Abstract
Postnatal growth failure is a common morbidity for preterm infants and is associated with adverse neurodevelopmental outcomes. Although sodium (Na) deficiency early in life impairs somatic growth, its impact on neurocognitive functions has not been extensively studied. We hypothesized that Na deficiency during early life is sufficient to cause growth failure and program neurobehavioral impairments in later life. C57BL/6J mice were placed on low- (0.4), normal- (1.5), or high- (3 g/kg) Na chow at weaning (PD22) and continued on the diet for 3 wk (to PD40). Body composition and fluid distribution were determined serially by time-domain NMR and bioimpedance spectroscopy, and anxiety, learning, and memory were assessed using the elevated plus maze and Morris water maze paradigms in later adulthood (PD63–PD69). During the diet intervention, body mass gains were suppressed in the low- compared with normal- and high-Na groups despite similar caloric uptake rates across groups. Fat mass was reduced in males but not in females fed low-Na diet. Fat-free mass and hydration were significantly reduced in both males and females fed the low-Na diet, although rapidly corrected after return to normal diet. Measures of anxiety-like behavior and learning in adulthood were not affected by diet in either sex, yet memory performance was modified by a complex interaction between sex and early life Na intake. These data support the concepts that Na deficiency impairs growth and that the amount of Na intake which supports optimal somatic growth during early life may be insufficient to fully support neurocognitive development.
Keywords: growth, neurobehavior, sodium
INTRODUCTION
The importance of sodium (Na) homeostasis for optimal growth in developing animals has long been recognized. Over 90 years ago, Mitchell and Carman (1) identified that rats and chicks demonstrated greater weight gain and nitrogen retention when provided rations with added NaCl and that the use of food energy for growth was impaired by inadequate NaCl intake. More recent studies in young, growing rats demonstrate that a Na-deficient diet impairs weight and length growth, diminishes nitrogen retention, and decreases muscle protein RNA synthesis (2). Weanling rats fed diets for 5 wk spanning a 30-fold range of Na concentrations demonstrate a linear relationship between rates of body weight gain and daily Na intake between 30 µmol/day and 300 µmol/day, whereas supplementation up to 900 µmol/day had no additional growth enhancing effect.
The need for adequate Na intake and the maintenance of a Na-replete state to optimize growth is also apparent in human infants. Preterm infants are at increased risk for Na deficiency because of high obligate urinary Na losses and the low Na concentration in breastmilk (3). We have previously hypothesized that a contributing factor to postnatal growth failure in preterm infants, which remains a significant morbidity in this population, is related to unrecognized total body Na depletion (4). We and others have shown that Na supplementation to preterm infants above that in their diet and above that currently recommended by many authorities results in improved postnatal growth (5, 6). The importance of this relationship is heightened by the strong association between in-hospital postnatal growth failure and impaired neurodevelopment (7–9).
Early life disturbances in Na homeostasis have been shown to be associated with cognitive and hearing impairments (10–12). However, understanding the relationship between Na and brain function is confounded in human studies by the presence of associated comorbidities and in animal studies by variation in models and duration of hyponatremia and lack of measurement of total body Na status. In addition, few neurobehavioral studies have addressed the impact of Na depletion remote from the period of depletion, with those studies in this area primarily focusing on alterations in Na appetite (13, 14). In a small, randomized trial performed in premature humans, infants receiving Na supplementation (4–5 mEq/kg/day) from postnatal days 4–14 had improved neurodevelopmental outcomes at 10–13 yr of age compared with nonsupplemented infants (1–1.5 mEq/kg/day) (15). However, impact of total body Na depletion during critical periods of development on later neurological and cognitive functions has not been extensively studied.
We therefore sought to establish a mouse model of early-life Na deficiency to further investigate the relationships among Na homeostasis, growth and metabolism, and neurobehavior. We hypothesized that Na deficiency during early life contributes to growth failure through altered energy flux and programs subsequent neurobehavioral impairment in later life.
MATERIALS AND METHODS
Animals
All animal use complied with the American Physiological Society’s Guiding Principles in the Care and Use of Laboratory Animals, the National Research Council’s Guide for the Care and Use of Laboratory Animals (16), and received prior approval from the Medical College of Wisconsin (MCW) Institutional Animal Care and Use Committee.
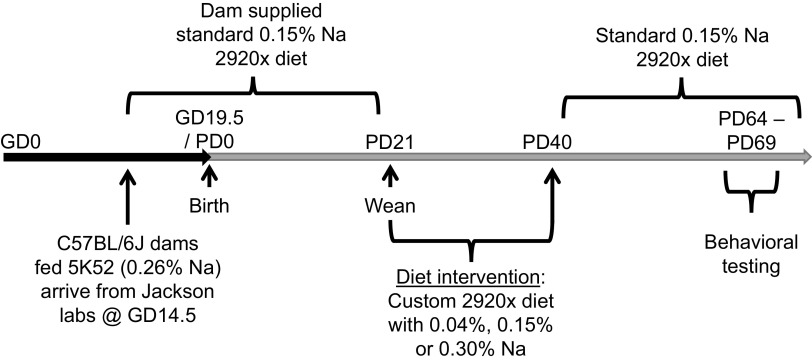
Timed-pregnant wild-type C57BL/6J dams were purchased from the Jackson Laboratories and arrived at MCW at gestational day 14.5. Animals at the Jackson Laboratories are maintained on LabDiet 5K52 (3.17 kcal/g metabolizable energy; 22% kcal from protein, 16% kcal from fat, and 62% kcal from carbohydrates; 0.26% Na, which is equivalent to 2.12 g Na/kg or 0.111 mEq Na/g). Upon arrival at MCW, dams were immediately switched to Envigo/Teklad 2920, which is a soy protein-free purified diet (3.13 kcal/g metabolizable energy; 24% kcal from protein, 16% kcal from fat, and 60% kcal from carbohydrates; 0.15% Na, which is equivalent to 1.5 g Na/kg or 0.065 mEq/g). All animals were maintained on a 14:10 light:dark cycle at standard room temperatures (∼22°C). Dams were allowed to deliver, and offspring were culled to 6 or 7 per dam by PD3 and then weaned at PD21 (Fig. 1).
Figure 1.
Study design. Graphic illustrating experiment design.
Dietary Intervention
At weaning on PD21, offspring from each litter were individually housed and randomly and evenly distributed within sex to one of three dietary interventions for the following 3 wk. Experimental diets included custom-modified versions of the 2920 diet, with either low (0.04% = 0.017 mEq Na/g), standard (0.15% = 0.065 mEq Na/g), or high (0.30% = 0.130 mEq Na/g) Na concentrations. Body masses and food intakes were assessed daily for the following 3 wk, and body composition was assessed weekly using time-domain nuclear magnetic resonance (TD-NMR). At PD40, mice were all returned to standard (0.15% Na) 2920 diet, and body masses and composition were assessed weekly until 10 wk of age (i.e., PD64–PD69) at which time anxiety, learning, and memory were assessed using the behavioral assays described in Elevated Plus Maze and Morris Water Maze. Feeding efficiency, representing the individual animal’s increase in body mass normalized to total calories ingested, was calculated by dividing the change in body mass from PD21 to PD40 by the cumulative caloric intake during this same timeframe.
Metabolic Cage Studies
In one cohort, mice were housed in individual single-mouse metabolic cages (Nalgene) for three overnight periods, to permit quantitative collection of urine and feces for analysis of Na and energy flux, as previously described (17).
Bomb Calorimetry
Energy density of food and fecal samples was determined by bomb calorimetry as previously described (17). Briefly, fecal samples collected during metabolic cage studies were desiccated, pressed into 200-mg pellets, and combusted to completion in a semimicro bomb calorimeter (Parr). Digestive efficiency was calculated as the fraction of calories that were absorbed (i.e., ingested but not present in fecal samples) divided by the total calories ingested, per day. Energy efficiency, representing the individual animal’s increase in body mass normalized to total calories absorbed, was calculated by dividing the change in body mass from PD21 to PD40 by the cumulative caloric absorption over this time period, which was estimated by multiplying measured food intake by the calculated digestive efficiency.
Time-Domain Nuclear Magnetic Resonance
Animals were weighed to the nearest 0.01 g. Body composition (fat mass, lean mass, and free water mass) was determined using TD-NMR via a Bruker LF110 system with mouse probe assembly, essentially as previously described (17). The TD-NMR system was calibrated by the manufacturer’s representative using mixes of oil for “fat,” lean chicken breast meat for “lean,” and saline or water for “free fluid” similarly to previous descriptions by other authors (18). Fat-free mass (FFM) was calculated as the total body mass minus fat mass as determined by TD-NMR. Total body water was calculated as 73.2% of FFM (19).
Bioimpedance Spectroscopy
Bioimpedance spectroscopy (BIS) was performed using an ImpediMed ImpediVET BIS1 system on a subset of animals within minutes after body composition analysis by TD-NMR, using a protocol that our team recently developed (20). Mice were anesthetized by isoflurane inhalant (2%) before electrodes were placed subcutaneously in locations recommended by the manufacturer and as previously described by others (21). Fluid compartmentalization in extracellular (ECF) and intracellular (ICF) spaces were then determined using the manufacturer’s recommended constants for mice (density = 1.05; proportion = 1; hydration = 0.732; males: RhoE = 998.9, RhoI = 1220.2; females: RhoE = 586.9, RhoI = 756.8). ECF and ICF data were then corrected using TD-NMR fat mass values. Total body osmotically active Na content was estimated in ECF and ICF spaces by multiplying ECF by 0.145 mol Na/L and ICF by 0.005 mol Na/L.
Elevated plus Maze
The elevated plus maze was performed essentially as previously (22, 23) using a maze consisting of two open arms (30 cm × 5 cm) and two enclosed arms (30 cm × 5 cm × 15 cm tall) extending from a central platform (5 cm × 5 cm) and suspended at 40 cm above the room floor. Mice were placed on the central platform facing an open arm, and distribution of behavior during the first 5 min of free exploration were recorded and analyzed using the AnyMaze software package (Stoelting Co.).
Morris Water Maze
The Morris water maze was performed essentially as previously described by others, with minor modifications (24). A circular pool with a diameter of 81.28 cm (32 in.; surface area = 5188 cm2) was filled with room temperature water (confirmed by NIST-traceable thermometer to be between 21°C and 24°C during training and testing phases) that was rendered opaque using a small amount of nontoxic water-soluble white paint. A square platform with sides of 7.62 cm (3 in.; surface area = 58 cm2, which represents 1.12% of the surface area of the pool) was anchored in the pool ∼16 cm away from the center of the circular pool. The pool was maintained in a room with various visual cues affixed to the walls. Starting at PD65, mice underwent daily training procedures which involved four 60-s trials in which animals were placed into the pool within the four cardinal quadrants in a randomized fashion and latency to find the platform was recorded. Animals that failed to find the platform within 60 s were gently guided to the platform and all animals were allowed to rest on the platform for 15 s. On PD65, the water depth was set so that the platform was visible above the surface of the water, and then for PD66–PD68, the water depth was set so the platform was submerged 1 cm below the surface. A single 30-s probe trial was performed 24 h after the final training session (i.e., on PD69), in which the platform was removed from the pool and time spent swimming within the area previously occupied by the platform was assessed. Animal behavior within the pool on training and probe trials was recorded by video and analyzed using the AnyMaze software package (Stoelting Co.).
Gene Expression
Renal gene expression was assessed in kidneys collected at PD70 by quantitative RT-PCR via SYBR Green following TRIzol-based RNA extraction, as previously described (25, 26). Primer sequences included renin (Ren): Forward 5′-TGAAGAAGGCTGTGCGGT-3′, Reverse 5′-TCCCAGGTCAAAGGAAATGTC-3′; the angiotensin II type 1a receptor (Agtr1a): Forward 5′-CCAAAGTCACCTGCATCATC-3′, Reverse 5′-CACAATCGCCATAATTATCCTA-3′; and remaining sequences were previously reported (26). β-Actin (Actb) was used as a loading control. Fold-change values were calculated using the method of Livak and Schmittgen (27), whereas analytical comparisons were performed on ΔCT values.
Statistics
Throughout, data are reported from individual animals where possible, and summary data are presented as means ± SE. Data were analyzed by simple linear regression, four-parameter nonlinear regression, one-sample t test, or two-way repeated-measures ANOVA followed by the Tukey’s multiple comparisons procedure, as indicated in individual figure legends, using GraphPad Prism v8.4. All comparisons were performed as two-tailed tests, and differences were considered significant with P < 0.05.
RESULTS
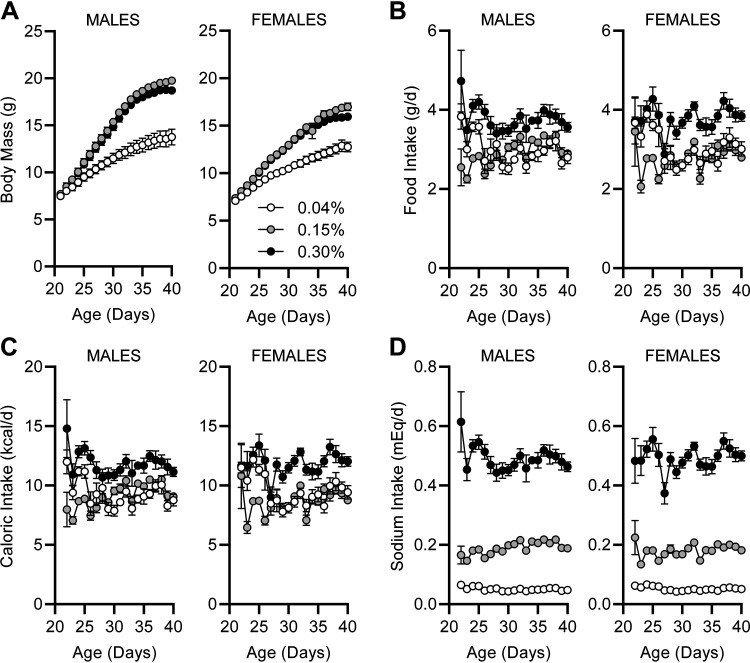
At weaning, offspring were randomly assigned to low (0.04%), standard (0.15%), or high (0.30%) Na versions of the 2920 diet. After randomization, body masses were indistinguishable at PD21 among mice assigned to each diet (Fig. 2A). Although growth rates were indistinguishable between 0.15% and 0.30% Na diets, mice of both sexes fed the 0.04% Na diet exhibited pronounced reductions in weight gain. Daily food intake of the animals was generally stable within each diet group across the following 3 wk, with the intake rates of 0.30% Na noticeably greater than the intake rates for 0.04% and 0.15% Na diets, regardless of sex (Fig. 2B). Because the metabolizable energy contents of the three diets were matched, caloric intake patterns paralleled the mass of food consumed (Fig. 2C). Importantly, Na intake patterns were stable over time in all three groups, with Na intake greatest in the 0.30% Na diet group, and lowest in the 0.04% Na diet group (Fig. 2D).
Figure 2.
Effects of postweaning dietary Na manipulation upon body mass and ingestive behaviors. A: body masses of mice while receiving custom-modified 2920 diets containing 0.04%, 0.15%, or 0.30% Na from weaning at PD21, for 3 wk. For both sexes, diet P < 0.05, age P < 0.005, and diet × age P < 0.05. By Tukey’s multiple comparisons procedure, 0.04% Na diet caused reduced weight gain (P < 0.05) vs. the other two diet groups starting at PD25. B: daily food intake by mass. For both sexes, diet P < 0.05, age P < 0.05, and diet × age P < 0.05. C: daily caloric ingestion. For both sexes, diet P < 0.05, age P < 0.05, and diet × age P < 0.05. D: daily Na ingestion. For males, diet P < 0.05, age P = 0.12, and diet × age P < 0.05, and for females, diet P < 0.05, age P = 0.05, and diet × age P = 0.08. For all panels, males: 0.04%, n = 13; 0.15%, n = 14; 0.30%, n = 14; and females: 0.04%, n = 14; 0.15%, n = 12; 0.30%, n = 13.
Comparison of body masses at PD40, at the end of the diet intervention, with masses at weaning (PD21) demonstrates that the 0.04% Na diet resulted in reduced weight gain compared with both 0.15% and 0.30% Na diets, regardless of sex (Fig. 3A). Interestingly, although differences between sexes are observed in mice fed the 0.15% and 0.30% Na diets, no effect of sex on body size is observed in mice fed the 0.04% Na diet. Total caloric intake, integrated over the 3-wk-intervention period, was similar between the 0.04% and 0.15% Na diets, but mice fed the 0.30% Na diet consumed significantly more calories regardless of sex (Fig. 3B). Calculation of feeding efficiency, which is the mass gained per calorie consumed and thereby considered a very rough and inverse metric of energy expenditure, was significantly reduced in mice fed 0.04% Na versus 0.15% Na. Mice fed 0.30% Na diet also unexpectedly exhibited a reduction in feeding efficiency relative to mice fed 0.15% Na diet (Fig. 3C).
Figure 3.
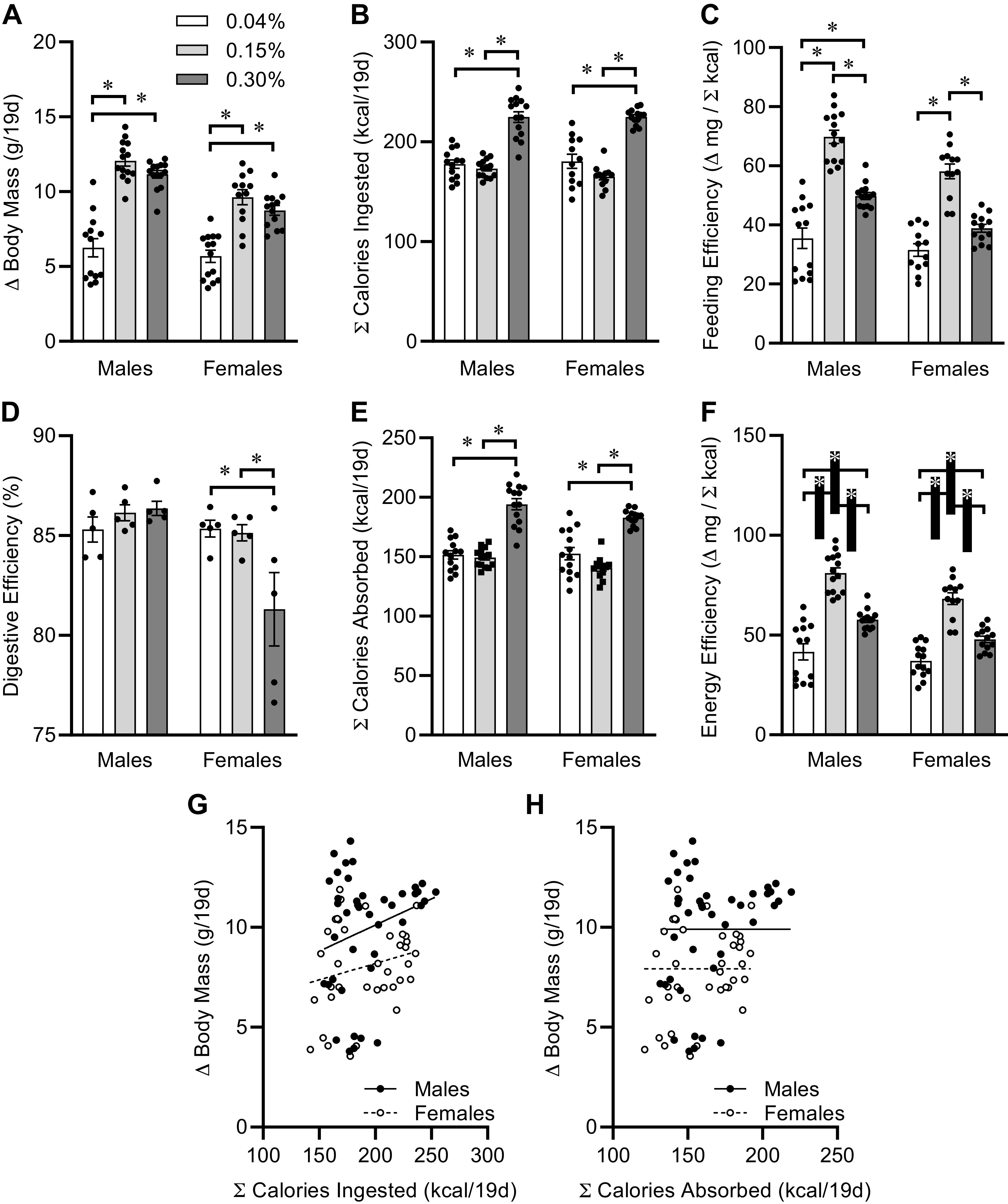
Contribution of caloric intake to postweaning growth. A: change in body masses from weaning at PD21 to PD40, of mice fed custom-modified 2920 diets containing 0.04%, 0.15%, or 0.30% Na. Diet P < 0.05, sex P < 0.05, diet × sex P < 0.05. B: total calories ingested PD21 to PD40. Diet P < 0.05, sex P = 0.63, diet × sex P = 0.43. C: feeding efficiency. Diet P < 0.05, sex P < 0.05, diet × sex P = 0.18. D: digestive efficiency determined on PD40 in a subset of animals. Diet P = 0.10, sex P < 0.05, diet × sex P < 0.05. E: estimate of total calories absorbed PD21 to PD40. Diet P < 0.05, sex P < 0.05, diet × sex P = 0.21. F: energy efficiency. Diet P < 0.05, sex P < 0.05, diet × sex P = 0.27. G: linear regression of change in body mass vs. total calories ingested, PD21 to PD40. Males R2 = 0.06, P = 0.12 vs. slope 0; females R2 = 0.05, P = 0.19 vs. slope 0. H: linear regression of change in body mass vs. total calories absorbed, PD21 to PD40. Males R2 = 0.07, P = 0.09 vs. slope 0; females R2 = 0.06, P = 0.14 vs. slope 0. For all panels, summary data presented as means ± SE, *P < 0.05 by Tukey’s multiple comparison procedure. Dots represent individual animals. For A–C and E–H, males: 0.04%, n = 13; 0.15%, n = 14; 0.30%, n = 14; and females: 0.04%, n = 12; 0.15%, n = 12; 0.30%, n = 13. For D, n = 5 for each group.
As we previously identified, an inverse effect of dietary Na concentration upon high-fat diet-induced weight gain that was mediated through the modulation of digestive efficiency (28), we next examined the effect of Na diets upon digestive efficiency in a subset of animals via bomb calorimetry. Interestingly, females fed 0.30% Na diet exhibited a reduction in digestive efficiency, but no effect of dietary Na was observed in males (Fig. 3D). Multiplying total caloric intake values for all mice by the average digestive efficiency rates determined for each diet and sex combination revealed the total caloric absorption rate (Fig. 3E). The resulting total caloric absorption rates largely paralleled the pattern of total caloric ingestion rate within each sex, with mice fed 0.30% Na absorbing more calories per unit time; but this correction for digestive efficiency uncovered a significant impact of sex on total calories entering the body. Finally, normalizing body mass gains for calories absorbed (i.e., energy efficiency) provided a more accurate inverse index of total energy expenditure. We observed that dietary Na modulated energy efficiency, indicating that dietary Na results in a complex inverted U-shaped effect upon caloric commitment toward growth (Fig. 3F). Water intake was assessed in this subset of mice, between PD37 and PD40, and no difference was observed among groups (data not shown) whether normalized for body mass (i.e., mL/day/g body mass; diet P = 0.12, sex P = 0.29, diet × sex P = 0.27) or not (i.e., mL/day; diet P = 0.65, sex P = 0.64, diet × sex P = 0.18).
To understand the relationships among body mass gains, caloric, and Na intake, we next performed regression analyses. First, within both sexes, no relationship was observed between body mass gains and total caloric intake behavior (Fig. 3G), as linear regression of these data sets yielded no mathematical relationships. Second, no statistically significant relationships were observed between body mass gains and total calories absorbed (Fig. 3H).
As expected, total Na consumption between PD21 and PD40 was dependent upon dietary Na content (Fig. 4A). Interestingly, although energy efficiency calculations indicated a complex U-shaped relationship between dietary Na intake and the commitment of calories toward growth (Fig. 3F), a similar calculation of Na efficiency (relating rates of somatic growth to Na intake) more clearly demonstrates a dose-dependent relationship (Fig. 4B). Animals fed lower concentrations of Na in the diet achieve greater somatic growth per amount of Na consumed. Furthermore, a complex asymptotic function was observed when comparing body mass gains versus Na intake between PD21 and PD40 (Fig. 4C). Although mass gains were indistinguishable within sex between mice fed 0.15% versus 0.30% Na diets, mice fed the 0.04% Na diet exhibited reduced somatic growth. Four-parameter sigmoidal curve fitting, with minimum constrained to 0, was applied to data within each sex. The hillslopes and EC50 inflection points of these lines of regression were indistinguishable between sexes, but the maximal growth rates were sex dependent and appear asymptotic. Collectively, these data support the concept that a minimum dietary concentration of Na, somewhere between 0.04% and 0.15% Na, is required for optimal somatic growth in young mice and that increased dietary Na concentration above 0.15% Na has no beneficial effect on this end point.
Figure 4.
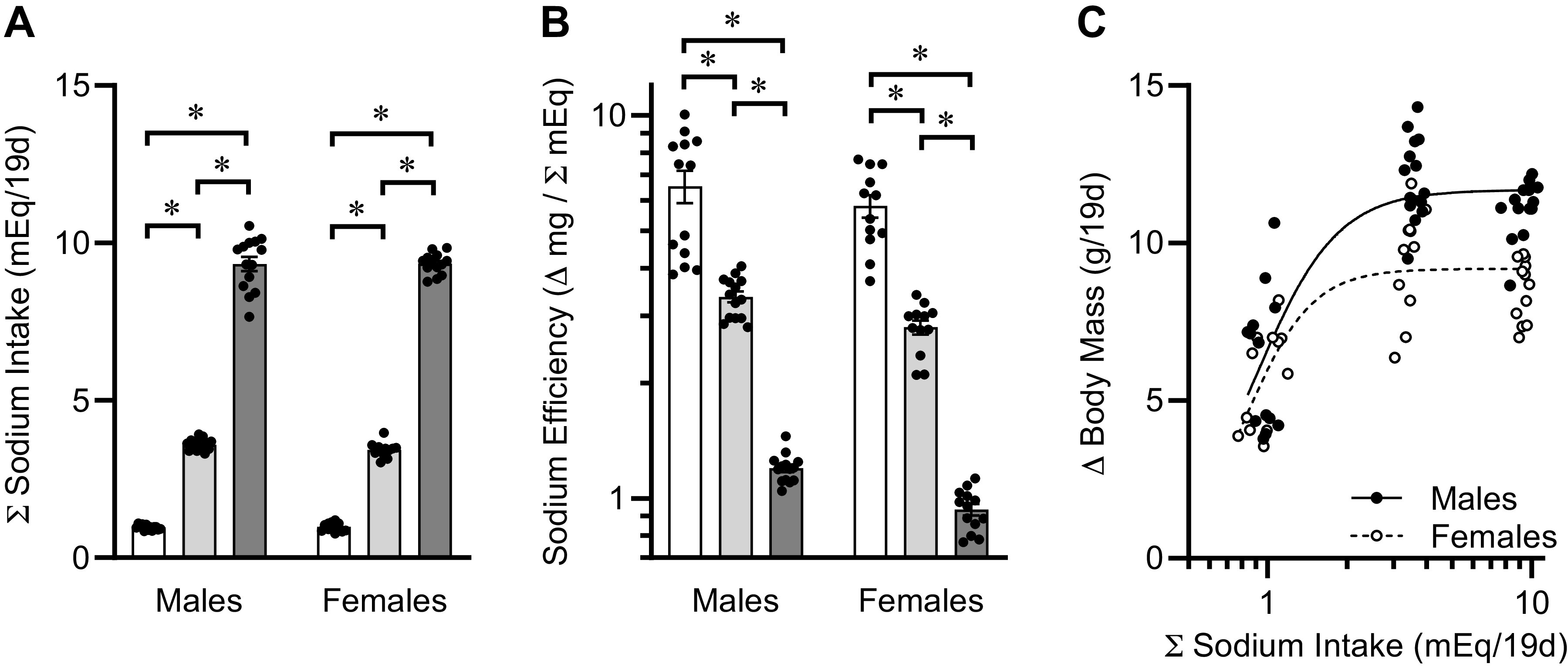
Contribution of Na intake to postweaning growth. A: total Na intake, PD21 to PD40, of mice fed custom-modified 2920 diets containing 0.04%, 0.15%, or 0.30% Na. Diet P < 0.05, sex P = 0.60, diet × sex P = 0.66. B: Na efficiency. Diet P < 0.05, sex P < 0.05, diet × sex P = 0.34. C: regression of body mass gains vs. total Na intake, PD21 to PD40. Nonlinear regression was performed using 4-parameter fit, with minimum constrained at 0. Hillslope for males = 2.85 ± 1.56 and females = 3.52 ± 1.63, P = 0.69. EC50 for males = 0.91 ± 0.06 and females = 0.84 ± 0.07, P = 0.40. Max growth for males = 11.69 ± 0.43 and females = 9.19 ± 0.31, P < 0.05. For all panels, summary data presented as means ± SE, *P < 0.05 by Tukey’s multiple comparison procedure. Dots represent individual animals. For all panels, males: 0.04%, n = 13; 0.15%, n = 14; 0.30%, n = 14; and females: 0.04%, n = 12; 0.15%, n = 12; 0.30%, n = 13.
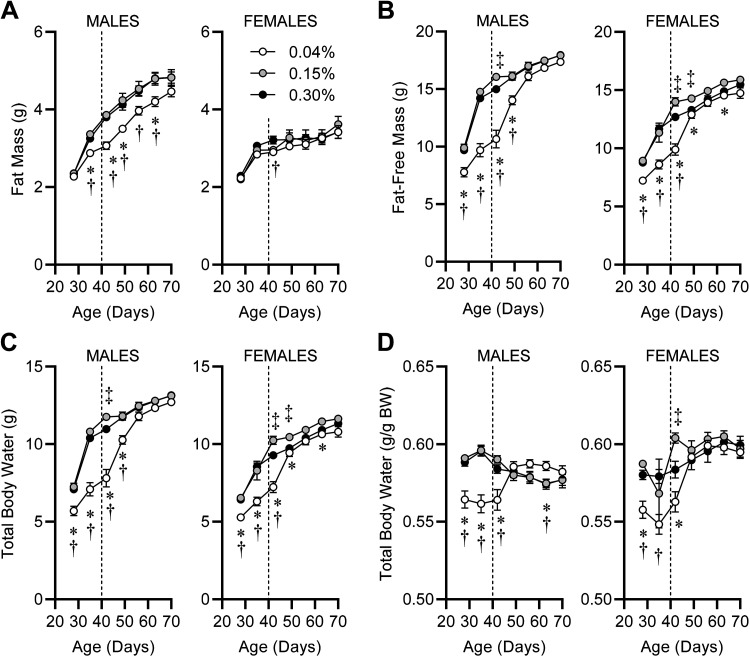
Analysis of body composition also highlighted dose-dependent effects of dietary Na, some of which were modified by sex. Total body fat mass was reduced in males fed 0.04% Na diet, but no difference in fat mass gains were observed in females regardless of diet (Fig. 5A). Fat-free mass was significantly reduced in both males and females fed the 0.04% Na diet, and this reduction was rapidly ameliorated upon return to standard 0.15% Na 2920 diet at PD40 (Fig. 5B). Many studies have demonstrated that postweaning animals of various species exhibit hydration at an essentially universal constant rate of 73.2% of fat-free mass (19, 29). Therefore, total body water was determined for the animals on each of the diets, and patterns were indistinguishable from fat-free mass data (Fig. 5C). Dividing total body water mass by total body mass demonstrated that animals fed 0.04% Na experienced dehydration (Fig. 5D). Although most healthy, lean animals exhibit total body water of ∼58%–60% of total body mass (21) and mice of both sexes in the current study fed 0.15% and 0.30% Na diets exhibited this typical range of hydration (Fig. 5D), mice of both sexes fed 0.04% Na exhibited significant dehydration during the dietary intervention (PD21–PD40) that rapidly corrected after return to standard 0.15% Na diet.
Figure 5.
Body composition during and following postwean dietary Na manipulation. A: fat masses from PD21 to PD70 of mice fed custom-modified 2920 diets containing 0.04%, 0.15%, or 0.30% Na. For males, diet P < 0.05, age P < 0.05, diet × age P < 0.05. For females, diet P = 0.41, age P < 0.05, diet × age P = 0.69. B: fat-free masses. For both sexes, diet P < 0.05, age P < 0.05, diet × age P < 0.05. C: total body water, estimated as 73.2% of fat-free mass. For both sexes, diet P < 0.05, age P < 0.05, diet × age P < 0.05. D: total body water normalized to total body mass. For males, diet P = 0.09, age P = 0.07, diet × age P < 0.05. For females, diet P = 0.05, age P < 0.05, diet × age P < 0.05. For all panels, *P < 0.05 for 0.04% vs. 0.15% Na diet; †P < 0.05 for 0.04% vs. 0.30% Na diet. ‡P < 0.05 for 0.15% vs. 0.30% Na diet by Tukey’s multiple comparison procedure. For all panels PD28–PD42, males: 0.04%, n = 13; 0.15%, n = 14; 0.30%, n = 14; and females: 0.04%, n = 14; 0.15%, n = 12; 0.30%, n = 13. For all panels PD49–PD70, n = 9 for all groups.
A closer examination of fluid balance at PD40, at the end of the 3-wk dietary intervention, illustrated the association between total body Na and somatic growth. Total body water at PD40 was significantly reduced in mice fed 0.04% Na versus the other two groups (Fig. 6A), and a small but statistically significant reduction in mice fed 0.30% versus 0.15% Na was also observed. These effects of diet were still present after normalization to total body mass (Fig. 6B). Dissection of extracellular (ECF) versus intracellular (ICF) fluid volumes by combined bioimpedance spectrometry and time-domain NMR demonstrated that total body water reductions in the extracellular (Fig. 6, C and D) and intracellular spaces (Fig. 6, E and F) generally paralleled observations of total body water. Importantly, quantification of these volumes allowed for estimation of total body osmotically active Na mass, by multiplication of ECF volume by the general constant of 145 mEq Na/L and the ICF volume by 5 mEq Na/L. Estimated total osmotically active Na throughout the body was significantly reduced in mice fed 0.04% Na diet, with no differences between mice fed 0.15% and 0.30% Na diets (Fig. 6G). Normalization of total body Na to total body mass generally eliminated this effect (Fig. 6H), leading to the concept that somatic growth is mathematically proportional to Na in the body, regardless of dietary Na concentration. Indeed, regression of total body mass, regardless of sex or diet, against total osmotically active Na throughout the body yielded a surprisingly tight linear relationship (R2 = 0.875, P < 0.001 vs. zero slope) (Fig. 6I). Together, these findings illustrate that regardless of dietary Na supply, total body Na stores are strongly associated with somatic growth.
Figure 6.
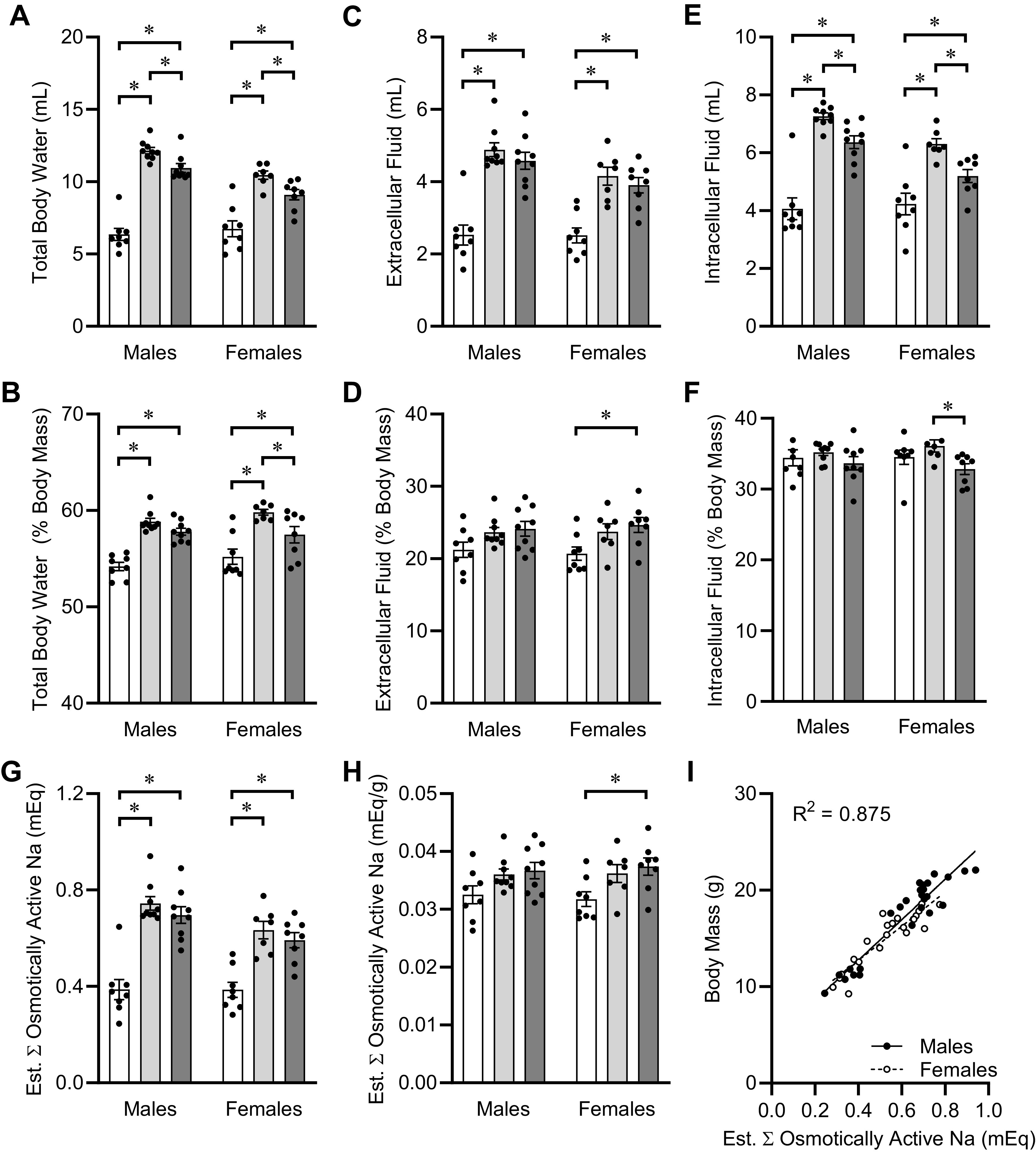
Fluid compartmentalization and total body Na content at PD40. A: total body water, estimated at 73.2% of fat-free mass by nuclear magnetic resonance (NMR). Diet P < 0.05, sex P < 0.05, diet × sex P < 0.05. B: total body water normalized to total body mass. Diet P < 0.05, sex P = 0.23, diet × sex P = 0.40. C: extracellular fluid, determined by bioimpedance spectroscopy (BIS). Diet P < 0.05, sex P < 0.05, diet × sex P = 0.24. D: extracellular fluid normalized to total body mass. Diet P < 0.05, sex P = 0.98, diet × sex P = 0.86. E: intracellular fluid, determined by BIS. Diet P < 0.05, sex P < 0.05, diet × sex P < 0.05. F: intracellular fluid normalized to total body mass. Diet P < 0.05, sex P = 0.94, diet × sex P = 0.61. G: estimated total osmotically active Na, assuming extracellular fluid at 145 mmol/L Na and intracellular fluid at 5 mmol/L Na. Diet P < 0.05, sex P < 0.05, diet × sex P = 0.21. H: osmotically active Na normalized to total body mass. Diet P < 0.05, sex P = 0.97, diet × sex P = 0.86. I: linear regression of total body mass vs. total osmotically active Na at PD40. Males R2 = 0.89, slope 20.98 ± 1.48 P < 0.05, vs. females R2 = 0.82, slope 17.66 ± 1.83 P < 0.05; comparison of slopes P = 0.18. For all panels, males: 0.04%, n = 8; 0.15%, n = 9; 0.30%, n = 9; and females: 0.04%, n = 8; 0.15%, n = 7; 0.30%, n = 8. *P < 0.05 by Tukey's multiple comparisons procedure.
In the 10th wk of age (i.e., PD64–PD69), mice underwent behavioral testing to assess programmed effects of early-life manipulations of dietary Na upon metrics of anxiety-like behaviors and spatial learning and memory during adulthood. Anxiety-like behaviors were assessed using the elevated plus-maze paradigm on PD64. During the single 5-min (300 s) trial, there were no differences in distribution of time spent between closed arms (0.04% Na diet, males 195.8 ± 7.8 and females 205.3 ± 7.5; 0.15% Na diet, males 183.4 ± 11.2 and females 201.4 ± 6.5; 0.30% Na diet, males 191.2 ± 14.0 and females 179.7 ± 7.9 s; diet P = 0.35, sex P = 0.52, diet × sex P = 0.42) versus the open arms plus center space among the Na-manipulated groups. Importantly, the mice in the current paradigm spent 65.0 ± 1.3% of the 5-min trial in the closed arm, which is indistinguishable from behaviors documented in previous publications involving exceptionally large cohorts (n > 900) of C57BL/6J mice (30).
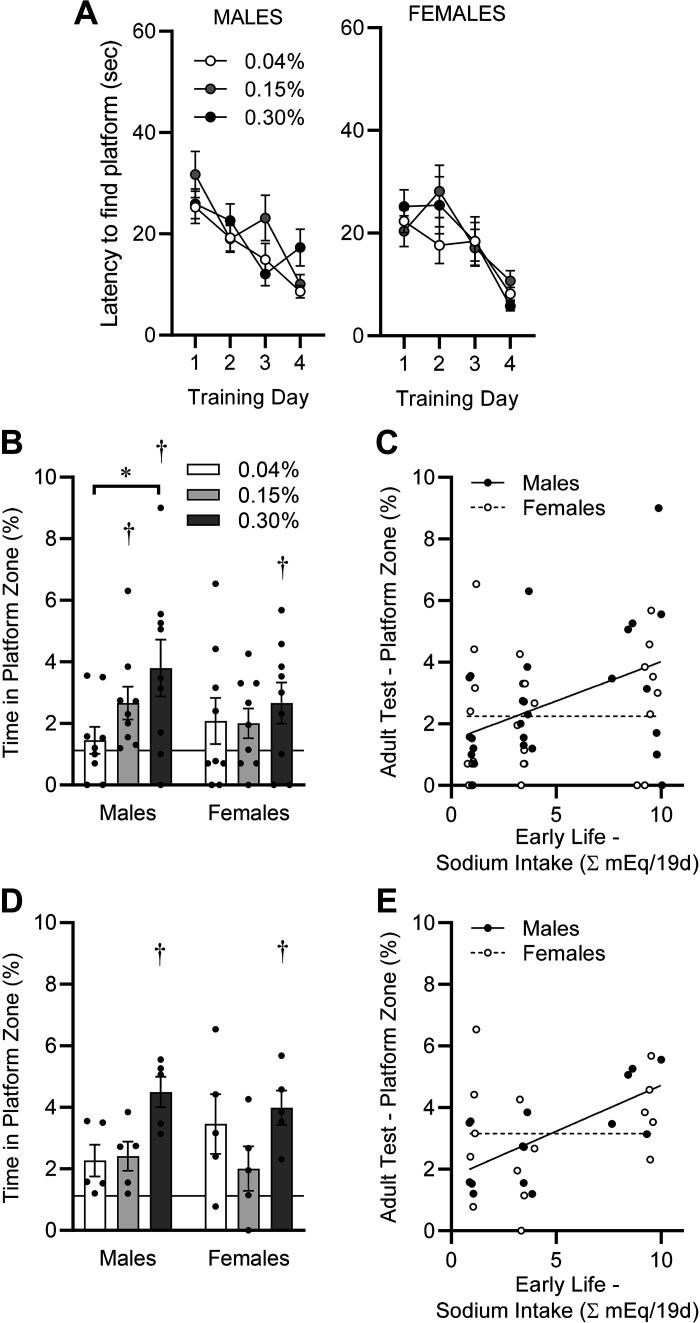
Spatial learning and memory were then assessed using the Morris water maze paradigm on PD65–PD69 using a round pool of 81.28 cm diameter and a square platform with sides of 7.62 cm (representing 1.12% of the surface area of the pool). Mice underwent four 60-s training trials on each of four consecutive days. On the first day, the platform was visible, and on the second through fourth days of training, the platform was submerged 1 cm within the opaque water. Performance (i.e., latency to swim to the platform) during the four training sessions within each day for each animal was averaged, and diet group performance was compared. All groups learned (acquired) the task across the 4 days of training as evidenced by a significant reduction in latency to reach the submerged platform, but no modulatory effects of dietary Na manipulations during the postweaning phase (PD21–PD40) were observed on acquisition rates (Fig. 7A). These data support similar spatial “learning” capabilities in mice of both sexes, regardless of early life Na exposure.
Figure 7.
Spatial learning and memory, assessed by Morris water maze paradigm, in adulthood (PD65–PD69). A: latency to find platform on training days. Platform was visible on training day 1 and submerged on subsequent training days. Males: diet P = 0.34, day P < 0.05, diet × day P = 0.05; females: diet P = 0.70, day P < 0.05, diet × day P = 0.36. B: time spent within area previously occupied by submerged platform during 30-s probe trial, performed 24 h after final training day. Horizontal line at 1.12% represents chance performance. Diet P = 0.09, sex P = 0.47, diet × sex P = 0.39. C: simple linear regression comparing probe trial performance on PD69 vs. integrated Na intake between PD21 and PD40. Males R2 = 0.18, and P = 0.03 vs. a slope of zero. Females R2 = 0.03 and P = 0.40 vs. a slope of zero. D: reanalysis of data from B, excluding animals that were exposed to isoflurane on PD40. Diet P = 0.01, sex P = 0.86, diet × sex P = 0.36. E: reanalysis of data from C, excluding animals that were exposed to isoflurane on PD40. Males R2 = 0.48 and P < 0.01 vs. a slope of zero. Females R2 = 0.06 and P = 0.39 vs. a slope of zero. For all panels, *P < 0.05 by Tukey’s multiple comparisons procedure, and †P < 0.05 vs. chance performance (1.12%) by one-sample t test. For A–C, n = 9 for all groups; for D and E, n = 5 for all groups.
On the 5th day, the submerged platform was removed from the pool, and swimming time within the area representing the same space as previously occupied by the platform was measured during a single 30-s probe trial. By one-sample t test, male and female mice previously fed 0.04% Na diet, and female mice previously fed 0.15% Na diet exhibited swimming time distributions that were statistically indistinguishable (P > 0.05) from chance (i.e., 1.12%), whereas male and female mice previously fed 0.30% Na and male mice previously fed 0.15% Na diet exhibited swimming time within the platform zone that was significantly greater than expected due to chance (P < 0.05). Furthermore, male mice exhibited an early-life dietary Na dose-dependent improvement in performance, as the 0.30% Na diet group spent significantly more time in the platform zone than the 0.04% Na diet group. In contrast, female mice appeared to exhibit no dose-dependent improvement (Fig. 7B). Underscoring these sex differences in the programming of adult performance on the Morris water maze task after early-life dietary Na intervention, simple linear regression of performance on this task at PD69 versus total Na intake between PD21 and PD40 in males uncovered a significantly non-zero relationship (P = 0.03) despite a relatively large coefficient of determination (R2 = 0.18) (Fig. 7C). This same comparison in females failed to detect any significant relationship (P = 0.40, R2 = 0.03).
It has previously been reported that exposure to isoflurane in young adulthood (3 mo of age) can program deficits specifically in spatial memory performance on the Morris water maze in rats, but notably that isoflurane has no negative effects on task acquisition/learning and retention effects in middle-aged rats (12 mo of age) are much less pronounced (31). Thus, we were concerned that exposure of a subset of animals to isoflurane at PD40, to enable bioimpedance spectroscopy measures, may have influenced performance of that subset of animals on the Morris water maze task. Reanalysis of the current data set (i.e., Fig. 7, B and C) was therefore performed using only animals that had not been exposed to isoflurane at PD40. Interestingly, with the removal of isoflurane-exposed animals from the data set, an effect of postweaning dietary Na manipulation was still clearly evident. Notably, in this reanalysis, male and female mice fed the 0.04% and 0.15% Na diets from PD21 to PD40 exhibited swimming time within the platform zone at PD69 that was indistinguishable from chance (1.12%), whereas male and female mice fed the 0.30% Na diet exhibited swimming time within the platform zone that was significantly greater than expected due to chance (P < 0.05) (Fig. 7D). Simple linear regression of swimming time within the platform zone at PD69 versus integrated Na intake from PD21 to PD40 within the no-isoflurane subset of animals again identified a significant nonzero relationship in males (R2 = 0.48, P < 0.01) but no relationship in females (R2 = 0.06, P = 0.39) (Fig. 7E).
At the conclusion of the study, animals were euthanized and masses of major cardiovascular tissues were assessed on PD70. A small but statistically significant difference in total body mass was noted between females that had previously received 0.04% versus 0.15% Na diets (Table 1). Although heart, liver, and adrenal masses were indistinguishable among diet groups within each sex, a notable effect of early-life Na manipulation upon total kidney mass persisted to PD70, with mice receiving the 0.04% Na diet from PD21 to PD40 exhibiting reduced kidney mass at PD70 even after normalization for total body mass.
Table 1.
Tissue masses at PD70
| End point | 0.04% | 0.15% | 0.30% | Diet P | Sex P | Diet × Sex P |
|---|---|---|---|---|---|---|
| Body Mass, g | M: 21.8 ± 0.4F: 18.2 ± 0.6 | M: 22.8 ± 0.4F: 19.5 ± 0.4 * | M: 22.8 ± 0.3F: 18.9 ± 0.3 | 0.0151 | <0.0001 | 0.7065 |
| Heart, mg | M: 100 ± 5F: 81 ± 6 | M: 98 ± 5F: 87 ± 5 | M: 95 ± 6F: 85 ± 4 | 0.6729 | 0.0021 | 0.5916 |
| Heart, mg/g | M: 4.6 ± 0.2F: 4.4 ± 0.3 | M: 4.3 ± 0.2F: 4.5 ± 0.2 | M: 4.2 ± 0.2F: 4.5 ± 0.2 | 0.6492 | 0.5697 | 0.5218 |
| Liver, g | M: 1.04 ± 0.05F: 0.88 ± 0.05 | M: 1.01 ± 0.06F: 0.94 ± 0.04 | M: 1.04 ± 0.02F: 0.85 ± 0.03 | 0.8912 | 0.0003 | 0.3994 |
| Liver, mg/g | M: 47.9 ± 2.2F: 48.4 ± 1.6 | M: 44.2 ± 2.3F: 47.8 ± 1.3 | M: 45.8 ± 0.8F: 45.0 ± 1.4 | 0.2367 | 0.4237 | 0.4254 |
| Avg. Kidney, mg | M: 121 ± 4F: 96 ± 5 | M: 131 ± 3F: 103 ± 2 | M: 133 ± 3*F: 106 ± 2 | 0.0047 | <0.0001 | 0.9053 |
| Avg. Kidney, mg/g | M: 5.54 ± 0.14F: 5.27 ± 0.14 | M: 5.76 ± 0.12F: 5.30 ± 0.10 | M: 5.83 ± 0.10F: 5.59 ± 0.08 | 0.0297 | 0.0011 | 0.6071 |
| Avg. Adrenal, mg | M: 3.07 ± 0.53F: 2.18 ± 0.36 | M: 3.28 ± 0.59F: 3.03 ± 0.60 | M: 2.77 ± 0.89F: 3.43 ± 0.78 | 0.6686 | 0.7618 | 0.4945 |
| Avg. Adrenal, mg/g | M: 0.14 ± 0.02F: 0.12 ± 0.02 | M: 0.15 ± 0.03F: 0.16 ± 0.03 | M: 0.12 ± 0.04F: 0.18 ± 0.04 | 0.7748 | 0.5169 | 0.4648 |
Data presented as means ± SE. For all groups, n = 9 males (M) and n = 9 females (F).
P < 0.05 vs. 0.04% Na diet within sex by Tukey’s multiple comparison procedure.
Finally, as a preliminary examination of the potential effects of early-life Na manipulations upon cardiovascular function in later adulthood, we examined expression of genes commonly associated with Na handling in kidney samples collected at PD70. Interestingly, we discovered that expression of Agtr1a (the angiotensin II type AT1A receptor), Slc12a3 (the Na-Cl co-transporter, NCC), Slc9a3 (the Na-H exchanger, NHE3), and Slc12a1 (the Na-K-Cl co-transporter, NKCC2) were significantly altered in kidneys of mice previously supplied varied Na diets (Table 2). Some gene expression patterns were altered by sex (i.e., Ren, Slc12a1, and Scnn1a) and others were subject to a complex interaction between diet × sex (i.e., Agtr1a). These findings support the concept that early-life dietary Na manipulation programs long-term changes in expression patterns of relevant genes within the kidney in addition to programming changes in spatial memory performance at this same age. Furthermore, these findings support the concept that the level of dietary Na supply in early life can program gene expression patterns in cardiovascular tissues in later life, and that most of these genes are influenced by a “dose” of dietary Na (i.e., >0.15%) that differs from the minimum amount of dietary Na required for normal somatic growth (i.e., between 0.04% and 0.15%). Future studies to fully understand the functional consequences of these programmed changes in gene expression patterns—and whether they confer cardiovascular protection or risk—are warranted.
Table 2.
Renal gene expression at PD70
|
Male |
Female |
DietP | SexP | Diet × SexP | |||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | 0.04%(n = 5) | 0.15%(n = 5) | 0.30%(n = 5) | 0.04%(n = 4) | 0.15%(n = 5) | 0.30%(n = 5) | |||
| Renin(Ren) | 1.34(1.18–1.52) | 1.00(0.87–1.15) | 1.05(0.89–1.24) | 1.95(1.56–2.44) | 2.18(1.89–2.51) | 1.64(1.53–1.75) | 0.37 | <0.01 | 0.34 |
| AT1A(Agtr1a) | 1.14 c(0.93–1.41) | 1.00 c(0.92–1.09) | 5.15a,b(3.15–8.43) | 0.85b(0.73–1.00) | 2.62a,c(2.10–3.25) | 2.39a(2.16–2.64) | <0.01 | 0.91 | <0.01 |
| NCC(Slc12a3) | 1.20c(1.06–1.35) | 1.00c(0.92–1.09) | 0.13a,b(0.10–0.16) | 1.54c(1.18–2.01) | 0.73c(0.47–1.15) | 0.19a,b(0.16–0.23) | <0.01 | 0.65 | 0.33 |
| NHE3(Slc9a3) | 1.13c(1.03–1.25) | 1.00c(0.94–1.07) | 1.64a,b(1.46–1.83) | 0.98c(0.86–1.12) | 1.22(1.12–1.33) | 1.37a(1.26–1.49) | <0.01 | 0.62 | 0.11 |
| Na-K-ATPase(Atp1a1) | 1.25(1.14–1.38) | 1.00(0.91–1.10) | 1.56(1.30–1.86) | 1.40(1.09–1.79) | 1.49(1.37–1.62) | 1.55(1.34–1.80) | 0.22 | 0.22 | 0.33 |
| NKCC2a(Slc12a1) | 1.33(1.25–1.41) | 1.00 c(0.95–1.06) | 1.65b(1.40–1.93) | 1.74(1.45–2.09) | 1.97(1.70–2.27) | 2.33(2.10–2.59) | 0.03 | <0.01 | 0.14 |
| ENaCα(Scnn1a) | 1.20(1.07–1.35) | 1.00(0.91–1.10) | 1.49(1.29–1.72) | 1.79(1.69–1.89) | 2.26(2.02–2.53) | 2.32(2.08–2.58) | 0.05 | <0.01 | 0.08 |
Data presented as means (±1 SE) fold of 0.15% Na diet male group.
aP < 0.05 vs. 0.04% within sex, bP < 0.05 vs. 0.15% group within sex, and cP < 0.05 vs. 0.30% group within sex by Tukey’s multiple comparison procedure.
DISCUSSION
The present study examined the relationships among decreased dietary Na intake during a 3-wk postweaning period, somatic growth, body composition, and behavior. Our data indicate that 1) somatic growth is restricted when Na intake is less than a critical value, but not enhanced by excess dietary Na, 2) Na depletion impairs energy efficiency but not caloric intake, resulting in growth failure independent of caloric intake, and 3) male but not female adult spatial memory is negatively programmed by reduced Na intake in early life. Importantly, the Na intakes that impact growth and memory appear to be different in males, with a higher Na intake necessary for optimal neurobehavioral development when compared with the Na intake necessary for optimal somatic growth. In females, growth is dependent on a similar minimum intake, however, spatial memory appears to be either unaffected, or may benefit from even higher intake than necessary for males (i.e., >0.30% Na diet) in early life. These findings support the concept that the amount of Na intake which supports optimal somatic growth during early life may be insufficient to fully support neurocognitive development, thereby programming cognitive deficits in later life even in animals with normal somatic growth.
Our findings on the effects of low-Na diet on growth and body composition complement and extend previous work in this area. In weanling rats, Na-deficient diets result in impaired weight gain and linear growth associated with decreased nitrogen and fat accretion, muscle protein, and RNA synthesis (1). In those studies, Na-deficient animals ingested similar or increased amounts of calories (total and per body weight) compared with Na-replete animals. We found both male and female mice on the 0.04% and 0.15% Na diets exhibited reduced caloric intake compared with 0.30% Na diet animals, though only animals fed the 0.04% Na diet displayed decreased weight gain. This dichotomy resulted from enhanced feeding and energy efficiency in the 0.15% Na diet group compared with the other groups. The inverted U-shaped pattern of feeding and energy efficiencies suggests these outcomes are very sensitive to the Na content of the diet and total Na intake. Reasons for Na-dependent differences in feeding and energy efficiency in newly weaned mice remain unclear but are unlikely to be related to an effect of digestive efficiency. Future detailed studies of energy expenditure mechanisms (including aerobic and anaerobic heat production, physical activity, etc.) in such animals will be necessary to better understand these relationships. Unfortunately, commonly employed equipment (such as, commercially available multiplexed systems for metabolic phenotyping) do not provide reliable detection of behaviors and respirometric gas exchange for mice weighing less than 10 g. Our team is working to develop appropriate technology to permit these types of mechanistic study.
The relationship between Na intake and changes in body mass in growing mammals appears asymptotic in nature. In the current study, total Na intakes of ∼100–150 µEq/day seen in animals placed on the 0.15% Na diet resulted in a similar increase in body mass as Na intakes of ∼450–525 µEq/day, observed in the 0.30% Na diet group. In contrast, Na intakes less than ∼50–80 µEq/day resulted in significantly decreased somatic growth. Similarly, Fine et al. (32) demonstrated a dose-response curve relating body weight gain and daily Na intake over a range of 30–300 µEq/day in weanling rats with no additional impact on growth with intakes of 600 µEq/day and 900 µEq/day. These findings suggest that a minimal Na intake is necessary for optimal growth (and that this minimal dose of Na is similar across rodent species), though excess Na has no growth-promoting effect.
Using NMR, we identified that growth impairment in the male 0.04% Na diet group was associated with decreased fat and fat-free masses, consistent with previous reports, whereas females showed only Na-dependent changes in fat-free mass. In contrast to previous reports, which found no effect on total body water (expressed as %BW), we found the 0.04% Na diet resulted in deceased total body water in both male and female animals. This was evident throughout the low Na diet period but normalized quickly upon return to standard 0.15% Na chow in both sexes. Not unexpectedly, the patterns of increased fat-free mass and total body water after return to normal Na chow were similar, reflective of water content of fat-free tissue. The mechanisms behind sex-dependent differences in fat versus fat-free mass gains may be related to differences in regional fat deposition or fatty acid metabolism, possibly secondary to differences in steroid hormones.
Using BIS coupled with TD-NMR, we examined the distribution of water within body compartments at the end of the dietary intervention period. The extracellular fluid compartment, expressed as % body mass, was similar in males across groups, though significantly decreased in females in 0.04% versus 0.30% Na diet groups. Intracellular fluid volume was also similar across all male groups and significantly decreased in 0.30% Na diet females compared with the 0.15%, but not 0.04% Na diet group. In weanling rats, Fine et al. (32) found extracellular fluid volume, expressed as % body mass, was significantly decreased and intracellular fluid volume increased in the most severely Na-restricted group (30 µEq/day), but not with diets providing 150–900 µEq/day of Na. A redistribution of body water from the extracellular to the intracellular space may be expected with severe Na depletion, as the vast majority of total body sodium is present within the extracellular fluid compartment and a decrease in effective extracellular osmoles would promote the movement of water from the extracellular to intracellular space. Reasons for the differences in our findings compared with those of Fine et al. may be related to species, duration and degree of sodium deprivation, and methodologies of the respective measurements. Furthermore, body water distribution results from a complex relationship among hydrodynamics, interstitial matrix biology, and solute concentrations which have not addressed in these studies (33).
The molecular mechanism(s) by which Na depletion results in growth failure have not been elucidated. Intestinal luminal Na participates in the absorption of glucose via the Na-glucose cotransporter (SGLT-1), thus a Na-deficient diet may impact carbohydrate absorption (34). However, in our series of studies, calories ingested, digestive efficiency, and calories absorbed were similar between animals on the 0.04% and 0.15% Na diets, despite significant differences in growth patterns, suggesting factors other than intestinal lumen Na concentration are responsible for the observed differences in growth among the groups. Haycock (35) hypothesized that reduced availability of Na in the extracellular fluid space results in decreased Na+/H+ antiporter activity across the cell membrane, increased intracellular H+ concentration, and decreased intracellular pH (pHi). Numerous growth factors normally stimulate Na+/H+ antiporter activity, which in turn raises pHi and stimulates biological mitogenic responses (36). In the presence of reduced availability of extracellular Na, antiporter activity is inhibited; decreasing pHi and impairing cell growth and division. Ray et al. (37) confirmed in young rats that Na deficiency significantly decreased muscle pHi associated with impaired weight and length growth. Knowing that changes as small as 0.01%–0.02% pH units may affect cellular responses to growth factors, Haycock’s hypothesis appears plausible (38). Alternatively or in addition, based upon previous work by us and others demonstrating critical roles of leptin in metabolic control and that perinatal sodium intake alters serum leptin levels, Na depletion may modify leptin signaling pathways to impact growth and body composition (39).
The programming effect of postweaning Na deprivation on neurobehavior was assessed at 9–10 wk of age. No effect of early life manipulations of dietary Na on anxiety-like behavior or spatial “learning” capabilities were observed in either sex. In contrast, animals of both sexes exhibited a Na diet-dependent effect on time in the platform zone during the Morris water maze probe trial phase, though sex-specific differences in the pattern of response exist. Specifically, males fed 0.04% Na diet in early life exhibited a time in the platform zone no different than that expected by chance, whereas males fed 0.15% and 0.30% Na diets during early life exhibited swimming times within the platform zone that are significantly greater than chance. Linear regression of Na intake in early life versus percent time in the platform zone underscored the early life Na dose-dependent effect on special “memory.” For females, only animals fed 0.30% Na diet in early life exhibited swimming time in the platform zone greater than chance, though by linear regression no effect of early life Na intake on measures of spatial memory was identified. Together, these findings support the conclusion that early life exposure to reduced dietary Na programs reductions in spatial memory during adulthood, despite normal metrics of anxiety and spatial learning. Furthermore, these initial studies of the growth and cognitive impacts of early-life Na manipulations hint that the dose dependencies of growth versus neurocognitive outcomes upon Na intake are distinct. For example, lower doses of Na (e.g., < 150 µEq/day) appear to inhibit both somatic growth and neurocognitive programming. Although somatic growth benefits from increased Na appear to plateau with early-life Na intakes between 150 µEq/day and 450 µEq/day, there may be continued increases in adult memory performance when animals are provided even higher Na in early life, at least in males.
Because of concerns regarding the potential impact of previous isoflurane exposure on Morris water maze performance (31), data from the initial cohort of animals that were never exposed to isoflurane were analyzed separately. Findings in this cohort were consistent were those of the combined cohorts; namely, animals of both sexes fed 0.30% Na diet displayed swimming time in platform zone during the probe trial greater than that expected due to chance, whereas the linear regression between Na intake and time in the platform zone was significant for males but not females. Collectively, these data support a modulatory effect of early life dietary Na exposure on adult spatial memory and point toward a robust modulatory effect of sex on this outcome. Furthermore, reanalysis of subgroups demonstrates that isoflurane exposure at PD40 programs significant reductions in performance on the Morris water maze recall task in C57BL/6J mice of both sexes, indicating that future studies should avoid and/or control for such confounding factors.
Given the established role of the hippocampus in spatial memory, future work should focus on the impact of early life Na deprivation upon synaptogenesis and signaling pathways in this area. The contribution of leptin pathways (which are impacted by early postnatal growth), the brain renin angiotensin system (which plays a recognized role in memory and cognition and may be programmed by early life Na depletion), and CREB and MAPK/ERK signaling cascades within the hippocampus (which are also implicated in spatial memory) (40–43), will all represent targets for future studies.
There are several limitations of our study that should be noted. The period of Na depletion examined in the current study appears to be rather late during brain development. It has been estimated that the weanling mouse hippocampus is at a developmental stage similar to the hippocampus of a term human infant (44). Thus, the period of Na deprivation in the present study may represent a developmental stage that occurs later than that which a premature infant would experience in a neonatal intensive care unit. Nonetheless, the links among Na deprivation, growth failure, and altered spatial memory are intriguing. Future studies should use a model of Na deprivation during murine maternal lactation. Additional measures of neurobehavioral outcomes are also necessary to more fully characterize the impact of Na intake on brain function and determine which domains of behavior are modified by early-life Na manipulations. Finally, in the current study, we took advantage of timed breeding program at the Jackson Laboratories to provide cohorts of dams on specific pregnancy timelines, but this may have contributed to some phenotypes due to the exposure of the dams to a diet with increased Na content during early pregnancy (vs. the 0.15% Na diet during late gestation and lactation), and due to any psychological and physiological stresses associated with shipping to our institution during gestation. We expect that because all dams experienced these stresses and offspring from each dam were included in every postweaning diet treatment group, that such early-gestational and shipping stresses are unlikely to explain observed differences in the offspring, but recognize the need to confirm the current results in future cohorts that are derived from local colonies that are maintained on a consistent diet throughout gestation and not exposed to additional stresses such as shipping.
Perspectives and Significance
Collectively, the findings presented here support the concept that somatic growth is associated with Na intake and total body Na content and that insufficient dietary Na supply reduces growth even when sufficient caloric intake is maintained. Furthermore, Na intake in early life programs specific domains of neurocognitive function, such as spatial memory. Interestingly, these effects of Na may be mediated through dissociable mechanisms, as the dose dependency of each effect of varied Na intake in males (i.e., growth vs. memory) is distinct. Our findings have important implications relative to the care of neonates and recognition of the importance of adequate Na intake to optimize growth and long-term neurodevelopment. In humans, Na depletion may occur in the face of unrecognized or unappreciated urine Na losses in excess of Na intake. We have argued in the past that this is a relatively common occurrence in extremely preterm infants and contributes to postnatal growth failure (4, 6). Although a relationship between postnatal growth failure and worse neurodevelopmental outcomes in the preterm infant population has been established, future studies may identify a cause-and-effect relationship.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL134850 and HL084207 and the American Heart Association Grant 18EIA33890055, the Medical College of Wisconsin Clinical & Translational Science Institute “Obesity” Ensemble Grant UL1TR001436, and the Advancing a Healthier Wisconsin Endowment to Medical College of Wisconsin.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L.S. and C.C.G. conceived and designed research; J.L.S., C.C.G., K.B., M.L.R., J.J.R., and J.L.G. performed experiments; J.L.S., C.C.G., K.B., M.L.R., J.J.R., and J.L.G. analyzed data; J.L.S., C.C.G., and J.J.R. interpreted results of experiments; J.L.S. drafted manuscript; J.L.S., C.C.G., and J.J.R. edited and revised manuscript; J.L.S., C.C.G., K.B., M.L.R., J.J.R., and J.L.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Kelsey Wackman, Jennifer Sterrett, Olivia Eckes, Ko-Ting Lu, Chetan N. Patil, Curt D. Sigmund, and Anne E. Kwitek along with the MCW Biomedical Resource Center, Comprehensive Rodent Metabolic Phenotyping Core, and the Neuroscience Research Center Rodent Behavior Core, for technical and intellectual assistance on the project.
REFERENCES
- 1.Mitchell HH, Carman GG. Does the addition of sodium chloride increase the value of a corn ration for growing animals? J Biol Chem 68: 165–181, 1926. doi: 10.1016/S0021-9258(18)84683-X. [DOI] [Google Scholar]
- 2.Wassner SJ. Altered growth and protein turnover in rats fed sodium-deficient diets. Pediatr Res 26: 608–613, 1989. doi: 10.1203/00006450-198912000-00019. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization, International Atomic Energy. Minor and Trace Elements in Breast Milk: Report of a Joint WHO/IAEA Collaborative Study. Atlanta, Geneva: World Health Organization, 1989. [Google Scholar]
- 4.Segar JL, Grobe CC, Grobe JL. Fetal storage of osmotically inactive sodium. Am J Physiol Regul Integr Comp Physiol 318: R512–R514, 2020. doi: 10.1152/ajpregu.00336.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segar DE, Segar EK, Harshman LA, Dagle JM, Carlson SJ, Segar JL. Physiological approach to sodium supplementation in preterm infants. Amer J Perinatol 35: 994–1000, 2018. doi: 10.1055/s-0038-1632366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segar JL. A physiological approach to fluid and electrolyte management of the preterm infant: review. J Neonatal Perinatal Med 13: 11–19, 2020. doi: 10.3233/NPM-190309. [DOI] [PubMed] [Google Scholar]
- 7.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 117: 1253–1261, 2006. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 8.Guellec I, Lapillonne A, Marret S, Picaud JC, Mitanchez D, Charkaluk ML, Fresson J, Arnaud C, Flamant C, Cambonie G, Kaminski M, Roze JC, Ancel PY; Étude Épidémiologique sur les Petits Âges Gestationnels (EPIPAGE; [Epidemiological Study on Small Gestational Ages]) Study Group. Effect of intra- and extrauterine growth on long-term neurologic outcomes of very preterm infants. J Pediatr 175: 93–99.e1, 2016. doi: 10.1016/j.jpeds.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Sammallahti S, Pyhälä R, Lahti M, Lahti J, Pesonen AK, Heinonen K, Hovi P, Eriksson JG, Strang-Karlsson S, Andersson S, Järvenpää AL, Kajantie E, Räikkönen K. Infant growth after preterm birth and neurocognitive abilities in young adulthood. J Pediatr 165: 1109–1115, 2014. doi: 10.1016/j.jpeds.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Baraton L, Ancel PY, Flamant C, Orsonneau JL, Darmaun D, Rozé JC. Impact of changes in serum sodium levels on 2-year neurologic outcomes for very preterm neonates. Pediatrics 124: e655–e661, 2009. doi: 10.1542/peds.2008-3415. [DOI] [PubMed] [Google Scholar]
- 11.Ertl T, Hadzsiev K, Vincze O, Pytel J, Szabo I, Sulyok E. Hyponatremia and sensorineural hearing loss in preterm infants. Biol Neonate 79: 109–112, 2001. doi: 10.1159/000047076. [DOI] [PubMed] [Google Scholar]
- 12.Lee HJ, Lee BS, Do HJ, Oh SH, Choi YS, Chung SH, Kim EA, Kim KS. Early sodium and fluid intake and severe intraventricular hemorrhage in extremely low birth weight infants. J Korean Med Sci 30: 283–289, 2015. doi: 10.3346/jkms.2015.30.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leshem M. The ontogeny of salt hunger in the rat. Neurosci Biobehav Rev 23: 649–659, 1999. doi: 10.1016/S0149-7634(98)00059-1. [DOI] [PubMed] [Google Scholar]
- 14.Leshem M, Maroun M, Del Canho S. Sodium depletion and maternal separation in the suckling rat increase its salt intake when adult. Physiol Behav 59: 199–204, 1996. doi: 10.1016/0031-9384(95)02059-4. [DOI] [PubMed] [Google Scholar]
- 15.Al-Dahhan J, Jannoun L, Haycock GB. Effect of salt supplementation of newborn premature infants on neurodevelopmental outcome at 10-13 years of age. Arch Dis Child Fetal Neonatal Ed 86: F120–F123, 2002. doi: 10.1136/fn.86.2.F120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Acadamies Press, 2011. [Google Scholar]
- 17.Grobe JL. Comprehensive assessments of energy balance in mice. Meth Mol Biol 1614: 123–146, 2017. doi: 10.1007/978-1-4939-7030-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morla L, Shore O, Lynch IJ, Merritt ME, Wingo CS. A noninvasive method to study the evolution of extracellular fluid volume in mice using time-domain nuclear magnetic resonance. Am J Physiol Renal Physiol 319: F115–F124, 2020. doi: 10.1152/ajprenal.00377.2019. [DOI] [PubMed] [Google Scholar]
- 19.Sheng HP, Huggins RA. A review of body composition studies with emphasis on total body water and fat. Am J Clin Nutr 32: 630–647, 1979. doi: 10.1093/ajcn/32.3.630. [DOI] [PubMed] [Google Scholar]
- 20.Segar JL, Balapattabi K, Reho JJ, Grobe CC, Burnett CML, Grobe JL. Quantification of body fluid compartmentalization by combined time-domain nuclear magnetic resonance and bioimpedance spectroscopy. Am J Physiol Regul Integr Comp Physiol 320: R44–R54, 2020. doi: 10.1152/ajpregu.00227.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman ME, Hu L, Plato CF, Kohan DE. Bioimpedance spectroscopy for the estimation of body fluid volumes in mice. Am J Physiol Renal Physiol 299: F280–F283, 2010. doi: 10.1152/ajprenal.00113.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, de Lange WJ, Li H, Sakai K, Thedens DR, Cassis LA, Rahmouni K, Mark AL, Johnson AK, Sigmund CD. The brain Renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab 12: 431–442, 2010. doi: 10.1016/j.cmet.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther 318: 304–311, 2006. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- 24.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1: 848–858, 2006. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claflin KE, Sandgren JA, Lambertz AM, Weidemann BJ, Littlejohn NK, Burnett CM, Pearson NA, Morgan DA, Gibson-Corley KN, Rahmouni K, Grobe JL. Angiotensin AT1A receptors on leptin receptor-expressing cells control resting metabolism. J Clin Invest 127: 1414–1424, 2017. doi: 10.1172/JCI88641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Littlejohn NK, Siel RB Jr, Ketsawatsomkron P, Pelham CJ, Pearson NA, Hilzendeger AM, Buehrer BA, Weidemann BJ, Li H, Davis DR, Thompson AP, Liu X, Cassell MD, Sigmund CD, Grobe JL. Hypertension in mice with transgenic activation of the brain renin-angiotensin system is vasopressin dependent. Am J Physiol Regul Integr Comp Physiol 304: R818–R828, 2013. doi: 10.1152/ajpregu.00082.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) Method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Weidemann BJ, Voong S, Morales-Santiago FI, Kahn MZ, Ni J, Littlejohn NK, Claflin KE, Burnett CM, Pearson NA, Lutter ML, Grobe JL. Dietary sodium suppresses digestive efficiency via the renin-angiotensin system. Sci Rep 5: 11123, 2015. doi: 10.1038/srep11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritz P. Body water spaces and cellular hydration during healthy aging. Ann NY Acad Sci 904: 474–483, 2000. doi: 10.1111/j.1749-6632.2000.tb06502.x. [DOI] [PubMed] [Google Scholar]
- 30.Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. J Vis Exp : 1088, 2008. doi: 10.3791/1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Callaway JK, Jones NC, Royse CF. Isoflurane induces cognitive deficits in the Morris water maze task in rats. Eur J Anaesthesiol 29: 239–245, 2012. doi: 10.1097/EJA.0b013e32835103c1. [DOI] [PubMed] [Google Scholar]
- 32.Fine BP, Ty A, Lestrange N, Levine OR. Sodium deprivation growth failure in the rat: alterations in tissue composition and fluid spaces. J Nutr 117: 1623–1628, 1987. doi: 10.1093/jn/117.9.1623. [DOI] [PubMed] [Google Scholar]
- 33.Bhave G, Neilson EG. Body fluid dynamics: back to the future. J Am Soc Nephrol 22: 2166–2181, 2011. doi: 10.1681/ASN.2011080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poulsen SB, Fenton RA, Rieg T. Sodium-glucose cotransport. Curr Opin Nephrol Hypertens 24: 463–469, 2015. doi: 10.1097/MNH.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haycock GB. The influence of sodium on growth in infancy. Pediatr Nephrol 7: 871–875, 1993. doi: 10.1007/BF01213376. [DOI] [PubMed] [Google Scholar]
- 36.Seifter JL, Aronson PS. Properties and physiologic roles of the plasma membrane sodium-hydrogen exchanger. J Clin Invest 78: 859–864, 1986. doi: 10.1172/JCI112671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray PE, Lyon RC, Ruley EJ, Holliday MA. Sodium or chloride deficiency lowers muscle intracellular pH in growing rats. Pediatr Nephrol 10: 33–37, 1996. doi: 10.1007/BF00863436. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka Y, Hayashi N, Kaneko A, Ito T, Horimoto M, Sasaki Y, Kasahara A, Fusamoto H, Kamada T. Characterization of signaling pathways to Na+/H+ exchanger activation with epidermal growth factor in hepatocytes. Hepatology 20: 966–974, 1994. doi: 10.1002/hep.1840200428. [DOI] [PubMed] [Google Scholar]
- 39.Lopes KL, Furukawa LN, de Oliveira IB, Dolnikoff MS, Heimann JC. Perinatal salt restriction: a new pathway to programming adiposity indices in adult female Wistar rats. Life Sci 82: 728–732, 2008. doi: 10.1016/j.lfs.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Dexter BC, Rahmouni K, Cushman T, Hermann GM, Ni C, Nopoulos PC, Thedens DL, Roghair RD. Neonatal leptin deficiency reduces frontal cortex volumes and programs adult hyperactivity in mice. Behav Brain Res 263: 115–121, 2014. doi: 10.1016/j.bbr.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer LR, Zhu V, Miller A, Roghair RD. Growth restriction, leptin, and the programming of adult behavior in mice. Behav Brain Res 275: 131–135, 2014. doi: 10.1016/j.bbr.2014.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peotta V, Rahmouni K, Segar JL, Morgan DA, Pitz KM, Rice OM, Roghair RD. Neonatal growth restriction-related leptin deficiency enhances leptin-triggered sympathetic activation and central angiotensin II receptor-dependent stress-evoked hypertension. Pediatr Res 80: 244–251, 2016. doi: 10.1038/pr.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinbrekera B, Colaizy TT, Vasilakos LK, Johnson KJ, Santillan DA, Haskell SE, Roghair RD. Origins of neonatal leptin deficiency in preterm infants. Pediatr Res 85: 1016–1023, 2019. doi: 10.1038/s41390-019-0359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stagni F, Giacomini A, Guidi S, Ciani E, Bartesaghi R. Timing of therapies for Down syndrome: the sooner, the better. Front Behav Neurosci 9: 265, 2015. doi: 10.3389/fnbeh.2015.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]