Abstract
Costello syndrome (CS) is a RASopathy caused by activating germline mutations in HRAS. Due to ubiquitous HRAS gene expression, CS affects multiple organ systems and individuals are predisposed to cancer. Individuals with CS may have distinctive craniofacial features, cardiac anomalies, growth and developmental delays, as well as dermatological, orthopedic, ocular, and neurological issues; however, considerable overlap with other RASopathies exists. Medical evaluation requires an understanding of the multifaceted phenotype. Subspecialists may have limited experience in caring for these individuals because of the rarity of CS. Furthermore, the phenotypic presentation may vary with the underlying genotype. These guidelines were developed by an interdisciplinary team of experts in order to encourage timely health care practices and provide medical management guidelines for the primary and specialty care provider, as well as for the families and affected individuals across their lifespan. These guidelines are based on expert opinion and do not represent evidence-based guidelines due to the lack of data for this rare condition.
Keywords: Costello syndrome, HRAS mutation, management guidelines, RAS/MAPK, RASopathy
1 |. INTRODUCTION
An apparently novel neurodevelopmental syndrome having distinctive craniofacial features, high birth weight with subsequent failure to thrive, and ectodermal anomalies including nasal papilloma was reported in two unrelated children in 1971 and 1977 (Costello, 1971; Costello, 1977). The eponym, Costello syndrome (CS; MIM 214080), was applied after a third patient with consistent clinical features was noted to have a similar phenotype (Der Kaloustian, Moroz, McIntosh, Watters, & Blaichman, 1991). The prevalence is estimated at ~1/300,000 live births (Abe et al., 2012; Goriely & Wilkie, 2012).
CS is one of the RASopathies which are a group of medical genetic syndromes caused by germ-line genetic mutations in components and regulators of the RAS/Mitogen-Activated Protein Kinase (MAPK) pathway (Rauen, 2013; Tidyman & Rauen, 2016a). The RAS/MAPK pathway is a well-studied signal transduction pathway with the RAS protein being a small guanosine triphosphate hydrolase, or GTPase, acting as an on–off signaling hub inside the cell. RAS proteins consist of a large family of GTPases of which KRAS, NRAS, and HRAS are the most commonly studied because they are frequently mutated in cancer. The RAS protein has numerous downstream pathway effectors of which the MAPK pathway is the best studied due to its role in tumorigenesis. The RAS/MAPK pathway has essential cellular functions including cell cycle progression, differentiation, transcription, proliferation, apoptosis, and motility. Because of the important nature of RAS in cellular function, perturbing the pathway during development has multisystem consequences (Rauen, 2007). The RASopathies have germline derived dysregulation of this pathway and include neurofibromatosis Type 1 (NF1), Noonan syndrome (NS), Noonan syndrome with multiple lentigines (NSML, formerly called LEOPARD syndrome), Noonan syndrome with loose anagen hair (NS-LAH), cardio-facio-cutaneous syndrome (CFC), capillary malformation-arteriovenous malformation syndrome (CM-AVM), Legius syndrome, and SYNGAP1-related intellectual disability (Tidyman & Rauen, 2016b). Although individually each syndrome may be rare, together the RASopathies represent a common group of neurodevelopmental syndromes affecting more than 1 in 1,000 individuals. CS affects multiple organ systems and shows phenotypic overlap with other RASopathies. For these reasons, an international panel of CS experts was convened to create management guidelines for health care professionals. The overarching goal of these guidelines is to assist in the clinical and molecular diagnoses, as well as the medical management of CS individuals across their lifespan by providing the most current medical and scientific information for families and medical providers. These guidelines were developed by expert opinion and do not represent evidence-based guidelines due to the lack of data for this rare condition.
2 |. MOLECULAR GENETICS
Costello syndrome is caused by specific heterozygous activating mutations in HRAS, a highly conserved gene located on 11p15.5 and encoding the Harvey rat sarcoma viral oncogene homologue, HRAS (Aoki et al., 2005). While somatically acquired HRAS mutations are associated with sporadic tumors, CS is typically the result of heterozygous, de novo germline mutations in HRAS (Estep, Tidyman, Teitell, Cotter, & Rauen, 2006; Gripp et al., 2006; Kerr et al., 2006; van Steensel et al., 2006). HRAS mutations associated with CS result in a gain-of-function, which causes constitutive activation of the RAS protein (Aoki et al., 2005) or more complex dysregulation (Gripp et al., 2015; Gripp, Kolbe, Brandenstein, & Rosenberger, 2017) of the RAS/MAPK pathway.
Most HRAS mutations are paternally derived and associated with advanced paternal age (Aoki et al., 2005; Estep et al., 2006; Giannoulatou et al., 2013; Sol-Church, Stabley, Nicholson, Gonzalez, & Gripp, 2006; Zampino et al., 2007). The identification of a known CS-associated germline HRAS mutation confirms the diagnosis of CS and may clarify the diagnosis in individuals whose phenotype overlaps with other RASopathies. For novel variants, a careful review and validation is necessary (Grant et al., 2018). Molecular confirmation of the clinical diagnosis assists in clarifying risks based upon genotype–phenotype correlations (Table 1). This implies that adults clinically diagnosed with CS prior to the identification of causative HRAS mutations should now be tested. Failure to identify an HRAS mutation is most commonly due to the individual being affected by a different syndrome, typically another RASopathy (Table 2). However, somatic mosaicism should be considered in individuals with clinical features consistent with CS or individuals with a milder presentation of phenotypic features involving only limited organ systems (Gripp et al., 2017; Gripp, Stabley, Nicholson, Hoffman, & Sol-Church, 2006; Sol-Church et al., 2009). Given that the majority of individuals with CS have a de novo mutation, the risk to siblings is low; however, affected siblings have been reported (Gripp et al., 2011) and gonadal mosaicism in a parent was confirmed in one family (Gripp, Stabley, et al., 2011).
TABLE 1.
Costello syndrome management recommendations
| Clinical specialty | Recommendations |
|---|---|
| Molecular genetics | At risk for: All individuals suspected or known to have Costello syndrome should have a thorough evaluation with medical genetics |
At diagnosis:
| |
Ongoing management:
| |
| Cardiology | At risk for: Pulmonic valve stenosis, hypertrophic cardiomyopathy (HCM), arrhythmia, septal defects, aortic dilation |
At diagnosis:
| |
Ongoing management:
| |
| Neurology | At risk for: Macrocephaly, hydrocephalus, Chiari I malformation, syrinx, tethered cord, seizures |
At diagnosis:
| |
Ongoing management:
| |
| Neurocognitive/psychological function | At risk for: Intellectual disability, speech and language impairment, orthopedic impairment, delayed/deficient fine- and gross-motor skills, impaired adaptive functioning |
At diagnosis:
| |
Ongoing management:
| |
| Endocrinology | At risk for: Failure to thrive, short stature, growth hormone deficiency, hypoglycemia, delayed or precocious puberty |
At diagnosis:
| |
Ongoing management:
| |
| Gastroenterology | At risk for: Failure to thrive due to feeding and/or swallowing difficulties, pyloric stenosis, gastroesophageal reflux, and increased resting metabolism, constipation |
At diagnosis:
| |
Ongoing management:
| |
| Respiratory/Otolaryngology | At risk for: Structural upper and lower airway anomalies, frequent infections, central and obstructive apnea, cardiopulmonary disease |
At diagnosis:
| |
Ongoing management:
| |
| Dental | At risk for: Delayed tooth eruption, malocclusion, cross bite, bruxism, and enamel erosion |
At diagnosis:
| |
Ongoing management:
| |
| Musculoskeletal | At risk for: Vertical talus, pectus, hip dysplasia/subluxation, tight heel cords, shoulder and elbow contractures, ulnar deviation of the wrists, scoliosis, osteopenia/osteoporosis, and weakness |
At diagnosis:
| |
Ongoing management:
| |
| Genitourinary | At risk for: Cryptorchidism or labial anomalies, kidney malformation, vesicoureteral reflux, inguinal hernia, transitional cell carcinoma of the bladder beginning in adolescence |
At diagnosis:
| |
Ongoing management:
| |
| Ophthalmology | At risk for: Amblyopia, ptosis, nystagmus, refractive error, strabismus, optic nerve hypoplasia, optic atrophy, cortical visual impairment and delayed visual maturation, keratoconus |
At diagnosis:
| |
Ongoing management:
| |
| Oncology | At risk for: Benign tumors, embryonal rhabdomyosarcoma, bladder carcinoma, neuroblastoma |
At diagnosis:
| |
Ongoing management:
| |
| Dermatology | At risk for: Papillomas, palmoplantar keratoderma, acanthosis nigricans |
At diagnosis:
| |
Ongoing management:
| |
| Adulthood |
Ongoing management:
|
TABLE 2.
Differential diagnosis of Costello syndrome
| Syndrome | Common features with CS | Differences with CS |
|---|---|---|
| Noonan syndrome | Hypertelorism, downslanting palpebral fissures, ptosis, short stature, relative macrocephaly, PVS, HCM, ASD, hypotonia, some with neurocognitive delay. | Facial features less coarse, lower incidence of severe feeding problems, fewer cutaneous features, lower incidence of neurocognitive delay. |
| Cardio-facio-cutaneous syndrome | Hypertelorism, downslanting palpebral fissures, curly hair, broad nasal bridge, epicanthal folds, PVS, HCM, pectus deformity, short stature, feeding difficulties, hypotonia, neurocognitive delay. | Facial features less coarse, higher incidence of neurocognitive delay, seizures, progressive mole formation, keratosis pilaris, ulerythema ophryogenes, |
| Noonan syndrome with multiple lentigenes (formerly LEOPARD syndrome) | Short stature, hypertelorism, PVS, HCM, conduction abnormalities, hypotonia, some with cognitive delay. | Multiple skin lentigines, frequent sensorineural deafness, conduction abnormalities. |
| Noonan syndrome with loose anagen hair | Triangular facies, macrocephaly, hypertelorism, high forehead, sparse thin hair, short stature, eczema, dry skin, hyperpigmentation, hypotonia. |
After infancy facial features less coarse, mitral valve dysplasia. |
| Beckwith-Wiedemann syndrome | Macrosomia at birth, coarse facial features, neonatal hypoglycemia, HCM, visceromegaly, hypotonia, embryonal tumors. | Ear creases/pits, macroglossia, omphalocele, renal abnormalities, hemihyperplasia. |
| Simpson-Golabi-Behmel syndrome | Macrosomia, coarse facial features, visceromegaly, developmental delay. | Macroglossia, renal anomalies, cleft lip, polydactyly. |
| Williams syndrome | Coarse facial features, full lips with large mouth, soft skin, ligamentous laxity, hyponotonia, feeding difficulties. | Elastin arteriopathy, peripheral pulmonary stenosis, supravalvar aortic stenosis, unique personality characteristics, hypercalcemia. |
| Lysosomal storage disorders | Coarse facial features, hypotonia. | Does not have serum and urine biochemical profile. |
3 |. MUTATIONS AND GENOTYPE–PHENOTYPE CORRELATIONS
Although data are limited given the rarity of CS, some genotype–phenotype correlations have been reported (Table 3). More than 95% of CS-causing HRAS gene mutations involve the amino acid glycine at Position 12 or 13 in HRAS (Sol-Church & Gripp, 2009). Glycine 12 and 13 are important for GTP-binding, affecting the activation of the RAS/MAPK signaling cascade (van Steensel et al., 2006). Approximately 80% of mutations result in a p.G12S missense change and as a result, this mutation is associated with the classic CS phenotype (Figure 1). p.G12A is the second most common missense mutation reported in CS. This mutation may have a higher rate of malignancy and individuals may have a more severe phenotype (Figure 2). A severe neonatal phenotype has been reported with p.G12D, p.G12C, and p.G12E missense mutations resulting in severe cardiomyopathy, pleural, and pericardial effusion, and lung abnormalities (Kerr et al., 2006; Lo et al., 2008; Weaver et al., 2014). The p.G12V missense mutation is associated with severe cardiomyopathy and tachycardia, as well as respiratory distress resulting in early death (Aoki et al., 2005; Quezada & Gripp, 2007; Sol-Church & Gripp, 2009). Detailed functional studies based on an unusual patient with a nonlethal phenotype due to a c.35_36GC>TG mutation (p.G12V) demonstrated the effect of alternative splicing on the phenotypic presentation (Hartung et al., 2017). The p.G13C mutation may be associated with a milder phenotype characterized by taller stature, absence of ulnar wrist deviation and a lower risk for malignant tumors or papillomata (Figure 2; Sol-Church & Gripp, 2009; Gripp et al., 2011).
TABLE 3.
Genotype–phenotype correlations in Costello syndrome
| HRAS mutation | Clinical phenotype |
|---|---|
| p.G12S | Classic features of CS |
| p.G12C | Severe neonatal phenotype—severe cardiomyopathy, pleural and pericardial effusion, and lung abnormalities |
| p.G12D | Severe neonatal phenotype—severe cardiomyopathy, pleural and pericardial effusion, and lung abnormalities |
| p.G12A | Higher rate of malignancy |
| p.G12V | Severe cardiomyopathy and tachycardia as well as respiratory distress; typically lethal |
| p.G13C | Milder symptoms with lower risk for malignant tumors or papillomata, taller stature, and absence of the classic CS ulnar wrist deviation |
| p.Q22K | Classic features of CS plus congenital myopathy |
| p.T58I | Facial features tend to be less coarse |
| p.G60D | Milder phenotype—reported with maternal transmission |
| p.G60V | Only one case reported—infant death |
| p.E63K | Classic features of CS plus congenital myopathy |
| p.E63_D69dup | Milder symptoms—milder intellectual disability, fewer feeding issues, and a lower risk of tumors |
| p.K117R | Facial features tend to be less coarse |
| p.A146P | Facial features tend to be less coarse |
| p.A146T | Milder symptoms—minor skin and joint involvement and milder growth restriction. Microcephaly and sparse, thin hair are also reported. |
| p.A146V | Facial features tend to be less coarse |
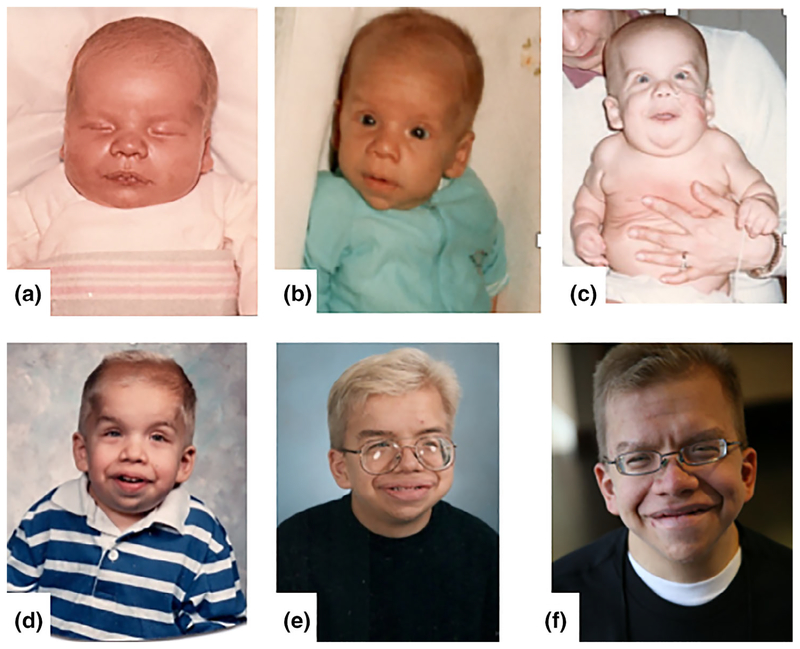
FIGURE 1.
Images of an individual male with the most common heterozygous HRAS p.G12S missense mutation. The images demonstrate the classical Costello syndrome craniofacial phenotype. This figure depicts the evolution of his features from birth (a), to 5 months of age (b), one and one-half years of age (c), four and one-half years of age (d), 15 years of age (e), and 23 years of age (f)
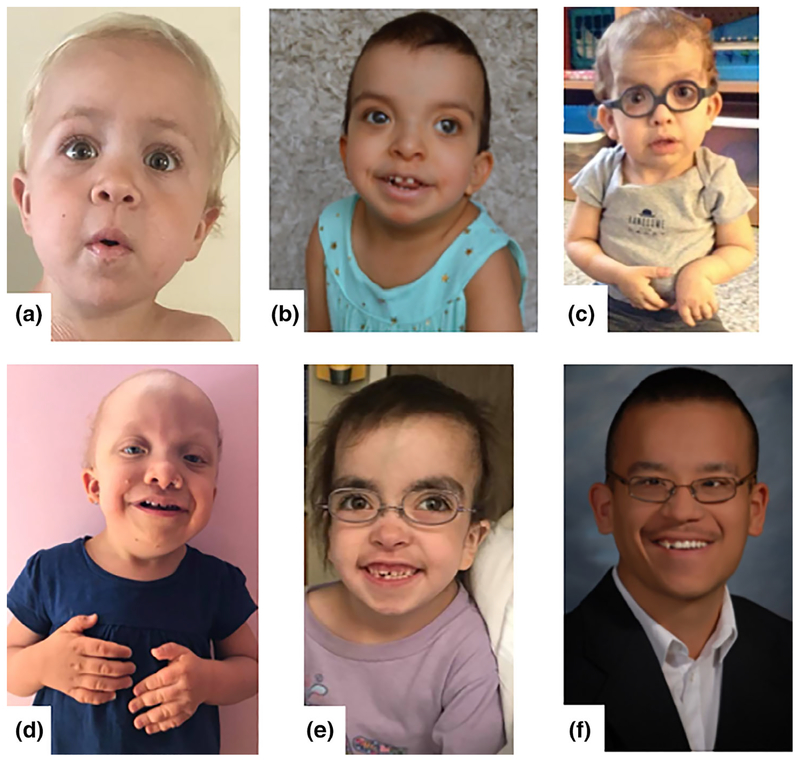
FIGURE 2.
Images of individuals with rarer HRAS missense mutations. (a) A 3-year-old boy with a heterozygous HRAS p.F156L missense mutation. (b) A 3-year-old girl with a heterozygous HRAS p.Q22K missense mutation. (c) A three and one-half-year-old boy with a heterozygous HRAS p.G12A missense mutation. (d) A five and one-half-year-old girl with a heterozygous HRAS p.G12C missense mutation. (e) A 6-year-old girl with a heterozygous HRAS p.G13C missense mutation. (f) A 26-year-old man with a heterozygous HRAS p.G13D missense mutation
Among the less common mutations, HRAS p.E63_D69dup causes an attenuated phenotype with milder intellectual disability, fewer feeding issues, and a lower tumor risk (Lorenz et al., 2013; Xu, Wang, Lin, & Yu, 2015). Facial features tend to be less coarse in individuals with the rarer HRAS missense mutations p.T58I, p.K117R, p.A146V, and p.A146P (Chiu et al., 2016; Gripp et al., 2008; Kerr et al., 2006). HRAS p.A146T has been associated with a milder presentation resulting in minor skin and joint involvement and milder growth restriction. HRAS p.G60D is associated with an overall milder phenotype and parental transmission (Gripp et al., 2015), whereas the only individual reported with HRAS p.G60V died in infancy (Gripp et al., 2017).
4 |. PRENATAL FINDINGS
A prenatal diagnosis of CS should be considered in fetuses with increased nuchal translucency (including cystic hygroma), polyhydramnios, ulnar deviation of the wrists, hypertrophic cardiomyopathy, or fetal tachycardia (Lin et al., 2009; Quezada & Gripp, 2007; Smith, Podraza, & Proud, 2009; Van den Bosch et al., 2002). Polyhydramnios is present in more than 70% and may be related to reduced fetal swallowing (Lin et al., 2009; Myers et al., 2014; Smith et al., 2009; Van den Bosch et al., 2002). Fetuses with CS tend to be large for gestational age which contrasts the failure to thrive and growth delays seen postnatally (Lin et al., 2009; Quezada & Gripp, 2007; Smith et al., 2009; Van den Bosch et al., 2002). Fetal tachyarrhythmia is fairly specific to CS (Myers et al., 2014). Preterm labor is common, as is the need for early delivery due to complications from fetal overgrowth, polyhydramnios, or fetal distress (Lin et al., 2009; Piccione et al., 2009; Smith et al., 2009). Ultrasound findings may include macrocephaly, ventriculomegaly, shortened long bones, and pyelectasis. Many fetal characteristics and prenatal ultrasound findings overlap with CFC or NS, emphasizing the importance of molecular testing.
5 |. CARDIOVASCULAR DISEASE
Cardiovascular disease is present in 85% of individuals including hypertrophic cardiomyopathy (HCM), congenital heart defects (CHD), dysrhythmias and/or hypertension. Cardiovascular disease is the major contributor to morbidity and mortality in the first years of life (Gelb, Roberts, & Tartaglia, 2015; Lin et al., 2011). HCM occurs in ~60%, accounting for 75% of cardiovascular pathology (Lin et al., 2011). Hypertrophy can be asymmetric/septal or concentric, with left ventricular or biventricular involvement (Lin et al., 2011). The clinical course varies from a rare severe neonatal lethal form, to the typical mild to moderate form of HCM seen in the majority (Burkitt-Wright et al., 2012; Lorenz et al., 2012). While long-term natural history data remain to be collected, follow-up of 146 patients ranging in age from 1 month to 40 years (with only 13 individuals >18 years) indicates that many had chronic or progressive hypertrophy (37%), a quarter had stable disease, and a fraction (14%) had improvement or even resolution (Lin et al., 2011).
A CHD is identified in 45% of individuals (Lin et al., 2011). Pulmonary valve stenosis (PVS) is the most frequent CHD (15–20%) and may be accompanied by subvalvar and supravalvar pulmonary stenosis, and double-chambered right ventricle. In most patients with PVS, the mild–moderate obstruction requires no intervention. Atrial septal defects (ASD) are uncommon (5–7%). Other infrequent defects include ventricular septal defect, mitral valve abnormalities, aortic thickening or stenosis, bicuspid aortic valve, coarctation of the aorta, patent ductus arteriosus, and aortic root dilation. Coronary artery anomalies have been reported in autopsy specimens, characterized as premature coronary disease, and coronary fibromuscular dysplasia (Kerr et al., 2006; Lin et al., 2011).
Atrial arrhythmias are very common in CS, seen in over 50%, and are typically characterized as nonreentrant atrial tachycardias (NRAT), ectopic atrial tachycardia, multifocal, or chaotic atrial tachycardia (Levin et al., 2018; Lin et al., 2011). Nonreentrant atrial tachycardias are often diagnosed in the first year of life and are stable or resolve in 70%. Later-onset atrial arrhythmias, namely atrial fibrillation and flutter, have been reported, whereas, ventricular arrhythmias appear to be rare.
Because of the high prevalence of cardiovascular disease, urgent pediatric cardiology consultation is indicated upon diagnosis of CS. The evaluation includes echocardiography (ECHO), electrocardiography (ECG), and continuous telemetry or Holter monitoring. HCM and NRATs are most likely to present in infancy, so frequent surveillance in the first 2 years of life is appropriate. Reevaluation in early childhood is dictated by abnormalities, with intervals determined by age in the absence of cardiovascular disease (Table 1). Treatment of HCM includes medical therapies to reduce heart rate and outflow obstruction as per published guidelines (Gersh et al., 2011). Severe outflow obstruction has been treated with septal myectomy. Conventional antiarrhythmic treatment of NRATs by a pediatric electrophysiologist is effective in most patients (Bradley, Fischbach, Law, Serwer, & Dick 2nd., 2001; Salerno, Kertesz, Friedman, & Fenrich, 2004). Surgical correction of CHDs is rarely necessary. Little is known about whether HCM and other myocardial disease can develop at older ages. All individuals with CS, even those who have had previous normal ECHO or underwent surgical repair of CHD as young children, should have periodic cardiac reevaluation by a cardiologist. Hypertension (Estep et al., 2006; Lin et al., 2011) and sudden death (presumed cardiac) is not uncommon (Lin et al., 2011). Given the risk for acquired and progressive cardiovascular abnormalities, screening for HCM with ECHO and ECG, early coronary disease, lipidopathy and hypertension is warranted throughout life (Gersh et al., 2011). Short and long-term outcomes of surgery in CS are not described, but higher surgical mortality related to comorbidities must be considered.
6 |. NEUROLOGIC FINDINGS
Neurologic findings are common and include structural and functional abnormalities. Structural central nervous system findings include absolute or relative macrocephaly, ventricular dilatation, crowding in the posterior fossa that may be severe enough to meet criteria for Chiari 1 malformation, and rarely Dandy-Walker malformation (Delrue, Chateil, Arveiler, & Lacombe, 2003; Gripp, Hopkins, Doyle, & Dobyns, 2010; Gripp & Lin, 2006; Gripp & Lin, 2012). While these findings may be present in early infancy, they can progress. Crowding in the posterior fossa is at least partially attributable to bony posterior fossa hypoplasia despite normal hindbrain volume (Calandrelli et al., 2015). A combination of typical infantile brain growth and possibly HRAS mutation driven overgrowth with decreased cerebellar fossa size and altered cranial shape predisposes to cerebellar tonsillar herniation that when severe presents as Chiari 1 malformation (Calandrelli et al., 2015; Paquin, Hordo, Kaplan, & Miller, 2009). Crowding in the posterior fossa and cerebellar tonsillar herniation through the foramen magnum can impede cerebellar spinal fluid flow, contributing to enlarged ventricles and syringomyelia formation. Syringomyelia may result in peripheral nervous system symptoms such as weakness, pain, or abnormal sensation. Tethered cord is more common than reflected in the literature and should be suspected in all individuals (Gripp et al., 2010). It is currently unclear if progressive Chiari I malformation, syringomyelia, or tethered cord and their neurologic consequences contribute to the development of scoliosis, developmental hip dysplasia, tight calcaneal tendons, and hand or foot positional abnormalities. Seizures occur with increased incidence (Kerr et al., 2006) and are treated as in the general population. No particular type or age of onset for seizures predominates. Due to the risk for hyperinsulinemic hypoglycemia in young infants (Gripp et al., 2015) or growth hormone deficiency related hypoglycemia in older individuals (Gripp, Scott Jr., Nicholson, & Figueroa, 2000), new onset seizures should prompt an evaluation for hypoglycemia.
Neurologic management in CS is life-long and referral to Neurology is important at diagnosis (Table 1). Serial clinical exams need to focus on gait abnormalities including toe-walking, tendon reflexes, and other signs of slowly progressive cord disease. When Chiari I malformation or syringomyelia is symptomatic or shows significant progression on imaging studies, neurosurgical consultation is indicated. This often leads to posterior fossa decompression, and sometimes repeat decompression (Gripp et al., 2010). For concerns of tethered cord, lower spine imaging at diagnosis or by age 1 year is indicated. Because tethered cord can be difficult to identify with certainty on imaging studies, a high index of suspicion should remain and symptomatic individuals should be re-imaged. Preliminary data suggest that many individuals with CS may have six lumbar vertebra and, therefore, it is important to count the vertebral level from the cervical spine down to appropriately ascertain the level of the conus.
7 |. NEUROCOGNITIVE FUNCTION
Intellectual disability occurs in ~80% of individuals with CS (Axelrad et al., 2009; Axelrad, Schwartz, Katzenstein, Hopkins, & Gripp, 2011; Cesarini et al., 2009). The majority fall in the mild to moderate range, with roughly one in five showing more severe impairment, and one in 10 showing low average-to-average performance. Nonverbal fluid reasoning is a relative strength, with about 20% of individuals falling in the low average range, whereas verbal reasoning and visual–spatial skills are areas of relative weakness. Preliminary evidence suggests that individuals with the p.G13C missense mutation have better cognitive and adaptive functioning (Axelrad et al., 2009, 2011; Gripp, Stabley, et al., 2011).
Most individuals with CS show speech/language delay, with first words generally occurring between the first and second year of life (Gripp, Stabley, et al., 2011). Speech onset often coincides with resolution of early feeding problems and toleration of oral feeds (Gripp & Lin, 2012). Speech/motor impairment persists (White et al., 2005), although a majority of individuals are able to speak in full sentences by adulthood (Hopkins et al., 2010) and some individuals successfully learn sign language (White et al., 2005). Standardized assessments reveal overall language abilities in the mild to moderate range of disability (Axelrad et al., 2009; Schwartz et al., 2013), though functional language comprehension may be better in familiar environments. Expressive language is typically worse than language comprehension, likely due to speech and/or articulation difficulties. Vocabulary development may accelerate slightly in adolescence or early adulthood.
Neuropsychological assessment of attention is challenging, as participants have had difficulty understanding task directions. Parent report indicates attention problems in approximately one-third of individuals with CS (Alfieri et al., 2014), though attention is probably commensurate with overall development. Verbal recall memory is generally in the mild to moderate range of disability (Axelrad et al., 2009; Dileone et al., 2010; Schwartz et al., 2013). In contrast, verbal recognition memory appears largely spared, falling in the low-average to average range (Schwartz et al., 2013). Memory for narrative information is better developed than memory for less structured information, such as word lists. Visual–spatial memory ranges from mild to severe disability (Axelrad et al., 2009; Axelrad, Nicholson, Stabley, Sol-Church, & Gripp, 2007; Dileone et al., 2010). Visual-motor abilities appear to be a relative weakness. Fine-motor deficits characterize most individuals and are compounded by positional hand anomalies and movement limitations.
Academic abilities are generally at an early grade-school level. Based on standardized testing, most individuals attain basic word reading and spelling skills between a kindergarten to second grade level, while a few progress to a 4th–6th grade level. Math calculation skills generally fall at a kindergarten to third grade level. Reading comprehension and applied math problem-solving skills tend to be less well developed (Schwartz et al., 2013).
Adaptive behavior is generally commensurate with intellectual functioning. Social skills tend to be better developed, whereas practical daily living skills tend to be weaker, in part due to orthopedic disability. Females tend to have better social and communication skills, and moderately better daily living skills. Most individuals with CS attain a limited degree of independence as adults, being able to feed, clean, and dress themselves with minimal help (White et al., 2005), and more than half can search the internet on their own; however, most are unable to complete more complex tasks such as managing money (Hopkins et al., 2010).
Most individuals with CS require specialized programming in school, typically in a life skills placement (Table 1). Children in the US should be given a comprehensive Individualized Education Plan. Learning may be facilitated by embedding content in narrative formats, and knowledge may be best assessed using multiple-choice formats, which are preferred to more open-ended questions. Children should be referred for speech/language evaluation to make recommendations for speech therapy, and for assistive technology evaluation to determine whether an assistive communication device might prove helpful. Use of an assistive communication device or Picture Exchange Communication System helps children with developmental disabilities communicate (Ganz, Davis, Lund, Goodwyn, & Simpson, 2012). Children with more severe speech problems can be taught sign language. For motor deficits, individuals should be referred for occupational and physical therapy and, in school, an orientation and mobility evaluation should also be completed.
8 |. SOCIAL, EMOTIONAL, AND BEHAVIORAL FUNCTION
Infants and toddlers with CS have been described as characteristically withdrawn, irritable, and hypersensitive to touch, which may be associated with underlying feeding and medical complications (Galéra et al., 2006; Gripp et al., 2010; Kawame et al., 2003). Early feeding difficulties and irritability both decrease over time (White et al., 2005). Many children younger than age 4 years also show elevated symptoms of autism-spectrum disorder (Adviento et al., 2014), though it is unclear if older children may emerge out of an autistic-like presentation (Schwartz et al., 2017; Young, Perati, Weiss, & Rauen, 2018). In later childhood and adolescence, social skills emerge as a relative strength, especially among females. Individuals have a distinct personality profile as they age, including agreeableness and sense of humor (Bizaoui, Gage, Brar, Rauen, & Weiss, 2018) and are often described as sociable and friendly (Gripp & Lin, 2012).
Individuals with CS experience elevated rates of internalizing problems, including separation anxiety and school anxiety (Axelrad et al., 2011; Galéra et al., 2006; Kawame et al., 2003). These symptoms tend to be most prevalent in males and persons with lower cognitive ability, suggesting that they find school stressful. Anxiety should be assessed in school-aged children. Children may respond well to family-based intervention for anxiety with an emphasis on exposure therapy. Parents may also benefit from support as having a medically complex child can be stressful.
About half of individuals show mild behavior problems such as temper tantrums and disobedience (Axelrad et al., 2009), although compared to normative samples, relatively few behavior problems are reported (Alfieri et al., 2014; Axelrad, Glidden, Nicholson, & Gripp, 2004). In CS adults, parents reported quality of life is inversely related to medical issues (Hopkins et al., 2010).
9 |. ENDOCRINOLOGIC FINDINGS
Endocrinopathies common in CS include neonatal hyperinsulinism, hypoglycemia, growth hormone (GH) deficiency, and problems with puberty. Neonates and infants are at high risk for hypoglycemia and should be screened immediately after birth and during ongoing medical care in the first year. Blood sugar levels less than 70 mg/dL should be addressed according to the Pediatric Endocrine Society recommendations (Thornton et al., 2015) and blood sugar equal to or less than 50 mg/dL should have a diagnostic sample (glucose, GH, insulin, cortisol, beta-hydroxybutyrate) obtained at that time to define the etiology of low blood sugar to guide management. Neonatal hyperinsulinism (Alexander et al., 2005; Sheffield et al., 2015), GH deficiency (Gregersen & Viljoen, 2004; Stein, Legault, Daneman, Weksberg, & Hamilton, 2004), and late dumping syndrome due to gastrostomy and fundoplication can cause or contribute to hypoglycemia (Calabria, Gallagher, Simmons, Blinman, & De León, 2011) with each requiring specific medical management. Infants or young individuals often present with low serum glucose due to GH deficiency and symptoms may include syncope or seizures. The incidence of GH deficiency as defined by an abnormal growth hormone stimulation test result may be as high as 30% (Estep et al., 2006; Gripp et al., 2010; unpublished data).
Delayed or dysregulated puberty may be due to the relationship between body fat stores and initiation of pubertal development. Individuals with CS typically have low body fat mass and delayed puberty (White et al., 2005). Current pediatric endocrine society guidelines dictate that females without development of secondary sex characteristics by age 14 and males without the development of secondary sex characteristics by age 15 should be evaluated for causes of hypogonadism, such as gonadotropin deficiency or gonadal failure. Precocious puberty has also been described (Kerr et al., 2006). Its etiology should be differentiated between an early rise in the central signal for puberty (early gonadotropin rise) or autonomous function of a sex steroid producing gonadal or other tumor. Different hormone producing tumors such as focal hyperinsulinism (Dickson et al., 2004; Gripp et al., 2016) and parathyroid adenoma (Cakir, Arici, Tacoy, & Karayalcin, 2004) have occurred.
Evaluation for GH deficiency includes measurement of insulin growth factor (IGF) levels followed by a GH stimulation test (Table 1). Once a diagnosis of GH deficiency is obtained, but before treatment with GH is begun, a thorough cardiac evaluation should be completed. Patients should be monitored every 6 months for the first year of GH replacement for development of hypertrophic cardiomyopathy. Individuals treated with GH should follow existing guidelines for surveillance for rhabdomyosarcoma (Gripp et al., 2002) since growth hormone is a mitogen which may affect the growth rate of neoplastic cells. The goal of GH replacement is the prevention of hypoglycemic episodes and the anecdotally reported increase in muscle tone and strength, rather than increased height growth. No systematically obtained outcome data are available to document the benefit of GH replacement.
Management for early life hyperinsulism may include diazoxide with close monitoring for cardiac status. Appropriate management for pubertal issues depends on the patient’s height and bone age and the initiation of sex steroid replacement may be postponed to augment final height. Bone mineral density must be investigated in patients with delayed puberty and, if low, may be treated with calcium, vitamin D, sex steroid replacement, or bisphosphonates. Gonadotropin-releasing hormone agonists may be used for central causes of precocious puberty and tumors may require excision.
10 |. GASTROENTEROLOGIC FINDINGS
Severe feeding problems and failure to thrive are almost universal in young children with CS. Feeding difficulties include suck and swallowing dysfunction, severe gastroesophageal reflux, and oral aversion. Weak suck and swallow dysfunction originate from the fetal period and continue into childhood. Contributing factors are macroglossia and oral hypersensitivity. Gastroesophageal reflux disease (GERD) with extensive vomiting, irritability, and disrupted sleep is a frequent and disabling issue (Kawame et al., 2003; Leoni et al., 2016). The combination of GERD and swallowing difficulties contribute to lack of weight gain and respiratory complications like choking and aspiration pneumonia. Oral aversion can be triggered by negative stimuli like choking, vomiting, nasogastric tube placement, and sensory integration difficulties. Poor general condition with cardiac and pulmonary manifestations of CS as well as generalized hypotonia can contribute to poor oral intake (Digilio et al., 2008; Lo et al., 2008; Myers et al., 2014). Pyloric stenosis is relatively common (5/58 in Gripp et al., 2008; 1/3 in Digilio et al., 2008) and should be considered in infants aged 2–4 months with progressive vomiting. Individuals can display gastrointestinal motility disorder with intestinal pseudo-obstruction and chronic constipation.
Despite extensive therapy and supplemental feeding, infants can have a characteristic appearance of malnutrition, which can disguise distinctive dysmorphic features, thus eluding the clinical recognition of the syndrome (Chiu et al., 2016; Zampino et al., 2007). Generally, feeding difficulties diminish over time and most children take oral feeds between age 2–4 years. Remarkably, the first acceptable tastes are often spicy and strong (Gripp & Lin, 2006). Most teenagers and adults eat independently (Abe et al., 2012; Hopkins et al., 2009; White et al., 2005). In individuals with the HRAS p.G13C mutation, feeding problems may be milder and of limited duration (Gripp, Stabley, et al., 2011).
The treatment of feeding difficulties is complex and requires a multidisciplinary team consisting of a pediatrician, gastroenterologist, dietitian, and feeding therapist (Table 1). Conservative measures like positioning, hypoallergenic or thickened formula, blended diet, and frequent or continuous feedings have limited success. Treatment with proton pump inhibitors may be of value. Prokinetic agents should be considered if gastrointestinal motility disorder is suspected. However, these drugs may have important adverse effects which may lead to arrhythmia. Swallow studies, including flexible endoscopy, and additional gastrointestinal imaging is often indicated in the evaluation and management of dysphagia, gastroesophageal reflux, and pulmonary aspiration. Most infants require a nasogastric tube or percutaneous gastrostomy (Leoni et al., 2016). Placement of gastroduodenal or gastrojejunal tube, jejunostomy, or fundoplication may be necessary due to severe reflux or impaired gastric motility (Lightdale & Gremse, 2013). Adult-onset GERD may be related to Chiari malformation (Hopkins et al., 2010; White et al., 2005). Because vomiting is a recognized Chiari malformation symptom, GERD in older individuals also merits neurological evaluation and possibly a brain MRI. Individuals with CS have an increased resting energy expenditure (Leoni et al., 2016) measured by indirect calorimetry, likely reflecting an increased cellular basal metabolism and contributing to failure to thrive despite normal to high daily caloric intake (Leoni et al., 2016). Normative growth charts for CS individuals receiving medical care have been published (Sammon et al., 2012).
11 |. RESPIRATORY AND OTOLARYNGOLOGIC FINDINGS
Complex pulmonary and airway co-morbidities are present in a significant proportion of neonates and infants with CS (Gomez-Ospina et al., 2016; Myers et al., 2014), and are more common and severe than in the general population, even accounting for prematurity. Upper and lower airway abnormalities as well as abnormalities of the lung parenchyma such as chronic lung disease occur. In infancy and early childhood, redundant nasal tissue, relative macroglossia, laryngomalacia, hypopharyngeal wall collapse, or nonspecific airway obstruction can require an epiglottoplasty or tracheostomy placement. Anecdotally, mucus production is increased and may compound respiratory problems due to hypotonia and swallowing dysfunction limiting mucus clearance from the airway. Chiari I malformation can result in dysphagia and central sleep apnea. Tracheobronchomalacia, chronic lung disease of infancy, and frequent respiratory tract infections tend to improve with age related growth and development. Other problems may arise, including adenoid and tonsillar hypertrophy. Nasal papillomata are common in older individuals and may require removal. Obstructive or central sleep apnea (Della Marca et al., 2006), ongoing parenchymal lung injury, or the evolution of cardiopulmonary disease require early recognition and treatment (Gomez-Ospina et al., 2016).
The pulmonary evaluation and management is individualized, but common tests may include chest and airway imaging, flexible (dynamic) and rigid (static) laryngoscopy and bronchoscopy, and polysomnography (Table 1). Chest and airway imaging can provide an evaluation of the lung parenchyma and evaluate for airway narrowing or tracheobronchomalacia. Airway endoscopy will confirm the presence or absence of a specific fixed airway lesion such as subglottic stenosis, or a dynamic airway upper or lower airway problem such as airway malacia. Polysomnography will assess control of breathing and confirm the presence or absence of sleep disordered breathing. Additional imaging may include CT scanning to provide a more detailed assessment of lung parenchyma and with IV contrast, a more detailed assessment of pulmonary blood flow. Assistance with anesthesia planning is an important role of the pulmonary consultant. A collaborative, multidisciplinary approach is preferred and involves the expertise and skills of a pulmonologist and otolaryngologist. Evaluation of potential cardiopulmonary interactions, particularly in patients with congenital heart disease, and ensuring a safe and efficacious feeding and nutritional plan may require input from cardiology, gastroenterology, speech therapy, and nutrition specialists.
12 |. DENTAL AND ORAL FINDINGS
Dental and oral issues in CS are perhaps the most severe of all RASopathies. Individuals with CS have oral habits including a secondary tongue thrust, open mouth posture, and excessive teeth grinding/bruxism, resulting in an anterior open bite with a posterior crossbite (Goodwin et al., 2014). Individuals have a significantly increased incidence of Class III malocclusion (37%) whereby the maxillary first molar is positioned posteriorly to the mandibular first molar (Goodwin, Oberoi, et al., 2014). The majority have a narrow, high-arched palate with thickening of the posterior maxillary and the anterior mandibular alveolar ridge. Malocclusion and palate issues can contribute to obstructive sleep apnea. Gingival hypertrophy is common (Hart et al., 2002). Most (93%) have delayed dental development with delayed eruption. They typically do not show increased dental crowding, hypodontia, supernumerary teeth, or abnormal tooth morphology. Microdontia has rarely been reported (Takahashi & Ohashi, 2013). Nearly all individuals with CS have an enamel defect characterized by demineralized white focal and striation lesions allowing pathologic wear due to increased susceptibility to abrasion and caries (Goodwin et al., 2014; Goodwin, Oberoi, et al., 2014).
Medical management includes regular dental exams with a general or pediatric dentist (Table 1). It is not uncommon for CS individuals to require anesthesia for dental visits. Special attention to oral hygiene is important since gingival hyperplasia makes cleaning difficult and enamel hypoplasia increases susceptibility to caries. Increased fluoride treatment can decrease caries. For bruxism, a custom mouth guard may be considered. Anticipatory guidance should include the possibility of delayed tooth development and eruption. Early referral to an orthodontist is recommended, especially for Class III malocclusion.
13 |. MUSCULOSKELETAL FINDINGS
Musculoskeletal findings are common and include scoliosis, pectus anomalies, osteopenia/osteoporosis, hip dysplasia/subluxation, vertical talus, tight Achilles tendons, large and small joint contractures, ulnar deviation of the wrists, hypotonia, joint laxity, and muscle weakness (Detweiler, Thacker, Hopkins, Conway, & Gripp, 2013; Reinker, Stevenson, & Tsung, 2011; Stevenson & Yang, 2011; Yassir, Grottkau, & Goldberg, 2003). Decreased bone mineral density has been reported in multiple RASopathies and is common in CS (Detweiler et al., 2013; Leoni et al., 2014; Stevenson et al., 2011; White et al., 2005). Osteopenia may be present and individuals may be symptomatic (White et al., 2005). However, the resultant impact of fractures due to osteoporosis in CS has not been well elucidated. Vitamin D deficiency has been documented in European groups (Leoni et al., 2014).
Scoliosis, as well as kyphosis, has been reported in 17–63% of individuals with CS (Detweiler et al., 2013; Reinker et al., 2011; Stevenson & Yang, 2011; Yassir et al., 2003). Scoliosis can be severe and progressive. Pectus abnormalities are frequent (6–30%), but rarely require intervention (Detweiler et al., 2013; Yassir et al., 2003). Reversal of the normal sagittal profile of the spine with thoracic lordosis and lumbar kyphosis may be seen (Detweiler et al., 2013).
Hip dysplasia affects 17–45% and may be seen early in infancy or during childhood and adolescence (Detweiler et al., 2013; Yassir et al., 2003). It is hypothesized that early on, hip dysplasia is usually bilateral and likely a result of hypotonia and ligamentous laxity. Hip dysplasia may be identified at routine screening and should prompt a referral to orthopedics. Late dysplasia of the hip during childhood/adolescence is almost always unilateral and is a poorly understood phenomenon in CS (Detweiler et al., 2013). These individuals may present with worsening of gait, hip pain or a limb-length discrepancy. Surgical reconstruction is often needed and can be challenging. Hip flexion contractures can be observed without concomitant hip dysplasia (Detweiler et al., 2013; Yassir et al., 2003).
Limbs are often described as thin and lacking musculature. Abnormalities on muscle biopsies suggest an underlying myopathy (Tidyman, Lee, & Rauen, 2011; van der Burgt et al., 2007) with in vitro biochemical studies demonstrating that CS mutations dysregulate skeletal myogenesis, providing further evidence that individuals with CS have an intrinsic myopathy (Tidyman et al., 2011). Almost three-quarters have Achilles tendon contractures which usually manifest as toe walking (Detweiler et al., 2013; Yassir et al., 2003). Congenital vertical talus (17–28% of individuals) is noted at or soon after birth. Other foot deformities such as talipes equinovarus (2%) or pes planus (53%) occur. Progressive unilateral foot abnormalities, especially in childhood, may indicate a tethered cord and should be investigated appropriately. Shoulder and elbow contractures occur in 65% and 55% of individuals, respectively (Detweiler et al., 2013). In addition to elbow flexion contractures, ulnar deviation at the wrist (63%) may be present. Radial head subluxation or dislocation at the elbow may occur. The wrist-hand deformities are characteristic and include short, broad hyperextensible digits as well as ulnar deviation. Handgrip weakness is common and may, in part, be due to the positioning of the hand and wrist, although intrinsic muscle weakness has been reported in other RASopathies (Stevenson et al., 2012).
Referral to orthopedics and physical therapy for, at minimum, base-line evaluation is indicated for all individuals with CS (Table 1). Radiographs are diagnostic for hip dysplasia and in such cases referral to orthopedics is indicated as there is a high likelihood of needing surgical intervention. At each physician visit, individuals should be evaluated for scoliosis with the Adam’s forward bend test (Adams, 1865). Referral to orthopedics as needed for the appropriate management for scoliosis. In general, rapid progression of scoliosis may be seen in individuals with central nervous system involvement. Given that central nervous system findings such as Chiari I malformation, syringomyelia, and tethered cord are common, MRI of the entire spine should be considered in individuals with scoliosis, particularly with rapid progression.
If fractures occur, dual energy x-ray absorptiometry should be considered, but the small stature of CS individuals needs to be considered when interpreting the results. Vitamin D supplementation may be needed to maintain sufficient serum 25-hydroxyvitamin D concentration. The orthopedist will be helpful for evaluation of the frequent joint abnormalities. Treatment for Achilles tendon tightness is often a combination of physical therapy and splinting, although botulism toxin injections have been tried anecdotally. Surgery may be required if it is refractory to these therapies. Recurrence of Achilles tendon tightness may be seen and may necessitate repeat surgery. For joint contractures, encouraging proper posture and stretching, as well as overhead activities may be beneficial. Occupational and physical therapy may be needed for stretching and bracing at an early age.
14 |. GENITOURINARY FINDINGS
Prenatally, renal anomalies are present in up to 83% of fetuses with CS and include echogenic kidneys as well as dilated renal pelvis and pyelectasis (Lorenz et al., 2012; Myers et al., 2014). Documented postnatal renal anomalies include echogenic kidneys, ectopic kidney(s), enlarged kidneys, dilated renal pelvis/pyelectasis/hydronephrosis, and renal collecting system anomalies (Dickson et al., 2004; Digilio et al., 2008; Gripp et al., 2012; Lin et al., 2009; Lo et al., 2008; Lorenz et al., 2012). Kidney stones may occur in children and adults (Assadi et al., 1999; Gripp, Stabley, et al., 2006; Sol-Church et al., 2009) and a bladder stone has been documented (Assadi et al., 1999). Additional genitourinary anomalies include cryptorchidism, as observed in other RASopathies, hydrocele, inguinal hernia, hypoplastic labia, or prominent labia minora (Cakir et al., 2004; Digilio et al., 2008; Gripp, Stabley, et al., 2011; Hennekam, 2003; Smith et al., 2009), and anecdotal reports of bladder diverticula. Bladder papillomata and transitional cell carcinoma of the bladder may arise from late childhood to adulthood, for screening recommendations see Hematology/Oncology section. Renal ultrasound should be considered at diagnosis with appropriate follow-up with urology as needed (Table 1).
15 |. OPHTHALMOLOGIC FINDINGS
The majority of individuals with CS have eye findings and vision problems with strabismus, nystagmus, and refractive errors such as myopia, hyperopia, and astigmatism (Estep et al., 2006; Gripp, Lin, et al., 2006). In a cross-sectional study the majority needed corrective lenses for refractive errors, myopia being most common (Shankar & Rauen, 2009). More than 50% had strabismus, lack of depth perception, and reduced visual acuity. Photophobia with avoidance of bright sun light was reported in a number of individuals. Ptosis is common with many having compensatory head posturing. Numerous individuals undergo strabismus correction surgery in early infancy or early childhood, with exotropia being more common. Nystagmus has been present in individuals without optic nerve or retinal problems and may diminish with age. Amblyopia is a common finding (Shankar & Rauen, 2009). Two individuals had keratoconus (Costello, 1996; Gripp & Demmer 2013). Posterior segment findings include optic nerve changes ranging from hypoplastic optic disks to small but normal appearing optic disks, tilted and irregular optic disk margins, and peripapillary pigmentation and atrophy. Retinal dystrophy occurred in two individuals (Pierpont, Richards, Engel, Mendelsohn, & Summers, 2017). The management of ocular issues in CS is life long and should begin early to prevent vision loss and amblyopia (Table 1). Refractive errors and some strabismus are managed by corrective lenses, and, ptosis and strabismus may need surgery. When optic nerve problems are noted, brain MRI is recommended and unexplained decreased vision may require electroretinogram to evaluate for retinal dystrophy. Pediatric ophthalmologic assessments should begin at birth or at the time of diagnosis and continue every 6 months for the first 2 years of life than annually or as recommended by an ophthalmologist.
16 |. HEMATOLOGIC AND ONCOLOGIC FINDINGS
Children and adults with CS have an increased risk of malignancy, predominantly for embryonal rhabdomyosarcoma in early childhood, bladder cancer in adolescence and early adult life, and neuroblastoma (Flores-Nava, Canun-Serrano, Moysen-Ramirez, Parraguirre-Martinez, & Escobedo-Chavez, 2000; Franceschini et al., 1999; Gripp et al., 2002; Kerr et al., 1998; Moroni et al., 2000; Sigaudy et al., 2000; Urakami et al., 2002). From a review of 268 published cases, the cumulative risk for cancer by age 20 was 15% (Kratz, Rapisuwon, Reed, Hasle, & Rosesnberg, 2011). Rhabdomyosarcoma had been reported in 19 patients (7%), bladder carcinoma in 4 (1%), and neuroblastoma in 5 (1%). The maximal risk for embryonal rhabdomyosarcoma occurs until age 6 years (Gripp et al., 2002) and the majority of tumors arise in the abdomen or pelvis (Robbins et al., 2016). The high childhood malignancy risk has been confirmed in a population-based study in children up to age 14 in Germany which demonstrated a standardized incidence ratio of 42.4 (5.1–153.2; Kratz et al., 2011).
It has not been firmly determined at this time if the malignancy risk varies with the underlying mutation. Given that 80% of individuals with CS have HRAS p.G12S, this missense mutation predominates also in patients with malignancy. In addition to malignancies, a number of benign lesions have been reported including ganglioneuroblastoma, a calcified epithelioma of the neck and epithelial paratubal cysts, acoustic neuroma, and stomach polyp (Di Rocco & Dodero, 2003; Martin & Jones, 1991; Suri & Garrett, 1998; Zampino et al., 1993). In adults, breast fibroadenomatosis, intraductal papilloma, a parathyroid papilloma, and choroid plexus papilloma have been reported (White et al., 2005).
As expected for such a rare disorder, there is no evidence on which to base a screening protocol, in terms of effect on mortality and morbidity. Based on literature recommendations, physical examination plus abdominal and pelvic ultrasounds are suggested every 3 months until age 8–10 years, and annual urinalysis from age 10 (Table 1; Gripp et al., 2002; Villani et al., 2017). Screening for neuroblastoma has been complicated by the demonstration of abnormal urinary catecholamines in the absence of neuroblastoma in patients with CS; as a result, this is no longer recommended (Bowron, Scott, Brewer, & Weir, 2005). Although the malignancies with a very high relative risk in CS have been well defined as outlined above, it remains unclear if there is an increased risk of other malignancies, but with a lower relative risk, as seen in NF1 (Narrod, Stiller, & Lenoir, 1991).
17 |. DERMATOLOGIC FINDINGS
The dermatologic features in CS are distinctive and several are unique to CS. The vast majority of individuals with CS have curly hair (95.7%) with frontotemporal alopecia (30.4%; Siegel, Mann, Krol, & Rauen, 2012). The hair tends to be sparse, brittle, and slow growing. In contrast, the fingernails and toenails grow quickly. The nails tend to be brittle and thin. The full, thick eyebrows are a common feature (47.8%) distinguishing CS from individuals with CFC who have thin, sparse eyebrows. Loose anagen hair syndrome has been reported in a subset of individuals with HRAS p.G13C (Gripp, Stabley, et al., 2011) and some had very long eyelashes, which required regular trimming.
Papillomas often begin to develop on the nasal ala and anterior nares, appearing from infancy through early adulthood, occurring in 71.7% of individuals. Other locations include the face, ear lobules, and perineal region. The papillomas tend to be soft, flesh colored, and small, often only 3–4 mm in size (Siegel et al., 2012). Hyperkeratosis develops in pressure areas on the palms and soles. This palmoplantar keratoderma becomes significant and symptomatic through the teen years in approximately three-quarters of patients. The palms and soles are notable for deep creases with loose, wrinkled, or redundant skin. It is common for patients to have a darker skin color compared to family members. The majority experience heat intolerance, excessive sweating and unusual body odor (Morice-Picard et al., 2013; Siegel et al., 2012). There are a few reported cases of severe generalized cutis laxa in the infantile period, which improved with time (Girisha, Lewis, Phadke, & Kutsche, 2010). Acanthosis nigricans, thick, hyper-pigmented, velvety skin on the dorsal neck, axilla and less frequently dorsal hands has been reported in approximately one-third. The age at presentation of acanthosis nigricans can range from early childhood through adolescence.
Regular evaluation by a pediatric dermatologist is important to monitor dermatologic findings, primarily papillomas, and palmoplantar keratoderma, which may require treatment (Table 1). The facial papillomas and palmoplantar keratoderma can have a negative impact on quality of life due to stigmatization, pain, and functional impairment. There are no FDA approved treatments for the papillomas or palmoplantar keratoderma. Treatments that have been used for papillomas include snip excision, cryotherapy, cautery and imiquimod cream; however, these generally provide only temporary benefit and the lesions frequently recur. Palmoplantar keratoderma is managed with topical tazarotene, urea cream and physical paring. One case report described improvement of acanthosis nigricans after treatment with isotretinoin for nodulocystic acne (Sriboonnark, Aurora, Falto-Aizpurua, Choudhary, & Connelly, 2015).
Individuals with CS have a high rate of sensitive skin and eczematous dermatitis. This can cause itching and discomfort. Eczema should be managed with sensitive skin care. The use of fragrance-free products (including soap, moisturizer, and laundry detergent) is beneficial to preventing skin irritation. Thick moisturizing creams and ointments are more effective as emollients than lotions. In some cases, prescription topical steroids are needed. Sun protection, including the use of hats, sun protective clothing, sunglasses, and sun screen, is important. Sun screen should be reapplied every 2 hr while outside, especially if swimming or sweating.
18 |. ISSUES IN ADULTHOOD
There is very little literature describing the health concerns specific to adults with CS. Two studies describe health concerns in 22 adults (16 years and older) with CS (Abe et al., 2012; White et al., 2005). Of these, 15 (68%) had cardiovascular pathology, eight individuals had isolated cardiomyopathy, five individuals had cardiomyopathy and arrhythmia, one individual had mitral valve prolapse and regurgitation, and one individual had pulmonic and tricuspid valve regurgitation. When comparing this group of 22 individuals to a cross sectional cohort of individuals with CS of all ages (61 individuals, mean age 12 years, 13 over the age of 18), the incidence of cardiomyopathy is constant (~65%), while arrhythmia seems to be common in the younger cohort (Abe et al., 2012; Levin et al., 2018; Lin et al., 2011; White et al., 2005). Age of onset of cardiac problems and longitudinal follow-up of cardiomyopathy is largely under-reported. Only four of the 22 adults referenced previously had age of onset of cardiomyopathy provided with two reportedly diagnosed as adults, age 16 and 26 years, respectively (Abe et al., 2012; White et al., 2005). Adult-onset GERD was reported in four of 17 adults (White et al., 2005) and three of them were later diagnosed with Chiari I malformation. While GERD is a common problem in the general population, the potential association with Chiari I malformation in an adult with CS is important. Among 14 adults with brain imaging, four were diagnosed with Chiari I malformation (Abe et al., 2012; White et al., 2005). Vision concerns continue into adulthood. Specific problems reported in adults include keratoconus in two individuals, and retinal dystrophy in two individuals, in addition to more common concerns such as myopia, astigmatism, amblyopia, nystagmus, and hypermetropia (Gripp & Demmer, 2013; White et al., 2005). Tumors and malignancies seem to be rare in adults. To date, the only malignancy reported in adults with CS has been transitional cell carcinoma of the bladder (Beukers, Hercegovac, & Zwarthoff, 2014; White et al., 2005). Low bone density may be an issue for adults with CS (Leoni et al., 2014; White et al., 2005). While individuals may have symptomatic low bone density, other comorbidities for low bone density may be present and, therefore, the causal relationship with CS is unclear. A recent study reported bone density in a group of individuals with CS, including four individuals over age 18. While bone density was low in three of the four individuals (lumbar spine and whole body z scores < −2), none of the individuals in the study had a fracture (Leoni et al., 2014). Orthopedic surgeries performed in older individuals include calcaneal tendon lengthening and spinal fusion. One individual had total hip replacement at age 19.
Physical features of individuals with CS change with age, with coarsening of facial features, and loss and thinning of hair. Quality of life is generally reported to be good in adults with CS (Hopkins et al., 2010). One major concern that deserves further investigation is anxiety symptoms (Weaver, unpublished observation). Cognitive function appears stable over time but has been poorly studied in adults. Among a group of 16 adults recently surveyed with mean age of 24.75 years (median 22.5 years, range 16–38 years, SD 6.5), 13 live with their parents, two live in a group home, and one lives semi-independently in an apartment near her parents. Three individuals attend a college program. All participate in daily activities such as volunteer work or part-time employment. In light of the high likelihood that an adult with CS will require life-long assistance with activities of daily living, it is important for parents or caregivers to begin planning early for continued support of their adult son or daughter with CS. The life expectancy is in need of further study, but as evidenced by the studies summarized above, many individuals survive into adulthood. Among 23 deceased individuals summarized in 2011, only two passed away as adults (Lin et al., 2011). Both were males who died suddenly, at age 27 and 47 years, respectively. The 27-year-old had known severe hypertrophic cardiomyopathy, arrhythmia, and ascending aortic dilation. The 47-year-old was the oldest reported living person with CS at the time of his death and had previously had a normal echocardiogram. In the event of unexpected death, it can be helpful to have previously considered whether autopsy or tissue/DNA preservation is desired and to have an appropriate plan in place. In addition to routine screening for the CS-specific concerns (Table 1), it is important to emphasize routine adult health maintenance recommendations, such as annual blood pressure and lipid panel screening in all individuals and mammography in women.
19 |. SUMMARY
Costello syndrome (CS) is a RASopathy due to activating germline mutations in the gene HRAS. Due to the ubiquitous nature in which HRAS is expressed, CS is a complex syndrome affecting multiple organ systems and individuals are predisposed to cancer. Like other RASopathies, CS individuals have distinctive craniofacial features, cardiac anomalies, growth and developmental delays, as well as dermatological, orthopedic, ocular, and neurological issues. It is essential that patients be evaluated by specialists and have continued follow-up in a regular, multidisciplinary approach. These recommendations were developed by an interdisciplinary team of experts with the overall goal to provide health care providers with the most timely health care practices and medical management guidelines for individuals with CS across their life span. However, because the full natural history of CS is unclear and systematically obtained data regarding the benefits of these management recommendations are currently lacking, these care guidelines will be refined in the future.
ACKNOWLEDGMENTS
The authors would like to thank the families and the past NIH supported RASopathy scientific meetings that led to interaction between the clinicians, families and researchers. In addition, the authors are grateful to the Costello Syndrome Family Network for their enthusiastic support, assistance, and thoughtful comments in the development of these guidelines. This work was partially support by the National Institute of Arthritis and Musculoskeletal and Skin Diseases R01AR062165 (K.A.R.) and the Children’s Miracle Network (S.P.S.).
Funding information
Childrens Miracle Network; National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant/Award Number: R01AR062165
Footnotes
CONFLICT OF INTEREST
None.
REFERENCES
- Abe Y, Aoki Y, Kuriyama S, Kawame H, Okamoto N, Kurosawa K, … Matsubara Y (2012). Prevalence and clinical features of Costello syndrome and cardio-facio-cutaneous syndrome in Japan: Findings from a nationwide epidemiological survey. American Journal of Medical Genetics. Part A, 158A(5), 1083–1094. 10.1002/ajmg.a.35292 [DOI] [PubMed] [Google Scholar]
- Adams W (1865). Lectures on the pathology and treatment of lateral and other forms of curvature of the spine. London: Churchill. [PMC free article] [PubMed] [Google Scholar]
- Adviento B, Corbin IL, Widjaja F, Desachy G, Enrique N, Rosser T, … Weiss LA (2014). Autism traits in the RASopathies. Journal of Medical Genetics, 51(1), 10–20. 10.1136/jmedgenet-2013-101951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander S, Ramadan D, Alkhayyat H, Al-Sharkawi I, Backer KC, El-Sabban F, & Hussain K (2005). Costello syndrome and hyperinsulinemic hypoglycemia. American Journal of Medical Genetics. Part A, 139(3), 227–230. 10.1002/ajmg.a.31011 [DOI] [PubMed] [Google Scholar]
- Alfieri P, Piccini G, Caciolo C, Perrino F, Gambardella ML, Mallardi M, … Vicari S (2014). Behavioral profile in RASopathies. American Journal of Medical Genetics. Part A, 164A(4), 934–942. 10.1002/ajmg.a.36374 [DOI] [PubMed] [Google Scholar]
- Aoki Y, Niihori T, Kawame H, Kurosawa K, Ohashi H, Tanaka Y, … Matsubara Y (2005). Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nature Genetics, 37(10), 1038–1040. 10.1038/ng1641 [DOI] [PubMed] [Google Scholar]
- Assadi FK, Scott CI Jr., McKay CP, Nicholson L, Cafone M, Hopp L, & Fattori DA (1999). Hypercalciuria and urolithiasis in a case of Costello syndrome. Pediatric Nephrology, 13(1), 57–59. [DOI] [PubMed] [Google Scholar]
- Axelrad ME, Glidden R, Nicholson L, & Gripp KW (2004). Adaptive skills, cognitive, and behavioral characteristics of Costello syndrome. American Journal of Medical Genetics Part A, 128A(4), 396–400. 10.1002/ajmg.a.30140 [DOI] [PubMed] [Google Scholar]
- Axelrad ME, Nicholson L, Stabley DL, Sol-Church K, & Gripp KW (2007). Longitudinal assessment of cognitive characteristics in Costello syndrome. American Journal of Medical Genetics. Part A, 143A(24), 3185–3193. 10.1002/ajmg.a.31968 [DOI] [PubMed] [Google Scholar]
- Axelrad ME, Schwartz DD, Fehlis J, Stabley D, Sol-Church K, & Gripp KW (2009). Longitudinal course of cognitive, adaptive, and behavioral characteristics in Costello syndrome. American Journal of Medical Genetics. Part A, 149A(12), 2666–2672. 10.1002/ajmg.a.33126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrad ME, Schwartz DD, Katzenstein JM, Hopkins E, & Gripp KW (2011). Neurocognitive, adaptive, and behavioral functioning of individuals with Costello syndrome: A review. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 157C (2), 115–122. 10.1002/ajmg.c.30299 [DOI] [PubMed] [Google Scholar]
- Beukers W, Hercegovac A, & Zwarthoff EC (2014). HRAS mutations in bladder cancer at an early age and the possible association with Costello syndrome. European Journal of Human Genetics, 22, 837–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizaoui V, Gage J, Brar R, Rauen KA, & Weiss LA (2018). RASopathies are associated with a distinct personality profile. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 177 (4), 434–446. 10.1002/ajmg.b.32632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowron A, Scott JG, Brewer C, & Weir P (2005). Increased HVA detected on organic acid analysis in a patient with Costello syndrome. Journal of Inherited Metabolic Disease, 28(6), 1155–1156. 10.1007/s10545-005-0124-8 [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Fischbach PS, Law IH, Serwer GA, & Dick M 2nd. (2001). The clinical course of multifocal atrial tachycardia in infants and children. Journal of the American College of Cardiology, 38(2), 401–408. [DOI] [PubMed] [Google Scholar]
- Burkitt-Wright EM, Bradley L, Shorto J, McConnell VP, Gannon C, Firth HV, … Kerr B (2012). Neonatal lethal Costello syndrome and unusual dinucleotide deletion/insertion mutations in HRAS predicting p.Gly12Val. American Journal of Medical Genetics. Part A, 158A(5), 1102–1110. 10.1002/ajmg.a.35296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir M, Arici C, Tacoy S, & Karayalcin U (2004). A case of Costello with parathyroid adenoma and hyperprolactinemia. American Journal of Medical Genetics. Part A, 124A(2), 196–199. 10.1002/ajmg.a.20361 [DOI] [PubMed] [Google Scholar]
- Calabria AC, Gallagher PR, Simmons R, Blinman T, & De León DD (2011). Postoperative surveillance and detection of postprandial hypoglycemia after fundoplasty in children. The Journal of Pediatrics, 159(4), 597–601.e1. 10.1016/j.jpeds.2011.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandrelli R, D’Apolito G, Marco P, Zampino G, Tartaglione T, & Colosimo C (2015). Costello syndrome: Analysis of the posterior cranial fossa in children with posterior fossa crowding. The Neuroradiology Journal, 28(3), 254–258. 10.1177/1971400915592549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarini L, Alfieri P, Pantaleoni F, Vasta I, Cerutti M, Petrangeli V, … Zampino G (2009). Cognitive profile of disorders associated with dysregulation of the RAS/MAPK signaling cascade. American Journal of Medical Genetics. Part A, 149A(2), 140–146. 10.1002/ajmg.a.32488 [DOI] [PubMed] [Google Scholar]
- Chiu AT, Zhu L, Mok GT, Leung GK, Chow CB, & Chung BH (2016). Before and after––Nutritional transformation of dysmorphism in a case of Costello syndrome. European Journal of Medical Genetics, 59(11), 573–576. 10.1016/j.ejmg.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Costello JM (1971). A new syndrome. The New Zealand Medical Journal, 74, 397. [Google Scholar]
- Costello JM (1977). A new syndrome: Mental subnormality and nasal papillomata. Australian Paediatric Journal, 13(2), 114–118. [DOI] [PubMed] [Google Scholar]
- Costello JM (1996). Costello syndrome: Update on the original cases and commentary. American Journal of Medical Genetics, 62(2), 199–201. 10.1002/ajmg.1320620203 [DOI] [PubMed] [Google Scholar]
- Della Marca G, Vasta I, Scarano E, Rigante M, De Feo E, Mariotti P, … Zampino G (2006). Obstructive sleep apnea in Costello syndrome. American Journal of Medical Genetics. Part A, 140(3), 257–262. 10.1002/ajmg.a.31076 [DOI] [PubMed] [Google Scholar]
- Delrue MA, Chateil JF, Arveiler B, & Lacombe D (2003). Costello syndrome and neurological abnormalities. American Journal of Medical Genetics. Part A, 123A(3), 301–305. 10.1002/ajmg.a.20330 [DOI] [PubMed] [Google Scholar]
- Der Kaloustian VM, Moroz B, McIntosh N, Watters AK, & Blaichman S (1991). Costello syndrome. American Journal of Medical Genetics, 41(1), 69–73. [DOI] [PubMed] [Google Scholar]
- Detweiler S, Thacker MM, Hopkins E, Conway L, & Gripp KW (2013). Orthopedic manifestations and implications for individuals with Costello syndrome. American Journal of Medical Genetics. Part A, 161A(8), 1940–1949. 10.1002/ajmg [DOI] [PubMed] [Google Scholar]
- Di Rocco M, & Dodero P (2003). Concerning “five additional Costello syndrome patients with rhabdomyosarcoma: Proposal for a tumour screening protocol”. American Journal of Medical Genetics, 118A(2), 199. [DOI] [PubMed] [Google Scholar]
- Dickson PI, Briones NY, Baylen BG, Jonas AJ, French SW, & Lin HJ (2004). Costello syndrome with pancreatic islet cell hyperplasia. American Journal of Medical Genetics. Part A, 130A(4), 402–405. 10.1002/ajmg.a.30288 [DOI] [PubMed] [Google Scholar]
- Digilio MC, Sarkozy A, Capolino R, Chiarini Testa MB, Esposito G, de Zorzi A, … Dallapiccola B (2008). Costello syndrome: Clinical diagnosis in the first year of life. European Journal of Pediatrics, 167(6), 621–628. 10.1007/s00431-007-0558-0 [DOI] [PubMed] [Google Scholar]
- Dileone M, Profice P, Pilato F, Alfieri P, Cesarini L, Mercuri E, … Di Lazzaro V (2010). Enhanced human brain associative plasticity in Costello syndrome. The Journal of Physiology, 588(Pt 18), 3445–3456. 10.1113/jphysiol.2010.191072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estep AL, Tidyman WE, Teitell MA, Cotter PD, & Rauen KA (2006). HRAS mutations in Costello syndrome: Detection of constitutional activating mutations in codon 12 and 13 and loss of wild-type allele in malignancy. American Journal of Medical Genetics. Part A, 140 (1), 8–16. 10.1002/ajmg.a.31078 [DOI] [PubMed] [Google Scholar]
- Flores-Nava G, Canun-Serrano S, Moysen-Ramirez SG, Parraguirre-Martinez S, & Escobedo-Chavez E (2000). Costello syndrome associated to a neuroblastoma. Presentation of a case. Gaceta Médica de México, 136(6), 605–609. [PubMed] [Google Scholar]
- Franceschini P, Licata D, Di Cara G, Guala A, Bianchi M, Ingrosso G, & Franceschini D (1999). Bladder carcinoma in Costello syndrome: Report on a patient born to consanguineous parents and review. American Journal of Medical Genetics, 86(2), 174–179. [PubMed] [Google Scholar]
- Galéra C, Delrue MA, Goizet C, Etchegoyhen K, Taupiac E, Sigaudy S, … Lacombe D (2006). Behavioral and temperamental features of children with Costello syndrome. American Journal of Medical Genetics. Part A, 140(9), 968–974. [DOI] [PubMed] [Google Scholar]
- Ganz JB, Davis JL, Lund EM, Goodwyn FD, & Simpson RL (2012). Meta-analysis of PECS with individuals with ASD: Investigation of targeted versus non-targeted outcomes, participant characteristics, and implementation phase. Research in Developmental Disabilities, 33 (2), 406–418. 10.1016/j.ridd.2011.09.023 [DOI] [PubMed] [Google Scholar]
- Gelb BD, Roberts AE, & Tartaglia M (2015). Cardiomyopathies in Noonan syndrome and the other RASopathies. Progress in Pediatric Cardiology, 39(1), 13–19. 10.1016/j.ppedcard.205.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, … American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons. (2011). 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. The Journal of Thoracic and Cardiovascular Surgery, 142(24), 2761–2796. 10.1161/CIR.0b013e318223e230 [DOI] [Google Scholar]
- Giannoulatou E, McVean G, Taylor IB, McGowan SJ, Maher GJ, Iqbal Z, … Goriely A (2013). Contributions of intrinsic mutation rate and selfish selection to levels of de novo HRAS mutations in the paternal germline. Proceedings of the National Academy of Sciences of the United States of America, 110(50), 20152–20157. 10.1073/pnas.1311381110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girisha KM, Lewis LE, Phadke SR, & Kutsche K (2010). Costello syndrome with severe cutis laxa and mosaic HRAS G12S mutation. American Journal of Medical Genetics. Part A, 152A(11), 2861–2864. 10.1002/ajmg.a.33687 [DOI] [PubMed] [Google Scholar]
- Gomez-Ospina N, Kuo C, Ananth AL, Myers A, Brennan M-L, Stevenson DA, … Hudgins L (2016). Respiratory system involvement in Costello syndrome. American Journal of Medical Genetics. Part A, 170(7), 1849–1857. 10.1002/ajmg.a.37655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin AF, Oberoi S, Landan M, Charles C, Massie JC, Fairley C, … Klein OD (2014). Craniofacial and dental development in Costello syndrome. American Journal of Medical Genetics. Part A, 164A(6), 1425–1430. 10.1002/ajmg.a.36475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin AF, Tidyman WE, Jheon AH, Sharir A, Zheng X, Charles C, … Klein OD (2014). Abnormal Ras signaling negatively regulates enamel formation. Human Molecular Genetics, 23(3), 682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriely A, & Wilkie AOM (2012). Paternal age effect mutations and selfish spermatogonial selection: Causes and consequences for human disease. American Journal of Human Genetics, 90(2), 175–200. 10.1016/j.ajhg.2011.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AR, Cushman BJ, Cavé H, Dillon MW, Gelb BD, Gripp KW, … Zenker M (2018). Assessing the gene-disease association of 19 genes with the RASopathies using the ClinGen gene curation framework. Human Mutation, 39, 1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen N, & Viljoen D (2004). Costello syndrome with growth hormone deficiency and hypoglycemia: A new report and review of the endocrine associations. American Journal of Medical Genetics. Part A, 129A(2), 115–171. 10.1002/ajmg.a.30189 [DOI] [PubMed] [Google Scholar]
- Gripp KW, Bifeld E, Stabley DL, Hopkins E, Meien S, Vinette K, … Rosenberer G (2012). A novel HRAS substitution (c.266C>G; p.S89C) resulting in decreased downstream signaling suggests a new dimension of RAS pathway dysregulation in human development. American Journal of Medical Genetics. Part A, 158A(9), 2106–2118. 10.1002/ajmg.a.35449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, & Demmer LA (2013). Keratoconus in Costello syndrome. American Journal of Medical Genetics. Part A, 161A(5), 1132–1136. 10.1002/ajmg.a.35816 [DOI] [PubMed] [Google Scholar]
- Gripp KW, Hopkins E, Doyle D, & Dobyns WB (2010). High incidence of progressive postnatal cerebellar enlargement in Costello syndrome: Brain overgrowth associated with HRAS mutations as the likely cause of structural brain and spinal cord abnormalities. American Journal of Medical Genetics. Part A, 152A(5), 1161–1168. 10.1002/ajmg.a.33391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Hopkins E, Sol-Church K, Stabley DL, Axelrad ME, Doyle D, … Lin AE (2011). Phenotypic analysis of individuals with Costello syndrome due to HRAS p.G13C. American Journal of Medical Genetics. Part A, 155A(4), 706–716. 10.1002/ajmg.a.33884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Innes AM, Axelrad ME, Gillan TL, Parboosingh JS, Davies C, … Sol-Church K (2008). Costello syndrome associated with novel germline HRAS mutations: An attenuated phenotype? American Journal of Medical Genetics. Part A, 146A(6), 683–690. 10.1002/ajmg.a.32227 [DOI] [PubMed] [Google Scholar]
- Gripp KW, Kolbe V, Brandenstein LI, & Rosenberger G (2017). Attenuated phenotype of Costello syndrome and early death in a patient with an HRAS mutation (c.179G>T; p.Gly60Val) affecting signalling dynamics. Clinical Genetics, 92(3), 332–337. 10.1111/cge.12980 [DOI] [PubMed] [Google Scholar]
- Gripp KW, & Lin A (2012). Costello syndrome: A Ras/mitogen activated protein kinase pathway syndrome (rasopathy) resulting from HRAS germline mutations. Genetics in Medicine, 14(3), 285–292. 10.1038/gim.0b013e31822dd91f [DOI] [PubMed] [Google Scholar]
- Gripp KW, & Lin AE (2006, 1993–2018). Costello Syndrome. In Adam MP, Ardinger HH, Pagon RA, Wallace SE, LJH B, Stephens K, & Amemiya A (Eds.), GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK1507/. [Google Scholar]
- Gripp KW, Lin AE, Stabley DL, Nicholson L, Scott CI Jr., Doyle D, … Sol-Church K (2006). HRAS mutation analysis in Costello syndrome: Genotype and phenotype correlation. American Journal of Medical Genetics. Part A, 140(1), 1–7. 10.1002/ajmg.a.31047 [DOI] [PubMed] [Google Scholar]
- Gripp KW, Robbins KM, Sheffield BS, Lee AF, Patel MS, Yip S, … Sol-Church K (2016). Paternal uniparental disomy 11p15.5 in the pancreatic nodule of an infant with Costello syndrome: Shared mechanism for hyperinsulinemic hypoglycemia in neonates with Costello and Beckwith-Wiedemann syndrome and somatic loss of heterozygosity in Costello syndrome driving clonal expansion. American Journal of Medical Genetics. Part A, 170(3), 559–564. 10.1002/ajmg.a.37471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Scott CI, Nicholson L, McDonald-McGinn DM, Ozeran JD, Jones MC, … Zackai EH (2002). Five additional Costello syndrome patients with rhabdomyosarcoma: Proposal for a tumor screening protocol. American Journal of Medical Genetics, 108(1), 80–87. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Scott CI Jr., Nicholson L, & Figueroa TE (2000). A second case of bladder carcinoma in a patient with Costello syndrome. American Journal of Medical Genetics, 90, 256–259. [DOI] [PubMed] [Google Scholar]
- Gripp KW, Sol-Church K, Smpokou P, Graham GE, Stevenson DA, Hanson H, … Rosenberger G (2015). An attenuated phenotype of Costello syndrome in three unrelated individuals with a HRAS c.179G>a (p.Gly60Asp) mutation correlates with uncommon functional consequences. American Journal of Medical Genetics Part A, 9, 2085–2097. 10.1002/ajmg.a.37128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Stabley DL, Geller PL, Hopkins E, Stevenson DA, Carey JC, & Sol-Church K (2011). Molecular confirmation of HRAS p.G12S in siblings with Costello syndrome. American Journal of Medical Genetics. Part A, 155A(9), 2263–2268. 10.1002/ajmg.a.34150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Stabley DL, Nicholson L, Hoffman JD, & Sol-Church K (2006). Somatic mosaicism for an HRAS mutation causes Costello syndrome. American Journal of Medical Genetics. Part A, 140(20), 2163–2169. 10.1002/ajmg.a.31456 [DOI] [PubMed] [Google Scholar]
- Hart TC, Zhang Y, Gorry MC, Hart PS, Cooper M, Marazita ML, … Pallos D (2002). A mutation in the SOS1 gene causes hereditary gingival fibromatosis type 1. American Journal of Human Genetics, 70, 943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung A-M, Swensen J, Uriz I, Lapin M, Kristjansdottir K, Peterson USS, … Andresen BS (2017). The splicing efficiency of activating HRAS mutations can determine Costello syndrome phenotype and frequency in cancer. PLoS Genetics, 12(5), e1006039. 10.1371/journal.pgen.1006039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekam RC (2003). Costello Syndrome: An Overview. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 117C(1), 42–48. 10.1002/ajmg.a.10019 [DOI] [PubMed] [Google Scholar]
- Hopkins E, Lin AE, Krepkovich KE, Axelrad ME, Sol-Church K, Stabley DL, … Gripp KW (2010). Living with Costello syndrome: Quality of life issues in older individuals. American Journal of Medical Genetics. Part A, 152A(1), 84–90. 10.1002/ajmg.a.33147 [DOI] [PubMed] [Google Scholar]
- Kawame H, Matsui M, Kurosawa K, Matsuo M, Masuno M, Ohashi H, … Fukushima Y (2003). Further delination of the behavioral and neurologic features in Costello syndrome. American Journal of Medical Genetics. Part A, 118A(1), 8–14. 10.1002/ajmg.a.10236 [DOI] [PubMed] [Google Scholar]
- Kerr B, Delrue MA, Sigaudy S, Perveen R, Marche M, Burgelin I, … Black G (2006). Genotype-phenotype correlation in Costello syndrome: HRAS mutation analysis in 43 cases. Journal of Medical Genetics, 43(5), 401–405. 10.1136/jmg.2005.040352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B, Eden OM, Dandamudi R, Shannon N, Quarrell O, Emmerson A, … Donnai D (1998). Costello syndrome: Two cases with embryonal rhabdomyosarcoma. Journal of Medical Genetics, 35 (12), 1036–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz CP, Rapisuwon S, Reed H, Hasle H, & Rosesnberg PS (2011). Cancer in Noonan, Costello, cardiofaciocutaneous and LEOPARD syndromes. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 157C(2), 83–89. 10.1002/ajmg.c.30300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni C, Onesimo R, Giorgio V, Diamanti A, Giorgio D, Martini L, … Zampino G (2016). Understanding growth failure in Costello syndrome: Increased resting energy expenditure. The Journal of Pediatrics, 2016(170), 322–324. 10.1016/j.jpeds.2015.11.076 [DOI] [PubMed] [Google Scholar]
- Leoni C, Stevenson DA, Martini L, De Sanctis R, Mascolo G, Pantaleoni F, … Zampino G (2014). Decreased bone mineral density in Costello syndrome. Molecular Genetics and Metabolism, 111(1), 41–45. 10.1016/j.ymgme.2013.08.007 [DOI] [PubMed] [Google Scholar]
- Levin MD, Saitta SC, Gripp KW, Wenger TL, Ganesh J, Kalish JM, … Lin AE (2018). Nonreentrant atrial tachycardia occurs independently of hypertophic cardiomyopathy in RASopathy patients. American Journal of Medical Genetics Part A, 176(8), 1711–1722. 10.1002/ajmg.a.38854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightdale JR, & Gremse DA. (2013). Gastroesophageal reflux: Management guidance for the pediatrician. Pediatrics, 131(5), e1684–e1695. 10.1542/peds.2013-0421. Section on Gastroenterology, Hepatology, and Nutrition [DOI] [PubMed] [Google Scholar]
- Lin AE, Alexander ME, Colan SD, Kerr B, Rauen KA, Noonan J, … Gripp KW (2011). Clinical, pathological, and molecular analyses of cardiovascular abnormalities in Costello syndrome: A Ras/MAPK pathway syndrome. American Journal of Medical Genetics. Part A, 155A(3), 486–507. 10.1002/ajmg.a.33857 [DOI] [PubMed] [Google Scholar]
- Lin AE, O’Brien B, Demmer LA, Almeda KK, Blanco CL, Glasow PF, … Gripp KW (2009). Prenatal features of Costello syndrome: Ultrasonographic findings and atrial tachycardia. Prenatal Diagnosis, 29(7), 682–690. 10.1002/pd.2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo IF, Brewer C, Shannon N, Shorto J, Tang B, Black G, … Kerr B (2008). Severe neonatal manifestations of Costello syndrome. Journal of Medical Genetics, 45(3), 167–171. 10.1136/jmg.2007.054411 [DOI] [PubMed] [Google Scholar]
- Lorenz S, Lissewski C, Simsek-Kiper PO, Alanay Y, Boduroglu K, Zenker M, & Rosenberger G (2013). Functional analysis of a duplication (p.E63_D69dup) in the switch II region of HRAS: New aspects of the molecular pathogenesis underlying Costello syndrome. Human Molecular Genetics, 22(8), 1643–1653. 10.1093/hmg/ddt014 [DOI] [PubMed] [Google Scholar]
- Lorenz S, Petersen C, Kordaß U, Seidel H, Zenker M, & Kutsche K (2012). Two cases with severe lethal course of Costello syndrome associated with HRAS p.G12C and p.G12D. European Journal of Medical Genetics, 55(11), 615–619. 10.1016/j.ejmg.2012.07.007 [DOI] [PubMed] [Google Scholar]
- Martin RA, & Jones KL (1991). Delineation of the Costello syndrome. American Journal of Medical Genetics, 41(3), 346–349. 10.1002/ajmg.1320410316 [DOI] [PubMed] [Google Scholar]
- Morice-Picard F, Ezzedine K, Delrue MA, Arveiler B, Fergelot P, Taïeb A, … Boralevi F (2013). Cutaneous manifestations in Costello and cardiofaciocutaneous syndrome: Report of 18 cases and literature review. Pediatric Dermatology, 30(6), 665–673. 10.1111/pde.12171 [DOI] [PubMed] [Google Scholar]
- Moroni I, Bedeschi F, Luksch R, Casanova M, D’Incerti L, Uziel G, & Selicorni A (2000). Costello syndrome: A cancer predisposing syndrome? Clinical Dysmorphology, 9(4), 265–268. [DOI] [PubMed] [Google Scholar]
- Myers A, Bernstein JA, Brennan ML, Curry C, Esplin ED, Fisher J, … Hudgins L (2014). Perinatal features of the RASopathies: Noonan syndrome, cardiofaciocutaneous syndrome and Costello syndrome. American Journal of Medical Genetics. Part A, 164A(11), 2814–2821. 10.1002/ajmg.a.36737 [DOI] [PubMed] [Google Scholar]
- Narrod SA, Stiller C, & Lenoir GM (1991). An estimate of the heritable fraction of childhood cancer. British Journal of Cancer, 63, 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquin A, Hordo C, Kaplan DR, & Miller FD (2009). Costello syndrome H-Ras alleles regulate cortical development. Developmental Biology, 330(2), 440–451. 10.1016/j.ydbio.2009.04.010 [DOI] [PubMed] [Google Scholar]
- Piccione M, Piro E, Pomponi MG, Matina F, Pietrobono R, Candela E, … Corsello G (2009). A premature infant with Costello syndrome due to a rare G13C HRAS mutation. American Journal of Medical Genetics. Part A, 149A(3), 487–489. 10.1002/ajmg.a.32674 [DOI] [PubMed] [Google Scholar]
- Pierpont ME, Richards M, Engel WK, Mendelsohn NJ, & Summers CG (2017). Retinal dystrophy in two boys with Costello syndrome due to the HRAS p.Gly13Cys mutation. American Journal of Medical Genetics. Part A, 173(5), 1342–1347. 10.1002/ajmg.a.38110 [DOI] [PubMed] [Google Scholar]
- Quezada E, & Gripp KW (2007). Costello syndrome and related disorders. Current Opinion in Pediatrics, 19(6), 636–644. 10.1097/MOP.0b013e3282f161dc [DOI] [PubMed] [Google Scholar]
- Rauen KA (2007). HRAS and the Costello syndrome. Clinical Genetics, 71 (2), 101–108. 10.1111/j.1399-0004.2007.00743.x [DOI] [PubMed] [Google Scholar]
- Rauen KA (2013). The RASopathies. Annual Review of Genomics and Human Genetics, 14, 355–369. 10.1146/annurev-genom-091212-153523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinker KA, Stevenson DA, & Tsung A (2011). Orthopaedic conditions in Ras/MAPK related disorders. Journal of Pediatric Orthopedics, 31(5), 599–605. 10.1097/BPO.0b013e318220396e [DOI] [PubMed] [Google Scholar]
- Robbins KM, Stabley DL, Holbrook J, Sahraoui R, Sadreameli A, Conard K, … Sol-Church K (2016). Paternal uniparental disomy with segmental loss of heterozygosity of chromosome 11 are hall-mark characteristics of syndromic and sporadic embryonal rhabdomyosarcoma. American Journal of Medical Genetics Part A, 170, 3197–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno JC, Kertesz NJ, Friedman RA, & Fenrich AL (2004). Clinical course of atrial ectopic tachycardia is age-dependent: Results and treatment in children <3 or > or =3 years of age. Journal of the American College of Cardiology, 43(3), 438–444. 10.1016/jack.2003.09.031 [DOI] [PubMed] [Google Scholar]
- Sammon MR, Doyle D, Hopkins E, Sol-Church K, Stabley DL, McGready J, … Gripp KW (2012). Normative growth charts for individuals with Costello syndrome. American Journal of Medical Genetics. Part A, 158A(11), 2692–2699. 10.1002/ajmg.a.35534 [DOI] [PubMed] [Google Scholar]
- Schwartz DD, Katzenstein JM, Highley EJ, Stabley DL, Sol-Church K, Gripp KW, & Axelrad ME (2017). Age-related differences in prevalence of autism Spectrum disorder symptoms in children and adolescents with Costello syndrome. American Journal of Medical Genetics. Part A, 173(5), 1294–1300. 10.1002/ajmg.a.38174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DD, Katzenstein JM, Hopkins E, Stabley DL, Sol-Church K, Gripp KW, & Axelrad ME (2013). Verbal memory functioning in adolescents and young adults with Costello syndrome: Evidence for relative preservation in recognition memory. American Journal of Medical Genetics. Part A, 161A(9), 2258–2265. 10.1002/ajmg.a.36078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar SP, Rauen KA. (2009). A Recurring Mutation G12S in HRAS causes Variable Ocular Phenotype in Costello Syndrome; (252/F PB#40) presented at The American Society of Human Genetics, Honolulu, HI, October 23, 2009. [Google Scholar]
- Sheffield BS, Yip S, Ruchelli ED, Dunham CP, Sherwin E, Brooks PA, … Lee A (2015). Fatal congenital hypertrophic cardiomyopathy and a pancreatic nodule morphologically identical to focal lesion of congenital hyperinsulinism in an infant with Costello syndrome. Case report and review of the literature. Pediatric and Developmental Pathology, 18(3), 237–244. 10.2350/14-07-1525-CR.1 [DOI] [PubMed] [Google Scholar]
- Siegel DH, Mann JA, Krol AL, & Rauen KA (2012). Dermatological phenotype in Costello syndrome: Consequences of Ras dysregulation in development. British Journal of Dermatology, 166(3), 601–607. 10.1111/j.1365-2133.2011.10744.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigaudy S, Vittu G, David A, Vigeron J, Lacombe D, Moncla A, … Philip N (2000). Costello syndrome: Report of six patients including one with an embryonal rhabdomyosarcoma. European Journal of Pediatrics, 159(3), 139–142. [DOI] [PubMed] [Google Scholar]
- Smith LP, Podraza J, & Proud VK (2009). Polyhydramnios, fetal overgrowth and macrocephaly: Prenatal ultrasound findings of Costello syndrome. American Journal of Medical Genetics. Part A, 149A(4), 779–784. 10.1002/ajmg.a.32778 [DOI] [PubMed] [Google Scholar]
- Sol-Church K, & Gripp KW (2009). In Zenker M (Ed.), Noonan syndrome and related disorders - a matter of deregulated Ras Signaling. Basel, Switzerland: Karger. [Google Scholar]
- Sol-Church K, Stabley DL, Demmer LA, Agbulos A, Lin AE, Smoot L, … Gripp KW (2009). Male-to-male transmission of Costello syndrome: G12S HRAS germline mutation inherited from a father with somatic mosaicism. American Journal of Medical Genetics. Part A, 149A(3), 315–321. 10.1002/ajmg.a.32639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol-Church K, Stabley DL, Nicholson L, Gonzalez IL, & Gripp KW (2006). Paternal bias in parental origin of HRAS mutations in Costello syndrome. Human Mutation, 27(8), 736–741. 10.1002/humu.20381 [DOI] [PubMed] [Google Scholar]
- Sriboonnark L, Aurora H, Falto-Aizpurua L, Choudhary S, & Connelly EA (2015). Costello syndrome with severe nodulocystic acne: Unexpected significant improvement of acanthosis nigricans after oral isotretinoin treatment. Case Reports in Pediatrics, 2015, 934864–934865. 10.1155/2015/934865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RI, Legault L, Daneman D, Weksberg R, & Hamilton J (2004). Growth hormone deficiency in Costello syndrome. American Journal of Medical Genetics. Part A, 129A(2), 166–170. 10.1002/ajmg.a.30187 [DOI] [PubMed] [Google Scholar]
- Stevenson DA, Allen S, Tidyman WE, Carey JC, Viskochil DH, Stevens A, … Rauen KA (2012). Peripheral muscle weakness in RASopathies. Muscle & Nerve, 46(3), 394–399. 10.1002/mus.23324 [DOI] [PubMed] [Google Scholar]
- Stevenson DA, Schwarz EL, Carey JC, Viskochil DH, Hanson H, Bauer S, … Pasquali M (2011). Bone resorption in syndromes of the Ras/MAPK pathway. Clinical Genetics, 80(6), 566–573. 10.1111/j.1399-0004.2010.01619.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson DA, & Yang FC (2011). The musculoskeletal phenotype of the RASopathies. American Journal of Medical Genetics Part C, 157C(2), 90–103. 10.1002/ajmg.c.30296 [DOI] [PubMed] [Google Scholar]
- Suri M, & Garrett C (1998). Costello syndrome with acoustic neuroma and cataract. Is the Costello locus linked to neurofibromatosis type 2 on 22q? Clinical Dysmorphology, 7(2), 149–151. [DOI] [PubMed] [Google Scholar]
- Takahashi M, & Ohashi H (2013). Craniofacial and dental malformations in Costello syndrome: A detailed evaluation by using multi-detector row computed tomography. Congenital Anomalies (Kyoto), 53(2), 67–72. 10.1111/cga.12004 [DOI] [PubMed] [Google Scholar]
- Thornton PS, Stanley CA, De Leon DD, Harris D, Haymond MW, Hussain K, … Pediatric Endocrine Society. (2015). Recommendations from the Pediatric Endocrine Society for evaluation and Management of Persistent Hypoglycemia in neonates, infants, and children. The Journal of Pediatrics, 167(2), 238–245. 10.1016/j.jpeds.2015.03.057 [DOI] [PubMed] [Google Scholar]
- Tidyman WE, Lee HS, & Rauen KA (2011). Skeletal muscle pathology in Costello and cardio-facio-cutaneous syndrome: Developmental consequences of germline Ras/MAPK activation on myogenesis. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 157C(2), 104–114. 10.1002/ajmg.c.30298 [DOI] [PubMed] [Google Scholar]
- Tidyman WE, & Rauen KA (2016a). Expansion of the RASopathies. Current Genetic Medicine Reports, 4(3), 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidyman WE, & Rauen KA (2016b). Pathogenetics of the RASopathies. Human Molecular Genetics, 25, R123–R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakami S, Igawa M, Shiina H, Shigeno K, Kikuno N, & Yoshino T (2002). Recurrent transitional cell carcinoma in a child with the Costello syndrome. The Journal of Urology, 168(3), 1133–1134. [DOI] [PubMed] [Google Scholar]
- Van den Bosch T, Van Schoubroeck D, Fryns JP, Naulaers G, Inion AM, & Devriendt K (2002). Prenatal findings in a monozygotic twin pregnancy with Costello syndrome. Prenatal Diagnosis, 22(5), 415–417. 10.1002/pd.333 [DOI] [PubMed] [Google Scholar]
- van der Burgt I, Kupsky W, Stassou S, Nadroo A, Barroso C, Diem A, … Zenker M (2007). Myopathy caused by HRAS germline mutations: Implications for disturbed myogenic differentiation in the presence of constitutive HRas activation. Journal of Medical Genetics, 44(7), 459–462. 10.1136/jmg.2007.049270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel MA, Vreeburg M, Peels C, van Ravenswaaij-Arts CM, Bijlsma E, Schrander-Stumpel CT, & van Geel M (2006). Recurring HRAS mutation G12S in Dutch patients with Costello syndrome. Experimental Dermatology, 15(9), 731–734. 10.1111/j.1600-0625.2006.0047.x [DOI] [PubMed] [Google Scholar]
- Villani A, Greer MC, Kaslish JM, Nakagawara A, Nathanson KL, Pajtler KW, … Kratz CP (2017). Recommendations for cancer surveillance in individuals with RASopathies and other rare genetic conditions with increased cancer risk. Clinical Cancer Research, 23(12), e83–e90. 10.1158/1078-0432.CCR-17-0631 [DOI] [PubMed] [Google Scholar]
- Weaver KN, Wang D, Cnota J, Gardner N, Stabley D, Sol-Church K, … Hopkin RJ (2014). Early-lethal Costello syndrome due to rare HRAS Tandem Base substitution (c.35_36GC>AA; p.G12E)-associated pulmonary vascular disease. Pediatric and Developmental Pathology, 17(6), 421–430. 10.2350/14-05-1488-OA.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SM, Graham JM Jr., Kerr B, Gripp K, Weksberg R, Cytrynbaum C, … Bankier A (2005). The adult phenotype in Costello syndrome. American Journal of Medical Genetics. Part A, 136(2), 128–135. 10.1002/ajmg.a.30747 [DOI] [PubMed] [Google Scholar]
- Xu F, Wang HJ, Lin ZM, & Yu B (2015). Recurrent duplication mutation in HRAS causing mild Costello syndrome in a Chinese patient. Clinical and Experimental Dermatology, 40(4), 404–407. 10.1111/ced.12571 [DOI] [PubMed] [Google Scholar]
- Yassir W, Grottkau BE, & Goldberg MJ (2003). Costello syndrome: Orthopaedic manifestations and functional health. Journal of Pediatric Orthopedics, 23(1), 94–98. [PubMed] [Google Scholar]
- Young O, Perati S, Weiss LA, & Rauen KA (2018). Age and ASD symptoms in Costello syndrome. American Journal of Medical Genetics. Part A, 176(4), 1027–1028. 10.1002/ajmg.a.38641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampino G, Mastroiacovo P, Ricci R, Zollini M, Segni G, Martini-Neri ME, & Neri G (1993). Costello syndrome: Further clinical delineation, natural history genetic definition and nosology. American Journal of Medical Genetics, 47(2), 176–183. 10.1002/ajmg.1320470210 [DOI] [PubMed] [Google Scholar]
- Zampino G, Pantaleoni F, Carta C, Cobellis G, Vasta I, Neri C, … Tartaglia M (2007). Diversity, parental germline origin, and phenotypic spectrum of de novo HRAS missense changes in Costello syndrome. Human Mutation, 28(3), 265–272. 10.1002/humu.20431 [DOI] [PubMed] [Google Scholar]