Abstract
The endothelial glycocalyx is a specialized extracellular matrix that covers the apical side of vascular endothelial cells, projecting into the lumen of blood vessels. The composition of the glycocalyx has been studied in great detail, and it is known to be composed of a mixture of proteoglycans, glycosaminoglycans, and glycoproteins. Although this structure was once believed to be a passive physical barrier, it is now recognized as a multifunctional and dynamic structure that participates in many vascular processes, including but not limited to vascular permeability, inflammation, thrombosis, mechanotransduction, and cytokine signaling. Because of its participation in many physiological and pathophysiological states, comprehensive knowledge of the glycocalyx will aid future vascular biologists in their research. With that in mind, this review discusses the biochemical structure of the glycocalyx and its function in many vascular physiological processes. We also briefly review a more recent discovery in glycocalyx biology, the placental glycocalyx.
Keywords: extracellular matrix, glycocalyx, vascular
INTRODUCTION
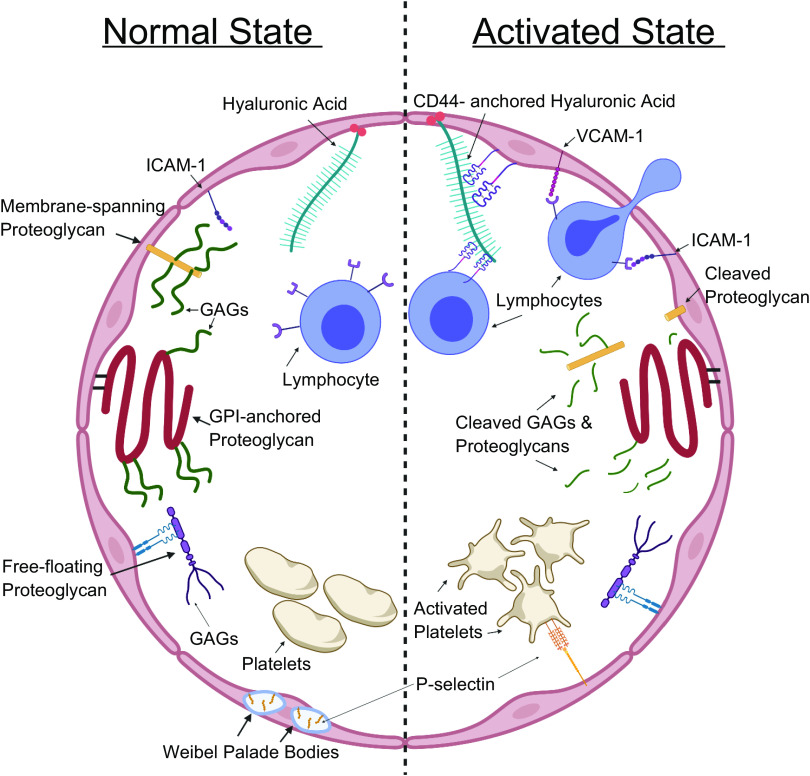
All eukaryotic cells possess an extracellular matrix consisting of heavily glycosylated proteins, sugars, and lipids. The extracellular matrix has multiple functions, including regulating cell signaling and intercellular adhesion, sensing the extracellular environment, and controlling the bioavailability of signaling molecules. In the last century, scientists discovered a specialized form of extracellular matrix located on the luminal side of endothelial cells. This specialized structure has been termed the endothelial glycocalyx, which is shown in detail in Fig. 1. Similar to the traditional functions of the extracellular matrix, the vascular endothelial glycocalyx regulates many extracellular functions, such as signal transduction, cell adhesion, and localized deposition of growth factors and other signaling molecules. In addition to these, the glycocalyx performs some functions unique to the vasculature, which include maintaining selective vascular permeability, modulating thrombosis, and regulating leukocyte extravasation. Because vascular dysfunction is associated with a wide variety of diseases and because of the crucial role of the glycocalyx in maintaining proper vascular function, the significance of the glycocalyx in disease has become a topic of intensive investigation. Altered endothelial glycocalyx thickness and composition are also associated with an increasing number of pathologies, of which sepsis, hypertension, diabetes, and diseases with endothelial dysfunction are included (1–3). Although the extent of the alterations in endothelial glycocalyx composition during diseases and the mediators of these alterations are not fully understood, they offer promising novel therapeutic targets to preserve endothelial function.
Figure 1.
A cartoon of the glycocalyx of a cross-sectioned blood vessel divided into a “normal state” side and an “activated state” side. Normal state: under normal conditions, the glycocalyx protrudes into the lumen of blood vessels and obscures adhesion molecules like intercellular adhesion molecule 1 (ICAM-1). Other adhesion molecules like P-selectin are stored in Weibel–Palade bodies. Its proteoglycans and glycosaminoglycans actively inhibit lymphocytes’ and platelets’ interactions with the underlying endothelial cells. Activated state: activated endothelium and lymphocytes degrade the glycocalyx, exposing underlying adhesion molecules. Other adhesion molecules such as vascular cell adhesion molecule 1 (VCAM-1) are upregulated in activated endothelium and Weibel–Palade bodies are mobilized to the surface of endothelial cells with their contents. GAGs, glycosaminoglycans; GPI, glycosylphosphatidylinositol.
More recently, a glycocalyx-like structure has been identified at the maternal blood/fetal tissue interface in the placenta. During pregnancy placental syncytiotrophoblasts produce a similar extracellular structure which lies in direct contact with maternal blood (4). In preeclampsia, a disease with well-characterized endothelial dysfunction and endothelial glycocalyx alterations, there are also alterations in this placental glycocalyx (5). However, the placental glycocalyx has not been thoroughly studied and the extent of its general, as well as its unique, characteristics and functions have yet to be elucidated. This review will describe the molecular structure of the major constituents of the glycocalyx as well as its noteworthy physiological functions.
DETERMINING THE STRUCTURAL DIMENSIONS OF THE GLYCOCALYX
One of the most difficult and contentious problems in studying the glycocalyx is determining its actual size. Most early attempts to visualize the glycocalyx failed to capture a complete image due to its hydrophilic nature and the fact that most histological techniques dehydrated and altered its appearance (6, 7). Upon dehydration, the glycocalyx, which normally “stands up” and protrudes into the vascular lumen, lies flat along the endothelial wall and cannot be seen or measured accurately. Because of these limitations, early estimates of the glycocalyx thickness were ∼20 nanometers (8). However, it was not long before theoretical estimations of the glycocalyx thickness began to contradict morphometric measurements. In Vivo studies attempting to measure capillary hematocrit soon theorized that a slow-moving plasma layer actually extended up to 1 micrometer into the lumen of capillaries (9). It is now understood that this slow plasma movement is because of solute and protein drag caused by molecular interactions with the glycocalyx.
Vink et al. (10) utilized an intravital microscopy technique to visualize the endothelial glycocalyx in hamster cremaster muscle capillaries. By observing red blood cell passage and visualizing distribution of the plasma with a large fluorescent dextran that would be incapable of penetrating the glycocalyx, a zone was found that did not include plasma or red blood cells. However, this zone extended farther into the lumen than the anatomically identified endothelium. By subtracting the diameter of the red blood cell and plasma zones from the fluorescently determined inner vessel diameter, they found the capillary glycocalyx thickness to be ∼0.5 micrometers (10). Interactions between the glycocalyx and plasma components, which can contribute greatly to the variability in the apparent functional thickness of the barrier, have also been described (11). Fluorescent imaging of the glycocalyx has advanced and now often utilizes FITC-labeled wheat germ agglutinin, as it specifically binds sugar residues and allows clearer resolution of glycocalyx boundaries (12). Methods such as this one are now frequently used to assess glycocalyx degradation in models of human disease. It is clear from these studies that the glycocalyx significantly protrudes into the vascular lumen and presents itself as a physiologically relevant structure.
BIOCHEMICAL COMPOSITION OF THE GLYCOCALYX
Proteoglycans
The endothelial glycocalyx is a luminal extracellular barrier composed of proteins, carbohydrates, and lipids that is in dynamic equilibrium with its own local microenvironment. The structure of the glycocalyx is primarily composed of proteoglycans, glycoasaminoglycans (GAGs), and glycoproteins (Fig. 1). Proteoglycans are either membrane bound or “free-floating” proteins that have long cores located in the extracellular space with unbranched carbohydrate side chains, called GAGs, attached to them. The composition of the COOH-terminal end of the proteoglycan determines whether it is membrane bound or free floating, while the composition of the peptide core determines what type of and how many GAG side chains will be attached to the proteoglycan.
The most commonly studied proteoglycans are membrane bound and are attached to cell membranes in one of two ways. The first is a simple single-span transmembrane domain, which anchors the proteoglycan to the cell membrane through hydrophilic interactions. Membrane-spanning proteoglycans can also possess short cytoplasmic domains that sometimes interact with internal cellular structures such as signaling cascade molecules or the cytoskeleton (13). Syndecans, first discovered by Saunders et al. (14), are a typical example of a membrane-spanning proteoglycan family and are one of the most abundant proteoglycan families in the endothelial glycocalyx. The second way proteoglycans can be attached to cell membranes is through a glycosylphosphatidylinositol (GPI) anchor on their COOH terminus (15). David et al. (16) were the first to identify the predominant GPI-anchored proteoglycan, glypican, in 1990. Furthermore, Brown et al. (17) and Lisanti et al. (18) have both described the importance of GPI anchors on localizing proteins to apical cell surfaces. Therefore, GPI anchors might play a large role in the localization of proteins specific to the luminal (apical) glycocalyx and could be a mechanism by which the glycocalyx is distinguished from the basolateral extracellular matrix.
Other free-floating proteoglycans can be located entirely in the extracellular space while having no direct interactions with cell membranes. They are, however, suspended by strong interactions with other extracellular components to which they are bound (19). Perlecan is a typical example of a free-floating proteoglycan and is synthesized by endothelial cells (20) and contains no transmembrane domains (21). Other free-floating vascular proteoglycans include mimecan, biglycan, decorin, and versican. By studying the murine and human sequences of perlecan, one can see that it possesses many unique domains in its protein structure that allow it to bind not only GAGs, but cell surface receptors, like fibroblast growth factor receptor, as well (21–23). The variety of proteoglycans is complemented by diverse structures that determine GAG interactions, membrane anchoring mechanisms, and intracellular signaling cascade interactions, among other properties.
Glycosaminoglycans
Most proteoglycans are covalently modified with GAG side chains, which create a mechanical barrier and provide binding sites for many growth factors and other molecules (24). GAGs are long, linear polysaccharide molecules composed of repeating disaccharide subunits. GAGs such as heparan sulfate, chondroitin sulfate, dermatan sulfate, and karatan sulfate are commonly found on proteoglycans in various combinations. For example, all syndecans bind three to five heparan sulfate molecules, but the larger syndecans, -1 and -3, have additional binding domains for chondroitin sulfate (25), and can therefore contain both of these GAGs. Approximately 50–90% of endothelial cell proteoglycans bind heparan sulfate (26), which is believed to be the most abundant GAG in humans and is often a centerpiece for glycocalyx research.
GAGs are synthesized on proteoglycans as posttranslational modifications in the Golgi apparatus before secretion, beginning with the addition of a tetrasaccharide linker molecule on a serine residue of the proteoglycan core (14). The “acidic-X-Ser-Gly-acidic” sequence has been suggested as the GAG-binding domain on proteoglycans, with slight variations between binding domains for different GAGs (27). GAGs are most commonly attached to proteoglycan cores by O-linked glycosylation, however keratan sulfate can be attached by O- or N-linked glycosylation (28). After linkage, polymerization of the polysaccharide chain is initiated with alternating additions of the two saccharide constituents specific to each GAG (29). The specific disaccharide sequences for each GAG are as follows: heparan sulfate is composed of glucuronic acid and N-acetylglucosamine; keratan sulfate is composed of galacturonic acid and N-acetylglucosamine; chondroitin sulfate is composed of glucuronic acid and N-acetylgalactosamine; and dermatan sulfate is also composed of glucuronic acid and N-acetylgalactosamine (30). Although they have the same disaccharide composition, chondroitin sulfate and dermatan sulfate are distinguished from one another through their unique structural modifications.
During or immediately after formation of the polysaccharide chain, structural modifications begin, the first of which is the deacetylation and sulfation of individual sugar residues. This process replaces many N-acetyl and hydroxyl groups of the saccharide units with sulfate groups, resulting in N- and O-linked sulfate groups (31–33). The extent of sulfation can vary greatly and has significant effects on function, as reviewed in depth by Soares da Costa et al. (34). Next, many of the glucuronic acid residues are epimerized to their chiral counterpart, iduronic acid, in soon-to-be heparan sulfate and dermatan sulfate molecules. N-deacetylation and subsequent N-sulfation are prerequisites for the epimerization of glucuronic acid to iduronic acid (35). The characteristic that distinguishes dermatan sulfate from chondroitin sulfate, both of which have the same initial disaccharide composition, is the conversion of many, if not most of, dermatan sulfate’s glucuronic acid residues to iduronic acid through epimerization (36). Because of its similarity to chondroitin sulfate, dermatan sulfate is sometimes referred to as chondroitin sulfate B, but in this review it will be denoted dermatan sulfate to avoid confusion. Due to the large number of acetyl and sulfate groups they all possess, all GAGs carry a massive net-negative charge. Negative charge is imperative for the biological activity of GAGs (37).
Hyaluronic acid, the longest GAG, is unique from other GAGs in many ways: it is not synthesized in the Golgi, is not sulfated, and is not bound to proteoglycans. Hyaluronic acid is synthesized by membrane-bound enzymes that secrete it into the extracellular matrix as it is synthesized (38). Because it is simultaneously synthesized and secreted into the extracellular matrix, hyaluronic acid does not undergo Golgi-based structural modifications like sulfation or epimerization. Although hyaluronic acid is not bound to proteoglycan cores, it interacts with other molecules such as CD44 (39) and hyaluronic acid synthases, which anchor and localize the molecule to the luminal side of endothelial cells. Hyaluronic acid is composed of repeating subunits of glucuronic acid and N-acetylglucosamine and still retains a substantial negative charge due to its carboxyl and acetyl groups.
Glycoproteins
Glycoproteins are another class of molecule that contributes to the structural make-up of the glycocalyx. Glycoproteins are membrane-bound proteins that differ from proteoglycans in that they are glycosylated with small, branched carbohydrate side chains instead of long, unbranched sidechains. Members of the glycoprotein class that are relevant to this review are mostly adhesion molecules, such as selectins, integrins, and immunoglobulin-like molecules.
Within the selectin family, E-selectin (40, 41) and P-selectin (42) are two members that are expressed on endothelial cells. Neither is constitutively expressed under basal conditions, but rather they are upregulated during endothelial activation by inflammatory processes. Selectins possess an NH2 terminus lectin domain, an epidermal growth factor-like domain, two to nine consensus repeats, a transmembrane domain, and a small cytoplasmic tail (41). The NH2 terminus lectin domains bind ligands, primarily carbohydrate polymers found on leukocytes, to initiate the characteristic “rolling” that is the first step to leukocyte extravasation (43). Additional importance has been attributed to the epidermal growth factor-like domain immediately adjacent to the lectin domain, which has been suggested as an additional modulator of selectin-ligand binding (44).
Integrins are named for their roles as integral membrane proteins. They connect the extracellular matrix with the cytoskeleton and are heavily implicated in signal transduction pathways (45). Heterodimeric integrin molecules are composed of one α- and one β-subunit, and specific integrin molecules are defined by their unique combinations of subunits. Varying combinations of the 18 α-subunits and 8 β-subunits give rise to functional integrins that bind collagen, laminin, and various ligands (46).
The last glycoproteins of concern are those of the immunoglobulin superfamily, of which vascular cell adhesion molecule 1 (VCAM-1), intracellular adhesion molecule 1 and 2 (ICAM-1 and -2), and platelet/endothelial cell adhesion molecule 1 (PECAM-1) are well known members. These adhesion molecules contain numerous repeats of immunoglobulin-like domains, a transmembrane domain, and cytoplasmic tails that activate intracellular signaling pathways (47). Immunoglobulin superfamily adhesion molecules serve as ligands for many growth factors and integrins located on the surfaces of leukocytes (48). Although there are additional glycoproteins that could be described, selectins, integrins, and immunoglobulin-like molecules illustrate the diverse biochemical composition of the glycocalyx and also constitute the majority of its composition.
PHYSIOLOGICAL FUNCTION
Early theories often underappreciated the significance of the glycocalyx, and many investigators deemed it a static, passive barrier. However, because of its systemic presence and its location between the blood stream and the endothelium, it would be reasonable to assume that the endothelial glycocalyx has an important role in many vascular processes. Currently, the glycocalyx is known to regulate vascular permeability, cellular interactions with the endothelium, mechanical signal transduction, and molecular bioavailability and signaling. Many of these functions have been thoroughly studied, and the molecules that contribute to them are well characterized, but there are still large knowledge gaps that leave an incomplete picture of how the glycocalyx functions as a whole.
Vascular Permeability
One of the first roles of the endothelial glycocalyx discovered was its maintenance of selective vascular permeability. Early studies in frog mesenteric capillaries revealed that degradation of the glycocalyx by protease mixtures increases capillary permeability (49), reinforcing previous notions that the glycocalyx could act in tandem with endothelial cells to control passage of fluid and solutes between the capillaries and interstitial spaces. Since then, the importance of the glycocalyx as a determinant of vascular permeability in capillaries has been solidified (50), and it has even been shown to contribute significantly to permeability in larger vessels such as arteries (51).
Recall the structure of GAGs, which extend far into the lumen of vessels and have a large net negative charge. It is through these mechanical and electrostatic properties that the glycocalyx limits passage of larger and/or more negatively charged particles out of blood vessels (52, 53). Many studies have demonstrated this through the use of variably sized, fluorescently labeled dextran molecules, demonstrating that large dextrans penetrate the glycocalyx layer much less, and at a greatly reduced rate, than smaller dextran molecules (51). Typically, dextrans of 70 kDa and larger are excluded form a bulky region of the glycocalyx extending as much as 0.5 micrometers from endothelial cells into the lumen of vessels (10). In reality, the permeability of the glycocalyx exists in a gradient where increasingly smaller molecules are restricted as they move closer to the endothelial cell membrane.
The endothelial glycocalyx also regulates passage of molecules on the basis of their electrostatic charge. By comparison of the penetration of anionic and neutral dyes of the same size, it has been shown that charge affects a molecule’s ability to penetrate into the glycocalyx as well as its rate of penetration (50). Specifically, the more negative a molecule’s charge is, the less permeable the glycocalyx will be to its passage. This is due to the repelling force generated by negatively charged GAGs. A study supporting this fact demonstrated that, when glycocalyx charge was neutralized with protamine sulfate, endothelial cells had increased uptake of albumin, which is usually repelled because of its negative charge (54). Together, these studies implicate the negative charge of the glycocalyx as an important deterrent for negatively charged plasma proteins.
Considering the properties that govern fluid filtration across a membrane, one can see that the glycocalyx plays a significant role in this process as well. Capillary filtration is determined by the sum of a capillary’s Starling forces, otherwise known as net filtration pressure, multiplied by the capillary filtration coefficient, which is an expression of the capacity of the capillary to filter water for a given net filtration pressure. As a constituent of the capillary physical barrier, the glycocalyx partially determines the capillary filtration coefficient by mechanically impeding the flow of fluids, but it also affects two of the four Starling forces (55). By limiting the passage of plasma proteins and other molecules, the glycocalyx aids the maintenance of capillary and interstitial oncotic forces, thus acting as an important factor for the determination of the net filtration pressure and, by extension, fluid filtration.
Although the contribution of the glycocalyx to maintaining selective vascular permeability is clear, it is still unknown whether or not alterations in glycocalyx structure play a role in normal physiological processes that regulate vascular permeability. Although stimuli such as atrial natriuretic peptide, vascular endothelial growth factor, and histamine are known to affect vascular permeability, which of these might work mechanistically through alterations in glycocalyx structure is still unknown.
Thrombomodulation
In addition to regulating solute interactions with blood vessel walls, the glycocalyx also restricts cellular interactions, primarily through mechanical impedance. In fact, some of the earliest studies that theorized the existence of a glycocalyx layer investigated its role in limiting the access of red blood cells to the endothelial layer. They found that modifications of glycocalyx thickness with heparinase (the bacterial equivalent of heparanase), which cleaves heparan sulfate chains, can alter capillary hematocrit and suggested that physiological stimuli that alter capillary hematocrit may do so through altering glycocalyx thickness (56). Since then, the cellular exclusion zone accredited to the glycocalyx has been proven to include platelets and leukocytes as well.
Platelets are excluded from contact with endothelial cells under normal conditions to prevent unnecessary clot formation. The thickness of the glycocalyx, which can reach up to 0.5 micrometers in capillaries (10) and 3 micrometers in arteries (51), causes steric hindrance of platelet interactions with endothelial cells. Adhesion molecules extend only nanometers from cell surface layers, making it nearly impossible for molecular interactions to occur without some initial perturbation of the overlying glycocalyx layer. By inhibiting platelet interactions with the endothelium in normal conditions, the glycocalyx prevents clot formation except in local environments where circumstances favor thrombosis (57).
Apart from steric properties, there are also molecular properties of the glycocalyx that regulate clot formation. For example, glypican has a high affinity for binding antithrombin III, which contributes to the antithrombotic properties of the endothelial glycocalyx by localizing antithrombin III to the surface of endothelial cells (58). Heparan sulfate is a GAG that directly interacts with and localizes antithrombin III (58). However, for unknown reasons, glypican’s affinity for antithrombin III is still higher than that of other proteoglycans that also contain these same GAGs (59). The difference in molecular affinities between the same GAGs located on different proteoglycans suggests that proteoglycan core structure may contribute to these molecular interactions. It is possible that a unique high-level structural arrangement of some proteoglycans provides ideal binding pockets for certain molecular interactions.
The glycocalyx’s role in clot formation is not limited to impairment, though, as it also contains adhesion molecules that aid in platelet binding and clot formation. P-selectin and PECAM mediate adhesion of platelets onto activated endothelial cells to initiate clot formation (60, 61). P-selectin is stored in endothelial cell secretory granules, called Weibel–Palade bodies, which are mobilized to the cell surface upon endothelial stimulation by inflammatory factors like histamine and thrombin (62, 63). Platelets also store P-selectin in secretory α-granules until they are activated (64). Platelets and some endothelial cells, under chronic inflammatory conditions, express P-selectin glycoprotein ligand 1, which mediates their binding to one another during clot formation (65, 66). During conditions that favor clotting, platelets release an enzyme, platelet endoglycosidase, that cleaves endothelial heparan sulfate (67). After heparan sulfate is cleaved by this enzyme, it is degraded further, leaving proteoglycan cores with little to none of the heparan sulfate chain left attached (68, 69). Endothelial ligands for platelet P-selectin molecules are short and are usually obscured by the glycocalyx; thus, degradation of the glycocalyx uncovers binding sites for these adhesion molecules. Therefore, the enzymatic cleavage of heparan sulfate chains, which also likely results in the loss of heparan sulfate-bound antithrombin III, provides platelets with a mechanism to alter the endothelial glycocalyx to facilitate clot formation. At least one luminal integrin, integrin αVβ3, is involved in clot formation. Endothelial integrin αVβ3 promotes activated platelet-endothelial cell binding by forming bridges with bound fibrinogen, fibronectin, and von Willebrand factor (70). Taken together, this information clearly demonstrates that the glycocalyx is crucial in the regulation of clotting.
Immunomodulation
Leukocyte interactions with the endothelium are also closely regulated by the glycocalyx. The glycocalyx is responsible for steric hindrance in leukocyte-endothelial cell interactions much in the same way it is for platelet interactions. Mulivor et al. demonstrated that glycocalyx degradation with heparinase increased the adhesion of fluorescently labeled microspheres coated with ICAM-1 antibody (71). These results suggest degradation of the glycocalyx, and the resulting exposure of adhesion molecules and ligands is an important step in the inflammatory process. Furthermore, Constantinescu et al. demonstrated that degradation of the glycocalyx by oxidized low-density lipoprotein increases the number of localized immobilized leukocytes in mouse cremaster venules (72). Interestingly, this process was attenuated through the administration of heparin and heparan sulfate. Experimental models of sepsis and ischemia/reperfusion have also strongly implicated degradation of the glycocalyx as a necessary process for leukocyte adhesion and infiltration (73, 74). Other studies have found that heparin and heparan sulfate exhibit anti-inflammatory actions through the binding and resultant blockade of P- and L-selectin, and that 6-O-sulfation of the heparin or heparan sulfate molecules is required for these effects (75, 76). Although these studies used exogenous heparin and heparan sulfate, it is possible that endogenous proteoglycan-bound heparan sulfate also interacts with and blocks P- and L-selectins to inhibit inflammation. Heparan sulfate degradation could therefore be a mechanism by which endothelial cells and leukocytes regulate specific, localized inflammation.
Additional inflammatory consequences of glycocalyx degradation result from the cleaved particles themselves. Circulating dermatan sulfate has been shown to activate NF-κB in endothelial cells (77). NF-κB regulates the expression of adhesion molecules like ICAM-1 (77) and VCAM-1 (14), as well as many important cytokines and chemokines associated with inflammation. Soluble heparan sulfate fragments act as ligands for Toll-like receptor 4, a strong inducer of proinflammatory cytokine production (78). The ability of shed glycocalyx constituents to activate Toll-like receptors and the NF-κB pathway provides another mechanism by which inflammation can be controlled through the degradation of glycocalyx constituents.
Several components of the glycocalyx are also involved in the processes of leukocyte rolling, adhesion, and diapedesis. Hyaluronic acid participates in the early stages of leukocyte extravasation and is an important factor in initiating and localizing inflammatory responses. Hyaluronic acid localization on endothelial cell surfaces is controlled by TNFα signaling through the expression of the hyaluronic acid-binding form of CD44 (79). Lymphoid cells can bind and roll onto CD44-anchored hyaluronic acid (80). E-selectin and P-selectin also mediate leukocyte recruitment and their binding to activated platelets and activated endothelial cells (81, 82). Additionally, endothelial VCAM-1 and ICAM-1 molecules participate in a tight binding to leukocytes, known as adhesion, which brings the cells into close contact with the endothelium (83, 84). Finally, PECAM regulates leukocyte transendothelial migration, which is the passage between adjacent endothelial cells, and leukocyte migration through the endothelial basement membrane (85). E-selectin, P-selectin, and VCAM-1 are not found on the surface of endothelial cells under normal conditions; rather, they are found only on activated endothelium, such as that stimulated by TNFα (82, 86). Activation of endothelium also upregulates some constitutively expressed adhesion molecules such as ICAM-1 and PECAM (82, 86). Upregulation of adhesion molecules by activated endothelial cells allows leukocytes to focus on specific areas of inflammation.
As stated above, endothelial NF-κB can be upregulated by particles of a degraded glycocalyx (77). NF-κB is a downstream molecule of the TNFα pathway and participates in activating endothelial cells, a process that changes endothelial expression patterns to favor inflammation (87). Degradation of glycocalyx constituents also exposes adhesion molecules and ligands for adhesion molecules (71–74). In this way, glycocalyx degradation acts a double-edged sword, whereby degradation of the glycocalyx not only exposes binding sites and ligands for adhesion molecules but also directly leads to activation of endothelial cells and upregulation of adhesion molecule expression through shed fragments. The ability of glycocalyx degradation not only to reveal binding sites on adhesion molecules but also to upregulate expression of adhesion molecules is a powerful proinflammatory mechanism.
There are also molecular-based anti-inflammatory actions of the glycocalyx, although few have been characterized. GAG-antithrombin III interactions appear to inhibit inflammation through nonantithrombotic pathways that are not fully understood but involve a reduction of leukocyte-endothelial cell contact (59, 88). There are likely other glycocalyx components that inhibit inflammation through molecular interactions, but they are not thoroughly studied.
Although angiogenesis may seem unrelated to inflammation, the process relies heavily on leukocytes and also utilizes some of the same glycocalyx components that are involved in inflammation. For example, E-selectin masking by heparan sulfate regulates angiogenesis through unique interactions with leukocytes. Also, E-selectin may be responsible for the recruitment of endothelial progenitor cells to areas of angiogenesis (89), and it has been suggested that E-selectin binding to endothelial cells may be regulated by heparan/chondroitin sulfate proteoglycans such as syndecan-1 (90). In summary, the glycocalyx regulates many inflammatory processes, especially those having to do with leukocyte recruitment and the activation of inflammatory pathways.
Mechanical Signal Transduction
Because of its luminal location, the glycocalyx is positioned to participate in sensing shear stress and mechanotransduction. The influence of shear stress on endothelial morphology and function is well characterized but has only recently begun to be attributed to the glycocalyx. For instance, conformational changes in the glycocalyx induce nitric oxide release, causing changes in vessel diameter to allow appropriate tissue perfusion (2). The molecular mechanisms of mechanosensation for many flow-sensitive signaling pathways, such as those attributed to G protein-coupled receptors, also result from conformational changes in the glycocalyx (91). More recent research has revealed that specific cell signaling events are dependent on which glycocalyx component acts as the mechanosensor. Mechanical signal transduction by glypican, a plasma membrane-associated molecule, regulates nitric oxide production, which explains its location in caveolae alongside endothelial nitric oxide synthase (92). Meanwhile, the COOH-terminal associations of transmembrane proteins like syndecans allow them to control cytoskeletal rearrangement when sensing changes in the extracellular environment (93).
Heparan sulfate proteoglycans mediate shear stress-induced gene expression of endothelial nitric oxide synthase, cyclooxygenase-2, von Willebrand factor, and VE-cadherin in mouse endothelial cells (94). Shear stress-induced expression of these genes is greatly reduced in the presence of heparinase III, indicating that proteoglycans containing heparan sulfate side chains participate either directly in mechanosensation or indirectly by mediating the response in some other way. In further support of the specific link between glycocalyx components and mechanical signal transduction, another study demonstrated that degradation of heparan sulfate, but not chondroitin sulfate, inhibited shear-induced nitric oxide production (95). This study brings forth an interesting question about whether the type of GAGs on a proteoglycan determine the signaling properties of the underlying proteoglycans or whether the proteoglycans have predetermined signaling pathways they are a part of and the GAGs act solely as accessories to sense the extracellular environment. Further work demonstrated that degradation of hyaluronic acid by hyaluronidase also inhibits shear-induced nitric oxide production (96).
Interestingly, the structure and thickness of the glycocalyx can also be influenced by shear stress. For example, hyaluronic acid production is upregulated in endothelial cells exposed to shear stress (97). It has also been observed that glycocalyx thickness in the divider region of the mouse carotid bifurcation is many times thicker than that in the sinus region of the carotid bifurcation (98). Though these regions lie immediately opposite each other in the internal carotid artery, the divider region experiences undisturbed high laminar flow. Higher shear stress due to this pattern of flow is likely the cause of the differences in glycocalyx thickness of the two regions.
The glycocalyx is an important intermediate between mechanical sensation of fluid shear stress and cell signaling pathways. The most common effect attributed to the glycocalyx’s mechanosensation is nitric oxide production, but many other actions are also regulated through its sensation of the extracellular environment. The composition of the glycocalyx can also be regulated by its own sensation of shear stress in a feed-forward process that may serve to protect endothelial cells from mechanical damage form high levels of shear stress.
Localization and Regulation of Soluble Factors
Although the glycocalyx is often characterized by its ability to repel molecules and prevent their interactions with endothelial cells, it also promotes many interactions through its binding sites for growth factors and other molecules. The binding sites provided by long, protruding GAGs allow storage and specific localization of such factors. Vascular endothelial growth factor (VEGF) contains a binding pocket for heparan sulfate (99, 100). It is also known, although less often considered, that the antiangiogenic factor sFlt-1 possesses a binding site for heparan sulfate (101, 102). VEGF is a key regulator of endothelial health and function and its actions are antagonized by sFlt-1 (103). Because they work in tandem to regulate endothelial health and function, VEGF and sFlt-1 bioavailability and localizations, which are regulated by their interactions with heparan sulfate, are of the utmost importance to endothelial health.
Other molecules contain binding sites for glycocalyx constituents and sometimes require these interactions to function properly. Several of these molecules are antithrombotic factors and growth factors. For example, antithrombin III, an inhibitor of coagulation, is tethered to the glycocalyx through interactions with glypican and heparan sulfate (58, 59). Great importance is attributed to antithrombin III’s localization, as its function needs to be specifically localized to the microenvironment of the endothelial surface to appropriately prevent clot formation. Tissue factor pathway inhibitor, an inhibitor of coagulation and suspected regulator of endothelial cell proliferation, associates with heparan sulfate to localize to endothelial cell surfaces (104, 105). Lipoprotein lipase also anchors itself to heparan sulfate (106). Because lipoprotein lipase catalyzes the hydrolysis of triglycerides from chylomicrons and very-low-density lipoprotein found in the blood stream, proper localization is imperative to its function. Fibroblast growth factor is localized to the glycocalyx by heparan sulfate as well (107). However, it is not clear whether other GAGs participate in the storage and localization of growth factors and other molecules, as most literature focuses on associations with heparan sulfate.
As additional interactions between heparan sulfate and other molecules have been revealed, it has become apparent that there must be some way GAGs regulate which molecules they interact with. A study of two myeloma cell lines demonstrated that different sulfation patterns of their heparan sulfate chains regulated their adhesion to collagen and that this was not dependent on total GAG charge or the core proteoglycan (108). Interestingly, this suggests that endothelial cells might regulate interactions of their GAGs with their environments in different ways by having unique sulfation patterns on their GAG chains. If we extrapolate this conclusion and apply it to other GAG interactions, it can be seen that the specificity of GAG sulfation could be of tremendous importance to cells’ abilities to interact with their microenvironments.
The Placental Glycocalyx
The endothelial glycocalyx has been the focus of glycobiology research for many years. However, placental syncytiotrophoblasts also possess a glycocalyx that lies in direct contact with maternal blood just as the endothelial glycocalyx does (4). Syncytiotrophoblasts form the outermost layer of placental chorionic villi by fusing their membranes together into a continuous sheet of cells. This sheet of syncytiotrophoblasts then functions as the interface between maternal blood and fetal tissue. Because of syncytiotrophoblasts’ location, epithelial nature, and their significant contributions to placental barrier function, one would reason that these cells maintain a glycocalyx that is not so different from the endothelial glycocalyx. Although there are relatively few studies examining the composition of the placental glycocalyx in depth, what is known is that it shares some of the same components found in the endothelium (109). For example, syndecans are expressed abundantly in both the placenta and the vasculature. Although the details on the composition and function of the placental glycocalyx are not yet fully clear, its similarities with the endothelial glycocalyx suggest that it could share many of the same roles in vascular and tissue homeostasis.
CONCLUSIONS
Lying at the interface of blood and the vascular wall, the glycocalyx acts as the primary functional barrier for endothelial cells. Its structure is a mesh of proteoglycans, glycosaminoglycans, and glycoproteins intertwined in a seemingly disorderly yet functional pattern. Recent decades of work have led to a newfound appreciation for the glycocalyx and its active role in vascular physiology. Acceptance that the glycocalyx plays a fundamental role in angiogenesis, inflammation, and coagulation is spreading as modern research continues to push this field forward. Although much of the basic knowledge pertaining to the structure and function of the glycocalyx has been studied in depth, there are still many unanswered questions about its role in maintaining proper cardiovascular function. The role of the glycocalyx in health and disease remains an area of fruitful research.
Perspectives and Significance
Because of its important role in vascular homeostasis, studies investigating the significance of the glycocalyx in vascular diseases such as atherosclerosis, cardiovascular disease, and hypertension could provide mechanistic insights into these prevalent and burdensome conditions. In this review, we have focused primarily on the physiological functions of the glycocalyx. However, pathological processes are known to alter and even degrade the glycocalyx, and it remains unclear exactly how the glycocalyx is restored after significant degradation. Two promising studies have suggested that growth factors may play a significant role in glycocalyx repair through increased production (110, 111), although it is still unknown whether or not too “thick” a glycocalyx would be detrimental or protective. An enhanced understanding of the glycocalyx will allow future research to further evaluate its alterations in disease and enable the development of therapeutic strategies aimed at preserving the glycocalyx and restoring endothelial health.
GRANTS
This work was supported in part by National Institutes of Health Grants P01HL51971, P20GM104357, T32HL105324, and R01HL137791.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.H.M. prepared figures; K.H.M. drafted manuscript; K.H.M., H.A.M., and E.M.G. edited and revised manuscript; K.H.M., H.A.M., and E.M.G. approved final version of manuscript.
REFERENCES
- 1.Chelazzi C, Villa G, Mancinelli P, De Gaudio AR, Adembri C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit Care 19: 26, 2015. doi: 10.1186/s13054-015-0741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolářová H, Ambrůzová B, Švihálková Šindlerová L, Klinke A, Kubala L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators Inflamm 2014: 694312, 2014. doi: 10.1155/2014/694312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarbell J, Cancel L. The glycocalyx and its significance in human medicine. J Intern Med 280: 97–113, 2016. doi: 10.1111/joim.12465. [DOI] [PubMed] [Google Scholar]
- 4.Fabre-Gray ACM, Down CJ, Neal CR, Foster RR, Satchell SC, Bills VL. Imaging the placental glycocalyx with transmission electron microscopy. Placenta 74: 59–61, 2018. doi: 10.1016/j.placenta.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Sukhikh GT, Ziganshina MM, Nizyaeva NV, Kulikova GV, Volkova JS, Yarotskaya EL, Kan NE, Shchyogolev AI, Tyutyunnik VL. Differences of glycocalyx composition in the structural elements of placenta in preeclampsia. Placenta 43: 69–76, 2016. doi: 10.1016/j.placenta.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Ebong EE, Macaluso FP, Spray DC, Tarbell JM. Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arterioscler Thromb Vasc Biol 31: 1908–1915, 2011. doi: 10.1161/ATVBAHA.111.225268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hempel C, Kapishnikov S, Perez‐Berna AJ, Werner S, Guttmann P, Pereiro E, Qvortrup K, Andresen TL. The need to freeze—dehydration during specimen preparation for electron microscopy collapses the endothelial glycocalyx regardless of fixation method. Microcirculation 27: e12643, 2020. doi: 10.1111/micc.12643. [DOI] [PubMed] [Google Scholar]
- 8.Luft JH. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc 25: 1773–1783, 1966. [PubMed] [Google Scholar]
- 9.Klitzman B, Duling BR. Microvascular hematocrit and red cell flow in resting and contracting striated muscle. Am J Physiol Heart Circ Physiol 237: H481–H490, 1979. doi: 10.1152/ajpheart.1979.237.4.H481. [DOI] [PubMed] [Google Scholar]
- 10.Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res 79: 581–589, 1996. doi: 10.1161/01.RES.79.3.581. [DOI] [PubMed] [Google Scholar]
- 11.Pries AR, Secomb TW, Sperandio M, Gaehtgens P. Blood flow resistance during hemodilution: effect of plasma composition. Cardiovasc Res 37: 225–235, 1998. doi: 10.1016/S0008-6363(97)00226-5. [DOI] [PubMed] [Google Scholar]
- 12.Kataoka H, Ushiyama A, Kawakami H, Akimoto Y, Matsubara S, Iijima T. Fluorescent imaging of endothelial glycocalyx layer with wheat germ agglutinin using intravital microscopy. Microsc Res Tech 79: 31–37, 2016. doi: 10.1002/jemt.22602. [DOI] [PubMed] [Google Scholar]
- 13.Couchman JR. Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol 26: 89–114, 2010. doi: 10.1146/annurev-cellbio-100109-104126. [DOI] [PubMed] [Google Scholar]
- 14.Saunders S, Jalkanen M, O'Farrell S, Bernfield M. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol 108: 1547–1556, 1989. doi: 10.1083/jcb.108.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulick MG, Bertozzi, CR. The glycosylphosphatidylinositol anchor: a complex membrane-anchoring structure for proteins. Biochemistry 47: 6991–7000, 2008. doi: 10.1021/bi8006324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.David G, Lories V, Decock B, Marynen P, Cassiman JJ, Van den Berghe H. Molecular cloning of a phosphatidylinositol-anchored membrane heparan sulfate proteoglycan from human lung fibroblasts. J Cell Biol 111: 3165–3176, 1990. doi: 10.1083/jcb.111.6.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown DA, Crise B, Rose JK. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science 245: 1499–1501, 1989. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- 18.Lisanti MP, Caras IW, Davitz MA, Rodríguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol 109: 2145–2156, 1989. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassell JR, Leyshon WC, Ledbetter SR, Tyree B, Suzuki S, Kato M, Kimata K, Kleinman HK. Isolation of two forms of basement membrane proteoglycans. J Biol Chem 260: 8098–8105, 1985. doi: 10.1016/S0021-9258(17)39569-8. [DOI] [PubMed] [Google Scholar]
- 20.Oohira A, Wight TN, Bornstein P. Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J Biol Chem 258: 2014–2021, 1983. doi: 10.1016/S0021-9258(18)33090-4. [DOI] [PubMed] [Google Scholar]
- 21.Noonan DM, Fulle A, Valente P, Cai S, Horigan E, Sasaki M, Yamada Y, Hassell JR. The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J Biol Chem 266: 22939–22947, 1991. doi: 10.1016/S0021-9258(18)54445-8. [DOI] [PubMed] [Google Scholar]
- 22.Murdoch AD, Dodge G, Cohen I, Tuan R, Iozzo R. Primary structure of the human heparan sulfate proteoglycan from basement membrane (HSPG2/perlecan). A chimeric molecule with multiple domains homologous to the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J Biol Chem 267: 8544–8557, 1992. doi: 10.1016/S0021-9258(18)42478-7. [DOI] [PubMed] [Google Scholar]
- 23.Patel VN, Knox SM, Likar KM, Lathrop CA, Hossain R, Eftekhari S, Whitelock JM, Elkin M, Vlodavsky I, Hoffman MP. Heparanase cleavage of perlecan heparan sulfate modulates FGF10 activity during ex vivo submandibular gland branching morphogenesis. Development 134: 4177–4186, 2007. doi: 10.1242/dev.011171. [DOI] [PubMed] [Google Scholar]
- 24.Proudfoot AE, Johnson Z, Bonvin P, Handel TM. Glycosaminoglycan interactions with chemokines add complexity to a complex system. Pharmaceuticals 10: 70, 2017. doi: 10.3390/ph10030070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rapraeger A, Jalkanen M, Endo E, Koda J, Bernfield M. The cell surface proteoglycan from mouse mammary epithelial cells bears chondroitin sulfate and heparan sulfate glycosaminoglycans. J Biol Chem 260: 11046–11052, 1985. doi: 10.1016/S0021-9258(17)39146-9. [DOI] [PubMed] [Google Scholar]
- 26.Ihrcke NS, Wrenshall LE, Lindman BJ, Platt JL. Role of heparan sulfate in immune system-blood vessel interactions. Immunol Today 14: 500–505, 1993. doi: 10.1016/0167-5699(93)90265-M. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann DR, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J 8: 2975–2981, 1989. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barry FP, Rosenberg LC, Gaw JU, Gaw JU, Koob TJ, Neame PJ. N-and O-linked keratan sulfate on the hyaluronan binding region of aggrecan from mature and immature bovine cartilage. J Biol Chem 270: 20516–20524, 1995. [Erratum in J Biol Chem 270: 31414, 1995]. doi: 10.1074/jbc.270.35.20516. [DOI] [PubMed] [Google Scholar]
- 29.Silbert JE. Incorporation of 14C and 3H from nucleotide sugars into a polysaccharide in the presence of a cell-free preparation from mouse mast cell tumors. J Biol Chem 238: 3542–3546, 1963. doi: 10.1016/S0021-9258(19)75305-8. [DOI] [PubMed] [Google Scholar]
- 30.Couchman JR, Pataki CA. An introduction to proteoglycans and their localization. J Histochem Cytochem 60: 885–897, 2012. doi: 10.1369/0022155412464638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Höök M, Lindahl U, Hallén A, Bäckström, G. Biosynthesis of heparin. Studies on the microsomal sulfation process. J Biol Chem 250: 6065–6071, 1975. doi: 10.1016/S0021-9258(19)41159-9. [DOI] [PubMed] [Google Scholar]
- 32.Lindahl U, Bäckström G, Jansson L, Hallén A. Biosynthesis of heparin II. Formation of sulfamino groups. J Biol Chem 248: 7234–7241, 1973. doi: 10.1016/S0021-9258(19)43383-8. [DOI] [PubMed] [Google Scholar]
- 33.Silbert JE. Biosynthesis of heparin III. Formation of a sulfated glycosaminoglycan with a microsomal preparation from mast cell tumors. J Biol Chem 242: 5146–5152, 1967. doi: 10.1016/S0021-9258(18)99487-1. doi:. [DOI] [PubMed] [Google Scholar]
- 34.Soares da Costa D, Reis RL, Pashkuleva I. Sulfation of glycosaminoglycans and its implications in human health and disorders. Annu Rev Biomed Eng 19: 1–26, 2017. doi: 10.1146/annurev-bioeng-071516-044610. [DOI] [PubMed] [Google Scholar]
- 35.Lindahl U, Jacobsson I, Höök M, Backström G, Feingold DS. Biosynthesis of heparin. Loss of C-5 hydrogen during conversion of D-glucuronic to L-iduronic acid residues. Biochem Biophys Res Commun 70: 492–499, 1976. doi: 10.1016/0006-291X(76)91073-1. doi:. [DOI] [PubMed] [Google Scholar]
- 36.Tykesson E, Hassinen A, Zielinska K, Thelin MA, Frati G, Ellervik U, Westergren-Thorsson G, Malmström A, Kellokumpu S, Maccarana M. Dermatan sulfate epimerase 1 and dermatan 4-O-sulfotransferase 1 form complexes that generate long epimerized 4-O-sulfated blocks. J Biol Chem 293: 13725–13735, 2018. doi: 10.1074/jbc.RA118.003875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gama CI, Tully SE, Sotogaku N, Clark PM, Rawat M, Vaidehi N, Goddard WA, Nishi A, Hsieh-Wilson LC. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol 2: 467–473, 2006. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- 38.Sasisekharan R, Raman R, Prabhakar V. Glycomics approach to structure-function relationships of glycosaminoglycans. Annu Rev Biomed Eng 8: 181–231, 2006. doi: 10.1146/annurev.bioeng.8.061505.095745. [DOI] [PubMed] [Google Scholar]
- 39.Yang B, Yang BL, Savani RC, Turley EA. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J 13: 286–296, 1994. doi: 10.1002/j.1460-2075.1994.tb06261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS, Gimbrone MA. Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci USA 84: 9238–9242, 1987. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bevilacqua MP, Stengelin S, Gimbrone MA Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science 243: 1160–1165, 1989. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- 42.Dore M, Korthuis R, Granger D, Entman M, Smith C. P-selectin mediates spontaneous leukocyte rolling in vivo. Blood 82: 1308–1316, 1993. doi: 10.1182/blood.V82.4.1308.1308. [DOI] [PubMed] [Google Scholar]
- 43.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell 74: 541–554, 1993. doi: 10.1016/0092-8674(93)80055-J. [DOI] [PubMed] [Google Scholar]
- 44.Huang K-S, Graves BJ, Wolitzky BA. Functional analysis of selectin structure. In: The Selectins: Initiators of Leukocyte Endothelial Adhesion. Amsterdam: Harwood Academic, 1997, p. 1–29. [Google Scholar]
- 45.Tamkun JW, DeSimone DW, Fonda D, Patel RS, Buck C, Horwitz AF, Hynes RO. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell 46: 271–282, 1986. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- 46.Haas TA, Plow EF. Integrin-ligarid interactions: a year in review. Curr Opin Cell Biol 6: 656–662, 1994. doi: 10.1016/0955-0674(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 47.Cybulsky MI, Fries J, Williams AJ, Sultan P, Eddy R, Byers M, Shows T, Gimbrone M, Collins T. Gene structure, chromosomal location, and basis for alternative mRNA splicing of the human VCAM1 gene. Proc Natl Acad Sci USA 88: 7859–7863, 1991. doi: 10.1073/pnas.88.17.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yap E-H, Rosche T, Almo S, Fiser A. Functional clustering of immunoglobulin superfamily proteins with protein–protein interaction information calibrated hidden Markov model sequence profiles. J Mol Biol 426: 945–961, 2014. doi: 10.1016/j.jmb.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adamson R. Permeability of frog mesenteric capillaries after partial pronase digestion of the endothelial glycocalyx. J Physiol 428: 1–13, 1990. doi: 10.1113/jphysiol.1990.sp018197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol 278: H285–H289, 2000. doi: 10.1152/ajpheart.2000.278.1.H285. [DOI] [PubMed] [Google Scholar]
- 51.van Haaren PM, VanBavel E, Vink H, Spaan JA. Localization of the permeability barrier to solutes in isolated arteries by confocal microscopy. Am J Physiol Heart Circ Physiol 285: H2848–H2856, 2003. doi: 10.1152/ajpheart.00117.2003. [DOI] [PubMed] [Google Scholar]
- 52.Fernández-Sarmiento J, Salazar-Peláez LM, Carcillo, JA. The endothelial glycocalyx: a fundamental determinant of vascular permeability in sepsis. Pediatr Crit Care Med 21: e291–e300, 2020. doi: 10.1097/PCC.0000000000002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rehm M, Zahler S, Lötsch M, Welsch U, Conzen P, Jacob M, Becker, BF. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology 100: 1211–1223, 2004. doi: 10.1097/00000542-200405000-00025. [DOI] [PubMed] [Google Scholar]
- 54.Ueda A, Shimomura M, Ikeda M, Yamaguchi R, Tanishita K. Effect of glycocalyx on shear-dependent albumin uptake in endothelial cells. Am J Physiol Heart Circ Physiol 287: H2287–H2294, 2004. doi: 10.1152/ajpheart.00808.2003. [DOI] [PubMed] [Google Scholar]
- 55.Crockett ES. Endothelial glycocalyx and the revised starling principle. PVRI Chronicle 1, 2014. [Google Scholar]
- 56.Desjardins C, Duling BR. Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Physiol Heart Circ Physiol 258: H647–H654, 1990. doi: 10.1152/ajpheart.1990.258.3.H647. [DOI] [PubMed] [Google Scholar]
- 57.Iba T, Levy J. Derangement of the endothelial glycocalyx in sepsis. J Thromb Haemost 17: 283–294, 2019. doi: 10.1111/jth.14371. [DOI] [PubMed] [Google Scholar]
- 58.Mertens G, Cassiman JJ, Van den Berghe H, Vermylen J, David G. Cell surface heparan sulfate proteoglycans from human vascular endothelial cells. Core protein characterization and antithrombin III binding properties. J Biol Chem 267: 20435–20443, 1992. doi: 10.1016/S0021-9258(19)88721-5. [DOI] [PubMed] [Google Scholar]
- 59.Hoffmann JN, Vollmar B, Römisch J, Inthorn D, Schildberg FW, Menger MD. Antithrombin effects on endotoxin-induced microcirculatory disorders are mediated mainly by its interaction with microvascular endothelium. Crit Care Medicine 30: 218–225, 2002. doi: 10.1097/00003246-200201000-00031. [DOI] [PubMed] [Google Scholar]
- 60.Meza D, Shanmugavelayudam SK, Mendoza A, Sanchez C, Rubenstein DA, Yin W. Platelets modulate endothelial cell response to dynamic shear stress through PECAM-1. Thromb Res 150: 44–50, 2017. doi: 10.1016/j.thromres.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Tseng CN, Chang YT, Yen CY, Lengquist M, Kronqvist M, Eriksson EE, Hedin U. Early inhibition of P-selectin/P-selectin glycoprotein ligand-1 reduces intimal hyperplasia in murine vein grafts through platelet adhesion. Thromb Haemost 119: 2014–2024, 2019. doi: 10.1055/s-0039-1697659. [DOI] [PubMed] [Google Scholar]
- 62.Geng JG, Bevilacqua MP, Moore KL, McIntyre TM, Prescott SM, Kim JM, Bliss GA, Zimmerman GA, McEver RP. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature 343: 757–760, 1990. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- 63.Hattori R, Hamilton K, Fugate R, McEver R, Sims P. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem 264: 7768–7771, 1989. doi: 10.1016/S0021-9258(18)83104-0. [DOI] [PubMed] [Google Scholar]
- 64.Israels S, Gerrard J, Jacques Y, McNicol A, Cham B, Nishibori M, Bainton D. Platelet dense granule membranes contain both granulophysin and P-selectin (GMP-140). Blood 80: 143–152, 1992. doi: 10.1182/blood.V80.1.143.143. [DOI] [PubMed] [Google Scholar]
- 65.Frenette PS, Denis CV, Weiss L, Jurk K, Subbarao S, Kehrel B, Hartwig JH, Vestweber D, Wagner DD. P-selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet–endothelial interactions in vivo. J Exp Med 191: 1413–1422, 2000. doi: 10.1084/jem.191.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laszik Z, Jansen PJ, Cummings RD, Tedder TF, McEver RP, Moore KL. P-selectin glycoprotein ligand-1 is broadly expressed in cells of myeloid, lymphoid, and dendritic lineage and in some nonhematopoietic cells. Thromb Haemost 88: 3010–3021, 1996. [PubMed] [Google Scholar]
- 67.Wasteson A, Glimelius B, Busch C, Westermark H, Heldin CH, Norling B. Effect of a platelet endoglycosidase on cell surface associated heparan sulphate of human cultured endothelial and glial cells. Thromb Res 11: 309–321, 1977. doi: 10.1016/0049-3848(77)90184-0. [DOI] [PubMed] [Google Scholar]
- 68.Pikas DS, Li J-P, Vlodavsky I, Lindahl U. Substrate specificity of heparanases from human hepatoma and platelets. J Biol Chem 273: 18770–18777, 1998. doi: 10.1074/jbc.273.30.18770. [DOI] [PubMed] [Google Scholar]
- 69.Vreys V, David G. Mammalian heparanase: what is the message? J Cell Mol Med 11: 427–452, 2007. doi: 10.1111/j.1582-4934.2007.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bombeli T, Schwartz BR, Harlan JM. Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), αvβ3 integrin, and GPIbα. J Exp Med 187: 329–339, 1998. doi: 10.1084/jem.187.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol 283: H1282–H1291, 2002. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- 72.Constantinescu AA, Vink H, Spaan JA. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. ATVB 23: 1541–1547, 2003. doi: 10.1161/01.ATV.0000085630.24353.3D. [DOI] [PubMed] [Google Scholar]
- 73.Chappell D, Dörfler N, Jacob M, Rehm M, Welsch U, Conzen P, Becker BF. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock 34: 133–139, 2010. doi: 10.1097/SHK.0b013e3181cdc363. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, Smith LP, Cheng SS, Overdier KH, Thompson KR, Geraci MW, Douglas IS, Pearse DB, Tuder RM. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 18: 1217–1223, 2012. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nelson RM, Cecconi O, Roberts WG, Aruffo A, Linhardt RJ, Bevilacqua MP. Heparin oligosaccharides bind L-and P-selectin and inhibit acute inflammation. Blood 82: 3253–3258, 1993. doi: 10.1182/blood.V82.11.3253.3253. [DOI] [PubMed] [Google Scholar]
- 76.Wang L, Brown JR, Varki A, Esko JD. Heparin’s anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of L-and P-selectins. J Clin Invest 110: 127–136, 2002. doi: 10.1172/JCI0214996. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Penc SF, Pomahac B, Eriksson E, Detmar M, Gallo RL. Dermatan sulfate activates nuclear factor-κb and induces endothelial and circulating intercellular adhesion molecule-1. J Clin Invest 103: 1329–1335, 1999. doi: 10.1172/JCI4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goodall KJ, Poon IK, Phipps S, Hulett MD. Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4. PloS One 9: e109596, 2014. doi: 10.1371/journal.pone.0109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mohamadzadeh M, DeGrendele H, Arizpe H, Estess P, Siegelman M. Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HA-dependent primary adhesion. J Clin Invest 101: 97–108, 1998. doi: 10.1172/JCI1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nandi A, Estess P, Siegelman MH. Hyaluronan anchoring and regulation on the surface of vascular endothelial cells is mediated through the functionally active form of CD44. J Biol Chem 275: 14939–14948, 2000. doi: 10.1074/jbc.275.20.14939. [DOI] [PubMed] [Google Scholar]
- 81.Diacovo TG, Puri KD, Warnock RA, Springer TA, Von Andrian UH. Platelet-mediated lymphocyte delivery to high endothelial venules. Science 273: 252–255, 1996. doi: 10.1126/science.273.5272.252. [DOI] [PubMed] [Google Scholar]
- 82.Müller AM, Hermanns MI, Cronen C, Kirkpatrick CJ. Comparative study of adhesion molecule expression in cultured human macro-and microvascular endothelial cells. Exp Mol Pathol 73: 171–180, 2002. doi: 10.1006/exmp.2002.2446. [DOI] [PubMed] [Google Scholar]
- 83.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell 51: 813–819, 1987. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 84.Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 59: 1203–1211, 1989. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 85.Liao F, Huynh HK, Eiroa A, Greene T, Polizzi E, Muller WA. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J Exp Med 182: 1337–1343, 1995. doi: 10.1084/jem.182.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jung U, Ley K. Regulation of E-selectin, P-selectin, and intercellular adhesion molecule 1 expression in mouse cremaster muscle vasculature. Microcirculation 4: 311–319, 1997. doi: 10.3109/10739689709146794. [DOI] [PubMed] [Google Scholar]
- 87.Serasanambati M, Chilakapati SR. Function of nuclear factor kappa B (NF-kB) in human diseases-a review. SIJBS 2: 368–387, 2016. doi: 10.22205/sijbs/2016/v2/i4/103443. [DOI] [Google Scholar]
- 88.Kaneider NC, Förster E, Mosheimer B, Sturn DH, Wiedermann CJ. Syndecan-4-dependent signaling in the inhibition of endotoxin-induced endothelial adherence of neutrophils by antithrombin. Thromb Haemost 90: 1150–1157, 2007. [Erratum in Thromb Haemost 2004 91: 415, 2003]. doi: 10.1160/TH03-03-0184. [DOI] [PubMed] [Google Scholar]
- 89.Oh IY, Yoon CH, Hur J, Kim JH, Kim TY, Lee CS, Park KW, Chae IH, Oh BH, Park YB, Kim HS. Involvement of E-selectin in recruitment of endothelial progenitor cells and angiogenesis in ischemic muscle. Blood 110: 3891–3899, 2007. doi: 10.1182/blood-2006-10-048991. [DOI] [PubMed] [Google Scholar]
- 90.Luo J, Kato M, Wang H, Bernfield M, Bischoff J. Heparan sulfate and chondroitin sulfate proteoglycans inhibit E‐selectin binding to endothelial cells. J Cell Biochem 80: 522–531, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 91.Perez-Aguilar S, Torres-Tirado D, Martell-Gallegos G, Velarde-Salcedo J, Barba-de la Rosa AP, Knabb M, Rubio R. G protein-coupled receptors mediate coronary flow-and agonist-induced responses via lectin-oligosaccharide interactions. Am J Physiol Heart Circ Physiol 306: H699–H708, 2014. doi: 10.1152/ajpheart.00481.2013. [DOI] [PubMed] [Google Scholar]
- 92.Tarbell JM, Pahakis M. Mechanotransduction and the glycocalyx. J Intern Med 259: 339–350, 2006. doi: 10.1111/j.1365-2796.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 93.Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc Natl Acad Sci USA 101: 16483–16488, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nikmanesh M, Shi ZD, Tarbell JM. Heparan sulfate proteoglycan mediates shear stress‐induced endothelial gene expression in mouse embryonic stem cell‐derived endothelial cells. Biotechnol Bioeng 109: 583–594, 2012. doi: 10.1002/bit.23302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pahakis M, Kosky J, Tarbell J. Sialic acids and heparan sulfate proteoglycans are mechanosensory components of the endothelial cell glycocalyx. In: Proceedings of the 2005 Summer Bioengineering Conference. Vail, CO, 22 June 2005, vol. 2005, P. 137–138. [Google Scholar]
- 96.Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JA, Kajiya F. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol 285: H722–H726, 2003. doi: 10.1152/ajpheart.00691.2002. [DOI] [PubMed] [Google Scholar]
- 97.Gouverneur M, Spaan JA, Pannekoek H, Fontijn RD, Vink H. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol 290: H458–H452, 2006. doi: 10.1152/ajpheart.00592.2005. [DOI] [PubMed] [Google Scholar]
- 98.van den Berg BM, Spaan JA, Rolf TM, Vink H. Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am J Physiol Heart Circ Physiol 290: H915–H920, 2006. doi: 10.1152/ajpheart.00051.2005. [DOI] [PubMed] [Google Scholar]
- 99.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 161: 851–858, 1989. doi: 10.1016/0006-291X(89)92678-8. doi:. [DOI] [PubMed] [Google Scholar]
- 100.Jakobsson L, Kreuger J, Holmborn K, Lundin L, Eriksson I, Kjellén L, Claesson-Welsh L. Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev Cell 10: 625–634, 2006. doi: 10.1016/j.devcel.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 101.Park M, Lee ST. The fourth immunoglobulin-like loop in the extracellular domain of FLT-1, a VEGF receptor, includes a major heparin-binding site. Biochem Biophys Res Commun 264: 730–734, 1999. doi: 10.1006/bbrc.1999.1580. [DOI] [PubMed] [Google Scholar]
- 102.Sela S, Natanson-Yaron S, Zcharia E, Vlodavsky I, Yagel S, Keshet E. Local retention versus systemic release of soluble VEGF receptor-1 is mediated by heparin-binding and regulated by heparanase. Circ Res 108: 1063–1070, 2011. doi: 10.1161/CIRCRESAHA.110.239665. [DOI] [PubMed] [Google Scholar]
- 103.Eichmann A, Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol 24: 188–193, 2012. doi: 10.1016/j.ceb.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ho G, Broze GJ, Schwartz AL. Role of heparan sulfate proteoglycans in the uptake and degradation of tissue factor pathway inhibitor-coagulation factor Xa complexes. J Biol Chem 272: 16838–16844, 1997. doi: 10.1074/jbc.272.27.16838. [DOI] [PubMed] [Google Scholar]
- 105.Kato H. Regulation of functions of vascular wall cells by tissue factor pathway inhibitor: basic and clinical aspects. Arterioscler Thromb Vasc Biol 22: 539–548, 2002. doi: 10.1161/01.ATV.0000013904.40673.CC. [DOI] [PubMed] [Google Scholar]
- 106.ParthaSarathy N, Goldberg IJ, Sivaram P, Mulloy B, Flory DM, Wagner WD. Oligosaccharide sequences of endothelial cell surface heparan sulfate proteoglycan with affinity for lipoprotein lipase. J Biol Chem 269: 22391–22396, 1994. doi: 10.1016/S0021-9258(17)31802-1. [DOI] [PubMed] [Google Scholar]
- 107.Allen BL, Filla MS, Rapraeger AC. Role of heparan sulfate as a tissue-specific regulator of FGF-4 and FGF receptor recognition. J Cell Biol 155: 845–858, 2001. doi: 10.1083/jcb.200106075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sanderson RD, Turnbull JE, Gallagher JT, Lander AD. Fine structure of heparan sulfate regulates syndecan-1 function and cell behavior. J Biol Chem 269: 13100–13106, 1994. doi: 10.1016/S0021-9258(17)36804-7. [DOI] [PubMed] [Google Scholar]
- 109.Hofmann-Kiefer KF, Chappell D, Knabl J, Frank HG, Martinoff N, Conzen P, Becker BF, Rehm M. Placental syncytiotrophoblast maintains a specific type of glycocalyx at the fetomaternal border: the glycocalyx at the fetomaternal interface in healthy women and patients with HELLP syndrome. Reprod Sci 20: 1237–1245, 2013. doi: 10.1177/1933719113483011. [DOI] [PubMed] [Google Scholar]
- 110.Desideri S, Onions KL, Baker SL, Gamez M, El Hegni E Hussien H, Russell A, Satchell SC, Foster RR. Endothelial glycocalyx restoration by growth factors in diabetic nephropathy. Biorheology 56: 163–179, 2019. doi: 10.3233/BIR-180199. [DOI] [PubMed] [Google Scholar]
- 111.Rizzo AN, Dudek SM. Endothelial Glycocalyx Repair: Building a Wall to Protect the Lung During Sepsis. New York: American Thoracic Society, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]