Abstract
Breast cancer is a highly heterogeneous disease, encompassing many subtypes that have distinct origins, behaviors, and prognoses. Although traditionally seen as a genetic disease, breast cancer is now also known to involve epigenetic abnormalities. Epigenetic regulators, such as DNA methyltransferases and histone-modifying enzymes, play essential roles in gene regulation and cancer development. Dysregulation of epigenetic regulator activity has been causally linked with breast cancer pathogenesis. Hairless (HR) encodes a 130-kDa transcription factor that is essential for development and tissue homeostasis. Its role in transcription regulation is partly mediated by its interaction with multiple nuclear receptors, including thyroid hormone receptor, retinoic acid receptor-related orphan receptors, and vitamin D receptor. HR has been studied primarily in epidermal development and homeostasis. Hr-mutant mice are highly susceptible to ultraviolet- or carcinogen-induced skin tumors. Besides its putative tumor suppressor function in skin, loss of HR function has also been implicated in increased leukemia susceptibility and promotes the growth of melanoma and brain cancer cells. HR has also been demonstrated to function as a histone H3 lysine 9 demethylase. Recent genomics studies have identified HR mutations in a variety of human cancers, including breast cancer. The anticancer function and mechanism of action by HR in mammary tissue remains to be investigated. Here, we review the emerging role of HR, its histone demethylase activity and histone methylation in breast cancer development, and potential for epigenetic therapy.
Keywords: breast cancer, histone methylation, histone demethylase, hairless, oncogenesis
According to the latest data from the International Agency for Research on Cancer, breast cancer surpassed lung cancer in 2020 to become the most commonly diagnosed cancer worldwide. In the United States alone, approximately 42 000 women die from breast cancer each year (1). Despite decades of intense research, significant gaps exist in our understanding of breast cancer etiology and associated genetic and epigenetic defects, which hinders breast cancer treatment progress (2-4). This is partly because breast cancer is a highly heterogeneous disease, encompassing many subtypes that have distinct origins, behaviors, and prognoses (5). Four major subtypes of breast cancer have been described, including basal-like, luminal A, luminal B, and HER2+ breast cancer, while additional subtypes may exist (6, 7). These subtypes differ significantly in their origins, behavior, and prognoses (8), underscoring the importance of precision medicine in guiding individualized treatment to improve patient survival and quality of life.
Cancer genetics and genomics studies have identified many high-risk breast cancer–predisposing genes among familial breast cancer cases such as BRCA1 and BRCA2 and medium- to low-risk genes such as CHEK2, ATM, PALB2, BRIP1, TP53, PTEN, CDH1, and MLL3 (9, 10). However, only about 10% of breast cancer patients have a clear family history that can be linked to known pathogenic mutations in these predisposition genes, whereas the majority of breast cancers are thought to occur sporadically with undefined causes (9). A major challenge facing breast cancer precision medicine is the lack of comprehensive understanding of breast cancer–associated genetic and nongenetic factors. Additional etiological factors that promote the initiation, growth, and progression both of familial and sporadic breast cancers remain to be identified. Such knowledge will enable targeted therapies directed at the specific mechanisms underpinning each breast cancer subtype.
Breast cancer research has focused extensively on genetic alterations. There is growing interest and emphasis on elucidating the role of epigenetic alterations in breast cancer, which may provide new mechanistic insights into breast cancer pathogenesis (11). Genetic mutations affecting the enzymatic activity of epigenetic regulators, such as DNA methyltransferases and histone-modifying enzymes, have been linked with cancer and other developmental disorders (12-14). Epigenetic regulators also modulate the interaction between genes and the environment to influence disease pathogenesis, thus carrying a great potential for addressing the missing heritability of breast cancers with environmental origins. More important, epigenetic alterations are often reversible, providing unique opportunities for cancer epigenetic therapies. Indeed, preclinical and clinical testing of inhibitors of epigenetic regulators demonstrates that such epigenetic drugs are effective when used alone or in combination with other therapies (15-21). However, mechanistic insights concerning the cause or consequence of epigenetic alterations in cancer are still limited. More studies are needed to determine how epigenetic regulators contribute to breast cancer development to understand the role of epigenetic abnormalities in breast cancer.
Here, we will review the mounting evidence supporting a versatile role of histone methylation in breast cancer development. We will discuss the function of the hairless (HR) gene, which encodes an epigenetic regulator with histone H3 lysine 9 (H3K9) demethylase activity, and its transcriptional regulatory and tumor suppressive activities in breast cancer. We will also discuss the therapeutic potential of targeting histone methylation as a new epigenetic therapy for breast cancer treatment.
Epigenetic Regulators and Histone Methylation in Cancer Development
Eukaryotic DNA is packaged into chromatin. The repeating unit of chromatin is the nucleosome, which contains 145 to 147 base pairs of DNA wrapped around core histones (H2A, H2B, H3, and H4). Chromatin is further condensed into high-order chromosomes, a process that is believed to impede gene transcription by default. Genes located within highly condensed chromatin regions are often transcriptionally inactive and require chromatin remodeling to alter chromatin conformation and facilitate gene activation (22). The mammalian genome encodes multiple epigenetic regulators, including DNA methyltransferases and histone-modifying enzymes, which modulate gene expression epigenetically by altering the chromatin conformation to either promote or suppress gene transcription (23, 24).
Histone C-terminal tails often undergo posttranslational modifications that can dynamically influence chromatin conformation and gene transcription, DNA replication, and repair (13, 25). Posttranslational modification of histones by acetylation or methylation plays an essential role in development and tissue homeostasis (13, 26). Acetylation of lysine residues in core histones produces a negative charge, resulting in a less tight interaction between DNA and histones to facilitate transcription. Histone methylation, on the other hand, does not alter the overall charge of the affected residues. Instead, histone methylation marks are recognized by a variety of effector protein factors (reader proteins) to modulate gene expression and activity.
Histone methylation usually occurs on basic residues including arginines, lysines, and histidines, although histidine methylation is thought to be rare and has not been well characterized compared with lysine or arginine methylation (27). Lysine and arginine residues both contain amino groups that confer basic and hydrophobic characteristics. Lysine can be monomethylated (me1), dimethylated (me2), or trimethylated (me3) with a methyl group replacing each hydrogen of its ε-NH3+ group. On the other hand, arginine can be monomethylated or dimethylated on its NH2+ group (28). Arginine methylation is catalyzed by protein arginine methyltransferase, while lysine methylation involves specific lysine methyltransferases (KMT) that often contain an evolutionarily conserved SET domain (29). The most extensively studied histone lysine methylation sites include H3 lysine 4 (H3K4), H3K9, H3K27, H3K36, H3K79, and H4K20 (30). The different forms and sites of histone lysine methylation occur in spatiotemporal-specific manners, which exert diverse effects on gene regulation in context-dependent manners (31).
Unlike the relatively straightforward functional relationship between histone acetylation and gene activation, the role of histone methylation in gene regulation is complex. Some individual methylation modifications can have different roles (activating or silencing) depending on the genetic context (31-33). H3K9me1, for example, is found to be associated with both actively transcribed and repressed genes (7, 31); whereas H3K9me2 and H3K9me3 are often associated with transcriptional repression or heterochromatin (13, 31, 34). It is possible that histone methylation serves as the chromatin marks, which influence genome organization and gene activity in the presence of other reader proteins including transcription factors. Readers of lysine methylation and epigenetic changes interpret the modifications in a context-dependent fashion, and binding of histone readers to their target posttranslational modifications is influenced by interactions with the surrounding residues (35, 36).
Based on the number of total lysines in the core histones H3 and H4, which can each exist in unmethylated, monomethylated, demethylated, or trimethylated forms, there are potentially billions of possible lysine methylation patterns in the human genome. The functional implications of such complex histone methylation patterns in development and human diseases are poorly understood. Extensive efforts are required to elucidate the importance of highly conserved patterns of histone modifications. Based on the prevalence of lysine methylation and its role in gene regulation, it is predictable that genetic mutations affecting the genes encoding the enzymes regulating lysine methylation have a profound impact on development and disease pathogenesis (12, 14, 37, 38).
Histone H3 Lysine 9 Methyltransferases and Demethylases
Several H3K9 methyltransferases have been identified in mammalian cells, including SUV39H1/H2 (KMT1A/1B), G9A (KMT1C), G9A-like protein (GLP, or KMT1D), SETDB1/2 (KMT1E/1F), and PR domain containing protein family members (PRDM3/16). SUV39H1/H2 catalyze H3K9me2/me3, which are often found in heterochromatin or transcriptionally silent regions (39). SETDB1 catalyzes H3K9me1 at the pericentromeric region and provides a substrate for SUV39H1/H2 to produce H3K9me3 (40). The heterodimer of G9A and GLP is the major KMT that catalyzes H3K9me1/me2 in euchromatin regions.(41) In vitro studies suggest that G9A or GLP homodimers also exhibit H3K9me1/me2 activity (42). PRDM3/16 are known to exhibit H3K9me1 activity (43), but whether PRDM protein-mediated H3K9 methylation is direct or indirect awaits further experimental verification.
Previously, lysine methylation was considered to be a permanent and irreversible modification due to the high thermodynamic stability of the N-CH3 bond (25). However, this view has changed since the discovery of histone demethylase enzymes. Dozens of lysine demethylases (KDMs) have been reported to date (44-46) that are classified into 2 main groups: the amine-oxidase type lysine-specific demethylases (LSDs) and the highly conserved Jumonji C (JmjC) domain–containing histone KDMs. LSD1 and LSD2 (KDM1A/B) are flavin adenine dinucleotide (FAD)-dependent amine oxidases that can demethylate monomethylated or dimethylated H3K4/K9. The JmjC KDMs, on the other hand, can remove methyl groups from all 3 lysine methylation states. The human genome encodes more than 30 JmjC KDMs, including KDM3 (JHDM2), KDM4 (JHDM3), KDM6a (UTX), and KDM7A/B (KIAA1718/PHF8). Three KDM3 family proteins (KDM3A-C) can demethylate H3K9me1/me2 and regulate hormone-dependent transcriptional activation (47, 48). KDM4 family proteins can demethylate H3K9me2 and H3K9me3 in addition to H3K36me2 and H3K36me3 (47, 48). KIAA1718 and PHF8 both harbor a plant homeodomain that binds H3K4me3 and demethylates either H3K9me2 or H3K27me2. The presence of H3K4me3 on the same peptide as H3K9me2 makes the doubly methylated peptide a better substrate of PHF8 while diminishing the H3K9me2 demethylase activity of KIAA1718 without adversely affecting its H3K27me2 activity (49). PHF8 depletion also leads to increased H4K20me1/H3K9me1 at transcription start sites and H3K9me2 at nontranscription start sites, respectively, suggesting differential substrate specificities at different target locations.
KMTs and KDMs have both been implicated in oncogenesis. These enzymes may be either overexpressed or inactivated in different cancer types (50, 51). Genetic mutations affecting the enzymatic activity of KMTs or KDMs have been linked with cancer and other developmental disorders (12-14). The effect of alteration in each specific KMT/KDM depends on the type of cancer involved. As the first KDM identified, LSD1 has been extensively studied. It can both activate and repress gene expression. Similarly, LSD1 can exhibit either protumor or antitumor activity in breast cancer development (52, 53), highlighting a context-dependent role in regulating different biological processes possibly by using different functional domains. The potential role of different KMTs and KDMs in breast cancer is summarized in Table 1 based on previous studies detailed in Cheng and colleagues’ 2019 review (51).
Table 1.
Tumor-promoting or -suppressing function of lysine methyltransferases and lysine demethylases that is implicated in breast cancer development
| Promoters | Suppressors | Dual |
|---|---|---|
| KMT1C(G9A) (54) KMT2B(MLL2) (55) KMT2D(MLL4) (56) KMT3C (SMYD2) KMT3E (SMYD3) (57) KMT4 (DOT1L) (58) KMT5A (SET8) (59) KMT7 (SET7) (60, 61) EZH1 (KIAA0388) (62) |
KMT2C (MLL3) KMT5C (SUV4-20H2) (63) KMT7 (SET7) (60) |
KMT1A(SUV39H1) (64, 65) KMT1E(SETDB1) (66) |
| KDM1B (LSD2) (67) KDM2A (68) KDM2B (69) KDM3A (70) KDM4B (71) KDM4C (72) KDM5A (JARID1A) (73) KDM6A (UTX) (56) KDM6B (74) KDM8 (75, 76) KDM9 (77) |
HR (56) | KDM1A (LSD1) (78, 79) KDM4A (80) |
Abbreviations: HR, hairless; KDM, lysine demethylases; KMT, lysine methyltransferases.
Furthermore, genomic histone methylation levels have been associated with clinical outcomes and progression of cancers (50). For example, immunohistochemical analyses have shown that low levels of H3Kme2 are associated with decreased survival in lung and renal carcinoma (81). Other cancers with altered levels of global histone modifications include prostate (82), lung (81), kidney (81), pancreas (83), hepatocellular carcinoma (84), and breast (85), among others. Different cancers can have opposite alterations in histone modifications; H3K9me3 is increased in gastric adenocarcinomas but decreased in prostate cancer compared to normal tissue (50), which highlights the complex function of histone methylation in cancer development. It has been proposed that the patterns of histone methylation may provide prognostic information. In addition to the global level of histone methylation and alterations in KMT and KDM activity, reader proteins of histone lysine methylation (effector proteins) have also been implicated in the development of various cancers (50), which will not be discussed in this review.
Transcriptional Regulation by Hairless
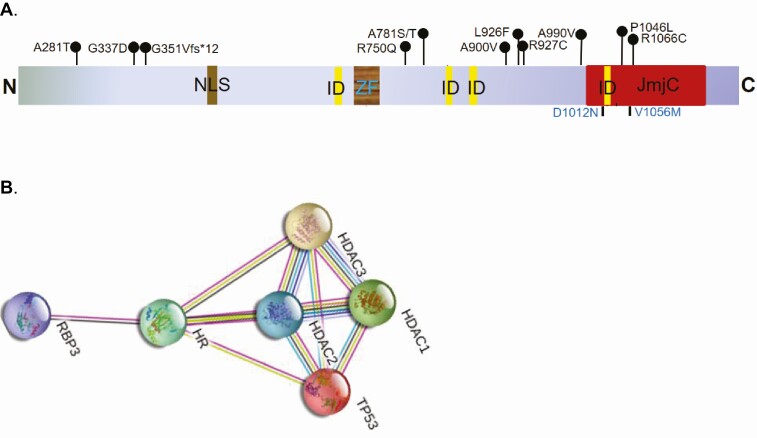
The HR gene encodes a transcription factor that regulates multiple pathways involved in cell proliferation, apoptosis, and inflammation. The HR gene structure and function are highly conserved between human, rat, and mouse, containing a nuclear localization signal and a zinc finger domain for DNA binding (Figure 1A) (86, 87). HR is essential for normal skin development and hair follicle cycling (86, 88). Humans and rodents with mutations in HR both suffer from congenital hair loss and epidermal abnormalities (89, 90). Mice with loss-of-function Hr mutations develop irreversible hair loss around postnatal day 18, followed by epidermal hyperplasia and hyperkeratosis (87, 91, 92). Intriguingly, reexpressing Hr in Hr-null mice can restore hair growth (93), providing strong evidence that Hr is necessary and sufficient for re-initiation of hair growth. Human patients with atrichia with papular lesions (APL) harbor inactivating mutations in HR and exhibit similar skin and hair disorders (88, 94). APL patients initially have normal hair growth after birth, but the hair sheds within a few years and does not grow back (88, 95, 96). The hairless phenotype is attributed to defective proliferation and migration of the hair follicle stem cells, which fail to respond to various signaling molecules in the absence of HR function (97).
Figure 1.
A, Schematic depiction of major hairless (HR) functional domains including a nuclear localization signal (NLS), a zinc finger (ZF), 4 protein-protein interaction domains (IDs), and a Jumonji C (JmjC) domain in the C terminus. Recurrent HR mutations identified in 3 or more human cancer types are indicated (top). Two atrichia with papular lesions (APL) patient mutations at amino acids 1012 and 1056 are also depicted (bottom). B, Illustration of the top 5 HR-interacting proteins based on the STRING interaction network database, including the critical tumor suppressor TP53 and several histone deacetylases (HDAC1 to 3). There is experimental evidence supporting HR-HDACs interaction, but HR-TP53 interaction awaits further experimental validation.
As a transcription factor, HR exerts its effects on gene transcription regulation though several mechanisms. The HR protein contains 4 motifs consisting of hydrophobic amino acids (2 LXXLL where L is leucine and X is any amino acid; 2 form ØXXØØ motifs where Ø can be leucine, isoleucine, or valine) (86, 87), which mediates its interactions with other proteins (see Figure 1A). HR has been shown to interact with various steroid receptors including vitamin D receptor (VDR), thyroid hormone receptor (TR), and receptor-related orphan receptors (87). Breast cancers are known to express VDR and TR, thus the interaction between HR and steroid receptors provides a potential role for HR in regulating breast cancer pathogenesis. VDR is expressed in most breast cancer cell lines (98), and an upregulation of VDR protein in breast carcinomas in comparison to healthy breast tissue (99). It has been suggested that expression of VDR in breast tumors may be a prognostic factor, with increased levels of VDR associated with lower risk of death (100, 101). TR is also expressed in breast carcinomas and has been associated with prognosis. Jerzak and colleagues examined expression of thyroid hormone receptor alpha (THRα1 and THRα2) in samples of patients with breast cancer and found high expression of THRα1 and THRα2 in 74% and 40% of samples, respectively. High THRα2 expression was associated with improved overall survival (102). In rodents, the interaction between HR and TR occurs strongly in the absence of thyroid hormone, and HR was shown to repress the transactivation activity of TRα and TRβ (86, 103, 104).
Other KDMs including KDM8, KDM2A, and JMJD3 interact with the nuclear hormone receptors that are expressed in breast tumors. For example, KDM8 is a H3K36me2 demethylase that is involved in the regulation of tumor metabolism and cell cycle in breast cancer cells (75). KDM8 interacts with the androgen receptor (AR) activating the AR response in the absence of androgen (105). Recent evidence has been emerging surrounding the positive role of androgens in breast cancer treatment for women with estrogen-receptor positive metastatic disease. Hickey and colleagues showed that AR activation leads to potent antitumor activity in multiple scenarios including resistance to estrogen receptor (ER) and CDK4/6 inhibitors (106). JMJD3 is an H3K27 demethylase involved in estrogen signaling (107). JMJD3 has been associated with breast cancer progression. Xun et al showed that ectopic expression of JMJD3 suppresses the stem cell–like characteristics of breast cancer cells (108). Taken together, HR and other KDMs may regulate breast cancer development and progression via their interactions with nuclear hormone receptors.
In addition to the steroid receptors, other interacting partners of HR include TP53, RBP3, and histone deacetylase (HDAC) 1 to 3 based on the STRING Protein Interaction database (Figure 1B) (109). There is clear evidence showing that HR interacts with HDACs, which partly accounts for its role in transcriptional repression (87, 110-112). A recent study has shown that HR recognizes and binds to TP53-response elements, therefore regulating several key TP53 target genes (PUMA, GADD45A, CDKN1A) and downstream effectors (BIRC5 and STMN1) (109, 113). These findings suggest a possible crosstalk between HR and p53 tumor-suppressor activities. Lastly, the HR protein exhibits KDM activity that can modulate gene activity epigenetically, which is discussed in detail subsequently (114).
The HR protein contains a JmjC domain, a highly conserved motif among more than 30 human proteins that catalyze demethylation of histones through an oxidative reaction using iron and α-ketoglutarate as cofactors (114, 115). HR contains an atypical JmjC domain and is structurally related to KDM3A (47). The metal-biding motifs of KDM3A and HR differ only by substitution of a cysteine residue for the first histidine in the HR JmjC domain. In vitro demethylation assays have shown that a partial HR protein containing the JmjC domain can demethylate H3K9me1 in a dose-dependent manner, but with a weaker effect on H3K9me2 and no effect on H3K9me3 or other methylated histone substrates (114). Additional demethylation experiments have shown that full-length HR can reduce the level of H3K9me1 and H3K9me2 in total native histones purified from HeLa cells. When human HeLa cells were transfected with either wild-type HR or HR with mutations in the metal-binding motif, immunofluorescence studies showed that expression of the mutant HR had no effect on H3K9 methylation, whereas the wild-type HR led to a dramatic loss of H3K9 methylation (114), confirming the H3K9 KDM activity of HR in human cells. To determine if missense mutations in the HR JmjC domain in APL patients could alter the HDM activity of HR, we introduced single-point mutations in the HR JmjC domain. In vitro demethylation analysis showed that 2 of the APL patient mutations in the HR JmjC domain (D1012N and V1056M; see Figure 1A) significantly reduced its H3K9 demethylase activity, suggesting that the KDM activity of HR may be linked to the clinical manifestation of APL patients. Taken together, these findings provided strong evidence that HR demethylase activity is necessary for hair cycling and skin homeostasis.
HR contains a zinc-finger domain that is highly conserved among mammalian species (116). Comparison with other zinc-finger proteins showed that this motif is similar to the GATA transcription factors, and thus it was proposed that HR may have DNA binding ability (97). By chromatin immunoprecipitation followed by sequencing analysis, we identified approximately 46 genomic regions that HR directly interacts with in human HEK293 kidney cells. The identified HR target genes cluster into 3 major functional categories, including hair biology, neural activity, and oncogenesis, which are consistent with the predicted biological functions of HR. Among these newly identified targets, HR may regulate cell growth, migration, and survival through COL25A1, COL6A1, CSNK2A2, DLEC1, FADD, FGF13, GPD1L, PREX2, and PVT1 (114). Demethylation of H3K9me1 often leads to gene repression, whereas demethylation of H3K9me2 leads to gene activation, consistent with the bimodal transcriptional regulation by HR in gene-specific or context-dependent manners (91, 114, 117).
Hairless Mutations and H3 Lysine 9 Methylation Alterations in Cancer Development
There are several naturally occurring loss-of-function Hr-mutant mouse strains, including the albino SKH1 mice, the Hrhr/hr mice, and the Hrrh-J mice (92). Loss of HR function in SKH1 and Hrhr/hr mice is due to a proviral insertion into Hr exon 6 (118, 119), and due to missense Hr mutations in Hrrh-J mice (120, 121). Iversen first showed that Hrhr/hr mice had increased skin tumor incidence compared to wild-type littermates when exposed to a chemical carcinogenesis protocol (122). Recent studies from our laboratory and others have confirmed that Hr deficiency alone drastically increases skin cancer susceptibility in response to ultraviolet irradiation or topical treatment with the carcinogen 7,12-dimethylbenz(a)anthracene (123-125). While previous studies on HR have mostly focused on understanding its role in skin development and hair follicle cycling, HR is widely expressed in other tissues including the brain and mammary tissues, where its function is unknown (97). Cancer genomics studies have revealed HR somatic alterations in a variety of human cancers. Surveying different cancer genomics data sets including The Cancer Genome Atlas, International Cancer Genome Consortium, the Catalog of Somatic Mutations in Cancer, and cBioPortal databases identified approximately 200 missense mutations across the HR gene locus in skin, colon, stomach, brain, breast, prostate, ovaries, and uterus, among other tissues (126-130). Importantly, a subset of the missense mutations is common among different cancer types (see Figure 1B).
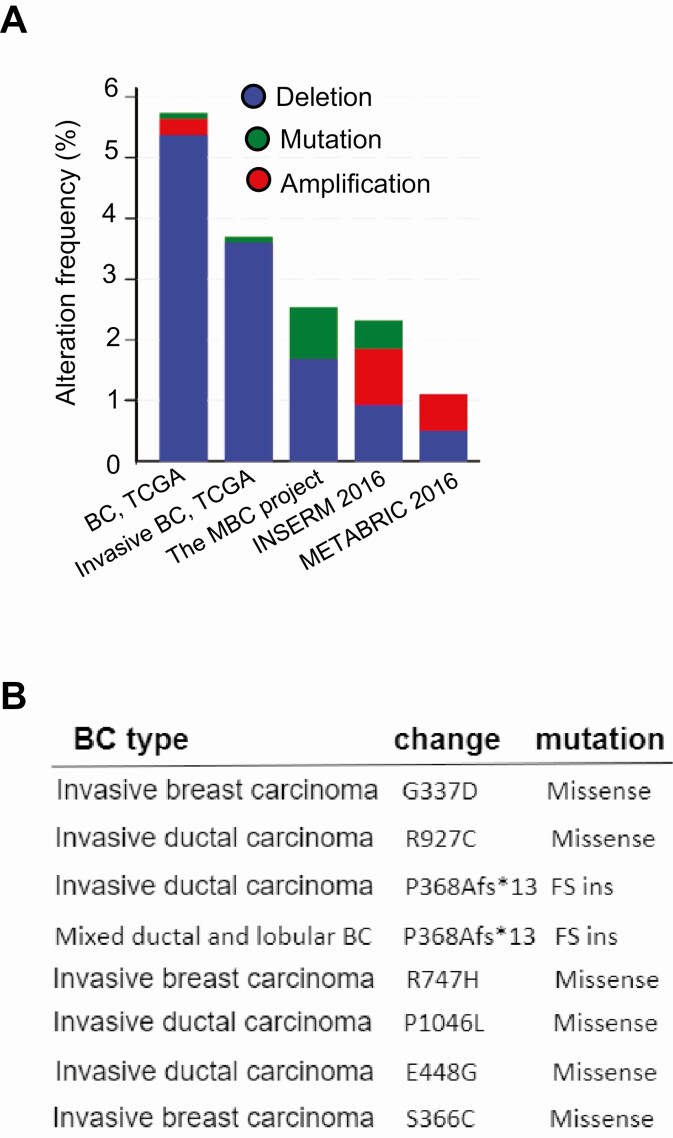
Interrogation of cancer genomics data sets has also revealed prevalent HR deletion or mutations in breast cancers (Figure 2A). Of 816 cases of breast cancer, 49 (~ 6%) of cases of invasive tumors harbored HR deletions or missense mutations (129). Multiple recurring HR mutations have been identified in human cancers, including G337D, R750Q, R927C, and P1046L in invasive breast tumors (Figure 2B). In addition to somatic mutations, copy number analysis of HR by digital polymerase chain reaction identifies frequent HR deletions in human cutaneous squamous cell carcinomas (personal communications). Consistent with this observation, querying the Cancer Cell Line Encyclopedia mutation database revealed that 17 out of 59 human breast cancer cell lines harbor HR gene copy number loss (Table 2). Most of these HR-deficient cell lines are derived from HER2+ or basal-like subtypes based on the PAM50 gene signature (131, 132), highlighting that loss of HR may be linked to the development of aggressive breast cancer subtypes. In addition to mutations and deletions, surveying The Cancer Genome Atlas breast cancer data sets also reveals a significant decreases of HR messenger RNA expression in luminal/HER2+-positive breast tumors (data not shown).
Figure 2.
A, Frequency of hairless (HR) deletion, amplification, and mutation in human breast cancers based on data from various genomics databases. According to The Cancer Genome Atlas (TCGA), HR deletion is a predominant genetic alteration in breast cancers, consistent with our unpublished targeted sequencing results. B, HR mutations identified in invasive breast cancers based on the cBioPortal for Cancer Genomics database. Among them, G337D, R927C, and P1046L are found in other human cancer types as depicted in Figure 1A.
Table 2.
A list of breast cancer cell lines with hairless (HR) copy number loss
| Cell line | Histology subtype | PAM50 subtype | Source |
|---|---|---|---|
| HCC1599 | Ductal carcinoma | Basal-like | ATCC |
| HCC70 | Ductal carcinoma | Basal-like | ATCC |
| CAL148 | Ductal carcinoma | Luminal B | DSMZ |
| HS343T | Adenocarcinoma | Basal-like | ATCC |
| EVSAT | Carcinoma Met | ND | DSMZ |
| HS281T | Adenocarcinoma | Basal-like | ATCC |
| HCC202 | Ductal carcinoma | Her2Amp | ATCC |
| CAL120 | Ductal carcinoma | Basal-like | DSMZ |
| ZR7530 | Ductal carcinoma met | Her2Amp | ATCC |
| HCC38 | Ductal carcinoma | Basal-like | ATCC |
| HCC1428 | Carcinoma Met | Luminal B | ATCC |
| MDAMB231 | Carcinoma Met | Basal-like | ATCC |
| MDAMB436 | Carcinoma Met | Basal-like | ATCC |
| HS274T | Carcinoma | Basal-like | ATCC |
| HMC18 | Carcinoma Met | ND | HSRRB |
| HCC1806 | Ductal carcinoma | Basal-like | ATCC |
| HCC1954 | Ductal carcinoma | Her2Amp | ATCC |
Abbreviation: ND, not determined.
Despite the strong evidence suggesting that mutational inactivation of HR promotes tumorigenesis, the mechanism underlying the putative tumor suppressor function of HR is not well understood. In skin carcinogenesis, Kim et al reported that there is a constitutive nuclear factor-κB activation in Hr-mutant epidermis, leading to activation of several downstream effectors including cyclin D1, cyclin E, Bcl-2, and the proinflammatory protein Cox-2 that contribute to uncontrolled epidermal proliferation and promote skin tumor development (123). It is proposed that HR is a crucial ultraviolet response gene and its loss creates a permissive environment for skin tumorigenesis (123). The human HR gene is located on chromosome 8p, a region that is frequently lost in breast and other cancer types (133). Analysis of the genes near the HR locus in the affected chromosome 8p region suggests no other known tumor suppressor genes except HR (unpublished observations). As mentioned earlier, the interaction between HR and TP53 may be one of the mechanisms by which HR suppresses cancer development, although further research is needed to elucidate the underlying mechanism and pathway (134).
Based on its activity in H3K9 demethylation, it is possible that loss of HR function perturbs genomic histone methylation, although no studies have explored this imporant epigenetic pathway in the tumor-suppressive function of HR. As described previously, G9A and GLP are the major KMTs that catalyze H3K9me1 and H3K9me2. Consistent with their opposite activities in H3K9 methylation, G9A is implicated to function as an oncogene whereas HR is implicated to function as a tumor suppressor gene. G9A is aberrantly upregulated in various human cancers and is found to be associated with enhanced cancer cell proliferation and metastasis. In addition to its role in H3K9 methylation, G9A can also methylate the TP53 protein and exhibit a negative regulation of UHRF1 and JAK2 transcription (135, 136). In breast cancer cells, Wang et al reported a novel mechanism by which G9A regulates breast cancer growth by modulating iron homeostasis through the repression of ferroxidase hephaestin activity (54). Although G9A/GLP and HR all target H3K9me1/me2, it remains to be determined whether and how many common target genes they share in the genome.
Therapeutic Targeting of H3 Lysine 9 Methylation in Breast Cancer
Dysregulation of KMTs or KDMs leads to altered histone methylation patterns, which may promote cancer pathogenesis via dysregulation of oncogenes and tumor suppressor genes to cause uncontrolled cell proliferation, invasion, and metastasis. Analyses of the large genomic data sets have shown that KMTs and KDMs are often mutated in cancer and represent 5% of the driver mutations identified in breast cancer (137). Acquired resistance to cancer treatment has also been associated with increased KMT expression (138, 139). Thus, it has been suggested that KMTs may serve as potential biomarkers or therapeutic targets for cancer patients. Among the breast cancer subtypes, basal-like breast cancers have the highest frequency of KMT mutations, amplications, or deletions, whereas luminal A tumors have the lowest mutation frequency in KMT genes (140).
Epigenetic alterations are often reversible, providing unique opportunities for cancer epigenetic therapies by using various drugs to target epigenetic regulators. 5-azaC (Vidaza, Celgene) and 5-aza-dC (Dacogen, Eisai Inc), which are inhibitors of DNA methyltransferases, are among the first epigenetic drugs applied to treat blood cancers (myelodysplastic syndrome and myelomonocytic leukemia). Shortly after, HDAC inhibitors suberoylanilide hydroxamic acid (Zolinza, Merck & Co) and romidepsin (Istodax, Celgene) received US Food and Drug Administration (FDA) approval for treating cutaneous or peripheral T-cell lymphoma patients. Whereas 5-azaC and 5-aza-dC are used as first-line chemotherapy for the treatment of leukemia patients, vorinostat and romidepsin are largely restricted for treatment of T-cell lymphoma refractory to other therapies (141). Following this initial success, more than 20 epigenetic drugs have entered clinical testing and validation, which led to approvals of 3 HDAC inhibitors (belinostat, panobinostat, and chidamide) (142). In 2015, panobinostat (Farydak, Novartis pharmaceuticals) received FDA approval for patients with refractory multiple myeloma who do not respond to other treatments. A major progress in cancer epigenetic therapy is success with the combination of epigenetic therapy with immunotherapy that produced promising potential in treating solid tumors including advanced breast cancer and metastatic lung cancer (143, 144).
The success with epigenetic drugs targeting DNA methylation and histone acetylation has sparked widespread interest and efforts to explore epigenetic writers, erasers, and readers involved in histone methylation as drug targets. There are more than 100 KMTs in human cells that use S-adenosyl methionine (SAM) as the substrate for methylation reactions. This offers a conceptual basis for KMT inhibitor design by using potent SAM mimetics. Tazemetostat (Tazverik, Epizyme, Inc.) is an orally available, small-molecule, selective inhibitor of EZH2, an H3K27 KMT. Activating mutations in EZH2 are found in human cancer patients (145, 146). Tazemetostat was first approved by the FDA for the treatment of metastatic or locally advanced epithelioid sarcoma in January 2020. In June 2020, the FDA granted accelerated approval to tazemetostat for the treatment of adult patients with relapsed or refractory follicular lymphoma whose tumors are positive for EZH2 mutations. Of note, prolonged EZH2 inhibition has been associated with tumor progression, therefore further studies should be conducted to understand the dual role of the drug in cancer growth and progression (147). Pinometostat is a first-in-class small-molecule inhibitor of DOT1L, an H3K79 KMT, and has been shown in a phase 1 clinical study to exhibit therapeutic potential for targeting DOT1L in genetically defined acute leukemia with recombinant multiple myeloma (148).
In addition to DOT1L and EZH2, G9A has also been actively explored as an epigenetic drug target because of its frequent upregulation in multiple human cancers. In a xenograft mouse model, knockdown of G9A suppressed breast tumor cell growth (149). Further, silencing of G9A led to the reexpression of cell-adhesion molecules such as E-cadherin (150), suggesting that G9A may regulate the epithelial-to-mesenchymal transition to promote breast cancer progression. Paradoxically, G9A has been shown to function as a coactivator for p21 transcription to trigger apoptosis (151). This is consistent with the observations that downregulation of G9A in breast cancer tissue is associated with breast cancer progression and metastasis (152), suggesting that G9A may also exhibit tumor-suppressive activity in certain settings. Further studies on G9A are necessary to understand its context-dependent role in breast cancer development and progression.
There are at least 8 different compounds that have been developed and tested to inhibit G9A and H3K9 methylation in human cells (Table 3). BIX-01294 is the first selective, small-molecule inhibitor of G9A discovered by high-throughput screening of 125 000 preselected compounds (42). The mechanism of action by BIX-01294 is through its competitive binding to the enzyme against the lysine substrate (154). BIX-01294 exhibits relatively high toxicity, which prompted further studies to develop other selective, small-molecule inhibitors with less toxicity for in vivo studies. Among them, UNC0642 exhibits both a high selectivity and potency in inhibiting G9A and reducing cellular H3K9me1/me2 levels in cell-based assays, while having excellent in vivo pharmacokinetic properties that enable preclinical testing in animal studies (159). Mechanistically, UNC0642 also functions as a substrate competitive inhibitor with a high selectivity for G9A and GLP over dozens of other KMTs (159). Despite this progress, the small-molecule inhibitors of G9A and H3K9 methylation have not reached the stage of clinical testing.
Table 3.
H3K9 lysine methyltransferase inhibitors in development for cancer therapies and associated mechanisms of action (18)
| Reference | Compound | KMT and methylation target | Selectivity | IC50 | Mechanism of action |
|---|---|---|---|---|---|
| Greiner (2005) (153) | Chaetocin | KMT1A, G9A H3K9me1/me2 | Low | 0.8 μM | Mixed disulfide linkages formed between cysteine residues of enzyme and inhibitor |
| Chang (2009) (154) | BIX-01338 | G9A, GLP, and other KMTs | Not selective | 5-15 μM | Unknown |
| Chang (2009) (154) | BIX-01294 | G9A and GLP, H3K9me2 | Selective | 1.7 μM | Binds to the substrate binding groove of the enzyme to prevent enzyme and substrate (SAH) interaction |
| Liu (2009) (155) | UNC0224 | G9A and GLP, H3K9me2 | Selective | 15-30 nM | Occupation of the G9A lysine binding channel by the 7-dimethylaminopropoxy group |
| Liu (2010) (156) | UNC0321 | G9A and GLP, H3K9me2 | Selective | 63 pM | Same as UNC0638 but with a longer ethoxyethyl chain instead of the 3-carbon chain of UNC0638. |
| Chang (2010) (157) | E72 | G9A and GLP | Selective | 100 nM | A lysine mimic added to the BIX-01294 structure to inhibit substrate binding |
| Vedadi (2011) (158) | UNC0638 | G9A and GLP, H3K9me1/me2 | Selective | 15-20 nM | Competition with the lysine substrate. This inhibitor occupies the substrate binding groove and does not interact with the SAM binding pocket |
| Liu (2013) (159) | UNC0642 | G9A and GLP, H3K9me1/me2 | Selective | < 2.5 nM | Same as UNC0638 but with optimized in vivo pharmacokinetic properties |
| Konze (2014) (160) | UNC0965 | G9A and GLP H3K9me1/me2 | Selective | 15-20 nM | A biotinylated derivative of UNC0638 |
| Yuan (2012) (161) | BRD4770 BRD9539 |
G9A, GLP, PRC2-EZH2, H3K9me2/me3 | Less selective | 6.3 nM | SAM-competitive inhibitor |
| Sweis (2014) (162) | A-366 | G9A and GLP, H3K9me1/me2 | Selective | 3.3-38 nM | Substrate-competitive inhibitor |
Abbreviations: GLP, G9A-like protein; IC50, half maximal inhibitory concentration; KMT, lysine methyltransferase; SAH, S-adenosylhomocysteine; SAM, S-adenosyl methionine.
Recently, a hybrid KDM inhibitor (MC3324) was developed as a pan-KDM inhibitor by coupling the chemical properties of tranylcypromine, an LSD1 inhibitor, with the 2-OG competitive moiety developed for JmjC KDM inhibition (163). Benedetti et al showed that MC3324 induced significant growth arrest and apoptosis of ER-positive breast cancer cells that was associated with significant increases in H3K4me2 and H3K27me3 (164). The antitumor action of MC3324 is linked to its ability to epigenetically downregulate ER and ER-responsive genes in breast cancer cells. Benedetti and colleagues further demonstrated a tumor-selective potential of MC3324 both in xenograft mouse and chicken embryo models, with insignificant toxicity and good oral efficacy (164). This hybrid pan-KDM inhibitor represents an exciting step in developing effective and selective epigenetic drugs for breast cancer treatment. Additionally, there are ongoing studies exploring engineered DNA binding domains to achieve selective targeting of specific genomic loci such as regulatory elements and noncoding genes. Engineered zinc-finger protein repressors have been shown to epigenetically silence oncogenes and decrease growth of breast cancer cells both in vitro and in mouse models (165, 166).
Conclusions
The hallmarks of sporadic breast cancer include both genetic mutations and epigenetic alterations in DNA methylation and histone modifications. Compared to DNA methylation and histone acetylation, histone methylation is much more complex and involves a significantly large number of factors that write, erase, and read the histone methylation marks to modulate genome organization and gene activity. Many KMTs and KDMs appear to have bimodal activities in either promoting or suppressing gene transcription in context-dependent manners. Not surprisingly, some of the well-studied KMTs and KDMs, such as LSD1 and G9A, have been implicated as oncogenes in certain settings but tumor suppressors in other settings. Despite the opposite roles of KMTs and KDMs in writing and erasing histone methylation marks, small-molecular inhibitors targeting both KMTs and KDMs are the major focus of current epigenetic drug development for cancer therapy. Many of the drugs developed to date show some promising potential in cell-based assays and preclinical models, but they remain to enter clinical testing. A better understanding of the specific mechanisms underlying epigenetic alterations will likely facilitate precision targeting of the driver epigenetic factors and pathways to improve therapeutic efficacy. We also discussed the function and mechanism of HR, an atypical JmjC KDM targeting H3K9me1/me2. There is strong evidence that HR functions as a tumor suppressor in skin tumorigenesis, but HR function in breast cancer has not been reported. Preliminary studies in our laboratory suggest a strong link of HR dysregulation to breast cancer development. Future studies are necessary to characterize the role of HR mutation and H3K9 demethylation in breast cancer pathogenesis, tumor growth, and progression. These studies will offer new insights into how genetic and epigenetic alterations cooperate to drive breast cancer development and may enable epigenetic therapy by targeting the H3K9 methylation pathway in breast cancer treatment.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health/National Cancer Institute (NIH/NCI grant No. R01CA196639), the NIH/Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS grant No. K01AR064315), the Karl Potach Foundation, and the Eagle’s Telethon Postdoctoral award (to B.S.). American Heart Association (AHA grant No. 18IPA34110189 to Z.C.).
Glossary
Abbreviations
- APL
atrichia with papular lesions
- AR
androgen receptor
- ER
estrogen receptor
- FAD
flavin adenine dinucleotide
- FDA
US Food and Drug Administration
- GLP
G9A-like protein
- H3K4
H3 lysine 4
- H3K9
H3 lysine 9
- HDAC
histone deacetylases
- HR
hairless
- JmjC
Jumonji C
- KDM
lysine demethylases
- KMT
lysine methyltransferases
- LSDs
lysine-specific demethylases
- me1
monomethylated lysine
- me2
dimethylated lysine
- me3
trimethylated lysine
- SAH
S-adenosylhomocysteine
- SAM
S-adenosyl methionine
- THRα
thyroid hormone receptor alpha
- TR
thyroid hormone receptor
- VDR
vitamin D receptor
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. [DOI] [PubMed] [Google Scholar]
- 2. Turashvili G, Brogi E. Tumor heterogeneity in breast cancer. Front Med (Lausanne). 2017;4:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ellsworth RE, Blackburn HL, Shriver CD, Soon-Shiong P, Ellsworth DL. Molecular heterogeneity in breast cancer: state of the science and implications for patient care. Semin Cell Dev Biol. 2017;64:65-72. [DOI] [PubMed] [Google Scholar]
- 4. Harris EER. Precision medicine for breast cancer: the paths to truly individualized diagnosis and treatment. Int J Breast Cancer. 2018;2018:4809183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dai X, Cheng H, Bai Z, Li J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J Cancer. 2017;8(16):3131-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747-752. [DOI] [PubMed] [Google Scholar]
- 7. Michalak EM, Visvader JE. Dysregulation of histone methyltransferases in breast cancer—opportunities for new targeted therapies? Mol Oncol. 2016;10(10):1497-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baslan T, Kendall J, Volyanskyy K, et al. Novel insights into breast cancer copy number genetic heterogeneity revealed by single-cell genome sequencing. Elife. 2020;9:e51480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellis MJ, Perou CM. The genomic landscape of breast cancer as a therapeutic roadmap. Cancer Discov. 2013;3(1):27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Apostolou P, Fostira F. Hereditary breast cancer: the era of new susceptibility genes. Biomed Res Int. 2013;2013:747318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flavahan WA, Gaskell E, Bernstein BE. Epigenetic plasticity and the hallmarks of cancer. Science. 2017;357(6348):eaal2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Albert M, Helin K. Histone methyltransferases in cancer. Semin Cell Dev Biol. 2010;21(2):209-220. [DOI] [PubMed] [Google Scholar]
- 13. Pedersen MT, Helin K. Histone demethylases in development and disease. Trends Cell Biol. 2010;20(11):662-671. [DOI] [PubMed] [Google Scholar]
- 14. D’Oto A, Tian QW, Davidoff AM, Yang J. Histone demethylases and their roles in cancer epigenetics. J Med Oncol Ther. 2016;1(2):34-40. [PMC free article] [PubMed] [Google Scholar]
- 15. Jones PA, Ohtani H, Chakravarthy A, De Carvalho DD. Epigenetic therapy in immune-oncology. Nat Rev Cancer. 2019;19(3):151-161. [DOI] [PubMed] [Google Scholar]
- 16. Bates SE. Epigenetic therapies for cancer. N Engl J Med. 2020;383(7):650-663. [DOI] [PubMed] [Google Scholar]
- 17. Kim Y, Lee HM, Xiong Y, et al. Targeting the histone methyltransferase G9A activates imprinted genes and improves survival of a mouse model of Prader-Willi syndrome. Nat Med. 2017;23(2):213-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaniskan HÜ, Konze KD, Jin J. Selective inhibitors of protein methyltransferases. J Med Chem. 2015;58(4):1596-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rowbotham SP, Li F, Dost AFM, et al. H3K9 methyltransferases and demethylases control lung tumor-propagating cells and lung cancer progression. Nat Commun. 2018;9(1):4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cao YP, Sun JY, Li MQ, et al. Inhibition of G9A by a small molecule inhibitor, UNC0642, induces apoptosis of human bladder cancer cells. Acta Pharmacol Sin. 2019;40(8):1076-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCabe MT, Mohammad HP, Barbash O, Kruger RG. Targeting histone methylation in cancer. Cancer J. 2017;23(5):292-301. [DOI] [PubMed] [Google Scholar]
- 22. Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074-1080. [DOI] [PubMed] [Google Scholar]
- 23. Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128(4):669-681. [DOI] [PubMed] [Google Scholar]
- 24. Chi P, Allis CD, Wang GG. Covalent histone modifications—miswritten, misinterpreted and mis-erased in human cancers. Nat Rev Cancer. 2010;10(7):457-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cloos PA, Christensen J, Agger K, Helin K. Erasing the methyl mark: histone demethylases at the center of cellular differentiation and disease. Genes Dev. 2008;22(9):1115-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feinberg AP, Koldobskiy MA, Göndör A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat Rev Genet. 2016;17(5):284-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13(5):343-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blanc RS, Richard S. Arginine methylation: the coming of age. Mol Cell. 2017;65(1):8-24. [DOI] [PubMed] [Google Scholar]
- 29. Green JP, Karras DJ. Update on emerging infections: news from the Centers for Disease Control and Prevention. Notes from the field: fatal fungal soft-tissue infections after a tornado—Joplin, Missouri, 2011. Ann Emerg Med. 2012;59(1):53-54. [DOI] [PubMed] [Google Scholar]
- 30. Hyun K, Jeon J, Park K, Kim J. Writing, erasing and reading histone lysine methylations. Exp Mol Med. 2017;49(4):e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barski A, Cuddapah S, Cui K, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823-837. [DOI] [PubMed] [Google Scholar]
- 32. Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693-705. [DOI] [PubMed] [Google Scholar]
- 33. Vakoc CR, Sachdeva MM, Wang H, Blobel GA. Profile of histone lysine methylation across transcribed mammalian chromatin. Mol Cell Biol. 2006;26(24):9185-9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shinkai Y, Tachibana M. H3K9 methyltransferase G9A and the related molecule GLP. Genes Dev. 2011;25(8):781-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yun M, Wu J, Workman JL, Li B. Readers of histone modifications. Cell Res. 2011;21(4):564-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Musselman CA, Lalonde ME, Côté J, Kutateladze TG. Perceiving the epigenetic landscape through histone readers. Nat Struct Mol Biol. 2012;19(12):1218-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Raghuraman S, Donkin I, Versteyhe S, Barrès R, Simar D. The emerging role of epigenetics in inflammation and immunometabolism. Trends Endocrinol Metab. 2016;27(11):782-795. [DOI] [PubMed] [Google Scholar]
- 38. Ballestar E, Li T. New insights into the epigenetics of inflammatory rheumatic diseases. Nat Rev Rheumatol. 2017;13(10):593-605. [DOI] [PubMed] [Google Scholar]
- 39. Bannister AJ, Zegerman P, Partridge JF, et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410(6824):120-124. [DOI] [PubMed] [Google Scholar]
- 40. Loyola A, Tagami H, Bonaldi T, et al. The HP1α-CAF1-SetDB1-containing complex provides H3K9me1 for Suv39-mediated K9me3 in pericentric heterochromatin. EMBO Rep. 2009;10(7):769-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tachibana M, Sugimoto K, Nozaki M, et al. G9A histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16(14):1779-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kubicek S, O’Sullivan RJ, August EM, et al. Reversal of H3K9me2 by a small-molecule inhibitor for the G9A histone methyltransferase. Mol Cell. 2007;25(3):473-481. [DOI] [PubMed] [Google Scholar]
- 43. Pinheiro I, Margueron R, Shukeir N, et al. Prdm3 and Prdm16 are H3K9me1 methyltransferases required for mammalian heterochromatin integrity. Cell. 2012;150(5):948-960. [DOI] [PubMed] [Google Scholar]
- 44. Cloos PA, Christensen J, Agger K, et al. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature. 2006;442(7100):307-311. [DOI] [PubMed] [Google Scholar]
- 45. Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7(9):715-727. [DOI] [PubMed] [Google Scholar]
- 46. Klose RJ, Yamane K, Bae Y, et al. The transcriptional repressor JHDM3A demethylates trimethyl histone H3 lysine 9 and lysine 36. Nature. 2006;442(7100):312-316. [DOI] [PubMed] [Google Scholar]
- 47. Yamane K, Toumazou C, Tsukada Y, et al. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell. 2006;125(3):483-495. [DOI] [PubMed] [Google Scholar]
- 48. Okada Y, Scott G, Ray MK, Mishina Y, Zhang Y. Histone demethylase JHDM2A is critical for Tnp1 and Prm1 transcription and spermatogenesis. Nature. 2007;450(7166):119-123. [DOI] [PubMed] [Google Scholar]
- 49. Horton JR, Upadhyay AK, Qi HH, Zhang X, Shi Y, Cheng X. Enzymatic and structural insights for substrate specificity of a family of jumonji histone lysine demethylases. Nat Struct Mol Biol. 2010;17(1):38-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Varier RA, Timmers HT. Histone lysine methylation and demethylation pathways in cancer. Biochim Biophys Acta. 2011;1815(1):75-89. [DOI] [PubMed] [Google Scholar]
- 51. Cheng Y, He C, Wang M, et al. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduct Target Ther. 2019;4:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fang Y, Liao G, Yu B. LSD1/KDM1A inhibitors in clinical trials: advances and prospects. J Hematol Oncol. 2019;12(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hu X, Xiang D, Xie Y, et al. LSD1 suppresses invasion, migration and metastasis of luminal breast cancer cells via activation of GATA3 and repression of TRIM37 expression. Oncogene. 2019;38(44):7017-7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang YF, Zhang J, Su Y, et al. G9A regulates breast cancer growth by modulating iron homeostasis through the repression of ferroxidase hephaestin. Nat Commun. 2017;8(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen J, Luo Q, Yuan Y, et al. Pygo2 associates with MLL2 histone methyltransferase and GCN5 histone acetyltransferase complexes to augment Wnt target gene expression and breast cancer stem-like cell expansion. Mol Cell Biol. 2010;30(24):5621-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim JH, Sharma A, Dhar SS, et al. UTX and MLL4 coordinately regulate transcriptional programs for cell proliferation and invasiveness in breast cancer cells. Cancer Res. 2014;74(6):1705-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fenizia C, Bottino C, Corbetta S, et al. SMYD3 promotes the epithelial-mesenchymal transition in breast cancer. Nucleic Acids Res. 2019;47(3):1278-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cho MH, Park JH, Choi HJ, et al. DOT1L cooperates with the c-Myc-p300 complex to epigenetically derepress CDH1 transcription factors in breast cancer progression. Nat Commun. 2015;6:7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu B, Zhang X, Song F, et al. MiR-502/SET8 regulatory circuit in pathobiology of breast cancer. Cancer Lett. 2016;376(2):259-267. [DOI] [PubMed] [Google Scholar]
- 60. Shen C, Wang D, Liu X, et al. SET7/9 regulates cancer cell proliferation by influencing β-catenin stability. FASEB J. 2015;29(10):4313-4323. [DOI] [PubMed] [Google Scholar]
- 61. Montenegro MF, Sánchez-Del-Campo L, González-Guerrero R, et al. Tumor suppressor SET9 guides the epigenetic plasticity of breast cancer cells and serves as an early-stage biomarker for predicting metastasis. Oncogene. 2016;35(47):6143-6152. [DOI] [PubMed] [Google Scholar]
- 62. Reijm EA, Timmermans AM, Look MP, et al. High protein expression of EZH2 is related to unfavorable outcome to tamoxifen in metastatic breast cancer. Ann Oncol. 2014;25(11):2185-2190. [DOI] [PubMed] [Google Scholar]
- 63. Tryndyak VP, Kovalchuk O, Pogribny IP. Loss of DNA methylation and histone H4 lysine 20 trimethylation in human breast cancer cells is associated with aberrant expression of DNA methyltransferase 1, Suv4-20h2 histone methyltransferase and methyl-binding proteins. Cancer Biol Ther. 2006;5(1):65-70. [DOI] [PubMed] [Google Scholar]
- 64. Mo W, Liu Q, Lin CC, et al. mTOR inhibitors suppress homologous recombination repair and synergize with PARP inhibitors via regulating SUV39H1 in BRCA-proficient triple-negative breast cancer. Clin Cancer Res. 2016;22(7):1699-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Khanal P, Kim G, Lim SC, et al. Prolyl isomerase Pin1 negatively regulates the stability of SUV39H1 to promote tumorigenesis in breast cancer. FASEB J. 2013;27(11):4606-4618. [DOI] [PubMed] [Google Scholar]
- 66. Ryu TY, Kim K, Kim SK, et al. SETDB1 regulates SMAD7 expression for breast cancer metastasis. BMB Rep. 2019;52(2):139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen L, Vasilatos SN, Qin Y, et al. Functional characterization of lysine-specific demethylase 2 (LSD2/KDM1B) in breast cancer progression. Oncotarget. 2017;8(47):81737-81753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen JY, Luo CW, Lai YS, Wu CC, Hung WC. Lysine demethylase KDM2A inhibits TET2 to promote DNA methylation and silencing of tumor suppressor genes in breast cancer. Oncogenesis. 2017;6(8):e369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kottakis F, Foltopoulou P, Sanidas I, et al. NDY1/KDM2B functions as a master regulator of polycomb complexes and controls self-renewal of breast cancer stem cells. Cancer Res. 2014;74(14):3935-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wade MA, Jones D, Wilson L, et al. The histone demethylase enzyme KDM3A is a key estrogen receptor regulator in breast cancer. Nucleic Acids Res. 2015;43(1):196-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang W, Oguz G, Lee PL, et al. KDM4B-regulated unfolded protein response as a therapeutic vulnerability in PTEN-deficient breast cancer. J Exp Med. 2018;215(11):2833-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Luo W, Chang R, Zhong J, Pandey A, Semenza GL. Histone demethylase JMJD2C is a coactivator for hypoxia-inducible factor 1 that is required for breast cancer progression. Proc Natl Acad Sci U S A. 2012;109(49):E3367-E3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang GJ, Wang W, Mok SWF, et al. Selective inhibition of lysine-specific demethylase 5A (KDM5A) using a Rhodium(III) complex for triple-negative breast cancer therapy. Angew Chem Int Ed Engl. 2018;57(40):13091-13095. [DOI] [PubMed] [Google Scholar]
- 74. Wang W, Lim KG, Feng M, et al. KDM6B counteracts EZH2-mediated suppression of IGFBP5 to confer resistance to PI3K/AKT inhibitor treatment in breast cancer. Mol Cancer Ther. 2018;17(9):1973-1983. [DOI] [PubMed] [Google Scholar]
- 75. Hsia DA, Tepper CG, Pochampalli MR, et al. KDM8, a H3K36me2 histone demethylase that acts in the cyclin A1 coding region to regulate cancer cell proliferation. Proc Natl Acad Sci U S A. 2010;107(21):9671-9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhao Z, Sun C, Li F, Han J, Li X, Song Z. Overexpression of histone demethylase JMJD5 promotes metastasis and indicates a poor prognosis in breast cancer. Int J Clin Exp Pathol. 2015;8(9):10325-10334. [PMC free article] [PubMed] [Google Scholar]
- 77. Abu-Jamous B, Buffa FM, Harris AL, Nandi AK. In vitro downregulated hypoxia transcriptome is associated with poor prognosis in breast cancer. Mol Cancer. 2017;16(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cao C, Vasilatos SN, Bhargava R, et al. Functional interaction of histone deacetylase 5 (HDAC5) and lysine-specific demethylase 1 (LSD1) promotes breast cancer progression. Oncogene. 2017;36(1):133-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang Y, Zhang H, Chen Y, et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell. 2009;138(4):660-672. [DOI] [PubMed] [Google Scholar]
- 80. Li LL, Xue AM, Li BX, et al. JMJD2A contributes to breast cancer progression through transcriptional repression of the tumor suppressor ARHI. Breast Cancer Res. 2014;16(3):R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Seligson DB, Horvath S, McBrian MA, et al. Global levels of histone modifications predict prognosis in different cancers. Am J Pathol. 2009;174(5):1619-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Seligson DB, Horvath S, Shi T, et al. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435(7046):1262-1266. [DOI] [PubMed] [Google Scholar]
- 83. Manuyakorn A, Paulus R, Farrell J, et al. Cellular histone modification patterns predict prognosis and treatment response in resectable pancreatic adenocarcinoma: results from RTOG 9704. J Clin Oncol. 2010;28(8):1358-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Magerl C, Ellinger J, Braunschweig T, et al. H3K4 dimethylation in hepatocellular carcinoma is rare compared with other hepatobiliary and gastrointestinal carcinomas and correlates with expression of the methylase Ash2 and the demethylase LSD1. Hum Pathol. 2010;41(2):181-189. [DOI] [PubMed] [Google Scholar]
- 85. Elsheikh SE, Green AR, Rakha EA, et al. Global histone modifications in breast cancer correlate with tumor phenotypes, prognostic factors, and patient outcome. Cancer Res. 2009;69(9):3802-3809. [DOI] [PubMed] [Google Scholar]
- 86. Potter GB, Beaudoin GM III, DeRenzo CL, Zarach JM, Chen SH, Thompson CC. The hairless gene mutated in congenital hair loss disorders encodes a novel nuclear receptor corepressor. Genes Dev. 2001;15(20):2687-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Thompson CC. Hairless is a nuclear receptor corepressor essential for skin function. Nucl Recept Signal. 2009;7:e010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ahmad W, Faiyaz ul Haque M, Brancolini V, et al. Alopecia universalis associated with a mutation in the human hairless gene. Science. 1998;279(5351):720-724. [DOI] [PubMed] [Google Scholar]
- 89. Ahmad W, Panteleyev AA, Christiano AM. The molecular basis of congenital atrichia in humans and mice: mutations in the hairless gene. J Investig Dermatol Symp Proc. 1999;4(3):240-243. [DOI] [PubMed] [Google Scholar]
- 90. Panteleyev AA, Ahmad W, Malashenko AM, et al. Molecular basis for the rhino Yurlovo (hrrhY) phenotype: severe skin abnormalities and female reproductive defects associated with an insertion in the hairless gene. Exp Dermatol. 1998;7(5):281-288. [DOI] [PubMed] [Google Scholar]
- 91. Zarach JM, Beaudoin GM III, Coulombe PA, Thompson CC. The co-repressor hairless has a role in epithelial cell differentiation in the skin. Development. 2004;131(17):4189-4200. [DOI] [PubMed] [Google Scholar]
- 92. Benavides F, Oberyszyn TM, VanBuskirk AM, Reeve VE, Kusewitt DF. The hairless mouse in skin research. J Dermatol Sci. 2009;53(1):10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Beaudoin GM 3rd, Sisk JM, Coulombe PA, Thompson CC. Hairless triggers reactivation of hair growth by promoting Wnt signaling. Proc Natl Acad Sci U S A. 2005;102(41):14653-14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ahmad W, Irvine AD, Lam H, et al. A missense mutation in the zinc-finger domain of the human hairless gene underlies congenital atrichia in a family of Irish travellers. Am J Hum Genet. 1998;63(4):984-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cichon S, Anker M, Vogt IR, et al. Cloning, genomic organization, alternative transcripts and mutational analysis of the gene responsible for autosomal recessive universal congenital alopecia. Hum Mol Genet. 1998;7(11):1671-1679. [DOI] [PubMed] [Google Scholar]
- 96. Sprecher E, Bergman R, Szargel R, et al. Atrichia with papular lesions maps to 8p in the region containing the human hairless gene. Am J Med Genet. 1998;80(5):546-550. [DOI] [PubMed] [Google Scholar]
- 97. Maatough A, Whitfield GK, Brook L, Hsieh D, Palade P, Hsieh JC. Human hairless protein roles in skin/hair and emerging connections to brain and other cancers. J Cell Biochem. 2018;119(1):69-80. [DOI] [PubMed] [Google Scholar]
- 98. Frampton RJ, Suva LJ, Eisman JA, et al. Presence of 1,25-dihydroxyvitamin D3 receptors in established human cancer cell lines in culture. Cancer Res. 1982;42(3):1116-1119. [PubMed] [Google Scholar]
- 99. Friedrich M, Axt-Fliedner R, Villena-Heinsen C, Tilgen W, Schmidt W, Reichrath J. Analysis of vitamin D-receptor (VDR) and retinoid X-receptor α in breast cancer. Histochem J. 2002;34(1-2):35-40. [DOI] [PubMed] [Google Scholar]
- 100. Huss L, Butt ST, Borgquist S, et al. Vitamin D receptor expression in invasive breast tumors and breast cancer survival. Breast Cancer Res. 2019;21(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ditsch N, Toth B, Mayr D, et al. The association between vitamin D receptor expression and prolonged overall survival in breast cancer. J Histochem Cytochem. 2012;60(2):121-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jerzak KJ, Cockburn J, Pond GR, et al. Thyroid hormone receptor α in breast cancer: prognostic and therapeutic implications. Breast Cancer Res Treat. 2015;149(1):293-301. [DOI] [PubMed] [Google Scholar]
- 103. Thompson CC, Bottcher MC. The product of a thyroid hormone-responsive gene interacts with thyroid hormone receptors. Proc Natl Acad Sci U S A. 1997;94(16):8527-8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Potter GB, Zarach JM, Sisk JM, Thompson CC. The thyroid hormone-regulated corepressor hairless associates with histone deacetylases in neonatal rat brain. Mol Endocrinol. 2002;16(11):2547-2560. [DOI] [PubMed] [Google Scholar]
- 105. Wang HJ, Pochampalli M, Wang LY, et al. KDM8/JMJD5 as a dual coactivator of AR and PKM2 integrates AR/EZH2 network and tumor metabolism in CRPC. Oncogene. 2019;38(1):17-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hickey TE, Selth LA, Chia KM, et al. The androgen receptor is a tumor suppressor in estrogen receptor-positive breast cancer. Nat Med. 2021;27(2):310-320. [DOI] [PubMed] [Google Scholar]
- 107. Biddie SC, John S. Minireview: conversing with chromatin: the language of nuclear receptors. Mol Endocrinol. 2014;28(1):3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Xun J, Wang D, Shen L, et al. JMJD3 suppresses stem cell-like characteristics in breast cancer cells by downregulation of Oct4 independently of its demethylase activity. Oncotarget. 2017;8(13):21918-21929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Brook L, Palade P, Maatough A, et al. Hairless regulates p53 target genes to exert tumor suppressive functions in glioblastoma. J Cell Biochem. 2019;120(1):533-543. [DOI] [PubMed] [Google Scholar]
- 110. Li H, Leo C, Schroen DJ, Chen JD. Characterization of receptor interaction and transcriptional repression by the corepressor SMRT. Mol Endocrinol. 1997;11(13):2025-2037. [DOI] [PubMed] [Google Scholar]
- 111. Burke LJ, Baniahmad A. Co-repressors 2000. FASEB J. 2000;14(13):1876-1888. [DOI] [PubMed] [Google Scholar]
- 112. Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14(2):121-141. [PubMed] [Google Scholar]
- 113. Brook L, Whitfield GK, Hsieh D, Bither RD, Hsieh JC. The mammalian hairless protein as a DNA binding phosphoprotein. J Cell Biochem. 2017;118(2):341-350. [DOI] [PubMed] [Google Scholar]
- 114. Liu L, Kim H, Casta A, Kobayashi Y, Shapiro LS, Christiano AM. Hairless is a histone H3K9 demethylase. FASEB J. 2014;28(4):1534-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mosammaparast N, Shi Y. Reversal of histone methylation: biochemical and molecular mechanisms of histone demethylases. Annu Rev Biochem. 2010;79:155-179. [DOI] [PubMed] [Google Scholar]
- 116. Lowry JA, Atchley WR. Molecular evolution of the GATA family of transcription factors: conservation within the DNA-binding domain. J Mol Evol. 2000;50(2):103-115. [DOI] [PubMed] [Google Scholar]
- 117. Chuma M, Endo-Umeda K, Shimba S, Yamada S, Makishima M. Hairless modulates ligand-dependent activation of the vitamin D receptor-retinoid X receptor heterodimer. Biol Pharm Bull. 2012;35(4):582-587. [DOI] [PubMed] [Google Scholar]
- 118. Schaffer BS, Grayson MH, Wortham JM, et al. Immune competency of a hairless mouse strain for improved preclinical studies in genetically engineered mice. Mol Cancer Ther. 2010;9(8):2354-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Stoye JP, Fenner S, Greenoak GE, Moran C, Coffin JM. Role of endogenous retroviruses as mutagens: the hairless mutation of mice. Cell. 1988;54(3):383-391. [DOI] [PubMed] [Google Scholar]
- 120. Ahmad W, Panteleyev AA, Henson-Apollonio V, Sundberg JP, Christiano AM. Molecular basis of a novel rhino (hrrhChr) phenotype: a nonsense mutation in the mouse hairless gene. Exp Dermatol. 1998;7(5):298-301. [DOI] [PubMed] [Google Scholar]
- 121. Ahmad W, Panteleyev AA, Sundberg JP, Christiano AM. Molecular basis for the rhino (hrrh-8J) phenotype: a nonsense mutation in the mouse hairless gene. Genomics. 1998;53(3):383-386. [DOI] [PubMed] [Google Scholar]
- 122. Iversen U, Iversen OH. The sensitivity of the skin of hairless mice to chemical carcinogenesis. Cancer Res. 1976;36(4):1238-1241. [PubMed] [Google Scholar]
- 123. Kim H, Casta A, Tang X, et al. Loss of hairless confers susceptibility to UVB-induced tumorigenesis via disruption of NF-kappaB signaling. PloS One. 2012;7(6):e39691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ha W, Hinde A, Xie L, Trager MH, Liu L. Biomarker function of HMGA2 in ultraviolet-induced skin cancer development. Exp Dermatol. 2020;29(10):1021-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Neagu M, Caruntu C, Constantin C, et al. Chemically induced skin carcinogenesis: updates in experimental models (review). Oncol Rep. 2016;35(5):2516-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cerami E, Gao J, Dogrusoz U, et al. The cBio Cancer Genomics Portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bettegowda C, Agrawal N, Jiao Y, et al. Exomic sequencing of four rare central nervous system tumor types. Oncotarget. 2013;4(4):572-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ciriello G, Gatza ML, Beck AH, et al. ; TCGA Research Network . Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163(2):506-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Barretina J, Caponigro G, Stransky N, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jiang GL, Zhang SJ, Yazdanparast A, et al. Comprehensive comparison of molecular portraits between cell lines and tumors in breast cancer. BMC Genom. 2016;17(Suppl 7):525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cai Y, Crowther J, Pastor T, et al. Loss of chromosome 8p governs tumor progression and drug response by altering lipid metabolism. Cancer Cell. 2016;29(5):751-766. [DOI] [PubMed] [Google Scholar]
- 134. Soussi T, Wiman KG. TP53: an oncogene in disguise. Cell Death Differ. 2015;22(8):1239-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Son HJ, Kim JY, Hahn Y, Seo SB. Negative regulation of JAK2 by H3K9 methyltransferase G9A in leukemia. Mol Cell Biol. 2012;32(18):3681-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Huang J, Dorsey J, Chuikov S, et al. G9A and Glp methylate lysine 373 in the tumor suppressor p53. J Biol Chem. 2010;285(13):9636-9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Nik-Zainal S, Davies H, Staaf J, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534(7605):47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Borley J, Brown R. Epigenetic mechanisms and therapeutic targets of chemotherapy resistance in epithelial ovarian cancer. Ann Med. 2015;47(5):359-369. [DOI] [PubMed] [Google Scholar]
- 139. Magnani L, Brunelle M, Gévry N, Lupien M. Chromatin landscape and endocrine response in breast cancer. Epigenomics. 2012;4(6):675-683. [DOI] [PubMed] [Google Scholar]
- 140. Liu L, Kimball S, Liu H, Holowatyj A, Yang ZQ. Genetic alterations of histone lysine methyltransferases and their significance in breast cancer. Oncotarget. 2014;6(4):2466-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Berdasco M, Esteller M. Clinical epigenetics: seizing opportunities for translation. Nat Rev Genet. 2019;20(2):109-127. [DOI] [PubMed] [Google Scholar]
- 142. Ganesan A, Arimondo PB, Rots MG, Jeronimo C, Berdasco M. The timeline of epigenetic drug discovery: from reality to dreams. Clin Epigenetics. 2019;11(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Connolly RM, Li H, Jankowitz RC, et al. Combination epigenetic therapy in advanced breast cancer with 5-azacitidine and entinostat: a phase II National Cancer Institute/Stand Up to Cancer Study. Clin Cancer Res. 2017;23(11):2691-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Mazzone R, Zwergel C, Mai A, Valente S. Epi-drugs in combination with immunotherapy: a new avenue to improve anticancer efficacy. Clin Epigenetics. 2017;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Bradley WD, Arora S, Busby J, et al. EZH2 inhibitor efficacy in non-Hodgkin’s lymphoma does not require suppression of H3K27 monomethylation. Chem Biol. 2014;21(11):1463-1475. [DOI] [PubMed] [Google Scholar]
- 146. Kim KH, Roberts CW. Targeting EZH2 in cancer. Nat Med. 2016;22(2):128-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. de Vries NA, Hulsman D, Akhtar W, et al. Prolonged Ezh2 depletion in glioblastoma causes a robust switch in cell fate resulting in tumor progression. Cell Rep. 2015;10(3):383-397. [DOI] [PubMed] [Google Scholar]
- 148. Stein EM, Garcia-Manero G, Rizzieri DA, et al. The DOT1L inhibitor pinometostat reduces H3K79 methylation and has modest clinical activity in adult acute leukemia. Blood. 2018;131(24):2661-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Dong C, Wu Y, Yao J, et al. G9A interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J Clin Invest. 2012;122(4):1469-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wozniak RJ, Klimecki WT, Lau SS, Feinstein Y, Futscher BW. 5-Aza-2′-deoxycytidine-mediated reductions in G9A histone methyltransferase and histone H3 K9 di-methylation levels are linked to tumor suppressor gene reactivation. Oncogene. 2007;26(1):77-90. [DOI] [PubMed] [Google Scholar]
- 151. Oh ST, Kim KB, Chae YC, Kang JY, Hahn Y, Seo SB. H3K9 histone methyltransferase G9A-mediated transcriptional activation of p21. FEBS Lett. 2014;588(5):685-691. [DOI] [PubMed] [Google Scholar]
- 152. Si W, Huang W, Zheng Y, et al. Dysfunction of the reciprocal feedback loop between GATA3- and ZEB2-nucleated repression programs contributes to breast cancer metastasis. Cancer Cell. 2015;27(6):822-836. [DOI] [PubMed] [Google Scholar]
- 153. Greiner D, Bonaldi T, Eskeland R, Roemer E, Imhof A. Identification of a specific inhibitor of the histone methyltransferase SU(VAR)3-9. Nat Chem Biol. 2005;1(3):143-145. [DOI] [PubMed] [Google Scholar]
- 154. Chang Y, Zhang X, Horton JR, et al. Structural basis for G9A-like protein lysine methyltransferase inhibition by BIX-01294. Nat Struct Mol Biol. 2009;16(3):312-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Liu F, Chen X, Allali-Hassani A, et al. Discovery of a 2,4-diamino-7-aminoalkoxyquinazoline as a potent and selective inhibitor of histone lysine methyltransferase G9A. J Med Chem. 2009;52(24):7950-7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Liu F, Chen X, Allali-Hassani A, et al. Protein lysine methyltransferase G9A inhibitors: design, synthesis, and structure activity relationships of 2,4-diamino-7-aminoalkoxy-quinazolines. J Med Chem. 2010;53(15):5844-5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Chang Y, Ganesh T, Horton JR, et al. Adding a lysine mimic in the design of potent inhibitors of histone lysine methyltransferases. J Mol Biol. 2010;400(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Vedadi M, Barsyte-Lovejoy D, Liu F, et al. A chemical probe selectively inhibits G9A and GLP methyltransferase activity in cells. Nat Chem Biol. 2011;7(8):566-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Liu F, Barsyte-Lovejoy D, Li F, et al. Discovery of an in vivo chemical probe of the lysine methyltransferases G9A and GLP. J Med Chem. 2013;56(21):8931-8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Konze KD, Pattenden SG, Liu F, et al. A chemical tool for in vitro and in vivo precipitation of lysine methyltransferase G9A. ChemMedChem. 2014;9(3):549-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Yuan Y, Wang Q, Paulk J, et al. A small-molecule probe of the histone methyltransferase G9A induces cellular senescence in pancreatic adenocarcinoma. ACS Chem Biol. 2012;7(7):1152-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Sweis RF, Pliushchev M, Brown PJ, et al. Discovery and development of potent and selective inhibitors of histone methyltransferase G9A. ACS Med Chem Lett. 2014;5(2):205-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Rotili D, Tomassi S, Conte M, et al. Pan-histone demethylase inhibitors simultaneously targeting Jumonji C and lysine-specific demethylases display high anticancer activities. J Med Chem. 2014;57(1):42-55. [DOI] [PubMed] [Google Scholar]
- 164. Benedetti R, Dell’Aversana C, De Marchi T, et al. Inhibition of histone demethylases LSD1 and UTX regulates ERα signaling in breast cancer. Cancers (Basel). 2019;11(12):2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Falahi F, Huisman C, Kazemier HG, et al. Towards sustained silencing of HER2/neu in cancer by epigenetic editing. Mol Cancer Res. 2013;11(9):1029-1039. [DOI] [PubMed] [Google Scholar]
- 166. Stolzenburg S, Beltran AS, Swift-Scanlan T, Rivenbark AG, Rashwan R, Blancafort P. Stable oncogenic silencing in vivo by programmable and targeted de novo DNA methylation in breast cancer. Oncogene. 2015;34(43):5427-5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”