ABSTRACT
The circulation of SARS-CoV-2 has resulted in the emergence of variants of concern (VOCs). It is currently unclear whether the previous infection with SARS-CoV-2 provides protection against reinfection with VOCs. Here, we show that low dose aerosol exposure to hCoV-19/human/USA/WA-CDC-WA1/2020 (WA1, lineage A), resulted in a productive mild infection. In contrast, a low dose of SARS-CoV-2 via fomites did not result in productive infection in the majority of exposed hamsters and these animals remained non-seroconverted. After recovery, hamsters were re-exposed to hCoV-19/South African/KRISP-K005325/2020 (VOC B.1.351) via an intranasal challenge. Seroconverted rechallenged animals did not lose weight and shed virus for three days. They had a little infectious virus and no pathology in the lungs. In contrast, shedding, weight loss and extensive pulmonary pathology caused by B.1.351 replication were observed in the non-seroconverted animals. The rechallenged seroconverted animals did not transmit the virus to naïve sentinels via direct contact transmission, in contrast to the non-seroconverted animals. Reinfection with B.1.351 triggered an anamnestic response that boosted not only neutralizing titres against lineage A, but also titres against B.1.351. Our results confirm that aerosol exposure is a more efficient infection route than fomite exposure. Furthermore, initial infection with SARS-CoV-2 lineage A does not prevent heterologous reinfection with B.1.351 but prevents disease and onward transmission. These data suggest that previous SARS-CoV-2 exposure induces partial protective immunity. The reinfection generated a broadly neutralizing humoral response capable of effectively neutralizing B.1.351 while maintaining its ability to neutralize the virus to which the initial response was directed against.
KEYWORDS: SARS-CoV-2, reinfection, Syrian hamster, aerosol, fomite, B.1.351, neutralization
Introduction
SARS-CoV-2 transmission appears largely driven by direct contact and aerosol exposures. In experimental SARS-CoV-2 animal models, the exposure route and dose are linked to disease severity [1, 2]. The evolution of SARS-CoV-2 has led to the emergence of several new variants. Within these novel emerging SARS-CoV-2 variants, there are variants of interest (VOIs) and variants of concern (VOCs). VOCs are defined by phenotypic changes including enhanced transmission, increased pathogenicity and decreased efficacy of prophylactic and therapeutic countermeasures [3, 4]. Some VOCs display specific mutations in the spike protein that reduce the binding affinity of neutralizing antibodies [5–8]. The relative protection induced by a previous SARS-CoV-2 infection against a homologous or heterologous challenge has not been demonstrated comprehensively.
VOC B.1.351 (20H/501Y.V2) was first detected in South Africa and has been shown to exhibit reduced susceptibility to neutralization by sera from COVID-19 patients and vaccinated individuals [9, 10]. Compared to the original Wuhan strain (lineage A), B.1.351 has nine amino acid substitutions and one deletion in the spike protein, including three changes in the receptor-binding domain (RBD); K417N/T, E484K and N501Y [11].
We previously demonstrated the susceptibility of the Syrian hamster to SARS-CoV-2 aerosol and fomite exposure [2]. Here, we show that hamsters can productively be infected with a very low dose of aerosolized SARS-CoV-2, whereas a comparable low dose of fomite exposure did not result in infection. We then rechallenged the animals with B.1.351 to examine the potential for SARS-CoV-2 reinfection and onward transmission.
Materials and methods
Ethics statement
Approval of animal experiments was obtained from the Institutional Animal Care and Use Committee of the Rocky Mountain Laboratories. Performance of experiments was done following the guidelines and basic principles in the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals. Work with infectious SARS-CoV-2 strains under BSL3 conditions was approved by the Institutional Biosafety Committee (IBC). Inactivation and removal of samples from high containment were performed per IBC-approved standard operating procedures.
Virus strains and cell lines
SARS-CoV-2 strain hCoV-19/human/USA/WA-CDC-WA1/2020 (WA1, lineage A) was provided by CDC, Atlanta, USA while SARS-CoV-2 variant hCoV-19/South Africa/KRISP-K005325/2020 (B.1.351) was obtained from Dr Tulio de Oliveira and Dr Alex Sigal at the Nelson R. Mandela School of Medicine, UKZN. Both variants were propagated in VeroE6 cells in DMEM supplemented with 2% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (DMEM2). VeroE6 cells were maintained in DMEM supplemented with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. VeroE6 cells were provided by Dr Ralph Baric. No mycoplasma was detected. Both strains were deep-sequenced using an Illumina NextSeq Platform, and no fixed mutations were found in nCoV-WA1-2020; the viral stock sequence was identical to the original patient sequence MN985325. However, for hCoV-19/South Africa/KRISP-K005325/2020 the viral stock contained the following non-fixed substitutions in the spike protein: Q677H (present at 88%) and R682W (present at 81%).
Animal exposure and inoculation
First, four to six-week-old female Syrian hamsters (n = 20, ENVIGO) were inoculated with a low dose of SARS-CoV-2 variant hCoV-19/human/USA/WA-CDC-WA1/2020 (WA1, lineage A) by aerosol or fomite. After 21 days, hamsters were rechallenged with a high dose of SARS-CoV-2 variant CoV-19/South African/KRISP-K005325/2020. For the initial inoculation, hamsters were exposed either via aerosol or fomite route as previously described by Port et al. 2020 [2]. For aerosol exposure non-anesthetized hamsters (4 per group) were placed into a stainless-steel wire mesh cage and subjected to 2.5 × 101 and 1.1 × 102 TCID50 SARS-CoV-2 aerosol particles, respectively, for 10 min using a 3-jet collision nebulizer (Biaera technologies, USA). Particles ranged from 1 to 5 µm in size. Dosage was confirmed by titration of the inoculum. Fomite exposure was conducted by exposing individually housed animals to a polypropylene dish containing 40 µl of 101, 102, and 103 TCID50 SARS-CoV-2 per hamster (4 hamsters per group). At 14 days post inoculation (DPI) with aerosolized virus or fomites, blood was obtained by retro-orbital bleeding and seroconversion was confirmed. At 21 DPI all seroconverted hamsters (9 hamsters) were intranasally inoculated with 40 µl sterile DMEM containing 8 × 104 TCID50 SARS-CoV-2 variant CoV-19/South African/KRISP-K005325/2020 (rechallenge). As a control, two randomly selected non-seroconverted hamsters were included in the rechallenge. At 5 days post rechallenge (DPR), 3 of the seroconverted and 2 non-seroconverted rechallenged hamsters were euthanized, and lungs and serum collected. The remaining 6 animals were euthanized at 14 DPR. To assess transmission, all rechallenged animals were co-housed with a naïve sentinel (1:1 ratio) in a new cage for 24 h at 2 DPR. In all experiments, hamsters were individually housed. Weights were collected and oropharyngeal swabs were taken in 1 ml DMEM with 200 U/ml penicillin and 200 µg/ml streptomycin. Sentinels were swabbed for three days.
Viral RNA detection
Swabs from hamsters were collected as described above. RNA was extracted (140 µl) using the QIAamp Viral RNA Kit (Qiagen) using QIAcube HT automated system (Qiagen) according to the manufacturer’s instructions. Sub-genomic (sg) viral RNA was detected by qRT-PCR [12]. Five μl RNA (150 µl elution volume) was tested with TaqMan™ Fast Virus One-Step Master Mix (Applied Biosystems) using QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems) according to the manufacturer’s instructions. Copy numbers/ml was calculated based on RNA standards.
Virus titration
Viable virus in lungs or swabs was determined as previously described [13]. Briefly, tissues were weighted, then homogenized in 1 ml of DMEM2. Virus titrations were performed by end-point titration in VeroE6 cells, inoculated with tenfold serial dilutions of hamster swabs or tissue homogenates in 96-well plates. For swabs, plates were spun down for 1 h at 1000 rpm before incubation. Cytopathic effect was scored at day 6. TCID50 was calculated by the method of Spearman–Karber and, if required, adjusted for tissue weight.
Histopathology
Necropsies and tissue sampling were performed according to IBC-approved protocols. Tissues were fixed for a minimum of 7 days in 10% neutral buffered formalin with 2 changes. Tissues were placed in cassettes and processed with a Sakura VIP-6 Tissue Tek, on a 12-hour automated schedule, using a graded series of ethanol, xylene, and ParaPlast Extra. Prior to staining, embedded tissues were sectioned at 5 µm and dried overnight at 42°C. Using GenScript U864YFA140-4/CB2093 NP-1 (1:1000) specific anti-CoV immunoreactivity was detected using the Vector Laboratories ImPress VR anti-rabbit IgG polymer (# MP-6401) as secondary antibody. The tissues were then processed using the Discovery Ultra automated processor (Ventana Medical Systems) with a ChromoMap DAB kit Roche Tissue Diagnostics (#760-159).
Serology – ELISA
Serum samples were inactivated with γ-irradiation (2 mRad). Maxisorp plates (Nunc) were coated with 50 ng spike protein per well and incubated overnight at 4°C. After blocking with casein in phosphate-buffered saline (PBS) (ThermoFisher) for 1 h at room temperature (RT), serially diluted 2-fold serum samples (duplicate, in casein) were incubated for 1 h at RT. Spike-specific antibodies were detected with goat anti-hamster IgG Fc (horseradish peroxidase (HRP)-conjugated, Abcam) for 1 h at RT and visualized with KPL TMB 2-component peroxidase substrate kit (SeraCare, 5120-0047). The reaction was stopped with KPL stop solution (Seracare) and read at 450 nm. Plates were washed 3× with PBS-T (0.1% Tween) in between steps. The threshold for positivity was calculated as the average plus 3× the standard deviation of negative control hamster sera.
Serology – virus neutralization
Heat-inactivated γ-irradiated sera were two-fold serially diluted in DMEM2. 100 TCID50 of SARS-CoV-2 strain nCoV-WA1-2020 or hCoV-19/South African/KRISP-K005325/2020 was added. After 1 h of incubation at 37°C and 5% CO2, the virus:serum mixture was added to VeroE6 cells. CPE was scored after 5 days at 37°C and 5% CO2. The virus neutralization titre was expressed as the reciprocal value of the highest dilution of the serum which still inhibited virus replication.
Results
Low-dose aerosol exposure is highly efficient and displays a mild disease phenotype in hamsters
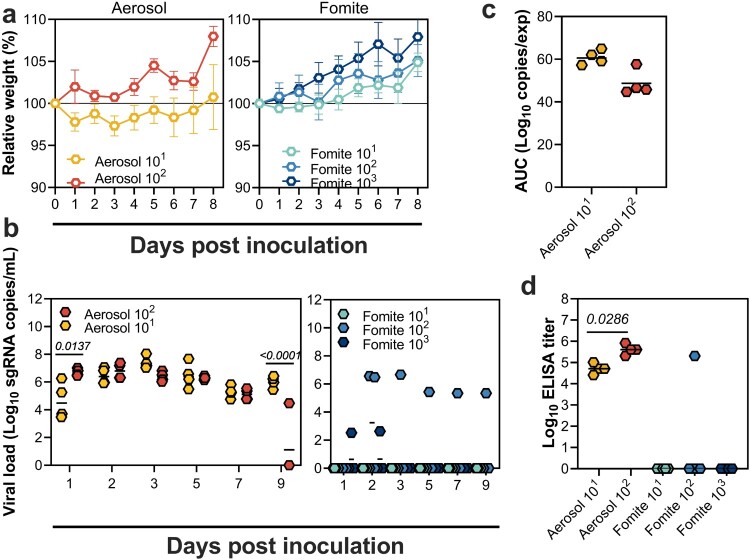
We previously demonstrated that hamsters become uniformly infected after an experimental aerosol or fomite exposure with a relatively high dose of 8 × 104 TCID50 SARS-CoV-2 [2]. To increase our understanding of the relationship between exposure route and infectious dose we exposed Syrian hamsters to SARS-CoV-2 administered via low dose aerosols or fomites. For the aerosol inoculation route, two groups (N = 4) of hamsters were exposed to 1.1 × 102 or 2.5 × 101 TCID50 of SARS-CoV-2 strain nCoV-WA1-2020 (WA1, lineage A; indicated as Aerosol 102 and 101). For the fomite inoculation route, three groups (N = 4) of hamsters were exposed to 103, 102 and 101 TCID50 of SARS-CoV-2 WA1 (indicated as Fomite 103, 102 and 101). No significant median weight loss was observed following inoculation for any of the groups (Figure 1(a)). To assess productive infection, we measured subgenomic RNA (sgRNA) in oropharyngeal swabs at 1, 2, 3, 5, 7, and 9 DPI. In the Aerosol 102 and 101 groups, respiratory shedding started at 1 DPI and lasted 9 days on average. In contrast, no prolonged shedding was observed in the Fomite 101 and 103 groups, and only one animal in the Fomite 102 group demonstrated prolonged shedding until 9 DPI (Figure 1(b)). To compare the magnitude of shedding between the two aerosol groups, we performed an area under the curve (AUC) analysis. No significant difference between the two groups was observed (median Aerosol 102 = 46.16 and Aerosol 101 = 60.57 AUC log10 copies/exp, N = 4, Mann–Whitney test, p = .0571) (Figure 1(c)). All aerosol-exposed hamsters seroconverted by 14 DPI, as measured by anti-spike ELISA. In contrast, seroconversion was only detected in the single animal which demonstrated prolonged shedding in the Fomite 102 group. The magnitude of the humoral response was higher in Aerosol 102 animals than in the Aerosol 101 animals (Figure 1(d), median Aerosol 102 = 409,600 and Aerosol 101 = 51,200 ELISA titre, N = 4, Mann Whitney test, p = .0286). This study confirms that aerosol exposure is a highly efficient infection route in hamsters, whereas fomite exposure is less so.
Figure 1.
Infection efficiency of low-dose SARS-CoV-2 aerosol and fomite exposure. (a) Relative weight in hamsters after SARS-CoV-2 inoculation via the aerosol route with 1.1 × 102 or 2.5 × 101 TCID50 (indicated as Aerosol 102 and 101) or fomite route with 103, 102 and 101 TCID50 (indicated as Fomite 103, 102 and 101) of hCoV-19/human/USA/WA-CDC-WA1/2020 (WA1, lineage A), (N = 4 per group). Symbols show mean and error bars show standard error of the mean (SEM). (b) Viral load in oropharyngeal swabs of individual animals. (c) Area under the curve (AUC) analysis of sgRNA detected in oropharyngeal swabs throughout the experiment. The lines represent the median. (d) Binding antibodies against spike protein of SARS-CoV-2 in serum obtained at 14 days post inoculation.
Previous low-dose aerosol exposure with SARS-CoV-2 lineage A protects hamsters from disease after high-dose B.1.351 challenge
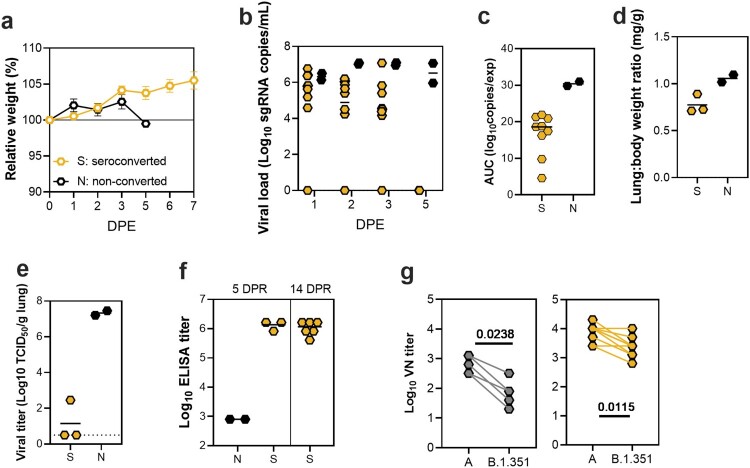
Next, we investigated whether reinfection with VOC B.1.351 would occur after a prior low-dose aerosol or fomite exposure with lineage A. At 21 DPI, the nine seroconverted animals (four of each aerosol exposed group and one Fomite 102 animal), and two non-seroconverted animals were each intranasally inoculated with 8 × 104 TCID50 VOC B.1.351. Seroconverted animals did not lose weight after rechallenge. In contrast, the two naive animals lost weight at 5 DPR (Figure 2(a)). Compared to the non-seroconverted animals, the seroconverted animals shed relatively low amounts of sgRNA and for a shorter duration. The magnitude of shedding was higher in non-seroconverted animals (median seroconverted = 16.62 (N = 9) and non-seroconverted = 30.42 AUC log10 copies/exp (N = 2)) (Figure 2(b,c)). At 5 DPR, three seroconverted animals and the two non-seroconverted animals were selected for necropsy. Lung:body weight ratio was increased in non-seroconverted animals compared to seroconverted animals (median seroconverted = 0.7256 mg/g (N = 3) and non-seroconverted = 1.057 mg/g (N = 2)) (Figure 2(d)). Up to 107 TCID50 SARS-CoV-2/g tissue was detected in the lungs of non-seroconverted animals while only one of the seroconverted animals was positive for infectious virus with a titre of 102.5 TCID50/g (Figure 2(e)). Spike protein amino acid substitutions Q677H and R682W were completely cleared from the virus population and no longer detected in lung tissue of virus positive animals.
Figure 2.
Heterologous B.1.351 challenge following SARS-CoV-2 lineage A exposure. (a) Relative weight in hamsters after 8 × 104 TCID50 VOC B.1.351 intranasal rechallenge over time (DPR = day post rechallenge) for the seroconverted animals (N = 9) and non-seroconverted animals (N = 2). Symbols show mean and error bars show standard error of the mean (SEM). (b) Viral load in oropharyngeal swabs from individual rechallenged animals. Line represents median. (c) Area under the curve (AUC) analysis of shedding as measured by viral load in swabs. Line represents median. (d) Lung:body weight ratio (mg/g) of hamsters euthanized at 5 DPR. Line represents median. (e) Infectious virus titre in lung tissue obtained at 5 DPR. Line represents median, dotted line represents limit of detection. (f) Binding antibodies against spike protein of SARS-CoV-2 in serum obtained 14 DPR. (g) Left panel: Virus neutralizing antibody titres against lineage A and VOC B.1.351. Right panel: Virus neutralizing antibody titres against lineage A and VOC B.1.351 in serum obtained from seroconverted animals after VOC B.1.351 rechallenge.
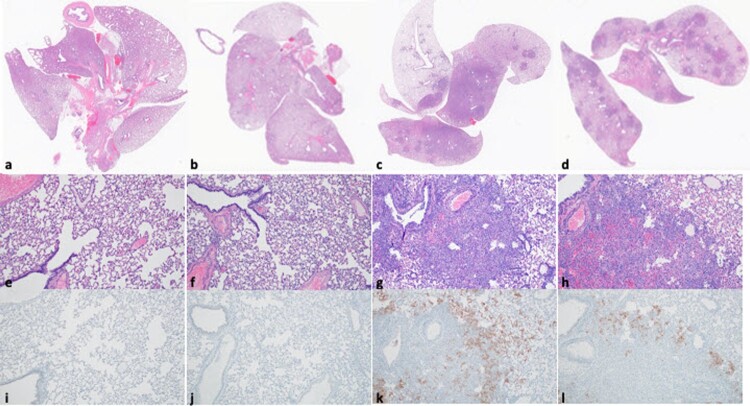
Upon histopathological analysis lung tissue from seroconverted animals was normal (Figure 3(a, b, e, and f)) while the lungs of the 2 non-seroconverted animals demonstrated alveolar inflammation consisting of macrophages and neutrophils, as well as variable amounts of hemorrhage, fibrin and edema (Figure 3(c, d, g, and h)) consistent with a lower respiratory tract infection with SARS-CoV-2. Immunohistochemistry using a monoclonal antibody against SARS-CoV-2 demonstrated viral antigen in bronchial and bronchiolar epithelium, type I and II pneumocytes as well as pulmonary macrophages within the non-seroconverted animals (Figure 3(k,l)), but not in the seroconverted animals (Figure 3(i, j)).
Figure 3.
Lung pathology after heterologous B.1.351 rechallenge. Comparison of SARS-CoV-2 pathology for seroconverted and non-seroconverted hamsters at 5 days post rechallenge (DPR). (a–d) HE 1×. Lungs of seroconverted hamsters (a and b) and non-seroconverted hamsters (c and d), lung tissue from seroconverted animals was normal whereas the non-seroconverted animals displayed pathology consistent of lower respiratory tract infection with SARS-CoV-2. (e–h) HE 100×. Normal lungs from seroconverted hamsters (e and f) and lungs from non-seroconverted hamsters demonstrated alveolar inflammation consisting of macrophages and neutrophils, as well as variable amounts of hemorrhage, fibrin and edema (g and h). (i–l) IHC staining against N protein SARS-CoV-2 (SARS-CoV-2 antigen is visible as red-brown staining), 100×. SARS-CoV-2 antigen is absent from lungs of the seroconverted hamsters (i and j) whereas lungs from non-seroconverted hamsters demonstrated viral antigen in bronchial and bronchiolar epithelium, type I and II pneumocytes as well as pulmonary macrophages (k and l). Scale bar indicates 50 µm.
Rechallenge with VOC B.1.351 led to an increase in the humoral response. ELISAs conducted at 5 DPR showed seroconverted rechallenged animals had increased levels of antibody titers against lineage A SARS-CoV-2 spike protein (median = 1,638,400 ELISA titer, N = 3) compared to the minimal levels of antibodies in the two non-seroconverted animals (median = 800, N = 2) (Figure 2(f)). Next, we investigated neutralizing antibody titres in the serum of initial and rechallenged animals against SARS-CoV-2 lineage A and B.1.351 variants. Neutralization of B.1.351 was significantly reduced compared to neutralization of SARS-CoV-2 lineage A (median = 80/640 VN titre, median 16-fold reduction, N = 5, Mann–Whitney test, p = .0238, Figure 2(g)). The rechallenge with VOC B.1.351 resulted in an increased neutralizing titre against both B.1.351 (median = 2560 VN titre, N = 9) and lineage A (median = 10,240 VN titre, N = 9). However, neutralization of B.1.351 was still significantly reduced compared to neutralization of SARS-CoV-2 lineage A (median 4-fold reduction, N = 9, Mann–Whitney test, p = .0115).
These data suggest that prior exposure does not protect hamsters from reinfection with an antigenically different SARS-CoV-2 virus. However, the humoral immune response generated by a low dose primary infection provides protection against disease and lower respiratory tract replication with an antigenically different SARS-CoV-2 virus and the replication remains confined to the upper respiratory tract.
Previous low-dose aerosol exposure with SARS-CoV-2 lineage A protects hamsters from transmitting VOC B.1.351 to naïve animals
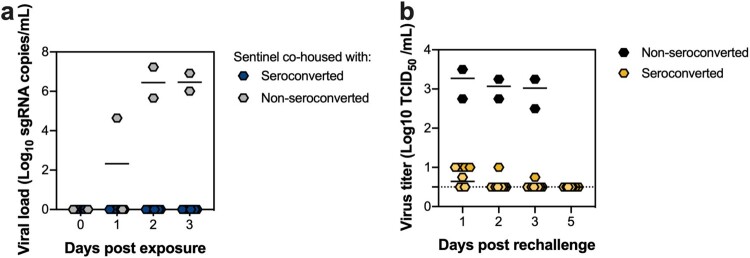
We next observed the ability of rechallenged hamsters to transmit VOC B.1.351 to naïve hamsters. Eight seroconverted and two non-seroconverted rechallenged hamsters (N = 10) were co-housed with naive sentinels at 2 DPR for 24 h. We assessed transmission by taking oropharyngeal swabs of the sentinels at day 1, 2 and 3 post exposure (DPE) and analysing them for the presence of sgRNA. In the two sentinels co-housed with a non-seroconverted rechallenged hamster, we observed shedding of sgRNA from the sentinel hamsters (Figure 4(a)). In contrast, in swabs obtained from sentinels co-housed with seroconverted rechallenged hamsters, no SARS-CoV-2 RNA was detected at any timepoint suggesting that no transmission occurred. The efficiency of transmission was also evaluated by serology at 14 DPE. Only the two sentinels co-housed with a non-seroconverted rechallenged hamster had seroconverted, as measured by anti-spike ELISA (Table 1). We thus conclude that none of the seroconverted animals transmitted B.1.351 to sentinels, whereas both non-seroconverted animals transmitted the virus to sentinels.
Figure 4.
Hamsters rechallenged with VOC B.1.351 do not transmit SARS-CoV-2 to naïve sentinels. VOC B.1.351 rechallenged animals were co-housed with a naïve sentinel (1:1 ratio) in a new cage for 24 h at 2 DPR. (a) Viral load of individual exposed sentinel animals measured by sgRNA in oropharyngeal swabs days post exposure (DPE). (b) Infectious virus titre of individual animals in oropharyngeal swabs from rechallenged animals. Dotted line = limit of detection.
Table 1.
Presence of SARS-CoV-2 spike IgG antibodies in sentinels co-housed with B.1.351 rechallenged hamsters in serum obtained 14 days post contact. negative (–): optical density (at 450 nm) < 0.124, positive (+) optical density (at 450 nm) ≥ 0.124.
| Donor | ID | Seroconversion status |
|---|---|---|
| Seroconverted | Sentinel 1 | (–) |
| Sentinel 2 | (–) | |
| Sentinel 3 | (–) | |
| Sentinel 4 | (–) | |
| Sentinel 5 | (–) | |
| Sentinel 6 | (–) | |
| Sentinel 7 | (–) | |
| Sentinel 8 | (–) | |
| Non-seroconverted | Sentinel 9 | (+) |
| Sentinel 10 | (+) |
To understand why transmission did not occur for sentinels co-housed with seroconverted hamsters, we determined the level of infectious virus in oropharyngeal swabs obtained from donor animals. Infectious virus could be detected in swabs from both non-seroconverted animals at 2 DPR (median = 1170 TCID50/ml, N = 2), while the infectious virus was detected in only one of the seroconverted animals and at a very low level (10 TCID50/ml) (Figure 4(b)). This indicates that previous challenge with lineage A by low-dose aerosol and fomite leads to rapid upper respiratory tract clearance of infectious virus after rechallenge and subsequently limits transmission potential.
Discussion
We investigated SARS-CoV-2 infection of Syrian hamsters after low dose exposure to aerosols or fomites. We demonstrated for the first time that aerosol exposure of only 2.5 × 101 TCID50 SARS-CoV-2 was sufficient to cause productive infection in Syrian hamsters. In addition, we confirmed the inefficiency of infection of hamsters after fomite exposure. These results are in line with previous experimental studies where infection via airborne SARS-CoV-2 or influenza A virus was more efficient than infection through contaminated environmental surfaces [14, 15]. In humans, the relative importance of each transmission route remains unclear, but the general consensus is that the principal mode of infection is via respiratory droplets and that the relative risk of fomite transmission of SARS-CoV-2 is considered low compared with direct contact, droplet transmission, or airborne transmission [16–20]. Our experimental data is in line with human epidemiological case studies which show that in closed spaces airborne transmission is highly efficient [21–23].
The emergence of VOC B.1.351 in South Africa has raised concerns with regards to its reinfection potential due to antigenic drift. VOC B.1.351 shows a significant reduction of neutralizing titres in human convalescent serum [24–30]. The degree of protective immunity by previous SARS-CoV-2 infection in humans is currently unknown, but several reports suggest that reinfection does occasionally occur [31–33]. In the Syrian hamster model, homologous challenge resulted in reduced virus shedding and reduced replication in the lungs [34, 35]. In addition, homologous rechallenged hamsters were unable to transmit to naïve animals [35]. Our reinfection data confirm the prior observations and also show that heterologous reinfection with VOC B.1.351 results in limited replication in the lungs, absence of pathology and prevention of onwards transmission in a direct contact transmission model.
Protection against VOC B.1.351 rechallenge was demonstrated in the Syrian hamster model after prior intranasal infection with a lineage A virus [36]. However, this study relied on a high-dose intranasal infection with lineage A. Our study evaluated reinfection potential of VOC B.1.351 after low-dosage fomite or aerosol exposure with lineage A. The B.1.351 virus stock used to challenge hamsters contained non-fixed amino acid variation at position Q677H and R682W (88% and 81%, respectively). These major variant amino acid SNPs were absent on 5 DPR, suggesting that they are rapidly selected against in the SARS-CoV-2 hamster model. Nonetheless, the substitutions which are thought to be important in immune evasion, such as E484K, are still present in the virus stock. Furthermore, efficient upper and lower respiratory tract replication and lung pathology were observed in infected hamsters. We thus do not believe that the presence of these additional amino acid substitutions, present as a quasispecies, affect the data interpretation.
Our results provide more experimental support to the consensus that aerosol exposure is a more efficient infection route than fomite exposure [37]. Whereas initial infection with SARS-CoV-2 lineage A did not directly prevent heterologous reinfection with VOC B.1.351, it did reduce shedding and did prevent disease and lower respiratory tract replication and onward transmission. Several studies have reported reduced neutralization of B.1.351 by convalescent plasma and vaccine-induced neutralizing antibodies [6, 38, 39, 40] and here we confirm this observation with hamster sera with a 16-fold drop in neutralizing titre between a lineage A virus and the B.1.351. Reinfection with B.1.351 triggered an anamnestic response that boosted not only the neutralizing titre against lineage A, but also against B.1.351, and the difference between neutralizing capacity was reduced from 16-fold to 4-fold. These data suggest that reinfection with B.1.351 not only boosts the existing antibody repertoire, but in addition generates a broadly neutralizing humoral response. This humoral response is capable of effectively neutralizing B.1.351 while maintaining its ability to neutralize the virus to which the initial response was generated against. Finally, the induction of partial protective immunity by previous SARS-CoV-2 exposure might reduce the risk for lower respiratory tract COVID-19 disease and onward transmission in humans.
Acknowledgements
The authors would like to thank Brandi Williamson, Craig Martens, Kent Barbian, Stacey Ricklefs, Sarah Anzick and the animal caretakers for their excellent assistance during the study and Sujatha Rashid, Ranjan Mukul, Kimberly Stemple for critical help with obtaining the SARS-CoV-2 isolates in this study. The following reagent was obtained through BEI Resources, NIAID, NIH: SARS-Related Coronavirus 2, Isolate hCoV-19/South Africa/KRISP-K005325/2020, NR-54009, contributed by Alex Sigal and Tulio de Oliveira.
Funding Statement
This research was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID) and National Institutes of Health (NIH).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Reference
- 1.Rosenke K, Meade-White K, Letko M, et al. . Defining the Syrian hamster as a highly susceptible preclinical model for SARS-CoV-2 infection. Emerg Microbes Infect. 2020;9(1):2673–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Port JR, Yinda CK, Owusu IO, et al. . SARS-CoV-2 disease severity and transmission efficiency is increased for airborne but not fomite exposure in Syrian hamsters. bioRxiv. 2020. 2020.12.28.424565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Public Health England VTG . SARS-CoV-2 variants of concern and variants under investigation in England, . Public Health England. 2021 11 March 2021; Technical briefing 7.
- 4.WHO . COVID-19 Weekly Epidemiological Update, 25 February 2021. Special edition: Proposed working definitions of SARS-CoV-2 Variants of Interest and Variants of Concern. Geneva, Switzerland. 2021 [Google Scholar]
- 5.Chen RE, Zhang X, Case JB, et al. . Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med. 2021;27:717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Supasa P, Zhou D, Dejnirattisai W, et al. . Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021;184(8):2201–2211.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stamatatos L, Czartoski J, Wan Y-H, et al. . Antibodies elicited by SARS-CoV-2 infection and boosted by vaccination neutralize an emerging variant and SARS-CoV-1. medRxiv. 2021. 2021.02.05.21251182. [Google Scholar]
- 8.Shen X, Tang H, McDanal C, et al. . SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021;29(4):529–539.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann M, Arora P, Groß R, et al. . SARS-CoV-2 variants B.1.351 and B.1.1.248: escape from therapeutic antibodies and antibodies induced by infection and vaccination. bioRxiv. 2021. 2021.02.11.430787. [Google Scholar]
- 10.Collier DA, De Marco A, Ferreira IATM, et al. . SARS-CoV-2 B.1.1.7 sensitivity to mRNA vaccine-elicited, convalescent and monoclonal antibodies. medRxiv. 2021. 2021.01.19.21249840. [Google Scholar]
- 11.Tegally H, Wilkinson E, Giovanetti M, et al. . Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv. 2020. 2020.12.21.20248640. [Google Scholar]
- 12.Corman VM, Landt O, Kaiser M, et al. . Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Doremalen N, Falzarano D, Ying T, et al. . Efficacy of antibody-based therapies against Middle East respiratory syndrome coronavirus (MERS-CoV) in common marmosets. Antiviral Res. 2017;143:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sia SF, Yan L-M, Chin AWH, et al. . Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583(7818):834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mubareka S, Lowen AC, Steel J, et al. . Transmission of influenza virus via aerosols and fomites in the Guinea pig model. J Infect Dis. 2009;199(6):858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman E. Exaggerated risk of transmission of COVID-19 by fomites. Lancet Infect Dis. 2020;20(8):892–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitol AK, Julian TR.. Community transmission of SARS-CoV-2 by fomites: risks and risk reduction strategies. medRxiv. 2020. 2020.11.20.20220749. [DOI] [PubMed] [Google Scholar]
- 18.CDC . How COVID-19 Spreads. 2021.
- 19.Zhang R, Li Y, Zhang AL, et al. . Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci USA. 2020;117(26):14857–14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boone SA, Gerba CP.. Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol. 2007;73(6):1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamagishi T. Environmental sampling for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during a coronavirus disease (COVID-19) outbreak aboard a commercial cruise ship. medRxiv. 2020. 2020.05.02.20088567. [Google Scholar]
- 22.Lu J, Gu J, Li K, et al. . COVID-19 Outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26(7):1628–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamner L, Dubbel P, Capron I, et al. . High SARS-CoV-2 attack rate following exposure at a Choir Practice — Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(19):606–610. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Liu J, Xia H, et al. . Neutralizing activity of BNT162b2-elicited serum. N Engl J Med. 2021;384(15):1466–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou D, Dejnirattisai W, Supasa P, et al. . Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine induced sera. Cell. 2021;184(9):2348–2361.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Planas D, Bruel T, Grzelak L, et al. . Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. bioRxiv. 2021. 2021.02.12.430472. [DOI] [PubMed] [Google Scholar]
- 27.Wang P, Nair MS, Liu L, et al. . Antibody Resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857):130–135. [DOI] [PubMed] [Google Scholar]
- 28.Wu K, Werner AP, Moliva JI, et al. . mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv. 2021. 2021.01.25.427948. [Google Scholar]
- 29.Xie X, Liu Y, Liu J, et al. . Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat Med. 2021;27(4):620–621. [DOI] [PubMed] [Google Scholar]
- 30.Wibmer CK, Ayres F, Hermanus T, et al. . SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med. 2021;27(4):622–625. [DOI] [PubMed] [Google Scholar]
- 31.Tillett RL, Sevinsky JR, Hartley PD, et al. . Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect Dis. 2021;21(1):52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.SeyedAlinaghi S, Oliaei S, Kianzad S, et al. . Reinfection risk of novel coronavirus (COVID-19): A systematic review of current evidence. World J Virol. 2020;9(5):79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J-S, Kim SY, Kim TS, et al. . Evidence of severe acute respiratory syndrome coronavirus 2 reinfection after recovery from mild coronavirus disease 2019. Clin Infect Dis. 2020;ciaa1421:1058–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imai M, Iwatsuki-Horimoto K, Hatta M, et al. . Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci U S A. 2020;117(28):16587–16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selvaraj P, Lien CZ, Liu S, et al. . SARS-CoV-2 infection induces protective immunity and limits transmission in Syrian hamsters. Life Sci Alliance. 2021;4(4):e202000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark JJ, Sharma P, Bentley EG, et al. . Naturally-acquired immunity in Syrian golden hamsters provides protection from re-exposure to emerging heterosubtypic SARS-CoV-2 variants B.1.1.7 and B.1.351. bioRxiv. 2021. [Google Scholar]
- 37.CDC . Science brief: SARS-CoV-2 and surface (fomite) transmission for indoor community environments. Atlanta, US: CDC. 2021. [PubMed] [Google Scholar]
- 38.Stamatatos L, Czartoski J, Wan Y-H, et al. . mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021. eabg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou D, Dejnirattisai W, Supasa P, et al. . Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184(9):2348–2361.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madhi SA, Baillie V, Cutland CL, et al. . Efficacy of the ChAdOx1 nCoV-19 covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1885–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]