Abstract
We have previously reported that several patients with idiopathic pulmonary hypertension (PH) had different types of G6PD deficiency. However, the role of G6PD in PH is multifactorial because G6PD is involved in controlling oxidative stress, metabolic switch, and red blood cell fragility. To delineate the contribution of G6PD to PH pathogenesis, we utilized a mouse line with decreased expression of G6PD (10% from wild-type level). We confirmed that mice with G6PD deficiency develop spontaneous pulmonary hypertension with pulmonary artery and right heart remodeling. G6PD deficiency resulted in increased free hemoglobin and activation of the p38 pathway, which we recently reported induces the development of PH in the sugen/hypoxia model via endothelial barrier dysfunction. Metabolomics analysis of G6PD deficient mice indicates the switch to alternative metabolic fluxes that feed into the pentose phosphate pathway (PPP), resulting in the upregulation of oxidative stress, fatty acid pathway, and reduction in pyruvate production. Thus, G6PD deficiency did not reduce PPP flux that is important for proliferation but activated collateral pathways at the cost of increased oxidative stress. Indeed, we found the upregulation of myo-inositol oxidase, reduction in GSH/GSSG ratio, and increased nitration in the lungs of G6PD-deficient mice. Increased oxidative stress also results in the activation of PI3K, ERK1/2, and AMPK that contribute to the proliferation of pulmonary vasculature. Therefore, G6PD deficiency has a multimodal effect, including hemolysis, metabolic reprogramming, and oxidative stress leading to the PH phenotype in mice.
Keywords: glycolysis, metabolism, oxidative stress, pentose phosphate pathway, pulmonary hypertension, vascular proliferation
INTRODUCTION
Pulmonary hypertension (PH) is a fatal vascular disease with proliferative vascular remodeling, blocking the lumen of the pulmonary arteries and leading to right ventricular (RV) dysfunction (1). Current therapeutic options are inadequate in reversing the complex mechanisms of PH pathogenesis. Vascular cell proliferation and vasoconstriction in the pulmonary artery are characterized as one of the major causes in the pathogenesis of PH (2). Predisposed genetic conditions in PH interferes and deludes the early diagnosis and treatment of the disease. Current PH diagnostic genetic panels include only a limited number of genes such as BMPR2, ACVRL1, CAV1, ENG, and SMAD9 (3). In our recent clinical investigation, 5 out of 22 patients with PH were found to be glucose-6-phosphate dehydrogenase (G6PD)-deficient (4). However, the mechanism of G6PD deficiency-related metabolic dysfunction in PH development reported in the case studies (5) is not well explored. Therefore, a better understanding of new genetic abnormalities, e.g., G6PD mutation in PH, is essential for the development of new diagnostic panels in the early detection of the disease.
Increased risk of PH was identified in some genetically predisposed disease conditions such as sickle cell anemia and thalassemia (6). It was shown that, in association with these diseases, hemolysis plays a pivotal role in the development of PH. There is an extreme disparity between the prevalence of PH in the general population and patients with hemolytic anemias. Idiopathic PH patients, who never experienced hemolytic conditions, exhibited a 5-fold increase in plasma free hemoglobin (Hb) levels as compared to nonPH patients (4). Hemolysis-released free heme was identified as a factor in barrier disruption leading to vascular leakage in human lung microvascular endothelial cells. Recently, we reported that hemolysis released free heme causes endothelial barrier dysfunction in the lungs (7, 8). Here, free heme was shown to activate the p38 mitogen-activated protein kinase (MAPK) pathway. Similarly, increased hemolysis induced vascular remodeling was also observed in the Sugen-5416/Hypoxia and MCT PH models (9, 10).
G6PD deficiency is shown to be linked with extensive hemolysis and increased reactive oxygen species (ROS) production (11). G6PD is the only enzyme that generates NADPH for the production of GSH in erythrocytes. Deficiency in G6PD, therefore, attenuates the erythrocyte's antioxidant capacity, resulting in increased ROS and augments erythrocyte fragility (12). Complementary to this, hemolysis released free heme was also shown to alter the cellular redox balance and generate ROS (13). Activation of proinflammatory pathways in G6PD deficiency was related to increased oxidative stress (11, 14, 15). People with G6PD deficiency are increasingly susceptible to acute hemolytic anemias with a number of drugs (16). In contrast with our previous clinical report (4), some studies have also reported that hypoxia-induced G6PD overexpression causes DNA methylation and PH development (17–19). Therefore, in order to delineate this discrepancy, we used a G6PD knockdown (G6PD KD) mouse model expressing only 10% G6PD to delineate the involvement of G6PD in the metabolic alterations and explore its link to vascular remodeling in PH.
METHODS
Human Subjects
Patients with PH with deidentified lung tissue of group I (idiopathic pulmonary arterial hypertension (IPAH) group, n = 10) and healthy control samples (control group, n = 10) were obtained through the Pulmonary Hypertension Breakthrough Initiative (PHBI). Institutional Review Boards approved the PHBI study protocol of the participating sites in the network, and all sites were adherent to the guidelines of the US Federal Policy for the Protection of Human Subjects (45 CFR, Part 46), and were also supported the general ethical principles of the Declaration of Helsinki.
Generation of G6pdxa-m1Neu/H Knockdown Mice
G6pdxa-m1Neu/H mice with a C3H/HeJ murine background were generated by Genetically Engineered Mouse Models (GEMM) Core, The University of Arizona (Tucson, AZ) using the G6pdxa-m1Neu/H embryo from European Mouse Mutant Archive Infrafrontier, Munich, Germany. These G6PD KD mice were achieved by a single point mutation by A to T transversion in the 5′ splice site consensus sequence at the 3′ terminal of exon 1 (15). The pups were genotyped by PCR with the following primers: ACACGCCCTCTTGcCGTTAAAT (forward) and GAGCACGTTTGATTGTGCAGAT (reverse, fluorescently labeled with 6-fluorescein amidite [FAM]) by University of Arizona Genetics Core, University of Arizona, Tucson, AZ). G6PD deficiency is an X‐linked, hereditary disease, mostly affecting males than females; therefore, male animals were selected for this study. This G6PD knockdown (KD) model showed only ∼10% of G6PD activity and protein expression compared to wild type (WT). Male animals (12 ± 1-wk-old; n = 7–9) were used for the study. All animals were maintained at 22°C, 12-h light/dark cycle, and had free access to water ad libitum and standard rodent food. All experimental protocols were approved by the University of Arizona Institutional Animal Care and Use Committee.
Measurement of Glucose-6-Phosphate Dehydrogenase Activity
G6PD activity was determined using the G6PD activity assay kit from Abcam (ab176722; Burlingame, CA). Samples and standards were prepared according to the manufacturer's instructions and activity was measured on a Synergy H1 microplate reader (BioTek, Winooski, VT). G6PD activity was normalized to the total protein concentration of samples and determined by the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL).
Cell-Free Hemoglobin Measurement
Analysis of cell-free hemoglobin in the plasma was carried out utilizing gel electrophoresis, and following this, the gel was excited at 488 nm (10). In brief, 1 µL of plasma was diluted in PBS (10×) and reconstituted with 6× nonreducing Laemmli sample buffer (Boston Bioproducts Inc., Ashland, MA). This was then loaded into 4%–20% SDS-PAGE Mini-PROTEAN TGXTM gels (Bio-Rad Laboratories Inc., Hercules, CA) and was separated by electrophoresis. The bands, which are autofluorescent, were visualized at 488 nm light emitted in a ChemiDoc MP Imaging System (Bio-Rad Laboratories Inc., Hercules, CA). Finally, the bands were analyzed using the Bio-Rad Image Lab software.
Metabolomics
Metabolomic analysis was done to quantify tissue and plasma metabolites like amino acids, carbohydrates, and nucleotides, by gas-chromatography time of flight (GC-TOF) mass spectrometry from the West Coast Metabolomics Center (UC Davis NIH West Coast Metabolomics, Davis CA) as described previously (4). Plasma and tissue samples of WT and G6PD KD animals were separately analyzed.
The metabolite abundance values were exported, and Perseus version 1.6.2.3 was used for visualization of the data (20). Z-scores were generated from the data by subtracting the mean of each column from each value in the column. The resultant value was then divided by the standard deviation of the row or column to obtain the final Z-score. A visual heat map represented the unsupervised hierarchical clustering; the distance was set to Euclidian and the linkage to average. The maximal numbers of clusters were set to 300. For the principal component analysis (PCA), the number of components was set to 5, and the Benjamini-Hochberg cut-off method was used (21).
Western Blotting
For the analysis of total proteins, lung tissue or pulmonary artery smooth muscle cells were homogenized in tissue permeabilization buffer containing Halt Protease and Phosphatase Inhibitor Cocktail (78444, Thermo Fisher Scientific, Rockford, IL), using a FisherBrand Homogenizer-850. Following this, the homogenate was centrifuged at 10,000 g for 10 min, and the supernatant was collected. The Pierce BCA Protein Assay Kit (23225, Thermo Fisher Scientific, Burlington, ONT) was used to determine protein concentrations. The samples were suspended in 6× Laemmli sample buffer (Boston Bioproducts Inc., Ashland, MA) and heated at 95°C for 5 min. 4–20% Mini-PROTEAN TGX stain-Free gels (Bio-Rad Laboratories Inc., Hercules, CA) were used to separate the proteins electrophoretically. The proteins were then transferred to a PVDF membrane using a PowerPac Universal power supply and Trans-Blot Turbo transferring system (Bio-Rad Laboratories Inc., Hercules, CA). Blocked membranes in 5% bovine serum albumin (37525, Thermo Scientific, Rockford IL) were probed using the following antibodies: Glut1 (1:1,000, sc-377228), PGAM1 (1:1,000, sc-130334), GPD1 (1:1,000, sc-376219), and GPD2 (1:1,000, sc-393620), MIOX from Santa Cruz Biotechnology; G6PD (1:1,000, 12263S), ZO-1 (1:1,000, 8193S), Afadin (1:1,000, 13531S), MKK3 (1:1,000, 8535S), PKM2 (1:1,000, 4053), phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (1:1,000, 9101S), phospho-p38 MAPK (Thr180/Tyr182) (1:1,000, 9211S), p38 MAPK (1:1,000, 9212S), phospho-AMPKα (Thr172) (1:1,000, 2535), AMPKα (1:1,000, 5831S), glutamate dehydrogenase 1/2 (1:1,000, 12793), p53 (1:1,000, 9282), and phospho-p53 (Ser315) (1:1,000, 2528) from Cell Signaling Technology; and phospho-MKK3 (189) (1:1,000, PA5-37700) from Thermo Scientific, Rockford IL; PKM1 (1:1,000, 15821-1-AP) from ProteinTech; anti-PI3 kinase, p85 (1:1,000, ABS234) from Sigma Aldrich, St. Louis, MO; and anti-nitrotyrosine antibodies were obtained from Calbiochem, La Jolla, CA (1:500,487923) (see Table 1). Reactive bands were imaged with the chemiluminescent protocol, recorded with the ChemiDoc MP Imaging System (Bio-Rad Laboratories Inc., Hercules, CA). Image Lab software was used to analyze the bands. Protein loading was determined from stain-free gels and was normalized with the total protein loaded as described previously (22). Some Western blot membranes were stripped using RestoreWestern Blot Stripping Buffer (21063, Thermo Fisher Scientific, Rockford, IL) and reprobed for more than one protein.
Table 1.
Summary of antibody validation
| Antibody Name | Company/Catalog Number | Validation | Reference |
|---|---|---|---|
| GLUT1 | Santa Cruz/sc- 377228 | Various cell lines with high/low protein expression | https://www.scbt.com/scbt/product/glut1-antibody-a-4 |
| PGAM1 | Santa Cruz/sc-130334 | Various cell lines with high/low protein expression | https://www.scbt.com/p/pgam1-antibody-6 |
| GPD1 | Santa Cruz/ sc-376219 | Various cell lines with high/low protein expression | https://www.scbt.com/p/gpd1-antibody-e-7 |
| GPD2 | Santa Cruz/sc-393620 | Various cell lines with high/low protein expression | https://www.scbt.com/p/gpd2-antibody-d-9 |
| MIOX | Santa Cruz/376080 | Positive/negative control (myo-inositol oxygenase transfected) | https://www.scbt.com/p/myo-inositol-oxygenase-antibody-e-11 |
| ZO-1 | Cell Signaling Technology/8193S | Various cell lines with high/low protein expression | https://www.cellsignal.com/products/primary-antibodies/zo-1-d7d12-rabbit-mab/8193 |
| Afadin | Cell Signaling Technology/13531S | Various cell lines with high/low protein expression | https://www.cellsignal.com/products/primary-antibodies/afadin-d1y3z-rabbit-mab/13531 |
| MKK3 | Cell Signaling Technology/8535S | Various cell lines with high/low protein expression | https://www.cellsignal.com/products/primary-antibodies/mkk3-d4c3-rabbit-mab/8535 |
| PKM2 | Cell Signaling Technology/4053 | Various cell lines with high/low protein expression | https://www.cellsignal.com/products/primary-antibodies/pkm2-d78a4-xp-rabbit-mab/4053 |
| Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) | Cell Signaling Technology/9101S | Positive/negative control (MEK1, MEK1/2 Inhibitors, PDGF) | https://www.cellsignal.com/products/primary-antibodies/phospho-p44-42-mapk-erk1-2-thr202-tyr204-antibody/9101 |
| Phospho-p38 MAPK (Thr180/Tyr182) | Cell Signaling Technology/9211S | Positive/negative control (UV- or anisomycin-treated) | https://www.cellsignal.com/products/primary-antibodies/phospho-p38-mapk-thr180-tyr182-antibody/9211 |
| P38 MAPK | Cell Signaling Technology/9212S | Positive/negative control (p38 MAPK siRNA, UV- or anisomycin-treated) | https://www.cellsignal.com/products/primary-antibodies/p38-mapk-antibody/9212 |
| Phospho-AMPKα (Thr172) | Cell Signaling Technology/2535 | Positive/negative control (oligomycin- or phenformin-treated) | https://www.cellsignal.com/products/primary-antibodies/phospho-ampka-thr172-40h9-rabbit-mab/2535 |
| AMPKα | Cell Signaling Technology/5831S | Various cell lines with high/low protein expression | https://www.cellsignal.com/products/primary-antibodies/ampka-d5a2-rabbit-mab/5831 |
| Phospho-MKK3 (189) | Thermo Scientific /PA5-37700 | Positive/negative control (treated with PMA) | https://www.thermofisher.com/antibody/product/Phospho-MEK3-Ser189-Antibody-Polyclonal/PA5-37700 |
| PKM1 | Proteintech/15821-1-AP | Various cell lines with high/low protein expression | https://www.ptglab.com/products/PKM1-specific-Antibody-15821-1-AP.htm |
| Anti-PI3 Kinase p85 | Sigma Aldrich /ABS234 | Various cell lines with high/low protein expression | https://www.sigmaaldrich.com/catalog/product/mm/abs234?lang=en®ion=US |
| Anti-nitrotyrosine | Calbiochem/487923 | Various cell lines with high/low protein expression | https://www.emdmillipore.com/US/en/product/Anti-Nitrotyrosine-Mouse-mAb-CC22.8C7.3, EMD_BIO-487923?ReferrerURL=https%3A%2F%2Fwww.google.com%2F" |
| G6PD | Cell Signaling Technology/12263S | Various cell lines with high/low protein expression | https://www.cellsignal.com/products/primary-antibodies/g6pd-d5d2-rabbit-mab/12263 |
Antibodies used in the present study have undergone previous validation. GLUT1, glucose transporter protein 1; GPD1, glucose phosphate dehydrogenase 1; G6PD, glucose-6-phosphate dehydrogenase; MIOX, myo-inositol oxygenase; MKK3, mitogen-activated protein kinase kinase 3; p38 MAPK, p38 mitogen-activated protein kinase; PGAM1, phosphoglycerate mutase; PKM, pyruvate kinase isozymes; ZO-1, zona occluden-1.
Measurement of Phosphoglycerate Mutase Activity
The PGAM activity assay was performed as per the manufacturer's instructions (K2007-100; Biovision Inc. CA). In brief, lung tissue or pulmonary artery smooth muscle cells (PASMCs) were lysed in PGAM lysis buffer mixed with appropriate volumes of the reaction mix (PGAM Assay Buffer, PGAM Substrate, PGAM Cofactor, PGAM Converter, PGAM Converter, PGAM Developer, and OxiRedTM Probe) following the kit protocol. Appropriate standards were used to quantify the total PGAM activity. The samples were read in a plate reader with fluorescence Ex/Em = 535/587 nm. The PGAM activity was then normalized according to the total protein.
Measurement of the Ratio of Reduced Glutathione to Oxidized Glutathione
The Abcam GSH/GSSG Ratio Detection Assay Kit (ab138881) was used to determine the reduced/oxidized glutathione ratios. In brief, 10 mg of the tissue was lysed in mammalian lysis buffer and then deproteinized by adding equal volumes of acetone, shaken vigorously for 2 min, and centrifuged at 10,000 g for 10 min. The supernatant was then collected and kept in open microfuge tubes at 37°C until the volume in the tubes was reduced to more than half, signifying the evaporation of acetone. The samples were then used to determine the GSH/GSSG ratios according to the manufacturer's instructions. All values were normalized and the total protein concentration of samples was determined by the Pierce BCA Protein Assay Kit.
Hemodynamic Measurement
Mice were anesthetized with Ketamine (Zetamine, 501072, MWI, Boise, ID) [100 mg/kg] + Xylazine (AnaSed, 510004, MWI, Boise, ID) [10 mg/kg]; IP. Right ventricular systolic pressure (RVSP) was assessed by a pressure transducer catheter (SPR-1000, Millar Instruments, Houston, TX). This catheter was inserted into the right jugular vein to reach the right ventricle (RV) to monitor the pressure. In brief, the pressure transducer catheter was used in conjunction with the Millar Transducer Control Unit TC-510 and PL3504 PowerLab 4/35 data acquisition system (AD Instruments, Colorado Springs, CO). Using this system, the RV pressure was screened. Systemic blood pressure was measured by inserting the pressure transducer catheter into the right carotid artery. After this procedure, the animals were then connected to a tracheal catheter ventilator system (MiniVent, Type-845; Harvard Apparatus, South Natick, MA), the thorax was opened and through the RV, the lungs were flushed by using a 0.9% sodium chloride solution. The heart and lungs were dissected from the animals; the RV free wall was separated from the left ventricle (LV) and the septum (S) and weighed. Fulton index (RV/LV + S ratio) was assessed as a parameter of RV hypertrophy. Following this, the left lungs were fixed in formalin and embedded in paraffin for histological studies. The remaining lung portions were snap-frozen in liquid nitrogen and stored at −80°C for further studies.
Plasma Nitrite Assay
The nitric oxide content in WT and G6PD KD plasma samples was measured with the Nitrite Assay Kit following the manufacturer’s protocol (Griess reagent) (K544-200, BioVision, CA).
Histopathological Analysis
For the morphometric assessment of pulmonary vessels, 5-μm tissue sections were dewaxed and stained with hematoxylin and eosin (H&E) by the Tissue Acquisition and Cellular/Molecular Analysis Shared Resource (TACMASR) of the University of Arizona Cancer Center (Tucson, AZ) using standard operating procedures. Ten transversely sectioned pulmonary arteries (PAs) (diameter < 300 μm to 50 μm) from each animal were selected randomly from the ×40 image (Leica DMI6000 multifunction motorized inverted microscope, Buffalo Grove, IL). The morphometric evaluation was performed using a blinded grouping fashion. All histology/microscopy experiments were performed at the same time under the same conditions. The wall thickness of the PA was assessed using the software Fiji ImageJ (Version-1.52p National Institutes of Health, Washington; http://fiji.sc/Fiji; in the public domain) (23).
Pulmonary Arterial Smooth Muscle Cell Isolation
Mouse pulmonary arterial smooth muscle cells (PASMC) were isolated from eight WT and G6PD KD animals as per the previously described protocol (24). Briefly, mice were euthanized, and the whole lungs were separated and immediately transferred in cold Dulbecco’s modified Eagle medium (DMEM) (11960-044, Gibco, Fisher Scientific, Pittsburgh, PA). After this, the vessels were cut into small segments and transferred to 2 mL of 1 mg/mL collagenase type-2 (LS004176, Worthington, Lakewood, NJ) for 20 min at 37°C. After enzyme digestion, the cells were precipitated by centrifugation at 2,000 g for 10 min. The supernatant was removed, and 10 mL of DMEM (11965-092, Gibco, Fisher Scientific, Pittsburgh, PA) containing 20% FBS (25–5144, Geneclone, San Diego, CA) was added. Cells were then plated in a 6-well plate covered with gelatin (Cat. No. ES-006-B, Millipore, Fisher Scientific, Pittsburgh, PA). Every 2–3 days, half of the medium was changed to DMEM. Then, cells were replated into a 10-cm dish and grew until they became confluent. Cell purity was confirmed by staining smooth muscle-actin (anti-α-SM actin (1A4), Alexa-Fluor488 ab184675, (Abcam Burlingame, CA), and Isotype IgG2a (Thermo Scientific, Rockford, IL) using FACS performed in a Novocyte 2000 (ACEA Biosciences, San Diego, CA) instrument. Cell purity for all experiments was determined to be >95%. For all cell culture experiments, cells from at least 3 different animals were pooled together.
Cell Growth Kinetics Measurement
Cell growth rates were determined in real-time using the iCELLigence System (ACEA Biosciences). This system utilizes a highly sensitive technique to derive real-time changes in electrical impedance, representing the cell growth index. The system consists of a 16-well E-Plate and a device station. The assays were performed as per the manufacturer's instructions, and the cell index (CI) was normalized as described previously (7).
Detection of Cellular Reactive Oxygen Species
Isolated PASMCs (50,000 cells/well in a 96-well plate) were grown in phenol red-free DMEM for 12 h. The media was then separated and used to detect hydrogen peroxide according to the Hydrogen Peroxide Cell-Based Assay Kit (600050, Cayman, Ann Arbor, Michigan) protocol. Values were normalized with the total protein determined by the Pierce BCA Protein Assay Kit.
Statistical Analysis
The GraphPad Prism software (version 8.3.1) was used to perform statistical analysis. The mean value (± SE) was calculated for all samples. After testing the data normality, the significance was determined by either the unpaired t test or the nonparametric Mann–Whitney U test. P < 0.05 was considered to be statistically significant. The Grubbs’ test (extreme studentized deviate) was used to determine the significant outliers before data analysis.
RESULTS
Glucose-6-Phosphate Dehydrogenase Expression and Activity
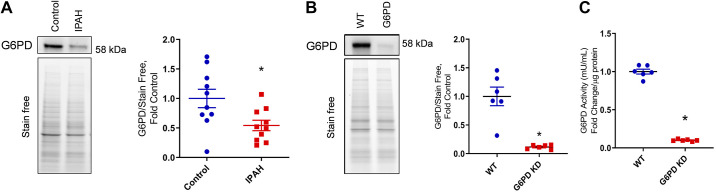
Screening of human lung tissue samples Group I PH patients (IPAH) from the PHBI cohort revealed decreased G6PD protein expression compared with healthy controls (Fig. 1A), indicating a strong association of G6PD deficiency with PH. IPAH patients demonstrated a 45.7% reduction in G6PD protein expression than control subjects. The role of G6PD deficiency in vascular proliferation and metabolic reprogramming was studied using G6pdxa-m1Neu/H mice. Specifically, this is a single point mutation by transversion of A to T in the 5′ splice site consensus sequence at the 3′ terminal of exon 1. G6PD is an important enzyme in the pentose phosphate pathway. G6PD knockdown (KD) mice showed a ∼90% reduction in G6PD protein expression (Fig. 1B). Also, in the lung tissue, mice displayed a similar 90% decrease in G6PD activity (Fig. 1C) in the erythrocytes.
Figure 1.
Western blot analysis of healthy controls and idiopathic pulmonary arterial hypertension (IPAH) patients indicated a significant decrease in glucose-6-phosphate dehydrogenase (G6PD) expression (A) in IPAH lung tissue. Data are expressed as means ± SE, n = 10. G6PD) in WT and deficient mice. G6PD protein expression (B) and enzyme activity (C) decreased significantly in G6PD deficient mice. Data are represented as means ± SE and normalized on stain-free gel total protein, n = 6, *P < 0.05 vs. control by unpaired t test or Mann–Whitney U t test. WT, wild-type.
G6PD Deficiency-Induced Hemodynamic Changes and Vascular Proliferation
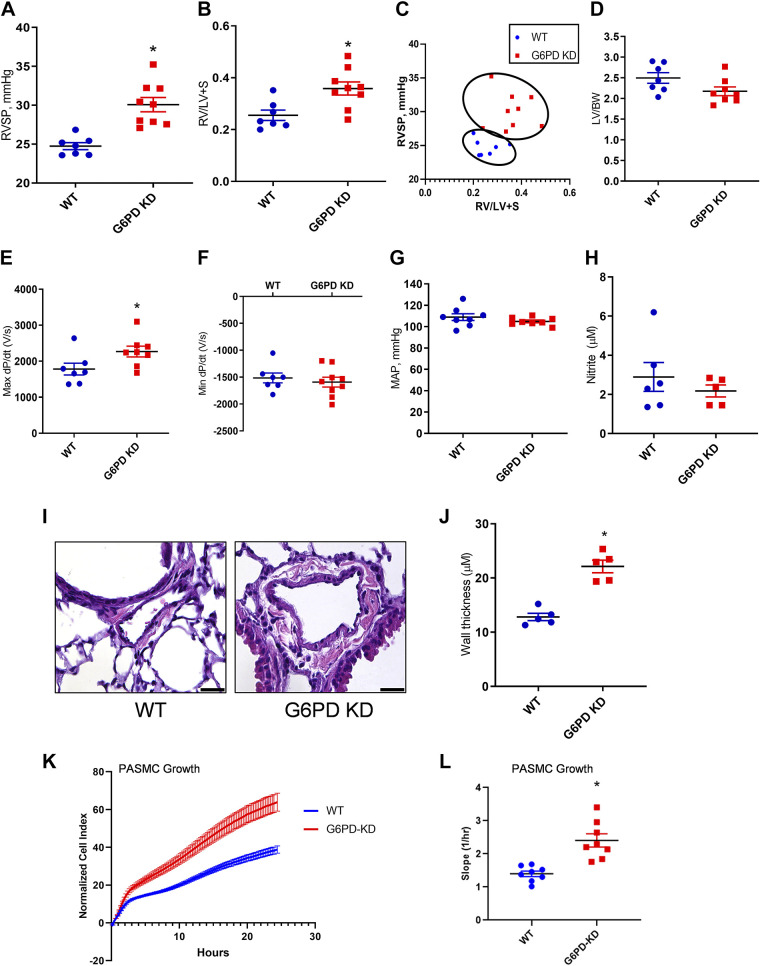
Hemodynamic changes contributing to PH development was assessed by RV catheterization in WT and G6PD KD 12-wk-old animals. Figure 2A shows increased right ventricular systolic pressure (RVSP) in G6PD KD mice. Fulton index, the ratio of RV weight to left ventricle plus septum, indicating right heart hypertrophy, was found increased in the G6PD KD group (Fig. 2B). Correlation analysis shows a strong association between increased RVSP and the hypertrophic changes in the heart (Fig. 2C). Increased RVSP directly corresponds with the increased Fulton index showing compensation of the right heart load due to increased pulmonary pressure. The ratio between left ventricle to body mass ratio was not altered (Fig. 2D). The value of Max dP/dT, an index of ventricular contractility, was also found to increase in the G6PD KD model (Fig. 2E), but no changes were observed in min dP/dT values (Fig. 2F) and systemic blood pressure (Fig. 2G). Unlike endothelial nitric oxide synthase (eNOS)-deficiency-related PH (25), the G6PD KD model does not show any significant changes in plasma NO production (Fig. 2H). Histopathological images demonstrate (Fig. 2, I and J) vascular proliferation in pulmonary arterioles of G6PD KD mice. In association with this, the cell growth kinetic assay in isolated PASMC demonstrated an increased growth rate in G6PD KD cells compared to WT PASMCs (Fig. 2, K and L). These results suggest that G6PD deficiency is closely associated with pulmonary vascular remodeling and PH development.
Figure 2.
Hemodynamic changes and histopathological alterations in G6PD KD mice. The G6PD KD model demonstrated a significant increase in right ventricular systolic pressure (RVSP) (A). Fulton index, the ratio of right to left ventricles plus septum (B), RVSP, and Fulton index correlation analysis showed the PH phenotype in the G6PD KD model (C). Left ventricle (LV) mass/body weight ratio (D) and mean arterial pressure (E) showed no change between WT and G6PD KD model. G6PD KD mice showed increased Max dP/dT (F), but Min dP/dT (G) was not altered. Plasma nitrite concentration was not found to be affected by G6PD deficiency (H). Hematoxylin and eosin staining showed media proliferation in pulmonary arteries of the G6PD KD model (scale is 100 µm) (I and J). Cell growth kinetic assay demonstrates an increased growth rate in pulmonary artery smooth muscle (PASMC) cells (K and L). Data are expressed as means ± SE, n = 6–9, *P < 0.05 vs. control by unpaired t test or Mann–Whitney U t test. G6PD KD, glucose-6-phosphate dehydrogenase knockdown; PH, pulmonary hypertension; WT, wild-type.
G6PD Deficiency-Induced Hemolysis and Barrier Dysfunction
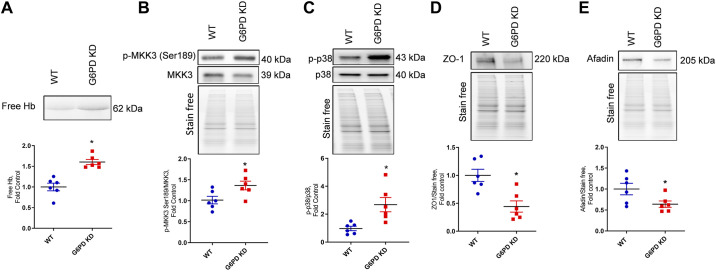
G6PD deficiency-induced hemolysis results in a concomitant release of free hemoglobin (Hb) from erythrocytes into the systemic circulation. As demonstrated in Fig. 3A, the in-gel Hb staining showed a high level of free Hb in the G6PD KD plasma compared to WT control samples. Heme-induced activation of the p38 pathway that we have shown previously (10) is induced by the activation of mitogen-activated protein kinase kinase-3 (MKK3) (Serine-189) (Fig. 3B). MKK3 is an upstream target of the p38 mitogen-activated protein kinase (MAPK) pathway. Thus, increased MKK3 activation caused the phosphorylation and the activation of the p38 signaling cascade (Fig. 3C). Activation of the p38 MAPK pathway leads to endothelial barrier dysfunction (10) and could disrupt tight junctions (26). Barrier proteins zona occluden-1 (ZO-1) and Afadin are significant regulators of vascular permeability (27). In G6PD KD lung tissue, the expression of peripheral membrane tight junction proteins ZO-1 and Afadin was significantly downregulated (Fig. 3, D and E). According to our recent finding, heme mediated barrier dysfunction is facilitated by activating the MKK3/p38 MAPK pathway (7). Therefore, according to our previous findings, these results clearly demonstrate that G6PD deficiency-induced hemolysis results in p38 pathway activation and endothelial dysfunction. These events could contribute to the development of vasculopathy and vascular remodeling, leading to PH pathogenesis (9, 10).
Figure 3.
G6PD deficiency-induced hemolysis and barrier dysfunction. G6PD deficiency resulted in increased hemolysis and increased free hemoglobin (Hb) in the G6PD KD model (A). As a result, phosphorylation of mitogen-activated protein kinase kinase 3 (MKK3) Ser189 (B) and phospho p38 (C) was found significantly upregulated in the G6PD KD model. Peripheral membrane tight junction proteins zona occluden-1 (ZO-1) and Afadin expressions were found downregulated in G6PD KD lung tissue (D and E). Data are expressed as means ± SE normalized on the total protein (stain-free), n = 6–7, *P < 0.05 vs. control by unpaired t test or Mann–Whitney U t test. G6PD KD, glucose-6-phosphate dehydrogenase knockdown.
G6PD Deficiency-Induced Metabolic Alterations
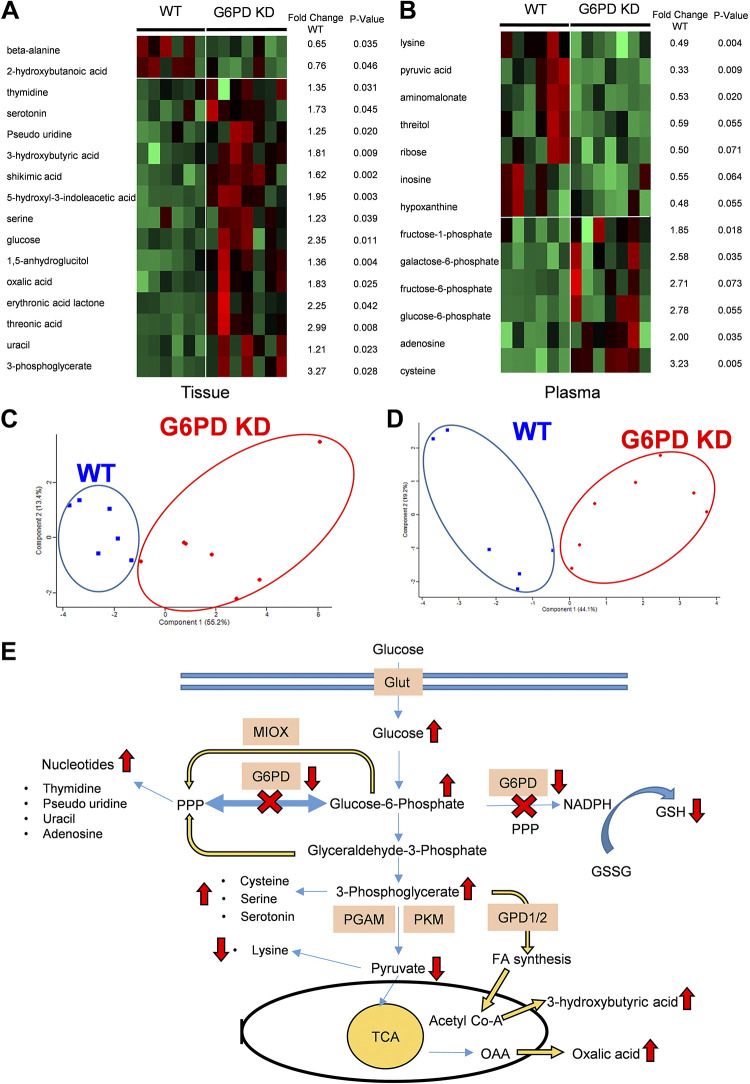
G6PD is an important enzyme in the pentose phosphate pathway (PPP), and its deficiency can induce metabolic alterations in the PPP, glycolytic pathway, and oxidative stress-regulated pathway. Metabolites in the G6PD KD model were studied by gas chromatography time of flight (GC-TOF) mass spectrometry in the lung tissue and plasma samples to quantify the primary metabolites, including carbohydrates, amino acids, and nucleotides. Two runs of separate profiling were done in G6PD KD versus WT samples. In the lung tissue of the WT and G6PD KD samples, out of 172 analyzed metabolites, 16 were significantly altered. In the plasma samples of the WT and G6PD KD mice, out of 172 analyzed metabolites, 7 metabolites showed significant differences and 6 metabolites were highly disturbed (>40% changes) with P value < 0.1. Figure 4, A and B, demonstrates the distinctive clustering of G6PD deficient samples from WT by heat maps and fold changes to the WT group with statistical significance for each metabolite. The principal component analyses (PCA) of altered metabolites displayed a significant separation and clustering either as WT or G6PD KD samples (Fig. 4, C and D). Thus, the markedly different tissue and plasma metabolomics profiling of G6PD KD and WT indicates metabolic dysregulation in G6PD deficiency. Metabolic reprogramming is considered as one of the causes of pulmonary vascular remodeling (7).
Figure 4.
Metabolic profiling of plasma and lung tissue of G6PD-deficient (G6PD KD; n = 7) mice, and comparison with control (WT; n = 6). The figure summarizes markedly changed metabolites in G6PD KD vs. WT analysis. Heat map analysis (A and B) and principal component analysis (PCA) (C and D) indicate the clustering of the G6PD-deficient animals from WT. Ellipses designate 0.95 probability area of the metabolic profiling samples from the same group were inside an ellipse. The fold changes to WT and P-value (unpaired t test) for each metabolite is given as a table with the heat map. E: summary of metabolic alterations in G6PD deficiency. G6PD KD plasma and tissue metabolic profiling showed increased nucleotide production, perhaps due to an alternative myo-inositol oxygenase (MIOX) pathway and a nonoxidative branch of pentose phosphate pathway (PPP) activation. Increased glucose and glycolytic intermediates 3-phosphoglycerate (3-PG), glucose-6-phosphate, and fructose-6-phosphate, thereby showed upregulation of glycolysis. Increased cysteine and serine levels indicate increased 3-PG-mediated amino acid synthesis. Decreased pyruvate concentration could be explained by inhibited glycolysis downstream of 3-PG. Increased 3-PG can also stimulate fatty acid synthesis via GPD1/2. G6PD KD, glucose-6-phosphate dehydrogenase knockdown; GPD1/2, glycerol-3-phosphate dehydrogenase 1/2; WT, wild-type.
Figure 4E depicts a schematic summary of G6PD deficiency-induced metabolic dysregulation, as observed in metabolomics profiling. In Fig. 4E, we mapped the markedly changed metabolites in G6PD KD mice, which were involved in PCA and heatmap clustering analysis, to monitor redistribution in metabolic fluxes. Knowing the alterations in metabolic fluxes, we could further guide our proteomic analysis of the lung tissue and isolated PASMC. The increased glucose and other glycolytic pathway intermediates (glucose-6-phosphate and 3-phosphoglycerate) observed in the lung tissue could be explained by increased PPP demand and correspond with the increased activation of glucose transporters. We have also observed that although 3-phosphoglycerate was significantly increased, the subsequent pyruvate production in the glycolytic pathway was notably decreased. Indeed, decreased pyruvate production corresponds with a reduction in lysine formation, supporting our observation in the metabolomics data. In addition to this, the increased 3-phosphoglycerate metabolite is confirmed by the observed increase in cysteine, serine, and serotonin. This dictates the inhibition of intermediate steps in glycolysis between 3-PG and pyruvate through either phosphoglycerate mutase or pyruvate kinase isozyme inhibition. The deficiency of G6PD displayed an increased production of nucleotide bases (thymidine pseudouridine, uracil, and adenosine), which is a counterintuitive observation. This could be explained by the activation of alternative pathways that bypass attenuated PPP to supply the demand for nucleotides. Importantly, driving the glycolysis in reverse can supplement PPP via the glyceraldehyde-3-phosphate shunt. PPP can also be supplied alternatively by myo-inositol oxidation, and we have recently reported the upregulation of myo-inositol in PH (28, 29). Both pathways can account for the substitution of G6PD.
Furthermore, G6PD-deficiency-attenuated NADPH synthesis could decrease GSH production, the primary substrate for glutathione peroxidases and glutaredoxins – the main antioxidant defense components. Thus, we could expect an increase in oxidative stress. In addition, G6PD deficient lung tissue showed increased 3-hydroxybutyric acid production, representing increased fatty acid metabolism (30). Moreover, the elevated level of oxalic acid, a known activator of intracellular calcium exclusively in preventing reendothelialization (31), was also found upregulated in G6PD deficiency. Thus, altogether, these metabolic abnormalities evidently support the idea that G6PD deficiency could potentially lead to a vascular proliferative phenotype in PH development.
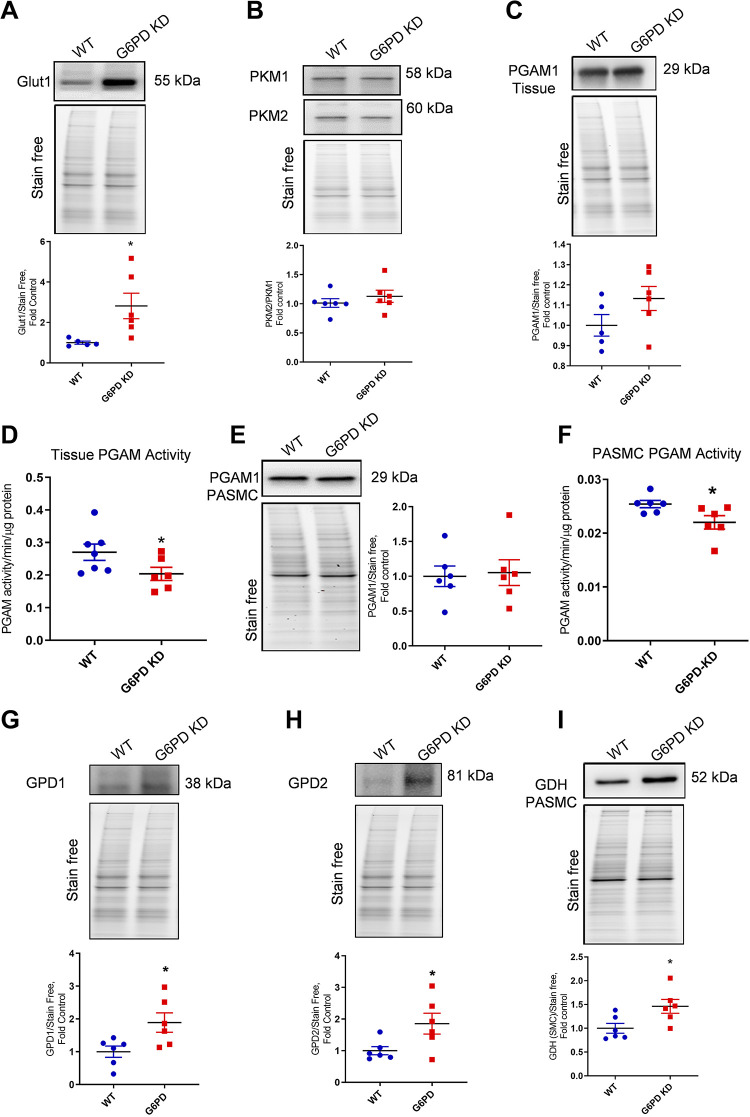
G6PD Deficiency Dysregulated Glycolytic Metabolism
To delineate the molecular mechanism of G6PD deficiency-induced metabolic dysregulation, we screened major targets based on our analysis of alterations in Fig. 4E. Increased glucose concentration in G6PD KD was found to be associated with the increased expression of glucose transporter Glut1 (Fig. 5A). Thus, the elevated level of cellular glucose could upregulate the glycolytic pathway, but the glycolytic end product, pyruvate, was found deceased. Investigating the pyruvate kinase (PKM) protein expression showed no change in PKM2/1 (Fig. 5B). However, we found that the activity of PGAM, which catalyzes the conversion of 3-phosphoglycerate to 2-phosphoglycerate, was significantly decreased in G6PD KD. Interestingly, PGAM protein expressions were not significantly altered in lung tissue and pulmonary artery smooth muscle cells (Fig. 5, C and E), whereas PGAM activity with respect to total protein concentration was found significantly reduced in both lung tissue and pulmonary smooth muscle cells (Fig. 5, D and F). This results in a reverse glycolytic flux to the metabolites, which may enter PPP via the glyceraldehyde-3-phosphate shunt. A higher concentration of 3-phosphoglycerate leads to increased fatty acid production from glycolytic intermediate 1,3-bis phosphoglycerate by glycerol-3-phosphate dehydrogenase (GPD)1 and 2 (Fig. 5, G and H), and this corresponds to the increased production of fatty acid metabolite 3-hydroxybutyric acid in the G6PD deficiency. G6PD deficient PASMC also showed increased glutaminolysis by increased expression of glutamate dehydrogenase (GDH), representing replenishment of alpha-ketoglutarate to the TCA cycle from amino acid glutamate (Fig. 5I). Previously, increased glutaminolysis was reported as one of the causes of pulmonary vascular cell migration and proliferation (32). Therefore, these observations suggest that increased glycolysis and attenuated PGAM channeled the metabolism towards the fatty acid pathway and glutaminolysis, therefore feeding into the increased vascular proliferative demands of PH.
Figure 5.
G6PD deficiency altered glycolytic metabolism. Increased glucose concentration and other intermediates in G6PD KD mice were found to be associated with the increased expression of glucose transporter Glut1 (A). Pyruvate kinase M (PKM) isoenzymes 1 and 2 were unaltered. B: phosphoglycerate mutase (PGAM1) protein expression was not significantly altered in the lung tissue (C) and pulmonary artery smooth muscle cells (PASMCs) (E), but PGAM enzyme activity in tissue (D) and PASMCs (F) was significantly decreased in G6PD KD lung tissue. Glycerol-3-phosphate dehydrogenase (GPD1 and 2) (G and H) expression was found to be significantly increased in the lung tissue of G6PD-deficient mice compared to WT control. Glutamate dehydrogenase (GDH) expression significantly increased in the lung tissue of G6PD KD animals (I). Data are expressed as means ± SE normalized on the total protein (stain-free), n = 6, *P < 0.05 vs. control by unpaired t test or Mann–Whitney U t test. G6PD KD, glucose-6-phosphate dehydrogenase knockdown; WT, wild-type.
G6PD Deficiency-Induced Oxidative Stress and Activation of Proliferative Signaling
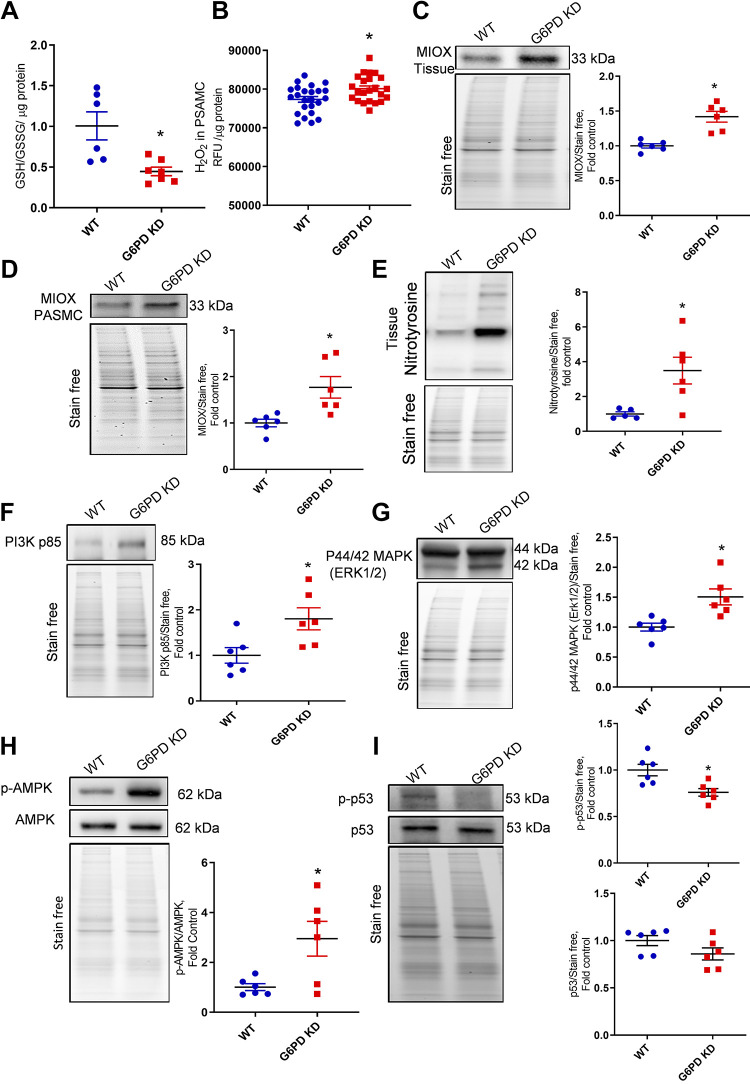
G6PD is a major enzyme that regulates the PPP and is an important source of GSH reduction via NADPH production to scavenge reactive oxygen species/reactive nitrogen species (ROS/RNS) (14). Our data indicate that G6PD deficiency attenuated GSH/GSSG ratios (Fig. 6A), which causes an increase in cellular ROS production. Figure 6B represents the increased rate of hydrogen peroxide production in isolated G6PD KD pulmonary artery SMCs. In association with this G6PD KD, lung tissue, and isolated PASMCs showed increased myo-inositol oxygenase (MIOX) expression (Fig. 6, C and D). It was recently reported that in PH, the inositol pathway was found to increase, and conversion of myo-inositol by MIOX could serve as an alternate pathway for PPP (29, 33). This could explain how G6PD KD animals overcome G6PD deficiency by increasing the expression of MIOX in lung tissue. MIOX helps to replenish the PPP intermediate xylulose-5-phosphate from glucose-6-phosphate, but this also causes increased oxidative stress due to the diminished GSH and increases ROS generation by MIOX. Increased oxidative stress produced more reactive nitrogen species (RNS), which was identified by the increased expression of global tyrosine nitration in G6PD KD lung tissue (Fig. 6E). Increased ROS/RNS upregulated redox-sensitive proliferative kinases PI3K p85, ERK1/2, and AMPK (Fig. 6, F–H) in G6PD KD lung tissue. Previous studies reported that increased vascular proliferation in PH was found to be related to the activation of PI3K, ERK1/2, and AMPK pathways (34, 35). Furthermore, tumor proapoptotic protein p53 phosphorylation was found significantly downregulated in G6PD deficient lung tissue (Fig. 6I). This also justifies the cause of vascular proliferative phenotype observed in the lung vasculature of the G6PD deficient mice (Fig. 2, I and J). Therefore, these findings suggest that G6PD deficiency upregulated oxidative stress could be an important factor for proliferative vascular remodeling in PH.
Figure 6.
G6PD deficiency-induced oxidative stress and proliferative signaling. G6PD deficiency attenuated GSH/GSSG ratios (A). Isolated pulmonary artery smooth muscle cells (PASMCs) showed increased production of reactive oxygen species (H2O2) than WT cells (B). G6PD KD lung tissue showed increased expression of myo-inositol oxygenase (MIOX) in lung tissue (C) and PASMCs (D). Elevated oxidative stress produced increased reactive nitrogen species (RNS), which was identified by increased expression of global tyrosine nitration in G6PD KD lung tissue (E). Increased ROS/RNS upregulated PI3K p85, ERK1/2, and AMPK pathways (F–H) in G6PD KD lung tissue. Proapoptotic protein p53 phosphorylation was significantly attenuated in G6PD KD lung tissue (I). Data are represented as means ± SE normalized on the total protein (stain-free), n = 6, *P < 0.05 vs. control by unpaired t test or Mann–Whitney U t test. G6PD KD, glucose-6-phosphate dehydrogenase knockdown; GSH, glutathione; GSSG, oxidized glutathione; ROS/RNS, reactive oxygen species/reactive nitrogen species; WT, wild-type.
DISCUSSION
The involvement of G6PD in pulmonary hypertension development and progression is controversial (4, 17–19). It was shown that G6PD is upregulated under hypoxia conditions in smooth muscle cells acquiring proliferative phenotype and contributing to vascular remodeling (17, 19). Indeed, the activation of PPP is important for nucleotide synthesis and cellular proliferation and reported in preclinical pulmonary hypertension models (PH) (36, 37). On the other side, hemolysis occurring due to G6PD deficiency may contribute to the development of PH via free heme-mediated signaling. Our group previously described G6PD deficient patients in the PH cohort (4). Interestingly in the present study, IPAH patient's lung tissue showed a >45% reduction in G6PD protein expression than healthy control subjects, presenting vital evidence of a link between G6PD and PH. Therefore, to delineate whether G6PD deficiency can contribute to PH, we used a G6PD-deficient model. Here we report that the G6PD-deficient mice develop mild pulmonary hypertension without any additional stimulation. Counterintuitively, the metabolomics data suggested that the flux into PPP in G6PD-deficient mice was higher than in wild-types. Thus, our data indicate that both views could be correct, and either increased or decreased G6PD activity from the basal level could be a tipping point for PH development.
We recently reported that the mechanism of heme induced endothelial barrier dysfunction is mediated by the activation of the MKK3/p38 MAPK axis (7). In the present study, G6PD KD animals showed increased hemolysis, and as a result, the MKK3/p38 MAPK pathway was upregulated. This reduces the expression of tight junction proteins ZO-1 and Afadin, which are key regulators of vascular permeability (27). These findings suggest that G6PD deficiency-induced hemolysis is one of the key contributors to barrier dysfunction leading to pulmonary vascular remodeling.
PPP is essential for nucleotide biosynthesis and the proliferation of pulmonary vasculature (38). Increased glycolysis is a well-known feature of PH (7). Glycolysis and PPP can be converged at three crucial metabolic points: at the oxidative branch of PPP via conversion of G6P by G6PD; at a nonoxidative branch of PPP via glyceraldehyde-3-phosphate (G3P) and fructose-6-phosphate (F6P) conversions (39). Thus, in the presence of G6PD deficiency, the increased glycolytic flux can be fed into PPP via G3P/F6P shunt. Indeed, our metabolomics data indicate increased 3-glycerol phosphate (3PG), fructose-6-phosphate, and glucose-6-phosphate but decreased pyruvate accumulation. Interestingly, we previously reported increased PPP intermediates in the PH model with decreased mitochondria respiration, despite decreased 6-phosphogluconate, the product of G6PD (40). Cells can overcome G6PD deficiency by reverting the glycolytic flux (11). Thus, we expected to find downregulation or decreased activity of glycolytic enzymes downstream of 3PG. We have examined all glycolytic enzymes and only found a decrease in PGAM activity. PGAM converts 3PG into 2PG; thus, a reduction in PGAM activity will increase 3PG under the condition of increased glycolytic flux (41). Indeed, we have observed not only increased 3PG in the metabolomics study but also increased downstream metabolites such as cysteine, serine, and products of fatty acids utilization. Moreover, in G6PD deficiency, increased glutaminolysis replenished the mitochondrial metabolites from amino acid glutamate. Importantly, glutaminolysis was reported as a significant contributor to vascular remodeling in PH (32).
Another pathway that can overcome G6PD deficiency and feed into PPP is the oxidation of myo-inositol by MIOX. Increased G6P accumulation stimulates the inositol synthesis pathway. G6P is converted by inositol-1-phosphate synthase and inositol phosphatase (IMPA) entering the inositol cycle (42). We have recently shown that IMPA interaction with the RAGE receptor plays a vital role in the PH-induced proliferation of vascular cells (29). Moreover, we reported increased myo-inositol in the plasma of patients with PH (43). Therefore, the availability of inositol and increased expression of MIOX results in oxidative replenishment of PPP bypassing G6PD. However, this collateral pathway leads to the induction of oxidative stress, and together with decreased GSH, produces extensive oxidative damage (44), as we found by the measurement of total protein nitration in the lungs. In turn, increased oxidative status stimulates redox-sensitive proliferative responses.
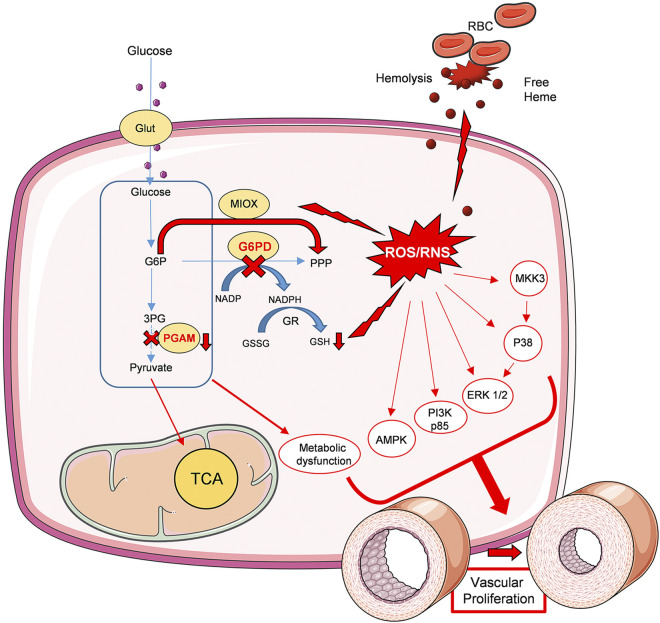
Figure 7 summarizes the overall contribution of G6PD deficiency to metabolic dysregulation and vascular remodeling. In the G6PD deficient model, hemolysis (a known contributor to the barrier dysfunction) in combination with oxidative stress and metabolic dysfunction developed vascular cell proliferation and PH pathogenesis. G6PD deficiency in erythrocytes induced increased hemolysis. Thus, the free heme released in the circulation upregulated oxidative stress and barrier dysfunction. On the other hand, G6PD deficiency upregulates glucose demand in cells resulting in the increased expression of the glucose transporter, Glut1. As a result, glycolysis increased, but the inhibition at PGAM redirected the glycolysis into PPP. The activated MIOX pathway, an alternative of PPP, decreased GSH pool in the cells, and in addition, free heme results in high ROS/RNS production. Collectively, increased oxidative stress and metabolic dysfunction resulted in the upregulation of proliferative signaling p38 MAPK, ERK1/2, PI3K, and AMPK pathways. In addition to this, the proapoptotic tumor antigen p-53 phosphorylation was significantly downregulated, and this could direct the proliferative signaling pathways towards pulmonary vascular cell proliferation and, consequently, increased pulmonary arterial pressure.
Figure 7.
Schematic diagram depicting the role of G6PD deficiency in vascular remodeling. G6PD deficiency in erythrocytes induced increased hemolysis and free hemoglobin release. Increased free heme in circulation upregulated oxidative stress and barrier dysfunction. Cellular glucose uptake is increased in the G6PD KD lungs. This results in increased glycolytic metabolism; however, the inhibition of PGAM redirected glycolysis towards a nonoxidative branch of PPP and fatty acid synthesis. Increased MIOX pathway, together with decreased GSH production, results in increased ROS/RNS generation. Thus, increased hemolysis and oxidative stress collectively contributed to the upregulation of proliferative signaling p38 MAPK, ERK1/2, PI3K, and AMPK pathways. This leads to vascular proliferation and remodeling. In summary, G6PD deficiency-induced metabolic dysfunction, oxidative stress, and vascular proliferation are the causes of PH pathogenesis in the G6PD KD model. Figure 7 was prepared using templates from the image bank of Servier Medical Art (http://smart.servier.com/) under CC-BY 3.0 license. G6PD KD, glucose-6-phosphate dehydrogenase knockdown; GSH, glutathione; MIOX, myo-inositol oxygenase; p3 MAPK, p38 mitogen-activated protein kinase; PGAM, phosphoglycerate mutase; PPP, pentose phosphate pathway; ROS/RNS, reactive oxygen species/reactive nitrogen species.
We and others have previously reported that stimulation of p38 MAPK is an important mechanism for vascular proliferation in PH (9, 10, 29). P38 mediated activation of ERK1/2, cytoskeleton rearrangement, and glycolysis activation via MK2 may be responsible for the proliferative phenotype of smooth muscle cells (45, 46). Stimulation of PI3K synergistically accelerates proliferation by engaging Akt signaling (47). The importance of Akt signaling in PH pathogenesis has been reported extensively (7, 48). Akt and AMPK enhance glycolysis, and their activation could be not only in response to oxidants but also due to the demand for decreased PPP. Importantly, activation of AMPK also can stimulate lipolysis that is possibly upregulated in G6PD knockdowns.
In conclusion, metabolic reprogramming is essential for vascular proliferation, similar to cancers. Redistribution of the glycolytic pathway may stimulate growth mechanisms by different scenarios. Thus, the upregulation or downregulation of G6PD may contribute to the development of the proliferative phenotype. It seems that increased oxidative stress is also a key player in the proliferative switch of vascular cells. Therefore, decreased G6PD contributes to oxidative stress in many ways, including reduced GSH homeostasis, hemolysis, and MIOX activation.
GRANTS
This work was supported by NIH Grants R01HL133085 (to O. Rafikova), R01HL151447 (to R. Rafikov), and R01HL132918 (to R. Rafikov). Clinical Data and tissue samples were obtained from PHBI under the Pulmonary Hypertension Breakthrough Initiative (PHBI). Funding for the PHBI is provided under an NHLBI R24 Grant R24HL123767 and by the Cardiovascular Medical Research and Education Fund (CMREF).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
O.R. and R.R. conceived and designed research; M.V.V. and J.J. performed experiments; M.V.V. and J.J. analyzed data; M.V.V. and J.J. interpreted results of experiments; M.V.V. and J.J. prepared figures; M.V.V., J.J., O.R., and R.R. drafted manuscript; R.R. edited and revised manuscript; M.V.V., J.J., O.R., and R.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Marina Zemskova for the cell isolations.
REFERENCES
- 1.Lai YC, Potoka KC, Champion HC, Mora AL, Gladwin MT. Pulmonary arterial hypertension: the clinical syndrome. Circ Res 115: 115–130, 2014. doi: 10.1161/CIRCRESAHA.115.301146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jernigan NL, Naik JS, Weise-Cross L, Detweiler ND, Herbert LM, Yellowhair TR, Resta TC. Contribution of reactive oxygen species to the pathogenesis of pulmonary arterial hypertension. PLoS One 12: e0180455, 2017. doi: 10.1371/journal.pone.0180455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Rivas G, Jerjes-Sanchez C, Rodriguez D, Garcia-Pelaez J, Trevino V. A systematic review of genetic mutations in pulmonary arterial hypertension. BMC Med Genet 18: 82, 2017. doi: 10.1186/s12881-017-0440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurdyukov S, Eccles CA, Desai AA, Gonzalez-Garay M, Yuan JX, Garcia JGN, Rafikova O, Rafikov R. New cases of glucose-6-phosphate dehydrogenase deficiency in pulmonary arterial hypertension. PLoS One 13: e0203493, 2018. doi: 10.1371/journal.pone.0203493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menon RG, Menon S, Valliyathu J, Al Delamie T. Methemoglobinemia masquerading as pulmonary hypertension. Interact Cardiovasc Thorac Surg 3: 44–45, 2004. doi: 10.1016/S1569-9293(03)00214-7. [DOI] [PubMed] [Google Scholar]
- 6.Kerstjens-Frederikse WS, Bongers EM, Roofthooft MT, Leter EM, Douwes JM, Van Dijk A, Vonk-Noordegraaf A, Dijk-Bos KK, Hoefsloot LH, Hoendermis ES, Gille JJ, Sikkema-Raddatz B, Hofstra RM, Berger RM. TBX4 mutations (small patella syndrome) are associated with childhood-onset pulmonary arterial hypertension. J Med Genet 50: 500–506, 2013. doi: 10.1136/jmedgenet-2012-101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James J, Srivastava A, Varghese MV, Eccles CA, Zemskova M, Rafikova O, Rafikov R. Heme induces rapid endothelial barrier dysfunction via the MKK3/p38MAPK axis. Blood 136: 749–754, 2020. doi: 10.1182/blood.2019003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machado RF, Gladwin MT. Pulmonary hypertension in hemolytic disorders: pulmonary vascular disease: the global perspective. Chest 137: 30S–38S, 2010. doi: 10.1378/chest.09-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rafikova O, James J, Eccles CA, Kurdyukov S, Niihori M, Varghese MV, Rafikov R. Early progression of pulmonary hypertension in the monocrotaline model in males is associated with increased lung permeability. Biol Sex Differ 11: 11, 2020. doi: 10.1186/s13293-020-00289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rafikova O, Williams ER, McBride ML, Zemskova M, Srivastava A, Nair V, Desai AA, Langlais PR, Zemskov E, Simon M, Mandarino LJ, Rafikov R. Hemolysis-induced lung vascular leakage contributes to the development of pulmonary hypertension. Am J Respir Cell Mol Biol 59: 334–345, 2018. doi: 10.1165/rcmb.2017-0308OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luzzatto L, Nannelli C, Notaro R. Glucose-6-phosphate dehydrogenase deficiency. Hematol Oncol Clin North Am 30: 373–393, 2016. doi: 10.1016/j.hoc.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Wu S, Wu G, Wu H. Hemolytic jaundice induced by pharmacological dose ascorbic acid in glucose-6-phosphate dehydrogenase deficiency: A case report. Medicine (Baltimore) 97: e13588, 2018. doi: 10.1097/MD.0000013588. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Tangudu NK, Alan B, Vinchi F, Worle K, Lai D, Vettorazzi S, Leopold K, Vujic SM. Scavenging reactive oxygen species production normalizes ferroportin expression and ameliorates cellular and systemic iron disbalances in hemolytic mouse model. Antioxid Redox Signal 29: 484–499, 2018. doi: 10.1089/ars.2017.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ham M, Lee J-W, Choi AH, Jang H, Choi G, Park J, Kozuka C, Sears DD, Masuzaki H, Kim JB. Macrophage glucose-6-phosphate dehydrogenase stimulates proinflammatory responses with oxidative stress. Mol Cell Biol 33: 2425–2435, 2013. doi: 10.1128/MCB.01260-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Zhang Z, Hu J, Stillman IE, Leopold JA, Handy DE, Loscalzo J, Stanton RC. Glucose-6-phosphate dehydrogenase-deficient mice have increased renal oxidative stress and increased albuminuria. FASEB J 24: 609–616, 2010. doi: 10.1096/fj.09-135731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bubp J, Jen M, Matuszewski K. Caring for glucose-6-phosphate dehydrogenase (G6PD)-deficient patients: implications for pharmacy. P T 40: 572–574, 2015. [PMC free article] [PubMed] [Google Scholar]
- 17.Chettimada S, Gupte R, Rawat D, Gebb SA, McMurtry IF, Gupte SA. Hypoxia-induced glucose-6-phosphate dehydrogenase overexpression and -activation in pulmonary artery smooth muscle cells: implication in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 308: L287–L300, 2015. doi: 10.1152/ajplung.00229.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupte RS, Rawat DK, Chettimada S, Cioffi DL, Wolin MS, Gerthoffer WT, McMurtry IF, Gupte SA. Activation of glucose-6-phosphate dehydrogenase promotes acute hypoxic pulmonary artery contraction. J Biol Chem 285: 19561–19571, 2010. doi: 10.1074/jbc.M109.092916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi SR, Kitagawa A, Jacob C, Hashimoto R, Dhagia V, Ramesh A, Zheng C, Zhang H, Jordan A, Waddell I, Leopold J, Hu CJ, McMurtry IF, D'Alessandro A, Stenmark KR, Gupte SA. Hypoxic activation of glucose-6-phosphate dehydrogenase controls the expression of genes involved in the pathogenesis of pulmonary hypertension through the regulation of DNA methylation. Am J Physiol Lung Cell Mol Physiol 318: L773–L786, 2020. doi: 10.1152/ajplung.00001.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyanova S, Cox J. Perseus: a bioinformatics platform for integrative analysis of proteomics data in cancer research. Methods Mol Biol 1711: 133–148, 2018. doi: 10.1007/978-1-4939-7493-1_7. [DOI] [PubMed] [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57: 289–300, 1995. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 22.Rivero-Gutierrez B, Anzola A, Martinez-Augustin O, de Medina FS. Stain-free detection as loading control alternative to Ponceau and housekeeping protein immunodetection in Western blotting. Anal Biochem 467: 1–3, 2014. doi: 10.1016/j.ab.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 23.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rafikov R, Sun X, Rafikova O, Meadows ML, Desai AA, Khalpey Z, Yuan JXJ, Fineman JR, Black SM. Complex I dysfunction underlies the glycolytic switch in pulmonary hypertensive smooth muscle cells. Redox Biol 6: 278–286, 2015. doi: 10.1016/j.redox.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood KC, Cortese-Krott MM, Kovacic JC, Noguchi A, Liu VB, Wang X, Raghavachari N, Boehm M, Kato GJ, Kelm M, Gladwin MT. Circulating blood endothelial nitric oxide synthase contributes to the regulation of systemic blood pressure and nitrite homeostasis. Arterioscler Thromb Vasc Biol 33: 1861–1871, 2013. doi: 10.1161/ATVBAHA.112.301068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu L, Gan X, Liu X, An R. Calcium oxalate crystals induces tight junction disruption in distal renal tubular epithelial cells by activating ROS/Akt/p38 MAPK signaling pathway. Ren Fail 39: 440–451, 2017. doi: 10.1080/0886022X.2017.1305968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta 1778: 794–809, 2008. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Rafikov R, James J, McClain N, Tofovic SP, Rafikova O. Role of gender in regulation of redox homeostasis in pulmonary arterial hypertension. Antioxidants (Basel ) 8: 135, 2019. doi: 10.3390/antiox8050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rafikov R, McBride ML, Zemskova M, Kurdyukov S, McClain N, Niihori M, Langlais PR, Rafikova O. Inositol monophosphatase 1 as a novel interacting partner of RAGE in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 316: L428–L444, 2019. doi: 10.1152/ajplung.00393.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazaki T, Honda A, Ikegami T, Iwamoto J, Monma T, Hirayama T, Saito Y, Yamashita K, Matsuzaki Y. Simultaneous quantification of salivary 3-hydroxybutyrate, 3-hydroxyisobutyrate, 3-hydroxy-3-methylbutyrate, and 2-hydroxybutyrate as possible markers of amino acid and fatty acid catabolic pathways by LC–ESI–MS/MS. Springerplus 4: 494, 2015. doi: 10.1186/s40064-015-1304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Recht PA, Tepedino GJ, Siecke NW, Buckley MT, Mandeville JT, Maxfield FR, Levin RI. Oxalic acid alters intracellular calcium in endothelial cells. Atherosclerosis 173: 321–328, 2004. doi: 10.1016/j.atherosclerosis.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 32.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang YY, Rehman S, Sugahara M, Qi Z, Gorcsan J 3rd, Vargas SO, Saggar R, Saggar R, Wallace WD, Ross DJ, Haley KJ, Waxman AB, Parikh VN, Marco TD, Hsue PY, Morris A, Simon MA, Norris KA, Gaggioli C, Loscalzo J, Fessel J, Chan SY . Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest 126: 3313–3335, 2016. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabhu KS, Arner RJ, Vunta H, Reddy CC. Up-regulation of human myo-inositol oxygenase by hyperosmotic stress in renal proximal tubular epithelial cells. J Biol Chem 280: 19895–19901, 2005. doi: 10.1074/jbc.M502621200. [DOI] [PubMed] [Google Scholar]
- 34.Pullamsetti SS, Savai R, Seeger W, Goncharova EA. Translational advances in the field of pulmonary hypertension. From cancer biology to new pulmonary arterial hypertension therapeutics. Targeting cell growth and proliferation signaling hubs. Am J Respir Crit Care Med 195: 425–437, 2017. doi: 10.1164/rccm.201606-1226PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhai C, Shi W, Feng W, Zhu Y, Wang J, Li S, Yan X, Wang Q, Zhang Q, Chai L, Li C, Liu P, Li M. Activation of AMPK prevents monocrotaline-induced pulmonary arterial hypertension by suppression of NF-kappaB-mediated autophagy activation. Life Sci 208: 87–95, 2018. doi: 10.1016/j.lfs.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 36.D'Alessandro A, El KK, Plecita-Hlavata L, Jezek P, Li M, Zhang H, Gupte SA, Stenmark KR. Hallmarks of pulmonary hypertension: mesenchymal and inflammatory cell metabolic reprogramming. Antioxid Redox Signal 28: 230–250, 2018. doi: 10.1089/ars.2017.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rafikova O, Meadows ML, Kinchen JM, Mohney RP, Maltepe E, Desai AA, Yuan JX, Garcia JG, Fineman JR, Rafikov R, Black SM. Metabolic changes precede the development of pulmonary hypertension in the monocrotaline exposed rat lung. PLoS One 11: e0150480, 2016. doi: 10.1371/journal.pone.0150480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smolders VF, Zodda E, Quax PHA, Carini M, Barbera JA, Thomson TM, Tura-Ceide O, Cascante M. Metabolic alterations in cardiopulmonary vascular dysfunction. Front Mol Biosci 5: 120, 2018. doi: 10.3389/fmolb.2018.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin L, Zhou Y. Crucial role of the pentose phosphate pathway in malignant tumors. Oncol Lett 17: 4213–4221, 2019. doi: 10.3892/ol.2019.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rafikova O, Srivastava A, Desai AA, Rafikov R, Tofovic SP. Recurrent inhibition of mitochondrial complex III induces chronic pulmonary vasoconstriction and glycolytic switch in the rat lung. Respir Res 19: 69, 2018. doi: 10.1186/s12931-018-0776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hitosugi T, Zhou L, Elf S, Fan J, Kang H-B, Seo JH, Shan C, Dai Q, Zhang L, Xie J, Gu TL, Jin P, Aleckovic M, Leroy G, Kang Y, Sudderth JA, DeBerardinis RJ, Luan CH, Chen GZ, Muller S, Shin DM, Owonikoko TK, Lonial S, Arellano ML, Khoury HJ, Khuri FR, Lee BH, Ye K, Boggon TJ, Kang S, He C, Chen J. Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth. Cancer Cell 22: 585–600, 2012. doi: 10.1016/j.ccr.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stein AJ, Geiger JH. The crystal structure and mechanism of 1-L-myo-inositol- 1-phosphate synthase. J Biol Chem 277: 9484–9491, 2002. doi: 10.1074/jbc.M109371200. [DOI] [PubMed] [Google Scholar]
- 43.Rafikov R, Coletta DK, Mandarino LJ, Rafikova O. Pulmonary arterial hypertension induces a distinct signature of circulating metabolites. J Clin Med 9: 217, 2020. doi: 10.3390/jcm9010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gabriella D, Chiara S, Marcantonio CF, Francesco B, Eugenio LG, Giulio R, Luciana C, Decio BA. Inositol administration reduces oxidative stress in erythrocytes of patients with polycystic ovary syndrome. Euro J Endocrinol 166: 703–710, 2012. doi: 10.1530/EJE-11-0840. [DOI] [PubMed] [Google Scholar]
- 45.Damarla M, Hasan E, Boueiz A, Le A, Pae HH, Montouchet C, Kolb T, Simms T, Myers A, Kayyali US, Gaestel M, Peng X, Reddy SP, Damico R, Hassoun PM. Mitogen activated protein kinase activated protein kinase 2 regulates actin polymerization and vascular leak in ventilator associated lung injury. PLoS One 4: e4600, 2009. doi: 10.1371/journal.pone.0004600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Yang H, Tachado SD, Capo-Aponte JE, Bildin VN, Koziel H, Reinach PS. Phosphatase-mediated crosstalk control of ERK and p38 MAPK signaling in corneal epithelial cells. Invest Ophthalmol Vis Sci 47: 5267–5275, 2006. doi: 10.1167/iovs.06-0642. [DOI] [PubMed] [Google Scholar]
- 47.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res 90: 1243–1250, 2002. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 48.Tang H, Chen J, Fraidenburg DR, Song S, Sysol JR, Drennan AR, Offermanns S, Ye RD, Bonini MG, Minshall RD, Garcia JGN, Machado RF, Makino A, Yuan JX-J. Deficiency of Akt1, but not Akt2, attenuates the development of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 308: L208–L220, 2015. doi: 10.1152/ajplung.00242.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]