Keywords: FOXO1, high-fat diet, MOTS-c, muscle atrophy, myostatin
Abstract
Obesity and type 2 diabetes are metabolic diseases, often associated with sarcopenia and muscle dysfunction. MOTS-c, a mitochondrial-derived peptide, acts as a systemic hormone and has been implicated in metabolic homeostasis. Although MOTS-c improves insulin sensitivity in skeletal muscle, whether MOTS-c impacts muscle atrophy is not known. Myostatin is a negative regulator of skeletal muscle mass and also one of the possible mediators of insulin resistance-induced skeletal muscle wasting. Interestingly, we found that plasma MOTS-c levels are inversely correlated with myostatin levels in human subjects. We further demonstrated that MOTS-c prevents palmitic acid-induced atrophy in differentiated C2C12 myotubes, whereas MOTS-c administration decreased myostatin levels in plasma in diet-induced obese mice. By elevating AKT phosphorylation, MOTS-c inhibits the activity of an upstream transcription factor for myostatin and other muscle wasting genes, FOXO1. MOTS-c increases mTORC2 and inhibits PTEN activity, which modulates AKT phosphorylation. Further upstream, MOTS-c increases CK2 activity, which leads to PTEN inhibition. These results suggest that through inhibition of myostatin, MOTS-c could be a potential therapy for insulin resistance-induced skeletal muscle atrophy as well as other muscle wasting phenotypes including sarcopenia.
NEW & NOTEWORTHY MOTS-c, a mitochondrial-derived peptide reduces high-fat-diet-induced muscle atrophy signaling by reducing myostatin expression. The CK2-PTEN-mTORC2-AKT-FOXO1 pathways play key roles in MOTS-c action on myostatin expression.
INTRODUCTION
Obesity and type 2 diabetes (T2D) are global pandemics, and high-fat diet (HFD)-induced insulin resistance has been recognized as a leading cause of obesity and T2D. In addition to the associated metabolic disorders, recent studies have suggested that insulin resistance accelerates the loss of skeletal muscle mass and strength (1, 2). Increased plasma free fatty acids are associated with insulin resistance in humans (3). Plasma free fatty acid concentrations are increased in obese younger female individuals (35–40 yr old) as well as in young (9-wk old) HFD-induced insulin-resistant mice (4–6). In such conditions, free fatty acid metabolism is altered; circulating free fatty acid results in accumulation of free fatty acid in the liver, pancreatic β cells, and skeletal muscles (6). The accumulation of free fatty acid interferes with normal cellular function of those cells. In skeletal muscle, free fatty acid overload induces insulin resistance, inflammation, and apoptosis (7, 8). This type of lipotoxicity in skeletal muscle induces muscle atrophy (8). For example, the exposure of differentiated skeletal myotubes to saturated palmitic acid (PA) leads to lipotoxicity-mediated myofiber loss (9, 10).
Myostatin, a transforming growth factor β (TGFβ) family member, is a negative regulator of skeletal muscle growth and development (11–13). Myostatin acts as an auto/paracrine inhibitor of muscle growth that binds to the activin A receptor type IIB, which couple to the type 1 receptors ALK4 and ALK5, in skeletal and cardiac muscle (14). Loss-of-function mutations in myostatin in mice, sheep, cattle, dogs, and humans result in extreme skeletal muscle hypertrophy, reflecting the loss of the inhibitory action of myostatin on muscle growth (15). Transgenic mice expressing high levels of follistatin, a natural circulating glycoprotein inhibitor of myostatin, exhibited a significant increase in muscle mass (13). Various studies strongly suggest that inhibiting the myostatin pathway is a potentially useful approach to block muscle wasting. Currently, monoclonal antibodies targeting myostatin-receptor and myostatin inhibitors are under clinical trials for treatment of sarcopenia and cancer-associated cachexia (16, 17).
Myostatin has been suggested as one of the possible mediators of insulin resistance-induced skeletal muscle wasting (18, 19). Myostatin accumulation is associated with insulin resistance and myostatin levels are elevated in obese individuals (20). Skeletal muscle cells produce and secrete myostatin and HFD upregulates the expression of myostatin mRNA levels in skeletal muscle (21). Myostatin mRNA expression is also elevated in palmitic acid-treated C2C12 myotubes (10). In addition, myostatin acts to modulate metabolic phenotypes such as insulin sensitivity and fat mass. The expression of myostatin protein in skeletal muscle is positively correlated with homeostatic model assessment for insulin resistance (HOMA-IR) and body mass index (BMI) (22). Mice lacking myostatin display reduced fat mass and improved insulin sensitivity (23). Thus, improving insulin resistance and lowering circulating and skeletal muscle myostatin expression is a potential therapy for HFD-induced skeletal muscle wasting (24).
Mitochondrial open reading frame of the 12S ribosomal RNA type-c (MOTS-c), a 16-amino acid peptide, is encoded in the 12S rRNA region of the mitochondria and well conserved between mammalian species (25). MOTS-c is found in different tissues, the skeletal muscle and fat tissues being its main organ targets, as well as in circulation, acting as a systemic hormone (25). MOTS-c plays an important role in metabolic homeostasis (25, 26). Plasma MOTS-c levels are negatively correlated with obesity and metabolic markers related to insulin resistance (27, 28). MOTS-c administration improves both the high-fat diet- and aging-induced insulin resistance (25). MOTS-c increases the glucose uptake in skeletal muscles and improves insulin signaling pathway such as PI3K/AKT signaling in HFD-fed and aged mice (25). MOTS-c is also connected with age-related and health-span processes, with its levels declining with age, both in skeletal tissue and in circulation (25). MOTS-c peptide levels increase in cellular senescent cells and impact the senescence-associated secretory phenotype production (29).
The transcription factor forkhead box protein O1 (FOXO1) directly binds to the myostatin promoter and upregulates myostatin mRNA expression in skeletal muscles (30). FOXO1 expression levels are tightly regulated by post-translational modification, subcellular localization, and ubiquitin-proteasome systems (31). FOXO1 binds to the promoter of target genes such as myostatin and atrogin-1 and increases transcription in the nucleus (30, 32). FOXO1 activation in the nucleus leads to muscle atrophy as a result of the upregulation of these muscle wasting genes. However, once FOXO1 becomes phosphorylated, it is excluded from the nucleus. The phosphorylated FOXO1 in the cytosol is further ubiquitinated and degraded by the proteasome. Thus, this phosphorylation plays key roles in the function and the levels of FOXO1. In skeletal muscle, the insulin signaling increases FOXO1 phosphorylation and inhibits the muscle wasting related genes (33). Insulin binds to the receptor, activates PDK1 which subsequently leads to the phosphorylation of AKT at Thr308. Additional phosphorylation at Ser473 fully activates AKT, and the activated AKT phosphorylates FOXO1. The insulin-PI3K-AKT-FOXO1 pathways can be less active during insulin-resistant conditions, which can partially explain the loss of skeletal muscle function and mass in HFD-fed mice (33).
Phosphatase and tensin homolog (PTEN) is a dual-specificity protein phosphatase that works on both proteins and lipids. PTEN catalyzes the conversion of phosphatidylinositol 3,4,5-trisphosphate (PIP3) into phosphatidylinositol 4,5-bisphosphate (PIP2). Since PIP3 allows PDK1 to phosphorylate AKT on Thr308, PTEN acts as a negative regulator of the PI3K/AKT signaling pathways. Moreover, PTEN dephosphorylates Ser473 of AKT and modulates AKT activation particularly in cells and tumors of skeletal muscle origin. For instance, the expression levels of PTEN is related to AKT phosphorylation on Ser 473, but not to Thr308 phosphorylation in C2C12 mouse myoblast cell line and seven IGF-II-overexpressing rhabdomyosarcomas cell lines (34). The phosphorylation of the cluster of Ser 380, Thr382, Thr283, and Ser385 at the C-terminal of PTEN is critical for its stability and activity (35). The phosphorylated C-terminus of PTEN favors a PTEN “closed” conformation and prevents the membrane association of the PTEN, thus the phosphorylated PTEN is less active (35, 36). Moreover, the phosphorylation of the C-terminus of PTEN leads to a “closed” conformation that is less prone to degradation, thus the less active form, phosphorylated PTEN, increases protein stability (35). The cluster of Ser 380, Thr382, Thr283, and Ser385 at the C-terminal of PTEN is phosphorylated by Casein kinase 2 (CK2) (37).
Here, we investigated the connection among MOTS-c, myostatin, and the potential beneficial effects of MOTS-c treatment on skeletal muscle wasting. We showed MOTS-c partly prevents HFD-induced muscle atrophy by decreasing myostatin expression in skeletal muscles and the circulation in HFD-fed mice. Specifically, we demonstrated that MOTS-c modulates the CK2-PTEN-AKT-FOXO1 pathway to inhibit myostatin expression and muscle wasting.
MATERIALS AND METHODS
Animals
Male CD-1 mice at 8 wk of age (n = 26) were obtained and assigned to one of the three experimental groups: a normal diet control group (ND), a high-fat diet (HFD, 60 kcal % fat) control group (sterilized water for 3 wk), and a HFD MOTS-c-treated group [0.5 mg/kg/day for 3 wk, intraperitoneal (ip) injection]. After a 3-wk experimental period, tissues of the liver, fat, soleus, gastrocnemius, and plantaris were collected from the mice. Next, the samples were weighed, flash-frozen in liquid nitrogen, and then stored at −80°C.
Male C57BL/6 mice at 10 wk of age (n = 24) were obtained from the Jackson Laboratory (Bar Harbor, ME) and fed a HFD (60 kcal % Fat, Research Diets, Cat No. D12492). Mice were randomly assigned to one of the experimental groups: a control group receiving daily intraperitoneal injections of vehicle (sterilized water) or a MOTS-c-treated group receiving daily ip injections (5 mg/kg/day for 8 wk). The mice were euthanized after 8 wk of treatment. Mice were under deep general anesthesia with isoflorane followed by euthanasia. The thigh muscle tissue was collected from the mice and then flash frozen and stored at −80°C. The whole blood was collected in EDTA-treated tubes, and the cells were removed by centrifugation for 10 min at 2,000 g at 4°C. The resulting supernatant was designated as the plasma. All animal procedures were carried out according to the University of Southern California and Juntendo University’s Animal Care and Use Committee and Animal Research.
Cell Culture
C2C12 mouse myoblasts were purchased from ATCC (Cat No. CRL-1772, Manassas, VA) and cultured in DMEM + 10% FBS (Thermo Fisher Scientific, Waltham, MA) at 37°C in 5% CO2. To differentiate C2C12 into myotubes, the media were replaced with DMEM + 2% horse serum every 48 h for 6 days until the cells were fully differentiated. To investigate the effect of MOTS-c on palmitic acid-induced myotube atrophy, the differentiated myotubes were incubated with or without 0.5 mM palmitic acid (PA; Sigma) and/or 50 μM MOTS-c (Genscript) for 48 h.
Immunofluorescence and Myotubes Diameter Measurements
For the immunofluorescent experiment, differentiated C2C12 myotubes were fixed in 4% paraformaldehyde for 15 min at room temperature. Cells were permeabilized in a 0.1% Triton X-100 in phosphate-buffered saline (PBS) and then blocked in a 0.2% bovine serum albumin in PBS for 1 h at room temperature. Myotubes were then stained with anti-myosin heavy chain (Sigma-Aldrich, Cat No. M1570) overnight at 4°C incubated with Alexa-488-conjugated goat anti-mouse secondary antibody (Thermo Fisher Scientific, Cat No. A-11001) for 1 h, and counter stained with Hoechst 33258 (2 mg/mL; Thermo Fisher Scientific). Images were acquired with a Keyence microscope (Keyence Corporation of America, Itasca, IL). A muscle cell that contained three or more nuclei was considered to be a myotube. For the myotube diameter analysis, six diameters were measured for each myotube and then averaged. Next, 10 myotubes were randomly selected in each image, and since four images were taken in each well, this step was repeated four times. If 10 myotubes were not present in the PA treatment group, all the remaining myotubes were measured.
Myostatin Measurement
Mouse circulating myostatin was measured using a commercial ELISA kit (Cat No.: DY788-05, R&D Systems) according to the manufacturer’s instruction. Before the ELISA procedure, the mouse plasma samples were activated by adding 1 N HCl, which was neutralized by adding 1.2 N NaOH/0.5 M HEPES and then diluted with the appropriate volume of the reagent diluent. Following activation, prepared standards (0, 31.3, 62.5, 125, 250, 500, 1,000, 2,000 pg/mL) and samples were added to a plate coated with rat anti-Myostatin capture antibody and incubated for 2 h on a shaker. Biotinylated anti-Myostatin detection antibody was added to each well after the plate was washed and then incubated for 2 h on a shaker. Streptavidin-horseradish peroxidase (HRP) conjugate was then added to the wells. Afterward, the plate was washed and further incubated for 30 min at room temperature. After the final wash, 100 µL of o-phenylenediamine dihydrochloride (OPD) solution (1 mg/mL in hydrogen peroxide substrate) was added to each well and incubated for 10–20 min. The reaction was stopped by the addition of 2 N H2SO4 and absorbance was measured on a plate spectrophotometer (Molecular Designs, Sunnyvale, CA) at 490 nm.
RNA Extraction and qRT-PCR
Total RNA was extracted from the mice skeletal muscle using the RNeasy Fibrous Tissue Kit (Qiagen, Redwood city, CA). The protocol was performed according to the manufacturer’s instructions and RNA was measured at 260 nm with the Nanodrop system (Thermo Fisher Scientific, Wilmington, DE). RNA purity and quality were evaluated by 260/280 and 260/230 ratios. The quantified RNA was then used as a template during reverse transcription to synthesize cDNA using the iScript cDNA Synthesis Kit (Biorad, Hercules, CA). Through a mixture of SsoAdvanced Universal SYBR Green Supermix (Biorad), myostatin, and B2M primers, quantitative polymerase chain reaction (qPCR) was performed to amplify the cDNA and determine if the expression of myostatin and B2M had been significantly altered by MOTS-c. B2M was used as a reference gene. The primers used for amplification are Myostatin Forward primer (5′- AGTGGATCTAAATGAGGGCAGT-3′) and Reverse primer (5′- GTTTCCAGGCGCAGCTTAC-3′); Atrogin-1 forward primer (5' - TCTCCAGACTCTCTACACATCC-3′) and reverse primer (5′- GAATGGTCTCCATCCGATACAC-3′); B2M forward primer (5' - CATGGCTCGCTCGGTGAC-3′), and reverse primer (5′- CAGTTCAGTATGTTCGGCTTCC-3′).
Western Blots
Skeletal muscle tissue was lysed with radioimmunoprecipitation assay buffer (RIPA) buffer containing 20 mM Tris-HCl, pH 7.8, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 1 mM dithiothreitol, 1 mM EDTA, 1% Triton X-100, 10% glycerol, and the Halt protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific). The mixture was then homogenized, followed by sonication. The supernatant was collected by centrifugation at 15,000 g for 15 min at 4°C. Protein content in the lysates was quantified using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Predetermined amounts of proteins (30 μg) were separated on 8%–16% or 4%–20% SDS-PAGE gels and blotted onto PVDF membranes (Biorad, Hercules, CA). Membranes were blocked with 5% bovine serum albumin Tris-buffered saline with 0.1% Tween 20 and incubated with diluted primary antibodies for phospho-Akt (Ser473, 1:1,000), Akt (1:1,000), phospho-FOXO1 (Ser256, 1:1,000), FOXO1 (1:1,000), phospho-PTEN (Ser380, 1:1,000), phospho-PTEN (Ser380/Thr382/383, 1:1,000), PTEN (1:1,000), Sin1 (1:1,000), phospho-mammalian target of rapamycin (mTOR) (Ser2448, 1:1,000), total mTOR (1:1,000), phospho-CK2 substrate [(pS/pT)DXE] (1:1,000) (all Cell Signaling Technology), GAPDH (1:2,000), and α-tubulin (1:1,000) (abcam) at 4°C overnight. After several washes with Tris-buffered saline containing 0.1% Tween-20, the membranes were incubated at room temperature for 1 h with the appropriate HRP-conjugated secondary antibody. Clarity Western ECL substrate (Biorad) was used for detecting specific bands. Membranes were imaged on a Bio-Rad ChemiDoc XRS+ imager and relative intensities of the bands were quantified using Image Lab software (Biorad).
Human Study
Participants were recruited through local newspaper advertisements and personal contacts around Tsukuba, a city located in Japan. A total of 105 adult Japanese men (age range from 24 to 82 yr) without a medical history of type 2 diabetes, myocardial infarction, or hypertension were analyzed. Body weight was measured to the nearest 0.1 kg with a digital scale. Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer. Body mass index (BMI) was calculated by dividing the participant’s weight by his/her height. Blood samples were taken in the morning after a 12-h overnight fast and stored at –80°C until the assay was performed. Serum concentrations of triglyceride, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and plasma concentration of glucose and HbA1c were determined using standard enzymatic techniques (LSI medience, Ibaraki, Japan). Plasma MOTS-c levels were measured by in-house ELISA as shown in previous studies (25). This study was approved by the ethics committee of the Faculty of Health and Sport Sciences at the University of Tsukuba. The study conformed to the principles outlined in the Declaration of Helsinki, and all participants were asked to provide written informed consent before inclusion in the study.
Statistical Analysis
The data are presented as the means ± SE. Significant differences were determined by unpaired Student’s t tests and one-way ANOVA, followed by Tukey’s post hoc test. Correlations between each value were determined using Spearman’s correlation coefficients. Independent correlates of plasma myostatin levels were examined by using a multivariate linear regression analysis (independent variables: age, body mass, and plasma MOTS-c levels). Statistical analyses were performed by using the GraphPad Prism 8 software or the JMP Pro version 12. Values of *<0.05, **<0.01, ***<0.001 were considered to be statistically significant.
RESULTS
Plasma MOTS-c Levels Are Inversely Correlated with Plasma Myostatin Levels in Human
MOTS-c is released in the circulatory system (25). The plasma levels of MOTS-c have been shown to be correlated with markers of insulin resistance and obesity (27). For example, plasma MOTS-c levels were reduced in obese male children (28). MOTS-c levels were inversely correlated with BMI, waist circumference, waist-to-hip ratio, HOMA-IR, and HbA1c (28). Circulating plasma myostatin were highly associated with the body mass and body composition (38). We initially analyzed the correlation between plasma MOTS-c and plasma myostatin levels in a healthy Japanese cohort (Supplemental Table S1; all Supplementary material is available at https://doi.org/10.6084/m9.figshare.13513596.v1). Plasma myostatin levels were inversely correlated to plasma MOTS-c levels (r = −0.25, P < 0.05) (Fig. 1). On the other hand, plasma myostatin levels were positively correlated to body mass (r = 0.20, P < 0.05) and tended to be correlated to BMI (r = 0.19, P = 0.054). The association between plasma MOTS-c and myostatin levels were still significant after considering confounders such as age and body mass (β = −0.23, P < 0.05).
Figure 1.
Plasma mitochondrial open reading frame of the 12S ribosomal RNA type-c (MOTS-c) levels are inversely correlated with plasma myostatin levels in human. Correlations between plasma myostatin levels and plasma MOTS-c levels in Japanese male subjects without a medical history of type 2 diabetes, myocardial infarction, or hypertension. The Spearman’s correlation coefficients were applied for the statistical analyses.
MOTS-c Prevents Palmitic Acid-Induced Atrophy in Differentiated C2C12 Myotubes
To understand the potential causal relationship between MOTS-c and myostatin and muscle atrophy, we used in vitro C2C12 myotubes. Increased accumulation of intramuscular lipids can lead to insulin resistance, dyslipidemia, and lipotoxicity (8, 39). Palmitate is the most abundant saturated fatty acid in the circulation, and therefore, it has been used frequently to examine the effects of saturated fatty acids in dyslipidemia on various tissues (40). Palmitate treatment in multiple cell types in vitro has resulted in apoptosis and has been found to inhibit AKT activity in response to insulin (41). For example, the exposure of differentiated C2C12 myotubes to saturated palmitic acid leads to lipotoxicity-mediated myofiber loss (9, 10). Since HFD is rich in saturated fatty acids such as palmitic acid, we examined whether MOTS-c could prevent palmitic acid-induced myofiber loss in differentiated C2C12 myotubes. We treated C2C12 myotubes with 0.5 mM palmitic acid in the absence or presence of 50 µM MOTS-c for 48 h. Immunostaining of the myosin heavy chain demonstrated that treatment of palmitate led to a decrease in the number and diameter of myotubes (Fig. 2, A–C). Cotreatment of MOTS-c prevents the loss of myotubes and also increased their diameter (Fig. 2, A–C). Moreover, the distribution of cell diameter was nearly identical in palmitic acid and MOTS-c-treated myotube as control myotubes (Fig. 2D). These results suggest that MOTS-c prevents palmitic acid-induced myotube atrophy as well as myotube loss.
Figure 2.
Mitochondrial open reading frame of the 12S ribosomal RNA type-c (MOTS-c) prevents palmitic acid-induced atrophy in differentiated C2C12 myotubes. Differentiated C2C12 myotubes were treated with 0.5 mM palmitic acid for 48 h in the absence or presence of 50 µM MOTS-c. A: representative images of myosin (green) and Hoechst 33258 (blue; nucleus) immunostaining in C2C12 myotubes. Scale bar, 100 µm. B: Myotube number per field was quantified. Myotube diameter (n = 240 myotubes) is depicted as mean diameter (C) and a histogram of diameter distribution (D). Significant differences were determined by one-way ANOVA followed by Tukey’s post hoc test. ***P < 0.001. PA, palmitic acid.
MOTS-c Decreases Myostatin Levels in Plasma and Skeletal Muscle from Diet-Induced Obese Mice
Myostatin is a key mediator of insulin resistance-induced skeletal muscle wasting. Myostatin levels are elevated in diet-induced mice and increased in obese individuals (22, 42). MOTS-c has been shown to be an exercise mimetic in high-fat-diet (HFD)-fed mice, improving insulin sensitivity and preventing weight gain (25). Facing the relationship between MOTS-c and myostatin levels (Fig. 1) and our in vitro data (Fig. 2), we next hypothesized that MOTS-c inhibits myostatin expression due to its insulin-sensitizing effects. We examined the circulating myostatin levels in HFD-fed mice following MOTS-c administration. C57/B6 mice fed a HFD were daily administered with MOTS-c (5 mg/kg/bodyweight) for 8 wk. The plasma myostatin levels were 40% lower in MOTS-c-treated mice compared with control mice (Fig. 3A). Myostatin is mainly expressed in skeletal muscle, but can also be found, to a lesser extent, in cardiac muscle. To identify which type of tissues are responsible for the reduction of plasma myostatin, we examined mRNA levels of myostatin in skeletal muscle and cardiac muscle. MOTS-c treatment decreased the levels of myostatin mRNA in skeletal muscle, but not in the heart (Fig. 3B and data not shown). These results suggest that MOTS-c probably lowers myostatin levels by inhibiting the production of myostatin in skeletal muscle. Although skeletal and cardiac muscles share several characteristics and aspects, both react differently in response to myostatin. Several recent papers have demonstrated that protein metabolism after myostatin treatment differs between muscle fibers/C2C12 myotubes and cardiomyocytes (43). Moreover, activin receptor type IIB blocking pretreatment in doxorubicin-treated mice had a more beneficial effect on skeletal muscle atrophy than it did for cardiac wasting (44).
Figure 3.
Mitochondrial open reading frame of the 12S ribosomal RNA type-c (MOTS-c) decreases myostatin levels in high-fat-diet-fed mice. C57BL/6J mice fed a high-fat diet were injected with MOTS-c peptide (5 mg/kg; ip) for 8 wk. Plasma myostatin levels (A) were measured by ELISA, whereas the myostatin mRNA expression (B) in skeletal muscle was measured by qRT-PCR. Data are reported as means ± SE of 7 or 12 mice per group. Significant differences were determined by Student’s t tests. *P < 0.05 and **P < 0.01.
MOTS-c Inhibits FOXO1 Transcription Activity in Skeletal Muscle from Diet-Induced Obese Mice
Next, we examine the signaling pathways that MOTS-c modulates to inhibit the myostatin expression in skeletal muscle taken from C57BL/6J mice fed with a HFD. We previously showed that MOTS-c improves insulin signaling pathways in HFD-fed mice and aged mice. MOTS-c increases AKT phosphorylation in vitro and in vivo. However, the effect of MOTS-c on FOXO1 expression has not yet been elucidated. We hypothesized that the lower expression of myostatin resulting from MOTS-c treatment in skeletal muscle is due to FOXO1 phosphorylation. We examined the FOXO1 phosphorylation at Ser256 and Thr24 residues, which are target sites of the AKT pathway. The phosphorylation of FOXO1 at both residues was significantly elevated by MOTS-c (Fig. 4, A–C). Total FOXO1 levels were lower in MOTS-c-treated mice compared with control mice, suggesting that the phosphorylated FOXO1 translocates into the cytosol and is degraded (Fig. 4D). FOXO1 in the nucleus increases not only myostatin but also Atrogin-1 expression. Atrogin-1 mRNA expression was also lower in MOTS-c-treated mice compared with control mice (Supplemental Fig. S1). We thus concluded that exclusion of FOXO1 from the nucleus due to its phosphorylation leads to decreased myostatin gene expression in skeletal muscle and muscle atrophy in HFD-fed mice.
Figure 4.
Mitochondrial open reading frame of the 12S ribosomal RNA type-c (MOTS-c) increases forkhead box protein O1 (FOXO1) phosphorylation in skeletal muscles taken from C57BL/6J mice fed a high-fat diet. A: total and phospho-FOXO1 levels (representative Western blots); GAPDH was used as a loading control. B–D: quantification of total and phospho-FOXO1 in Western blots. Data are reported as means ± SE of 12 mice per group. Significant differences were determined by Student’s t tests. *P < 0.05 and ***P < 0.001.
MOTS-c Increases AKT Phosphorylation and mTOR Complex 2 Activity
AKT phosphorylates FOXO1. Phosphorylation at Ser 473, along with Thr 308 of AKT’s activation loop, is necessary for AKT function. MOTS-c elevated the phosphorylation of AKT at Ser 473, although the phosphorylation of AKT at Thr308 was not altered (Figs. 5A and 6B). The phosphorylation of AKT at Ser 473 and Thr308 is mainly mediated by mTOR complex 2 (mTORC2) and phosphoinositide-dependent kinase 1 (PDK1), respectively. Since the phosphorylation of AKT at Ser 473 is upregulated by MOTS-c, we examined the mTORC2 activity in MOTS-c-treated mice. Mammalian target of rapamycin (mTOR) plays important roles in cell growth, proliferation, apoptosis, metabolism, and PI3K/PTEN/AKT signaling pathways via the raptor-mTOR (mTORC1) and rictor-mTOR (mTORC2). mTORC2 consists of mTOR, rictor, protor, GβL, and SIN1. SIN1 is a component of mTORC2 but not mTORC1, and SIN1 is an essential mTORC2 subunit because the mTORC2 is activated when SIN1 is bound to mTOR (45). SIN1 knockout diminishes AKT-Ser473 phosphorylation, and disrupts rictor-mTOR interaction but maintains Thr308 phosphorylation (45). The expression levels of SIN1 are correlated with AKT phosphorylation at Ser473 (46). We found that SIN1 levels were significantly elevated in MOTS-c-treated mice compared with control mice (Fig. 5, C and D). The higher AKT phosphorylation might be attributed to SIN1 overexpression that regulates mTORC2 enzymatic activity.
Figure 5.
Mitochondrial open reading frame of the 12S ribosomal RNA type-c (MOTS-c) activates protein kinase B (AKT) via mammalian target of rapamycin (mTOR) complex 2 in skeletal muscle samples from C57BL/6J mice fed a high-fat diet. A: representative blots of total and phospho-AKT levels. Glyceraldhyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. B: quantification of phospho-AKT (Ser 473) in Western blots. C and D: the expression levels of a rate-limiting protein of the mTOR complex 2, SIN 1 were examined. C: representative blots of SIN1. α-Tubulin was used as a loading control. D: quantification of SIN 1 in Western blots. Data are reported as means ± SE of 12 mice per group. Significant differences were determined by Student’s t tests. **P < 0.01, ***P < 0.001.
Figure 6.
Mitochondrial open reading frame of the 12S ribosomal RNA type-c (MOTS-c) increases casein kinase 2 (CK2) activity and its targets phosphatase and tensin homolog (PTEN) phosphorylation in skeletal muscle samples from C57BL/6J mice fed a high-fat diet. A: total and phospho-PTEN (Ser380/Thr382/383) levels (representative Western blots); α-tubulin was used as a loading control. B and C: quantification of phospho- and total-PTEN in Western blots. D: phosphorylated-CK2 substrate levels (representative Western blots); α-tubulin was used as a loading control. E: quantification of phospho-CK2 substrate in Western blots. Data are reported as means ± SE of 12 mice per group. Significant differences were determined by Student’s t tests. *P < 0.05, **P < 0.01, ***P < 0.001.
MOTS-c Reduces Phosphatase and Tensin Homolog (PTEN) Activity in Skeletal Muscle from Diet-Induced Obese Mice
PTEN dephosphorylates Ser473 of AKT and modulates AKT activation. We thus hypothesized that MOTS-c may inhibit the action of PTEN, and therefore the phosphorylation of AKT at Ser 473 increased. We focus on the phosphorylation of the cluster of Ser 380, Thr382, Thr283, and Ser385 at the C-terminal of PTEN, which is critical for its stability and activity (35). MOTS-c increased the phosphorylation of the c-terminal cluster of PTEN (Fig. 6, A and B). As it has been shown in previous studies, the phosphorylation at the c-terminal cluster increases the PTEN protein stability, the total level of PTEN were elevated in MOTS-c-treated mice compared with control mice (Fig. 6, A and C). Taken together, MOTS-c increases the phosphorylation in the cluster of Ser 380, Thr382, Thr283, and Ser385 at the C-terminal of PTEN, which stabilizes PTEN, resulting in increased protein abundance but suppressed PTEN phosphatase activity. Next, we examined the CK2 kinase activity by investigating the phosphorylation levels of CK2 substrates by Western blot (Fig. 6D). In MOTS-c-treated mice, the total levels of phosphorylated CK2 substrate were elevated compared with control mice, suggesting CK2 kinase activity is higher in MOTS-c treated mice (Fig. 6, D and E). These results suggest that MOTS-c increases CK2 activity and counteracts the PTEN action on AKT via phosphorylation of the c-terminal cluster and thus increases AKT phosphorylation.
MOTS-c Prevents Muscle Atrophy in Short-Term Diet-Induced Obese Mice
So far, we showed that MOTS-c reduces muscle atrophy signaling in HFD-fed mice for 8 wk, in the same rodent model in which MOTS-c was able to maintain metabolic homeostasis and increase insulin sensitivity (25). Unfortunately, we were not able to examine whether MOTS-c prevented HFD-induced muscle atrophy in this mouse model because we originally did not collect the muscle weight data from the animals. Therefore, we instead looked at results from a previously performed study in which we administered MOTS-c to CD-1 mice fed with HFD for a shorter period, namely 3 wk. Similar to C57BL/6 mice, MOTS-c increased insulin sensitivity in CD-1 mice fed with a HFD, again showing MOTS-c has insulin sensitizing effects in both inbred C57BL/6 and outbred CD-1 mouse. We administered MOTS-c (0.5 mg/kg/day, ip injection) in HFD (60 kcal% fat)-fed mice for 3 wk and examined gastrocnemius, soleus, and plantaris muscle mass. HFD feeding of CD-1 mice for 3 wk resulted in increased body weight and abdominal fat mass compared with control diet-fed mice (Fig. 7, A and B). Muscle mass was also adjusted for body weight. Although no differences were found between any of the three treatment groups when considering total, gastrocnemius, soleus, and plantaris absolute muscle mass, differences were more visible when performing an adjustment calculation with body weight. Total and gastrocnemius muscle mass was significantly decreased in HFD-fed mice, with, MOTS-c administration preventing the decrease in those parameters in HFD-fed mice (Fig. 7C and Supplemental Fig. S2). Due to the short-term of the HFD-feeding, the major differences were, as described, in skeletal muscle index, a useful and important parameter used to access muscle atrophy but not in absolute muscle mass changes. In addition, our data show that the skeletal muscle index change might be derived mainly by gastrocnemius but not by soleus and plantaris muscle.
Figure 7.
Mitochondrial open reading frame of the 12S ribosomal RNA type-c (MOTS-c) prevents muscle atrophy in mice fed a high-fat diet. CD-1 mice fed either normal diet (ND) or high-fat diet (HFD) were injected with the MOTS-c peptide (0.5 mg/kg/day ip) for 3 wk. Body weight (A), abdominal fat (B), and total mass (mg) (C) of gastrocnemius, soleus, and plantaris muscle per bodyweight (mg/g) were measured. D: myostatin mRNA expression in skeletal muscle was measured by quantitative reverse transcription-polymerase chain reaction (qRT-PCR). E: the correlation between myostatin mRNA expression and muscle mass/body weight (BW). The data are reported as means ± SE of mice per group. Normal diet group, n = 6; high-fat-diet group, n = 10. Significant differences were determined by one-way ANOVA followed by Tukey’s post hoc test. *P < 0.05 and **P < 0.01. The Spearman’s correlation coefficients were applied for the statistical analyses of E.
Myostatin is a key regulator of muscle wasting. HFD feeding increases the myostatin mRNA expression in skeletal muscle, whereas MOTS-c inhibits the myostatin gene expression during HFD feeding (Fig. 7D). Correlation analysis of myostatin mRNA expression and muscle mass showed that myostatin gene expression is inversely correlated with muscle mass in mice (Fig. 7E). We did not see a significant correlation between myostatin gene expression and body weight or fat mass (Supplemental Fig. S3, A and B). These results suggest that myostatin is associated with muscle atrophy in HFD-fed mice.
DISCUSSION
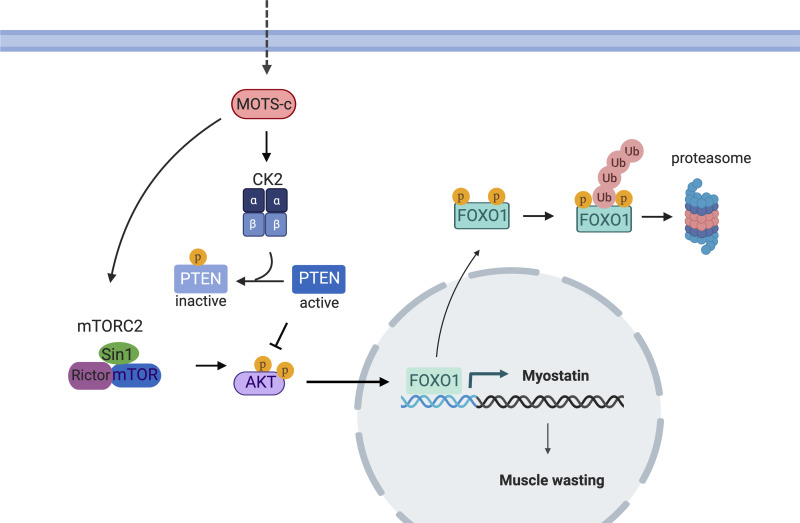
In the present study, we showed MOTS-c, a mitochondrial-derived peptide has the potential to treat muscle wasting conditions as a novel myostatin inhibitor. MOTS-c treatment leads to a decrease in the systemic and tissue specific levels of myostatin, which can prevent muscle wasting in HFD-fed mice. Moreover, plasma MOTS-c levels were inversely correlated with circulating myostatin levels in humans. AKT-FOXO1 pathways play key roles in muscle wasting gene expression. MOTS-c inhibits FOXO1 transcription activity by increasing AKT activity via increasing mTORC2 activity and inhibiting PTEN action through CK2 (Fig. 8). The molecular analysis revealed a novel, important signaling pathway of MOTS-c. Taken together, this research shows MOTS-c improves not only metabolic function but also muscle mass. We previously proposed that MOTS-c acts as an exercise mimetic hormone (25). The exercise mimetic effect could be derived from MOTS-c’s action as a myostatin inhibitor.
Figure 8.
Schematic diagram how mitochondrial open reading frame of the 12S ribosomal RNA type-c (MOTS-c) leads to a decrease in myostatin expression.
PTEN is an important regulator of the PI3K-AKT pathway. In highly active metabolic tissues, including liver and adipose tissues, PTEN negatively regulates insulin-stimulated glucose uptake and insulin sensitivity. The deletion of PTEN, thus, leads to enhanced insulin sensitivity and glucose homeostasis. In skeletal muscle, the deletion of PTEN not only protects mice from insulin resistance and diabetes but also improves muscle development (47, 48). Muscle-specific PTEN knockout regulates muscle protein degradation in diabetes mouse models through suppressing the ubiquitin-proteasome system and the activation of caspase-3 (49). PTEN also plays a critical role in maintaining satellite cell quiescence and homeostasis (50). Studies showed that PTEN plays critical roles in HFD-induced muscle defects and PTEN deletion blocked the impaired growth of muscle and abnormalities in the regeneration of injured muscle (51). Therefore, the suppression of PTEN activity has been proposed as a therapeutic strategy to improve the repair of damaged muscle in patients with diabetes. Here, we showed that MOTS-c, an anti-diabetic hormone, inhibits PTEN activity by increasing the phosphorylation at the C-terminal cluster. The PTEN inhibition leads to the suppression of myostatin expression in skeletal muscle. We newly identified that PTEN play a role in MOTS-c action on enhanced insulin sensitivity as well as reduced myostatin expression in skeletal muscle. Furthermore, we demonstrated MOTS-c increases CK2 activity, which phosphorylates PTEN. Further studies examining whether MOTS-c directly binds and modulates CK2 activity will provide a better understanding of the direct target molecules of MOTS-c.
Myostatin modulates insulin sensitivity, fat mass, muscle mass, and function (18, 19). Mice lacking myostatin display reduced fat mass and improved insulin sensitivity (23). Previously, MOTS-c showed improved insulin signaling in HFD-fed mice and this current study showed that MOTS-c reduced myostatin expression via AKT-FOXO1 pathways. These results suggest that improving insulin sensitivity can be a therapeutic strategy for HFD-induced skeletal muscle wasting.
Previous studies suggest that inhibiting the myostatin pathway is a useful approach to block muscle wasting. Indeed, monoclonal antibodies targeting the myostatin-receptor and myostatin inhibitors are under clinical trials for treatment of sarcopenia and cancer-associated cachexia (16, 17). HFD-fed mice showed decreased muscle mass and muscle strength due to mitochondria impairment and lipid accumulation in the skeletal muscles (52–54). Although we expect the inhibition of myostatin levels to lead to improvement of muscle function in HFD-fed mice, we did not measure the MOTS-c effect on muscle function directly in this study. Reynolds et al. (55) showed MOTS-c treatment indeed improves muscle strength and exercise capacity in HFD-fed mice and aged mice. In addition, further studies using long-term HFD-fed mice treated with MOTS-c will provide us a better understanding of whether muscle atrophy signaling change by MOTS-c affects muscle mass in various types and during long-time period stimuli. A MOTS-c analog is in phase-1 clinical trials by CohBar Inc. for the treatment of nonalcoholic steatohepatitis (NASH). The insights from studies using muscle wasting mice models can be rapidly translated into clinical trials of MOTS-c and related analogs. MOTS-c analogs could potentially treat conditions such as insulin resistance-induced skeletal muscle atrophy as well as other muscle wasting phenotypes, including sarcopenia.
In summary, our research suggests that MOTS-c acts as a novel physiological myostatin inhibitor. The CK2-PTEN-mTORC2-AKT-FOXO1 pathways play key roles in MOTS-c action on myostatin expression. These results suggest that MOTS-c can be a potential therapy for sarcopenia and other muscle wasting phenotypes.
GRANTS
This work was supported by a Glenn/AFAR Postdoctoral Fellowship Program for Translational Research on Aging to S. J. Kim, by R01AG061834, P01AG034906, R56AG062693, an AFAR BIG AWARD Grants to P. Cohen, by the Fundação Luso-Americana para o Desenvolvimento (FLAD) Healthcare 2020 Grant to P. J. Oliveira, and by a PhD fellowship from Portuguese FCT (SFRH/BD/103399/2014) to A. R. Coelho.
DISCLOSURES
Pinchas Cohen is a consultant and stockholder of CohBar Inc. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
H.K. and S-J.K. conceived and designed research; H.K., A.R.C., J.W., H.H.M., K.Y., A.H., H.Z., N.F., and S-J.K. performed experiments; H.K., S.M., and S-J.K. analyzed data; H.K. and S-J.K. interpreted results of experiments; S-J.K. prepared figures; H.K., A.R.C., A.H., and S-J.K. drafted manuscript; H.K., A.R.C., J.W., H.H.M., K.Y., H.Z., N.F., P.J.O., P.C., and S-J.K. edited and revised manuscript; H.K., A.R.C., J.W., H.H.M., K.Y., A.H., H.Z., N.F., S.M., P.J.O., P.C., and S-J.K. approved final version of manuscript.
REFERENCES
- 1.Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA, Newman AB. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes 55: 1813–1818, 2006. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- 2.Park SW, Goodpaster BH, Strotmeyer ES, Kuller LH, Broudeau R, Kammerer C, de Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA, Cho Y-W, Newman AB; Health, Aging, and Body Composition Study. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care 30: 1507–1512, 2007. doi: 10.2337/dc06-2537. [DOI] [PubMed] [Google Scholar]
- 3.Boden G. Effects of free fatty acids (FFA) on glucose metabolism: significance for insulin resistance and type 2 diabetes. Exp Clin Endocrinol Diabetes 111: 121–124, 2003. doi: 10.1055/s-2003-39781. [DOI] [PubMed] [Google Scholar]
- 4.Boden G. Free fatty acids and insulin secretion in humans. Curr Diab Rep 5: 167–170, 2005. doi: 10.1007/s11892-005-0004-5. [DOI] [PubMed] [Google Scholar]
- 5.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes 18: 139–143, 2011. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sobczak AIS, Blindauer CA, Stewart AJ. Changes in plasma free fatty acids associated with type-2 diabetes. Nutrients 11: 2022, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estadella D, da Penha Oller do Nascimento CM, Oyama LM, Ribeiro EB, Dâmaso AR, de Piano A. Lipotoxicity: effects of dietary saturated and transfatty acids. Mediators Inflamm 2013: 137579, 2013. doi: 10.1155/2013/137579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turpin SM, Ryall JG, Southgate R, Darby I, Hevener AL, Febbraio MA, Kemp BE, Lynch GS, Watt MJ. Examination of “lipotoxicity” in skeletal muscle of high-fat fed and ob/ob mice. J Physiol 587: 1593–1605, 2009. doi: 10.1113/jphysiol.2008.166033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henique C, Mansouri A, Fumey G, Lenoir V, Girard J, Bouillaud F, Prip-Buus C, Cohen I. Increased mitochondrial fatty acid oxidation is sufficient to protect skeletal muscle cells from palmitate-induced apoptosis. J Biol Chem 285: 36818–36827, 2010. doi: 10.1074/jbc.M110.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee H, Lim J-Y, Choi S-J. Oleate prevents palmitate-induced atrophy via modulation of mitochondrial ROS production in skeletal myotubes. Oxid Med Cell Longev 2017: 2739721, 2017. doi: 10.1155/2017/2739721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogdanovich S, Krag TOB, Barton ER, Morris LD, Whittemore L-A, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature 420: 418–421, 2002. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- 12.Bogdanovich S, Perkins KJ, Krag TOB, Whittemore L-A, Khurana TS. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J 19: 543–549, 2005. doi: 10.1096/fj.04-2796com. [DOI] [PubMed] [Google Scholar]
- 13.McPherron AC, Lawler AM, Lee S-J. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature 387: 83–90, 1997. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 14.Pasteuning-Vuhman S, Boertje-van der Meulen JW, van Putten M, Overzier M, Dijke Ten P, Kiełbasa SM, Arindrarto W, Wolterbeek R, Lezhnina KV, Ozerov IV, Aliper AM, Hoogaars WM, Aartsma-Rus A, Loomans CJM. New function of the myostatin/activin type I receptor (ALK4) as a mediator of muscle atrophy and muscle regeneration. FASEB J 31: 238–255, 2017. doi: 10.1096/fj.201600675R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carnac G, Vernus B, Bonnieu A. Myostatin in the pathophysiology of skeletal muscle. Curr Genomics 8: 415–422, 2007. doi: 10.2174/138920207783591672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Argilés JM, López-Soriano FJ, Stemmler B, Busquets S. Therapeutic strategies against cancer cachexia. Eur J Transl Myol 29: 7960, 2019. doi: 10.4081/ejtm.2019.7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith RC, Lin BK. Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders. Curr Opin Support Palliat Care 7: 352–360, 2013. doi: 10.1097/SPC.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol 229: R67–R81, 2016. doi: 10.1530/JOE-15-0533. [DOI] [PubMed] [Google Scholar]
- 19.Roy B, Curtis ME, Fears LS, Nahashon SN, Fentress HM. Molecular mechanisms of obesity-induced osteoporosis and muscle atrophy. Front Physiol 7: 439, 2016. doi: 10.3389/fphys.2016.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buehring B, Binkley N. Myostatin—the holy grail for muscle, bone, and fat? Curr Osteoporos Rep 11: 407–414, 2013. doi: 10.1007/s11914-013-0160-5. [DOI] [PubMed] [Google Scholar]
- 21.Ruan J, Zhang Y, Yuan J, Xin L, Xia J, Liu N, Mu Y, Chen Y, Yang S, Li K. A long-term high-fat, high-sucrose diet in Bama minipigs promotes lipid deposition and amyotrophy by up-regulating the myostatin pathway. Mol Cell Endocrinol 425: 123–132, 2016. doi: 10.1016/j.mce.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Hittel DS, Berggren JR, Shearer J, Boyle K, Houmard JA. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes 58: 30–38, 2009. doi: 10.2337/db08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong J, Dong Y, Dong Y, Chen F, Mitch WE, Zhang L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int J Obes (Lond) 40: 434–442, 2016. doi: 10.1038/ijo.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 14: 513–537, 2018. doi: 10.1038/s41574-018-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R, Cohen P. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab 21: 443–454, 2015. doi: 10.1016/j.cmet.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SJ, Miller B, Mehta HH, Xiao J, Wan J, Arpawong TE, Yen K, Cohen P. The mitochondrial-derived peptide MOTS-c is a regulator of plasma metabolites and enhances insulin sensitivity. Physiol Rep 7: e14171, 2019. doi: 10.14814/phy2.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cataldo LR, Fernández-Verdejo R, Santos JL, Galgani JE. Plasma MOTS-c levels are associated with insulin sensitivity in lean but not in obese individuals. J Investig Med 66: 1019–1022, 2018. doi: 10.1136/jim-2017-000681. [DOI] [PubMed] [Google Scholar]
- 28.Du C, Zhang C, Wu W, Liang Y, Wang A, Wu S, Zhao Y, Hou L, Ning Q, Luo X. Circulating MOTS-c levels are decreased in obese male children and adolescents and associated with insulin resistance. Pediatr Diabetes 19: 1058–1064, 2018. doi: 10.1111/pedi.12685. [DOI] [PubMed] [Google Scholar]
- 29.Kim SJ, Mehta HH, Wan J, Kuehnemann C, Chen J, Hu J-F, Hoffman AR, Cohen P. Mitochondrial peptides modulate mitochondrial function during cellular senescence. Aging (Albany NY) 10: 1239–1256, 2018. doi: 10.18632/aging.101463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen DL, Unterman TG. Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Physiol Cell Physiol 292: C188–C99, 2007. doi: 10.1152/ajpcell.00542.2005. [DOI] [PubMed] [Google Scholar]
- 31.Huang H, Tindall DJ. Regulation of FOXO protein stability via ubiquitination and proteasome degradation. Biochim Biophys Acta 1813: 1961–1964, 2011. doi: 10.1016/j.bbamcr.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng Z, White MF. Targeting Forkhead box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxid Redox Signal 14: 649–661, 2011. doi: 10.1089/ars.2010.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wan X, Helman LJ. Levels of PTEN protein modulate Akt phosphorylation on serine 473, but not on threonine 308, in IGF-II-overexpressing rhabdomyosarcomas cells. Oncogene 22: 8205–8211, 2003. doi: 10.1038/sj.onc.1206878. [DOI] [PubMed] [Google Scholar]
- 35.Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol 20: 5010–5018, 2000. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahdar M, Inoue T, Meyer T, Zhang J, Vazquez F, Devreotes PN. A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc Natl Acad Sci USA 106: 480–485, 2009. doi: 10.1073/pnas.0811212106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem 276: 993–998, 2001. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 38.Fife E, Kostka J, Kroc Ł, Guligowska A, Pigłowska M, Sołtysik B, Kaufman-Szymczyk A, Fabianowska-Majewska K, Kostka T. Relationship of muscle function to circulating myostatin, follistatin and GDF11 in older women and men. BMC Geriatr 18: 1–10, 2018. doi: 10.1186/s12877-018-0888-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brons C, Grunnet LG. Mechanism in endocrinology: skeletal muscle lipotoxicity in insulin resistance and type 2 diabetes: a causal mechanism or an innocent bystander. Eur J Endocrinol 176: R67–R78, 2017. [DOI] [PubMed] [Google Scholar]
- 40.Carta G, Murru E, Banni S, Manca C. Palmitic acid: physiological role, metabolism and nutritional implications. Front Physiol 8: 902, 2017. doi: 10.3389/fphys.2017.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson JM, Wang Y, Bryner RW, Williamson DL, Alway SE. Bax signaling regulates palmitate-mediated apoptosis in C2C12 myotubes. Am J Physiol Endocrinol Metab 295: E1307–E1314, 2008. doi: 10.1152/ajpendo.00738.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyons J-A, Haring JS, Biga PR. Myostatin expression, lymphocyte population, and potential cytokine production correlate with predisposition to high-fat diet induced obesity in mice. PLoS One 5: e12928, 2010. doi: 10.1371/journal.pone.0012928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manfredi LH, Paula-Gomes S, Zanon NM, Kettelhut IC. Myostatin promotes distinct responses on protein metabolism of skeletal and cardiac muscle fibers of rodents. Braz J Med Biol Res 50: e6733, 2017. doi: 10.1590/1414-431X20176733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hulmi JJ, Nissinen TA, Räsänen M, Degerman J, Lautaoja JH, Hemanthakumar KA, Backman JT, Ritvos O, Silvennoinen M, Kivelä R. Prevention of chemotherapy-induced cachexia by ACVR2B ligand blocking has different effects on heart and skeletal muscle. J Cachexia Sarcopenia Muscle 9: 417–432, 2018. doi: 10.1002/jcsm.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127: 125–137, 2006. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 46.Moraitis D, Karanikou M, Liakou C, Dimas K, Tzimas G, Tseleni-Balafouta S, Patsouris E, Rassidakis GZ, Kouvaraki MA. SIN1, a critical component of the mTOR-Rictor complex, is overexpressed and associated with AKT activation in medullary and aggressive papillary thyroid carcinomas. Surgery 156: 1542–1549, 2014. doi: 10.1016/j.surg.2014.08.095. [DOI] [PubMed] [Google Scholar]
- 47.Shan T, Liu J, Xu Z, Wang Y. Roles of phosphatase and tensin homolog in skeletal muscle. J Cell Physiol 234: 3192–3196, 2019. doi: 10.1002/jcp.26820. [DOI] [PubMed] [Google Scholar]
- 48.Wijesekara N, Konrad D, Eweida M, Jefferies C, Liadis N, Giacca A, Crackower M, Suzuki A, Mak TW, Kahn CR, Klip A, Woo M. Muscle-specific Pten deletion protects against insulin resistance and diabetes. Mol Cell Biol 25: 1135–1145, 2005. doi: 10.1128/MCB.25.3.1135-1145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu Z, Lee IH, Wang X, Sheng H, Zhang L, Du J, Mitch WE. PTEN expression contributes to the regulation of muscle protein degradation in diabetes. Diabetes 56: 2449–2456, 2007. doi: 10.2337/db06-1731. [DOI] [PubMed] [Google Scholar]
- 50.Yue F, Bi P, Wang C, Shan T, Nie Y, Ratliff TL, Gavin TP, Kuang S. PTEN is necessary for the quiescence and maintenance of adult muscle stem cells. Nat Commun 8: 14328–14313, 2017. doi: 10.1038/ncomms14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu Z, Wang H, Lee IH, Modi S, Wang X, Du J, Mitch WE. PTEN inhibition improves muscle regeneration in mice fed a high-fat diet. Diabetes 59: 1312–1320, 2010. doi: 10.2337/db09-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abrigo J, Rivera JC, Aravena J, Cabrera D, Simon F, Ezquer F, Ezquer M, Cabello-Verrugio C. High fat diet-induced skeletal muscle wasting is decreased by mesenchymal stem cells administration: implications on oxidative stress, ubiquitin proteasome pathway activation, and myonuclear apoptosis. Oxid Med Cell Longev 2016: 9047821, 2016. doi: 10.1155/2016/9047821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sishi B, Loos B, Ellis B, Smith W, Toit Du EF, Engelbrecht A-M. Diet-induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Exp Physiol 96: 179–193, 2011. doi: 10.1113/expphysiol.2010.054189. [DOI] [PubMed] [Google Scholar]
- 54.Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SA. High-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 54: 1926–1933, 2005. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- 55.Reynolds J, Lai RW, Woodhead JST, Joly JH, Mitchell CJ, Cameron-Smith D, Lu R, Cohen P, Graham NA, Benayoun BA, Merry TL, Lee C. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. bioRxiv 12: 470, 2019. doi: 10.1038/s41467-020-20790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]