opposing effects of estrogens on neural activity could be due to heterogeneity in the responses to estrogens at the level of neuron type, interactions with modulatory inputs from upstream circuit nodes, or species differences. Nonetheless, the global effects of estrogens on body temperature likely reflect the integration of estrogen’s effects at multiple nodes of the thermoregulatory circuitry.
Thus, to explicitly understand the regulation of temperature by estrogens, or other hormones, it is important to dissect the individual neuron populations, neuronal connections, and circuit-level interactions. Fortunately, emerging circuit-based approaches and single-cell resolution analyses are facilitating such nuanced approaches.
Effects of Estrogens on Facets of Thermoregulation
Heat Dissipation
Estrogens affect heat dissipation by regulating cutaneous vasoconstriction (CVC) and fluid vaporization. In rodents, Ttail is greatly influenced by CVC, which is controlled by the release of norepinephrine from sympathetic fibers innervating vascular smooth muscle in the skin (57) and has been used as an indication of heat loss. In rats, Ttail is lower on the night of proestrus, when E2 is high, compared with other stages when housed at 22 oC (58); however, this difference has not been observed in mice using the same method for temperature measurement at ambient temperatures between 22 and 24 oC (59). Depleting circulating estrogens by ovariectomy increases Ttail in rats (60-62) and sometimes in mice (3-5 weeks after ovariectomy) (63, 64). Although the effects of the estrous cycle and ovariectomy differ to some degree in rats and mice, E2 replacement decreases tail skin temperature in ovariectomized rats (60-62, 65) and in mice when ambient temperature is below the thermoneutral point (59, 64). Collectively, these studies suggest that estrogens regulate CVC to suppress heat loss and promote heat conservation. Moreover, the effects of estrogens may also differ by species, time of estrogen depletion, and housing temperature.
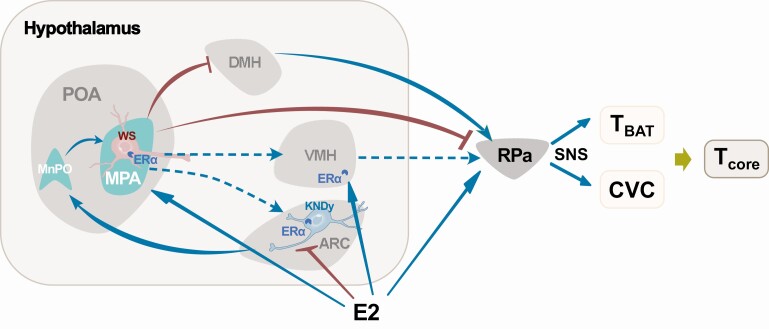
The precise mechanisms underlying the effects of estrogens on heat dissipation remain largely unknown but the evidence to date suggests that neurons in the median preoptic nucleus (MnPO) play an important role. Neurons in the MnPO receive cutaneous thermosensory inputs and project both directly and indirectly to sympathetic premotor neurons in RPa that regulate sympathetic vasoconstrictor nerves (36). These MnPO neurons are regulated by estrogens: E2 replacement in ovariectomized rats reduced cFos expression in the MnPO and decreased tail skin heat loss (66). The inhibitory effect of E2 on MnPO neurons may be mediated by estrogen receptor expression in the MnPO (67, 68) or via inputs from a subpopulation of estrogen responsive neurons in the ARC (69) that co-express kisspeptin, neurokinin B (NKB), and dynorphin (KNDy) and are negatively inhibited by E2 through ERα (70, 71). Ablation of KNDy neurons reduces tail skin temperature and blocks E2-induced heat loss in ovariectomized rats (56), whereas optogenetic activation of kisspeptin neurons in the ARC of mice upregulates cFos expression in the MnPO and increases heat loss at the tail (72). In addition, warm-responsive neurons in the MPA send inhibitory inputs to the RPa to modulate sympathetic CVC (73). ERα neurons in the MPA are mostly GABAergic (27) and temperature-responsive (50), indicating that E2 may also regulate heat dissipation via temperature- and estrogen-sensitive neurons in the MPA. Together, these findings identify multiple estrogen-sensitive regions (e.g., ARC, MnPO, and MPA) in the hypothalamus and an estrogen-sensitive circuit (ARC-MnPO-MPA-RPa) that controls heat dissipation at the skin (Fig. 1).
Figure 1.
Estrogen-sensitive nodes and circuits involved in thermoregulation. E2 acts on the ARC to suppress the activity of KNDy neurons that project to the MnPO. The warm-sensitive neurons in the MPA receive afferent inputs from the MnPO and inhibit premotor sympathetic neurons in the RPa controlling cutaneous vascular constriction (CVC). E2 acts at the VMH to increase brown adipose tissue temperature (TBAT) or may act at the MPA warm-sensitive neurons to suppress TBAT and CVC. Brown lines with bar head indicate inhibitory inputs and cyan lines with triangle head indicate stimulatory inputs. Blue dashed lines indicate potential estrogen-sensitive thermoregulatory projections (37, 132, 133). Abbreviations: ARC, arcuate nucleus of the hypothalamus; DMH, dorsomedial nucleus of the hypothalamus; KNDy, neurons co-expressing kisspeptin, neurokinin B, and dynorphin; MnPO, median preoptic nucleus; MPA, medial preoptic area; POA, preoptic area of hypothalamus; RPa, raphe pallidus; SNS, sympathetic nervous system; VMH, ventromedial nucleus of the hypothalamus; WS, warm-sensitive neurons.
Estrogens also alter evaporative heat loss, which includes sweating in humans, panting in dogs, and salivation in rodents. E2 replacement does not seem to change evaporative heat loss in ovariectomized rats (74) but does alter the threshold of evaporative heat defense, increasing evaporative water loss during heat stress at a lower core temperature compared to untreated ovariectomized rats (75). Similarly, young women in the high E2 preovulatory phase of the menstrual cycle exhibited a lower esophageal temperature threshold for sweating during exercise compared to women in the low E2 early follicular phase (76). Accordingly, estrogen therapy is associated with a lower body temperature threshold for sweating during exercise (77). Together, these studies suggest that estrogens decrease dry heat loss from skin vasodilation and increase vaporizing heat loss at a lower temperature threshold. The precise neural circuits controlling evaporative heat loss are less clear but may involve the warm-sensitive neurons in the POA and the downstream areas in the medulla, such as the superior salivatory nucleus and rostral ventromedial medulla (10, 78, 79).
In summary, it appears that E2 modulates different types of heat loss (skin vasodilation and evaporative cooling). We would speculate that this could happen through distinct neuron populations or neural circuits within the brain. Dissections of these estrogen-sensitive regions and their potential interaction is essential for understanding the effects of estrogens on heat dissipation.
Effects of Estrogens on Heat Production
BAT is a specialized thermogenic organ that can effectively burn fatty acid into heat by uncoupling protein 1 (UCP1)-mediated oxidation (80). BAT is most commonly found in rodents and humans at a very young age (81), but BAT is recruitable and active in adult humans as well (82, 83). Thermogenic activity within BAT is strongly dependent on sympathetic inputs, including norepinephrine that binds to beta-adrenergic receptors in brown adipocytes (80, 84). Aside from the therapeutic potential for BAT as a metabolic furnace to combat obesity, BAT has a critical role in adaptive thermogenesis, is responsive to estrogens, and may contribute to thermoregulatory dysfunction in perimenopause (85, 86). Female rodents and humans exhibit more BAT and higher thermogenic capacity in BAT than males (87, 88). E2 treatment induces thermogenic gene expression in BAT from mice fed a high fat diet (12, 89) or ovariectomized rats (53). ERα is expressed in brown adipocytes (90) and regulates thermogenesis directly within BAT (91, 92). In addition to the direct effect of estrogens on BAT thermogenesis, E2 delivery to the brain increases the sympathetic activation of BAT thermogenesis (53), pointing to a role for central estrogen signaling in the regulation of BAT. In line with these findings, ablation of ERα in the central nervous system reduces BAT activity and metabolic heat production (93). Further studies indicate that the effect of E2 on BAT is mediated by ERα in the VMH. Microinjection of E2 in the VMH, but not the adjacent ARC, inhibits AMP-activated protein kinase (AMPK) and ceramide-induced endoplasmic reticulum stress which in turn increases sympathetic nerve activity in the BAT and increases core body temperature (53, 54). By contrast, inhibition of ERα or activation of AMPK in the VMH suppresses sympathetic BAT activation (53). This AMPK–sympathetic nervous system–BAT pathway may also contribute to the fluctuation of body temperature observed over the estrous cycle (53). Interestingly, activating rat-insulin-promoter neurons in the VMH promotes thermogenesis in white adipose tissue also via AMPK and sympathetic outflow but has no effect on BAT (94), suggesting that white adipose tissue and BAT thermogenesis can be regulated by the VMH. However, whether estrogens employ both of these neural pathways to modulate heat production remains unknown. Notably, AMPK is involved in p53-induced autophagy (95) and we recently uncovered a role for Reprimo (Rprm), another p53-responsive gene, in the regulation of thermogenesis. Silencing Rprm gene function in the VMH increases core temperature and BAT thermogenesis selectively in females and ectopic Rprm expression in males is associated with reduced core temperature (96). It will be interesting to determine whether Rprm or AMPK in the VMH are required for the changes in thermogenesis or body temperature induced by the estrous cycle, ovariectomy, or estrogen treatment.
Estrogen signaling in the hypothalamus may also modulate or interact with other signaling systems to alter heat production. For example, estrogens can increase central leptin sensitivity (97) and leptin signaling in the hypothalamus can alter BAT thermogenesis (98). In the POA, estradiol could induce synthesis of prostaglandin E2 (99), which is a critical mediator of the febrile response (44, 45). Estrogens also increase physical activity (100, 101), which may contribute to elevated heat production during cold exposure, particularly in mice (102). However, recent studies suggest that increased movement and BAT thermogenesis might be separately regulated by distinct estrogen-sensitive neurons (96, 101). Indeed, temperature analyses that correct for changes in movement suggest that motor activity does not fully explain the elevated body temperature observed in mice during estrus, even though activity is increased (103). Similarly, E2 replacement in ovariectomized rats increases heat production regardless of ambient temperature even when the locomotor activity is restricted (74). Therefore, it is unlikely that estrogens employ motor activity as a means to increase heat production, at least at room temperature. Together, these studies point to a molecular axis (ERα–AMPK/Rprm–sympathetic nervous system–BAT) that mediates the effects of estrogens on heat production. Further study is needed to understand how other thermogenic effectors, such as metabolic and muscle shivering thermogenesis, may be integrated in the coordination of estrogen-induced heat production.
Effects of Estrogens on Body Temperature
As discussed above, estrogens modulate both heat dissipation and heat production, 2 key determinants of body temperature balance. However, it is not firmly established how estrogens affect core temperature. Core temperature shows regular fluctuations that coincide with changes in ovarian hormones in humans (104) and mice (103, 105), and female mice exhibit a higher core temperature than males, a difference that disappears after gonadectomy (105). In women, endogenous or exogenous increases in E2 have been shown to reduce core body temperature (76, 77, 106), while some studies show no difference (107). Animal studies find that E2 replacement following ovariectomy may increase (34, 53, 65), have no influence (58, 63, 74, 108), or reduce (59, 75, 109) core body temperature. These conflicting results may be due to the complex interplay between heat dissipation and thermogenesis which are both affected by estrogens. For example, an early study showed that E2 simultaneously increased heat production and heat loss, resulting in increased metabolic heat production but no effect on core body temperature in ovariectomized rats (74). It is unknown whether the concurrent increases in heat production and heat loss are due to compensatory effects or a selective activation of each effector by E2. Indeed, increased BAT thermogenesis can be a compensatory and secondary consequence of an impairment in the control of vasoconstriction (110). However, the existing data generally support the notion that E2 suppresses heat loss and increases heat production, which would synergistically increase but not decrease core body temperature (56, 58, 66, 109). Studies to investigate the interaction between these opposing or overlapping effects of estrogens would help rationalize the apparent conflicts in the literature. In addition, careful dissection of the effects at distinct thermoregulatory regions could clarify the direct and indirect consequences of estrogen treatment that contribute to changes core body temperature.
In addition, ambient temperature is an essential variable that influences the effect of estrogens on body temperature. Vasodilation may predominate when ambient temperature is within or below the thermoneutral point, whereas evaporative heat loss becomes more efficient during heat exposure. Indeed, studies in rats indicate that E2 decreases core body temperature only when ambient temperature is above the thermoneutral point (66, 75, 109). Changes in ambient temperature may recruit temperature-sensitive neurons in the POA (47), an effect that could be mediated through rapid estrogen actions of ERα or ERβ (111). In addition, upon cold exposure, both exogenous and endogenous E2 enhances cFos expression in the MPA and the DMH (52), 2 key sites for cold-defense thermogenesis. Together these lines of evidence suggest that warm- or cold-responsive signals may integrate with estrogens at multiple brain sites to mobilize specific thermal effectors that regulate core body temperature. Therefore, future studies should carefully consider the effects of estrogen manipulations across a range of ambient temperatures and thermoregulatory responses.
Estrogen-Sensitive Neurons and Hot Flashes
Hot flashes are often preceded by a small increase in core temperature and followed by a dip in core temperature due to increased heat loss (112, 113). In line with the crucial role of estrogens in thermoregulation, varying levels of estrogen are highly associated with hot flashes although a direct causational relationship remains uncertain. It is suggested that estrogen withdrawal rather than low estrogen levels determines the onset of hot flashes, as differences in plasma estrogen levels are not detected between menopausal women with and without hot flashes (112) and people with chronic estrogen deficiencies do not experience hot flashes (114). It remains unclear how vasomotor symptoms are precipitated by fluctuating estrogens during the menopausal transition. Pioneering studies in women and rodents have identified that NKB signaling through the NK3 receptor (NK3R) as a potential therapeutic option (reviewed in (8)). Treating rats with an NK3R agonist causes vasodilation at the tail (115). In contrast, ablating NK3R-expressing neurons in the POA reduces tail vasodilation (116). NK3R signaling also modulates tail vasodilation in mice (72) and vasomotor symptoms in women (117, 118). NKB administration in healthy women induces hot flashes with increased skin temperature (117) and NKB receptor antagonists markedly reduce hot flash symptoms (118-120). Current studies are evaluating the specificity of NK3R antagonists as therapeutics. NK3R antagonist treatment can disrupt ovarian function in cycling humans (121). It is unclear if the effects on fertility are due to central or peripheral actions, as NK3R also is expressed in visceral tissues (122). Nevertheless, this exciting breakthrough demonstrates the power and therapeutic potential of neuroendocrine studies in rodents. Despite the promise of treatments that target NKB signaling, emerging evidence suggests that estrogens modulate vasodilation and heat dissipation responses (33, 50, 123). A full understanding of hot flashes will require a dissection and mechanistic interrogation of the effects of estrogens on the neural circuits that maintain temperature homeostasis.
Estrogen-Sensitive Neurons and Torpor
The control of body temperature is energetically costly and a major component of energy homeostasis. Increased thermogenesis during cold exposure consumes up to 60% of total energy expenditure in mice (124). With insufficient food availability, many species cease to defend their body temperature and enter a hypothermic and hypometabolic state known as torpor (125). In the POA, the temperature-sensitive neurons are not only targets for the thermal inputs but also influenced by other “temperature-insensitive” neurons, hormones, and metabolic signals. Single-cell RNA profiling of the POA has revealed that Esr1 transcripts are expressed within many of the neuronal clusters implicated in the control of body temperature and metabolism (27). The overlapping functions of estrogens and the MPA on thermoregulation and energy balance suggest a potential role of ERα positive MPA neurons in the coordination of thermoregulation and metabolism. We recently demonstrated that ablation of ERα neurons in the MPA increased core temperature in female but not male mice (50), suggesting a female-specific effect on basal body temperature. It is unclear if the effect on basal body temperature is due to sex-specific roles of ERα neurons in the MPA or more POA neurons being ablated in females than in males. In complementary studies, chemogenetic activation of ERα MPA neurons drives a hypometabolic state similar to torpor, the natural activity of ERα neurons in the MPA is altered during fasting-induced torpor, and ablating ERα MPA neurons attenuates torpor expression in a sex-dependent manner (50). These results suggest that ERα neurons in the MPA serve as an integrative center for thermoregulation and metabolic homeostasis. Our findings are concordant with other studies showing that deep hypothermia and hypometabolism in torpor or hibernation is controlled by POA neurons in mice (48, 49). Although it is tempting to speculate that POA neurons could be used to induce therapeutic hypothermia, the potential application to species that do not exhibit torpor, including humans, requires further study. Additionally, it is important to note that activating ERα neurons is different from activating ERα signaling and may not reflect the natural effects of estrogens on thermoregulation and metabolic rate. Future studies are required to understand how torpor-regulating ERα neurons and the associated circuitry are sensitive to estrogens or other signals of metabolic reserves.
Strategies to Dissect the Effects of Estrogens on Thermoregulation
There is mounting evidence that estrogen-sensitive areas of the brain regulate distinct aspects of thermoregulation or metabolism; however, the effects of estrogens on these brain regions are not in isolation. Endogenous estrogens are widely distributed and can act at multiple estrogen-sensitive regions simultaneously. Systemic manipulations of E2 signaling may overlook the interactions between these brain regions and the synergistic or compensatory actions of E2. Therefore, dissecting the effects of estrogens on individual regions or circuit connections will be necessary for a better understanding of the modulatory effects of estrogens on thermoregulation. Emerging viral and genetic tools are facilitating circuit-based studies. Here, we outline the most commonly used approaches for projection-specific or multiplexed manipulations and highlight several opportunities for combining these systems neuroscience approaches with endocrine manipulations.
DREADDs (designer receptors exclusively activated by designer drugs) are G protein–coupled receptors that can be activated by the synthetic ligand clozapine-N-oxide (CNO) (126). Depending on the receptor types, a Gq- or Gi-coupled receptor can increase (hM3Dq) or decrease (hM4Di) neuronal excitability. When combined with a double-floxed inverse open reading frame (DIO) DREADDs can specifically target a subpopulation of neurons that express Cre recombinase. Similarly, optogenetic tools employ opsins that control ion channels or proton pumps upon activation by specific wavelengths of light (127). Similar to DREADDs, both excitatory and inhibitory opsins are available for bidirectional modulation of neuronal activity. Because both DREADDs and opsins can be expressed at the axon terminals, local infusion of CNO or light illumination on the projecting fibers enables selective activation of circuit connections. For example, we recently identified differential effects on body temperature by activating distinct projections of ERα neurons from the MPA. By delivering CNO to regions that receive neuronal projections, we found that local activation of projections to the DMH led to a partial decrease in core body temperature, whereas local activation of projections to the ARC recapitulated the effect of systemic CNO delivery on core body temperature (50). Similarly, activating terminals in the POA from kisspeptin neurons in the ARC increases tail skin temperature (72). Alternatively, optogenetic or chemogenetic tools can be used in combination with a retrogradely delivered Cre to selectively manipulate connections within a circuit. By delivering Cre-dependent DREADDs or opsins to one region and retrograde Cre to a region that receives neuronal projections, only the neurons with functional connections will be activated or inhibited. However, this method loses the specificity for neuron types as all the neurons in the 2 regions that form connections will be affected, unless combinatorial genetic tools (eg, Cre and Flp dependence) are used to label neuronal subsets more specifically. In addition, because the same neurons may project to multiple structures, the possible activation of collaterals should be taken into consideration when manipulating regions that receive neuronal projections.
Neuron-specific or projection-selective manipulations can be combined with endocrine or genetic manipulations to reveal the modulatory effects of hormones. For example, ovariectomy or ERα ablation can severely blunt the effects of activating neurons of the ventrolateral subregion of the VMH (101), revealing a permissive role for ERα signaling in output of the associated circuits. On the other hand, estradiol delivered to the brain or locally to a specific hypothalamic region can help to reveal the role of the nucleus within a neural circuit (53, 128). Because thermoregulation is modulated by other estrogen receptors, similar studies to test the effects of ERβ or GPER1 ablation in the context of neuron activation within the thermoregulatory circuitry would be of great interest. The approach of combining circuit manipulations with endocrine or genetic manipulations might also help us unravel opposing effects of estradiol within a circuit. There is evidence that E2 can activate MPA neurons to facilitate heat loss while inhibiting KNDy neurons in the ARC to reduce heat dissipation (Fig. 1). These antagonistic effects could be dissected by multiplexing DREADD with KORD, another type of DREADD that silences neurons using a different ligand, salvinorin B (129). If neurons in the ARC were inhibited via KORD while simultaneously activating the MPA neurons via DREADD, one would predict that this simultaneous inhibition and activation might phenocopy the effects of E2 on skin vasoconstriction. Alternatively, activation or inhibition could be performed in conjunction with estrogen receptor ablation in the ARC, MPA, or other thermoregulatory regions beyond the hypothalamus (eg, nucleus of solitary tract and dorsal raphe nucleus). Eliminating the effects of estrogens specifically in one region while maintaining the effects elsewhere could help unravel the respective contributions of estrogen signaling throughout the thermoregulatory neurocircuitry.
Conclusion
Several estrogen-sensitive regions have been revealed as candidates for mediating the effects of estrogens on body temperature, but evidence suggests that these areas work synergistically, antagonistically, and/or in parallel to modulate whole-body thermoregulation. Whereas estrogens affect body temperature by modulating both heat production and dissipation, many of these effects are mediated specifically or simultaneously via different estrogen-sensitive regions (13). We recommend a systems-level, circuit-based approach to dissect the distinct thermal effectors modulated by estrogens. Further, it is important to note that other hormones, such as leptin, insulin, and thyroid hormone, potently affect metabolism and thermoregulation through these estrogen-sensitive sites. Thus, it is of interest to decipher the potential interactions of estrogens with these hormones and their coordinated effects on temperature and energy balance. Future studies taking a circuit-based approach should also pay heed to connections and crosstalk between the thermoregulation and energy homeostasis systems, particularly as they both exhibit circuit-level modulation by estrogens.
Given the widespread impact of menopausal hot flashes on quality of life, it has become imperative to try to better understand the neural circuits responsible for the physiological effects of estrogens on body temperature. A deeper understanding of the modular and circuit-level consequences of estrogens will help to identify new potential targets for the development of therapeutics that could mimic the thermoregulatory effects of estrogens and avoid the detrimental side effects of hormone therapy.
Acknowledgments
The authors acknowledge support from M. Massa, N. Sandoval, and C. Goodpaster, who provided feedback on the manuscript.
Financial Support: National Institutes of Health grants (R01 AG066821 and R21 CA249338) to S.M.C., an American Heart Association Postdoctoral Fellowship (18POST33960457) to Z.Z., Pilot Awards to Z.Z. and S.M.C. from the Iris Cantor-UCLA Women’s Health Center/UCLA National Center of Excellence in Women’s Health and NIH National Center for Advancing Translational Science (NCATS) UCLA Clinical and Translational Science Institute (CTSI) (UL1TR001881).
Glossary
Abbreviations
- AMPK
adenosine monophosphate–activated protein kinase
- ARC
arcuate nucleus of the hypothalamus
- BAT
brown adipose tissue
- CNO
clozapine-N-oxide
- DMH
dorsomedial nucleus of the hypothalamus
- DREADD
designer receptor exclusively activated by designer drugs
- E2
17β-estradiol
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- GPER1
G protein-coupled estrogen receptor
- KNDy
kisspeptin/neurokinin B/dynorphin
- MnPO
median preoptic nucleus
- MPA
medial preoptic area
- NK3R
NK3 receptor
- NKB
neurokinin B
- POA
preoptic area
- RPa
raphe pallidus
- Ttail
tail skin temperature
- VMH
ventromedial nucleus of the hypothalamus
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21(9):924-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stearns V, Ullmer L, López JF, Smith Y, Isaacs C, Hayes D. Hot flushes. Lancet. 2002;360(9348):1851-1861. [DOI] [PubMed] [Google Scholar]
- 3. Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collaborative Group on Epidemiological Studies of Ovarian C. Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. The Lancet. 2015;385(9980):1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ayers B, Smith M, Hellier J, Mann E, Hunter MS. Effectiveness of group and self-help cognitive behavior therapy in reducing problematic menopausal hot flushes and night sweats (MENOS 2): a randomized controlled trial. Menopause. 2012;19(7):749-759. [DOI] [PubMed] [Google Scholar]
- 6. Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med. 2014;174(7):1058-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franco OH, Chowdhury R, Troup J, et al. Use of plant-based therapies and menopausal symptoms: a systematic review and meta-analysis. JAMA. 2016;315(23):2554-2563. [DOI] [PubMed] [Google Scholar]
- 8. Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. López M, Tena-Sempere M. Estrogens and the control of energy homeostasis: a brain perspective. Trends Endocrinol Metab. 2015;26(8):411-421. [DOI] [PubMed] [Google Scholar]
- 10. Madden CJ, Morrison SF. Central nervous system circuits that control body temperature. Neurosci Lett. 2019;696:225-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan CL, Knight ZA. Regulation of body temperature by the nervous system. Neuron. 2018;98(1):31-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu Y, López M. Central regulation of energy metabolism by estrogens. Mol Metab. 2018;15:104-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwabe MR, Taxier LR, Frick KM. It takes a neural village: circuit-based approaches for estrogenic regulation of episodic memory. Front Neuroendocrinol. 2020;59:100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hojo Y, Kawato S. Neurosteroids in adult hippocampus of male and female rodents: biosynthesis and actions of sex steroids. Front Endocrinol (Lausanne). 2018;9:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21(1):1-56. [DOI] [PubMed] [Google Scholar]
- 16. Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM. Brain aromatase is neuroprotective. J Neurobiol. 2001;47(4):318-329. [DOI] [PubMed] [Google Scholar]
- 17. Jensen EV. On the mechanism of estrogen action. Perspect Biol Med. 1962;6:47-59. [DOI] [PubMed] [Google Scholar]
- 18. Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93(12):5925-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153(7):2953-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20(3):279-307. [DOI] [PubMed] [Google Scholar]
- 21. Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276(40):36869-36872. [DOI] [PubMed] [Google Scholar]
- 22. Micevych PE, Mermelstein PG, Sinchak K. Estradiol Membrane-Initiated Signaling in the Brain Mediates Reproduction. Trends Neurosci. 2017;40(11):654-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29(5):241-249. [DOI] [PubMed] [Google Scholar]
- 24. Stincic TL, Rønnekleiv OK, Kelly MJ. Diverse actions of estradiol on anorexigenic and orexigenic hypothalamic arcuate neurons. Horm Behav. 2018;104:146-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507-525. [DOI] [PubMed] [Google Scholar]
- 26. Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473(2):270-291. [DOI] [PubMed] [Google Scholar]
- 27. Moffitt JR, Bambah-Mukku D, Eichhorn SW, et al. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science. 2018;362(6416):eaau5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roesch DM. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav. 2006;87(1):39-44. [DOI] [PubMed] [Google Scholar]
- 29. Ohlsson C, Hellberg N, Parini P, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun. 2000;278(3):640-645. [DOI] [PubMed] [Google Scholar]
- 30. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frank A, Brown LM, Clegg DJ. The role of hypothalamic estrogen receptors in metabolic regulation. Front Neuroendocrinol. 2014;35(4):550-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Opas EE, Scafonas A, Nantermet PV, et al. Control of rat tail skin temperature regulation by estrogen receptor-beta selective ligand. Maturitas. 2009;64(1):46-51. [DOI] [PubMed] [Google Scholar]
- 33. Fleischer AW, Schalk JC, Wetzel EA, et al. Long-term oral administration of a novel estrogen receptor beta agonist enhances memory and alleviates drug-induced vasodilation in young ovariectomized mice. Horm Behav. 2021;130:104948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roepke TA, Bosch MA, Rick EA, et al. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology. 2010;151(10):4926-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boulant JA, Dean JB. Temperature receptors in the central nervous system. Annu Rev Physiol. 1986;48:639-654. [DOI] [PubMed] [Google Scholar]
- 36. Blessing W, McAllen R, McKinley M. Control of the cutaneous circulation by the central nervous system. Compr Physiol. 2016;6(3):1161-1197. [DOI] [PubMed] [Google Scholar]
- 37. Contreras C, Nogueiras R, Diéguez C, Rahmouni K, López M. Traveling from the hypothalamus to the adipose tissue: the thermogenic pathway. Redox Biol. 2017;12:854-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Caron A, Richard D. Neuronal systems and circuits involved in the control of food intake and adaptive thermogenesis. Ann N Y Acad Sci. 2017;1391(1):35-53. [DOI] [PubMed] [Google Scholar]
- 39. McAllen RM. Preoptic thermoregulatory mechanisms in detail. Am J Physiol Regul Integr Comp Physiol. 2004;287(2):R272-R273. [DOI] [PubMed] [Google Scholar]
- 40. Tan CL, Cooke EK, Leib DE, et al. Warm-Sensitive Neurons that Control Body Temperature. Cell. 2016;167(1):47-59.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008;11(1):62-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang WZ, Du X, Zhang W, et al. Parabrachial neuron types categorically encode thermoregulation variables during heat defense. Sci Adv. 2020;6(36):eabb9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu S, François M, Huesing C, Münzberg H. The hypothalamic preoptic area and body weight control. Neuroendocrinology. 2018;106(2):187-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lazarus M, Yoshida K, Coppari R, et al. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10(9):1131-1133. [DOI] [PubMed] [Google Scholar]
- 45. Machado NLS, Bandaru SS, Abbott SBG, Saper CB. EP3R-expressing glutamatergic preoptic neurons mediate inflammatory fever. J Neurosci. 2020;40(12):2573-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Krueger JM, Takahashi S. Thermoregulation and sleep. Closely linked but separable. Ann N Y Acad Sci. 1997;813:281-286. [DOI] [PubMed] [Google Scholar]
- 47. Silva NL, Boulant JA. Effects of testosterone, estradiol, and temperature on neurons in preoptic tissue slices. Am J Physiol. 1986;250(4 Pt 2):R625-R632. [DOI] [PubMed] [Google Scholar]
- 48. Hrvatin S, Sun S, Wilcox OF, et al. Neurons that regulate mouse torpor. Nature. 2020;583(7814):115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takahashi TM, Sunagawa GA, Soya S, et al. A discrete neuronal circuit induces a hibernation-like state in rodents. Nature. 2020;583(7814):109-114. [DOI] [PubMed] [Google Scholar]
- 50. Zhang Z, Reis FMCV, He Y, et al. Estrogen-sensitive medial preoptic area neurons coordinate torpor in mice. Nat Commun. 2020;11(1):6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hori T, Nakashima T, Koga H, Kiyohara T, Inoue T. Convergence of thermal, osmotic and cardiovascular signals on preoptic and anterior hypothalamic neurons in the rat. Brain Res Bull. 1988;20(6):879-885. [DOI] [PubMed] [Google Scholar]
- 52. Uchida Y, Kano M, Yasuhara S, Kobayashi A, Tokizawa K, Nagashima K. Estrogen modulates central and peripheral responses to cold in female rats. J Physiol Sci. 2010;60(2):151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20(1):41-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. González-García I, Contreras C, Estévez-Salguero Á, et al. Estradiol regulates energy balance by ameliorating hypothalamic ceramide-induced ER stress. Cell Rep. 2018;25(2):413-423.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686-3692. [DOI] [PubMed] [Google Scholar]
- 56. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci U S A. 2012;109(48):19846-19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ootsuka Y, Tanaka M. Control of cutaneous blood flow by central nervous system. Temperature (Austin). 2015;2(3):392-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Williams H, Dacks PA, Rance NE. An improved method for recording tail skin temperature in the rat reveals changes during the estrous cycle and effects of ovarian steroids. Endocrinology. 2010;151(11):5389-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Krajewski-Hall SJ, Blackmore EM, McMinn JR, Rance NE. Estradiol alters body temperature regulation in the female mouse. Temperature (Austin). 2018;5(1):56-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Berendsen HH, Weekers AH, Kloosterboer HJ. Effect of tibolone and raloxifene on the tail temperature of oestrogen-deficient rats. Eur J Pharmacol. 2001;419(1):47-54. [DOI] [PubMed] [Google Scholar]
- 61. Kobayashi T, Tamura M, Hayashi M, et al. Elevation of tail skin temperature in ovariectomized rats in relation to menopausal hot flushes. Am J Physiol Regul Integr Comp Physiol. 2000;278(4):R863-R869. [DOI] [PubMed] [Google Scholar]
- 62. Opas EE, Rutledge SJ, Vogel RL, Rodan GA, Schmidt A. Rat tail skin temperature regulation by estrogen, phytoestrogens and tamoxifen. Maturitas. 2004;48(4):463-471. [DOI] [PubMed] [Google Scholar]
- 63. Zhao L, Mao Z, Schneider LS, Brinton RD. Estrogen receptor β-selective phytoestrogenic formulation prevents physical and neurological changes in a preclinical model of human menopause. Menopause. 2011;18(10):1131-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Opas EE, Gentile MA, Kimmel DB, Rodan GA, Schmidt A. Estrogenic control of thermoregulation in ERalphaKO and ERbetaKO mice. Maturitas. 2006;53(2):210-216. [DOI] [PubMed] [Google Scholar]
- 65. Hosono T, Chen XM, Zhang YH, Kanosue K. Effects of estrogen on thermoregulatory responses in freely moving female rats. Ann N Y Acad Sci. 1997;813:207-210. [DOI] [PubMed] [Google Scholar]
- 66. Dacks PA, Krajewski SJ, Rance NE. Ambient temperature and 17β-estradiol modify Fos immunoreactivity in the median preoptic nucleus, a putative regulator of skin vasomotion. Endocrinology. 2011;152(7):2750-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76-95. [DOI] [PubMed] [Google Scholar]
- 68. Mufson EJ, Cai WJ, Jaffar S, et al. Estrogen receptor immunoreactivity within subregions of the rat forebrain: neuronal distribution and association with perikarya containing choline acetyltransferase. Brain Res. 1999;849(1-2):253-274. [DOI] [PubMed] [Google Scholar]
- 69. Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166(2):680-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147(3):1154-1158. [DOI] [PubMed] [Google Scholar]
- 71. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Padilla SL, Johnson CW, Barker FD, Patterson MA, Palmiter RD. A neural circuit underlying the generation of hot flushes. Cell Rep. 2018;24(2):271-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tanaka M, McKinley MJ, McAllen RM. Preoptic-raphé connections for thermoregulatory vasomotor control. J Neurosci. 2011;31(13):5078-5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Laudenslager ML, Wilkinson CW, Carlisle HJ, Hammel HT. Energy balance in ovariectomized rats with and without estrogen replacement. Am J Physiol. 1980;238(5):R400-R405. [DOI] [PubMed] [Google Scholar]
- 75. Baker MA, Dawson DD, Peters CE, Walker AM. Effects of estrogen on thermoregulatory evaporation in rats exposed to heat. Am J Physiol. 1994;267(3 Pt 2):R673-R677. [DOI] [PubMed] [Google Scholar]
- 76. Stephenson LA, Kolka MA. Esophageal temperature threshold for sweating decreases before ovulation in premenopausal women. J Appl Physiol (1985). 1999;86(1):22-28. [DOI] [PubMed] [Google Scholar]
- 77. Tankersley CG, Nicholas WC, Deaver DR, Mikita D, Kenney WL. Estrogen replacement in middle-aged women: thermoregulatory responses to exercise in the heat. J Appl Physiol (1985). 1992;73(4):1238-1245. [DOI] [PubMed] [Google Scholar]
- 78. Farrell MJ, Trevaks D, McAllen RM. Preoptic activation and connectivity during thermal sweating in humans. Temperature (Austin). 2014;1(2):135-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shafton AD, McAllen RM. Location of cat brain stem neurons that drive sweating. Am J Physiol Regul Integr Comp Physiol. 2013;304(10):R804-R809. [DOI] [PubMed] [Google Scholar]
- 80. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277-359. [DOI] [PubMed] [Google Scholar]
- 81. Ponrartana S, Hu HH, Gilsanz V. On the relevance of brown adipose tissue in children. Ann N Y Acad Sci. 2013;1302:24-29. [DOI] [PubMed] [Google Scholar]
- 82. Carpentier AC, Blondin DP, Virtanen KA, Richard D, Haman F, Turcotte ÉE. Brown adipose tissue energy metabolism in humans. Front Endocrinol (Lausanne). 2018;9:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58(7):1526-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Labbé SM, Caron A, Lanfray D, Monge-Rofarello B, Bartness TJ, Richard D. Hypothalamic control of brown adipose tissue thermogenesis. Front Syst Neurosci. 2015;9:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Richard MA, Pallubinsky H, Blondin DP. Functional characterization of human brown adipose tissue metabolism. Biochem J. 2020;477(7):1261-1286. [DOI] [PubMed] [Google Scholar]
- 86. Frank AP, Palmer BF, Clegg DJ. Do estrogens enhance activation of brown and beiging of adipose tissues? Physiol Behav. 2018;187:24-31. [DOI] [PubMed] [Google Scholar]
- 87. Rodriguez-Cuenca S, Pujol E, Justo R, et al. Sex-dependent thermogenesis, differences in mitochondrial morphology and function, and adrenergic response in brown adipose tissue. J Biol Chem. 2002;277(45):42958-42963. [DOI] [PubMed] [Google Scholar]
- 88. Pfannenberg C, Werner MK, Ripkens S, et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59(7):1789-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Al-Qahtani SM, Bryzgalova G, Valladolid-Acebes I, et al. 17β-Estradiol suppresses visceral adipogenesis and activates brown adipose tissue-specific gene expression. Horm Mol Biol Clin Investig. 2017;29(1):13-26. [DOI] [PubMed] [Google Scholar]
- 90. Rodriguez-Cuenca S, Monjo M, Frontera M, Gianotti M, Proenza AM, Roca P. Sex steroid receptor expression profile in brown adipose tissue. Effects of hormonal status. Cell Physiol Biochem. 2007;20(6):877-886. [DOI] [PubMed] [Google Scholar]
- 91. Rodríguez-Cuenca S, Monjo M, Gianotti M, Proenza AM, Roca P. Expression of mitochondrial biogenesis-signaling factors in brown adipocytes is influenced specifically by 17beta-estradiol, testosterone, and progesterone. Am J Physiol Endocrinol Metab. 2007;292(1):E340-E346. [DOI] [PubMed] [Google Scholar]
- 92. Zhou Z, Moore TM, Drew BG, et al. Estrogen receptor alpha controls metabolism in white and brown adipocytes by regulating Polg1 and mitochondrial remodeling. Sci Transl Med. 2020;12(555):eaax8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Xu Y, Nedungadi TP, Zhu L, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang B, Li A, Li X, et al. Activation of hypothalamic RIP-Cre neurons promotes beiging of WAT via sympathetic nervous system. EMBO Rep. 2018;19(4):e44977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jing K, Song KS, Shin S, et al. Docosahexaenoic acid induces autophagy through p53/AMPK/mTOR signaling and promotes apoptosis in human cancer cells harboring wild-type p53. Autophagy. 2011;7(11):1348-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. van Veen JE, Kammel LG, Bunda PC, et al. Hypothalamic estrogen receptor alpha establishes a sexually dimorphic regulatory node of energy expenditure. Nat Metab. 2020;2(4):351-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55(4):978-987. [DOI] [PubMed] [Google Scholar]
- 98. Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31(34):12189-12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7(6):643-650. [DOI] [PubMed] [Google Scholar]
- 100. Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144(1):230-239. [DOI] [PubMed] [Google Scholar]
- 101. Correa SM, Newstrom DW, Warne JP, et al. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep. 2015;10(1):62-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gordon CJ. Temperature Regulation in Laboratory Rodents. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- 103. Weinert D, Waterhouse J, Nevill A. Changes of body temperature and thermoregulation in the course of the ovarian cycle in laboratory mice. Biol Rhythm Res. 2004;35(3):171-185. [Google Scholar]
- 104. Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8(6):613-622. [DOI] [PubMed] [Google Scholar]
- 105. Sanchez-Alavez M, Alboni S, Conti B. Sex- and age-specific differences in core body temperature of C57Bl/6 mice. Age (Dordr). 2011;33(1):89-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Brooks EM, Morgan AL, Pierzga JM, et al. Chronic hormone replacement therapy alters thermoregulatory and vasomotor function in postmenopausal women. J Appl Physiol (1985). 1997;83(2):477-484. [DOI] [PubMed] [Google Scholar]
- 107. Freedman RR, Blacker CM. Estrogen raises the sweating threshold in postmenopausal women with hot flashes. Fertil Steril. 2002;77(3):487-490. [DOI] [PubMed] [Google Scholar]
- 108. Hosono T, Chen XM, Miyatsuji A, et al. Effects of estrogen on thermoregulatory tail vasomotion and heat-escape behavior in freely moving female rats. Am J Physiol Regul Integr Comp Physiol. 2001;280(5):R1341-R1347. [DOI] [PubMed] [Google Scholar]
- 109. Dacks PA, Rance NE. Effects of estradiol on the thermoneutral zone and core temperature in ovariectomized rats. Endocrinology. 2010;151(3):1187-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Warner A, Rahman A, Solsjö P, et al. Inappropriate heat dissipation ignites brown fat thermogenesis in mice with a mutant thyroid hormone receptor α1. Proc Natl Acad Sci U S A. 2013;110(40):16241-16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Abrahám IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145(7):3055-3061. [DOI] [PubMed] [Google Scholar]
- 112. Freedman RR, Norton D, Woodward S, Cornélissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metab. 1995;80(8):2354-2358. [DOI] [PubMed] [Google Scholar]
- 113. Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertil Steril. 1998;70(2):332-337. [DOI] [PubMed] [Google Scholar]
- 114. Kronenberg F. Hot flashes: phenomenology, quality of life, and search for treatment options. Exp Gerontol. 1994;29(3-4):319-336. [DOI] [PubMed] [Google Scholar]
- 115. Dacks PA, Krajewski SJ, Rance NE. Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology. 2011;152(12):4894-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mittelman-Smith MA, Krajewski-Hall SJ, McMullen NT, Rance NE. Neurokinin 3 receptor-expressing neurons in the median preoptic nucleus modulate heat-dissipation effectors in the female rat. Endocrinology. 2015;156(7):2552-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jayasena CN, Comninos AN, Stefanopoulou E, et al. Neurokinin B administration induces hot flushes in women. Sci Rep. 2015;5:8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Prague JK, Roberts RE, Comninos AN, et al. Neurokinin 3 receptor antagonism rapidly improves vasomotor symptoms with sustained duration of action. Menopause. 2018;25(8):862-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Skorupskaite K, George JT, Veldhuis JD, Millar RP, Anderson RA. Neurokinin 3 Receptor Antagonism Reveals Roles for Neurokinin B in the Regulation of Gonadotropin Secretion and Hot Flashes in Postmenopausal Women. Neuroendocrinology. 2018;106(2):148-157. [DOI] [PubMed] [Google Scholar]
- 120. Depypere H, Timmerman D, Donders G, et al. Clinical evaluation of the NK3 receptor antagonist fezolinetant (a.k.a. ESN364) for the treatment of menopausal hot flashes. Maturitas. 2017;103:89-90. [Google Scholar]
- 121. Skorupskaite K, George JT, Veldhuis JD, Anderson RA. Neurokinin B regulates gonadotropin secretion, ovarian follicle growth, and the timing of ovulation in healthy Women. J Clin Endocrinol Metab. 2018;103(1):95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Szeliga A, Czyzyk A, Podfigurna A, Genazzani AR, Genazzani AD, Meczekalski B. The role of kisspeptin/neurokinin B/dynorphin neurons in pathomechanism of vasomotor symptoms in postmenopausal women: from physiology to potential therapeutic applications. Gynecol Endocrinol. 2018;34(11):913-919. [DOI] [PubMed] [Google Scholar]
- 123. Fredette NC, Meyer MR, Prossnitz ER. Role of GPER in estrogen-dependent nitric oxide formation and vasodilation. J Steroid Biochem Mol Biol. 2018;176:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Abreu-Vieira G, Xiao C, Gavrilova O, Reitman ML. Integration of body temperature into the analysis of energy expenditure in the mouse. Mol Metab. 2015;4(6):461-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ruf T, Geiser F. Daily torpor and hibernation in birds and mammals. Biol Rev Camb Philos Soc. 2015;90(3):891-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Roth BL. DREADDs for neuroscientists. Neuron. 2016;89(4):683-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71(1):9-34. [DOI] [PubMed] [Google Scholar]
- 128. Zhang Z, Liu J, Veldhuis-Vlug AG, et al. Effects of chronic estrogen administration in the ventromedial nucleus of the hypothalamus (VMH) on fat and bone metabolism in ovariectomized rats. Endocrinology. 2016;157(12):4930-4942. [DOI] [PubMed] [Google Scholar]
- 129. Vardy E, Robinson JE, Li C, et al. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron. 2015;86(4):936-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Krajewski-Hall SJ, Miranda Dos Santos F, McMullen NT, Blackmore EM, Rance NE. Glutamatergic neurokinin 3 receptor neurons in the median preoptic nucleus modulate heat-defense pathways in Female mice. Endocrinology. 2019;160(4):803-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Prague JK, Roberts RE, Comninos AN, et al. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10081):1809-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Fahrbach SE, Morrell JI, Pfaff DW. Studies of ventromedial hypothalamic afferents in the rat using three methods of HRP application. Exp Brain Res. 1989;77(2):221-233. [DOI] [PubMed] [Google Scholar]
- 133. Imai-Matsumura K, Matsumura K, Nakayama T. Involvement of ventromedial hypothalamus in brown adipose tissue thermogenesis induced by preoptic cooling in rats. Jpn J Physiol. 1984;34(5):939-943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.