INTRODUCTION
Asthma, the most common chronic respiratory condition worldwide, afflicts more than 300 million people and is characterized by wheezing, coughing, and chest tightness as a result of aberrant inflammation and airway remodeling processes such as airway smooth muscle hyperplasia and mucus production (1). Symptoms are well controlled in many patients with inhaled corticosteroids, which reduce inflammation, and long-acting β2 adrenergic receptor agonists, which relax airway smooth muscle. Unfortunately, available treatments are not effective for all patients, and a subset of disease termed severe asthma is refractory to current therapies and therefore of intense research interest (2). Further, severe asthma with fungal sensitization (SAFS) is a phenotype of allergic asthma in which patients are sensitized to environmental fungi such as Aspergillus fumigatus, which is associated with increased severity of disease (3–5). Individuals with SAFS often have uncontrolled asthma, more steroid usage, higher IgE levels, and increased intensive care unit admissions (3–5).
Activation of host immunity via recognition of cell wall components of A. fumigatus is critical for clearance of the organisms from the lung, yet can also contribute to pathogenesis of fungal asthma. Along these lines, our laboratory has previously reported such a role for β-glucan recognition via the C-type lectin receptor Dectin-1, which is protective during acute exposure to A. fumigatus, but contributes to asthma severity during chronic exposure (6). Similarly, we have recently studied the role of chitin degradation in acute and chronic A. fumigatus exposure via investigation of acidic mammalian chitinase (AMCase), which is capable of binding and cleaving chitin, and which we found to contribute to disease severity in A. fumigatus-associated allergic asthma (7). Chitin, the second most abundant polysaccharide in nature, is a critical component of the fungal cell wall (8) and the exoskeletons of aeroallergens such as house dust mite and cockroach (9). Early studies demonstrated that chitin induces the accumulation of IL-4-expressing innate immune cells, including eosinophils and basophils (10). Fungal chitin derived from home environments has been shown to induce acute eosinophilic inflammation in the lung (11). Other studies have shown that acute chitin exposure elicits patterns of innate cytokines (IL-25, IL-33, and thymic stromal lymphopoietin [TSLP] as well as IL-1β and IL-23) that target distinct populations of resident lymphoid cells, revealing divergent pathways underlying the tissue accumulation of specific types of inflammatory myeloid cells (12). Despite these observations, a knowledge gap exists regarding immunoresponsiveness to chitin in a chronic exposure setting and the impact on allergic asthma. This is primarily the result of studies often employing models that administer shellfish-derived chitin (or beads coated with chitin) acutely (i.e., once or twice) and examine responses a day later (10–12). Other studies have employed the model antigen ovalbumin (13) or a commercial fungal hyphal extract (14) co-administered with shellfish-derived chitin to infer causality/pathology associated with chitin exposure. Collectively, prior research on a role for chitin in asthmatic responses has employed models that may only be tangentially related to responses observed in allergic asthma.
As a continuation of our previous study on chitin and associated host pathways (7), we report on the role of chitinase 3-like-1/BRP-39 (Chi3l1). Chitinase 3-like-1/BRP-39 is a chitinase-like protein that can bind but not cleave chitin and that has been associated with increased asthma risk and severity in humans (15). Study of chitinase 3-like-1/BRP-39 in ovalbumin and house dust mite mouse models of asthma revealed a role in promotion of Th2 inflammatory responses and airway hyperresponsiveness (16). In the current report, we sought to define a role for chitinase 3-like-1/BRP-39 in fungal asthma severity. Unexpectedly, our data indicate that chitinase 3-like-1/BRP-39 maintains lung function during chronic fungal exposure, which outweighs its contribution to lung inflammation in relation to its effect on AHR.
MATERIALS AND METHODS
Subjects, BALF Collection, and Processing, Luminex Analysis
Patients with mild-to-severe asthma were comprehensively characterized according to the National Heart, Lung, and Blood Institute (NHLBI) SARP phenotype protocol at Wake Forest School of Medicine as previously described (17), and a description of this cohort was recently reported (18). The sputum induction method was adopted from the NHLBI Asthma Clinical Research Network and used in SARP (17). Bronchoalveolar lavage fluid (BALF) and sputum samples were derived from both the SARP 1 and 2 cohorts. Per SARP protocol, BALF and sputum were not collected within a week of each other. Sputum was processed immediately after collection, and cell cytospins were stained for differential counts of at least 500 nonsquamous cells/subject slide. Total white blood cell (WBC) count in sputum was determined via trypan blue staining and enumeration using a hemacytometer. Cell-free supernatants were aliquoted and stored at −80°C before use in Luminex analyses (see Whole Lung and BALF Cytokine and Chemokine Analysis). Biospecimens were randomly selected without a priori selection based on asthma severity, rather than based on whether they were skin test-positive or test-negative for Alternaria, Aspergillus, and/or Cladosporium. Sputum supernatants were assayed for different inflammatory cytokine, chemokine, and growth factor protein concentrations using Milliplex Human Cytokine/Chemokine Panels I, II, III, and IV (catalogs HCYTOMAG-60K-PX41, HCYP2MAG-62K-PX23, HCYP3MAG-63K, and HCY4MG-64K-PX21, respectively; MilliporeSigma). Standards for determination of linear curve plus two control samples representing high and low levels of each cytokine/chemokine were included in each assay. Human subjects were enrolled with written informed consent under approved Wake Forest School of Medicine IRB BG01-425 (Wake Forest University IRB). Human samples were analyzed under UAB IRB X130827009 (UAB IRB for Human Use).
Mice
Male and female, age-matched WT Balb/c mice were obtained from Charles River (Wilmington, MA). Chi3l1−/− mice were a kind gift from Dr. Alison Humbles, MedImmune (Gaithersburg, MD), and bred at Tulane University. All animals were housed in a specific pathogen-free, Association for Assessment and Accreditation of Laboratory Animal Care-certified facility and handled according to Public Health Service Office of Laboratory Animal Welfare policies after review by the Tulane Institutional Animal Care and Use Committee.
Preparation of A. fumigatus, Acute and Chronic in Vivo Challenge, Lung Fungal Burden Assessment
A. fumigatus isolate 13073 (ATCC, Manassas, VA) was maintained on potato dextrose agar for 5–7 days at 37°C. Conidia were harvested by washing the culture flask with 50 mL of sterile phosphate-buffered saline supplemented with 0.1% Tween-20. The conidia were then passed through a sterile 40-μm nylon membrane to remove hyphal fragments and enumerated on a hemacytometer. The invasive aspergillosis model consisted of one intratracheal administration of 7 × 107 conidia. For lung fungal burden analysis, left lungs were collected and homogenized in 1 mL of PBS. Total RNA was extracted from 0.1 mL of unclarified lung homogenate using the MasterPure yeast RNA purification kit (Lucigen, Madison, WI), which includes a DNAse treatment step to eliminate genomic DNA as previously reported because DNA is not predictive of organism viability in this assay (19). Lung A. fumigatus burden was analyzed with real-time PCR measurement of the A. fumigatus 18S rRNA (GenBank accession number AB008401) and quantified using a standard curve of A. fumigatus 18S rRNA produced in a bacterial plasmid and copy number calculated based on spectrophotometry. The repeated A. fumigatus-associated allergic airway inflammation model was performed as we have extensively reported (7, 18, 20, 21). Briefly, mice were lightly anesthetized with isoflurane and administered 1 × 107 live A. fumigatus conidia in a volume of 50 μL of PBS intratracheally (it). After resting for 7 days, mice were challenged intratracheally with 1 × 106 live A. fumigatus conidia in 50 μL of PBS daily for 5 consecutive days (days 7, 8, 9, 10, and 11), allowed to rest for 2 consecutive days (days 12 and 13), and then challenged intratracheally with 1 × 106 live A. fumigatus conidia in 50 μL of PBS daily for 3 consecutive days (days 14, 15, and 16). Twenty-four hours after the last A. fumigatus challenge (day 17), mice were euthanized for analysis via ketamine/xylazine overdose and aortic exsanguination.
Whole Lung and BALF Cytokine and Chemokine Analysis
Following euthanasia, the right lung was homogenized in PBS supplemented with Complete, Mini Protease Inhibitor Tablets (Roche Diagnostics), clarified by centrifugation (12,000 g for 10 min at 4°C), and stored at −80°C. From lung homogenate supernatants, IL-33, IL-38, CCL17, and CCL22 levels were quantified by ELISA (R&D Systems). For lung BALF cytokine analysis, a bronchoalveolar lavage was performed on individual mice as previously described (6, 22). Clarified lavage supernatants were analyzed for the protein levels of 32 cytokines and chemokines using the Luminex-based Milliplex multiplex suspension cytokine array (MilliporeSigma), according to the manufacturer’s instructions. The data were analyzed using Bio-Plex Manager software (Bio-Rad). IL-22 and TGF-β1 levels in lavage fluid were quantified by ELISA (R&D Systems). The TGF-β1 assay included an acid activation step to convert latent TGF-β1 to its detectable, immunoreactive form.
Lung Cell Flow Cytometry
Lung cells were isolated via BAL as previously described (6). Cells were washed, and Fc receptors were blocked with Mouse BD Fc Block (BD Biosciences) at 4°C for 20 min. Thereafter, cells were stained with a single-color LIVE/DEAD Fixable Dead Cell Stain (Invitrogen), followed by labeling with specific immune cell surface markers. The following staining parameters were employed: eosinophils as CD45 + CD11b+Siglec F+ Ly-6G– using anti-CD45 (BioLegend, catalog 103115, clone 30-F11), anti-CD11b (BioLegend, catalog 101237, clone M1/70), anti-Siglec F (BioLegend, catalog 155507, clone S17007L), and anti-Ly-6G (BioLegend, catalog 127621, clone 1A8); neutrophils as CD45+ CD11b+Ly6G+ Siglec F– using anti-CD45, anti-CD11b, anti-Ly-6G, and anti-Siglec F; samples were acquired using a 4-laser, 20-parameter analytic BD LSRFortessa, and data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Unstained lung leukocytes served as a control for background fluorescence and gating. Appropriately stained UltraComp eBeads (Thermo Fisher Scientific, Waltham, MA) served as single-color controls.
Pulmonary Function Assessment
Individual anesthetized A. fumigatus-exposed mice were intubated, and each animal was attached to a computer-controlled volume ventilator (flexiVent; SCIREQ). Regular breathing was set at 150 breaths per minute, with volume and pressure controlled by the flexiVent system based on individual animal weights. Positive end-expiratory pressure was set to 2 cm H2° and measured during each breath stroke. The single-frequency forced oscillation technique was employed to measure total/dynamic lung resistance (Rrs). The low-frequency/broadband forced oscillation technique was employed to measure Newtonian resistance (Rn; also known as airway hyperreactivity). All measurements were collected at baseline and after a linear dose response with methacholine challenge (10–40 mg/mL), as previously described (6, 7, 18, 21). Lung function was also assessed in naive WT and mutant mice, which confirmed no baseline anomalies and no differences between groups (data not shown).
Lung Edema and Mucin Analysis
Lungs were collected, weighed, dried for 24 h at 60°C, and then reweighed. For real-time PCR analysis of mucin gene expression, left lungs were collected into TRIzol Reagent (Life Technologies, Carlsbad, CA), homogenized, and RNA isolated according to the manufacturer’s protocol. mRNA levels were then measured via TaqMan assay for Muc5ac and Gob5 and normalized to Hprt (Applied Biosystems, Waltham, MA)
Bone Marrow Chimeras
WT and Chi3l1−/− reciprocal chimeric mice were generated by irradiating the indicated recipient mice with 950 Rads from an X-ray source delivered in 2 equal doses administered 4–5 h apart (23). Following irradiation, mice were intravenously injected with 5 million total bone marrow cells (100% from either WT or Chi3l1−/− mice) and were allowed to reconstitute for 8–10 wk before being subjected to experimental fungal asthma.
Histology
Lungs were inflated and fixed in 4% formalin. The fixed lungs were paraffin embedded and then processed and stained by GNO Histology Consultants (New Orleans, LA). Imaging was performed using a Swift Optical Instruments M10T-P Trinocular LED Microscope equipped with a Motic Moticam 5 + 5-megapixel digital camera.
Statistics
Data were analyzed using GraphPad Prism Version 7.0 statistical software (GraphPad Software). Comparisons between groups when data were normally distributed were made with the two-tailed unpaired Student’s t test or two-way ANOVA. Significance was accepted at a value of P < 0.05.
RESULTS
Chitinase 3-like-1/BRP-39 Is Dispensable for Lung Fungal Clearance During Acute A. fumigatus Exposure
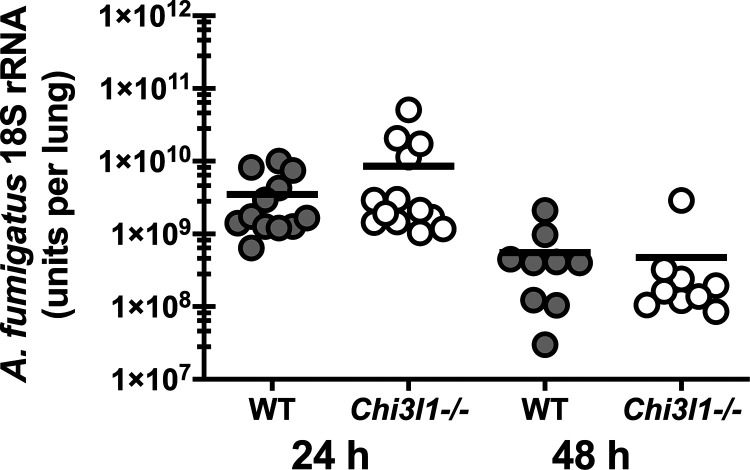
Chitin comprises up to 20% of the A. fumigatus cell wall depending on the morphology, i.e., conidia (lower percentage) versus hyphae (higher percentage) (24). Mammalian chitinases, which include acidic mammalian chitinase (AMCase) and chitotriosidase, were initially described as having antifungal properties against clinically relevant pathogens such as Cryptococcus neoformans and Candida albicans (25). We recently reported, however, that mice deficient in AMCase clear A. fumigatus from the lungs more efficiently that WT control mice (7), suggesting that AMCase-mediated degradation of chitin does not function in a traditional host defense capacity against A. fumigatus. We therefore asked the question whether binding of chitin by chitinase-3-like-1 affected host defense against A. fumigatus. Results in Fig. 1 show that mice deficient in chitinase 3-like-1/BRP-39 (Chi3l1−/−) had no impairments in clearing A. fumigatus from the lung. Thus, BRP-39 is not required for lung clearance of A. fumigatus.
Figure 1.
Chitinase 3-like-1/BRP-39 is dispensable for lung fungal clearance during acute A. fumigatus exposure. Balb/c wild-type (WT) and BRP-39-deficient (Chi3l1−/−) mice were challenged intratracheally with 7 × 107 A. fumigatus conidia, and 24 or 48 h after exposure, lung fungal burden was assessed by real-time PCR analysis of A. fumigatus 18S rRNA levels. The figure represents cumulative data from three independent studies per time point (n = 4 or 6 mice per group, per study). Each symbol represents an individual mouse. Line within a given group represents the mean.
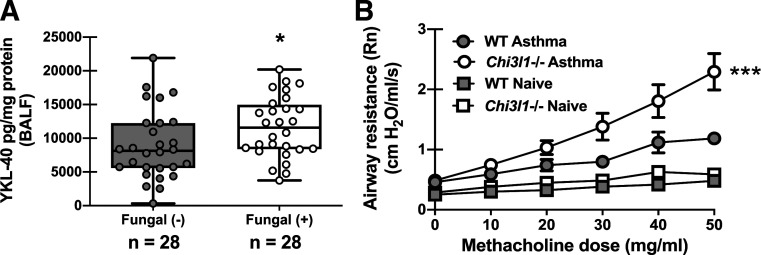
Chitinase 3-like-1 (YKL-40/BRP-39) Is Elevated in Humans With Asthma Sensitized to Fungi but Protects Against AHR in an Experimental Animal Model of Fungal Asthma
In previous reports, we identified immune mediators that were increased in humans with asthma with allergic sensitization to fungi and subsequently pursued mechanistic investigation of these mediators in an experimental animal model of A. fumigatus-associated allergic airway inflammation (fungal asthma) (18, 21). This work uncovered immunopathogenic roles for mediators such as IL-7 (18) and IL-1α and IL-1β (21) and an immunoprotective role for CX3CL1/fractalkine (26) during fungal asthma. The chitinase-like protein YKL-40 (chitinase 3-like-1, BRP-39 in mice) is elevated in humans with asthma and positively correlates with more severe asthma (27, 28). Further, specific Chi3l1 SNPs are associated with increased levels of YKL-40 in sputum of patients with asthma, which correlated with decreased lung function (29). Analysis of bronchoalveolar lavage fluid from humans with asthma revealed that YKL-40 levels were significantly higher in fungal-sensitized versus nonsensitized asthmatics (Fig. 2A). This association suggests that YKL-40 may be induced as part of the immune response during fungal sensitization and led us to question whether it played a causal role in fungal asthma pathogenesis. Unexpectedly, mice deficient in chitinase 3-like-1 (Chi3l1−/−, BRP-39) demonstrated significantly worse airway hyperresponsiveness (AHR) during A. fumigatus-associated allergic airway inflammation (Fig. 2B). There were no differences between naïve WT and Chi3l1−/− mice (Fig. 2B). Thus, BRP-39 is required for optimal lung function during experimental fungal asthma.
Figure 2.
Chitinase 3-like-1 (YKL-40/BRP-39) is elevated in humans with asthma sensitized to fungi but protects against AHR in an experimental animal model of fungal asthma. A: bronchoalveolar lavage fluid (BALF) was collected from subjects with atopic asthma who were sensitized (n = 28) or were not sensitized (n = 28; sputum) to fungi. YKL-40 levels were quantified in clarified BALF supernatants by Milliplex. Data were normalized to the total protein content of each sample and expressed as mean pg/mg protein (each symbol represents a single subject). *P value <0.05 (two-tailed Student’s t test). B: Balb/c wild-type (WT) and BRP-39-deficient (Chi3l1−/−) mice were chronically exposed to A. fumigatus as described in the materials and methods. Twenty-four hours after the last organism challenge, airway (Newtonian) resistance was analyzed via mechanical ventilation using the flexiVent pulmonary function system. Naïve mice served as controls for baseline lung function. The figures illustrate cumulative data from two independent studies (n = 6 mice per group per study). Data are expressed as means ± SE. ***P value < 0.001 (two-way ANOVA).
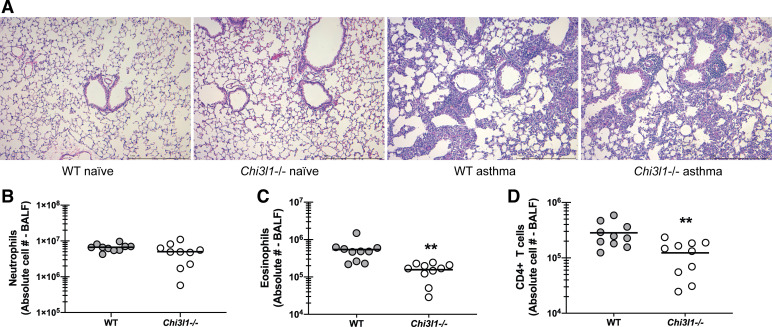
Chitinase 3-like-1/BRP-39 Promotes Increased Lung Cellularity During Experimental Fungal Asthma
We have previously reported that differences in AHR often correlated with the magnitude of lung inflammation and cellularity, specifically increased neutrophil numbers, and, in some instances, increased eosinophil numbers (18, 21). Histological analysis of lung sections from WT and Chi3l1−/− mice revealed an increase in inflammatory cells surrounding the blood vessels and the airways as well as infiltration into the alveolar spaces in WT mice (Fig. 3A) over that observed in Chi3l1−/− mice (Fig. 3A). There were no differences between naïve WT and Chi3l1−/− mice (Fig. 3A). Quantifying these differences by flow cytometric analysis of cells isolated via bronchoalveolar lavage demonstrated that Chi3l1−/− mice had significantly lower eosinophil (Fig. 3B) and CD4 T-cell (Fig. 3C), but not neutrophil (Fig. 3D), numbers. Thus, despite worse AHR in Chi3l1−/− mice, cells that often contribute to AHR, i.e., eosinophils and CD4 T cells, were surprisingly lower.
Figure 3.
Chitinase 3-like-1/BRP-39 promotes increased lung cellularity during experimental fungal asthma. A: Balb/c wild-type (WT) and BRP-39-deficient (Chi3l1−/−) mice were chronically exposed to A. fumigatus as described in the materials and methods. Representative hematoxylin and eosin (H&E)-stained lung sections from naïve and asthmatic WT and Chi3l1−/− mice. Original magnification ×10. Bar = 1 mm. B: Balb/c wild-type (WT) and BRP-39-deficient (Chi3l1−/−) mice were chronically exposed to A. fumigatus as described in the materials and methods. Twenty-four hours after last challenge, lung cells from WT and Chi3l1−/− mice were isolated by bronchoalveolar lavage, enumerated, Fc-blocked, stained with a live/dead staining kit, then stained for neutrophils (CD45+CD11b+Ly-6G+; B), eosinophils (CD45+CD11b+SiglecF+; C), and CD4 T cells (CD45+CD3+CD4+; D), and quantified via flow cytometry. The figure illustrates cumulative data from two independent studies (n = 5 mice per group, per study). Each dot presents a single mouse. The middle line represents the mean. Data are expressed as absolute cell number in lavage fluid. **P value < 0.01 (two-tailed Student’s t test).
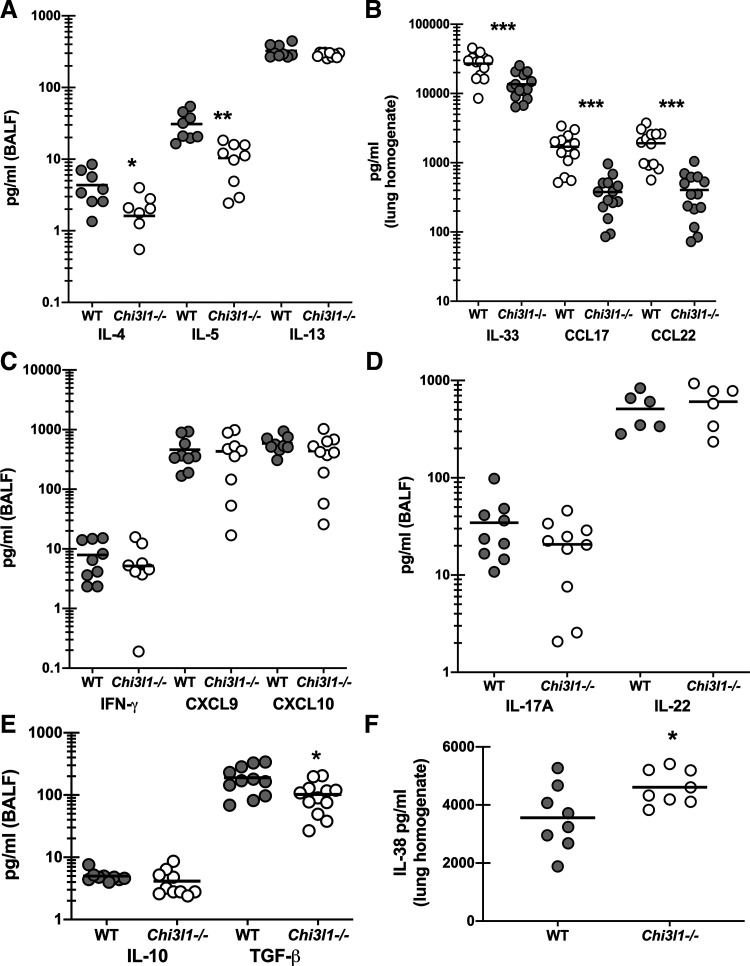
Chitinase 3-like-1/BRP-39 Promotes Type 2, but Not Type 1 or Type 17, Responses During Experimental Fungal Asthma
We and others have previously reported that type 1/Th1 and type 17/Th17 responses correlate with and contribute to disease severity during experimental fungal asthma (7, 18, 21). However, type 2/Th2 responses are also robustly induced during experimental fungal asthma and have been shown by others to also contribute to severity (30, 31). As there was a specific change in lung cellularity during experimental fungal asthma, i.e., decreased eosinophils and CD4 T cells, but not neutrophils, we questioned whether these changes correlated with changes in various pro- and anti-inflammatory responses. Results in Fig. 4A show that the type 2 cytokines IL-4 and IL-5 were significantly lower in Chi3l1−/− mice during fungal asthma, whereas IL-13 levels were unchanged. This correlated with reductions in the pro-type 2 cytokine IL-33 as well as the type 2 associated chemokines CCL17 and CCL22 (Fig. 4B). In contrast, the type 1 cytokine IFN-γ and the associated type 1 chemokines CXCL9 and CXCL10 were not different between WT and Chi3l1−/− mice (Fig. 4C). Likewise, the type 17 cytokines IL-17A and IL-22 were not different (Fig. 4D) nor was the anti-inflammatory cytokine IL-10 (Fig. 4E). The immunoregulatory cytokine TGF-β1 can drive multiple aspects of airway remodeling during asthma including airway smooth muscle proliferation and synthesis of extracellular matrix proteins (32). An increase in TGF-β1 could potentially contribute to increased AHR in Chi3l1−/− mice; however, we found that TGF-β1 levels were decreased in the absence of BRP-39 (Fig. 4E). In contrast, the IL-1 family cytokine IL-38, which functions in an antagonistic manner after receptor binding (33), was found to be increased in Chi3l1−/− mice (Fig. 4F). Thus, despite worse AHR in Chi3l1−/− mice, cytokines that often contribute to AHR were either lower or not different in the absence of BRP-39.
Figure 4.
Chitinase 3-like-1/BRP-39 promotes type 2, but not type 1 or type 17, responses during experimental fungal asthma. Balb/c wild-type (WT) and BRP-39-deficient (Chi3l1−/−) mice were chronically exposed to A. fumigatus as described in the materials and methods. Twenty-four hours after last challenge, a bronchoalveolar lavage was performed and BAL fluid was clarified by centrifugation. A: IL-4, IL-5, and IL-13 levels were quantified in clarified BALF by Milliplex. B: IL-33, CCL17, and CCL22 levels were quantified in clarified lung homogenates by ELISA. C: IFN-γ, CXCL9, and CXCL10 were quantified in clarified BALF by Milliplex. D: IL-17A and IL-22 levels were quantified in clarified BALF by Milliplex and ELISA, respectively. E: IL-10 and TGF-β were quantified in clarified BALF by Milliplex and ELISA, respectively. F: IL-38 levels were quantified in clarified lung homogenates by ELISA. The figures illustrate cumulative data from two independent studies (n = 5 mice per group, per study). Each dot presents a single mouse. The middle line represents the mean. Data are expressed as pg/mL in lavage fluid or lung homogenate. *P value < 0.05; **P value < 0.01; ***P value < 0.001 (two-tailed Student’s t test).
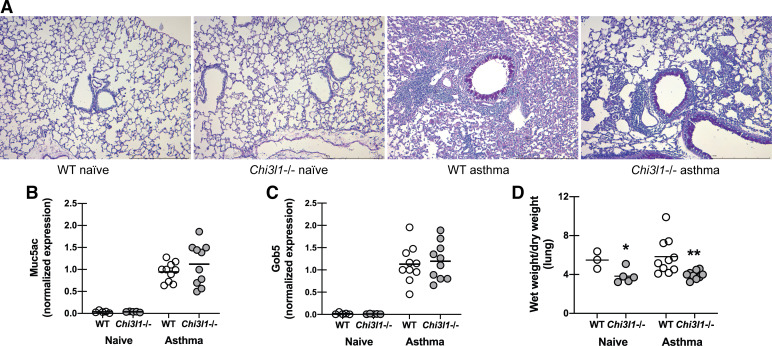
Chitinase 3-like-1/BRP-39 Deficiency Does Not Affect Production of Mucins but Decreases Lung Edema During Fungal Asthma
As differences in inflammation in terms of cell recruitment and cytokine profiles failed to explain increased AHR in Chi3l1−/− mice, we assessed mucus production by examining periodic acid-Schiff (PAS)-stained lung sections. This analysis revealed no differences in goblet cell hyperplasia and airway mucus between WT control (Fig. 5A) and Chi3l1−/− mice (Fig. 5A). There were also no differences between naïve WT and Chi3l1−/− mice (Fig. 5A). This was further supported by real-time PCR assessment of the mucus-associated genes Muc5ac (Fig. 5B) and Gob5 (Fig. 5C), which showed no differences in mRNA expression between WT and Chi3l1−/− mice. Though more commonly measured in acute lung injury, pulmonary edema can also contribute to AHR in animal models (34) and is present in very severe clinical cases of asthma (35). We analyzed wet/dry ratios of WT and Chi3l1−/− mice and observed decreased ratios in the latter during fungal asthma. This decreased wet/dry ratio implies decreased edema, or potentially decreased blood volume, in the absence of BRP-39. Thus, despite worse AHR in Chi3l1−/− mice, there were no differences in mucus gene expression or mucus appearance in the lung. Surprisingly, lung wet/dry ratio was lower in the absence of BRP-39, thus eliminating lung edema as a possible cause of increased AHR.
Figure 5.
Chitinase 3-like-1/BRP-39 deficiency does not affect production of mucins but decreases lung edema during fungal asthma. Balb/c wild-type (WT) and BRP-39-deficient (Chi3l1−/−) mice were chronically exposed to A. fumigatus as described in the materials and methods. A: representative periodic acid-Schiff-stained lung sections from naïve and asthmatic WT and Chi3l1−/− mice. Original magnification ×10. Bar = 1 mm. B and C: twenty-four hours after the last challenge, the left lungs were collected and Muc5ac (B) and Gob5 (C) gene expression was quantified by real-time PCR and normalized to HPRT. Data are expressed as 2−ΔΔCt. The figure illustrates cumulative data from two independent studies (n = 2–3 mice per group per study for naïve mice and n = 5 mice per group per study for asthmatic mice). Each dot presents a single mouse. The middle line represents the mean. D: twenty-four hours after the last challenge, lungs were harvested, weighed, dried for 24 h, and then reweighed. Data are expressed as wet weight/dry weight. The figure illustrates cumulative data from two independent studies (n = 2 or 3 mice per group per study for naïve mice and n = 5 mice per group per study for asthmatic mice). Each dot presents a single mouse. The middle line represents the mean. *P value < 0.05; **P value < 0.01 (two-tailed Student’s t test).
Presence of Chitinase 3-like-1/BRP-39 in Either the Hematopoietic or Nonhematopoietic Compartment Is Sufficient to Regulate Increased AHR during Fungal Asthma
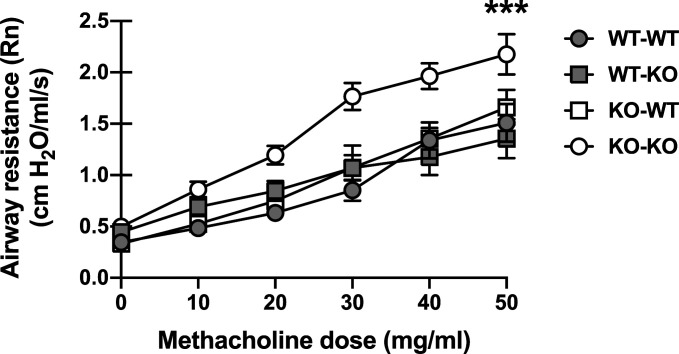
BRP-39 is broadly expressed in both hematopoietic cells, including neutrophils and macrophages, and nonhematopoietic cells, including fibroblasts and epithelial cells (36). As a step toward understanding the discordant airway function and inflammatory features of Chi3l1−/− mice, we determined in which group of cells BRP-39 expression was protective. To answer this question, we created reciprocal bone marrow chimeras in which either the hematopoietic or nonhematopoietic compartment expressed BRP-39, as well as control chimeras in which both compartments (analogous to WT) or neither compartment (analogous to Chi3l1−/− mice) expressed BRP-39. Following reconstitution, these chimeras were subjected to experimental fungal asthma and airway function was assessed. We found that expression of BRP-39 in either the hematopoietic compartment (WT → KO) or the nonhematopoietic compartment (KO → WT) was sufficient to regulate the magnitude of AHR (Fig. 6), indicating that BRP-39 expression in either compartment is sufficient to control AHR.
Figure 6.
Presence of chitinase 3-like-1/BRP-39 in either the hematopoietic or nonhematopoietic compartment is sufficient to regulate increased AHR during fungal asthma. Bone marrow chimeras were generated in which mice expressed BRP-39 in either only the hematopoietic compartment (C57BL/6-Chi3l1−/−; WT-KO), only the nonhematopoietic compartment (KO-WT), both compartments (WT-WT) or neither compartment (KO-KO) as described in the materials and methods. Eight to ten weeks following reconstitution, chimeras were chronically exposed to A. fumigatus as described in the materials and methods. Airway (Newtonian) resistance was analyzed via flexiVent. The figure illustrates cumulative data from two independent studies (n = 5 mice per group, per study). Data are expressed as means ± SE. ***P value < 0.001 (two-way ANOVA). KO, knockout; WT, wild-type.
DISCUSSION
Studies have shown that YKL-40/chitinase 3-like-1/BRP-39 levels correlate with thickening of the epithelial basal membrane (37) and may function in airway smooth muscle remodeling in human asthma (38). Experimentally, although somewhat limited, mice deficient in BRP-39 (chitinase-3-like protein 1; Chi3l1) have attenuated type 2 responses and better lung function during chitin-negative ovalbumin-associated asthma and chitin-containing house dust mite-associated asthma (16, 39). Although chitooligosaccharides are the dominant ligands for the glycoside hydrolase 18 family (chitinases and chitinase-like proteins) (28, 40), IL-13Rα2 has been identified as a receptor for BRP-39 (23). Via IL-13Rα2, BRP-39 has been shown to promote resistance to hyperoxia-induced apoptosis, S. pneumoniae-induced pyroptosis and inflammasome activation, and lung melanoma metastasis (41). However, a previous study has reported that IL-13Rα2 is not expressed in the lung during experimental A. fumigatus-associated asthma (42), suggesting that IL-13Rα2 is not functioning as ligand for BRP-39 during fungal asthma. Other studies have shown that BRP-39 induces inflammatory mediator production during lung metastasis, which can be reversed by treatment with chitin (43), whereas BRP-39 expressed on colonic epithelial cells binds chitin particles, which can be simultaneously bound by chitin-binding protein-expressing bacteria, leading to enhanced bacterial adhesion (44). Collectively, these studies suggest that chitin binding is a critical factor in the function of BRP-39. However, BRP-39 binding to fungal chitin and how this influences responses or severity of allergic fungal asthma has not been examined. Here, we sought to fill this gap in knowledge by elucidating the role of BRP-39 in an experimental animal model of fungal-associated allergic airway inflammation. Firstly however, data suggest that BRP-39 may play a role in lung host defense. Previous work has demonstrated that BRP-39 promotes clearance of Streptococcus pneumoniae from the lung by augmenting bactericidal activity of macrophages and limiting excessive inflammation (27). During Pseudomonas aeruginosa pneumonia, BRP-39 also functions to limit excessive inflammation and improve survival (45). In contrast, we confirmed that BRP-39 was not required for host defense against A. fumigatus as evidenced by similar lung fungal burden at both 24 and 48 h following infection. We did not assess inflammatory responses in these experiments, as any effect was not sufficient to affect fungal burden or survival. A noteworthy difference between the reported bacterial models and the A. fumigatus infection model employed here is much higher lethality in WT control mice in the bacterial infections compared to fungal infection. This difference also suggests that BRP-39 may be primarily required to regulate inflammation during lung infections that causes significant mortality due to hyperinflammatory responses. Nevertheless, BRP-39 is dispensable for fungal clearance from the lung during acute A. fumigatus challenge.
It is increasingly appreciated that the underlying pathogenic processes that result in asthma phenotypes are extremely diverse and that development of more effective and targeted treatments will likely require precise knowledge of these processes. To this end, we have sought to define pathways that contribute to fungal asthma pathogenesis by identifying factors that are modulated in humans with fungal (+) asthma and exploring the roles of these factors in experimental models. Our work has identified IL-7 (18) and IL-1R signaling (21) as immunopathogenic factors in fungal asthma. In contrast, we demonstrated that IL-1RA was an attractive and efficacious immunoprotective therapeutic for improving fungal asthma severity (21). Ongoing work in our laboratory has also identified CX3CL1 as an immunoprotective mediator during fungal asthma (26). YKL-40 has been implicated in non-type 2 asthma due to its relationship with mediators associated with IL-17-mediated inflammation (15, 46). Indeed, higher circulating YKL-40 consistently associates with more severe asthma, with clusters of patients exhibiting features of non-type 2-driven inflammation (47, 48). Our finding of higher YKL-40 levels in fungal-sensitized asthmatics may be intuitive based on YKL-40’s known ability to bind chitin (28) as well as the fact that fungal asthma is often characterized by induction of type 17 responses (21, 49, 50). We therefore applied our model of fungal asthma to WT and Chi3l1−/− mice and measured airway resistance to understand the effect of BRP-39 on lung function. While the role of BRP-39 in fungal asthma has not been previously investigated, study of Chi3l1−/− mice in ovalbumin (OVA) and house dust mite (HDM) models of asthma reported decreased airway resistance (improved lung function) (16). In an alternative study design where bone marrow-derived dendritic cells overexpressing BRP-39 were transferred into mice that subsequently underwent the OVA asthma model, BRP-39 was also found to drive AHR (51). OVA and HDM models of asthma primarily drive a Th2 asthma phenotype, whereas the fungal asthma model employed here attempts to recapitulate a particular endotype of human asthma (severe asthma with fungal sensitization). Through the use of live A. fumigatus conidia, our model generates a complex and broad host response in addition to the typical Th2 asthmatic responses (6, 18). Thus, it is somewhat surprising that, in contrast to the findings in the OVA and HDM models described above, our experiment revealed a contrasting, protective/beneficial role for BRP-39 during fungal asthma. YKL-40 generally correlates with worse disease in human asthma (15, 47, 52, 53). Indeed, we show that YKL-40 levels were elevated in fungal-sensitized asthmatics (Fig. 2A), a cohort that we have reported to have more severe disease (18). Although the data are limited, in addition to the bacterial pneumonia studies mentioned previously, other studies also support a beneficial role for BRP-39 in some experimental lung disease models. In hypoxia-associated acute lung injury, BRP-39 is required for survival in conjunction with regulating the magnitude of neutrophil recruitment and inflammation (54). In a murine model of bleomycin-induced fibrosis, BRP-39 ameliorated inflammation and cell death as well as played a profibrotic role in the repair phase by augmenting alternative macrophage activation, fibroblast proliferation, and matrix deposition (55). Therefore, there is a precedence for BRP-39 functioning in a protective capacity. The contrast between the pathogenic effect of BRP-39 in Th2-centric models of asthma and the protective effect of BRP-39 in our model of fungal asthma raises intriguing and potentially therapeutically important questions about the function of this molecule in different allergic and lung disease contexts.
BRP-39 promotes type 2/Th2 responses in the aforementioned OVA and HDM asthma models (16), and our data further support this finding during fungal asthma. We further found that numbers of eosinophils and CD4 T cells, classic contributors to Th2 asthma, were decreased. In addition to decreased IL-4 and IL-5 in BALF, our data expand the role of BRP-39 in the induction of type 2/Th2 responses by showing that the absence of BRP-39 resulted in lower levels of the pro-type 2/Th2 mediator IL-33. We further show that the levels of CCL17 and CCL22, chemokines that we have previously found to be associated with disease severity in our model (6, 7, 18), were also reduced in the absence of BRP-39. Therefore, our data support a central role of BRP-39 in both the initiation of type 2/Th2 responses via IL-33 induction and the recruitment of eosinophils (56) and CD4 T cells (57), which express the receptor for CCL17 and CCL22 (CCR4). BRP-39 has been shown to suppress differentiation of naïve T cells into Th1 cells (58) and to support IL-17A production by γδ T cells (59). We have reported that type 1/Th1 and type 17/Th17 responses correlate with neutrophil levels and the severity of fungal asthma (7, 18, 21). Enhanced type 1/Th1 or type 17/Th17 responses could explain the exacerbation observed in the absence of BRP-39. However, levels of IFN-γ and IL-17A were unaffected by the absence of BRP-39 during fungal asthma as were the levels of neutrophils in the lung. The lone mediator we observed to be increased in the absence of BRP-39 was IL-38. IL-38 is not well studied in allergic asthma, although reports suggest it may regulate the production of some inflammatory cytokines as well as induction of Th2 cytokines and eosinophil recruitment (60). Our data support IL-38 functioning in the latter during fungal asthma; however, we cannot exclude an immunopathogenic role for IL-38, which will be the subject of future studies. Together, these data indicate that BRP-39 plays a role in the induction of select Th2 responses during fungal asthma, as has been reported in other models, but does not affect potentially immunopathogenic type 1/Th1 or type 17/Th17 responses. Overall, our findings are in accordance with BRP-39 as a driver of allergic inflammation, but paradoxical considering the phenotype of impaired lung function in our model.
Though the mechanism of increased AHR in Chi3l1−/− mice during fungal asthma remains to be determined, we have excluded numerous potential drivers of immunopathogenesis. In fact, all the potentially immunopathogenic mediators that were modulated in the absence of BRP-39 were either not different or decreased, making increased AHR in this context all the more compelling. Therefore, we investigated nonimmune functions of BRP-39 that might explain the increase in AHR in its absence. Excessive production of mucins is an additional feature of asthma pathogenesis that we considered as a possible correlate of increased AHR in Chi3l1−/− mice. We assessed mucus production via qPCR analysis of Muc5ac and Gob5 and via histological periodic acid-Schiff staining. These analyses indicated the transcription and presence of mucins in the airways of WT and Chi3l1−/− during fungal asthma compared to naïve controls, but no difference between WT and Chi3l1−/− mice. We further excluded increased lung edema as a driver of AHR in the absence of BRP-39 via analysis of lung wet weight/dry weight ratios. Although lung wet:dry weight did not correlate with AHR, it did correlate with lower inflammatory cell numbers (eosinophils and CD4 T cells) and lower cytokine values (in our model, lower type 2 cytokines). This finding is similar to recent data showing that Chi3l1−/− mice exposed to RSV also have lower inflammatory cell numbers in the lung and lower type 2 cytokines (61). Therefore, lung injury is often a direct correlate of the level of inflammation. In regard to Chi3l1−/− mice, this appears to be pathogen/model-specific, as bacterial infection contributes to elevated lung injury whereas fungal and viral exposure/infection does not.
We next utilized reciprocal bone marrow chimeras to discriminate between the functions of BRP-39 in relevant hematopoietic cells (leukocytes) versus nonhematopoietic cells (lung epithelial cells, airway smooth muscle cells), in hopes of guiding future studies into how BRP-39 suppresses AHR during fungal asthma. We found that chimeras expressing BRP-39 in either the hematopoietic or nonhematopoietic compartments exhibited lung function similar to chimeras expressing BRP-39 in both compartments. Only chimeras lacking BRP-39 entirely exhibited impaired lung function. BRP-39 is a secreted protein, and these results indicate that any effect of the source of BRP-39 secretion (hematopoietic or nonhematopoietic cells) is secondary to its downstream effects in terms of AHR. Other groups have recapitulated BRP-39 phenotypes with administration of BRP-39, also supporting its function as a secreted molecule (27, 41). BRP-39 can also bind to collagen (2) and other endogenous ligands including hyaluronan (62), leaving open the possibility of multiple potential nonimmune functions of secreted BRP-39. Recent studies suggest that low molecular weight hyaluronan fragments can drive AHR, whereas high molecular weight hyaluronan fragments can reduce AHR (63, 64). Although speculative, the absence of BRP-39 may result in hyaluronan-mediated events during fungal asthma that negatively affect AHR. Future studies will determine if during fungal asthma, there is an imbalance between low and high molecular weight hyaluronan fragments in Chi3l1−/− mice. Further, a key point of interest in our pursuit of the role of BRP-39 in fungal asthma is to unravel the complex role of chitin during fungal asthma. In terms of the function of BRP-39, one possibility is its binding to chitin during exposure to a chitin-rich pathogen potentially, which inhibits binding to other endogenous ligands and thus interrupts protective or pathogenic processes. More broadly, mammals express multiple chitinases and chitinase-like proteins including BRP-39, all of which may affect either the amount of chitin in the lung or how it interacts with the immune system. Along these lines, acidic mammalian chitinase has been shown to prevent long-term damage caused by accumulation of environmental chitin in the lung (65) and to promote chitin-dependent eosinophilia following exposure to Aspergillus niger preparations (11). Chitin cleavage by another true chitinase, chitotriosidase, supports development of Th2 responses during infection with the chitin-rich fungal pathogen, Cryptococcus neoformans (66). BRP-39 is reported to be constitutively expressed at higher levels in the lung than either chitotriosidase or acidic mammalian chitinase (67), and though it lacks chitinolytic activity, it retains the ability to bind chitin (28). As already discussed, during fungal asthma Chi3l1−/− mice phenocopy selects aspects of other asthma models, including some containing chitin (primarily the induction of Th2 responses) but has the opposite effect on AHR. Given the complexity of the effects of chitin, particularly fungal chitin, on immune responses, we are currently undertaking studies to understand if BRP-39, in concert with active chitinases, modulates chitin levels or exposure of chitin to other recognition factors during fungal asthma.
In conclusion, we report that increased levels of chitinase 3-like-1/BRP-39 in humans with asthma are associated with allergic sensitization to fungi. Interrogating of the role of BRP-39 in a mouse model of Aspergillus-associated allergic airway inflammation revealed that BRP-39 is protective for lung function, yet paradoxically also drives type 2/Th2 inflammation. This correlation has not been observed in other models; thus, our data add another important aspect to our knowledge of the biology of this molecule. This effect occurs via a yet to be determined mechanism but likely occurs via a function of BRP-39 as a secreted molecule, which potentially binds multiple endogenous substrates as well as exogenous chitin, to currently unknown effect. These results add to our previous work on acidic mammalian chitinase to expand our understanding of the role of chitin-associated proteins and set the stage for further study of the role of fungal chitin in allergic asthma.
GRANTS
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute Grants HL122426 and HL136211 (both to C.S.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.J.M., A.T.H., D.A.M. and C.S. conceived and designed research; J.J.M., A.T.H., D.A.M., C.S., J.M.G., M.J., D.A.E., J.P.B., Z.Y., S.M. and M.C. performed experiments; J.J.M., A.T.H., D.A.M., C.S., J.M.G., S.M., and F.E.L. analyzed data; J.J.M., A.T.H., D.A.M., C.S., J.M.G., and F.E.L. interpreted results of experiments; J.J.M. and C.S. prepared figures; J.J.M., A.T.H., D.A.M. and C.S. drafted manuscript; J.J.M., A.T.H., D.A.M. and C.S. edited and revised manuscript; J.J.M., A.T.H., D.A.M. and C.S. approved final version of manuscript.
REFERENCES
- 1.Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD. Asthma. Nat Rev Dis Primers 1: 15025, 2015. doi: 10.1038/nrdp.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bigg HF, Wait R, Rowan AD, Cawston TE. The mammalian chitinase-like lectin, YKL-40, binds specifically to type I collagen and modulates the rate of type I collagen fibril formation. J Biol Chem 281: 21082–21095, 2006. doi: 10.1074/jbc.M601153200. [DOI] [PubMed] [Google Scholar]
- 3.Denning DW, O'Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J 27: 615–626, 2006. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 4.Goh KJ, Yii ACA, Lapperre TS, Chan AK, Chew FT, Chotirmall SH, Koh MS. Sensitization to Aspergillus species is associated with frequent exacerbations in severe asthma. J Asthma Allergy 10: 131–140, 2017. doi: 10.2147/JAA.S130459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medrek SK, Kao CC, Yang DH, Hanania NA, Parulekar AD. Fungal sensitization is associated with increased risk of life-threatening asthma. J Allergy Clin Immunol Pract 5: 1025–1031, 2017. doi: 10.1016/j.jaip.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Lilly LM, Gessner MA, Dunaway CW, Metz AE, Schwiebert L, Weaver CT, Brown GD, Steele C. The beta-glucan receptor dectin-1 promotes lung immunopathology during fungal allergy via IL-22. J Immunol 189: 3653–3660, 2012. doi: 10.4049/jimmunol.1201797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garth JM, Mackel JJ, Reeder KM, Blackburn JP, Dunaway CW, Yu Z, Matalon S, Fitz L, Steele C. Acidic mammalian chitinase negatively affects immune responses during acute and chronic Aspergillus fumigatus exposure. Infect Immun 86: e00944–e01017, 2018. doi: 10.1128/IAI.00944-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagener J, Malireddi RK, Lenardon MD, Koberle M, Vautier S, MacCallum DM, Biedermann T, Schaller M, Netea MG, Kanneganti TD, Brown GD, Brown AJ, Gow NA. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathog 10: e1004050, 2014. doi: 10.1371/journal.ppat.1004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim LK, Morita R, Kobayashi Y, Eisenbarth SC, Lee CG, Elias J, Eynon EE, Flavell RA. AMCase is a crucial regulator of type 2 immune responses to inhaled house dust mites. Proc Natl Acad Sci USA 112: E2891–E2899, 2015. doi: 10.1073/pnas.1507393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reese TA, Liang HE, Tager AM, Luster AD, van RN, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 447: 92–96, 2007. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Dyken SJ, Garcia D, Porter P, Huang X, Quinlan PJ, Blanc PD, Corry DB, Locksley RM. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J Immunol 187: 2261–2267, 2011. doi: 10.4049/jimmunol.1100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Dyken SJ, Mohapatra A, Nussbaum JC, Molofsky AB, Thornton EE, Ziegler SF, McKenzie AN, Krummel MF, Liang HE, Locksley RM. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and gammadelta T cells. Immunity 40: 414–424, 2014. doi: 10.1016/j.immuni.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arae K, Morita H, Unno H, Motomura K, Toyama S, Okada N, Ohno T, Tamari M, Orimo K, Mishima Y, Suto H, Okumura K, Sudo K, Miyazawa H, Taguchi H, Saito H, Matsumoto K, Nakae S. Chitin promotes antigen-specific Th2 cell-mediated murine asthma through induction of IL-33-mediated IL-1beta production by DCs. Sci Rep 8: 11721, 2018. doi: 10.1038/s41598-018-30259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy RM, Paes HC, Nanjappa SG, Sorkness R, Gasper D, Sterkel A, Wuthrich M, Klein BS. Complement component 3C3 and C3a receptor are required in chitin-dependent allergic sensitization to Aspergillus fumigatus but dispensable in chitin-induced innate allergic inflammation. mBio 4: e00162–e00213, 2013. doi: 10.1128/mBio.00162-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Zhang X, Liu Y, Zhang L, Zheng J, Wang J, Hansbro PM, Wang L, Wang G, Hsu AC. Chitinase-like protein YKL-40 correlates with inflammatory phenotypes, anti-asthma responsiveness and future exacerbations. Respir Res 20: 95, 2019. doi: 10.1186/s12931-019-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, Humbles A, Kearley J, Coyle A, Chupp G, Reed J, Flavell RA, Elias JA. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med 206: 1149–1166, 2009. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, Wenzel SE, Peters SP, Meyers DA, Bleecker ER, National Heart, Lung, and Blood Institute's Severe Asthma Research Program. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol 133: 1557–1563, 2014. doi: 10.1016/j.jaci.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeder KM, Dunaway CW, Blackburn JP, Yu Z, Matalon S, Hastie AT, Ampleford EJ, Meyers DA, Steele C. The common gamma-chain cytokine IL-7 promotes immunopathogenesis during fungal asthma. Mucosal Immunol 11: 1352–1362, 2018. doi: 10.1038/s41385-018-0028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattila PE, Metz AE, Rapaka RR, Bauer LD, Steele C. Dectin-1 Fc targeting of Aspergillus fumigatus beta-glucans augments innate defense against invasive pulmonary aspergillosis. Antimicrob Agents Chemother 52: 1171–1172, 2008. doi: 10.1128/AAC.01274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gessner MA, Werner JL, Lilly LM, Nelson MP, Metz AE, Dunaway CW, Chan YR, Ouyang W, Brown GD, Weaver CT, Steele C. Dectin-1-dependent interleukin-22 contributes to early innate lung defense against Aspergillus fumigatus. Infect Immun 80: 410–417, 2012. doi: 10.1128/IAI.05939-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godwin MS, Reeder KM, Garth JM, Blackburn JP, Jones M, Yu Z, Matalon S, Hastie AT, Meyers DA, Steele C. IL-1RA regulates immunopathogenesis during fungal-associated allergic airway inflammation. JCI Insight 4: e129055, 2019. doi: 10.1172/jci.insight.129055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson MP, Metz AE, Li S, Lowell CA, Steele C. The absence of Hck, Fgr and Lyn tyrosine kinases augments lung innate immune responses to Pneumocystis murina. Infect Immun 77: 1790–1797, 2009. doi: 10.1128/IAI.01441-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guedes AG, Jude JA, Paulin J, Rivero-Nava L, Kita H, Lund FE, Kannan MS. Airway responsiveness in CD38-deficient mice in allergic airway disease: studies with bone marrow chimeras. Am J Physiol Lung Cell Mol Physiol 308: L485–L493, 2015. doi: 10.1152/ajplung.00227.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Rubio R, de Oliveira HC, Rivera J, Trevijano-Contador N. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front Microbiol 10: 2993, 2019. doi: 10.3389/fmicb.2019.02993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bussink AP, van Eijk M, Renkema GH, Aerts JM, Boot RG. The biology of the Gaucher cell: the cradle of human chitinases. Int Rev Cytol 252: 71–128, 2006. doi: 10.1016/S0074-7696(06)52001-7. [DOI] [PubMed] [Google Scholar]
- 26.Godwin MS, Jones M, Blackburn JP, Yu Z, Matalon S, Hastie AT, Meyers DA, Steele C. The chemokine CX3CL1/fractalkine regulates immunopathogenesis during fungal-associated allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol 320: L393–L404, 2021. doi: 10.1152/ajplung.00376.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dela Cruz CS, Liu W, He CH, Jacoby A, Gornitzky A, Ma B, Flavell R, Lee CG, Elias JA. Chitinase 3-like-1 promotes Streptococcus pneumoniae killing and augments host tolerance to lung antibacterial responses. Cell Host Microbe 12: 34–46, 2012. doi: 10.1016/j.chom.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fusetti F, Pijning T, Kalk KH, Bos E, Dijkstra BW. Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J Biol Chem 278: 37753–37760, 2003. doi: 10.1074/jbc.M303137200. [DOI] [PubMed] [Google Scholar]
- 29.Gomez JL, Crisafi GM, Holm CT, Meyers DA, Hawkins GA, Bleecker ER, Jarjour N, Severe Asthma Research Program I, Cohn L, Chupp GL, Severe Asthma Research Program (SARP) Investigators. Genetic variation in chitinase 3-like 1 (CHI3L1) contributes to asthma severity and airway expression of YKL-40. J Allergy Clin Immunol 136: 51–58 e10, 2015. doi: 10.1016/j.jaci.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murdock BJ, Shreiner AB, McDonald RA, Osterholzer JJ, White ES, Toews GB, Huffnagle GB. Coevolution of TH1, TH2, and TH17 responses during repeated pulmonary exposure to Aspergillus fumigatus conidia. Infect Immun 79: 125–135, 2011. doi: 10.1128/IAI.00508-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramaprakash H, Shibata T, Duffy KE, Ismailoglu UB, Bredernitz RM, Moreira AP, Coelho AL, Das AM, Fursov N, Chupp GL, Hogaboam CM. Targeting ST2L potentiates CpG-mediated therapeutic effects in a chronic fungal asthma model. Am J Pathol 179: 104–115, 2011. doi: 10.1016/j.ajpath.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makinde T, Murphy RF, Agrawal DK. The regulatory role of TGF-beta in airway remodeling in asthma. Immunol Cell Biol 85: 348–356, 2007. doi: 10.1038/sj.icb.7100044. [DOI] [PubMed] [Google Scholar]
- 33.Catalan-Dibene J, McIntyre LL, Zlotnik A. Interleukin 30 to interleukin 40. J Interferon Cytokine Res 38: 423–439, 2018. doi: 10.1089/jir.2018.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans RL, Changani KK, Hotee S, Pindoria K, Campbell S, Nials AT, Ford WR, Broadley KJ, Kidd EJ. Pulmonary edema measured by MRI correlates with late-phase response to allergen challenge. Exp Lung Res 41: 189–198, 2015. doi: 10.3109/01902148.2014.985407. [DOI] [PubMed] [Google Scholar]
- 35.D'Amato G, Vitale C, Lanza M, Sanduzzi A, Molino A, Mormile M, Vatrella A, Bilo MB, Antonicelli L, Bresciani M, Micheletto C, Vaghi A, D'Amato M. Near fatal asthma: treatment and prevention. Eur Ann Allergy Clin Immunol 48: 116–122, 2016. [PubMed] [Google Scholar]
- 36.Lee CG, Da Silva CA, la CC, Ahangari F, Ma B, Kang MJ, He CH, Takyar S, Elias JA. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol 73: 479–501, 2011. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konradsen JR, James A, Nordlund B, Reinius LE, Soderhall C, Melen E, Wheelock AM, Lodrup CKC, Lidegran M, Verhoek M, Boot RG, Dahlen B, Dahlen SE, Hedlin G. The chitinase-like protein YKL-40: a possible biomarker of inflammation and airway remodeling in severe pediatric asthma. J Allergy Clin Immunol 132: 328–335, 2013.doi: 10.1016/j.jaci.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Bara I, Ozier A, Girodet PO, Carvalho G, Cattiaux J, Begueret H, Thumerel M, Ousova O, Kolbeck R, Coyle AJ, Woods J, Tunon de Lara JM, Marthan R, Berger P. Role of YKL-40 in bronchial smooth muscle remodeling in asthma. Am J Respir Crit Care Med 185: 715–722, 2012. doi: 10.1164/rccm.201105-0915OC. [DOI] [PubMed] [Google Scholar]
- 39.Nikota JK, Botelho FM, Bauer CM, Jordana M, Coyle AJ, Humbles AA, Stampfli MR. Differential expression and function of breast regression protein 39 (BRP-39) in murine models of subacute cigarette smoke exposure and allergic airway inflammation. Respir Res 12: 39, 2011. doi: 10.1186/1465-9921-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schimpl M, Rush CL, Betou M, Eggleston IM, Recklies AD, van Aalten DM. Human YKL-39 is a pseudo-chitinase with retained chitooligosaccharide-binding properties. Biochem J 446: 149–157, 2012. doi: 10.1042/BJ20120377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He CH, Lee CG, Dela Cruz CS, Lee CM, Zhou Y, Ahangari F, Ma B, Herzog EL, Rosenberg SA, Li Y, Nour AM, Parikh CR, Schmidt I, Modis Y, Cantley L, Elias JA. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor alpha2. Cell Rep 4: 830–841, 2013. [Erratum in Cell Rep 5: 1156, 2013] doi: 10.1016/j.celrep.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blease K, Jakubzick C, Westwick J, Lukacs N, Kunkel SL, Hogaboam CM. Therapeutic effect of IL-13 immunoneutralization during chronic experimental fungal asthma. J Immunol 166: 5219–5224, 2001. doi: 10.4049/jimmunol.166.8.5219. [DOI] [PubMed] [Google Scholar]
- 43.Libreros S, Garcia-Areas R, Shibata Y, Carrio R, Torroella-Kouri M, Iragavarapu-Charyulu V. Induction of proinflammatory mediators by CHI3L1 is reduced by chitin treatment: decreased tumor metastasis in a breast cancer model. Int J Cancer 131: 377–386, 2012. doi: 10.1002/ijc.26379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawada M, Chen CC, Arihiro A, Nagatani K, Watanabe T, Mizoguchi E. Chitinase 3-like-1 enhances bacterial adhesion to colonic epithelial cells through the interaction with bacterial chitin-binding protein. Lab Invest 88: 883–895, 2008. doi: 10.1038/labinvest.2008.47. [DOI] [PubMed] [Google Scholar]
- 45.Marion CR, Wang J, Sharma L, Losier A, Lui W, Andrews N, Elias JA, Kazmierczak BI, Roy CR, Dela Cruz CS. Chitinase 3-like 1 (Chil1) regulates survival and macrophage-mediated interleukin-1beta and tumor necrosis factor alpha during Pseudomonas aeruginosa pneumonia. Infect Immun 84: 2094–2104, 2016. doi: 10.1128/IAI.00055-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hinks TSC, Brown T, Lau LCK, Rupani H, Barber C, Elliott S, Ward JA, Ono J, Ohta S, Izuhara K, Djukanovic R, Kurukulaaratchy RJ, Chauhan A, Howarth PH. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J Allergy Clin Immunol 138: 61–75, 2016. doi: 10.1016/j.jaci.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez JL, Yan X, Holm CT, Grant N, Liu Q, Cohn L, Nezgovorova V, Meyers DA, Bleecker ER, Crisafi GM, Jarjour NN, Rogers L, Reibman J, Chupp GL, Investigators S. Characterisation of asthma subgroups associated with circulating YKL-40 levels. Eur Respir J 50: 1700800, 2017. doi: 10.1183/13993003.00800-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ilmarinen P, Tuomisto LE, Niemela O, Hamalainen M, Moilanen E, Kankaanranta H. YKL-40 and adult-onset asthma: elevated levels in clusters with poorest outcome. J Allergy Clin Immunol Pract 7: 2466–2468, 2019. doi: 10.1016/j.jaip.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 49.Fei M, Bhatia S, Oriss TB, Yarlagadda M, Khare A, Akira S, Saijo S, Iwakura Y, Fallert Junecko BA, Reinhart TA, Foreman O, Ray P, Kolls J, Ray A. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc Natl Acad Sci USA 108: 5360–5365, 2011. doi: 10.1073/pnas.1015476108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murdock BJ, Falkowski NR, Shreiner AB, Sadighi Akha AA, McDonald RA, White ES, Toews GB, Huffnagle GB. IL-17 drives pulmonary eosinophilia following repeated Aspergillus fumigatus conidia exposure. Infect Immun 80: 1424–1436, 2012. doi: 10.1128/IAI.05529-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Q, Chai SJ, Qian YY, Zhang M, Wang K. Breast regression protein-39 (BRP-39) promotes dendritic cell maturation in vitro and enhances Th2 inflammation in murine model of asthma. Acta Pharmacol Sin 33: 1525–1532, 2012. doi: 10.1038/aps.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knihtila H, Kotaniemi-Syrjanen A, Pelkonen AS, Savinko T, Malmberg LP, Makela MJ. Serum chitinase-like protein YKL-40 is linked to small airway function in children with asthmatic symptoms. Pediatr Allergy Immunol 30: 803–809, 2019. doi: 10.1111/pai.13119. [DOI] [PubMed] [Google Scholar]
- 53.Specjalski K, Chełmińska M, Jassem E. YKL-40 protein correlates with the phenotype of asthma. Lung 193: 189–194, 2015. doi: 10.1007/s00408-015-9693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sohn MH, Kang MJ, Matsuura H, Bhandari V, Chen NY, Lee CG, Elias JA. The chitinase-like proteins breast regression protein-39 and YKL-40 regulate hyperoxia-induced acute lung injury. Am J Respir Crit Care Med 182: 918–928, 2010. doi: 10.1164/rccm.200912-1793OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Y, Peng H, Sun H, Peng X, Tang C, Gan Y, Chen X, Mathur A, Hu B, Slade MD, Montgomery RR, Shaw AC, Homer RJ, White ES, Lee CM, Moore MW, Gulati M, Lee CG, Elias JA, Herzog EL. Chitinase 3-like 1 suppresses injury and promotes fibroproliferative responses in mammalian lung fibrosis. Sci Transl Med 6: 240ra276, 2014. doi: 10.1126/scitranslmed.3007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu LY, Jarjour NN, Busse WW, Kelly EA. Chemokine receptor expression on human eosinophils from peripheral blood and bronchoalveolar lavage fluid after segmental antigen challenge. J Allergy Clin Immunol 112: 556–562, 2003. doi: 10.1016/s0091-6749(03)01798-6. [DOI] [PubMed] [Google Scholar]
- 57.Solari R, Pease JE. Targeting chemokine receptors in disease–a case study of CCR4. Eur J Pharmacol 763: 169–177, 2015. doi: 10.1016/j.ejphar.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim DH, Park HJ, Lim S, Koo JH, Lee HG, Choi JO, Oh JH, Ha SJ, Kang MJ, Lee CM, Lee CG, Elias JA, Choi JM. Regulation of chitinase-3-like-1 in T cell elicits Th1 and cytotoxic responses to inhibit lung metastasis. Nat Commun 9: 503, 2018. doi: 10.1038/s41467-017-02731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sutherland TE, Logan N, Ruckerl D, Humbles AA, Allan SM, Papayannopoulos V, Stockinger B, Maizels RM, Allen JE. Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat Immunol 15: 1116–1125, 2014. doi: 10.1038/ni.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsang MS, Sun X, Wong CK. The role of new IL-1 family members (IL-36 and IL-38) in atopic dermatitis, allergic asthma, and allergic rhinitis. Curr Allergy Asthma Rep 20: 40, 2020. doi: 10.1007/s11882-020-00937-1. [DOI] [PubMed] [Google Scholar]
- 61.Kim MJ, Shim DH, Cha HR, Moon KY, Yang CM, Hwang SJ, Kim KW, Park JH, Lee CG, Elias JA, Sohn MH, Lee JM. Chitinase 3-like 1 protein plays a critical role in respiratory syncytial virus-induced airway inflammation. Allergy 74: 685–697, 2019. doi: 10.1111/all.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kognole AA, Payne CM. Inhibition of mammalian glycoprotein YKL-40: identification of the physiological ligand. J Biol Chem 292: 2624–2636, 2017. doi: 10.1074/jbc.M116.764985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson CG, Stober VP, Cyphert-Daly JM, Trempus CS, Flake GP, Cali V, Ahmad I, Midura RJ, Aronica MA, Matalon S, Garantziotis S. High molecular weight hyaluronan ameliorates allergic inflammation and airway hyperresponsiveness in the mouse. Am J Physiol Lung Cell Mol Physiol 315: L787–L798, 2018. doi: 10.1152/ajplung.00009.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lazrak A, Song W, Zhou T, Aggarwal S, Jilling T, Garantziotis S, Matalon S. Hyaluronan and halogen-induced airway hyperresponsiveness and lung injury. Ann NY Acad Sci 1479: 29–43, 2020. doi: 10.1111/nyas.14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Dyken SJ, Liang HE, Naikawadi RP, Woodruff PG, Wolters PJ, Erle DJ, Locksley RM. Spontaneous chitin accumulation in airways and age-related fibrotic lung disease. Cell 169: 497–509, 2017. doi: 10.1016/j.cell.2017.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wiesner DL, Specht CA, Lee CK, Smith KD, Mukaremera L, Lee ST, Lee CG, Elias JA, Nielsen JN, Boulware DR, Bohjanen PR, Jenkins MK, Levitz SM, Nielsen K. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog 11: e1004701, 2015. doi: 10.1371/journal.ppat.1004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bohr S, Patel SJ, Vasko R, Shen K, Golberg A, Berthiaume F, Yarmush ML. The role of CHI3L1 (chitinase-3-Like-1) in the pathogenesis of infections in burns in a mouse model. PLoS One 10: e0140440, 2015. doi: 10.1371/journal.pone.0140440. [DOI] [PMC free article] [PubMed] [Google Scholar]