Abstract
The nucleus tractus solitarii (nTS) is the first central site for the termination and integration of autonomic and respiratory sensory information. Sensory afferents terminating in the nTS as well as the embedded nTS neurocircuitry release and utilize glutamate that is critical for maintenance of baseline cardiorespiratory parameters and initiating cardiorespiratory reflexes, including those activated by bouts of hypoxia. nTS astrocytes contribute to synaptic and neuronal activity through a variety of mechanisms, including gliotransmission and regulation of glutamate in the extracellular space via membrane-bound transporters. Here, we aim to highlight recent evidence for the role of astrocytes within the nTS and their regulation of autonomic and cardiorespiratory processes under normal and hypoxic conditions.
Keywords: autonomic nervous system, blood pressure, excitatory amino acid transporters, respiration, synaptic plasticity
INTRODUCTION
Astrocytes are part of the glial family that includes microglia and oligodendrocytes (1). Named for their star-like morphology, astrocytes within the central nervous system (CNS) do not function in isolation but interact, often bidirectionally, with neurons, other glia, and blood vessels (1, 2). Although the complete role of astrocytes has not been fully defined, several functions are now well-accepted including ion homeostasis, synapse formation, metabolic-neural (neurovascular) coupling, neuronal communication, and neurotransmitter clearance (3). Although many astrocytic functions have been identified at the cellular level, it is increasingly recognized that astrocytes are involved in central control of the autonomic nervous and respiratory systems. Here, we review the contribution of astrocytes within the brainstem nucleus tractus solitarii (nTS) on cardiorespiratory function in health and disease with a focus on astrocyte control of glutamate release, concentration, and activation of their receptors.
NUCLEUS TRACTUS SOLITARII
The dorsal brainstem nTS is the first central synaptic termination site for numerous visceral sensory inputs for integration and modulation (4–6). Within the caudal nTS, the medial subnucleus receives substantial input from baroafferents originating from vessels near the heart to modulate blood pressure, whereas the commissural and medial subnuclei receive input originating from carotid body chemoreceptors to control respiratory and autonomic reflexes. However, substantial overlap exists between these inputs and other modalities (5, 7).
Sensory afferents whose central processes terminate within the nTS have their somas in peripheral autonomic ganglia. Baroafferents, such as those innervating the vessels, have their somas in the paired nodose ganglia. Carotid body chemoreceptors, which sense arterial oxygen, CO2, and pH, activate chemoafferents that travel through the glossopharyngeal nerve and whose somas lie in the paired petrosal ganglia adjacent to the nodose ganglia. These first-order afferents release glutamate into the nTS (8, 9) which binds to glutamate receptors (GluRs) on second-order nTS neurons, specifically ionotropic N-methyl-d-aspartate receptors (NMDARs) and non-NMDARs (e.g., α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, AMPA receptors) as well as metabotropic glutamate receptors (mGluRs). Such afferent information is relayed to other nuclei to ultimately produce an efferent signal. It is through these multisynaptic pathways that the nTS regulates resting and reflex control of cardiorespiratory parameters.
The nTS plays a role in many pathologies including hypertension (10), obstructive sleep apnea (11), Rett syndrome (12, 13), and neurocardiogenic syncope (14, 15). Often these dysfunctions are due to alterations in synaptic signaling. For example, a decrease in glutamate (16) and increase in GABA (17) augment sympathetic nervous system activity and are implicated in neurogenic hypertension. Because of the critical role of the nTS in visceral signal integration and overall homeostasis, understanding the cellular mechanisms mediating normal function and maladaptation, including those mediated by astrocytes, is critical to developing rational therapeutic approaches.
nTS ASTROCYTES AND AFFERENT INPUT
Astrocytes interact with multiple cell types and contribute to several neurophysiological processes within the central nervous system, including the nTS. Markers for mature astrocytes include protoplasmic S100 calcium-binding protein B (S100β) and glial fibrillar acidic protein (GFAP) (18). GFAP is an intermediate filament in mature astrocytes that serves a cyto-architectural function, contributes to astrocyte mobility, and is often used to examine morphological alterations. GFAP-labeled astrocytes are readily observed throughout the nTS with the volume fraction of nTS astrocytic processes reaching 15% (19). Although GFAP represents only a small fraction of astrocyte volume (20), individual nTS astrocytes have overlapping domains with GFAP-labeled astrocyte fibers extensively branching and occupying a volume of ∼7,000 µm3 and total surface area of ∼2,600 µm2 (21). Given the structural and functional overlap of sensory afferent innervation within the nTS and onto neurons (22, 23), and the wide occupational space of nTS astrocytes, such organization suggests that astrocytes influence a multitude of interacting synaptic inputs and reflex arcs.
Close association of nTS astrocytes to the central terminals of viscerosensory afferents innervating nTS neurons (the tripartite synapse, Fig. 1) likely contributes to the importance of astrocytes in peripheral information processing. Astrocytic membranes express Ca2+ permeable NMDARs (24) and AMPARs (25), although not all studies agree (26). Solitary tract (TS)-glutamatergic afferent fiber stimulation induces nTS astrocyte depolarization and increases intracellular Ca2+ largely through AMPAR activation (6, 25, 27). In the intact rat, astrocyte lesion reduces GFAP expression and attenuates several reflexes including baroreflex and chemoreflex (28), indicating nTS astrocytes are critical for proper information transfer and/or integration of sensory signals.
Figure 1.
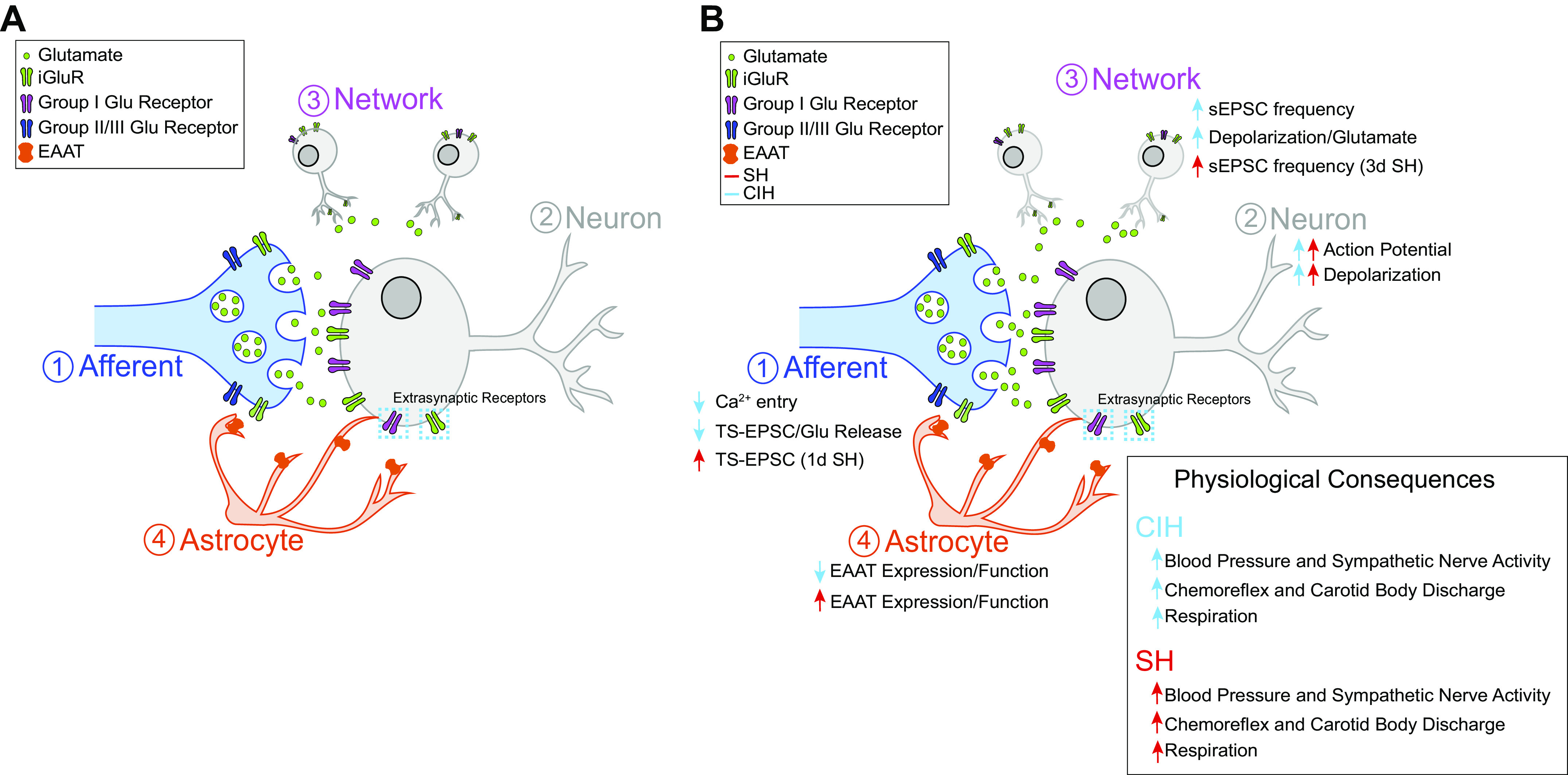
Astrocytes in the nucleus tractus solitarii (nTS) modulate synaptic and neuronal activity. A: the tripartite synapse is composed of afferent terminals (1), nTS neurons (2+3), and astrocytes (4). Afferent terminals release glutamate, which is buffered by astrocyte excitatory amino acid transporters (EAATs), to activate postsynaptic and presynaptic iGluRs and metabotropic glutamate receptors (mGluRs). B: chronic intermittent hypoxia (CIH, blue) and sustained hypoxia (SH, red) differentially alter astrocytic function. CIH decreases EAAT function to result in reduced presynaptic Ca2+ entry, Glu release and tractus solitarii-excitatory postsynaptic current (TS-EPSC) amplitude (1), somal depolarization (2), and elevated spontaneous EPSC (sEPSC) frequency (3). SH increases EAAT expression and function to induce time-dependent changes in TS-EPSC amplitude between 1 and 7 days (1), somal depolarization (2), and increases in sEPSC frequency after 3 days of SH (3). Physiological consequences of CIH and SH shown (inset).
Astrocyte coverage of somas in the nTS increases with development with up to ∼66% of small somas ensheathed by postnatal day 21 (29). Glutamatergic synapses are also not completely ensheathed. Glutamatergic synapses are ∼50%–58% covered by astrocytic processes depending upon their single or multisynaptic arrangements (19). Astrocyte coverage dynamically changes in response to numerous stimuli and changes to the extracellular milieu composition. For instance, astrocyte activation increases GFAP expression (30) and overall branching, suggesting elevated astrocytic synaptic coverage. Such coverage of nTS somas and glutamatergic synapses by glia highlights the potential for glia to modulate neuronal and synaptic activity and their plasticity via a multitude of mechanisms.
nTS ASTROCYTES INFLUENCE (NEURO)PHYSIOLOGICAL FUNCTION
nTS astrocytes play a multitude of roles important for autonomic homeostasis. nTS astrocytes contribute to feeding control by acting as glucodetectors under hypoglycemic conditions. For example, nutritional excess via a high-fat diet upregulates GFAP (31). During glucoprivation, increased astrocytic intracellular Ca2+ drives nTS activity and gastric motility (27, 32). Pretreatment with the astrocyte inhibitor fluorocitrate suppressed the effects of glucoprivation on the gastric reflex circuits (33). This counter-regulatory response is suggested to occur through nTS astrocyte communication with nearby catecholaminergic neurons (34). In addition, exogenous nTS thrombin increases glycemia and suppresses respiratory rhythm through an astrocyte-dependent mechanism (35). nTS astrocytes also detect pH changes to modulate nTS synaptic and neuronal function (36). Acidification induces astrocyte depolarization, subsequently limits glutamate uptake, and potentiates glutamatergic excitatory postsynaptic potentials (EPSPs). The effect of astrocyte acidification on cardiorespiratory parameters requires further study but may enhance nTS activity to downstream nuclei.
ASTROCYTE GLIOTRANSMITTER RELEASE AND CARDIORESPIRATORY CONTROL
Gliotransmission is the release of signaling chemicals from astrocytes that allows for communication to nearby neurons. Astrocytes release gliotransmitters such as glutamate, adenosine 5′-triphosphate (ATP), and d-serine among others (37, 38) that bind to receptors on adjacent neurons. Gliotransmitter release may be initiated via multiple processes, including activation of astrocytic ionotropic GluRs and mGluRs (39, 40), and elevation of intracellular Ca2+ through inositol (1,4,5)-trisphosphate activation following astrocytic activity (37, 41). Exocytosis may occur via fusion of synaptic-like vesicles in a Ca2+-dependent manner, reversal of glutamate uptake transporters, or opening of anion transporters following cell swelling. Coupled with neurotransmission, this bidirectional communication within synapses positions glia to actively influence synaptic strength, plasticity, and network dynamics (42).
nTS gliotransmission shapes presynaptic and postsynaptic activity. For instance, activation of astrocytic proteinase-activated receptor 1 (PAR1) induces endovanilloid-like release that subsequently triggers presynaptic glutamate release via transient receptor potential cation channel subfamily V member 1 (TRPV1). This glutamate release influences respiratory motor control as a subset of nTS neurons that are excited directly project to the rostral ventral respiratory group (43). Astrocytic PAR1 activation also induces astrocytic glutamate release through a Ca2+-dependent mechanism to activate postsynaptic NMDARs and augment neuronal intracellular Ca2+ (44). This increase of neuronal but not astrocytic Ca2+ was GluR dependent (45). Finally, afferent glutamate and serotonin release triggers astrocytic Ca2+-dependent ATP release to modulate baroreflex sensitivity (46). Altogether, gliotransmission modifies nTS activity and subsequent downstream centers to modulate cardiorespiratory control.
ASTROCYTE GLUTAMATE RE-UPTAKE TRANSPORTERS AND CARDIORESPIRATORY CONTROL
Afferent information from the periphery, including the chemo- and baroreflex, is transmitted to nTS neurons via glutamate release. Although the concentration of glutamate in the nTS requires clarification, basal glutamate has been reported to be ∼3 µM and increases 70% in response to hypoxic chemoafferent activation (47). Glutamate within the nTS evokes both tonic and phasic excitation to depolarize nTS neurons and influence cardiorespiratory activity. Therefore, within the nTS, spatial-temporal concentration of glutamate requires precise control (48) to maintain not only autonomic and respiratory homeostasis but also its reflexes within physiological working range. Failure to maintain control of nTS excitation may result in sympathetic and respiratory overexcitation observed in many diseases.
Excitatory amino acid transporters (EAATs) are responsible for the uptake and regulation of extracellular glutamate (49). Of the five identified transporters, EAAT-1 and EAAT-2 [encoded by the genes Slc1a3 (Eaat1, GLAST) and Slc1a2 (Eaat2, GLT-1)] represent the majority of EAATs expressed in CNS (50) and nTS astrocytes (19, 51). These transporters operate by removing one glutamate with three Na+ and one H+ in exchange for one K+ (52). It is generally accepted that EAAT-2 is more effective in glutamate uptake than EAAT-1 (53). Once removed from the synaptic cleft, glutamate is recycled into glutamine then shuttled into the presynaptic terminal for glutamate synthesis (54).
Astrocytic transporters are critical for normal nTS synaptic and neuronal activity and for their influence on cardiorespiratory function via their restraint of GluRs (55–57) (Fig. 1B). In the nTS brainstem slice, EAAT-1 and -2 block with threo-β-benzyloxyaspartic acid (TBOA) elevates glutamate in the synaptic cleft, depolarizes nTS neurons, increases action potential firing, and augments spontaneous (s) excitatory postsynaptic current (EPSC) frequency. Paradoxically, TS afferent-evoked EPSC amplitude decreases following EAAT-block. These neurophysiological effects in response to EAAT-block are due, in part, to activation of NMDAR, AMPAR, and mGluRs.
Within the nTS, the expression of EAAT-2 is greater than EAAT-1 (19, 58) and thus likely contributes to the majority of neuronal responses when blocked by the nonspecific inhibitor TBOA. Specific block of EAAT-2 with dihydrokainic acid (DHK) produced comparable postsynaptic responses as EAAT-1 and -2 block with TBOA; nTS neurons depolarized, action potential discharge increased, and sEPSC frequency was enhanced. Such similarities suggest at the functional level that EAAT-2 is the primary glutamate transporter responsible for uptake in the nTS. The neuronal responses to DHK were blocked by AMPAR antagonism with minor effect of NMDAR inhibition indicating EAAT-2 primarily regulates postsynaptic AMPARs. Afferent TS-EPSCs were not altered by EAAT-2 block via DHK, unlike general EAAT-block with TBOA. This may be attributed to a greater increase of extracellular glutamate when EAAT-1 and -2 are inhibited compared with EAAT-2 block alone, or a unique spatial distribution of EAAT-1 versus EAAT-2 near afferent terminals.
In addition to NMDAR and AMPAR, mGluRs profoundly modify nTS activity. mGluRs are generally classified into three groups. In the nTS, Group I is postsynaptic whereas Groups II/III are typically localized to the presynaptic terminal (59–61). Not surprising, EAATs are crucial to controlling the activation of mGluRs and their influence of neuronal functon. Group I is activated in response to synaptically released glutamate to depolarize neurons and enhance their excitability (59). Recent studies suggest EAATs limit activation of Group I mGluRs and its resulting neuronal depolarization and subsequent increase in action potential-dependent spontaneous EPSCs (55). In response to high-afferent activity and presumably elevated glutamate concentrations (60, 62), Groups II/III presynaptic mGluRs are activated to decrease TS-EPSC amplitude. EAATs also reduce activation of these presynaptic mGluRs and the resulting reduction in synaptic transmission, which occurs through modulation of Ca2+ influx (55).
Pharmacological block of EAAT-1 and/or EAAT-2 at the synaptic and neuronal level ultimately effects cardiorespiratory parameters. Specifically, EAAT-block reduced basal blood pressure, heart rate, and sympathetic nerve activity, as well as reduced respiratory (phrenic) output. These responses to baseline function are similar to that seen with nTS microinjection of glutamate (63, 64). As observed in vitro, AMPARs (56) and Group I mGluRs (55) play a prominent role in mediating these physiological effects as prior receptor inhibition attenuated or eliminated the physiological response to excess Glu following EAAT-block. Although EAATs modulate baseline cardiorespiratory parameters, they also influence the responses to several reflexes consistent with astrocyte ablation studies (65). Activation of the carotid body chemoreflex induces pressor, tachycardia, and sympathoexcitation (66, 67). These responses are enhanced by EAAT-2 block and its resulting increase in glutamate (56). In vivo block of all EAATs or EAAT-2 alone reduced the cardiac and sympathetic baroreflex (68). Altogether, astrocytes contribute to nTS glutamatergic regulation to maintain baseline cardiovascular parameters and reflex control.
ASTROCYTES CONTRIBUTE TO NEURONAL SIGNALING IN RESPONSE TO HYPOXIA
Periods of low oxygen or hypoxia occur due to environmental (i.e., high-altitude ascent) or disease states. Hypoxic exposure increases breathing and sympathetic nervous system activity via activation of the carotid body and its adjacent chemoafferent fibers (69, 70), nTS glutamate release (47), and elevated nTS activity (6). Intermittent hypoxia is characterized by brief (10 to >100 s) but frequent hypoxic episodes (5 to >30 per hour) between 10% and 6% O2 for 8–12 h/day (71). When occurring over days to weeks, chronic intermittent hypoxia (CIH) is a model for the hypoxic episodes that occur during obstructive sleep apnea (OSA) (72). Sustained hypoxia (SH) occurs during exposure to a persistent level of hypoxia over several days and is associated with long-term altitude ascent and chronic lung disease (73). Exposure to CIH and SH induces persistent changes in central control of cardiorespiratory function in that the effects of hypoxia occur on several time scales and persist upon returning to normoxia (21% O2) (74, 75). Recent studies have demonstrated that nTS astrocytes and their transporters contribute to hypoxia responses.
OSA affects ∼34% of males, ∼17% of females, and ∼8% of juveniles (76, 77). OSA occurs due to recurrent upper airway collapses for short periods during sleep causing intermittent episodes of hypoxia and hypercapnia (elevated CO2). Recurrent apneic episodes cause daytime elevation of blood pressure and sympathetic nerve activity. Patients with untreated OSA develop hypertension associated with increased sympathetic nervous system activity as well as tonic activation and increased chemoreflex sensitization. Similar to OSA, CIH elevates breathing, chemoreflex, and sympathetic nerve activity; induces hypertension; and is associated with stroke, arrhythmia, and increased mortality. Carotid body discharge increases with each hypoxic episode after CIH (78). CIH also enhances the central gain of chemosensory activity (79), neuronal nTS activity as demonstrated by Fos immunoreactivity (80), and AMPAR and NMDAR expression (73) and function (72). CIH increases nTS basal spontaneous synaptic activity as well as synaptic currents that occur during and following an afferent stimulus train (asynchronous release). However, CIH also decreases solitary tract-evoked EPSC amplitude (11, 81). We have suggested that CIH differentially affects the presynaptic vesicular pools responsible for spontaneous and evoked transmitter release via Ca2+-dependent mechanisms (11), while others suggest the depressed afferent-evoked postsynaptic currents occurs via reduction in the number of active syanpses (81). In addition, alterations in nTS postsynaptic GluR expression and function have been reported (82). Thus, the contribution of the nTS in CIH is likely multifaceted; recent work suggests astrocytic dysfunction may also contribute.
Interestingly, the neuronal and synaptic responses following EAAT-block (55, 57) are similar to that after CIH (11), specifically the elevated spontaneous current frequency, decreased TS-EPSC amplitude, and augmented action potential discharge. Several lines of evidence suggest expressional and/or functional changes in astrocytic EAATs contribute to CIH effects (83). CIH decreased EAAT-1 protein expression, whereas mRNA transcript was similar to normoxia. The expression differences between protein and mRNA may result from stabilization of the EAAT mRNA or protein, or reduction in their breakdown. Functionally, after CIH, general EAAT-block was less effective in inducing somal depolarization, increasing sEPSC frequency, and reducing TS-EPSC amplitude. The reduced synaptic events after CIH were due to presynaptic Ca2+-dependent mechanisms as their responses were also attenuated following EAAT block. Although our data suggests decreased EAAT expression and function, we do not know the consequence of this decreased role of EAAT on ionotropic GluR or mGluR function or its overall influence on altered reflex function described in OSA and CIH. In addition, EAAT function is modulated via post-translational modifications (84); how hypoxia may further contribute is unknown. Identifying and understanding the role of such EAAT modifications on influencing neurotransmission and neuronal discharge, including the GluR responsible, requires further study.
nTS dysfunction due to changes in neuron-astrocyte interaction may also cause some of the pathologies seen in the cardiorespiratory system during sustained hypoxia. SH activates and enhances the peripheral chemoreflex thereby elevating respiration (85–87). Seven days of SH enhances the central gain of sensory afferents (88), suggesting that pathways downstream from primary sensory afferents, such as the nTS, contribute to tachypnea. In rats, SH activates nTS astrocytes as demonstrated by increased GFAP expression within the first 24 h of exposure (89, 90). SH also increases postsynaptic excitability via enhanced NMDAR- and AMPAR-mediated currents as well as reduced transient outward potassium (IA) and ATP-dependent potassium (KATP) currents that normally reduce neuronal excitability (85, 86, 91).
SH induces time- and EAAT-dependent alteration in nTS synaptic transmission (58). One day (1 day) of sustained 10% O2 exposure increases TS-EPSC amplitude, which returns to normal after 3 days of SH and remains at this level for up to 7 days of SH. Although changes in TS-EPSC amplitude occurred early, sEPSC frequency only increased at 3 days of SH. EAAT-2 block with DHK decreased TS-EPSC amplitude only after 3 days of SH. On the other hand, neuronal depolarization after EAAT-2 block with DHK was absent only after 3 days of SH. Potential mechanisms for this shift include activation of Groups II/III mGluRs that inhibit presynaptic glutamate release (55) or a shift in EAAT expression to the synaptic cleft from the extracellular space. Support for changes in EAAT expression in response to SH was demonstrated by the elevated EAAT-2 mRNA following 3 days and protein after 3 and 7 days of SH. EAAT-1 protein was only elevated after 7 days of SH (58). Additional studies are needed to define how hypoxia alters the ultrastructural location of EAATs and the role of GluRs in these EAAT effects.
WHERE DO WE GO FROM HERE?
This review highlighted some of the important roles that nTS astrocytes play in modulating synaptic transmission, neuronal activity, and cardiorespiratory activity. Nevertheless, further work is needed to completely elucidate the mechanisms by which astrocytes support these functions, and many questions also remain. For instance, do astrocyte morphology alterations lead to (neuro)physiological changes, or vice versa? How do astrocytes alter their gliotransmission or neurochemical uptake in response to elevated afferent activity such as during hypoxic exposure? How does activation of specific astrocytic receptors lead to increased gliotransmission or astrocyte activity to modify sensory function? Why is there redundancy of nTS EAAT-1 and -2? What is each of their unique role(s) in cardiorespiratory health and disease? These questions remain along with the need to study GABA and glycine inhibition that counterbalance glutamate excitation. GABA and glycine possess their own set of re-uptake transporters whose function in the nTS is relatively unknown. Answers to these and many others questions are becoming increasingly easier to discover through many emerging tools that will help establish the ever-expanding role of nTS astrocytes in cardiorespiratory health.
GRANTS
Collected data reported in this manuscript was supported by National Heart, Lung, and Blood Institute (NHLBI) HL128454 (D. D. Kline), NHLBI Grant HL-132836 (E. M. Hasser), and American Heart Postdoctoral Grant 19POST34380079 (D. Martinez).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.M. prepared figures; D.M. and D.D.K. drafted manuscript; D.M. and D.D.K. edited and revised manuscript; D.M. and D.D.K. approved final version of manuscript.
ACKNOWLEDGMENTS
Current address for D. Martinez: Cooper Medical School of Rowan University, Camden, NJ.
REFERENCES
- 1.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60: 430–440, 2008. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 2.MacVicar BA, Newman EA. Astrocyte regulation of blood flow in the brain. Cold Spring Harb Perspect Biol 7: a020388, 2015. doi: 10.1101/cshperspect.a020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung WS, Allen NJ, Eroglu C. Astrocytes control synapse formation, function, and elimination. Cold Spring Harb Perspect Biol 7: a020370, 2015. doi: 10.1101/cshperspect.a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastrointestinal function. Auton Neurosci 161: 6–13, 2011. doi: 10.1016/j.autneu.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kline DD. Plasticity in glutamatergic NTS neurotransmission. Respir Physiol Neurobiol 164: 105–111, 2008. doi: 10.1016/j.resp.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zoccal DB, Furuya WI, Bassi M, Colombari DS, Colombari E. The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Front Physiol 5: 238, 2014. doi: 10.3389/fphys.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andresen MC, Kunze DL. Nucleus tractus solitarius—gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- 8.Brew S, de Castro D, Housley GD, Sinclair JD. The role of glutamate in the transmission of the hypoxic input to respiration through the nucleus of the tractus solitarius. In: Chemoreceptors and Chemoreceptor Reflexes, edited by Acker H, Trzebski A, O’Regan RG.. Boston, MA: Springer, 1990, p. 331–338. [Google Scholar]
- 9.Talman WT, Granata AR, Reis DJ. Glutamatergic mechanisms in the nucleus tractus solitarius in blood pressure control. Fed Proc 43: 39–44, 1984. [PubMed] [Google Scholar]
- 10.Sved AF. Peripheral pressor systems in hypertension caused by nucleus tractus solitarius lesions. Hypertension 8: 742–747, 1986. doi: 10.1161/01.HYP.8.9.742. [DOI] [PubMed] [Google Scholar]
- 11.Kline DD, Ramirez-Navarro A, Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J Neurosci 27: 4663–4673, 2007. doi: 10.1523/JNEUROSCI.4946-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CY, Di Lucente J, Lin YC, Lien CC, Rogawski MA, Maezawa I, Jin LW. Defective GABAergic neurotransmission in the nucleus tractus solitarius in Mecp2-null mice, a model of Rett syndrome. Neurobiol Dis 109: 25–32, 2018. doi: 10.1016/j.nbd.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kline DD, Ogier M, Kunze DL, Katz DM. Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice. J Neurosci 30: 5303–5310, 2010. doi: 10.1523/JNEUROSCI.5503-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beacher FD, Gray MA, Mathias CJ, Critchley HD. Vulnerability to simple faints is predicted by regional differences in brain anatomy. Neuroimage 47: 937–945, 2009. doi: 10.1016/j.neuroimage.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zurak N, Bilic E. Syncope: facts and fiction. Med Hypotheses 63: 394–401, 2004. doi: 10.1016/j.mehy.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 16.Costa-Silva JH, Zoccal DB, Machado BH. Chronic intermittent hypoxia alters glutamatergic control of sympathetic and respiratory activities in the commissural NTS of rats. Am J Physiol Regul Integr Comp Physiol 302: R785–R793, 2012. doi: 10.1152/ajpregu.00363.2011. [DOI] [PubMed] [Google Scholar]
- 17.Mei L, Zhang J, Mifflin S. Hypertension alters GABA receptor-mediated inhibition of neurons in the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 285: R1276–R1286, 2003. doi: 10.1152/ajpregu.00255.2003. [DOI] [PubMed] [Google Scholar]
- 18.Verkhratsky A, Nedergaard M. Physiology of astroglia. Physiol Rev 98: 239–389, 2018. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chounlamountry K, Kessler JP. The ultrastructure of perisynaptic glia in the nucleus tractus solitarii of the adult rat: comparison between single synapses and multisynaptic arrangements. Glia 59: 655–663, 2011. doi: 10.1002/glia.21135. [DOI] [PubMed] [Google Scholar]
- 20.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22: 183–192, 2002. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheikh Bahaei S, Morris B, Collina J, Anjum S, Znati S, Gamarra J, Zhang R, Gourine AV, Smith JC. Morphometric analysis of astrocytes in brainstem respiratory regions. J Comp Neurol 526: 2032–2047, 2018. doi: 10.1002/cne.24472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciriello J, Hochstenbach SL, Roder S. Central projections of baroreceptor and chemoreceptor afferent fibers in the rat. In: Nucleus of the Solitary Tract, edited by Barraco IRA. Boca Raton, FL: CRC Press, 1994, p. 35–50. [Google Scholar]
- 23.Felder RB, Miffin SW. Baroreceptor and chemoreceptor afferent processing in the solitary tract nucleus. In: Nucleus of the Solitary Tract, edited by Barraco IRA. London: CRC Press, 1984, p. 169–185. [Google Scholar]
- 24.Aicher SA, Sharma S, Pickel VM. N-Methyl-d-aspartate receptors are present in vagal afferents and their dendritic targets in the nucleus tractus solitarius. Neuroscience 91: 119–132, 1999. doi: 10.1016/S0306-4522(98)00530-2. [DOI] [PubMed] [Google Scholar]
- 25.McDougal DH, Hermann GE, Rogers RC. Vagal afferent stimulation activates astrocytes in the nucleus of the solitary tract via AMPA receptors: evidence of an atypical neural-glial interaction in the brainstem. J Neurosci 31: 14037–14045, 2011. doi: 10.1523/JNEUROSCI.2855-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baude A, Strube C, Tell F, Kessler JP. Glutamatergic neurotransmission in the nucleus tractus solitarii: structural and functional characteristics. J Chem Neuroanat 38: 145–153, 2009. doi: 10.1016/j.jchemneu.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 27.McDougal DH, Viard E, Hermann GE, Rogers RC. Astrocytes in the hindbrain detect glucoprivation and regulate gastric motility. Auton Neurosci 175: 61–69, 2013. doi: 10.1016/j.autneu.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin LH, Moore SA, Jones SY, McGlashon J, Talman WT. Astrocytes in the rat nucleus tractus solitarii are critical for cardiovascular reflex control. J Neurosci 33: 18608–18617, 2013. doi: 10.1523/JNEUROSCI.3257-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tashiro Y, Kawai Y. Glial coverage of the small cell somata in the rat nucleus of tractus solitarius during postnatal development. Glia 55: 1619–1629, 2007. doi: 10.1002/glia.20577. [DOI] [PubMed] [Google Scholar]
- 30.Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol 4: 229–237, 1994. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald AJ, Holmes FE, Beall C, Pickering AE, Ellacott KLJ. Regulation of food intake by astrocytes in the brainstem dorsal vagal complex. Glia 68: 1241–1254, 2020. doi: 10.1002/glia.23774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDougal DH, Hermann GE, Rogers RC. Astrocytes in the nucleus of the solitary tract are activated by low glucose or glucoprivation: evidence for glial involvement in glucose homeostasis. Front Neurosci 7: 249, 2013. doi: 10.3389/fnins.2013.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermann GE, Viard E, Rogers RC. Hindbrain glucoprivation effects on gastric vagal reflex circuits and gastric motility in the rat are suppressed by the astrocyte inhibitor fluorocitrate. J Neurosci 34: 10488–10496, 2014. doi: 10.1523/JNEUROSCI.1406-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers RC, McDougal DH, Ritter S, Qualls-Creekmore E, Hermann GE. Response of catecholaminergic neurons in the mouse hindbrain to glucoprivic stimuli is astrocyte dependent. Am J Physiol Regul Integr Comp Physiol 315: R153–R164, 2018. doi: 10.1152/ajpregu.00368.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers RC, Hasser EM, Hermann GE. Thrombin action on astrocytes in the hindbrain of the rat disrupts glycemic and respiratory control. Am J Physiol Regul Integr Comp Physiol 318: R1068–R1077, 2020. doi: 10.1152/ajpregu.00033.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huda R, McCrimmon DR, Martina M. pH modulation of glial glutamate transporters regulates synaptic transmission in the nucleus of the solitary tract. J Neurophysiol 110: 368–377, 2013. doi: 10.1152/jn.01074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13: 54–63, 2007. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Koizumi S. Synchronization of Ca2+ oscillations: involvement of ATP release in astrocytes. FEBS J 277: 286–292, 2010. doi: 10.1111/j.1742-4658.2009.07438.x. [DOI] [PubMed] [Google Scholar]
- 39.Panatier A, Vallee J, Haber M, Murai KK, Lacaille JC, Robitaille R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146: 785–798, 2011. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 40.Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci 17: 7817–7830, 1997. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savtchouk I, Volterra A. Gliotransmission: beyond black-and-white. J Neurosci 38: 14–25, 2018. doi: 10.1523/JNEUROSCI.0017-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron 81: 728–739, 2014. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huda R, Chang Z, Do J, McCrimmon DR, Martina M. Activation of astrocytic PAR1 receptors in the rat nucleus of the solitary tract regulates breathing through modulation of presynaptic TRPV1. J Physiol 596: 497–513, 2018. doi: 10.1113/JP275127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vance KM, Rogers RC, Hermann GE. PAR1-activated astrocytes in the nucleus of the solitary tract stimulate adjacent neurons via NMDA receptors. J Neurosci 35: 776–785, 2015. doi: 10.1523/JNEUROSCI.3105-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hermann GE, Van Meter MJ, Rood JC, Rogers RC. Proteinase-activated receptors in the nucleus of the solitary tract: evidence for glial-neural interactions in autonomic control of the stomach. J Neurosci 29: 9292–9300, 2009. doi: 10.1523/JNEUROSCI.6063-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mastitskaya S, Turovsky E, Marina N, Theparambil SM, Hadjihambi A, Kasparov S, Teschemacher AG, Ramage AG, Gourine AV, Hosford PS. Astrocytes modulate baroreflex sensitivity at the level of the nucleus of the solitary tract. J Neurosci 40: 3052–3062, 2020. doi: 10.1523/JNEUROSCI.1438-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Kurosawa H, Okabe S, Takishima T, Shirato K. In vivo release of glutamate in nucleus tractus solitarii of the rat during hypoxia. J Physiol 478: 55–66, 1994. doi: 10.1113/jphysiol.1994.sp020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanai Y, Hediger MA. The glutamate and neutral amino acid transporter family: physiological and pharmacological implications. Eur J Pharmacol 479: 237–247, 2003. doi: 10.1016/j.ejphar.2003.08.073. [DOI] [PubMed] [Google Scholar]
- 49.Rose CR, Ziemens D, Untiet V, Fahlke C. Molecular and cellular physiology of sodium-dependent glutamate transporters. Brain Res Bull 136: 3–16, 2018. doi: 10.1016/j.brainresbull.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 50.Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci USA 89: 10955–10959, 1992. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Had-Aissouni L. Toward a new role for plasma membrane sodium-dependent glutamate transporters of astrocytes: maintenance of antioxidant defenses beyond extracellular glutamate clearance. Amino Acids 42: 181–197, 2012. doi: 10.1007/s00726-011-0863-9. [DOI] [PubMed] [Google Scholar]
- 52.Levy LM, Warr O, Attwell D. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J Neurosci 18: 9620–9628, 1998. doi: 10.1523/JNEUROSCI.18-23-09620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci 14: 5559–5569, 1994. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaudhry FA, Reimer RJ, Edwards RH. The glutamine commute: take the N line and transfer to the A. J Cell Biol 157: 349–355, 2002. doi: 10.1083/jcb.200201070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinez D, Rogers RC, Hermann GE, Hasser EM, Kline DD. Astrocytic glutamate transporters reduce the neuronal and physiological influence of metabotropic glutamate receptors in nucleus tractus solitarii. Am J Physiol Regul Integr Comp Physiol 318: R545–R564, 2020. doi: 10.1152/ajpregu.00319.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matott MP, Kline DD, Hasser EM. Glial EAAT2 regulation of extracellular nTS glutamate critically controls neuronal activity and cardiorespiratory reflexes. J Physiol 595: 6045–6063, 2017. doi: 10.1113/JP274620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matott MP, Ruyle BC, Hasser EM, Kline DD. Excitatory amino acid transporters tonically restrain nTS synaptic and neuronal activity to modulate cardiorespiratory function. J Neurophysiol 115: 1691–1702, 2016. doi: 10.1152/jn.01054.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matott MP, Hasser EM, Kline DD. Sustained hypoxia alters nTS glutamatergic signaling and expression and function of excitatory amino acid transporters. Neuroscience 430: 131–140, 2020. doi: 10.1016/j.neuroscience.2020.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Austgen JR, Fong AY, Foley CM, Mueller PJ, Kline DD, Heesch CM, Hasser EM. Expression of Group I metabotropic glutamate receptors on phenotypically different cells within the nucleus of the solitary tract in the rat. Neuroscience 159: 701–716, 2009. doi: 10.1016/j.neuroscience.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen C-Y, Ling E-H, Horowitz JM, Bonham AC. Synaptic transmission in nucleus tractus solitarius is depressed by group II and III but not group I presynaptic metabotropic glutamate receptors in rats. J Physiol 538: 773–786, 2002. doi: 10.1113/jphysiol.2001.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sekizawa S, Bechtold AG, Tham RC, Bonham AC. A novel postsynaptic group II metabotropic glutamate receptor role in modulating baroreceptor signal transmission. J Neurosci 29: 11807–11816, 2009. doi: 10.1523/JNEUROSCI.2617-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glaum SR, Miller RJ. Metabotropic glutamate receptors mediate excitatory transmission in the nucleus of the solitary tract. J Neurosci 12: 2251–2258, 1992. doi: 10.1523/JNEUROSCI.12-06-02251.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonham AC, McCrimmon DR. Neurones in a discrete region of the nucleus tractus solitarius are required for the Breuer–Hering reflex in rat. J Physiol 427: 261–280, 1990. doi: 10.1113/jphysiol.1990.sp018171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foley CM, Moffitt JA, Hay M, Hasser EM. Glutamate in the nucleus of the solitary tract activates both ionotropic and metabotropic glutamate receptors. Am J Physiol 275: R1858–R1866, 1998. doi: 10.1152/ajpregu.1998.275.6.R1858. [DOI] [PubMed] [Google Scholar]
- 65.Talman WT, Dragon DN, Lin LH. Reduced responses to glutamate receptor agonists follow loss of astrocytes and astroglial glutamate markers in the nucleus tractus solitarii. Physiol Rep 5: e13158, 2017. doi: 10.14814/phy2.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Franchini KG, Krieger EM. Cardiovascular responses of conscious rats to carotid body chemoreceptor stimulation by intravenous KCN. J Auton Nerv Syst 42: 63–69, 1993. doi: 10.1016/0165-1838(93)90342-R. [DOI] [PubMed] [Google Scholar]
- 67.Machado BH. Neurotransmission of the cardiovascular reflexes in the nucleus tractus solitarii of awake rats. Ann NY Acad Sci 940: 179–196, 2001. doi: 10.1111/j.1749-6632.2001.tb03676.x. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto K, Mifflin S. Inhibition of glial glutamate transporter GLT1 in the nucleus of the solitary tract attenuates baroreflex control of sympathetic nerve activity and heart rate. Physiol Rep 6: e13877, 2018. doi: 10.14814/phy2.13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol (1985) 90: 1986–1994, 2001. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- 70.Vidruk EH, Olson EB Jr, Ling L, Mitchell GS. Responses of single-unit carotid body chemoreceptors in adult rats. J Physiol 531: 165–170, 2001. doi: 10.1111/j.1469-7793.2001.0165j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tietjens JR, Claman D, Kezirian EJ, De Marco T, Mirzayan A, Sadroonri B, Goldberg AN, Long C, Gerstenfeld EP, Yeghiazarians Y. Obstructive sleep apnea in cardiovascular disease: a review of the literature and proposed multidisciplinary clinical management strategy. J Am Heart Assoc 8: e010440, 2019. doi: 10.1161/JAHA.118.010440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kline DD. Chronic intermittent hypoxia affects integration of sensory input by neurons in the nucleus tractus solitarii. Respir Physiol Neurobiol 174: 29–36, 2010. doi: 10.1016/j.resp.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reeves SR, Gozal E, Guo SZ, Sachleben LR Jr, Brittian KR, Lipton AJ, Gozal D. Effect of long-term intermittent and sustained hypoxia on hypoxic ventilatory and metabolic responses in the adult rat. J Appl Physiol (1985) 95: 1767–1774, 2003. doi: 10.1152/japplphysiol.00759.2002. [DOI] [PubMed] [Google Scholar]
- 74.Pamenter ME, Powell FL. Time domains of the hypoxic ventilatory response and their molecular basis. Compr Physiol 6: 1345–1385, 2016. doi: 10.1002/cphy.c150026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998. doi: 10.1016/S0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- 76.Czeisler CA. Duration, timing and quality of sleep are each vital for health, performance and safety. Sleep Health 1: 5–8, 2015. doi: 10.1016/j.sleh.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 77.Dudley S, Wood J. Pediatric obstructive sleep apnea. In: Pediatric Otolaryngology: A Concise Guide to Pediatric Ear, Nose and Throat, edited by Thompson JW. Newcastle upon Tyne, UK: Cambridge Scholar Publishing, 2020, p. 338. [Google Scholar]
- 78.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA 100: 10073–10078, 2003. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci 21: 5381–5388, 2001. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Knight WD, Little JT, Carreno FR, Toney GM, Mifflin SW, Cunningham JT. Chronic intermittent hypoxia increases blood pressure and expression of FosB/DeltaFosB in central autonomic regions. Am J Physiol Regul Integr Comp Physiol 301: R131–R139, 2011. doi: 10.1152/ajpregu.00830.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Almado CE, Machado BH, Leao RM. Chronic intermittent hypoxia depresses afferent neurotransmission in NTS neurons by a reduction in the number of active synapses. J Neurosci 32: 16736–16746, 2012. doi: 10.1523/JNEUROSCI.2654-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Paula PM, Tolstykh G, Mifflin S. Chronic intermittent hypoxia alters NMDA and AMPA-evoked currents in NTS neurons receiving carotid body chemoreceptor inputs. Am J Physiol Regul Integr Comp Physiol 292: R2259–R2265, 2007. doi: 10.1152/ajpregu.00760.2006. [DOI] [PubMed] [Google Scholar]
- 83.Martinez D, Rogers RC, Hasser EM, Hermann GE, Kline DD. Loss of excitatory amino acid transporter restraint following chronic intermittent hypoxia contributes to synaptic alterations in nucleus tractus solitarii. J Neurophysiol 123: 2122–2135, 2020. doi: 10.1152/jn.00766.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peterson AR, Binder DK. Post-translational regulation of GLT-1 in neurological diseases and its potential as an effective therapeutic target. Front Mol Neurosci 12: 164, 2019. doi: 10.3389/fnmol.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Accorsi-Mendonca D, Almado CE, Bonagamba LG, Castania JA, Moraes DJ, Machado BH. Enhanced firing in NTS induced by short-term sustained hypoxia is modulated by glia-neuron interaction. J Neurosci 35: 6903–6917, 2015. doi: 10.1523/JNEUROSCI.4598-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Accorsi-Mendonca D, Bonagamba LGH, Machado BH. Astrocytic modulation of glutamatergic synaptic transmission is reduced in NTS of rats submitted to short-term sustained hypoxia. J Neurophysiol 121: 1822–1830, 2019. doi: 10.1152/jn.00279.2018. [DOI] [PubMed] [Google Scholar]
- 87.Powell FL, Huey KA, Dwinell MR. Central nervous system mechanisms of ventilatory acclimatization to hypoxia. Respir Physiol 121: 223–236, 2000. doi: 10.1016/S0034-5687(00)00130-4. [DOI] [PubMed] [Google Scholar]
- 88.Dwinell MR, Powell FL. Chronic hypoxia enhances the phrenic nerve response to arterial chemoreceptor stimulation in anesthetized rats. J Appl Physiol (1985) 87: 817–823, 1999. doi: 10.1152/jappl.1999.87.2.817. [DOI] [PubMed] [Google Scholar]
- 89.Stokes JA, Arbogast TE, Moya EA, Fu Z, Powell FL. Minocycline blocks glial cell activation and ventilatory acclimatization to hypoxia. J Neurophysiol 117: 1625–1635, 2017. doi: 10.1152/jn.00525.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tadmouri A, Champagnat J, Morin-Surun MP. Activation of microglia and astrocytes in the nucleus tractus solitarius during ventilatory acclimatization to 10% hypoxia in unanesthetized mice. J Neurosci Res 92: 627–633, 2014. doi: 10.1002/jnr.23336. [DOI] [PubMed] [Google Scholar]
- 91.Zhang W, Carreno FR, Cunningham JT, Mifflin SW. Chronic sustained and intermittent hypoxia reduce function of ATP-sensitive potassium channels in nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 295: R1555–R1562, 2008. doi: 10.1152/ajpregu.90390.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


