ABSTRACT
Understanding the dynamics of the immune microenvironment is critical to the development of immuno-based strategies for the prevention of oral potentially malignant disorders transformation to oral squamous cell carcinoma (OSCC). We used laser capture microdissection and RNA-sequencing to profile the expression of 13 matched pairs of epithelial versus stromal compartments from normal mucosa, hyperplasia, dysplasia, and invasive tumors in the 4-nitroquinolein (4-NQO) murine model of oral carcinogenesis. Genes differentially expressed at each step of transformation were defined. Immune cell deconvolution and enrichment scores of various biological processes including immune-related ones were computed. Immunohistochemistry was also performed to characterize the immune infiltrates by T-cells (T-cells CD3+, helper CD4+, cytotoxic CD8+, regulatory FoxP3+), B-cells (B220+), and macrophages (M1 iNOS+, M2 CD163+) at each histological step. Enrichment of three independent M2 macrophages signatures were computed in 86 oral leukoplakia with available clinical outcome. Most gene expression changes were observed in the stromal compartment and related to immune biological processes. Immune cell deconvolution identified infiltration by the macrophage population as the most important quantitatively especially at the stage of dysplasia. In 86 patients with oral leukoplakia, three M2 macrophages signatures were independently associated with improved oral cancer-free survival. This study provides a better understanding of the dynamics of the immune microenvironment during oral carcinogenesis and highlights an unexpected association of M2 macrophages gene expression signatures with oral cancer free survival in patients with oral leukoplakia.
KEYWORDS: Oral carcinogenesis, oral potentially malignant disorders, oral leukoplakia, immune microenvironment, M2 macrophages, stroma, 4-NQO model
Introduction
Oral cavity is the most common site of Head and Neck Squamous Cell Carcinoma (HNSCC) which is ranked as the 8th most common cancer worldwide.1 Oral SCC (OSCC) is associated with significant morbidity and mortality. Because OSCC may develop from oral premalignant disorders (OPMD), prevention of OPMD malignant transformation may improve patients outcomes.2 However, although several oncogenic-driven chemoprevention strategies have been proposed, no prevention strategy can be considered as a standard of care.3 Identification of OPMD with a risk of malignant transformation is needed to develop relevant preventive strategies addressing the cancerization field. Besides clinical (inhomogeneity) and histological (severe dysplasia) OPMD characteristics,4 several biomarkers5,6 have been proposed to identify patients at high risk of OSCC development. Loss of heterozygosity (LOH) at specific chromosomal sites has been validated prospectively as the most robust biomarker of risk of oral cancer development in patients with OPMD. Using the 4-NQO murine model of oral carcinogenesis,7 we have previously shown the role of early epithelial gene expression changes during OPMD malignant transformation.8
The role of the immune microenvironment (IME) during head and neck carcinogenesis has been recently emphasized. In particular, T-cells infiltrate has been associated with improved outcomes in patients with HNSCC, one of the most immune-infiltrated cancer types.9 Recent clinical trials investigating immunotherapies represent an unprecedented advance in HNSCC.10,11 Within this context, the role of the IME during early steps of oral carcinogenesis has been reported,12 and immunoprevention strategies proposed.13 We have previously identified two distinct gene expression based OPMD subtypes named immunological and classical respectively.14 The immunological class was characterized by an activation of immune signaling pathways, an increased immune infiltrate and an overexpression of miR-142-5p, a microRNA involved in M1/M2 macrophage switch. Intriguingly, we found that a high miR-142-5p expression level was associated with improved oral cancer-free survival (OCFS), contrasting with the typical protumorigenic role of M2 macrophages in established tumors.
Herein, using the 4-NQO murine model of oral carcinogenesis, we show that early gene expression changes in the stroma underlying lesions, were mainly associated to immune biological processes, mainly involving the macrophage population. Moreover, using immunohistochemistry, significant changes in T-cells, B-cells, and macrophages stroma infiltration were observed at premalignant stages. In particular, M2 macrophages infiltrate changes were among the most important ones, especially at the stage of dysplasia. Surprisingly, three independently published M2 macrophages gene expression signatures were associated and highly predictive of improved OCFS in 86 OPMD. These results support the need to revisit the role of M2 macrophage during oral tumorigenesis.
Methods
Details are provided in Supplementary Methods for the 4-NQO model, laser microdissection, RNA extraction, sequencing, immunohistochemistry staining protocols, and bioinformatic analysis.
The 4-NQO mice model of oral carcinogenesis
As previously described,8 the experiments were performed in accordance with the animal care guidelines and were validated by the local Animal Ethic Evaluation Committee (CECCAPP_CLB_2016_014). Briefly, six-week-old mice were exposed to the chemical carcinogen 4-NQO. The 4-NQO treatment is given ad libidum at a dose of 100 μg/mL for 8 weeks. The 4-NQO was then replaced by normal drinking water. Few weeks after the cessation of the 4-NQO, mice developed progressively premalignant lesions (hyperplasia and dysplasia) and later tumors. Mice were sacrificed every 4 weeks after the cessation of the 4-NQO. In all the manuscript, the specified times (in weeks) refer to the time of the experiment.
Based on the histological mapping of a veterinary pathologist (A.T.) examining the paraffin-embedded (FFPE) tongues of CBA mice from this model, we selected several biological replicates of each histological stage: 3 normal samples from control mice (at week 16, 20, and 24), 3 hyperplastic lesions (at week 20, 24, and 32), 4 dysplastic lesions (at week 16, 24, 28 and 32), and 3 tumor samples (at week 28 and 2 at week 32) from the 4-NQO treated mice (Supplementary Figure S1 and S2). Only typical pathological diagnoses were selected from mice that were sacrificed at different time points to avoid a potential bias due to the biological effect of age.
Laser Capture microdissection (LCM)
Using three FFPE sections of each histological stage, we performed LCM (PALM MicroBeam IV Zeiss®) of epithelial cells (E-samples) as well as underlying stroma (S-samples) to generate gene expression profiles (Supplementary Figure S3–S5; Supplementary Video).
Total RNA extraction and RNA-sequencing
After total RNA extraction (QIAGEN RNeasy® FFPE-kit), samples (n = 13 epithelial and n = 13 stromal, subsequently referred as E-samples and S-samples, respectively) were sequenced (NextSeq 550 Illumina®) (quality controls in Supplementary Table 1). Raw data were deposited at Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) (GSE164619).
Immunohistochemistry (IHC)
The HALO™ software system (Indica Labs, v.2.0.1145.19) was used to quantify immune infiltrates for T-cells (CD3, CD4, CD8, FoxP3), B-cells (CD45R0/B220), and macrophages (M1 iNOS1 and M2 CD163) in the epithelium, the underlying stroma and muscle, of both normal (N) mucosa, hyperplasia (H), dysplasia (D) and tumor (T) samples (6 replicates for each condition totalizing 24 samples for each antibody). Data were normalized by the surface analyzed (n cells/mm2). In addition, to control for a potential inflammatory effect of 4-NQO, normal areas at distance of hyperplasia, dysplasia, and tumor lesions were analyzed (Supplementary Figures S6–S11).
Bioinformatics analysis
We defined sets of genes differentially expressed between tumor and normal mucosa called the “tumor gene set” (TGS), for both E- and S-samples i.e., TGSE and TGSS respectively (Supplementary Table 2 and Supplementary Figure S12). TGSE and TGSS were divided into four non-overlapping gene subsets to study the dynamics of gene expression changes during oral tumorigenesis, as previously described:8 the “early” (EGS), the “intermediate” (IGS) and the “late” (LGS) gene subsets, representing changes observed at each step (i.e. H vs. N, D vs. H and T vs. D, respectively). The fourth gene subset, named “progressive” gene set (PGS), included genes changing progressively overtime but not included in other subsets (Supplementary Table 3–5 and Supplementary Figure S12 and S13).
The single-sample Gene Set Enrichment Analysis (ssGSEA) projection tool from GenePattern (ssGSEA v8) was used to compute separate enrichment scores (ES) for each sample using different gene sets (TGS, EGS, IGS, LGS, and PGS) in various datasets downloaded from GEO (GSE75421; GSE9844; and GSE30784). Standard GSEA analysis (GSEA v6.3) was performed to compute the ES of 4 762 curated gene sets (C2 gene set, MSigDB database v6.1, default parameters) using the log2 fold-change in tumor versus normal mucosa as the input.
Seq-ImmuCC (http://218.4.234.74:3200/immune/) was used for immune cell populations deconvolution and infer the relative proportions of ten major immune cells (B-cells, CD4 and CD8 T-cells, Dendritic cells, Eosinophils, Macrophages, Mast cells, Monocytes, Neutrophils, and NK cells).
M2 macrophages signatures and oral cancer risk
We have evaluated the association of some M2 macrophages gene expression signatures with Oral Cancer Free Survival (OCFS) using data previously published from 86 patients with OPMD, in particular, oral leukoplakia15 (GSE26549) (Supplementary Table 6). Briefly, gene expression profile was measured in 86 Oral leukoplakia patients who were enrolled in a clinical chemoprevention trial that used the incidence of oral cancer development as a prespecified endpoint.16 After inclusion, the patients were randomly assigned to intervention (13-cis- retinoic acid versus retinyl palmitate with or without beta-carotene). OCFS was defined as the time from random assignment until diagnosis of any OSCC. Analysis of OCFS was performed in the entire intent-to-treat population and was analyzed by the Kaplan-Meier method. The median follow-up time was 7.11 years and 35 of the 86 patients developed oral cancer over the course. The average time between the OL and OSCC diagnosis is 3.11 years (0.18–14.33).
Immunological and classical subtypes of OPMD
We have recently identified two distinct gene expression based OPMD subtypes named immunological and classical respectively.14 Briefly, based on genome-wide expression profiles of 86 oral leucoplakias (GSE26549)15 and using the 2,500 most variable genes, we performed an unsupervised and unbiased clustering of these samples (discovery dataset). Two main clusters, including 42 (cluster 1) and 44 (cluster 2) OPMD, respectively, were identified. Cluster 1, showing a strong and significant enrichment of immune pathways was named ‘immunological’ while cluster 2, characterized by a moderate enrichment of pathways involved in xenobiotic metabolism as well as EGFR signaling, was named ‘classical’. By comparing genome-wide expression profiles of immunological as well as classical OPMD, we have identified the 200 most overexpressed genes and the 200 most under-expressed genes. These genes were used to build an “Enrichment Score-based classifier” allowing the classification of OPMD as being immunological or classical if the score was >0 or <0, respectively (ssGSEA). This classifier was then validated using OPMD samples from three independent datasets, including 17 (GSE30784),13 (GSE10174), and 15 (GSE85195) OPMD.
Statistical analysis
Statistical analyses and plots were performed using R 4.0.0 and the GSVA_1.36.2, the survival_3.1–12, survminer_0.4.7, PMCMRplus_1.5.0, pROC_1.16.2, and ggplot2 packages. Non-parametric Kruskal-Wallis test was used to compare to compare GSEA ES as well as immune cell counts between more than two groups and Mann Whitney for two groups’ comparisons. Post hoc Steel-Dwass-Flinger test was used for comparisons with false discovery rate adjusted P-value <.05. Survival distributions were estimated using the Kaplan-Meier method and compared with the log-rank test between two groups (clustered by a Gaussian mixture model). Correlations between the ES of two signatures were computed using the Spearman test. Prediction of oral cancer risk was computed using the multivariate Cox model including ES normalized by inter-quartile range. Three-folds cross validated AUC was performed, and 95% confidence intervals were computed by bootstrap (DeLong method). For Cox models, proportional hazards assumption was tested. P-values ≤0.05 were considered to be statistically significant.
Results
Gene expression changes in stroma discriminate OSCC from normal mucosa
A total of three control mice and nine mice treated by 4-NQO and sacrificed at different ages were included (Supplementary Figure S1 and S2). The histological mapping of 270 FFPE-tongues H&E sections from the nine 4-NQO treated mice showed an increasing incidence of histological lesions (i.e., Hyperplasia (H), Dysplasia (D), and Tumor (T)) over time (Figure 1). Preneoplastic lesions (H and D) were more common during the first 24 weeks. At week 28, histological lesions were observed in 97% of the sections analyzed. At week 32, T were observed in 72% of the sections analyzed.
Figure 1.
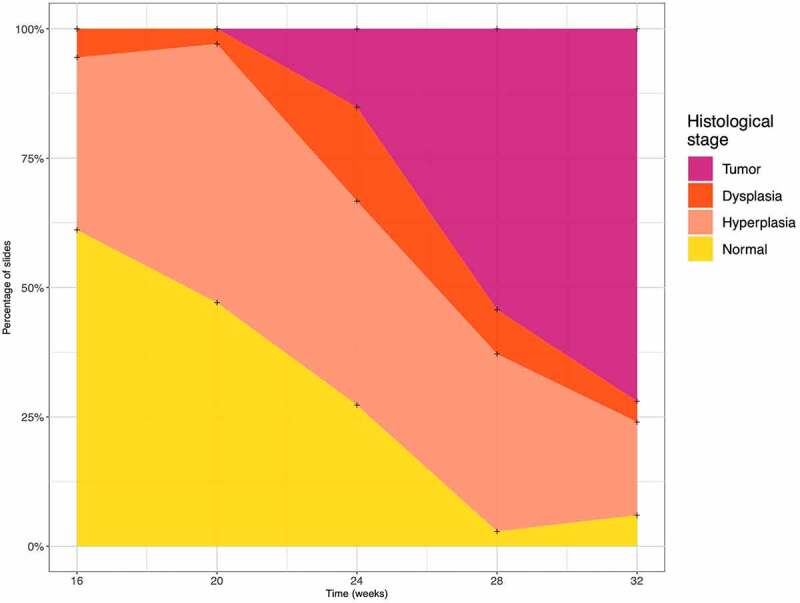
Histological changes in the 4-nitroquinoleine-1-oxyde (4-NQO) treated mice. A total of 270 formalin-fixed paraffin-embedded tongues slides from the nine mice were treated with 4-NQO
Gene expression profiles were generated in 12 selected mice from microdissected epithelium (E-samples) and underlying stroma (S-samples), including 3 N, 3 H, 4 D, and 3 T. Using whole genome expression profiles, E-samples and S-samples were divided into two distinct clusters (Figure 2a).
Figure 2.

Global gene expression changes in the epithelial and stromal compartments. (a) Principal Component Analyses using the 1% genes with the highest mediation absolute deviation. Two distinct patterns of distribution were observed. (b) TGSE and TGSS enrichment scores computed using ssGSEA in murine (GSE75421) and human (GSE9844, GSE30784) independent datasets. Significant changes related to histological stages are observed. (TGSE: tumor gene set Epithelium; TGSS: tumor gene set Stroma; UP: version for genes upregulated; DN: version for genes downregulated; UP+DN: combined version; ssGSEA: single sample gene set enrichment analysis)
We identified two sets of genes differentially expressed between T and N in E-samples and S-samples, respectively called TGSE and TGSS that included 837 and 1,519 genes respectively (Supplementary Table 4). Both gene sets were divided in a UP and DOWN subsets including genes which were coordinately up- or down-regulated within a sample. The overlap between TGSE and TGSS was 373 (16%). TGSE and TGSS were able to discriminate cancer from normal samples in three independents murine (GSE75421) and human (GSE9844 and GSE30784) datasets (Figure 2b).
In summary, gene expression changes were more abundant in the stroma and different from those occurring in the epithelial compartment and the 4-NQO model is relevant to understand the dynamic changes observed during oral carcinogenesis.
Gene expression changes in the stroma and mainly related to immune biological processes
To study the dynamics of gene expression changes during oral carcinogenesis, we divided the TGSS as well as the TGSE into four non-overlapping gene subsets associated with histological changes i.e., “early” (EGS), “intermediate” (IGS), “late” (LGS) and “progressive” (PGS) gene subsets either in the epithelial or the stroma compartment (Supplementary Tables 2–5, Supplementary Figures S12 and S13). In the epithelium, the number of genes specifically and differentially expressed was higher between normal mucosa to hyperplasia while it was more evenly distributed during tumorigenesis in the stromal compartment
In order to get some insight into the biological significance of gene expression changes during oral carcinogenesis, we first used GSEA. In the stroma, among the 34 gene sets significantly enriched between tumor vs normal mucosa (FDR q-value <1e-5; Supplementary Table 7), more than half were involved in immune and inflammation regulation processes, including a signature up-regulated in macrophages (Figure 3a). In the epithelium, most of the 122 significantly enriched pathways were related to carcinogenesis and invasiveness processes (Supplementary Table 7, Figure 3b). Interestingly, in the epithelium, Interferon related pathways, especially Interferon-gamma, were highly enriched in the early stage of oral carcinogenesis (Hyperplasia vs. Normal) while head and neck carcinogenesis pathways were enriched during malignant transformation of Dysplasia into Tumor in both the Epithelium and underlying Stroma.
Figure 3.

Graphical representation of some Enrichment Scores of genes specifically and differentially expressed between tumor and normal mucosa in the Stroma (a) and the Epithelium (b) for the 4,762 of the C2 “curated gene sets”. High enriched scores (peak of the plot) for signatures related to immune regulation and cancer invasiveness were observed (https://software.broadinstitute.org/gsea/doc/GSEAUserGuideFrame.html)
We then used the five gene sets previously proposed to determine the cancer immune subtypes17 and computed an ES using ssGSEA in our samples. Among them, in both the epithelium and the stroma compartments, the “wound healing” and “TGF-beta” gene sets were the most positively enriched, while the “leukocyte infiltration” gene set was the most negatively enriched, but the three of them had limited variation during tumorigenesis. The “Interferon gamma” gene set ES increased overtime in both compartments but remained negative. Interestingly, the “CSF1 response” gene set ES, an M2 macrophage related signature, was increasing overtime in both compartments, but became positively enriched in the stroma only (Supplementary Table 8, Supplementary Figure S14).
The dynamics of immune cell populations during oral carcinogenesis
Using gene expression profiles generated in the stroma, we performed immune cell populations deconvolution (http://wap-lab.org:3200/immune/) to infer the relative proportions of ten major immune cells (Figure 4). While macrophages represent less than 20% of immune infiltrating cells in normal/hyperplastic tissues, immune infiltrates in dysplasia and tumor tissues are dominated by macrophages (almost 35% and >50% of immune infiltrate respectively).
Figure 4.

Deconvolution of immune cell populations in stroma samples. Each immune cell type is given as a percentage (T-cells: T lymphocytes; B-cells: B lymphocytes; NK: Natural Killer)
In order to get a direct insight into the dynamics of immune cell populations during oral carcinogenesis, we performed immunostaining for T- and B-cells, and macrophages populations. Results were analyzed by combining the epithelium and stroma compartments or by considering them separately.
When combining the epithelium and stroma compartments, immune cells infiltrates were increasing overtime for T-cells (T-cells CD3+, helper CD4+, cytotoxic CD8+, regulatory FoxP3+), B-cells (B220+), and macrophages (M1 iNOS+, M2 CD163+) (Figure 5). For almost all immune cell populations, the infiltrate was the highest in dysplasia. Of note, these changes were related to histological changes and not to a potential inflammatory effect of 4-NQO (Supplementary Figure S11). Except for CD3 + T-cells, the increase in CD163+ M2 infiltrate was the most important one quantitatively. When analyzing the epithelial and stroma compartments separately, the increase of the immune cells infiltrate, including the CD163+ M2 population, was mainly seen in the stroma compartment (Figure 6).
Figure 5.

Quantification of immune cells infiltrate at each stage of oral tumorigenesis in the 4-nitroquinoleine-1-oxyde model. T-cells (total CD3+, helper CD4+, cytotoxic CD8+, regulatory FoxP3+), B-cells (B220+) and macrophages (M1 iNOS+, M2 CD163+) were immunostained and quantified in normal mucosa (n), hyperplasia (h), dysplasia (d) and established tumor (t)
Figure 6.
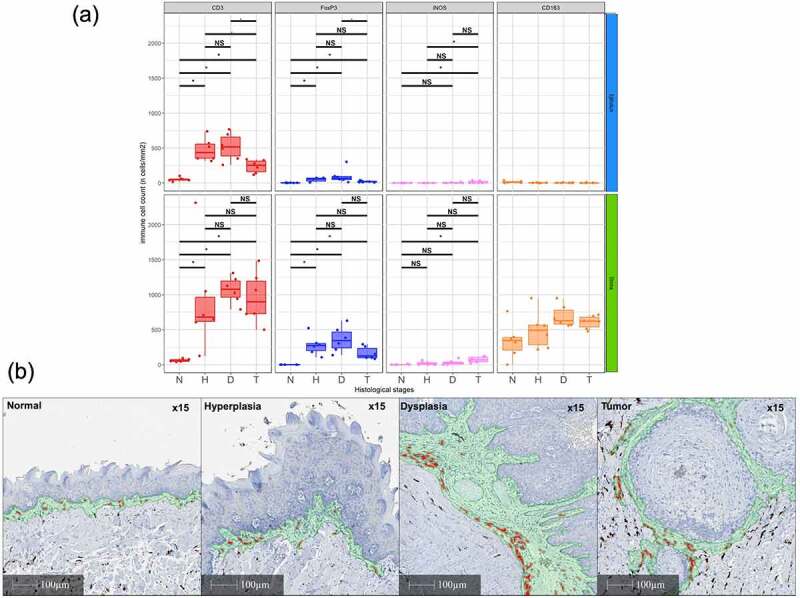
Quantification of immune cells infiltrate in the epithelial and underlying stroma compartment (a) and changes in the CD163+ M2 macrophages infiltrate in the stromal compartment in the in the 4-nitroquinoleine-1-oxyde model during oral tumorigenesis (b). T-cells (total CD3, regulatory FoxP3+) and macrophages (M1 iNOS+, M2 CD163+) were immunostained and quantified in normal mucosa (n), hyperplasia (h), dysplasia (d) and established tumor (t)
M2 macrophages signatures and oral cancer risk
To investigate the precise role of M2 macrophages during oral carcinogenesis, we identified 13 murine and human gene expression signatures previously reported in the literature18–28 (Supplementary Figure S15a). Surprisingly, virtually no overlap was observed between genes included in these signatures (Supplementary Figure S15b).
We then evaluated the association of each M2 signature with OCFS using data from 86 patients with OPMD (oral leukoplakia, GSE26549, Supplementary Table 6).15 ssGSEA was used to compute an ES of each individual sample. Analysis was first done in the overall population in which none of the M2 macrophages signatures was associated with oral cancer-free survival (Supplementary Figure 16).
We then explored the signatures in the immunological (n = 42) versus classical (n = 44) subtypes of OPMD. As expected, the ES of 11/13 signatures was significantly higher in OPMD of the immunological subtype than in OPMD of the classical subtype (Supplementary Figure S17). A similar trend for improved OCFS in immunological OPMD with high ES was observed for a majority of M2 signatures (Supplementary Figure S18) and with a P-value <0.01 for 3 signatures (Figure 7). No such association was observed when considering classical OPMD (data not shown) or when considering the 4-NQO gene sets as tested signatures (Supplementary Figures S19 and S20).
Figure 7.
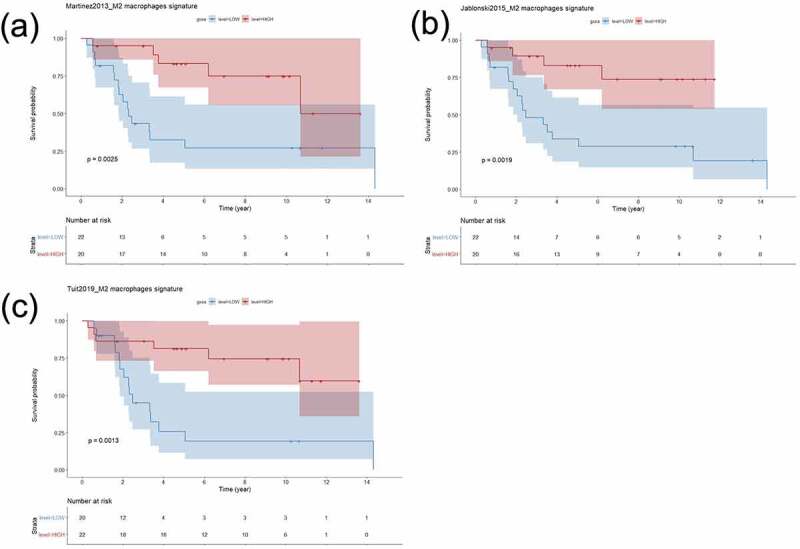
Association of three independent M2 macrophages gene expression signature with oral cancer-free survival of patients with oral potentially malignant disorders of the immunological subtype
We next tested the ability of these signatures to predict OSCC development in immunological OPMD and if combining them do improve this prediction (Cox model, 3-folds cross validated AUC) (Figure 8). We found AUCs significantly higher to 0.5, supporting the excellent prediction ability of these signatures. Given that there were no statistical differences between AUCs of different models as well as the associated Hazard ratios, we found that all signatures were predictive of better OCFS in immunological OPMD, in particular the “Martinez2013_M2”. Overall, these results showed an unexpected association between M2 macrophages infiltrates and OCFS in the immunological subtype of OPMD.
Figure 8.
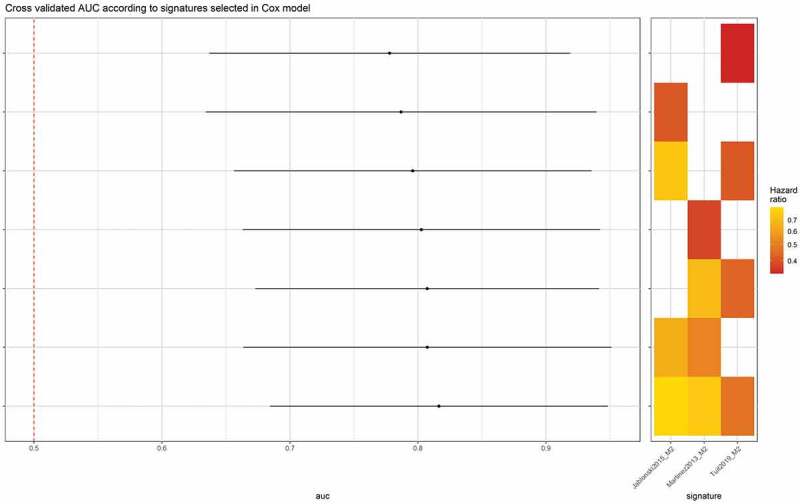
Three independent M2 macrophages gene expression signatures are predictive of oral cancer-free survival. Each signature (Martinez2013, Jablonski2015, Tuit2019) demonstrates an excellent prediction (AUC ≫ 0.5, Hazard Ratio [0.3–0.8]) of improved oral cancer-free survival in patients with oral potentially malignant disorders of the immunological subtype (Cox model, 3-folds cross validated AUC)
Discussion
In this work, by carefully analyzing, in the 4-NQO model, of the epithelial and the underlying stromal compartments, using gene expression profiles as well as immunostaining and semi-quantitative assessment of different immune cell populations, we report that immunological changes are observed in early stages of oral tumorigenesis. Using different data mining approaches, we identify changes associated with M2 macrophages as being the most prominent quantitatively. Intriguingly, M2 macrophages gene expression signatures were consistently associated with a decreased oral cancer-risk in patients with an immunological OPMD subtype that we have previously reported.14
Our study shows that all immune cell populations (T- and B cells, macrophages) are increasing early during mucosal transformation. It is striking that most of the changes seen in terms of the immune cell infiltration were observed at the stage of hyperplasia and did not dramatically change afterward. This is contrasting with stromal gene expression changes that were distributed across the different stages of tumorigenesis. This difference may be explained by immune cells functional changes rather than changes in the immune cell subtypes.
As expected, the dynamic changes observed were mostly seen in the stromal compartments. However, it is interesting to note that some significant changes were also observed in the epithelial compartment, especially for T-cells. Their impacts need to be better understood. Several authors have recently reported that a decrease in CD3+, CD4+, (helper) and CD8+ (cytotoxic) T-cells infiltrates was associated with malignant transformation of patients with OPMD.29–31 In our model, CD4 + T-cells were significantly increased in hyperplasia vs. normal mucosa, while CD8 + T-cells were significantly increased at the stage of dysplasia vs. normal mucosa, but none of them were decreasing significantly in established tumors. This might be related to the small number of lesions analyzed.
The precise role of M2 macrophages during oral carcinogenesis is unclear.32 While they have been largely reported to be associated with worse prognosis in patients with established tumors9 and besides the paucity of reports studying M2 macrophages in OPMD patients, conflicting results have been published. Some authors reported an increased M2 infiltrate in OPMD that subsequently transformed into OSCC compared to those that did not transform.30,33,34 Among them, Yagyuu et al. found that subepithelial CD163-positive cell count was significantly associated with malignant-free survival; of note, only 8/120 (7%) of the patients developed OSCC in their cohort.34 Weber et al. recently published the same conclusions in a large cohort of 103 OPMD with long term follow-up (60 months), including 50 patients that developed OSCC. Interestingly, the M2 infiltrate found in the epithelium was prognostic, while the one in the subepithelial compartment was not found to be associated with prognosis.33 In our study of the 4-NQO model as well as in another report,35 M2 macrophages infiltrate within the epithelial cells compartment was very limited. More recently, Yagyuu et al. reported a series including n = 200 OPMD in which the subepithelial CD163+ cell count was not associated with malignant transformation of OPMD.36
On the other hand, the CD163/CD68 expression ratio in the stroma, which can be considered as a good indicator of M2 macrophages polarization, was significantly associated with non-transformed OPMD in the Weber et al. as well as the Stasikowska-Kanicka et al. studies.33,35 These results are consistent with our recent observation that miRNA 142–5p, that promotes M1 to M2 switch,37 is associated with improved OCFS in immunological OPMD.14
Overall, the precise role of M2 macrophages during oral carcinogenesis is not yet established.38 Some discrepancies may be related to the tissue compartment that is being analyzed (epithelial versus underlying stroma). As stated by Shigeoka et al., in their recent systematic review “the biological features of macrophages in oral carcinogenesis differ drastically depending on the anatomical compartment that they infiltrate”.39
Also, as recently reported at the transcriptional level, macrophages are recognized as being extremely plastic during macrophage polarization,40 and intermediate stages may not be properly recapitulated by the “classical” M1 or M2 subtypes and impact differently oral tumorigenesis.
Herein, we report three independent M2 macrophages gene expression signatures18,19,21 that are associated with better OCFS in the subgroup of patients harboring an OPMD of the immunological subtype. While our results are not fitting conventional role of M2 macrophages and remain to be validated by further biological investigations, they underline that the “classic” association between M2 macrophages infiltrate and worse prognosis in established tumors may not translate in early stages of tumorigenesis.9,41 It is interesting to note that the impact of M2 macrophages signature was not observed in patients with the classical subtype that is characterized by a “cold” OPMD. We believe this is an important observation in the context of the ongoing effort of the scientific community to develop immunoprevention strategies and argues in favor of proper patient stratification as those strategies are being or will be evaluated in clinical trials.
In summary, our study improves our understanding of early changes in the immune microenvironment during oral tumorigenesis and reveals an unexpected association of M2 macrophages with OCFS in immunologically active OPMD. It provides a detailed description of dynamic changes in the 4-NQO murine model that will be important to consider when evaluating new immune-prevention strategies in this model.42
Supplementary Material
Acknowledgments
To the French association “les Chirurgiens Maxillo-Faciaux” and the Equipex ANR-11-EQPX-0035 PHENOCAN.
Funding Statement
Fondation Nuovo Soldati 2019 (JB); ITMO Cancer 2020, “Formation à la Recherche Fondamentale et Translationnelle en Cancérologie” (JB); Ligue contre le cancer 2017, comité du Rhône (PS); 2017-2020: INCa PLBIO17-171 (PS); 2017-INCa-DGOS-Inserm_12563: INCa SIRIC-LYriCAN INCa-DGOS-Inserm_12563 (PS).
Highlights
Oral carcinogenesis is characterized by early immune-related gene expression and immune infiltrate changes.
In oral leukoplakia, enrichment in M2 macrophage signatures is associated with improved oral cancer-free survival.
Disclosure of potential conflicts of interest
PS is a member of HTG Diagnostics Scientific Advisory Board and receives research grants from HTG Diagnostics, Inivata, ArcherDx, Bristol-Myers Squibb, Roche Molecular Diagnostics, Roche, AstraZeneca, Novartis, Bristol-Myers Squibb Foundation, and Illumina.
Author contributions statement
All authors have participated to the conception and design, acquisition, analysis, interpretation of data and to the drafting the article or revising it critically for important intellectual content; All authors have approved the final version to be published.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2018;68(6):394–11. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Foy J-P, Bertolus C, Saintigny P. Oral cancer prevention worldwide: challenges and perspectives. Oral Oncol. 2019;88:91–94. doi: 10.1016/j.oraloncology.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Lodi G, Franchini R, Warnakulasuriya S, Varoni EM, Sardella A, Kerr AR, Carrassi A, MacDonald LCI, Worthington HV. Interventions for treating oral leukoplakia to prevent oral cancer. Cochrane Database Syst Rev. 2016;(7)CD001829. doi: 10.1002/14651858.CD001829.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speight PM, Khurram SA, Kujan O. Oral potentially malignant disorders: risk of progression to malignancy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(6):612–627. doi: 10.1016/j.oooo.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Saintigny P, William WN, Foy J-P, Papadimitrakopoulou V, Lang W, Zhang L, Fan YH, Feng L, Kim ES, El-Naggar AK, et al. Met receptor tyrosine kinase and chemoprevention of oral cancer. J Natl Cancer Inst. 2018;110(3):250–257. doi: 10.1093/jnci/djx186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saintigny P, El-Naggar AK, Papadimitrakopoulou V, Ren H, Fan Y-H, Feng L, Lee JJ, Kim ES, Hong WK, Lippman SM, et al. DeltaNp63 overexpression, alone and in combination with other biomarkers, predicts the development of oral cancer in patients with leukoplakia. Clin Cancer Res. 2009;15(19):6284–6291. doi: 10.1158/1078-0432.CCR-09-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouaoud J, De Souza G, Darido C, Tortereau A, Elkabets M, Bertolus C, Saintigny P. The 4-NQO mouse model: an update on a well-established in vivo model of oral carcinogenesis. In: Methods in cell biology.Vol. 163. United States: Academic Press; 2021. pp. 197–229. (Carcinogen-driven mouse models of oncogenesis). [DOI] [PubMed] [Google Scholar]
- 8.Foy J-P, Tortereau A, Caulin C, Le Texier V, Lavergne E, Thomas E, Chabaud S, Perol D, Lachuer J, Lang W, et al. The dynamics of gene expression changes in a mouse model of oral tumorigenesis may help refine prevention and treatment strategies in patients with oral cancer. Oncotarget. 2016;7(24):35932–35945. doi: 10.18632/oncotarget.8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandal R, Şenbabaoğlu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, Lee K-W, Ganly I, Hakimi AA, Chan TA, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1(17). doi: 10.1172/jci.insight.89829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, De Castro G, Psyrri A, Basté N, Neupane P, Bratland Å, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet . 2019;394(10212):1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 12.Ai R, Tao Y, Hao Y, Jiang L, Dan H, Ji N, Zeng X, Zhou Y, Chen Q. Microenvironmental regulation of the progression of oral potentially malignant disorders towards malignancy. Oncotarget. 2017;8(46):81617–81635. doi: 10.18632/oncotarget.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutkind JS, Bui JD. The next frontier: head and neck cancer immunoprevention. Cancer Prev Res Phila Pa. 2017;10(12):681–683. doi: 10.1158/1940-6207.CAPR-17-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foy J-P, Bertolus C, Ortiz-Cuaran S, Albaret M-A, Williams WN, Lang W, Destandau S, Souza GD, Sohier E, Kielbassa J, et al. Immunological and classical subtypes of oral premalignant lesions. OncoImmunology. 2018;7(12):e1496880. doi: 10.1080/2162402X.2018.1496880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saintigny P, Zhang L, Fan Y-H, El-Naggar AK, Papadimitrakopoulou VA, Feng L, Lee JJ, Kim ES, Ki Hong W, Mao L. Gene expression profiling predicts the development of oral cancer. Cancer Prev Res Phila Pa. 2011;4(2):218–229. doi: 10.1158/1940-6207.CAPR-10-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadimitrakopoulou VA, Lee JJ, William WN, Martin JW, Thomas M, Kim ES, Khuri FR, Shin DM, Feng L, Hong WK, et al. Randomized Trial of 13-cis Retinoic Acid Compared With Retinyl Palmitate With or Without Beta-Carotene in Oral Premalignancy. J Am Soc Clin Oncol. 2009;27(4):599–604. doi: 10.1200/JCO.2008.17.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Yang T-HO, Porta-Pardo E, Gao GF, Plaisier CL, Eddy JA, et al. The immune landscape of cancer. Immunity. 2018;48(4):812–830.e14. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez FO, Helming L, Milde R, Varin A, Melgert BN, Draijer C, Thomas B, Fabbri M, Crawshaw A, Ho LP, et al. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood. 2013;121(9):e57–69. doi: 10.1182/blood-2012-06-436212. [DOI] [PubMed] [Google Scholar]
- 19.Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado J de D, Popovich PG, Partida-Sanchez S, Guerau-de-Arellano M. Novel markers to delineate murine M1 and M2 macrophages. PLoS ONE. 2015;10(12):e0145342. doi: 10.1371/journal.pone.0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jha AK, Huang S-C-C, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42(3):419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Tuit S, Salvagno C, Kapellos TS, Hau C-S, Seep L, Oestreich M, Klee K, de Visser KE, Ulas T, Schultze JL. Transcriptional signature derived from murine tumor-associated macrophages correlates with poor outcome in breast cancer patients. Cell Rep. 2019;29(5):1221–1235.e5. doi: 10.1016/j.celrep.2019.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H-J, Li Yim AYF, Griffith GR, de Jonge WJ, Mannens MMAM, Ferrero E, Henneman P, de Winther MPJ. Meta-analysis of in vitro-differentiated macrophages identifies transcriptomic signatures that classify disease macrophages in vivo. Front Immunol. 2019;10(10). doi: 10.3389/fimmu.2019.02887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker M, De Bastiani MA, Parisi MM, Guma FTCR, Markoski MM, Castro MAA, Kaplan MH, Barbé-Tuana FM, Klamt F. Integrated transcriptomics establish macrophage polarization signatures and have potential applications for clinical health and disease. Sci Rep. 2015;5(1):13351. doi: 10.1038/srep13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buscher K, Ehinger E, Gupta P, Pramod AB, Wolf D, Tweet G, Pan C, Mills CD, Lusis AJ, Ley K. Natural variation of macrophage activation as disease-relevant phenotype predictive of inflammation and cancer survival. Nat Commun. 2017;8(1). doi: 10.1038/ncomms16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, Soong DYH, Cotechini T, Anur P, Lin EY, et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell. 2019;35(4):588–602.e10. doi: 10.1016/j.ccell.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS–) vs. alternatively activated macrophages. Front Immunol. 2019:10. doi: 10.3389/fimmu.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaves ALF, Silva AG, Maia FM, Lopes GFM, de Paulo LFB, Muniz LV, Dos Santos HB, Soares JMA, Souza AA, de Oliveira Barbosa LA, et al. Reduced CD8+ T cells infiltration can be associated to a malignant transformation in potentially malignant oral epithelial lesions. Clin Oral Investig. 2019;23(4):1913–1919. doi: 10.1007/s00784-018-2622-8. [DOI] [PubMed] [Google Scholar]
- 30.Kouketsu A, Sato I, Oikawa M, Shimizu Y, Saito H, Tashiro K, Yamashita Y, Takahashi T, Kumamoto H. Regulatory T cells and M2-polarized tumour-associated macrophages are associated with the oncogenesis and progression of oral squamous cell carcinoma. Int. J Oral Maxillofac Surg. 2019;48(10):1279–1288. doi: 10.1016/j.ijom.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Öhman J, Mowjood R, Larsson L, Kovacs A, Magnusson B, Kjeller G, Jontell M, Hasseus B. Presence of CD3-positive T-cells in oral premalignant leukoplakia indicates prevention of cancer transformation. Anticancer Res. 2015;35:311–317. [PubMed] [Google Scholar]
- 32.Qualls JE, Murray PJ. Chapter ten - tumor macrophages: protective and pathogenic roles in cancer development. In: Dyer MA, editor. Current topics in developmental biology. Vol. 94. United States: Academic Press; 2011. pp. 309–328. (Cancer and Development). [DOI] [PubMed] [Google Scholar]
- 33.Weber M, Wehrhan F, Baran C, Agaimy A, Büttner-Herold M, Öztürk H, Neubauer K, Wickenhauser C, Kesting M, Ries J. Malignant transformation of oral leukoplakia is associated with macrophage polarization. J Transl Med. 2020;18(1). doi: 10.1186/s12967-019-02191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yagyuu T, Hatakeyama K, Imada M, Kurihara M, Matsusue Y, Yamamoto K, Obayashi C, Kirita T. Programmed death ligand 1 (PD-L1) expression and tumor microenvironment: implications for patients with oral precancerous lesions. Oral Oncol. 2017;68:36–43. doi: 10.1016/j.oraloncology.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Stasikowska-Kanicka O, Wągrowska-Danilewicz M, Danilewicz M. T cells are involved in the induction of macrophage phenotypes in oral leukoplakia and squamous cell carcinoma-a preliminary report. J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 2018;47(2):136–143. doi: 10.1111/jop.12657. [DOI] [PubMed] [Google Scholar]
- 36.Yagyuu T, Funayama N, Imada M, Kirita T. Effect of smoking status and programmed death-ligand 1 expression on the microenvironment and malignant transformation of oral leukoplakia: a retrospective cohort study. PLOS ONE. 2021;16(4):e0250359. doi: 10.1371/journal.pone.0250359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su S, Zhao Q, He C, Huang D, Liu J, Chen F, Chen J, Liao J-Y, Cui X, Zeng Y, et al. miR-142-5p and miR-130a-3p are regulated by IL-4 and IL-13 and control profibrogenic macrophage program. Nat Commun. 2015;6(1):8523. doi: 10.1038/ncomms9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grigolato R, Bizzoca ME, Calabrese L, Leuci S, Mignogna MD, Lo Muzio L. Leukoplakia and immunology: new chemoprevention landscapes? Int. J Mol Sci. 2020;21(18):6874. doi: 10.3390/ijms21186874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shigeoka M, Koma Y, Nishio M, Akashi M, Yokozaki H. Alteration of macrophage infiltrating compartment: a novel view on oral carcinogenesis. Pathobiology. 2021:1–11. doi: 10.1159/000515922. [DOI] [PubMed] [Google Scholar]
- 40.Liu SX, Gustafson HH, Jackson DL, Pun SH, Trapnell C. Trajectory analysis quantifies transcriptional plasticity during macrophage polarization. Sci Rep. 2020;10(1):12273. doi: 10.1038/s41598-020-68766-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balermpas P, Rödel F, Liberz R, Oppermann J, Wagenblast J, Ghanaati S, Harter PN, Mittelbronn M, Weiss C, Rödel C, et al. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br J Cancer. 2014;111(8):1509–1518. doi: 10.1038/bjc.2014.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palladini A, Landuzzi L, Lollini P-L, Nanni P. Cancer immunoprevention: from mice to early clinical trials. BMC Immunol. 2018;19(1). doi: 10.1186/s12865-018-0253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


