Abstract
(Pro)renin receptor (PRR), a 350-amino acid receptor initially thought of as a receptor for the binding of renin and prorenin, is multifunctional. In addition to its role in the renin-angiotensin system (RAS), PRR transduces several intracellular signaling molecules and is a component of the vacuolar H+-ATPase that participates in autophagy. PRR is found in the kidney and particularly in great abundance in the cortical collecting duct. In the kidney, PRR participates in water and salt balance, acid-base balance, and autophagy and plays a role in development and progression of hypertension, diabetic retinopathy, and kidney fibrosis. This review highlights the role of PRR in the development and function of the kidney, namely, the macula densa, podocyte, proximal and distal convoluted tubule, and the principal cells of the collecting duct, and focuses on PRR function in body fluid volume homeostasis, blood pressure regulation, and acid-base balance. This review also explores new advances in the molecular mechanism involving PRR in normal renal health and pathophysiological states.
Keywords: blood pressure, kidney, prorenin receptor, sodium balance, water balance
INTRODUCTION
Since its identification in 2002, the (pro)renin receptor (PRR) has been shown to play an important role for physiological and pathophysiological processes in most body organs. Particularly, PRR is central for the development and function of the kidney and renal cells such as podocytes, mesangial cells, and principal and intercalated cells (Fig. 1).
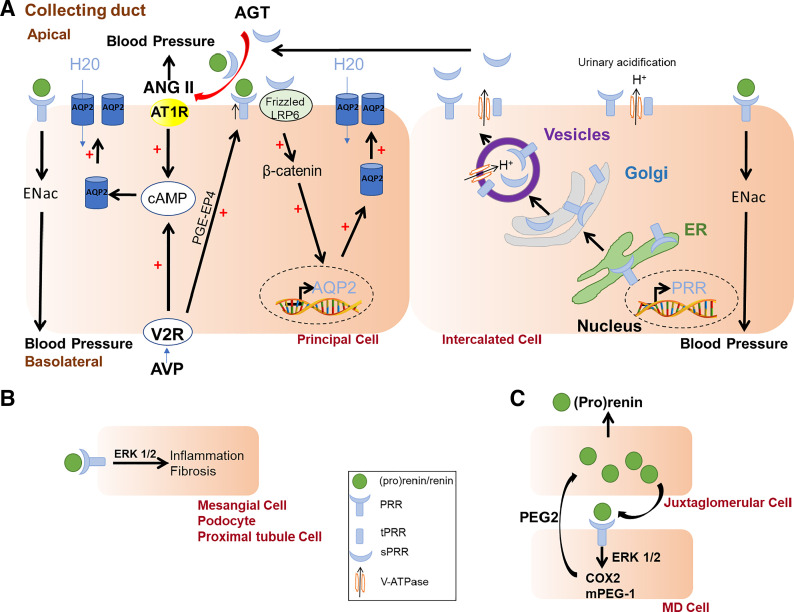
Figure 1.
Influence of renal prorenin receptor (PRR) on water and sodium homeostasis and blood pressure. A: in the principal cells of the collecting duct, the binding of vasopressin (AVP) to the vasopressin receptor (V2R) stimulates cAMP and upregulates PRR [via the activation of the prostaglandin (PGE2) pathway], which increases of the expression of aquaporin (AQP2) and water reabsorption. The intercalated cells secrete soluble PRR (sPRR) that binds to the frizzled-8 (FZD8) /LDL receptor related protein 6 (LRP6), increases AQP2 gene expression, and participates in water reabsorption in the principal cells. Both PRR and sPRR bind prorenin and renin and increase ANG II generation, which, in turn, stimulates angiotensin type 1 receptor (AT1R) and influences AQP2 trafficking. PRR influences Na+ homeostasis and blood pressure regulation via modulation of epithelial Na+ channel (ENaC) expression. B: PRR, via ERK1/2 signaling, has proinflammatory and fibrotic effects in podocyte and mesangial, and proximal tubule cells. C: PRR activates ERK1/2 pathway in the macula densa (MD) cells, which increases the synthesis of prostaglandin (PGE2) via the activation of cyclooxygenase-2 (COX2) and PGE-synthetase (mPEG-1), and increases (pro)renin release by juxtaglomerular cells.
GENERAL PHYSIOLOGY AND FUNCTIONS OF PRR
The PRR is encoded by the ATP6AP2 (ATPase-associated protein-2) gene located on the X chromosome. Currently, three forms of the receptor have been identified: a 35- to 39-kDa full-length receptor or PRR, a 28-kDa amino-terminal fragment named the soluble PRR (sPRR), and a truncated 8.9-kDa carboxy-terminal fragment also known as M8-9 or truncated PRR (tPRR).
PRR is present in many different tissues, including the adipose tissue (1–3), kidney (4), heart (5), brain (6), eye (7), vessel wall (8), placenta (4), and different cell types such as macrophages (9) or smooth muscle cells (10). PRR is a single transmembrane receptor composed of 350 amino acids and binds both renin and prorenin. Interestingly, the cleavage of angiotensinogen (AGT) into angiotensin I (ANG I) is five times more efficient when renin is bound to PRR (kcat/Km = 11.2 µM − 1·s−1) than when renin is free in solution (kcat/Km = 2.2 µM −1·s−1) (11). PRR also binds prorenin, the inactive form of renin, and induces a conformational change of prorenin that leads to its activation without the cleavage of its prosegment (11). In addition, the interaction between PRR and renin/prorenin activates a transient intracellular signaling pathway through the kinase, ERK1/2 pathways, and the mitogen-activated protein kinase (p38 MAPK) (4). Independent of the renin-angiotensin system (RAS), PRR also affects developmental processes through the Wnt β-catenin pathway (12).
sPRR is released from the cleavage of PRR by enzymes including furin, ADAM 19, and site 1 protease (13–15). sPRR is secreted into the plasma (13–15) and urine (16), whereas tPRR remains attached to the cellular membrane. Previous studies demonstrated that similar to the full-length PRR, sPRR binds both prorenin and renin, which, in turn, activates the renin-angiotensin system (RAS) cascade, thereby directly influencing body fluid volume homeostasis (12). M8-9 or tPRR has been established as an accessory subunit of the vacuolar H+-ATPase (V-ATPase) involved in lysosomal acidification (17, 18).
THE IMPORTANCE OF KIDNEY PRR IN RENAL DEVELOPMENT
The absence of PRR can be detrimental to the normal development and function of the kidney and to embryogenesis (19). The expression of renal PRR is elevated during gestation but decreases with gestational age and after birth in mouse. In humans, renal PRR is present in the glomeruli, proximal tubules, collecting ducts, and arteries and is higher in neonates than in older children (20). Stromal progenitors PRR knock out (KO) mice died within 24–48 h after birth (21). Conditional deletion of PRR in renal stromal progenitor cells led to fewer glomeruli per kidney section, a delay in nephrogenesis measured by the apparent absence of mature S-shaped nephrons in the outer cortex, thicker nephron progenitor layers and widened progenitor caps, impaired renal vesicle formation and nephron segmentation, and reduced formation of proximal tubules (21). Stromal progenitors PRR KO also had a decrease in α-smooth muscle actin (a marker of vascular smooth muscle cells), in renin (a marker of juxtaglomerular cells in the renal vasculature), and an impairment of the development of the renal arterial vasculature (21).
In the collecting duct, the PRR KO caused mild hypoplasia of the renal medulla, smaller kidney size, and fewer glomeruli than wild-type mice (22).
The knockdown of PRR in the podocyte led to proteinuria, albuminuria, and elevated total serum cholesterol and creatinine levels, whereas total serum protein and albumin levels reduced (23). The podocyte-specific PRR KO mice also had enlarged podocytes, aggregation of actin filament, and extensive effacement of the foot process in early postnatal life. By postnatal day 14, podocyte-specific PRR KO mice developed diffuse glomerular changes, which consisted of hypertrophic changes to parietal epithelial cells without crescent formation, expansion of the mesangium matrix, segmental sclerosis, and some tubule-interstitial changes. Because PRR serves as an accessory subunit of V-ATPase, these drastic deformities were attributed to a lack of lysosomal acidification that was the result of a drastic reduction (>90%) of the mRNA of PRR.
The deletion of PRR in the uretic bud reduced the number of uretic bud tips and induced lower kidney size, kidney weight, and kidney-to-body weight ratio than in control mice (24). The uretic bud PRR KO mice also had a decreased nephron number, thin cortex, occasional cysts in the collecting duct, dilation of tubules from a proximal tubule origin, an increase in apoptotic cells, and poor acid-loading handling. In this study, the authors demonstrated that the inactivation of the ERK 1/2 pathway mediated by PRR KO likely accounted for the lack of uretic bud branching (24).
In contrast, studies demonstrated that when renal PRR is deleted at midstage of life, very few, if not negligible, negative effects on development are seen (25).
Overall, PRR appears to exert a pleiotropic effect on renal development that depends on the specific function of the different renal segment and of the cell types present in the kidney. These studies showed the importance of PRR in nephrogenesis and fetal and newborn growth.
KIDNEY PRR IN WATER HOMEOSTASIS
Several reports have demonstrated the importance of the PRR in the regulation of urine flow and showed a close relationship between PRR and molecules involved in water homeostasis, particularly in regard to vasopressin (AVP) and the arginine vasopressin 2 receptor (V2R)-mediated water channels such as aquaporin 2 (AQP2). During water deprivation, PRR and sPRR protein expression markedly increased in kidneys of rats and mice (26, 27). In addition, PRR deletion in the whole nephron modulated the responsiveness of V2R to the binding of AVP by reducing AVP-stimulated cyclic adenosine monophosphate (cAMP), causing a significant reduction in the abundance of AQP2 (26).
In collecting ducts, selective deletion of PRR increased urinary flow rate and decreased urine osmolality in both mice and rats, leading to the inability of the kidney to concentrate urine (22, 26–28). To investigate the cell types involved, Ramkumar et al. (28) deleted PRR either in principal cells or in intercalated cells of the collecting duct. With the absence of renal histological abnormalities, the deletion of PRR in principal cells decreased urinary concentrating ability coupled with elevated urine flow rate and reduced urine osmolality (28). The changes in the ability to concentrate the urine were not observed when PRR was deleted in intercalated cells even though the deletion of PRR was more pronounced in intercalated cells than in principal cells (28). Together, those findings indicated that the functional role of PRR in the regulation of normal water balance occurred mainly in the principal cells.
In determining possible mechanisms for PRR modulation of water balance, Wang et al. (27) have shown that prostaglandin EP4 promoted PRR expression through a cyclic adenosine monophosphate/protein kinase A (cAMP/PKA)-dependent mechanism, which, in turn, modulates AQP2 expression (27). Indeed, water deprivation in mice, upregulated PRR protein expression and the administration of ONO-AE3-208, a P4 receptor antagonist, significantly reduced PRR protein expression. In addition, AVP-induced upregulation of PRR was blunted by treatment with either a PRR decoy peptide (PRO20), a PRR antibody (anti-PRR-N antibody), an interfering RNA (PRR siRNA), or the EP4 receptor antagonist (ONO-AE3-208).
Recent findings have also involved sPRR in water homeostasis (29). Lu et al. (12) demonstrated that sPRR activated the frizzled-8 (FZD8)/Wnt/β-catenin pathway. Using coimmunoprecipitation studies, Lu et al. (12) demonstrated that sPRR exerts an antidiuretic effect via its binding to FZD8. Treatment with histidine-tagged recombinant sPRR triggered Wnt-responsive luciferase activity and markedly increased AQP2 expression in rat inner medulla collecting duct cells. They demonstrated that the silencing of FZD8 blunted the sPRR-induced Wnt signaling pathway and AQ2 expression. In line with these findings, in vivo studies showed that water deprivation for 24 h increased β-catenin protein in the nuclear fraction from both cortex and inner medulla of mice. Moreover, the treatment with an FZD8 inhibitor increased urine flow rate and decreased urine osmolality (12). The short-term exposure (30 min) of inner medullary collecting duct (IMCD) cells to AVP caused a rapid rise in cellular cAMP and a redistribution of AQP2 from the cytosol to the cell membranes, which was unaffected by FZD8 inhibitor. A long-term exposure (24 h), however, caused increased cAMP and mRNA level and a total protein abundance of AQP2, which were all attenuated by the FZD8 inhibitor treatment. Thus, it appears that sPRR via its interaction with FZD8 activates Wnt/β-catenin pathways and upregulates AQP2 gene transcription, whereas AVP directly controls AQP2 trafficking (12).
Recently, our laboratory showed that the deletion of PRR in adipocyte induced a counter-regulatory increase of circulating sPRR, an elevation of urinary AVP and body water in male and female mice, and a decrease in urinary volume in female mice supporting the concept that sPRR participates in water homeostasis (30, 31). Interestingly, the infusion of mouse recombinant sPRR increased renal and hepatic AGT, circulating renin, and doubled urinary AVP excretion in female mice fed a standard diet, but did not change urine volume and water intake (31, 32). Therefore, because sPRR regulated renin and AGT, one could speculate that elevated renin, or the activation of circulating RAS, particularly ANG II, could act on the circumventricular organs of the brain (e.g., subforical organ) and induce the release of AVP. Together, our data suggest that sPRR could directly or indirectly regulate AVP release from the posterior pituitary. Moreover, sPRR-mediated increase in urinary AVP seems to be more pronounced in female mice than in male mice, indicating a potential sex difference.
Finally, within the RAS-dependent pathway, it is well established that ANG II modulates AVP and AQP2 trafficking via cAMP/PKA and calcium-calmodulin signaling pathways (33). ANG II administration increased AQP2 in a dose-dependent manner at the basolateral membrane of collecting duct cells (mpkCCDc14), and increased AQP2 density at apical membranes after only 30 min of incubation. This ANG II-mediated AQP2 trafficking was blunted by selective inhibition of PKC, PKA, or calmodulin (33). Taken together, these data indicate that ANG II may also influence AQP2 trafficking by several different but connected intracellular signaling pathways. Interestingly, Gonzalez et al. (16) demonstrated that chronic ANG II infusion in rats augmented sPRR in the collecting duct and urine. Elevated sPRR, in turn, increased renin synthesis in collecting ducts and elevated intratubular ANG II formation, forming a sPRR/ANG II/renin-mediated positive feedback loop (16). Therefore, the close relationship between ANG II and PRR/sPRR could participate in AQP2 trafficking and vasopressin regulation. Overall, several pathways and signaling mechanisms involving PRR and/or sPRR mediating urine flow and water balance have been proposed, but further attention should be paid to the relative contribution of PRR versus sPRR to water balance in physiological and pathophysiological conditions.
KIDNEY PRR IN THE MODULATION OF SODIUM BALANCE
Maintenance of normal sodium balance may be critically coupled with water 2Cl− cotransporter (NKCC2), Na+-Cl− cotransporter (NCC), and the epithelial Na+ channel (ENaC) (34). The deletion of PRR in early nephron segments decreased the protein expression of NHE3, NKCC2, and NCC, whereas there was no effect of the deletion of PRR in the collecting duct on the expression of these transporters (22, 25). ENaC is recognized as a critical rate-limiting step for the transepithelial movement of sodium in the distal convoluted tubule and the collecting duct (34); therefore, the relationship between PRR and ENaC expression has been extensively investigated to understand the role of PRR in the control of Na+ excretion. Indeed, α-ENaC expression was markedly reduced in nephron-wide PRR knockout mice or in principal cells-specific PRR knockout mice compared with that in their wild-type littermates, whereas β- and γ-ENaC channels were significantly increased in PRR knockout mice (26, 28). In contrast, the deletion of PRR in intercalated cells increased α-ENaC expression but decreased β- and γ-ENaC expression (Fig. 1A) (28). Because the ENaC subunit expression is similar in the nephron-wide PRR knockout mice and in the principal cell-specific PRR knockout mice, these studies suggested that principal cells PRR is critical to the modulation of ENaC expression in the nephron (Fig. 1A). In line with these findings, in principal cells-specific PRR knockout mice fed a low sodium chloride diet, which is known to increase renin and PRR expression (35), α-ENaC expression remained low, whereas β- and γ-ENaC expression remained elevated.
Furthermore, ANG II has been shown to modulate ENaC expression through its receptor angiotensin type 1 receptor (AT1R) (35, 36), raising the question of whether PRR modulation of ENaC is RAS dependent or independent. In mouse collecting duct cells, prorenin stimulated the transepithelial Na+ current, whereas a Nox4 inhibitor or PRO20 blocked the epithelial Na+ channel activity (37). Interestingly, Losartan, an AT1R antagonist, had no effect on epithelial Na+ channel activity. These findings indicated that prorenin elicited PRR-activation, which, in turn, stimulated ENaC activity through an NADPH oxidase 4-derived H2O2-dependent/AT1-independent mechanism. Moreover, the silencing of PRR reduced ANG II-induced serine/threonine-protein kinase-1 and phosphorylated Nedd4-2 (SGK1-Nedd4-2) activation (37, 38).
Together, these results indicate that PRR-Nox4 and PRR-SGK1-Nedd4-2 pathways are two RAS-independent pathways that could modulate ENaC. These studies also showed the importance of the presence of PRR in the principal cells in the control of α-ENaC expression and possibly the regulation of sodium balance. A potential negative feedback loop might occur between the intercalated and the principal cells that could affect the overall function and expression of ENaC. This would give credence to an autocrine/paracrine regulation of PRR-ENaC pathway in intercalated and principal cells. If sPRR likely contributes to water homoeostasis, studies investigating the relative contribution of sPRR to sodium balance are limited and requires further investigation.
KIDNEY PRR IN BLOOD PRESSURE REGULATION
Kidneys possess all the components of the RAS required for ANG II generation and, therefore, these RAS components are critical to overall body fluid volume control and blood pressure regulation (39, 40). Because PRR participates in ANG I formation, significant research has also examined the role of renal PRR in blood pressure control.
In mice lacking PRR in the collecting duct, systolic blood pressure (SBP), and mean arterial pressure (MAP) were markedly reduced compared with control mice (22). Despite chronic ANG II infusion, collecting duct PRR knockout mice had attenuated SBP and DBP (22, 41). In addition, SBP and DBP were significantly lower in nephron-wide PRR knockout mice infused with ANG II (25). Increasing evidence suggests that the mechanism involved depends on PRR mediated-increase of ENaC expression through an ANG II-dependent pathway that will further increase blood pressure (25, 41). Indeed, the MAP of uninephrectomized Sprague-Dawley rats infused with amiloride, an ENaC inhibitor, declined and remained lower than that of control rats (41). The diuretic blunted the increased ENaC expression, thereby preventing sodium reabsorption and attenuating the rise in blood pressure. Because PRR increased the expression of ENaC through ANG II, leading to hypertension, the treatment with amiloride prevented the action of PRR on ENaC even in the presence of ANG II.
Being a physiological regulator of renin release from the juxtaglomerular apparatus, the macula densa (MD) is important for blood pressure control. The MD senses low NaCl concentration and triggers prostaglandin (PGE2) synthesis and release via cyclooxygenase-2 (COX2) and mPEGS-1 activation and, consequently, increases renin release. Interestingly, PRR is expressed in the MD. Riquier-Brison et al. (42) demonstrated that PRR activates ERK1/2 signaling and stimulates PGE2 in cultured MD cells and participates in the release of renin in juxtaglomerular tissues (Fig. 1C) (42). They suggested that elevated renin could, in turn, activate PRR to keep the pathway activated. In vivo, the deletion of PRR in the MD reduced renin and systolic blood pressure, which dropped further when mice were treated with a low-salt diet combined with an angiotensin-converting enzyme (ACE) inhibitor treatment to challenge the RAS. Together, this body of work suggests an essential role of MD PRR in the physiological regulatory loop of low salt- and MD-induced renin secretion, and in blood pressure control (42).
KIDNEY PRR IN ACID-BASE BALANCE
As mentioned previously (4), the gene encoded for PRR, ATP6AP2, is also a subunit component of V-ATPase. Because the V-ATPase is responsible for the acidification of several organelles in the cell (43), changes in PRR expression influences the expression of the V-ATPase and its function. PRR has been shown to strongly localize with the B2 subunit of the V-ATPase and a knockdown of PRR significantly reduced prorenin-stimulated increase in V-ATPase proton translocating activity (44). Kidney-wide knockdown of PRR led to severe metabolic acidosis in mice with very low urinary ammonium and titratable acid excretion when challenged with a HCl-enriched diet (45). V-ATPase-dependent proton extrusion in intercalated cells, which are critical for acid-base balance, was blunted in renal PRR KO mice, suggesting that PRR expression in intercalated cells is important for urine acidification (45). In addition, the knockdown of PRR in the podocyte, induced an increase in vesicular pH that caused an alteration in cytoskeleton and podocyte morphology (46). Moreover, knockdown of PRR in Madin-Darby Canine Kidney (MDCK) cells decreased the V0 and V1 subunit of V-ATPase expression, although the α4 and δ2 subunits of V0 domain and Β2 and Β1 subunits of the V1 domain remained unchanged (17). The treatment of MDCK cells with bafilomycin, a V-ATPase inhibitor, blunted prorenin/renin-induced ERK1/2 phosphorylation, indicating that PRR is important for acid-base balance via its role in the maintenance of V-ATPase integrity independently of its RAS function (47).
PRR IN RENAL PATHOGENESIS
An accumulating body of evidence indicated that renal PRR expression increased markedly in different types of progressive renal injury, either acute or chronic kidney disease, such as ischemic acute kidney injury, polycystic kidney disease, and unilateral ureteral obstruction (44, 48, 49). Similarly, high levels of PRR have been found in the progression of diabetes and its complication such as nephropathy (50, 51) and the inhibition of PRR, via PRO20, protected against nephropathy (52).
High glucose and diabetes upregulated PRR in the podocytes and in mesangial cells of the kidney (53, 54). Siragy et al. (54) demonstrated that high glucose induced podocytes’ injury via the upregulation of PRR, which activated the canonical Wnt3a-catenin snail signaling pathway and the silencing of PRR inhibited glucose-induced podocyte dysfunction and injury (54).
Li et al. (55) observed that the induction of kidney injury by ischemia-reperfusion or by ANG II increased renal PRR expression through Wnt/β-catenin pathway (55). The kidney injury and fibrosis improved after knockdown of PRR expression. The silencing of PRR abolished Wnt/β-catenin activity, improving kidney function. They also demonstrated that the treatment with bafilomycin A1 treatment, an inhibitor of V-ATPase activity, abolished PRR/Wnt/β-catenin pathway, suggesting that PRR required V-ATPase to potentiate Wnt/β-catenin pathway (55). PRR also activated the intrarenal RAS in the pathogenesis of adriamycin-induced nephropathy, which activated NADPH oxidase 4 (Nox4), and increased reactive oxygen species (ROS) and the transient receptor potential channel C6 (TRPC6) expression, whereas PRO20 protected against podocyte damage and fibrosis (52).
Inflammation is an important pathogenic mechanism for kidney disease and injury, and in vivo and in vitro studies have involved PRR in renal inflammation via both RAS-dependent and RAS-independent pathways. Indeed, PRR is upregulated in the clipped kidney of Goldblatt hypertensive rats and renal administration of PRR shRNA attenuated the upregulation of proinflammatory markers: tumor necrosis factor-α, nuclear factor-κB pathways, COX2, monocyte chemoattractant protein-1, and collagen type (56). In vitro studies have shown that upregulation of PRR stimulated NF-κB pathways, plasminogen activator inhibitor 1, collagen type 1, and fibronectin in collecting duct cells, which was blunted by the silencing of PRR (57, 58). In line with those findings, the overexpression of human PRR in transgenic rat increased TGFβ and induced glomerulosclerosis, which was reversed by the handle region decoy peptide, horseradish peroxidase (HRP) (59).
In humans, serum sPRR levels could be an interesting marker of renal damage. Several studies demonstrated that plasma sPRR positively correlated with tubulointerstitial fibrosis (60) and mesangial injury markers and matrix expansion (61). Serum sPRR levels were elevated in patients with chronic kidney disease (CKD) (60–62). Moreover, serum sPRR levels correlated positively with serum creatinine, blood urea nitrogen (BUN), uric acid, and urine protein-to-creatinine ratio, and correlated inversely with estimated GFR (eGFR) across participants with normal kidney function and those with renal damage/disease (62). Therefore, one could speculate that PRR contributes to renal pathogenesis and the potential preventive effect of blocking PRR and its use as a therapeutic target merits further study.
CONCLUSIONS
Renal PRR affects the morphology of the kidney and influences the renal function by contributing to blood pressure control, water and sodium balance, and acid-base balance through both RAS-dependent and RAS-independent mechanism. PRR plays a role in the progression of diseases such as hypertension and renal pathogenesis. An apparent lack of water homeostasis is seen when PRR/sPRR are deficient. Polyuria is largely observed when PRR is deficient in the nephron, specifically in the intercalated cells of the collecting duct, reaffirming the role of PRR and sPRR as an antidiuretic. The control of salt balance by PRR seems centered on its control of the α-ENaC subunit in the principal cells of the collecting duct. However, the complexity of PRR lies in its multifaceted forms, function, and upstream/downstream regulation. Further investigation is required to better understand the relative contribution of PRR, sPRR, and tPRR and their autocrine and paracrine effects to physiological and physiopathological renal conditions in both men and women.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grant R01-HL-142969 (to F. B. Yiannikouris).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.A. and F.B.Y. prepared figures; G.A. and F.B.Y. drafted manuscript; G.A., J.L.O., and F.B.Y. edited and revised manuscript; G.A., J.L.O., and F.B.Y. approved final version of manuscript.
REFERENCES
- 1..Achard V, Boullu-Ciocca S, Desbriere R, Nguyen G, Grino M. Renin receptor expression in human adipose tissue. Am J Physiol Regul Integr Comp Physiol 292: R274–R282, 2007. doi: 10.1152/ajpregu.00439.2005. [DOI] [PubMed] [Google Scholar]
- 2.Achard V, Tassistro V, Boullu-Ciocca S, Grino M. Expression and nutritional regulation of the (pro)renin receptor in rat visceral adipose tissue. J Endocrinol Invest 34: 840–846, 2011. doi: 10.3275/7627. [DOI] [PubMed] [Google Scholar]
- 3.Yiannikouris F, Karounos M, Charnigo R, English VL, Rateri DL, Daugherty A, Cassis LA. Adipocyte-specific deficiency of angiotensinogen decreases plasma angiotensinogen concentration and systolic blood pressure in mice. Am J Physiol Regul Integr Comp Physiol 302: R244–R251, 2012. doi: 10.1152/ajpregu.00323.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer J-D. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burcklé AC, Danser HJA, Müller ND, Garrelds MI, Gasc MJ-M, Popova ME, Plehm MR, Peters MJ, Bader MM, Nguyen MG. Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension 47: 552–556, 2006. doi: 10.1161/01.HYP.0000199912.47657.04. [DOI] [PubMed] [Google Scholar]
- 6.Contrepas A, Walker J, Koulakoff A, Franek KJ, Qadri F, Giaume C, Corvol P, Schwartz CE, Nguyen G. A role of the (pro)renin receptor in neuronal cell differentiation. Am J Physiol Regul Integr Comp Physiol 297: R250–R257, 2009. doi: 10.1152/ajpregu.90832.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White AJR, Cheruvu SC, Sarris M, Liyanage SS, Lumbers E, Chui J, Wakefield D, McCluskey PJ. Expression of classical components of the renin-angiotensin system in the human eye. J Renin Angiotensin Aldosterone Syst 16: 59–66, 2015. doi: 10.1177/1470320314549791. [DOI] [PubMed] [Google Scholar]
- 8.Feldman DL, Jin L, Xuan H, Contrepas A, Zhou Y, Webb RL, Mueller DN, Feldt S, Cumin F, Maniara W, Persohn E, Schuetz H, Jan Danser AH, Nguyen G. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension 52: 130–136, 2008. doi: 10.1161/HYPERTENSIONAHA.107.108845. [DOI] [PubMed] [Google Scholar]
- 9.Satofuka S, Ichihara A, Nagai N, Noda K, Ozawa Y, Fukamizu A, Tsubota K, Itoh H, Oike Y, Ishida S. (Pro)renin receptor promotes choroidal neovascularization by activating its signal transduction and tissue renin-angiotensin system. Am J Pathol 173: 1911–1918, 2008. doi: 10.2353/ajpath.2008.080457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batenburg WW, Krop M, Garrelds IM, de Vries R, de Bruin RJ, Burckle CA, Muller DN, Bader M, Nguyen G, Danser AH. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. J Hypertens 25: 2441–2453, 2007. doi: 10.1097/HJH.0b013e3282f05bae. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen G. The (pro)renin receptor: pathophysiological roles in cardiovascular and renal pathology. Curr Opin Nephrol Hypertens 16: 129–133, 2007. doi: 10.1097/MNH.0b013e328040bfab. [DOI] [PubMed] [Google Scholar]
- 12.Lu X, Wang F, Xu C, Soodvilai S, Peng K, Su J, Zhao L, Yang KT, Feng Y, Zhou S-F, Gustafsson J-Å, Yang T. Soluble (pro)renin receptor via β-catenin enhances urine concentration capability as a target of liver X receptor. Proc Natl Acad Sci USA 113: E1898–E1906, 2016. doi: 10.1073/pnas.1602397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayumu Y, Yoshimi A, Ken-Ichi K, Fukuko K, Teruo S, Shinichiro I, Shoichi I, Shigeyuki N, Masayoshi S, Takaaki S. The (pro)renin receptor is cleaved by ADAM19 in the golgi leading to its secretion into extracellular space. Hypertens Res 34: 599, 2011. doi: 10.1038/hr.2010.284. [DOI] [PubMed] [Google Scholar]
- 14.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension 53: 1077–1082, 2009. doi: 10.1161/HYPERTENSIONAHA.108.127258. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa T, Suzuki-Nakagawa C, Watanabe A, Asami E, Matsumoto M, Nakano M, Ebihara A, Uddin MN, Suzuki F. Site-1 protease is required for the generation of soluble (pro)renin receptor. J Biochem 161: 369–379, 2017. doi: 10.1093/jb/mvw080. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez AA, Lara SL, Luffman MC, Seth CD, Prieto CM. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II–dependent hypertensive rats. Hypertension 57: 859–864, 2011. doi: 10.1161/HYPERTENSIONAHA.110.167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu X, Garrelds I, Wagner C, Jan Danser A, Meima M. (Pro)renin receptor is required for prorenin-dependent and -independent regulation of vacuolar H+-ATPase activity in MDCK.C11 collecting duct cells. Am J Physiol Renal Physiol 305: F417–F425, 2013. doi: 10.1152/ajprenal.00037.2013. [DOI] [PubMed] [Google Scholar]
- 18.Ludwig J, Kerscher S, Brandt U, Pfeiffer K, Getlawi F, Apps DK, Schagger H. Identification and characterization of a novel 9.2-kDa membrane sector-associated protein of vacuolar proton-ATPase from chromaffin granules. J Biol Chem 273: 10939–10947, 1998. doi: 10.1074/jbc.273.18.10939. [DOI] [PubMed] [Google Scholar]
- 19.Yosypiv I. Prorenin receptor in kidney development. Pediatr Nephrol 32: 383–392, 2017. doi: 10.1007/s00467-016-3365-x. [DOI] [PubMed] [Google Scholar]
- 20.Yosypiv IV. Renin-angiotensin system in mammalian kidney development. Pediatr Nephrol 36: 479–489, 2021. doi: 10.1007/s00467-020-04496-5. [DOI] [PubMed] [Google Scholar]
- 21.Yosypiv IV, Sequeira-Lopez MLS, Song R, De Goes Martini A. Stromal prorenin receptor is critical for normal kidney development. Am J Physiol Regul Integr Comp Physiol 316: R640–R650, 2019. doi: 10.1152/ajpregu.00320.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prieto M, Reverte V, Mamenko M, Kuczeriszka M, Veiras L, Rosales C, McLellan M, Gentile O, Jensen V, Ichihara A, McDonough A, Pochynyuk O, Gonzalez A. Collecting duct prorenin receptor knockout reduces renal function, increases sodium excretion, and mitigates renal responses in ANG II-induced hypertensive mice. Am J Physiol Renal Physiol 313: F1243–F1253, 2017. doi: 10.1152/ajprenal.00152.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oshima Y, Kinouchi K, Ichihara A, Sakoda M, Kurauchi-Mito A, Bokuda K, Narita T, Kurosawa H, Sun-Wada G-H, Wada Y, Yamada T, Takemoto M, Saleem MA, Quaggin SE, Itoh H. Prorenin receptor is essential for normal podocyte structure and function. J Am Soc Nephrol 22: 2203–2212, 2011. doi: 10.1681/ASN.2011020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song R, Preston G, Ichihara A, Yosypiv I. Deletion of the prorenin receptor from the ureteric bud causes renal hypodysplasia. PLoS One 8: e63835-undefined, 2013. doi: 10.1371/journal.pone.0063835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramkumar N, Stuart D, Mironova E, Bugay V, Wang S, Abraham N, Ichihara A, Stockand JD, Kohan DE. Renal tubular epithelial cell prorenin receptor regulates blood pressure and sodium transport. Am J Physiol Renal Physiol 311: F186–F194, 2016. doi: 10.1152/ajprenal.00088.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramkumar N, Stuart D, Calquin M, Quadri S, Wang S, Van Hoek A, Siragy H, Ichihara A, Kohan D. Nephron-specific deletion of the prorenin receptor causes a urine concentration defect. Am J Physiol Renal Physiol 309: F48–F56, 2015. doi: 10.1152/ajprenal.00126.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang F, Lu X, Peng K, Fang H, Zhou L, Su J, Nau A, Yang KT, Ichihara A, Lu A, Zhou S-F, Yang T. Antidiuretic action of collecting duct (pro)renin receptor downstream of vasopressin and PGE2 receptor EP4. J Am Soc Nephrol 27: 3022–3034, 2016. doi: 10.1681/ASN.2015050592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramkumar N, Stuart D, Mironova E, Abraham N, Gao Y, Wang S, Lakshmipathi J, Stockand JD, Kohan DE. Collecting duct principal, but not intercalated, cell prorenin receptor regulates renal sodium and water excretion. Am J Physiol Renal Physiol 315: F607–F617, 2018. doi: 10.1152/ajprenal.00122.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Q, Yang T. Enzymatic sources and physio-pathological functions of soluble (pro)renin receptor. Curr Opin Nephrol Hypertens 27: 77–82, 2018. doi: 10.1097/MNH.0000000000000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gatineau E, Cohn D, Poglitsch M, Loria A, Gong M, Yiannikouris F. Losartan prevents the elevation of blood pressure in adipose-PRR deficient female mice while elevated circulating sPRR activates the renin-angiotensin system. Am J Physiol Heart Circ Physiol 316: H506–H515, 2019. doi: 10.1152/ajpheart.00473.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C-H, Mohammadmoradi S, Thompson J, Su W, Gong M, Nguyen G, Yiannikouris F. Adipocyte (pro)renin-receptor deficiency induces lipodystrophy, liver steatosis and increases blood pressure in male mice. Hypertension 68: 213–219, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gatineau CE, Gong CM, Yiannikouris CF. Soluble prorenin receptor increases blood pressure in high fat–fed male mice. Hypertension 74: 1014–1020, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C, Wang W, Rivard CJ, Lanaspa MA, Summer S, Schrier RW. Molecular mechanisms of angiotensin II stimulation on aquaporin-2 expression and trafficking. Am J Physiol Renal Physiol 300: F1255–F1261, 2011. doi: 10.1152/ajprenal.00469.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PMT, Kohan DE. Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol 10: 135–146, 2015. doi: 10.2215/CJN.05760513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez AA, Womack PJ, Liu ML, Seth CD, Prieto CM. Angiotensin II increases the expression of (pro)renin receptor during low-salt conditions. Am J Med Sci 348: 416–422, 2014. doi: 10.1097/MAJ.0000000000000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol 13: 1131–1135, 2002. doi: 10.1097/01.ASN.0000013292.78621.FD. [DOI] [PubMed] [Google Scholar]
- 37.Lu X, Wang F, Liu M, Yang KT, Nau A, Kohan DE, Reese V, Richardson RS, Yang T. Activation of ENaC in collecting duct cells by prorenin and its receptor PRR: involvement of Nox4-derived hydrogen peroxide. Am J Physiol Renal Physiol 310: F1243–F1250, 2016. doi: 10.1152/ajprenal.00492.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quadri MS, Siragy MH. (Pro)renin receptor contributes to regulation of renal epithelial sodium channel. J Hypertens 34: 486–494, 2016. doi: 10.1097/HJH.0000000000000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim H-S, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest 115: 1092–1099, 2005. doi: 10.1172/JCI23378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical renin-angiotensin system in kidney physiology. Compr Physiol 4: 1201–1228, 2014. doi: 10.1002/cphy.c130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng K, Lu X, Wang F, Nau A, Chen R, Zhou S-F, Yang T. Collecting duct (pro)renin receptor targets ENaC to mediate angiotensin II-induced hypertension. Am J Physiol Renal Physiol 312: F245–F253, 2017. doi: 10.1152/ajprenal.00178.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riquier-Brison A, Sipos A, Prókai Á, Vargas S, Toma I, Meer E, Villanueva K, Chen J, Gyarmati G, Yih C, Tang E, Nadim B, Pendekanti S, Garrelds I, Nguyen G, Danser AH, Peti-Peterdi J. The macula densa prorenin receptor is essential in renin release and blood pressure control. Am J Physiol Renal Physiol 315: F521–F534, 2018. doi: 10.1152/ajprenal.00029.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roy A, Al-Bataineh MM, Pastor-Soler NM. Collecting duct intercalated cell function and regulation. Clin J Am Soc Nephrol 10: 305–324, 2015. doi: 10.2215/CJN.08880914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yun L, Sujun Z, Xiaoyan L, Jinjin F, Xueqin C, Xueqing Y, Qiongqiong Y. Interaction between V-ATPase B2 and (Pro) renin receptors in promoting the progression of renal tubulointerstitial fibrosis. Sci Rep 6: 27677, 2016. doi: 10.1038/srep27677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trepiccione F, Gerber S, Grahammer FF, López-Cayuqueo K, Baudrie V, Păunescu TT, Capen DE, Picard N, Alexander RT, Huber T, Chambrey R, Brown D, Houillier P, Eladari D, Simons M. Renal Atp6ap2/(Pro)renin receptor is required for normal vacuolar H + -ATPase function but not for the renin-angiotensin system. J Am Soc Nephrol 27: 3320–3330, 2016. doi: 10.1681/ASN.2015080915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riediger F, Quack I, Qadri F, Hartleben B, Park J-K, Potthoff SA, Sohn D, Sihn G, Rousselle A, Fokuhl V, Maschke U, Purfürst B, Schneider W, Rump LC, Luft FC, Dechend R, Bader M, Huber TB, Nguyen G, Muller DN. Prorenin receptor is essential for podocyte autophagy and survival. J Am Soc Nephrol 22: 2193–2202, 2011. doi: 10.1681/ASN.2011020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Advani JA, Kelly JD, Cox EA, White LK, Advani AS, Thai MK, Connelly AK, Yuen ED, Trogadis EJ, Herzenberg EA, Kuliszewski EM, Leong-Poi EH, Gilbert ER. The (pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 54: 261–269, 2009. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 48.Ono M, Tsuji T, Ohashi N, Yasuda H, Sakao Y, Kato A, Nishiyama A, Fujigaki Y. Role of intrarenal (pro)renin receptor in ischemic acute kidney injury in rats. Clin Exp Nephrol 19: 185–196, 2015. doi: 10.1007/s10157-014-0979-9. [DOI] [PubMed] [Google Scholar]
- 49.Saigusa T, Dang Y, Bunni MA, Amria MY, Steele SL, Fitzgibbon WR, Bell PD. Activation of the intrarenal renin‐angiotensin‐system in murine polycystic kidney disease. Physiol Rep 3: e12405, 2015. doi: 10.14814/phy2.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang H, Deng M, Zhang L, Lu A, Su J, Xu C, Zhou L, Wang L, Ou J-S, Wang W, Yang T. Role of (pro)renin receptor in albumin overload-induced nephropathy in rats. Am J Physiol Renal Physiol 315: F1759–F1768, 2018. doi: 10.1152/ajprenal.00071.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matavelli LC, Huang J, Siragy HM. (Pro)renin receptor contributes to diabetic nephropathy by enhancing renal inflammation. Clin Exp Pharmacol Physiol 37: 277–282, 2010. doi: 10.1111/j.1440-1681.2009.05292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo R, Yang KT, Wang F, Xu C, Yang T. A (pro)renin receptor decoy peptide PRO20 protects against adriamycin-induced nephropathy by targeting intrarenal renin-angiotensin system. Am J Physiol Renal Physiol 319: F930–F940, 2020. doi: 10.1152/ajprenal.00279.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helmy MS, Jiqian H. Renal (pro)renin receptor upregulation in diabetic rats through enhanced angiotensin AT1 receptor and NADPH oxidase activity. Exp Physiol 93: 709–714, 2008. doi: 10.1113/expphysiol.2007.040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li C, Siragy HM. High glucose induces podocyte injury via enhanced (pro)renin receptor-Wnt-β-catenin-snail signaling pathway. PloS One 9: e89233–e89233, 2014. doi: 10.1371/journal.pone.0089233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Z, Zhou L, Wang Y, Miao J, Hong X, Hou FF, Liu Y. (Pro)renin receptor is an amplifier of Wnt/β-catenin signaling in kidney injury and fibrosis. J Am Soc Nephrol 28: 2393–2408, 2017. doi: 10.1681/ASN.2016070811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quadri SS, Culver S, Siragy HM. Prorenin receptor mediates inflammation in renal ischemia. Clin Exp Pharmacol Physiol 45: 133–139, 2018. doi: 10.1111/1440-1681.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez AA, Zamora L, Reyes-Martinez C, Salinas-Parra N, Roldan N, Cuevas CA, Figueroa S, Gonzalez-Vergara A, Prieto MC. (Pro)renin receptor activation increases profibrotic markers and fibroblast‐like phenotype through MAPK‐dependent ROS formation in mouse renal collecting duct cells. Clin Exp Pharmacol Physiol 44: 1134–1144, 2017. doi: 10.1111/1440-1681.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su J, Liu X, Xu C, Lu X, Wang F, Fang H, Lu A, Qiu Q, Li C, Yang T. NF-κB-dependent upregulation of (pro)renin receptor mediates high-NaCl-induced apoptosis in mouse inner medullary collecting duct cells. Am J Physiol Cell Physiol 313: C612–C620, 2017. [Erratum in Am J Physiol Cell Physiol 314: C254, 2018]. doi: 10.1152/ajpcell.00068.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T, Itoh H. Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol 18: 1789–1795, 2007. doi: 10.1681/ASN.2006091062. [DOI] [PubMed] [Google Scholar]
- 60.Ohashi N, Isobe S, Ishigaki S, Suzuki T, Iwakura T, Ono M, Fujikura T, Tsuji T, Otsuka A, Ishii Y, Furuse H, Kato A, Ozono S, Yasuda H, Joles JA. Plasma soluble (pro)renin receptor reflects renal damage. PLoS One 11: e0156165, 2016. doi: 10.1371/journal.pone.0156165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narumi K, Sato E, Hirose T, Yamamoto T, Nakamichi T, Miyazaki M, Sato H, Ito S. (Pro)renin receptor is involved in mesangial fibrosis and matrix expansion. Sci Rep 8: 16, 2018. doi: 10.1038/s41598-017-18314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamada K, Taniguchi Y, Shimamura Y, Inoue K, Ogata K, Ishihara M, Horino T, Fujimoto S, Ohguro T, Yoshimoto Y, Ikebe M, Yuasa K, Hoshino E, Iiyama T, Ichihara A, Terada Y. Serum level of soluble (pro)renin receptor is modulated in chronic kidney disease. Clin Exp Nephrol 17: 848–856, 2013. doi: 10.1007/s10157-013-0803-y. [DOI] [PubMed] [Google Scholar]