Keywords: β-cell heterogeneity, β-cell subtype, diabetes, insulin secretion, β-cell heterogeneity, β-cell subtype, insulin secretion
Abstract
Pancreatic β-cells perform glucose-stimulated insulin secretion, a process at the center of type 2 diabetes etiology. Efforts to understand how β-cells behave in healthy and stressful conditions have revealed a wide degree of morphological, functional, and transcriptional heterogeneity. Sources of heterogeneity include β-cell topography, developmental origin, maturation state, and stress response. Advances in sequencing and imaging technologies have led to the identification of β-cell subtypes, which play distinct roles in the islet niche. This review examines β-cell heterogeneity from morphological, functional, and transcriptional perspectives, and considers the relevance of topography, maturation, development, and stress response. It also discusses how these factors have been used to identify β-cell subtypes, and how heterogeneity is impacted by diabetes. We examine open questions in the field and discuss recent technological innovations that could advance understanding of β-cell heterogeneity in health and disease.
INTRODUCTION
Pancreatic islet β-cells are optimized to secrete insulin, a process critical for maintaining appropriate blood glucose concentration. This requires coordination of glucose sensing and metabolism, ATP production via oxidative phosphorylation, insulin biosynthesis, and [Ca2+]-dependent insulin release (1). In type 2 diabetes, peripheral insulin resistance promotes initial β-cell expansion, but ultimately results in β-cell death and dysfunction (2–4). Therapies include metformin, sulfonylureas, and injection of exogenous insulin, which do not improve long-term β-cell function, and have been associated with weight gain, cancer, and hypoglycemic events (5–7). Current research strategies include ameliorating disrupted β-cell signaling pathways, removing dysfunctional β-cells, and improving the β-cell microenvironment (8–12). Triggering β-cell neogenesis, transdifferentiation of other cell types, and inducing β-cell replication are also being explored (13–16). Many of the therapeutic agents that improve β-cell function are in various stages of development (17). These strategies require understanding the physiological mechanisms controlling β-cell function and are complicated by observations that not all β-cells are alike.
Cell sorting, imaging, and sequencing technologies have allowed for analysis of multiple β-cell properties, revealing that β-cells vary in morphology, function, and gene expression. Drivers of heterogeneity include β-cell topography, developmental origin, maturation state, and stress response (18–21). Several groups have proposed that distinct β-cell subtypes exist, and play unique roles within the islet (22–25). The importance of heterogeneity is reinforced by observations that β-cells are subject to variable fates in diabetes including apoptosis, replication, and dedifferentiation (2, 4, 26, 27). Characterization of β-cell heterogeneity has led to questions about its role in diabetic etiology and therapy (28). This review examines current understanding of the breadth and sources of β-cell heterogeneity and its relevance to diabetes. Examining heterogeneity lends itself to questions about the applications and limitations of β-cell plasticity, which can focus research aimed at improving β-cell function.
β-CELLS ARE MORPHOLOGICALLY AND FUNCTIONALLY HETEROGENEOUS
Histological assessment of the pancreatic islets show β-cells vary in basic morphology, including larger cells with large nuclei that localize near the islet center (29, 30). Increased nuclear size is attributed to polyploidy; β-cells are the only endocrine cell type observed in tetra- and octoploid states (31). The cause of polyploidy in β-cells is linked to metabolic stress, as mean nuclear size and incidence of polyploidy increase in diabetes (31–34). β-Cells exhibit morphological variation in other organelles, with central β-cells exhibiting enlarged endoplasmic reticulum (ER) and Golgi apparatuses following short glucose exposure (35). Other features that distinguish central from peripheral β-cells include mitochondrial morphology, number of insulin granules, and number of cell-cell contacts (33, 36–39). Most β-cells exhibit polarity, consisting of an apical face where primary cilia project into extracellular space, a lateral face where glucose uptake and insulin secretion occur, and a basal face connected to vasculature, forming a rosette-like structure around capillaries (40–43). Furthermore, β-cells with atypical cellular organization have also been identified, including nonpolar cells and cells with apical mRNA localization (22, 23). β-Cells display observable cell cycle variability, with <0.5% of adult human β-cells and 2% of adult mouse β-cells displaying markers of mitosis (44, 45). This replication rate is increased in obesity, pregnancy, and with glucocorticoids (46–49). Clearly, β-cells vary in basic cellular features, and experiments reveal that β-cell function is equally diverse.
Glucose-stimulated insulin secretion has been characterized in depth (50–53). Glucose is converted to pyruvate through glycolysis initiated by glucokinase (Gck), then promotes ATP production through the citric acid cycle and oxidative phosphorylation, which triggers membrane depolarization and [Ca2+]-dependent insulin release. Fluorescence-activated cell sorting (FACS) based on metabolic activity of individual β-cells using NADPH autofluorescence revealed 25% of rat β-cells do not increase activity in high (20 mM) glucose conditions (54). A follow-up study examined metabolic responders and non-responders at 7.5 mM glucose (55). Responders enter first phase insulin secretion at lower glucose concentration and secrete more insulin, despite having similar insulin stores. This is attributed to preferential secretion of newly synthesized insulin over stored insulin and is supported by work showing insulin biosynthesis is stimulated by glucose and that responsive cells are enriched for proinsulin-rich granules (56, 57). Thus, glucose-sensitive cells produce and release mature insulin granules rapidly, resulting in an elevated proinsulin/insulin ratio. Dispersed human β-cells display variation in insulin secretion, with 20% of cells secreting 70–90% of insulin (58, 59). Sorting β-cells based on metabolic activity at 16.7 mM glucose revealed metabolically responsive β-cells are larger in size (60). Controlling for cell size shows metabolically active and inactive cells secrete similar amounts of insulin. Thus, insulin secretion is influenced by cell size, linking β-cell morphology and insulin secretion. This evidence was corroborated by observations that larger, central β-cells degranulate faster than peripheral cells in vivo in response to hyperglycemic conditions (35). Observations that insulin secretion varies between β-cells has been confirmed by a variety of techniques including patch-clamp measurements and 3 D imaging (61–63).
Glucose sensitivity is associated with higher expression of glycolytic and protein biosynthesis genes and correlates with the glucose phosphorylation activity of Gck (64, 65). Treatment with glyceraldehyde bypasses glucose phosphorylation to induce metabolic activity in glucose-unresponsive cells at low glucose concentrations but cannot exceed maximum glucose-induced insulin biosynthesis, suggesting Gck activity is the rate limiting step in insulin synthesis and secretion (66). Giordano et al. (67) assessed the stability of glucose sensitivity during repeat glucose exposure using a hemolytic plaque technique to measure insulin secretion. They found 75% of β-cells were consistently responsive or unresponsive following 3 glucose exposures, whereas 25% of β-cells switched between states, suggesting glucose sensitivity fluctuates in a subset of cells. Glucose sensitivity is predictive of susceptibility to mitotic stimuli and protection against oxidative damage and apoptosis, hinting at how cells would fare in diabetes (47, 54, 68). Collectively, these observations highlight the breadth of phenotypic variation in β-cells, motivating studies aimed at understanding the mechanisms underlying these differences.
HETEROGENEITY IN GENE AND PROTEIN EXPRESSION
Many genes in the insulin secretion pathway display heterogeneous expression and have a direct impact on β-cell function. Glucose import through Glut2 is the first step in the insulin secretion pathway and is inhibited in STZ-induced diabetes in mice (69). Heterogeneous Glut2 expression has been reported in several studies and may be indicative of maturation state and replicative potential (see below) (22, 25, 70–74). The rate-limiting enzyme Gck is variably expressed among β-cells, correlating with glucose sensitivity, Glut2 levels, and number of insulin granules (64, 75). Metabolic perturbations influence Gck expression, which precede changes in Ins2 expression, suggesting a regulatory role in insulin production (76). Variation in insulin gene expression and protein levels has been observed in many studies (23, 37, 73, 76, 77). Central β-cells contain less insulin protein than peripheral β-cells, a distinction that disappears with fasting (76). Using an insulin promoter construct linked with GFP, Katsuta et al. (37) used FACS to separate β-cells containing low, medium, and high levels of insulin protein. Medium level β-cells comprised 70% of cells, and the low insulin population had diminished insulin gene expression and secretion. Variable expression of these components may directly contribute to the heterogeneity seen at the phenotypic level.
Other gene markers of β-cell heterogeneity with a less direct connection to insulin secretion have been identified. These include cell-cell adhesion genes like the sialylated form of neural cell adhesion molecule (PSA-NCAM) (78). FACs sorting β-cells into PSA-NCAM high and low populations revealed that expression correlates with glucose-stimulated insulin secretion (71, 79, 80). PSA-NCAM low cells have less Ca2+ influx following glucose exposure, reduced ATP levels, and decreased expression of Glut2 and Gck. Morphologically, PSA-NCAM knockout cells differ in F-actin distribution, altering cell polarity (78). The proportion of PSA-NCAM high cells increases in diabetes (71). Whether NCAM is heterogeneously expressed in humans is unknown. β-Cells can be sorted based on expression of E-cadherin, which promotes islet formation and compaction (81–83). Like NCAM, sorting β-cells into E-cadherin high and low populations revealed that higher expression is associated with increased glucose-stimulated insulin secretion and insulin expression (81). Furthermore, glucose induces E-cadherin expression and causes dispersed β-cells to aggregate in vitro (82). Other variably expressed genes include Npy, Th, Vmat2, and Dickkopf-3 (84–87).
Several studies have identified β-cell subpopulations based on variation in global gene expression. Baron et al. (88) found two subpopulations in nondiabetic donors driven by variable expression of ER-stress response genes including HERPUD1, HSPA5, and DDIT3, which correspond with low expression of genes associated with β-cell function including UCN3, MAFA, and NEUROD1. This heterogeneity was lost in hyperglycemic samples. Similarly, Muraro et al. (89) identified 3 β-cell populations, one of which had elevated expression of stress response genes HERPUD1 and HSPA1B. Importantly, Muraro et al. (89) validated these findings using RNA-FISH on intact islets to rule out isolation-induced stress. Xin et al. (21) identified four subpopulations in human β-cells, one of which differentially expressed 431 genes. This subpopulation had low insulin expression and elevated expression of ER-stress and UPR-associated genes. Fang et al. (90) profiled 9,964 β-cells from healthy and diabetic donors and identified a subpopulation enriched for expression of heat shock proteins. Last, Segerstolpe et al. (91) identified 5 subgroups based on expression of RBP4, FFAR/GPR120, and ID family transcription factors. RBP4 expression is associated with insulin resistance, whereas ID transcription factors are associated with cell cycle activity (92, 93). Several groups have failed to detect β-cell subpopulations; however, many were limited by the number of β-cells analyzed (94–99). Despite little consensus in the genes that may drive heterogeneity, an emerging pattern suggests β-cells differ in expression of genes related to stress response.
The limitations of single cell technology explain some of the discordance among studies. These shortcomings are highlighted in a meta-analysis from Mawla and Huising (100), who examined discrepancies across five single cell RNA sequencing studies (88, 89, 91, 94, 98, ). Pooling β-cells across all studies revealed 2 populations of β-cells driven by differential expression of 52 genes. These include genes associated with other endocrine and exocrine cell types, but not stress response. Only 3 genes had previously been associated with β-cell heterogeneity: RBP4, DLK1, and HERPUD1. When subsets were identified in cells from healthy donors, only 24 genes were differentially expressed across β-cells. Among these, only NPY has been previously associated with β-cell heterogeneity. This lack of correspondence among studies is a concern. Only 86 genes were detected in all β-cells within the aggregated data. This contrasts with the hundreds of housekeeping genes required for cellular function (101). β-Cells express at least 18,000 genes, thus 99.5% of genes are inconsistently captured (21, 91). With current methodology, the capture of most genes is likely due to technical artifacts or random chance, and not intrinsic biological variation (102). Methods for preprocessing and noise removal are being developed to aid in quality control, including tools that remove cell doublets, remove ambient RNA, assess transcript coverage, and provide imputation for gene dropout (103–106). In human studies, donor age and isolation procedure likely contribute to discordance among studies but are seldom controlled for. Furthermore, many groups report differential expression of genes between subsets of β-cells, but size effect is rarely reported or discussed. As technological advances increase single cell sequencing read depth, profiling thousands of cells all but guarantees statistically significant differential expression based on P values alone. Discussions about whether these differences are meaningful will be increasingly important, and will be aided by measuring effect size (107).
Dorrell et al. (108) used FACS to identify 4 subpopulations based on expression of human β-cell surface markers CD9 and ST8SIA1. They designated these populations as β1–4, where β1/β2 cells are positive for ST8SIA1 and β3/β4 are ST8SIA1 negative. There are 125 genes differentially expressed across groups, whereas gene ontology enrichment analysis revealed β1/β2 cells are enriched for protein secretion, whereas β3/β4 cells are enriched for neurogenesis. This corresponds with β1/β2 populations having a greater glucose-stimulated insulin secretion, whereas β3/β4 had elevated basal insulin secretion. The proportions of these populations are altered in diabetic individuals, suggesting they differ in susceptibility to metabolic stress, with the caveat that inter-patient variation is much higher than variation between healthy and diabetic donors. How these markers of heterogeneity are tied to β-cell function remain areas of interest. It is possible that these populations have separate roles within the islet niche, with β1/β2 cells specializing in glucose-stimulated insulin secretion, and β3/β4 cells specializing in basal insulin secretion.
The potential existence of specialized basal insulin secreting β-cells is supported by Farack et al. (23), who used single molecule FISH (smFISH) to identify a subpopulation of mouse β-cells with elevated insulin expression. These cells, termed “extreme” β-cells, represent ∼7% of β-cells, and localize near the islet center. Extreme β-cells have a distinct polarization pattern, high pro-insulin and ribosomal RNA content, and less mature insulin protein. They have elevated expression of Ins1, Iapp, Chga, and Pcsk2, which are associated with insulin secretion. Thus, the authors suggest that these cells are producing and quickly secreting high levels of insulin, so they maintain a high proinsulin/insulin ratio despite having other markers of β-cell maturity. The proportion of extreme β-cells increase in db/db mice, suggesting they may play a role in glycemic stress response. These findings are summarized in Fig. 1. Could these extreme β-cells overlap with the β3 and β4 populations identified by Dorrell et al. (108)? Or with PSA-NCAM low and E-cadherin low cell populations? Answers to these questions may provide insight into how to remedy the dysregulated glucose-stimulated insulin secretion seen in diabetic islets. Discussed below are 4 factors that influence β-cell behavior: topography, development, maturation, and stress response. These factors provide insight into how β-cell heterogeneity is maintained, and how it may be harnessed for therapeutic purposes.
Figure 1.
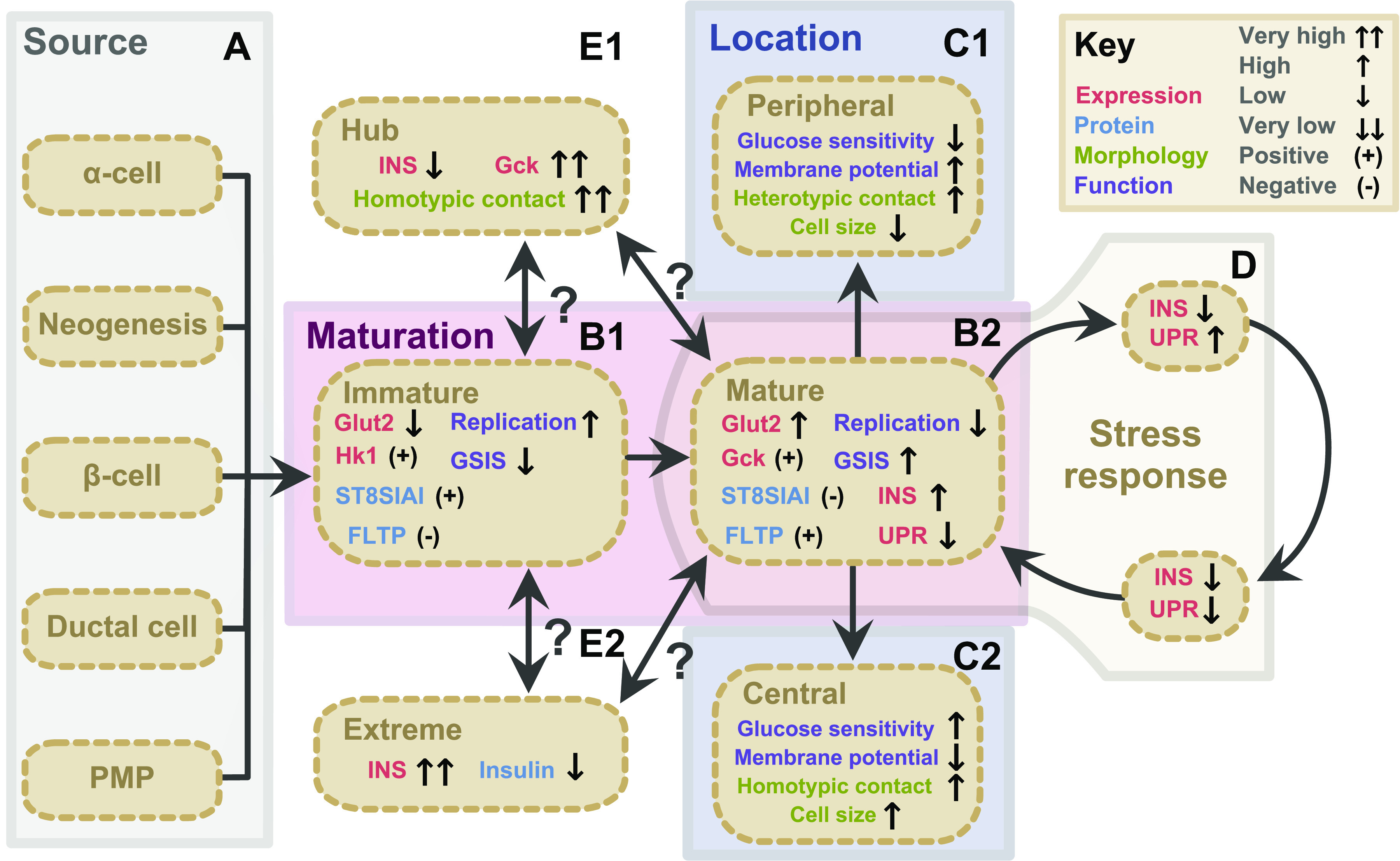
Classes, sources, and subtypes of β-cell heterogeneity. β-Cells display variation in gene expression (red), protein level (blue), morphology (green), and function (purple). Heterogeneity is influenced by β-cell source (A), maturation state (B), topology (C), and stress response (D). A: β-cell sources include neogenesis during development, transdifferentiation of α- and ductal cells, β-cell replication, and differentiation PMPs. B: immature β-cells (B1) and mature β-cells (B2) present distinct gene expression, protein, and functional characteristics. C: topography influences β-cells through the core-mantel structure, where peripheral β-cells (C1) have more heterotypic contacts, resulting in low glucose sensitivity and insulin release compared to core β-cells (C2). D: β-cell stress response causes β-cells to cycle between states of high insulin with low UPR, low insulin with high UPR, and a period of low insulin with low UPR. Two subtypes of adult β-cells have been identified: Hub cells (E1) are characterized by very high Gck expression but low insulin expression, and high number of homotypic contacts. Extreme β-cells (E2) are located near the islet core, characterized by very high insulin expression but low mature insulin protein stores. It is unclear if hub cells and extreme β-cells stem from a mature or immature population. PMP, pancreatic multipotent progenitor; INS, insulin expression; insulin, insulin protein; UPR, unfolded protein response; FLTP, Flattop.
TOPOGRAPHY INFLUENCES β-CELL HETEROGENEITY
In addition to β-cells, islets contain α-cells, δ-cells, ε-cells, and γ-cells, as well as non-endocrine cells including endothelial cells, macrophages, glia, fibroblasts, and pericytes (58, 109). Mice and humans have different islet compositions, which may explain why few discoveries from rodents validate in humans (41, 58, 110, 111). Mouse islets are comprised of 60–80% β-cells and 15–20% α-cells. β-Cells localize to the center, or core, of the islet, whereas α- and δ-cells localize to the periphery, or mantle. Human islets are comprised of 50% β-cells and 40% α-cells. Recent imaging efforts suggest human islets comprise small repeating clusters of the β-cell core, α-cell mantle pattern (41, 110, 112). Furthermore, location within the pancreas influences islet cell composition. Islets in the pancreatic head are enriched for γ-cells, whereas islets enriched in α-cells are found in the neck. The proportion of β-cells per islet increases from head to tail of the pancreas (113–115). Islets in the tail of the pancreas degranulate faster than head islets in vivo following prolonged glucose exposure, and have higher rates of replication, insulin synthesis, and secretion (35, 116–118). Human islets near the pancreatic head tend to be less spherical than those found in the tail, which may influence cell-cell communication (119). NCAM plays a role in islet organization, as NCAM knockout mice have altered islet cell composition, including α-cell infiltration into the islet core (78). Both humans and mice display altered islet composition in diabetes, including deviation from the mantle-core arrangement (120, 121). β-Cell loss is more pronounced in γ-cell-rich islets within the head of the pancreas in diabetes (118). β-Cells in the splenic region of the pancreas undergo proliferation during diabetes, whereas those near the head do not (54).
As previously discussed, β-cells near the islet core exhibit morphological and functional differences from those in the periphery (29, 35, 76, 122–124). These differences are attributed to the homo- and heterotypic connections maintained by each cell, a result of the mantle-core architecture of the islet, in which core β-cells are enriched for homotypic contacts (125). Dispersed β-cells secrete less insulin in response to glucose than β-cells in an intact islet (126, 127). β-Cells coupled with other β-cells secrete more insulin than individual β-cells, whereas β-α-cell couplings produce more insulin than individual β-cells, but less than β-β-cell coupling (59, 126, 128). Peripheral β-cells have more heterotypic contacts with somatostatin-secreting δ-cells, which inhibit insulin secretion (124, 129–132). Importantly, comparisons between core and peripheral β-cells in islets cultured ex vivo are confounded by the rapid onset of hypoxia in the islet core following isolation, particularly in large islets (133–135). It is critical that observations comparing each population are verified using complementary methods. Primary cilia influence β-cell function by facilitating communication with neighboring cells, and further exploration is needed to see if they play a role in maintaining heterogeneity (136, 137). The morphological and functional differences between core and peripheral β-cells may be derived from connections to other β-cells, α-cells, or δ-cells, which in turn are influenced by the location of the islet within the pancreas (summarized in Fig. 1C).
Within each islet, small clusters of β-cells synchronize Ca2+ fluctuations (138). This is attributed to gap junctions between β-cells coordinating insulin release and may explain why coupled cells secrete more insulin than isolated cells (36, 124, 139, 140). Gap junctions are heterogeneously expressed across the population (36, 38, 140, 141).
A subpopulation of hyperconnected β-cells has been proposed (24). These “hub” cells were identified by quantifying the number of significant pairwise correlations of glucose-stimulated Ca2+ oscillations that each cell maintained. These cells represent 1–10% of all β-cells and control the activity of “follower” cells by coordinating Ca2+ signaling through gap junctions. These cells express high levels of Gck and genes involved in glucose oxidation, but low levels of insulin, and have accelerated and extended Ca2+ signaling during glucose stimulation. Ablation of hub cells disrupts Ca2+ synchronization across the islet, although ablating follower cells had no effect. These cells are sensitive to diabetic insults, which could explain why Ca2+ coordinated insulin release is disrupted in diabetes. These findings are summarized in Fig. 1E. Salem et al. (142) built upon this model, showing transplanted islets contain hyperconnected β-cells, termed “leader” cells, that trigger a wave of calcium release across the islet. The role of hub/leader cells have been challenged, because the mechanisms through which hyperpolarizing hub cells inhibit Ca2+ coordination among other cells are unclear (143). The relevance of such a subpopulation has been questioned, as disrupting Ca2+ signaling in ∼70% of β-cells does not alter insulin secretion, suggesting other mechanisms are of greater importance (144–146). Furthermore, knockout of the gap junction protein Cx36 in mice results in altered calcium synchronization, but has minimal effect on overall insulin release, suggesting Ca2+ signaling is influential but not critical for β-cell function (147, 148).
Vascularization and innervation also vary among islets and provide a template for heterogeneity. Capillaries provide nutrients, oxygen, and ECM support to islets, which improve insulin secretion and promote cell survival (149–151). Small islets have 2–3 vascular penetrations, large islets have >3, resulting in β-cells receiving unequal access (149). Islets in the pancreatic periphery receive capillary branches, whereas islets at the center directly contact large blood vessels (149). Several groups have proposed the existence of two classes of islets based on vascularization, high-perfused and low-perfused (152–154). High-perfused populations represent 75% of islets, have higher glucose-stimulated insulin secretion and greater rates of β-cell replication, but are susceptible to cytokine-induced cell death and hypoxia (152–154). These differences are attributed to capillaries increasing oxygen tension within β-cells (153). The low-perfused islets appear to serve as reserves and are recruited to activity when metabolic demand increases (152, 153). β-Cells organize themselves around capillaries in rosette structures, with insulin granules localizing toward the capillary and driving polarity (40, 41). Evidence suggests blood typically flows from islet core to mantle, from β-cells, to α-cells, then δ-cells (149, 155–157). This may bias directionality of cell-cell communication among endocrine cells (157, 158). Innervation also impacts islet development, maturation, mass, and function (159–161). Although only a small number of β-cells receive axon terminals, which tend to reside in the islet periphery, neurotransmitters including serotonin and GABA promote β-cell replication (162–165). Human islets have less innervation than mouse islets, which may explain why they have lower regenerative capacity (166). However, it is unclear if differences in biological age between mice and human donors confound these findings. Clearly the location of the β-cell within the islet, and the islet within the pancreas, influences β-cell exposure to nutrients and potentially harmful milieu. As β-cell heterogeneity is explored in greater depth, topography may aid in understanding why cells behave differently.
DEVELOPMENTAL ORIGIN INFLUENCES β-CELL HETEROGENEITY
The pancreas arises from two epithelial evaginations in the foregut, termed the dorsal and ventral buds, which give rise to the head and tail regions, respectively (167). Despite differences in blood supply and innervation, both buds contain endocrine progenitor-precursor cells, which give rise to nascent β-cells (167–170). Using chimeric mice, Deltour et al. (171) showed that individual islets contain β-cells with different developmental origins, suggesting β-cells are not monoclonal within the islet. Nascent β-cells undergo an initial period of replication, peaking at 20 weeks gestation (172). β-Cell replication remains high at birth, then declines rapidly with age, resulting in a severalfold expansion of β-cell mass between birth and adulthood (173). β-Cell telomere length diminishes over time, particularly during the first two months of life, when β-cell proliferation is at its highest (174). Furthermore, 4-month-old mice display greater variability in telomere length among β-cells compared to 12-day-old mice, suggesting replication is not uniform across the population. In adult mice, β-cell replication is the principle mechanism for maintenance of β-cell mass, driven by a small number of mitotically competent cells, which declines with age (45, 175–177). The number of replicating β-cells increases under metabolic stress and inflammation, but this effect is also restricted by age (47, 48, 173, 178–180). Several other markers have been associated with β-cell aging and functional decline, including expression of IGF1R and P53BP1, and increased lipid storage (181, 182). The degree of heterogeneity displayed by these markers is unknown. Although β-cell replication plays a central role in generating and maintaining β-cell mass, the notion that it is the sole mechanism for β-cell replacement has been challenged.
Insulin-positive cells are observed in ductal tissue following partial pancreatectomy and overexpression of gastrin and transforming growth factor (TGF) α in ductal cells (183–186). Prolonged glucose infusion induces ductal clusters to become PDX1 positive, with some expressing insulin (187). These extra-insular β-cells are also proliferative, as they are observed in aggregates <50 µm (188–190). Like low-perfused islets, ductal cells may provide a reserve pool of insulin production during glycemic stress. Several groups have identified a pancreatic multipotent progenitor (PMP) population (25, 70, 191, 192). These cells express insulin but lack Glut2 expression, likely preventing them from performing glucose-stimulated insulin secretion. Interestingly, these cells have the capacity to proliferate and generate multiple endocrine cell types, including functional β-cells. Furthermore, replication of these cells is induced by hyperglycemic conditions, suggesting they too may play a role in glycemic stress response. The existence of PMPs has been heavily challenged, as other studies fail to identify such populations, even following substantial β-cell loss (175, 193, 194). Much of the argument centers on the weaknesses of lineage tracing, reviewed in Ref. 195, and the low likelihood that these cells contribute meaningfully to functional β-cell mass. What relationship, if any, exists between ductal-derived β-cells and PMP’s remains to be characterized.
α-Cells have been shown to acquire β-cell-like properties in response to extreme β-cell loss and genetic perturbations (196–200). However, the utility of these observations is questioned because of lack of evidence that this phenomenon occurs in normal islet homeostasis. A study by van der Meulen et al. (74) addressed some of these criticisms by identifying a Glut2-low population of β-cells residing within the islet periphery. Using lineage tracing, they showed that these “virgin” β-cells do not express Glut2 because they are an intermediate in α- to β-cell transdifferentiation. These cells do not import meaningful amounts of glucose following 50 min of exposure and are resistant to STZ-induced β-cell death, likely due to lack of Glut2 expression. These cells transition into Glut2-high β-cells over time, making them indistinguishable from conventional β-cells. A recent study challenged that Glut2-low cells represent α-β transitional cells, and that massive β-cell loss induces transdifferentiation (201). Feng et al. (201) used an STZ model of diabetes to assess the fates of Glut2-high and Glut2-low β-cells using single cell RNAseq. Although Glut2-low β-cells survive STZ treatment, they do not mature into Glut2-high cells in 9 months post-treatment, contradicting the findings of van der Meulen et al. (74). Furthermore, they did not find evidence for α-β-cell transdifferentiation at the transcriptional level. However, these mice were given insulin pellets to ensure long-term survival, which may confound interpretation. Regardless of their relevance to normal insulin homeostasis, the transdifferentiation of ductal or α-cells into β-cells remains an appealing therapeutic approach for diabetes. Neither ductal or α-cell populations are reduced by diabetes, providing a steady source of potential β-cells. Furthermore, α-cells display a high degree of resistance to viral and metabolic induced stress, suggesting that β-cells derived from α-cells could retain these properties (202, 203). The potential sources for β-cells are summarized in Fig. 1A. At this junction, it is not clear how β-cells from different developmental sources display meaningful heterogeneity. Given the influence of the β-cell microenvironment on function, there is no reason to believe β-cells from dorsal or ventral islets, β-cell replication, differentiation of PMPs, or transdifferentiation of β-cells would behave identically. Understanding how β-cells functionally mature will provide insight into how β-cell location, age, and function interconnect.
MATURATION INFLUENCES β-CELL HETEROGENEITY
How β-cells acquire specialized functionality has been characterized in depth. In mice, initial β-cell maturation proceeds in two developmental phases (204–207). The first phase lasts two weeks following birth, a period of rapid β-cell proliferation and simultaneous waves β-cell apoptosis, which coordinate to set the foundation for β-cell mass (208, 209). Surviving cells begin to express transcription factors required for β-cell function and are considered immature (168, 206, 210). The second developmental phase starts at weaning, characterized by changes in metabolic pathways and functional maturation (207, 211). Comparing β-cells from 4-week-old mice and 16-week-old mice reveals differential expression of thousands of genes, whereas only 193 genes were differentially expressed between 3- and 26-month-old mice, suggesting β-cells change substantially early in life and little in adulthood (99, 212). Pseudotime analysis of individual β-cells reveals differential expression of genes associated with amino acid uptake, reactive oxygen species (ROS) production, and cellular respiration during functional maturation (95, 177). Markers of mature β-cells include Glut2, Ins2, Mafa, Nkx6.1, NeuroD, and Ucn3, whereas immature β-cells express Hk1, PaxA, Pax6, and Mafb (3, 213–216). In humans, MAFB is expressed in mature β-cells and knockout of MAFB results in β-cell dysfunction, suggesting it plays a nonredundant role from MAFA (217–219). A set of “disallowed” genes including Hk1, Ldha, Mct1, Rest, and Pdgfrα have been identified as being prohibited from expression in mature β-cells (211, 220, 221). Many of these genes are involved in glucose metabolism and exocytosis and would interfere with glucose-stimulated insulin release (222). The molecular machinery driving insulin release is similar in both mature and immature β-cells, with a few key differences (213). Neonatal islets have elevated basal insulin secretion due to high glucose sensitivity (213, 223). Interestingly, when immature and mature β-cells are depolarized independently of glucose sensing, both release similar levels of insulin, suggesting their differences lie in glucose sensing and metabolism (213). Immature β-cells express Hk1, which has a higher affinity for glucose than Gck, which is expressed in mature β-cells (224–226). Thus, it takes higher concentrations of glucose to trigger glycolysis and downstream oxidative phosphorylation in mature β-cells, which may explain differences in insulin secretion between these two classes of cells. Other components of the insulin secretion pathway, including genes involved in glycolysis, the TCA cycle, oxidative phosphorylation, and exocytosis are also differentially expressed between mature and immature β-cells (207, 211, 227). An overview of immature β-cell characteristics is shown in Fig. 2A.
Figure 2.
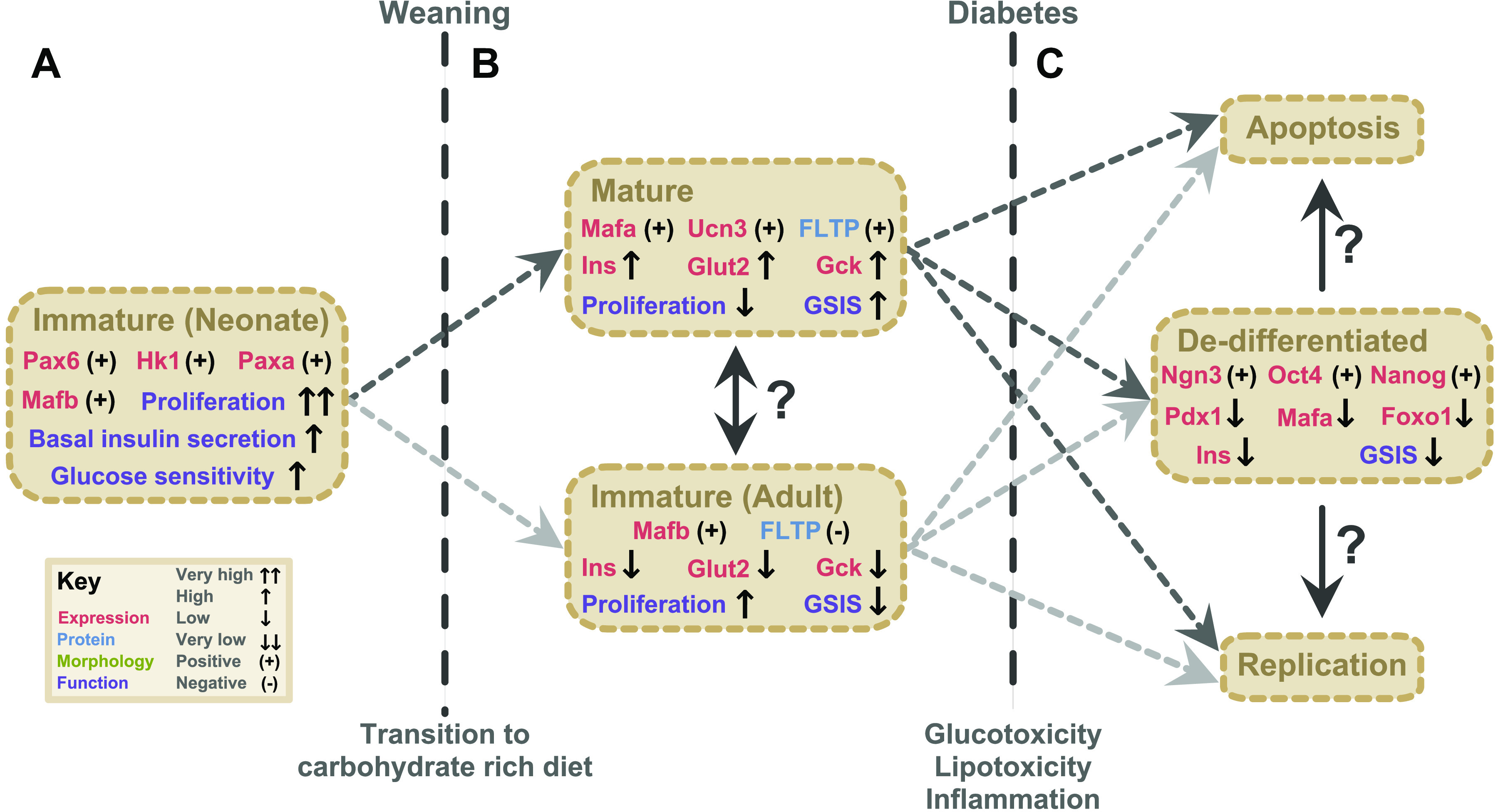
Maturation state heterogeneity in pancreatic beta β-cells. A: following birth, neonatal β-cells are transcriptionally and functionally immature, characterized by Pax6, Hk1, Paxa, and Mafb expression, very high proliferation rates, very high basal insulin secretion, and high glucose sensitivity. Maturation is facilitated by transition to a carbohydrate-rich diet at weaning. B: mature β-cells are characterized by expression of Mafa, Ucn3, and Fltp, high expression of insulin, Glut2, and Gck. These β-cells have low proliferation rates but high glucose-stimulated insulin secretion. A subset of adult β-cells maintain an immature-like profile, including Mafb expression, low expression of insulin, Glut2, and Gck, and lack Fltp expression. These cells have elevated proliferation rates but low glucose-simulated insulin secretion. Whether these cells differ in developmental origin or can interconvert is unknown. C: in diabetes, glucotoxicity, lipotoxicity, and inflammation induces variable fates in β-cells, including dedifferentiation, apoptosis, and replication. Dedifferentiated β-cells have expression of developmental genes including Ngn3, Oc4, and Nanog, low expression of genes associated with mature β-cells including Pdx1, Mafa, Foxo1, and insulin, and low glucose-stimulated insulin secretion. Other fates for β-cells in diabetes include replication and apoptosis. The relationship among adult mature and immature cells and the three fates in diabetes remain to be described. FLTP, Flattop.
The maturation state appears to be a heterogeneous trait in adult β-cells and is associated with replicative capacity (212, 228, 229). Szabat et al. (73) used a Pdx1/Insulin dual reporter to discover that 25% of β-cells in humans and mice are Pdx1 positive but have low insulin expression. These cells have an immature gene expression profile, including elevated Mafb expression, and low Gck and Glut2 expression. Immature cells have high proliferative capacity and poor insulin secretion, and functionally mature without proliferation in vitro. Bader et al. (22) reinforced these findings by identifying a subpopulation of immature β-cells based on lack of Fltp expression (discussed below). These cells have high replicative capacity at the expense of mature β-cell function. The replication/maturation dichotomy was illustrated by Klochendler et al. (228), who used a fluorescent reporter mouse for cyclin B1, which is active only during mitosis. This allowed sorting of cells based on a mitotic state. Performing RNAseq on these populations revealed a global increase in cell cycle genes, but a distinct down-regulation of genes associated with β-cell function, including Glut2 and Ucn3, as well as enrichment for genes regulated by mature β-cell transcription factors. In human β-cells, a clustering approach to simultaneously assess intracellular Ca2+ dynamics in ∼300 islet cells revealed two distinct populations based on Ca2+ flux frequency and amplitude, suggesting mature and immature states (230). The dichotomy between replication and maturation is associated with ER stress response (see below) (177, 231). These observations have implications for understanding postnatal β-cell expansion; individual β-cells cannot simultaneously replicate and be functional.
Observations that β-cell replication and maturation are at odds were coalesced into a unified theory by Bader et al. (22), who separated β-cells into mature and immature populations based on expression of Flattop (FLTP). FLTP is expressed in tissues where the Wnt/Planar cell polarity pathway is active and is necessary for epithelial organization and architecture (232). Bader et al. (22) used an islet-specific FLTP reporter and found expression increases throughout β-cell maturation, resulting in 80% of cells positive for FLTP in adult mice. Transcriptional analysis revealed 997 genes differentially expressed between populations, with positive cells expressing markers of maturation, including Ucn3, Mafa, mitochondrial oxidative phosphorylation genes, and insulin secretion genes, whereas negative cells expressed genes associated with MAP kinases, Gcprs, and had low Glut2 expression. FLTP(−) cells are more proliferative, with 8% of negative cells staining Ki67(+) at postnatal day 11, compared to 2% of FLTP(+) cells. In high-fat diet-induced islet hypertrophy, the FLTP(+) population expanded in line with heightened metabolic demand, suggesting that the progeny of FLTP(−) cells can become FLTP(+). Morphologically, FLTP(+) cells are polarized and found in rosette structures. Re-aggregated FLTP(−) cells in vitro have lower glucose-stimulated insulin secretion than positive cells, suggesting they are functionally immature. Knockout of FLTP resulted in very mild functional changes, suggesting FLTP is a passive marker for maturation. In humans, islet FLTP expression correlates with glucose tolerance, with healthy donors expressing higher levels of FLTP than prediabetic and diabetic donors. Thus, FLTP distinguishes proliferative and metabolically active β-cells and suggests β-cell maturation could be driven by the polarization process. The specific mechanism through which the wnt/PCP pathway effects β-cell function is unknown, but it may involve cytoskeleton components and gene expression changes. These findings raise questions about β-cell heterogeneity. Are FLTP(−) cells part of the 25% of β-cells that do not increase metabolic activity in high glucose (54)? Could these be the Glut2-low cells reported in PSA-NCAM low cells or E-cadherin low cells (71, 79–81)? Or the poor functioning β3 and β4 cells identified by Dorrell et al. (108)? It is possible that many of these studies are converging on overlapping populations of cells. The differences between immature and mature β-cells are highlighted in Fig. 1B and Fig. 2B.
Dedifferentiation of mature β-cell identity is a central component of β-cell dysfunction in type 2 diabetes (215, 233–237). This is attributed to many factors, including oxidative stress, glucose and lipid toxicity, and inflammation (4, 233, 238, 239). Dedifferentiated β-cells have decreased insulin expression and decreased insulin secretion, thus contributing to loss of glycemic control (3, 91, 238). Dedifferentiation is characterized by decreased expression of β-cell-specific transcription factors Pdx1, Nkx6.1, and MafA, increased expression of developmental genes, Ngn3, Oct4, Nanog, and L-myc, and expression of disallowed genes, Ldha, Mct1, G6pc, and Hk1 (26, 221, 239–241). These changes are linked to decreased expression of Foxo1, which mediates Pdx1 activity (3, 26, 242–244). An overview of the characteristics of dedifferentiated β-cells is shown in Fig. 2C. Moving hyperglycemia-induced dedifferentiated β-cells into a healthy environment can reverse the dedifferentiation phenotype (215, 235, 245). Insulin administration can also return immature cells to a functional state, suggesting therapeutic intervention is possible (237). We recently published findings on obese SM/J mice, which spontaneously transition from hyperglycemic-obese to normoglycemic-obese with age (246). Underlying this transition is improved β-cell function, including increased insulin content and glucose-stimulated insulin secretion. This suggests SM/J β-cells are likely undergoing maturation and show that β-cell functionality can be improved in vivo in the context of obesity. Evidence for dedifferentiated β-cells in diabetic humans based on single cell RNA sequencing data has been established (96). The parallels between nascent β-cell maturation and diabetes-induced dedifferentiation have not been fully explored. Greater understanding of the β-cell maturation process may provide insight into how dedifferentiated β-cells may be rescued, opening exciting new avenues for therapeutic interventions.
STRESS RESPONSE INFLUENCES β-CELL HETEROGENEITY
An emerging paradigm suggests that β-cells undergo cycles of insulin production and stress response, due to the unique physiological demands they face. Insulin synthesis accounts for 10% of total protein during basal conditions, compared to 50% in stimulated conditions (57, 247). Insulin is highly prone to misfolding (248–250). Once misfolded, insulin can be refolded or degraded. Under demanding conditions, misfolded proteins accumulate, and the UPR machinery is activated to clear the misfolded proteins (251). The high rate of oxidative phosphorylation in β-cells also generates ROS, which can damage nascent proteins (252). β-Cells have been characterized as having low antioxidant defense, and thus are more susceptible to stress (253, 254). This combination of factors make the insulin secretion responsibilities of β-cells a unique cellular challenge.
Many single cell studies identified subpopulations of β-cells based on an ER stress response signature, at the expense of genes related to mature β-cell function (21, 88–90). Xin et al. (21) performed pseudotime analysis on human β-cells and identified the transcriptional trajectory between the three β-cell states: high insulin/low unfolded protein response (UPR), low insulin/high UPR, and low insulin/low UPR. This suggests mature β-cells undergo a cycle: elevated insulin production, followed by a period of low insulin production and UPR activation to clear misfolded insulin protein, then a rest period of low insulin and low UPR activity. Under this hypothesis, only a fraction of β-cells produce insulin at a given time while others are resting/recuperating (255). This is supported by evidence from isolated human and mouse β-cells, where a small fraction of cells are responsible for the majority of insulin secretion (55, 58, 59). Xin et al. (21) also found low insulin/high UPR cells were more proliferative, suggesting they are given the chance to replicate during recovery. Cells under glycemic stress would produce more insulin, thus would have a great degree of UPR activation. Whether replication is induced by the intensity or length of UPR response is unknown. This theory is supported by Szabat et al. (255), who used a Pdx1/INS dual reporter to show that β-cells transition between periods of high and low insulin in ∼27 h cycles. Activation of UPR in human β-cells also stimulates proliferation (231). A summary of β-cell stress response cycling is summarized in Fig. 1D. Could the interplay between these metabolic states explain the morphological, functional, and transcriptional heterogeneity seen in β-cells, particularly the identification of β-cell subpopulations in single cell RNAseq data? Loss of β-cell mass by apoptosis occurs in diabetes, driven in part by failure of the ER stress response. In response to prolonged stress, what drives β-cells to undergo apoptosis, dedifferentiation, or replication? Identifying cells that are prone to a specific fate may help prevent loss of β-cell mass.
Conclusions
Our understanding of β-cell heterogeneity has increased over decades, revealing several trends. First, there is a morphological and functional dichotomy between peripheral and core β-cells (29, 35, 123). This is attributed to topography including homotypic contacts, heterotypic contacts, vascularization, and proximity to axon terminals (59, 124–126, 149, 165). Furthermore, islet topography changes in diabetes, highlighting the importance of understanding the role of the β-cell microenvironment (118, 120, 121). The developmental origins of the β-cell, including neogenesis, β-cell replication, and differentiation from another cell may play a role in heterogeneity. These different sources produce adult β-cells that vary in cellular age, which is associated with functional decline (174, 181, 182). How the distribution of young and old β-cells influences islet function and stress response is unknown, but it clearly influences replicative capacity (175–177). The existence and relevance of ductal cell-, α-cell-, and PMP-derived β-cells remains controversial, but they are appealing avenues for diabetic therapy because of their stress resistance characteristics (70, 74, 183, 201–203). β-Cell function and replication are at odds during the initial maturation process, resulting in decreased mitotic index and improved glucose-stimulated insulin secretion (168, 207, 211, 214, 215). In adults, a subpopulation of cells retain immature gene expression and functional characteristics, including higher replication rates (22, 73, 108, 228, 230). Loss of mature β-cell identity is a hallmark of diabetes and disrupts the insulin-glucose axis (215, 233, 235, 236). Can studying the differences between immature and mature β-cell populations in healthy adults inform diabetic therapy? Variation in β-cell metabolic activity is associated with differences in stress susceptibility, suggesting mature and immature populations likely succumb to different fates in diabetes (47, 54, 152–154). The relationship between β-cell maturation, replication, and stress response is complicated by observations that mature β-cells undergo a stress response cycle, and that UPR activation can stimulate replication (21, 177, 231, 255). The relationships among classes of variation, sources of variation, and proposed subtypes are summarized in Fig. 1. These likely contribute to the variance seen in single cell RNA sequencing studies, many of which cluster cells based on expression of stress response genes (21, 88–90).
Assessment of variation in gene expression, protein levels, morphology, and function has led several groups to identify β-cell subpopulations. Given the frequency of these populations, there is likely substantial overlap. This includes FLTP(−), ST8SIA1 (+), PSA-NCAM low, E-cadherin low, and Glut2-low “virgin” β-cells all possibly identifying adult immature β-cells based on gene expression and functional characteristics (22, 74, 80, 81, 108). How much of the transcriptional heterogeneity identified in single cell RNA sequencing is driven by the ER stress response cycling (21, 88, 89, 255)? Furthermore, does ER stress response cycling influence the immature β-cell population? This could explain the elevated, but not dramatic increase in β-cell replication seen in immature cells. These observations raise questions over requirements for subtype classification. Are subtype traits under unique selective pressure, different from the general population? What are the temporal requirements for a subtype? What about cell plasticity, the ability of subtypes to interconvert? Should a subtype require a unique functional and transcriptional profile? Answering these questions will clarify which heterogeneous traits are critical for building subtype identities.
Techniques including FACS and single cell RNA sequencing are mainstays in shaping understanding of β-cell heterogeneity. However, these techniques have limitations, as they fail to capture functional, transcriptional, and topographical information simultaneously. Furthermore, questions about the temporal regulation of β-cell heterogeneity are becoming unavoidable. Lineage tracing is the gold standard for understanding developmental trajectories but has received considerable criticism for its shortcomings including promoter leakage, low labeling efficiency, and inability to recapitulate native expression patterns (195). Dual trace techniques may overcome some of these issues (256). Several new techniques promise to improve our understanding of β-cell heterogeneity, including preserving live sections using perfluorocarbon (257, 258). This will allow temporal and topographical assessment of β-cells and provide the opportunity to test the effects of various compounds on β-cell growth and function in vivo. This will also provide a new platform for lineage tracing. SeqFISH allows one to probe the expression of hundreds of genes simultaneously in intact tissue, which can couple extensive transcriptional information with topography (259). Patch-Seq couples β-cell ion-channel activity with single cell RNA sequencing, linking functionality with gene expression (61). This is an exciting time to be in the field of β-cell heterogeneity, as many of the discoveries made over decades of research are slowly coalescing into a unified understanding of how and why β-cells differ. Whether these discoveries will make meaningful improvements in the way diabetes is studied and treated remains to be seen.
GRANTS
This work was supported by the Washington University Department of Genetics, the Diabetes Research Center at Washington University Grant P30DK020579, and National Institutes of Health Grant K01 DK095003 to H.A.L., Grant T32 DK108742 to M.A.M., and Grant F31 DK125023-01 to M.A.M.).
DISCLOSURE
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.F.M. prepared figures; M.A.M. and H.A.L. drafted manuscript; M.A.M. and H.A.L. edited and revised manuscript; M.A.M., J.F.M., and H.A.L. approved final version of manuscript.
REFERENCES
- 1.Fu Z, R. Gilbert E, Liu D. Regulation of insulin synthesis and secretion and pancreatic beta-cell dysfunction in diabetes. Curr Diabetes Rev DR 9: 25–53, 2012. doi: 10.2174/1573399811309010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102–110, 2003. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 3.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150: 1223–1234, 2012. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 53: S16–S21, 2004. doi: 10.2337/diabetes.53.suppl_3.S16. [DOI] [PubMed] [Google Scholar]
- 5.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G, ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355: 2427–2443, 2006. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 6.Mannucci E. Insulin therapy and cancer in type 2 diabetes. ISRN Endocrinol 2012: 240634–240612, 2012. doi: 10.5402/2012/240634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merlotti C, Morabito A, Pontiroli AE. Prevention of type 2 diabetes; a systematic review and meta-analysis of different intervention strategies. Diabetes Obes Metab 16: 719–727, 2014. doi: 10.1111/dom.12270. [DOI] [PubMed] [Google Scholar]
- 8.Aguayo-Mazzucato C, Andle J, Lee TB Jr, Midha A, Talemal L, Chipashvili V, Hollister-Lock J, van Deursen J, Weir G, Bonner-Weir S. Acceleration of β cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab 30: 129–142.e4, 2019. doi: 10.1016/j.cmet.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alismail H, Jin S. Microenvironmental stimuli for proliferation of functional islet β-cells. Cell Biosci 4: 12, 2014. doi: 10.1186/2045-3701-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang WJ, Peng YC, Yang KM. Cellular signaling pathways regulating β-cell proliferation as a promising therapeutic target in the treatment of diabetes (review). Exp Ther Med 16: 3275–3285, 2018. doi: 10.3892/etm.2018.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muhammad SA, Raza W, Nguyen T, Bai B, Wu X, Chen J. Cellular signaling pathways in insulin resistance-systems biology analyses of microarray dataset reveals new drug target gene signatures of type 2 diabetes mellitus. Front Physiol 8, 13, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 52: 581–587, 2003. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- 13.Aguayo-Mazzucato C, Bonner-Weir S. Pancreatic β cell regeneration as a possible therapy for diabetes. Cell Metab 27: 57–67, 2018. doi: 10.1016/j.cmet.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tritschler S, Theis FJ, Lickert H, Böttcher A. Systematic single-cell analysis provides new insights into heterogeneity and plasticity of the pancreas. Mol Metab 6: 974–990, 2017. doi: 10.1016/j.molmet.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong F, Jiang Y. Endogenous pancreatic β cell regeneration: a potential strategy for the recovery of β cell deficiency in diabetes. Front Endocrinol (Lausanne) 10, 101, 2019. doi: 10.3389/fendo.2019.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Q, Melton DA. Pancreas regeneration. Nature 557: 351–358, 2018. [Erratum in Nature. 560: E34, 2018]. doi: 10.1038/s41586-018-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vetere A, Choudhary A, Burns SM, Wagner BK. Targeting the pancreatic β-cell to treat diabetes. Nat Rev Drug Discov 13: 278–289, 2014. doi: 10.1038/nrd4231. [DOI] [PubMed] [Google Scholar]
- 18.Puri S, Folias AE, Hebrok M. Plasticity and dedifferentiation within the pancreas: development, homeostasis, and disease. Cell Stem Cell 16: 18–31, 2015. doi: 10.1016/j.stem.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roscioni SS, Migliorini A, Gegg M, Lickert H. Impact of islet architecture on β-cell heterogeneity, plasticity and function. Nat Rev Endocrinol 12: 695–709, 2016. doi: 10.1038/nrendo.2016.147. [DOI] [PubMed] [Google Scholar]
- 20.Salinno C, Bastidas P, Tarquis M, Lickert B. β-Cell maturation and identity in health and disease. Int J Mol Sci 20, 5417, 2019. doi: 10.3390/ijms20215417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xin Y, Dominguez Gutierrez G, Okamoto H, Kim J, Lee A-H, Adler C, Ni M, Yancopoulos GD, Murphy AJ, Gromada J. Pseudotime ordering of single human β-cells reveals states of insulin production and unfolded protein response. Diabetes 67: 1783–1794, 2018. doi: 10.2337/db18-0365. [DOI] [PubMed] [Google Scholar]
- 22.Bader E, Migliorini A, Gegg M, Moruzzi N, Gerdes J, Roscioni SS, Bakhti M, Brandl E, Irmler M, Beckers J, Aichler M, Feuchtinger A, Leitzinger C, Zischka H, Wang-Sattler R, Jastroch M, Tschöp M, Machicao F, Staiger H, Häring H-U, Chmelova H, Chouinard JA, Oskolkov N, Korsgren O, Speier S, Lickert H. Identification of proliferative and mature β-cells in the islets of Langerhans. Nature 535: 430–434, 2016. doi: 10.1038/nature18624. [DOI] [PubMed] [Google Scholar]
- 23.Farack L, Golan M, Egozi A, Dezorella N, Bahar Halpern K, Ben-Moshe S, Garzilli I, Tóth B, Roitman L, Krizhanovsky V, Itzkovitz S. Transcriptional heterogeneity of beta cells in the intact pancreas. Dev Cell 48: 115–125.e4, 2019. doi: 10.1016/j.devcel.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Johnston NR, Mitchell RK, Haythorne E, Pessoa MP, Semplici F, Ferrer J, Piemonti L, Marchetti P, Bugliani M, Bosco D, Berishvili E, Duncanson P, Watkinson M, Broichhagen J, Trauner D, Rutter GA, Hodson DJ. Beta cell hubs dictate pancreatic islet responses to glucose. Cell Metab 24: 389–401, 2016. doi: 10.1016/j.cmet.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smukler SR, Arntfield ME, Razavi R, Bikopoulos G, Karpowicz P, Seaberg R, Dai F, Lee S, Ahrens R, Fraser PE, Wheeler MB, van der Kooy D. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell 8: 281–293, 2011. doi: 10.1016/j.stem.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Cinti F, Bouchi R, Kim-Muller JY, Ohmura Y, Sandoval PR, Masini M, Marselli L, Suleiman M, Ratner LE, Marchetti P, Accili D. Evidence of β-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab 101: 1044–1054, 2016. doi: 10.1210/jc.2015-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgia S, Bhushan A. β Cell replication is the primary mechanism for maintaining postnatal β cell mass. J Clin Invest 114: 963–968, 2004. doi: 10.1172/JCI22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudish LI, Reusch JE, Sussel L. β Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J Clin Invest 129: 4001–4008, 2019. doi: 10.1172/JCI129188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hellerström C, Petersson B, Hellman B. Some properties of the beta cells in the islets of langerhans studied with regard to the position of the cells. Acta Endocrinol (Copenh) XXXIV, XXXIV: 449–456, 1960. doi: 10.1530/acta.0.XXXIV0449. [DOI] [PubMed] [Google Scholar]
- 30.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. Turnover in humans: effects of obesity and aging. Diabetes Care 36: 111–117, 2013. doi: 10.2337/dc12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehrie MG, Swartz FJ. Diploid, tetraploid and octaploid beta cells in the islets of Langerhans of the normal human pancreas. Diabetes 23: 583–588, 1974. doi: 10.2337/diab.23.7.583. [DOI] [PubMed] [Google Scholar]
- 32.Ehrie MG, Swartz FJ. Polyploidy in the pancreas of the normal and diabetic mutant mouse. Diabetologia 12: 167–170, 1976. doi: 10.1007/BF00428984. [DOI] [PubMed] [Google Scholar]
- 33.Park K-S, Wiederkehr A, Kirkpatrick C, Mattenberger Y, Martinou J-C, Marchetti P, Demaurex N, Wollheim CB. Selective actions of mitochondrial fission/fusion genes on metabolism-secretion coupling in insulin-releasing cells. J Biol Chem 283: 33347–33356, 2008. doi: 10.1074/jbc.M806251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoenfelder KP, Fox DT. The expanding implications of polyploidy. J Cell Biol 209: 485–491, 2015. doi: 10.1083/jcb.201502016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stefan Y, Meda P, Neufeld M, Orci L. Stimulation of insulin secretion reveals heterogeneity of pancreatic B cells in vivo. J Clin Invest 80: 175–183, 1987. doi: 10.1172/JCI113045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benninger RKP, Piston DW. Cellular communication and heterogeneity in pancreatic islet insulin secretion dynamics. Trends Endocrinol Metab 25: 399–406, 2014. doi: 10.1016/j.tem.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katsuta H, Aguayo-Mazzucato C, Katsuta R, Akashi T, Hollister-Lock J, Sharma AJ, Bonner-Weir S, Weir GC. Subpopulations of GFP-marked mouse pancreatic β-cells differ in size, granularity, and insulin secretion. Endocrinology 153: 5180–5187, 2012. doi: 10.1210/en.2012-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meda P, Denef JF, Perrelet A, Orci L. Nonrandom distribution of gap junctions between pancreatic beta-cells. Am J Physiol Cell Physiol 238: C114–C119, 1980. doi: 10.1152/ajpcell.1980.238.3.C114. [DOI] [PubMed] [Google Scholar]
- 39.Molina AJA, Wikstrom JD, Stiles L, Las G, Mohamed H, Elorza A, Walzer G, Twig G, Katz S, Corkey BE, Shirihai OS. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes 58: 2303–2315, 2009. doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonner-Weir S. Morphological evidence for pancreatic polarity of beta-cell within islets of Langerhans. Diabetes 37: 616–621, 1988. doi: 10.2337/diabetes.37.5.616. [DOI] [PubMed] [Google Scholar]
- 41.Bonner-Weir S, Sullivan BA, Weir GC. Human islet morphology revisited. J Histochem Cytochem 63: 604–612, 2015. doi: 10.1369/0022155415570969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gan WJ, Zavortink M, Ludick C, Templin R, Webb R, Webb R, Ma W, Poronnik P, Parton RG, Gaisano HY, Shewan AM, Thorn P. Cell polarity defines three distinct domains in pancreatic β-cells. J Cell Sci 130: 143–151, 2017. doi: 10.1242/jcs.185116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geron E, Boura-Halfon S, Schejter ED, Shilo BZ. The edges of pancreatic islet β cells constitute adhesive and signaling microdomains. Cell Rep 10: 317–325, 2015. doi: 10.1016/j.celrep.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 44.Köhler CU, Kreuter A, Rozynkowski MC, Rahmel T, Uhl W, Tannapfel A, Schmidt WE, Meier JJ. Validation of different replication markers for the detection of beta-cell proliferation in human pancreatic tissue. Regul Pept 162: 115–121, 2010. doi: 10.1016/j.regpep.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 45.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of β-cells in aged adult mice. Diabetes 54: 2557–2567, 2005. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- 46.Aerts L, Assche FA. Ultrastructural changes of the endocrine pancreas in pregnant rats. Diabetologia 11: 285–289, 1975. doi: 10.1007/BF00422393. [DOI] [PubMed] [Google Scholar]
- 47.Assefa Z, Akbib S, Lavens A, Stangé G, Ling Z, Hellemans KH, Pipeleers D. Direct effect of glucocorticoids on glucose-activated adult rat β-cells increases their cell number and their functional mass for transplantation. Am J Physiol Endocrinol Metab 311: E698–E705, 2016. doi: 10.1152/ajpendo.00070.2016. [DOI] [PubMed] [Google Scholar]
- 48.Linnemann AK, Baan M, Davis DB. Pancreatic β-cell proliferation in obesity. Adv Nutr 5: 278–288, 2014. doi: 10.3945/an.113.005488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology 130: 1459–1466, 1992. doi: 10.1210/endo.130.3.1537300. [DOI] [PubMed] [Google Scholar]
- 50.Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 295: E1287–E1297, 2008. doi: 10.1152/ajpendo.90604.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang T, Jensen P, Huang H, Lund Christensen G, Billestrup N, Larsen MR. Characterization of the molecular mechanisms underlying glucose stimulated insulin secretion from isolated pancreatic β-cells using post-translational modification specific proteomics (PTMomics). Mol Cell Proteomics 17: 95–110, 2018. doi: 10.1074/mcp.RA117.000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meda P, Schuit F. Glucose-stimulated insulin secretion: the hierarchy of its multiple cellular and subcellular mechanisms. Diabetologia 56: 2552–2555, 2013. doi: 10.1007/s00125-013-3073-z. [DOI] [PubMed] [Google Scholar]
- 53.Newsholme P, Krause M. Nutritional regulation of insulin secretion: implications for diabetes. Clin Biochem Rev . 33: 35–47, 2012. [PMC free article] [PubMed] [Google Scholar]
- 54.Van De Winkel M, Pipeleers D. Autofluorescence-activated cell sorting of pancreatic islet cells: purification of insulin-containing B-cells according to glucose-induced changes in cellular redox state. Biochem Biophys Res Commun 114: 835–842, 1983. doi: 10.1016/0006-291x(83)90857-4. [DOI] [PubMed] [Google Scholar]
- 55.Van Schravendijk CFH, Kiekens R, Pipeleers DG. Pancreatic β cell heterogeneity in glucose-induced insulin secretion. J Biol Chem 267: 21344–21348, 1992. doi: 10.1016/S0021-9258(19)36615-3. [DOI] [PubMed] [Google Scholar]
- 56.Gutierrez GD, Gromada J, Sussel L. Heterogeneity of the pancreatic beta cell. Front Genet 8: 22, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuit FC, In't Veld PA, Pipeleers DG. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc Natl Acad Sci USA 85: 3865–3869, 1988. doi: 10.1073/pnas.85.11.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA 103: 2334–2339, 2006. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wojtusciszyn A, Armanet M, Morel P, Berney T, Bosco D. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia 51: 1843–1852, 2008. doi: 10.1007/s00125-008-1103-z. [DOI] [PubMed] [Google Scholar]
- 60.Giordano E, Cirulli V, Bosco D, Rouiller D, Halban P, Meda P. B-cell size influences glucose-stimulated insulin secretion. Am J Physiol Physiol 265: C358–C364, 1993. doi: 10.1152/ajpcell.1993.265.2.C358. [DOI] [PubMed] [Google Scholar]
- 61.Camunas-Soler J, Dai XQ, Hang Y, Bautista A, Lyon J, Suzuki K, Kim SK, Quake SR, MacDonald PE. Patch-Seq links single-cell transcriptomes to human islet dysfunction in diabetes. Cell Metab 31: 1017–1031.e4, 2020. doi: 10.1016/j.cmet.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Low JT, Zavortink M, Mitchell JM, Gan WJ, Do OH, Schwiening CJ, Gaisano HY, Thorn P. Insulin secretion from beta cells in intact mouse islets is targeted towards the vasculature. Diabetologia 57: 1655–1663, 2014. doi: 10.1007/s00125-014-3252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tarasov AI, Galvanovskis J, Rorsman O, Hamilton A, Vergari E, Johnson PRV, Reimann F, Ashcroft FM, Rorsman P. Monitoring real-time hormone release kinetics: via high-content 3-D imaging of compensatory endocytosis. Lab Chip 18: 2838–2848, 2018. doi: 10.1039/c8lc00417j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heimberg H, De Vos A, Vandercammen A, Van Schaftingen E, Pipeleers D, Schuit F. Heterogeneity in glucose sensitivity among pancreatic beta-cells is correlated to differences in glucose phosphorylation rather than glucose transport. EMBO J 12: 2873–2879, 1993. doi: 10.1002/j.1460-2075.1993.tb05949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martens GA, Jiang L, Verhaeghen K, Connolly JB, Geromanos SG, Stangé G, Van Oudenhove L, Devreese B, Hellemans KH, Ling Z, Van Schravendijk C, Pipeleers DG, Vissers JPC, Gorus FK. Protein markers for insulin-producing beta cells with higher glucose sensitivity. PLoS One 5: e14214, 2010. doi: 10.1371/journal.pone.0014214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martens GA, Wang Q, Kerckhofs K, Stangé G, Ling Z, Pipeleers D. Metabolic activation of glucose low-responsive β-cells by glyceraldehyde correlates with their biosynthetic activation in lower glucose concentration range but not at high glucose. Endocrinology 147: 5196–5204, 2006. doi: 10.1210/en.2006-0580. [DOI] [PubMed] [Google Scholar]
- 67.Giordano E, Bosco D, Cirulli V, Meda P. Repeated glucose stimulation reveals distinct and lasting secretion patterns of individual rat pancreatic B cells. J Clin Invest 87: 2178–2185, 1991. doi: 10.1172/JCI115251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoorens A, Van de Casteele M, Klöppel G, Pipeleers D. Glucose promotes survival of rat pancreatic beta cells by activating synthesis of proteins which suppress a constitutive apoptotic program. J Clin Invest 98: 1568–1574, 1996. doi: 10.1172/JCI118950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schnedl WJ, Ferber S, Johnson JH, Newgard CB. STZ transport and cytotoxicity: specific enhancement in GLUT2-expressing cells. Diabetes 43: 1326–1333, 1994. doi: 10.2337/diab.43.11.1326. [DOI] [PubMed] [Google Scholar]
- 70.Beamish CA, Strutt BJ, Arany EJ, Hill DJ. Insulin-positive, Glut2-low cells present within mouse pancreas exhibit lineage plasticity and are enriched within extra-islet endocrine cell clusters. Islets 8: 65–82, 2016. doi: 10.1080/19382014.2016.1162367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karaca M, Castel J, Tourrel-Cuzin C, Brun M, Géant A, Dubois M, Catesson S, Rodriguez M, Luquet S, Cattan P, Lockhart B, Lang J, Ktorza A, Magnan C, Kargar C. Exploring functional β-cell heterogeneity in vivo using PSA-NCAM as a specific marker. PLoS One 4: e5555, 2009. doi: 10.1371/journal.pone.0005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pang K, Mukonoweshuro C, Wong GG. Beta cells arise from glucose transporter type 2 (Glut2)-expressing epithelial cells of the developing rat pancreas. Proc Natl Acad Sci USA 91: 9559–9563, 1994. doi: 10.1073/pnas.91.20.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szabat M, Luciani DS, Piret JM, Johnson JD. Maturation of adult β-cells revealed using a Pdx1/Insulin dual-reporter lentivirus. Endocrinology 150: 1627–1635, 2009. doi: 10.1210/en.2008-1224. [DOI] [PubMed] [Google Scholar]
- 74.van der Meulen T, Mawla AM, DiGruccio MR, Adams MW, Nies V, Dólleman S, Liu S, Ackermann AM, Cáceres E, Hunter AE, Kaestner KH, Donaldson CJ, Huising MO. Virgin beta cells persist throughout life at a neogenic niche within pancreatic islets. Cell Metab 25: 911–926.e6, 2017. doi: 10.1016/j.cmet.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jetton TL, Magnuson MA. Heterogeneous expression of glucokinase among pancreatic beta cells. Proc Natl Acad Sci USA 89: 2619–2623, 1992. doi: 10.1073/pnas.89.7.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jörns A, Tiedge M, Lenzen S. Nutrient-dependent distribution of insulin and glucokinase immunoreactivities in rat pancreatic beta cells. Virchows Arch 434: 75–82, 1999. doi: 10.1007/s004280050308. [DOI] [PubMed] [Google Scholar]
- 77.Serre-Beinier V, Bosco D, Zulianello L, Charollais A, Caille D, Charpantier E, Gauthier BR, Diaferia GR, Giepmans BN, Lupi R, Marchetti P, Deng S, Buhler L, Berney T, Cirulli V, Meda P. Cx36 makes channels coupling human pancreatic β-cells, and correlates with insulin expression. Hum Mol Genet 18: 428–439, 2009. doi: 10.1093/hmg/ddn370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Esni F, Täljedal IB, Perl AK, Cremer H, Christofori G, Semb H. Neural cell adhesion molecule (N-CAM) is required for cell type segregation and normal ultrastructure in pancreatic islets. J Cell Biol 144: 325–337, 1999. doi: 10.1083/jcb.144.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bernard-Kargar C, Kassis N, Berthault MF, Pralong W, Ktorza A. Sialylated form of the neural cell adhesion molecule (NCAM): a new tool for the identification and sorting of beta-cell subpopulations with different functional activity. Diabetes 50: S125–S130, 2001. doi: 10.2337/diabetes.50.2007.S125. [DOI] [PubMed] [Google Scholar]
- 80.Kiss JZ, Wang C, Olive S, Rougon G, Lang J, Baetens D, Harry D, Pralong WF. Activity-dependent mobilization of the adhesion molecule polysialic NCAM to the cell surface of neurons and endocrine cells. EMBO J 13: 5284–5292, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bosco D, Rouiller DG, Halban PA. Differential expression of E-cadherin at the surface of rat β-cells as a marker of functional heterogeneity. J Endocrinol 194: 21–29, 2007. doi: 10.1677/JOE-06-0169. [DOI] [PubMed] [Google Scholar]
- 82.Dahl U, Sjødin A, Semb H. Cadherins regulate aggregation of pancreatic β-cells in vivo. Development 122: 2895–2902, 1996. [DOI] [PubMed] [Google Scholar]
- 83.Wakae-Takada N, Xuan S, Watanabe K, Meda P, Leibel RL. Molecular basis for the regulation of islet beta cell mass in mice: the role of E-cadherin. Diabetologia 56: 856–866, 2013. doi: 10.1007/s00125-012-2824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hermann M, Pirkebner D, Draxl A, Berger P, Untergasser G, Margreiter R, Hengster P. Dickkopf-3 is expressed in a subset of adult human pancreatic beta cells. Histochem Cell Biol 127: 513–521, 2007. doi: 10.1007/s00418-007-0278-6. [DOI] [PubMed] [Google Scholar]
- 85.Rodnoi P, Rajkumar M, Moin ASM, Georgia SK, Butler AE, Dhawan S. Neuropeptide Y expression marks partially differentiated β cells in mice and humans. JCI Insight 2, 2017. doi: 10.1172/jci.insight.94005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saisho Y, Harris PE, Butler AE, Galasso R, Gurlo T, Rizza RA, Butler PC. Relationship between pancreatic vesicular monoamine transporter 2 (VMAT2) and insulin expression in human pancreas. J Mol Histol 39: 543–551, 2008. doi: 10.1007/s10735-008-9195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Teitelman G, Alpert S, Polak JM, Martinez A, Hanahan D. Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development 118: 1031–1039, 1993. [DOI] [PubMed] [Google Scholar]
- 88.Baron M, Veres A, Wolock SL, Faust AL, Gaujoux R, Vetere A, Ryu JH, Wagner BK, Shen-Orr SS, Klein AM, Melton DA, Yanai I. A single-cell transcriptomic map of the human and mouse pancreas reveals inter- and intra-cell population structure. Cell Syst 3: 346–360.e4, 2016. doi: 10.1016/j.cels.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Muraro MJ, Dharmadhikari G, Grün D, Groen N, Dielen T, Jansen E, van Gurp L, Engelse MA, Carlotti F, de Koning EJP, van Oudenaarden A. A single-cell transcriptome atlas of the human pancreas. Cell Syst 3: 385–394.e3, 2016. doi: 10.1016/j.cels.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fang Z, Weng C, Li H, Tao R, Mai W, Liu X, Lu L, Lai S, Duan Q, Alvarez C, Arvan P, Wynshaw-Boris A, Li Y, Pei Y, Jin F, Li Y. Single-cell heterogeneity analysis and CRISPR screen identify key β-cell-specific disease genes. Cell Rep 26: 3132–3144.e7, 2019. doi: 10.1016/j.celrep.2019.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Segerstolpe Å, Palasantza A, Eliasson P, Andersson E-M, Andréasson A-C, Sun X, Picelli S, Sabirsh A, Clausen M, Bjursell MK, Smith DM, Kasper M, Ämmälä C, Sandberg R. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 Diabetes. Cell Metab 24: 593–607, 2016. doi: 10.1016/j.cmet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roschger C, Cabrele C. The Id-protein family in developmental and cancer-associated pathways. Cell Commun Signal 15: 7, 2017. doi: 10.1186/s12964-016-0161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436: 356–362, 2005. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 94.Lawlor N, George J, Bolisetty M, Kursawe R, Sun L, Sivakamasundari V, Kycia I, Robson P, Stitzel ML. Single-cell transcriptomes identify human islet cell signatures and reveal cell-type–specific expression changes in type 2 diabetes. Genome Res 27: 208–222, 2017. doi: 10.1101/gr.212720.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qiu W-L, Zhang Y-W, Feng Y, Li L-C, Yang L, Xu C-R. Deciphering pancreatic islet β cell and α cell maturation pathways and characteristic features at the single-cell Level. Cell Metab 25: 1194–1205.e4, 2017. [Erratum in Cell Metab. 27: 702, 2018]. doi: 10.1016/j.cmet.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 96.Wang YJ, Schug J, Won K-J, Liu C, Naji A, Avrahami D, Golson ML, Kaestner KH. Single-cell transcriptomics of the human endocrine pancreas. Diabetes 65: 3028–3038, 2016. doi: 10.2337/db16-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xin Y, Kim J, Ni M, Wei Y, Okamoto H, Lee J, Adler C, Cavino K, Murphy AJ, Yancopoulos GD, Lin HC, Gromada J. Use of the fluidigm C1 platform for RNA sequencing of single mouse pancreatic islet cells. Proc Natl Acad Sci USA 113: 3293–3298, 2016. doi: 10.1073/pnas.1602306113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xin Y, Kim J, Okamoto H, Ni M, Wei Y, Adler C, Murphy AJ, Yancopoulos GD, Lin C, Gromada J. RNA sequencing of single human islet cells reveals type 2 diabetes genes. Cell Metab 24: 608–615, 2016. doi: 10.1016/j.cmet.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 99.Xin Y, Okamoto H, Kim J, Ni M, Adler C, Cavino K, Na E, Murphy AJ, Yancopoulos GD, Lin C, Gromada J. Single-cell RNAseq reveals that pancreatic β-cells from very old male mice have a young gene signature. Endocrinology 157: 3431–3438, 2016. doi: 10.1210/en.2016-1235. [DOI] [PubMed] [Google Scholar]
- 100.Mawla AM, Huising MO. Navigating the depths and avoiding the shallows of pancreatic islet cell transcriptomes. Diabetes 68: 1380–1393, 2019. doi: 10.2337/dbi18-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eisenberg E, Levanon EY. Human housekeeping genes, revisited. Trends Genet 29: 569–574, 2013. [Erratum in Trends Genet. 30: 119-120, 2014]. doi: 10.1016/j.tig.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 102.Stegle O, Teichmann SA, Marioni JC. Computational and analytical challenges in single-cell transcriptomics. Nat Rev Genet 16: 133–145, 2015. doi: 10.1038/nrg3833. [DOI] [PubMed] [Google Scholar]
- 103.DePasquale EAK, Schnell DJ, Van Camp PJ, Valiente-Alandí Í, Blaxall BC, Grimes HL, Singh H, Salomonis N. Doublet decon: deconvoluting doublets from single-cell RNA-sequencing data. Cell Rep 29: 1718–1727.e8, 2019. doi: 10.1016/j.celrep.2019.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Enge M, Arda HE, Mignardi M, Beausang J, Bottino R, Kim SK, Quake SR. Single-cell analysis of human pancreas reveals transcriptional signatures of aging and somatic mutation patterns. Cell 171: 321–330.e14, 2017. doi: 10.1016/j.cell.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Linderman GC, Zhao J, Kluger Y. Zero-preserving imputation of scRNA-seq data using low-rank approximation. bioRxiv 397588: 2018. doi: 10.1101/397588. [DOI] [Google Scholar]
- 106.Wang L, Nie J, Sicotte H, Li Y, Eckel-Passow JE, Dasari S, Vedell PT, Barman P, Wang L, Weinshiboum R, Jen J, Huang H, Kohli M, Kocher J-PA. Measure transcript integrity using RNA-seq data. BMC Bioinformatics 17: 58, 2016. doi: 10.1186/s12859-016-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sullivan GM, Feinn R. Using effect size: or why the P value is not enough. J Grad Med Educ 4: 279–282, 2012. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dorrell C, Schug J, Canaday PS, Russ HA, Tarlow BD, Grompe MT, Horton T, Hebrok M, Streeter PR, Kaestner KH, Grompe M. Human islets contain four distinct subtypes of β cells. Nat Commun 7: 11756, 2016. doi: 10.1038/ncomms11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arrojo e Drigo R, Ali Y, Diez J, Srinivasan DK, Berggren PO, Boehm BO. New insights into the architecture of the islet of Langerhans: a focused cross-species assessment. Diabetologia 58: 2218–2228, 2015. doi: 10.1007/s00125-015-3699-0. [DOI] [PubMed] [Google Scholar]
- 110.Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, Giovannoni L, Parnaud G, Berney T. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes 59: 1202–1210, 2010. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steiner DJ, Kim A, Miller K, Hara M. Pancreatic islet plasticity: Interspecies comparison of islet architecture and composition. Islets 2: 135–145, 2010. doi: 10.4161/isl.2.3.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Migliorini A, Bader E, Lickert H. Islet cell plasticity and regeneration. Mol Metab 3: 268–274, 2014. doi: 10.1016/j.molmet.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gersell DJ, Gingerich RL, Greider MH. Regional distribution and concentration of pancreatic polypeptide in the human and canine pancreas. Diabetes 28: 11–15, 1979. doi: 10.2337/diabetes.28.1.11. [DOI] [PubMed] [Google Scholar]
- 114.Malaisse-Lagae F, Stefan Y, Cox J, Perrelet A, Orci L. Identification of a lobe in the adult human pancreas rich in pancreatic polypeptide. Diabetologia 17: 361–365, 1979. doi: 10.1007/BF01236270. [DOI] [PubMed] [Google Scholar]
- 115.Olehnik SK, Fowler JL, Avramovich G, Hara M. Quantitative analysis of intra- and inter-individual variability of human beta-cell mass. Sci Rep 7: 16398, 2017. doi: 10.1038/s41598-017-16300-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ellenbroek JH, Töns HA, de Graaf N, Loomans CJ, Engelse MA, Vrolijk H, Voshol PJ, Rabelink TJ, Carlotti F, de Koning EJ. Topologically heterogeneous beta cell adaptation in response to high-fat diet in mice. PLoS One 8: e56922, 2013. doi: 10.1371/journal.pone.0056922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Orci L, Unger R. Functional subdivision of islets of Langerhans and possible role of D cells. Lancet 2: 1243–1244, 1975. doi: 10.1016/S0140-6736(75)92078-4. [DOI] [PubMed] [Google Scholar]
- 118.Wang X, Misawa R, Zielinski MC, Cowen P, Jo J, Periwal V, Ricordi C, Khan A, Szust J, Shen J, Millis JM, Witkowski P, Hara M. Regional differences in islet distribution in the human pancreas: preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS One 8: e67454, 2013. doi: 10.1371/journal.pone.0067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ionescu-Tirgoviste C, Gagniuc PA, Gubceac E, Mardare L, Popescu I, Dima S, Militaru M. A 3D map of the islet routes throughout the healthy human pancreas. Sci Rep 5: 14634, 2015. doi: 10.1038/srep14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baetens D, Stefan Y, Ravazzola M, Malaisse-Lagae F, Coleman DL, Orci L. Alteration of islet cell populations in spontaneously diabetic mice. Diabetes 27: 1–7, 1978. doi: 10.2337/diabetes.27.1.1. [DOI] [PubMed] [Google Scholar]
- 121.Kilimnik G, Zhao B, Jo J, Periwal V, Witkowski P, Misawa R, Hara M. Altered islet composition and disproportionate loss of large islets in patients with type 2 diabetes. PLoS One 6: e27445, 2011. doi: 10.1371/journal.pone.0027445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beigelman PM, Ribalet B, Atwater I. Electrical activity of mouse pancreatic beta cells: II. effects of glucose and arginine. J Physiol (Paris) 73: 201–217, 1977. [PubMed] [Google Scholar]
- 123.Dean PM, Matthews EK. Electrical activity in pancreatic islet cells. Nature 219: 389–390, 1968. doi: 10.1038/219389a0. [DOI] [PubMed] [Google Scholar]
- 124.Pipeleers D. The biosociology of pancreatic B cells. Diabetologia 30: 277–291, 1987. doi: 10.1007/BF00299019. [DOI] [PubMed] [Google Scholar]
- 125.Orci L, Malaisse-Lagae F, Baetens D, Perrelet A. Pancreatic-polypeptide-rich regions in human pancreas. Lancet 312: 1200–1201, 1978. doi: 10.1016/S0140-6736(78)92181-5. [DOI] [PubMed] [Google Scholar]
- 126.Pipeleers D, in't Veld PI, Maes E, Van De Winkel M. Glucose-induced insulin release depends on functional cooperation between islet cells. Proc Natl Acad Sci USA 79: 7322–7325, 1982. doi: 10.1073/pnas.79.23.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Weir GC, Halban PA, Meda P, Wollheim CB, Orci L, Renold AE. Dispersed adult rat pancreatic islet cells in culture: A, B, and D cell function. Metabolism 33: 447–453, 1984. doi: 10.1016/0026-0495(84)90146-X. [DOI] [PubMed] [Google Scholar]
- 128.Bosco D, Orci L, Meda P. Homologous but not heterologous contact increases the insulin secretion of individual pancreatic B-cells. Exp Cell Res 184: 72–80, 1989. doi: 10.1016/0014-4827(89)90365-0. [DOI] [PubMed] [Google Scholar]
- 129.Curry DL, Bennett LL. Does somatostatin inhibition of insulin secretion involve two mechanisms of action? Proc Natl Acad Sci USA 73: 248–251, 1976. doi: 10.1073/pnas.73.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hauge-Evans AC, Bowe J, Franklin ZJ, Hassan Z, Jones PM. Inhibitory effect of somatostatin on insulin secretion is not mediated via the CNS. J Endocrinol 225: 19–26, 2015. doi: 10.1530/JOE-14-0709. [DOI] [PubMed] [Google Scholar]
- 131.Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptor subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology 141: 111–117, 2000. doi: 10.1210/endo.141.1.7263. [DOI] [PubMed] [Google Scholar]
- 132.Trimble ER, Halban PA, Wollheim CB, Renold AE. Functional differences between rat islets of ventral and dorsal pancreatic origin. J Clin Invest 69: 405–413, 1982. doi: 10.1172/jci110464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Giuliani M, Moritz W, Bodmer E, Dindo D, Kugelmeier P, Lehmann R, Gassmann M, Groscurth P, Weber M. Central necrosis in isolated hypoxic human pancreatic islets: evidence for postisolation ischemia. Cell Transplant 14: 67–76, 2005. doi: 10.3727/000000005783983287. [DOI] [PubMed] [Google Scholar]
- 134.Komatsu H, Cook C, Wang C-H, Medrano L, Lin H, Kandeel F, Tai Y-C, Mullen Y. Oxygen environment and islet size are the primary limiting factors of isolated pancreatic islet survival. PLoS One 12: e0183780, 2017. doi: 10.1371/journal.pone.0183780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Komatsu H, Kandeel F, Mullen Y. Impact of oxygen on pancreatic islet survival. Pancreas 47: 533–543, 2018. doi: 10.1097/MPA.0000000000001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gerdes JM, Christou-Savina S, Xiong Y, Moede T, Moruzzi N, Karlsson-Edlund P, Leibiger B, Leibiger IB, Östenson C-G, Beales PL, Berggren PO. Ciliary dysfunction impairs beta-cell insulin secretion and promotes development of type 2 diabetes in rodents. Nat Commun 5: 5308, 2014. doi: 10.1038/ncomms6308. [DOI] [PubMed] [Google Scholar]
- 137.Hughes JW, Cho JH, Conway HE, DiGruccio MR, Ng XW, Roseman HF, Abreu D, Urano F, Piston DW. Primary cilia control glucose homeostasis via islet paracrine interactions. Proc Natl Acad Sci USA 117: 8912–8923, 2020. doi: 10.1073/pnas.2001936117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Quesada I, Todorova MG, Alonso-Magdalena P, Beltrá M, Carneiro EM, Martin F, Nadal A, Soria B. Glucose induces opposite intracellular Ca2+ concentration oscillatory patterns in identified α- and β-cells within intact human islets of Langerhans. Diabetes 55: 2463–2469, 2006. doi: 10.2337/db06-0272. [DOI] [PubMed] [Google Scholar]
- 139.Almaça J, Liang T, Gaisano HY, Nam HG, Berggren PO, Caicedo A. Spatial and temporal coordination of insulin granule exocytosis in intact human pancreatic islets. Diabetologia 58: 2810–2818, 2015. doi: 10.1007/s00125-015-3747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bertram R, Sherman A, Satin LS. Metabolic and electrical oscillations: partners in controlling pulsatile insulin secretion. Am J Physiol Endocrinol Metab 293: E890–E900, 2007. doi: 10.1152/ajpendo.00359.2007. [DOI] [PubMed] [Google Scholar]
- 141.Dean PM, Matthews EK, Sakamoto Y. Pancreatic islet cells: effects of monosaccharides, glycolytic intermediates and metabolic inhibitors on membrane potential and electrical activity. J Physiol 246: 459–478, 1975. doi: 10.1113/jphysiol.1975.sp010899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Salem V, Silva LD, Suba K, Georgiadou E, Neda Mousavy Gharavy S, Akhtar N, Martin-Alonso A, Gaboriau DCA, Rothery SM, Stylianides T, Carrat G, Pullen TJ, Singh SP, Hodson DJ, Leclerc I, Shapiro AMJ, Marchetti P, Briant LJB, Distaso W, Ninov N, Rutter GA. Leader β-cells coordinate Ca2+ dynamics across pancreatic islets in vivo. Nat Metab 1: 615–629, 2019. doi: 10.1038/s42255-019-0075-2. [DOI] [PubMed] [Google Scholar]
- 143.Satin LS, Zhang Q, Rorsman P. “Take me to your leader”: an electrophysiological appraisal of the role of hub cells in pancreatic islets. Diabetes 69: 830–836, 2020. doi: 10.2337/dbi19-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Koster JC, Remedi MS, Flagg TP, Johnson JD, Markova KP, Marshall BA, Nichols CG. Hyperinsulinism induced by targeted suppression of beta cell KATP channels. Proc Natl Acad Sci USA 99: 16992–16997, 2002. doi: 10.1073/pnas.012479199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu JSE, Hebrok M. All mixed up: defining roles for β-cell subtypes in mature islets. Genes Dev 31: 228–240, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rocheleau JV, Remedi MS, Granada B, Head WS, Koster JC, Nichols CG, Piston DW. Critical role of gap junction coupled KATP channel activity for regulated insulin secretion. PLoS Biol 4: e26, 2006. doi: 10.1371/journal.pbio.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Benninger RK, Head WS, Zhang M, Satin LS, Piston DW. Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet. J Physiol 589: 5453–5466, 2011. doi: 10.1113/jphysiol.2011.218909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Head WS, Orseth ML, Nunemaker CS, Satin LS, Piston DW, Benninger RK. Connexin-36 gap junctions regulate in vivo first- and second-phase insulin secretion dynamics and glucose tolerance in the conscious mouse. Diabetes 61: 1700–1707, 2012. doi: 10.2337/db11-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.El-Gohary Y, Sims-Lucas S, Lath N, Tulachan S, Guo P, Xiao X, Welsh C, Paredes J, Wiersch J, Prasadan K, Shiota C, Gittes GK. Three-dimensional analysis of the islet vasculature. Anat Rec (Hoboken) 295: 1473–1481, 2012. doi: 10.1002/ar.22530. [DOI] [PubMed] [Google Scholar]
- 150.Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fässler R, Gu G, Gerber H-P, Ferrara N, Melton DA, Lammert E. The vascular basement membrane: a niche for insulin gene expression and β cell proliferation. Dev Cell 10: 397–405, 2006. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 151.Peiris H, Bonder CS, Coates PTH, Keating DJ, Jessup CF. The beta-cell/EC axis: how do islet cells talk to each other? Diabetes 63: 3–11, 2014. doi: 10.2337/db13-0617. [DOI] [PubMed] [Google Scholar]
- 152.Lau J, Svensson J, Grapensparr L, Johansson Å, Carlsson P-O. Superior beta cell proliferation, function and gene expression in a subpopulation of rat islets identified by high blood perfusion. Diabetologia 55: 1390–1399, 2012. doi: 10.1007/s00125-012-2476-6. [DOI] [PubMed] [Google Scholar]
- 153.Olsson R, Carlsson P-O. A low-oxygenated subpopulation of pancreatic islets constitutes a functional reserve of endocrine cells. Diabetes 60: 2068–2075, 2011. doi: 10.2337/db09-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ullsten S, Lau J, Carlsson P-O. Vascular heterogeneity between native rat pancreatic islets is responsible for differences in survival and revascularisation post transplantation. Diabetologia 58: 132–139, 2015. doi: 10.1007/s00125-014-3385-7. [DOI] [PubMed] [Google Scholar]
- 155.Nyman LR, Ford E, Powers AC, Piston DW. Glucose-dependent blood flow dynamics in murine pancreatic islets in vivo. Am J Physiol Endocrinol Metab 298: E807–E814, 2010. doi: 10.1152/ajpendo.00715.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nyman LR, Wells KS, Head WS, McCaughey M, Ford E, Brissova M, Piston DW, Powers AC. Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets. J Clin Invest 118: 3790–3797, 2008. doi: 10.1172/JCI36209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stagner JI, Samols E. The vascular order of islet cellular perfusion in the human pancreas. Diabetes 41: 93–97, 1992. doi: 10.2337/diab.41.1.93. [DOI] [PubMed] [Google Scholar]
- 158.Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 74: 2296–2299, 1984. doi: 10.1172/JCI111658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ahrén B. Autonomic regulation of islet hormone secretion: implications for health and disease. Diabetologia 43: 393–410, 2000. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 160.Borden P, Houtz J, Leach SD, Kuruvilla R. Sympathetic innervation during development is necessary for pancreatic islet architecture and functional maturation. Cell Rep 4: 287–301, 2013. doi: 10.1016/j.celrep.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Di Cairano ES, Moretti S, Marciani P, Sacchi VF, Castagna M, Davalli A, Folli F, Perego C. Neurotransmitters and neuropeptides: new players in the control of islet of Langerhans’ cell mass and function. J Cell Physiol 231: 756–767, 2016. doi: 10.1002/jcp.25176. [DOI] [PubMed] [Google Scholar]
- 162.Burris RE, Hebrok M. Pancreatic innervation in mouse development and β-cell regeneration. Neuroscience 150: 592–602, 2007. doi: 10.1016/j.neuroscience.2007.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Goyvaerts L, Lemaire K, Arijs I, Auffret J, Granvik M, Van Lommel L, Binart N, in’t Veld P, Schuit F, Schraenen A. Prolactin receptors and placental lactogen drive male mouse pancreatic islets to pregnancy-related mRNA changes. PLoS One 10: e0121868, 2015. doi: 10.1371/journal.pone.0121868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ligon B, Yang J, Morin SB, Ruberti MF, Steer ML. Regulation of pancreatic islet cell survival and replication by γ-aminobutyric acid. Diabetologia 50: 764–773, 2007. doi: 10.1007/s00125-007-0601-8. [DOI] [PubMed] [Google Scholar]
- 165.Rodriguez-Diaz R, Caicedo A. Neural control of the endocrine pancreas. Best Pract Res Clin Endocrinol Metab 28: 745–756, 2014. doi: 10.1016/j.beem.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 166.Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren P-O, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 14: 45–54, 2011. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Edlund H. Pancreatic organogenesis: developmental mechanisms and implications for therapy. Nat Rev Genet 3: 524–532, 2002. doi: 10.1038/nrg841. [DOI] [PubMed] [Google Scholar]
- 168.Bastidas-Ponce A, Scheibner K, Lickert H, Bakhti M. Cellular and molecular mechanisms coordinating pancreas development. Development 144: 2873–2888, 2017. doi: 10.1242/dev.140756. [DOI] [PubMed] [Google Scholar]
- 169.Bastidas-Ponce A, Tritschler S, Dony L, Scheibner K, Tarquis-Medina M, Salinno C, Schirge S, Burtscher I, Böttcher A, Theis FJ, Lickert H, Bakhti M. Comprehensive single cell mRNA profiling reveals a detailed roadmap for pancreatic endocrinogenesis. Development 146: dev173849, 2019. doi: 10.1242/dev.173849. [DOI] [PubMed] [Google Scholar]
- 170.Mamidi A, Prawiro C, Seymour PA, de Lichtenberg KH, Jackson A, Serup P, Semb H. Mechanosignalling via integrins directs fate decisions of pancreatic progenitors. Nature 564: 114–118, 2018. doi: 10.1038/s41586-018-0762-2. [DOI] [PubMed] [Google Scholar]
- 171.Deltour L, Leduque P, Paldi A, Ripoche MA, Dubois P, Jami J. Polyclonal origin of pancreatic islets in aggregation mouse chimaeras. Development 112: 1115–1121, 1991. [DOI] [PubMed] [Google Scholar]
- 172.Bouwens L, Lu WG, De Krijger R. Proliferation and differentiation in the human fetal endocrine pancreas. Diabetologia 40: 398–404, 1997. doi: 10.1007/s001250050693. [DOI] [PubMed] [Google Scholar]
- 173.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 57: 1584–1594, 2008. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Peng S, Zhu L, Chen M, Zhang M, Li D, Fu Y, Chen S, Wei C. Heterogeneity in mitotic activity and telomere length implies an important role of young islets in the maintenance of islet mass in the adult pancreas. Endocrinology 150: 3058–3066, 2009. doi: 10.1210/en.2008-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–46, 2004. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 176.Wang YJ, Golson ML, Schug J, Traum D, Liu C, Vivek K, Dorrell C, Naji A, Powers AC, Chang K-M, Grompe M, Kaestner KH. Single-cell mass cytometry analysis of the human endocrine pancreas. Cell Metab 24: 616–626, 2016. doi: 10.1016/j.cmet.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zeng C, Mulas F, Sui Y, Guan T, Miller N, Tan Y, Liu F, Jin W, Carrano AC, Huising MO, Shirihai OS, Yeo GW, Sander M. Pseudotemporal ordering of single cells reveals metabolic control of postnatal β cell proliferation. Cell Metab 25: 1160–1175.e11, 2017. doi: 10.1016/j.cmet.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Meier JJ, Butler AE, Galasso R, Rizza RA, Butler PC. Increased islet beta cell replication adjacent to intrapancreatic gastrinomas in humans. Diabetologia 49: 2689–2696, 2006. doi: 10.1007/s00125-006-0410-5. [DOI] [PubMed] [Google Scholar]
- 179.Tschen S-I, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes 58: 1312–1320, 2009. doi: 10.2337/db08-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Veld PI, De Munck N, Van Belle K, Buelens N, Ling Z, Weets I, Haentjens P, Pipeleers-Marichal M, Gorus F, Pipeleers D. Cell replication is increased in donor organs from young patients after prolonged life support. Diabetes 59: 1702–1708, 2010. doi: 10.2337/db09-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Aguayo-Mazzucato C, van Haaren M, Mruk M, Lee TB, Crawford C, Hollister-Lock J, Sullivan BA, Johnson JW, Ebrahimi A, Dreyfuss JM, Van Deursen J, Weir GC, Bonner-Weir S. β Cell aging markers have heterogeneous distribution and are induced by insulin resistance. Cell Metab 25: 898–910.e5, 2017. doi: 10.1016/j.cmet.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cnop M, Grupping A, Hoorens A, Bouwens L, Pipeleers-Marichal M, Pipeleers D. Endocytosis of low-density lipoprotein by human pancreatic β cells and uptake in lipid-storing vesicles, which increase with age. Am J Pathol 156: 237–244, 2000. doi: 10.1016/S0002-9440(10)64724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas: a possible recapitulation of embryonic development. Diabetes 42: 1715–1720, 1993. doi: 10.2337/diabetes.42.12.1715. [DOI] [PubMed] [Google Scholar]
- 184.Brockenbrough JS, Weir GC, Bonner-Weir S. Discordance of exocrine and endocrine growth after 90% pancreatectomy in rats. Diabetes 37: 232–236, 1988. doi: 10.2337/diabetes.37.2.232. [DOI] [PubMed] [Google Scholar]
- 185.Song SY, Gannon M, Washington MK, Scoggins CR, Meszoely IM, Goldenring JR, Marino CR, Sandgren EP, Coffey RJ, Wright CVE, Leach SD. Expansion of Pdx1-expressing pancreatic epithelium and islet neogenesis in transgenic mice overexpressing transforming growth factor α. Gastroenterology 117: 1416–1426, 1999. doi: 10.1016/S0016-5085(99)70292-1. [DOI] [PubMed] [Google Scholar]
- 186.Wang TC, Bonner-Weir S, Oates PS, Chulak M, Simon B, Merlino GT, Schmidt EV, Brand SJ. Pancreatic gastrin stimulates islet differentiation of transforming growth factor alpha-induced ductular precursor cells. J Clin Invest 92: 1349–1356, 1993. doi: 10.1172/JCI116708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jetton TL, Everill B, Lausier J, Roskens V, Habibovic A, LaRock K, Gokin A, Peshavaria M, Leahy JL. Enhanced β-cell mass without increased proliferation following chronic mild glucose infusion. Am J Physiol Endocrinol Metab 294: E679–E687, 2008. doi: 10.1152/ajpendo.00569.2007. [DOI] [PubMed] [Google Scholar]
- 188.Chintinne M, Stangé G, Denys B, Ling Z, In‘t Veld P, Pipeleers D. Beta cell count instead of beta cell mass to assess and localize growth in beta cell population following pancreatic duct ligation in mice. PLoS One 7: e43959, 2012. doi: 10.1371/journal.pone.0043959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA 105: 19915–19919, 2008. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xu X, D'Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H. β Cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132: 197–207, 2008. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 191.Razavi R, Najafabadi HS, Abdullah S, Smukler S, Arntfield M, van der Kooy D. Diabetes enhances the proliferation of adult pancreatic multipotent progenitor cells and biases their differentiation to more β-cell production. Diabetes 64: 1311–1323, 2015. doi: 10.2337/db14-0070. [DOI] [PubMed] [Google Scholar]
- 192.Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol 22: 1115–1124, 2004. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- 193.Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, Stoffers DA. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet β cell, regeneration. J Clin Invest 117: 971–977, 2007. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xiao X, Chen Z, Shiota C, Prasadan K, Guo P, El-Gohary Y, Paredes J, Welsh C, Wiersch J, Gittes GK. No evidence for β cell neogenesis in murine adult pancreas. J Clin Invest 123: 2207–2217, 2013. doi: 10.1172/JCI66323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Domínguez-Bendala J, Qadir MMF, Pastori RL. Pancreatic progenitors: there and back again. Trends Endocrinol Metab 30: 4–11, 2019. doi: 10.1016/j.tem.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ackermann AM, Wang Z, Schug J, Naji A, Kaestner KH. Integration of ATAC-seq and RNA-seq identifies human alpha cell and beta cell signature genes. Mol Metab 5: 233–244, 2016. doi: 10.1016/j.molmet.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ben-Othman N, Vieira A, Courtney M, Record F, Gjernes E, Avolio F, Hadzic B, Druelle N, Napolitano T, Navarro-Sanz S, Silvano S, Al-Hasani K, Pfeifer A, Lacas-Gervais S, Leuckx G, Marroquí L, Thévenet J, Madsen OD, Eizirik DL, Heimberg H, Kerr-Conte J, Pattou F, Mansouri A, Collombat P. Long-term GABA administration induces alpha cell-mediated beta-like cell neogenesis. Cell 168: 73–85.e11, 2017. doi: 10.1016/j.cell.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 198.Bramswig NC, Everett LJ, Schug J, Dorrell C, Liu C, Luo Y, Streeter PR, Naji A, Grompe M, Kaestner KH. Epigenomic plasticity enables human pancreatic α to β cell reprogramming. J Clin Invest 123: 1275–1284, 2013. doi: 10.1172/JCI66514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into α and subsequently β cells. Cell 138: 449–462, 2009. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic α-cells to Β-cells after extreme Β-cell loss. Nature 464: 1149–1154, 2010. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Feng Y, Qiu WL, Yu XX, Zhang Y, He MY, Li LC, Yang L, Zhang W, Franti M, Ye J, Hoeck JD, Xu CR. Characterizing pancreatic β-cell heterogeneity in the streptozotocin model by single-cell transcriptomic analysis. Mol Metab 37: 100982, 2020. doi: 10.1016/j.molmet.2020.100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Marroqui L, Lopes M, dos Santos RS, Grieco FA, Roivainen M, Richardson SJ, Morgan NG, Op de Beeck A, Eizirik DL. Differential cell autonomous responses determine the outcome of coxsackievirus infections in murine pancreatic α and β cells. Elife 4, 2015. doi: 10.7554/eLife.06990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Marroqui L, Masini M, Merino B, Grieco FA, Millard I, Dubois C, Quesada I, Marchetti P, Cnop M, Eizirik DL. Pancreatic α cells are resistant to metabolic stress-induced apoptosis in type 2 diabetes. EBioMedicine 2: 378–385, 2015. doi: 10.1016/j.ebiom.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bonner-Weir S, Aguayo-Mazzucato C, Weir GC. Dynamic development of the pancreas from birth to adulthood. Ups J Med Sci 121: 155–158, 2016. doi: 10.3109/03009734.2016.1154906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dore BA, Grogan WM, Madge GE, Webb SR. Biphasic development of the postnatal mouse pancreas. Biol Neonate 40: 209–217, 1981. doi: 10.1159/000241494. [DOI] [PubMed] [Google Scholar]
- 206.Martens GA, Motté E, Kramer G, Stangé G, Gaarn LW, Hellemans K, Nielsen JH, Aerts JM, Ling Z, Pipeleers D. Functional characteristics of neonatal rat β cells with distinct markers. J Mol Endocrinol 52: 11–28, 2014. doi: 10.1530/JME-13-0106. [DOI] [PubMed] [Google Scholar]
- 207.Stolovich-Rain M, Enk J, Vikesa J, Nielsen FC, Saada A, Glaser B, Dor Y. Weaning triggers a maturation step of pancreatic β cells. Dev Cell 32: 535–545, 2015. [Erratum in Dev Cell 33: 238-239, 2015]. doi: 10.1016/j.devcel.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 208.Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology 138: 1736–1741, 1997. doi: 10.1210/endo.138.4.5069. [DOI] [PubMed] [Google Scholar]
- 209.Trudeau JD, Dutz JP, Arany E, Hill DJ, Fieldus WE, Finegood DT. Neonatal beta-cell apoptosis: a trigger for autoimmune diabetes? Diabetes 49: 1–7, 2000. doi: 10.2337/diabetes.49.1.1. [DOI] [PubMed] [Google Scholar]
- 210.Sharon N, Chawla R, Mueller J, Vanderhooft J, Whitehorn LJ, Rosenthal B, Gürtler M, Estanboulieh RR, Shvartsman D, Gifford DK, Trapnell C, Melton D. A peninsular structure coordinates asynchronous differentiation with morphogenesis to generate pancreatic islets. Cell 176: 790–804.e13, 2019. doi: 10.1016/j.cell.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jacovetti C, Matkovich SJ, Rodriguez-Trejo A, Guay C, Regazzi R. Postnatal β-cell maturation is associated with islet-specific microRNA changes induced by nutrient shifts at weaning. Nat Commun 6: 8084, 2015. doi: 10.1038/ncomms9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Avrahami D, Li C, Zhang J, Schug J, Avrahami R, Rao S, Stadler MB, Burger L, Schübeler D, Glaser B, Kaestner KH. Aging-dependent demethylation of regulatory elements correlates with chromatin state and improved β cell function. Cell Metab 22: 619–632, 2015. doi: 10.1016/j.cmet.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Blum B, Hrvatin S, Schuetz C, Bonal C, Rezania A, Melton DA. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotechnol 30: 261–264, 2012. doi: 10.1038/nbt.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gu C, Stein GH, Pan N, Goebbels S, Hörnberg H, Nave KA, Herrera P, White P, Kaestner KH, Sussel L, Lee JE. Pancreatic β cells require NeuroD to achieve and maintain functional maturity. Cell Metab 11: 298–310, 2010. doi: 10.1016/j.cmet.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ross Laybutt D, Sharma A, Sgroi DC, Gaudet J, Bonner-Weir S, Weir GC. Genetic regulation of metabolic pathways in β-cells disrupted by hyperglycemia. J Biol Chem 277: 10912–10921, 2002. doi: 10.1074/jbc.M111751200. [DOI] [PubMed] [Google Scholar]
- 216.van der Meulen T, Huising MO. Maturation of stem cell-derived beta-cells guided by the expression of urocortin 3. Rev Diabet Stud 11: 115–132, 2014. doi: 10.1900/RDS.2014.11.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Akerman I, Tu Z, Beucher A, Rolando DMY, Sauty-Colace C, Benazra M, Nakic N, Yang J, Wang H, Pasquali L, Moran I, Garcia-Hurtado J, Castro N, Gonzalez-Franco R, Stewart AF, Bonner C, Piemonti L, Berney T, Groop L, Kerr-Conte J, Pattou F, Argmann C, Schadt E, Ravassard P, Ferrer J. Human pancreatic β cell lncRNAs control cell-specific regulatory networks. Cell Metab 25: 400–411, 2017. doi: 10.1016/j.cmet.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cyphert HA, Walker EM, Hang Y, Dhawan S, Haliyur R, Bonatakis L, Avrahami D, Brissova M, Kaestner KH, Bhushan A, Powers AC, Stein R. Examining how the MAFB transcription factor affects islet β-cell function postnatally. Diabetes 68: 337–348, 2019. doi: 10.2337/db18-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Morán I, Akerman İ, van de Bunt M, Xie R, Benazra M, Nammo T, , et al. Human β cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metab 16: 435–448, 2012. doi: 10.1016/j.cmet.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dhawan S, Tschen SI, Zeng C, Guo T, Hebrok M, Matveyenko A, Bhushan A. DNA methylation directs functional maturation of pancreatic β cells. J Clin Invest 125: 2851–2860, 2015. doi: 10.1172/JCI79956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Thorrez L, Laudadio I, Van Deun K, Quintens R, Hendrickx N, Granvik M, Lemaire K, Schraenen A, Van Lommel L, Lehnert S, Aguayo-Mazzucato C, Cheng-Xue R, Gilon P, Van Mechelen I, Bonner-Weir S, Lemaigre F, Schuit F. Tissue-specific disallowance of housekeeping genes: the other face of cell differentiation. Genome Res 21: 95–105, 2011. doi: 10.1101/gr.109173.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Schuit F, Van Lommel L, Granvik M, Goyvaerts L, de Faudeur G, Schraenen A, Lemaire K. β-Cell–specific gene repression: a mechanism to protect against inappropriate or maladjusted insulin secretion? Diabetes 61: 969–975, 2012. doi: 10.2337/db11-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Henquin JC, Nenquin M. Immaturity of insulin secretion by pancreatic islets isolated from one human neonate. J Diabetes Investig 9: 270–273, 2018. doi: 10.1111/jdi.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Liang Y, Jetton TL, Zimmerman EC, Najafi H, Matschinsky FM, Magnuson MA. Effects of alternate RNA splicing on glucokinase isoform activities in the pancreatic islet, liver, and pituitary. J Biol Chem 266: 6999–7007, 1991. [PubMed] [Google Scholar]
- 225.Moukil MA, Veiga-da-Cunha M, Van Schaftingen E. Study of the regulatory properties of glucokinase by site-directed mutagenesis: conversion of glucokinase to an enzyme with high affinity for glucose. Diabetes 49: 195–201, 2000. doi: 10.2337/diabetes.49.2.195. [DOI] [PubMed] [Google Scholar]
- 226.Piston DW, Knobel SM, Postic C, Shelton KD, Magnuson MA. Adenovirus-mediated knockout of a conditional glucokinase gene in isolated pancreatic islets reveals an essential role for proximal metabolic coupling events in glucose-stimulated insulin secretion. J Biol Chem 274: 1000–1004, 1999. doi: 10.1074/jbc.274.2.1000. [DOI] [PubMed] [Google Scholar]
- 227.Yoshihara E, Wei Z, Lin CS, Fang S, Ahmadian M, Kida Y, Tseng T, Dai Y, Yu RT, Liddle C, Atkins AR, Downes M, Evans RM. ERRγ is required for the metabolic maturation of therapeutically functional glucose-responsive β cells. Cell Metab 23: 622–634, 2016. doi: 10.1016/j.cmet.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Klochendler A, Caspi I, Corem N, Moran M, Friedlich O, Elgavish S, Nevo Y, Helman A, Glaser B, Eden A, Itzkovitz S, Dor Y. The genetic program of pancreatic β-cell replication in vivo. Diabetes 65: 2081–2093, 2016. doi: 10.2337/db16-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Leiter EH, Premdas F, Harrison DE, Lipson LG. Aging and glucose homeostasis in C57BL/6J male mice. FASEB J 2: 2807–2811, 1988. doi: 10.1096/fasebj.2.12.3044905. [DOI] [PubMed] [Google Scholar]
- 230.Wills QF, Boothe T, Asadi A, Ao Z, Warnock GL, Kieffer TJ, Johnson JD. Statistical approaches and software for clustering islet cell functional heterogeneity. Islets 8: 48–56, 2016. doi: 10.1080/19382014.2016.1150664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sharma RB, O'Donnell AC, Stamateris RE, Ha B, McCloskey KM, Reynolds PR, Arvan P, Alonso LC. Insulin demand regulates β cell number via the unfolded protein response. J Clin Invest 125: 3831–3846, 2015. doi: 10.1172/JCI79264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gegg M, Böttcher A, Burtscher I, Hasenoeder S, Van Campenhout C, Aichler M, Walch A, Grant SGN, Lickert H. Flattop regulates basal body docking and positioning in mono- and multiciliated cells. Elife 3, 2014. doi: 10.7554/eLife.03842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bensellam M, Jonas JC, Laybutt DR. Mechanisms of β-cell dedifferentiation in diabetes: recent findings and future research directions. J Endocrinol 236: R109–R143, 2018. doi: 10.1530/JOE-17-0516. [DOI] [PubMed] [Google Scholar]
- 234.Diedisheim M, Oshima M, Albagli O, Huldt CW, Ahlstedt I, Clausen M, Menon S, Aivazidis A, Andreasson AC, Haynes WG, Marchetti P, Marselli L, Armanet M, Chimienti F, Scharfmann R. Modeling human pancreatic beta cell dedifferentiation. Mol Metab 10: 74–86, 2018. doi: 10.1016/j.molmet.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patanè G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic β cell differentiation in an animal model of diabetes. J Biol Chem 274: 14112–14121, 1999. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- 236.Laybutt DR, Glandt M, Xu G, Ahn YB, Trivedi N, Bonner-Weir S, Weir GC. Critical reduction in β-cell mass results in two distinct outcomes over time. J Biol Chem 278: 2997–3005, 2003. doi: 10.1074/jbc.M210581200. [DOI] [PubMed] [Google Scholar]
- 237.Wang Z, York NWW, Nichols CGG, Remedi MSS. Pancreatic β cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab 19: 872–882, 2014. doi: 10.1016/j.cmet.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Brereton MF, Iberl M, Shimomura K, Zhang Q, Adriaenssens AE, Proks P, Spiliotis II, Dace W, Mattis KK, Ramracheya R, Gribble FM, Reimann F, Clark A, Rorsman P, Ashcroft FM. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat Commun 5: 4639, 2014. doi: 10.1038/ncomms5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest 123: 3305–3316, 2013. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Pullen TJ, Khan AM, Barton G, Butcher SA, Sun G, Rutter GA. Identification of genes selectively disallowed in the pancreatic islet. Islets 2: 89–95, 2010. doi: 10.4161/isl.2.2.11025. [DOI] [PubMed] [Google Scholar]
- 241.Weir GC, Aguayo-Mazzucato C, Bonner-Weir S. β-Cell dedifferentiation in diabetes is important, but what is it? Islets 5: 233–237, 2013. doi: 10.4161/isl.27494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bastidas-Ponce A, Roscioni SS, Burtscher I, Bader E, Sterr M, Bakhti M, Lickert H. Foxa2 and Pdx1 cooperatively regulate postnatal maturation of pancreatic β-cells. Mol Metab 6: 524–534, 2017. doi: 10.1016/j.molmet.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sacco F, Seelig A, Humphrey SJ, Krahmer N, Volta F, Reggio A, Marchetti P, Gerdes J, Mann M. Phosphoproteomics reveals the GSK3-PDX1 axis as a key pathogenic signaling node in diabetic islets. Cell Metab 29: 1422–1432.e3, 2019. doi: 10.1016/j.cmet.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 244.Spaeth JM, Gupte M, Perelis M, Yang Y-P, Cyphert H, Guo S, Liu J-H, Guo M, Bass J, Magnuson MA, Wright C, Stein R. Defining a novel role for the Pdx1 transcription factor in islet β-cell maturation and proliferation during weaning. Diabetes 66: 2830–2839, 2017. doi: 10.2337/db16-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Dahan T, Ziv O, Horwitz E, Zemmour H, Lavi J, Swisa A, Leibowitz G, Ashcroft FM, In’t Veld P, Glaser B, Dor Y. Pancreatic β-cells express the fetal islet hormone gastrin in rodent and human diabetes. Diabetes 66: 426–436, 2017. doi: 10.2337/db16-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Miranda MA, Carson C, St. Pierre CL, Macias‐Velasco JF, Hughes JW, Kunzmann M, Schmidt H, Wayhart JP, Lawson HA. Spontaneous restoration of functional β‐cell mass in obese SM/J mice. Physiol Rep 8, e14573, 2020. doi: 10.14814/phy2.14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with β-cell failure and diabetes. Endocr Rev 29: 317–333, 2008. [Erratum in Endocr Rev. 29: 631, 2008]. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Liu M, Haataja L, Wright J, Wickramasinghe NP, Hua QX, Phillips NF, Barbetti F, Weiss MA, Arvan P. Correction: Mutant INS-gene induced diabetes of youth: proinsulin cysteine residues impose dominant-negative inhibition on wild-type proinsulin transport. PLoS One 5, e13333, 2010. [Erratum in PLoS One 5: 10.1317, 2010].doi: 10.1371/journal.pone.0013333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Liu M, Li Y, Cavener D, Arvan P. Proinsulin disulfide maturation and misfolding in the endoplasmic reticulum. J Biol Chem 280: 13209–13212, 2005. doi: 10.1074/jbc.C400475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang J, Chen Y, Yuan Q, Tang W, Zhang X, Osei K. Control of precursor maturation and disposal is an early regulative mechanism in the normal insulin production of pancreatic β-cells. PLoS One 6: e19446, 2011. doi: 10.1371/journal.pone.0019446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol Metab 22: 266–274, 2011. doi: 10.1016/j.tem.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hasnain SZ, Prins JB, McGuckin MA. Oxidative and endoplasmic reticulum stress in β-cell dysfunction in diabetes. J Mol Endocrinol 56: R33–R54, 2016. doi: 10.1530/JME-15-0232. [DOI] [PubMed] [Google Scholar]
- 253.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 20: 463–466, 1996. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 254.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 46: 1733–1742, 1997. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 255.Szabat M, Pourghaderi P, Soukhatcheva G, Verchere CB, Warnock GL, Piret JM, Johnson JD. Kinetics and genomic profiling of adult human and mouse β-cell maturation. Islets 3: 175–187, 2011. doi: 10.4161/isl.3.4.15881. [DOI] [PubMed] [Google Scholar]
- 256.Chen C, Shiota C, Agostinelli G, Ridley D, Jiang Y, Ma J, Prasadan K, Xiao X, Gittes GK. Evidence of a developmental origin for β-cell heterogeneity using a dual lineage-tracing technology. Development 146: dev164913, 2019. doi: 10.1242/dev.164913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Qadir MMF, Álvarez-Cubela S, Weitz J, Panzer JK, Klein D, Moreno-Hernández Y, Cechin S, Tamayo A, Almaça J, Hiller H, Beery M, Kusmartseva I, Atkinson M, Speier S, Ricordi C, Pugliese A, Caicedo A, Fraker CA, Pastori RL, Domínguez-Bendala J. Long-term culture of human pancreatic slices as a model to study real-time islet regeneration. Nat Commun 11: 3265, 2020. [Erratum in Nat Commun 11: 3742, 2020]. doi: 10.1038/s41467-020-17040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Salg GA, Giese NA, Schenk M, Hüttner FJ, Felix K, Probst P, Diener MK, Hackert T, Kenngott HG. The emerging field of pancreatic tissue engineering: a systematic review and evidence map of scaffold materials and scaffolding techniques for insulin-secreting cells. J Tissue Eng 10: 204173141988470, 2019. doi: 10.1177/2041731419884708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lohoff T, Ghazanfar S, Missarova A, Koulena N, Pierson N, Griffiths JA, Bardot ES, Eng C-HL, Tyser RV, Argelaguet R, Guibentif C, Srinivas S, Briscoe J, Simons BD, Hadjantonakis A-K, Göttgens B, Reik W, Nichols JC, Ai L, Marioni JC. Highly multiplexed spatially resolved gene expression profiling of mouse organogenesis. bioRxiv 11.20.391896, 2020. 10.1101/2020.11.20.391896. [DOI] [Google Scholar]


