Keywords: cell-free mRNA, classification biomarker, NASH, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis
Abstract
Hepatic fibrosis stage is the most important determinant of outcomes in patients with nonalcoholic fatty liver disease (NAFLD). There is an urgent need for noninvasive tests that can accurately stage fibrosis and determine efficacy of interventions. Here, we describe a novel cell-free (cf)-mRNA sequencing approach that can accurately and reproducibly profile low levels of circulating mRNAs and evaluate the feasibility of developing a cf-mRNA-based NAFLD fibrosis classifier. Using separate discovery and validation cohorts with biopsy-confirmed NAFLD (n = 176 and 59, respectively) and healthy subjects (n = 23), we performed serum cf-mRNA RNA-Seq profiling. Differential expression analysis identified 2,498 dysregulated genes between patients with NAFLD and healthy subjects and 134 fibrosis-associated genes in patients with NAFLD. Comparison between cf-mRNA and liver tissue transcripts revealed significant overlap of fibrosis-associated genes and pathways indicating that the circulating cf-mRNA transcriptome reflects molecular changes in the livers of patients with NAFLD. In particular, metabolic and immune pathways reflective of known underlying steatosis and inflammation were highly dysregulated in the cf-mRNA profile of patients with advanced fibrosis. Finally, we used an elastic net ordinal logistic model to develop a classifier that predicts clinically significant fibrosis (F2–F4). In an independent cohort, the cf-mRNA classifier was able to identify 50% of patients with at least 90% probability of clinically significant fibrosis. We demonstrate a novel and robust cf-mRNA-based RNA-Seq platform for noninvasive identification of diverse hepatic molecular disruptions and for fibrosis staging with promising potential for clinical trials and clinical practice.
NEW & NOTEWORTHY This work is the first study, to our knowledge, to utilize circulating cell-free mRNA sequencing to develop an NAFLD diagnostic classifier.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease in the world, affecting one-quarter of the global population (1, 2). The more common form of NAFLD is nonalcoholic fatty liver (NAFL) or simple steatosis, whereas a subset of patients with NAFLD develop the progressive form, nonalcoholic steatohepatitis (NASH). NASH is characterized by steatosis, lobular inflammation, and hepatocyte ballooning and is associated with increasing levels of liver fibrosis, which can subsequently progress to liver cirrhosis, liver failure requiring transplant, and hepatocellular carcinoma (3). Identification of patients with NASH and advanced hepatic fibrosis is critical due to their greater risk for developing complications of chronic liver disease (4). Although liver biopsy remains the preferred reference method for NASH and NAFLD diagnosis (5), biopsies are invasive, expensive, subjective due to pathology review, and prone to sampling error. In addition, although numerous pharmacotherapies are in development, none is yet approved for NASH, and many have failed in recent trials (6–9). Intensive lifestyle intervention and bariatric surgery have demonstrated reductions in steatosis and fibrosis but would benefit from noninvasive efficacy metrics beyond just weight loss, an imperfect correlate of liver steatosis (10–13). Therefore, there is a critical need for accurate noninvasive tests for NASH and advanced fibrosis.
Currently, there are two distinct approaches for noninvasive assessment of liver fibrosis, a “physical” approach based on the assessment of liver stiffness using elastography techniques and a “biological” approach based on the quantification of biomarkers in serum samples (14). For imaging-based approaches, vibration-controlled transient elastography (VCTE) and magnetic resonance elastography are the most widely used clinical tools (15–17). As for quantitative biological approaches, blood-based tests such as fibrosis 4 (FIB-4) index, which accounts for aspartate aminotransferase and alanine aminotransferase levels, the platelet count, and age to give a fibrosis index, is used commonly in the clinics (7, 18). Furthermore, several circulating protein-based tests including the enhanced liver fibrosis (ELF) test as well as NIS4, which combines protein markers and circulating microRNA, miR-34a, levels, are also available (15, 19). Although these diagnostic approaches have been useful to estimate the severity of fibrosis in patients with NAFLD, there are limited data on their utility in monitoring fibrosis progression/regression (20).
Over the past decade, an increasing research focus on “liquid biopsy” has resulted in the development of several circulating nucleic acid-based diagnostic tests for multiple diseases (21). Accordingly, various types of circulating nucleic acids, including cell-free DNA (cf-DNA), methylated cf-DNAs, and noncoding RNAs, especially microRNAs, have been examined as potential noninvasive biomarker candidates for NAFLD (22–25). In recent gene-expression profiling studies of liver tissue acquired from patients with NAFLD/NASH, gene-expression signatures for patients with NAFLD/NASH and transcriptionally regulated pathways associated with increased disease activity and fibrosis were discovered (26). Therefore, messenger RNAs appear to be promising biomarker candidates for NAFLD/NASH diagnosis and monitoring. Although quantification of cell-free messenger RNAs (cf-mRNAs) was considered challenging due to their low abundance in the circulation, we and others have developed next-generation sequencing (NGS)-based platforms and demonstrated that circulating cf-mRNA profiling may be used for diagnosis and monitoring of multiple diseases (27–29). Studies have shown that rather than cf-mRNA reflecting debris from cellular apoptosis or necrosis as with cfDNA, these selective actively released circulating nucleic acids provide lines of intercellular communication to inform proximal and distal cells of ongoing active transcriptional processes (30, 31). Furthermore, beyond a reflection of ongoing cellular processes, cf-mRNA has been demonstrated to be biologically functional such that cells that take up these nucleic acids have altered transcription (32).
Here, using a robust NGS platform developed to measure transcript levels in circulation, we sequenced the cf-mRNA transcriptomes from the serum of 247 well-characterized patients with NAFLD and 23 healthy control individuals. We first established the potential of our technology to differentiate NAFLD from healthy controls to identify dysregulated pathological processes. Next, we identified cf-mRNA transcriptomic signatures associated with the continuum of liver fibrosis, many of which coincide with previously known biological processes of disease (33, 34). Using these differentially expressed genes, we developed a cf-mRNA-based classifier to stratify patients according to the severity of liver fibrosis and assessed performance in an independent cohort of patients with NAFLD. Our study demonstrates the potential of using cf-mRNA profiling as a “liquid-biopsy” to intercept the intercellular communication signals and interrogate the molecular changes associated with NALFD progression.
MATERIALS AND METHODS
Patients and Sample Information
Samples from two NAFLD cohorts (one retrospective, “discovery cohort” and one prospective, “validation cohort”) and one prospective healthy cohort were evaluated (Supplemental Table S1 and S2; all supplemental material is available at https://doi.org/10.5061/dryad.1jwstqjt1). A total of 188 serum samples were retrospectively randomly selected from the Indiana University School of Medicine biobank (IU) to obtain a balanced representation of liver fibrosis (F0–F4), of which 176 were evaluable and had matched liver biopsy evaluated by central pathological reading. These 176 samples were allocated as the “discovery cohort.” A total of 89 serum samples were collected prospectively from IU and the biorepository at the University of Florida (UF), using a standardized collection protocol. From UF, 59 were evaluable due to having matched liver biopsy evaluated by a local pathologist. Inclusion criteria for the both retrospective and prospective cohort specified suspected NAFLD and NASH diagnosed via biopsy, which was to be performed within 30 days of blood collection. Patients were also required to fast for 8 h before blood draw. Patients with other forms of liver disease (hepatitis B or C, alcoholic hepatitis, etc.), patients taking drugs known to cause hepatic steatosis, and pregnant patients were excluded. In brief, blood samples were collected in BD Vacutainer clotting tubes (BD No. 367820) and processed within 2 h after the blood draw. All samples were centrifuged at 1,900 g for 10 min; then, serum was separated into new tubes and stored at −80°C. All 59 biopsy-confirmed prospective NAFLD samples were used as the “validation cohort.” In addition, serum samples from 23 control healthy individuals were prospectively collected from the San Diego Blood Bank, CA, using the same standardized protocol. Written informed consent was obtained from all patients, and the study was approved by the institutional review boards of all the participating institutions. Clinical data used in the study were obtained from the individual institutions in March 2018 and were collected and verified by each of the participating institutions.
Histology and Biochemical Tests
All biopsies were routinely stained with hematoxylin and eosin and Masson’s trichrome. Liver pathologists at the individual institute scored stained sections using the NASH Clinical Research Network scoring system (35). A fasting blood sample was obtained at the time of biopsy, and routine biochemical tests were performed using standard methods and assays. Biochemical tests included aminotransferase (ALT) and aspartate aminotransferase (AST), and additional blood samples were drawn for serum processing.
Library Preparation and Whole Transcriptome RNA-Seq
RNA was extracted using the QIAamp Circulating Nucleic Acid kit (Qiagen) from up to 1 mL of serum and eluted in a 16 µL volume. Then, 1 µL of extracted RNA was analyzed on an Agilent RNA 6000 Pico chip (Agilent Technologies) for presence of observable RNA traces. In total, 5 µL of the extracted RNA was then converted into a sequencing library as described previously (30). Qualitative and quantitative analyses of the NGS library preparation process were conducted using a chip-based electrophoresis, and libraries were quantified using a qPCR-based quantification kit (Roche, Cat. No. KK4824). Sequencing was performed using the Illumina NextSeq500 platform (Illumina Inc.), using paired-end sequencing, 75-cycle sequencing. For all sequencing data, we obtained a median of 8.7 million pass-filter reads per sample (range: 8.1–16.2 million reads). Base-calling was performed on an Illumina BaseSpace platform (Illumina Inc.), using the FASTQ Generation Application. For sequencing data analysis, adaptor sequences were removed, and low-quality bases were trimmed using cutadapt (v1.11). Reads shorter than 15 base-pairs were excluded from subsequent analysis. Read sequences greater than 15 base-pairs were compared with the human reference genome GRCh38 using STAR (v2.5.2b) with GENCODE v24 gene models. Duplicated reads were removed using the samtools (v1.3.1) rmdup command. Gene-expression levels were calculated from deduplicated BAM files using RSEM (v1.3.0).
Multiplex qPCR
cDNA was generated by primer annealing 3 µL of RNA with 10 mM dNTPs (Thermo Fisher Scientific, 10297018) and random hexamers (IDT) at 60°C for 5 min, followed by reverse transcription using the SuperScript IV Reverse Transcriptase kit (Invitrogen, 18090200) according to the manufacturer’s instruction. Subsequently, cDNA was amplified using Platinum Taq DNA polymerase (Invitrogen, 10966034) and preamplification primers (Supplemental Table S3). The cDNA samples were then treated with Exonucleus I (Thermo Fischer Scientific, EN0582) to remove primers used for the nested PCR reactions according to the manufacturer’s instruction. Multiplex qPCR was conducted with the Fluidigm BioMark HD system (Fluidigm) using 96.96 Dynamic array IFC (Fluidigm) with SsoFast EvaGreen Supermixes (Bio-Rad, 1725200) with target gene primers (primer sequences are listed in Supplemental Table S3).
Differential Expression Analysis and Correlation
Differential expression (DE) analysis was implemented with DESeq2 (v1.12.4) (36) using read counts as input. Genes with fewer than 200 total reads across the entire cohort were excluded from subsequent analysis. Technical replicates were averaged before the DE analysis, which applies a negative binomial model with raw read counts as the dependent variable. For NAFLD versus healthy control analysis, the independent variables were NAFLD/healthy and sex. For analysis of fibrosis, fibrosis was treated as a continuous variable. The Benjamini–Hochberg correction was used to correct for multiple testing and to obtain adjusted P values in differential expression analysis. Gene Ontology and Reactome Pathway enrichment analyses were conducted using Metascape (37) using Homo sapiens as the input and analysis species.
Cf-mRNA Transcriptome Decomposition Using Nonnegative Matrix Factorization
Nonnegative matrix factorization (NMF) was performed to decompose normalized gene-expression profiles from cf-mRNA into 12 components. In NMF decomposition, genes sharing similar expression patterns across samples are grouped together in an unsupervised manner. NMF decomposition was performed using the scikit-learn Python machine-learning library. Genes with >40% loading attributable to a particular component were considered enriched in the component. For each component, we selected genes enriched in the component and examined associated pathways and biological processes using Gene Ontology via Metascape (37). To robustly perform Gene Ontology analysis, we selected clusters with at least 200 genes as a criterion for further analysis. We determined the title of the cluster by preferentially using the top category identified in the Gene Ontology biological processes. If the terminology provided by the biological process category was nonspecific, we evaluated the top term identified in the pathway analysis and named the cluster according to the pathway term.
Bioinformatics Analysis/Classifier
In each sample for each gene, we took the arithmetic average of the TPMs of the replicates. Because of sample size limitations in the discovery cohort, fibrosis stage was aggregated to three categories: F0/F1, F2, and F3/F4. We applied an ordinal logistic regression model with an elastic net penalty (equal weighting to ridge and lasso) to classify fibrosis stage. Predictiveness curves were generated for the validation cohort. These curves depict the probability of clinically significant fibrosis (F2–F4) on the y-axis and the percentile of the biomarker score on the x-axis.
RESULTS
Cohort Descriptions
Evaluable samples from three cohorts of patients were included in analyses: 176 NASH/NAFL patient samples from a retrospective cohort (discovery cohort), 59 NASH/NAFL patient samples from a prospective cohort (validation cohort), and 23 healthy control samples collected prospectively from the San Diego Blood Bank (Fig. 1 and Supplemental Table S1). The full fibrosis spectrum of disease was observed in both NAFLD cohorts (Supplementary Table S2). The age and BMI distributions were reflective of the general NAFLD population and were similar in both NAFLD cohorts. Of note, the proportion of females was higher in the discovery cohort compared with the validation and healthy control cohorts (Supplemental Table S1). Furthermore, the proportion of females increased with higher fibrosis in the discovery cohort (Supplemental Table S2). The distributions of some demographics and baseline factors were imbalanced across cohorts and/or across fibrosis stage. Common comorbidities within this cohort were examined. In this study, 19% of patients with NAFLD were confirmed diabetics, and the median BMI among NAFLD patients was 34 kg/m2, which is considered obese.
Figure 1.
Consort diagram. Summary of patient samples that were collected initially and subsequent samples that were used for the analysis. Samples that were excluded from the study are also summarized.
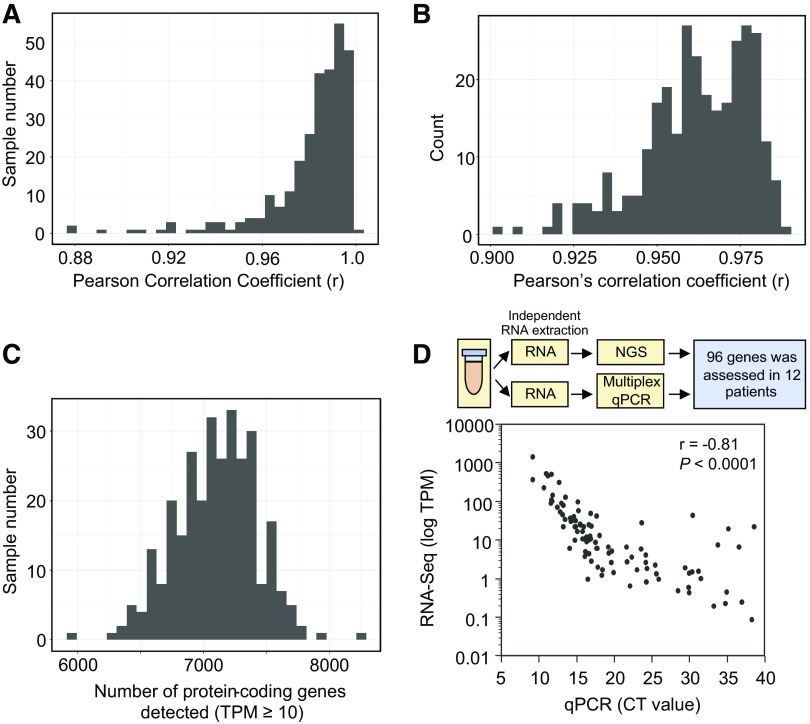
Technical Performance of cf-mRNA NGS Assay in Human Serum Specimens
We have previously demonstrated the potential of cf-mRNA as a platform for diagnosis and monitoring disease status in patients with hematological cancers (30) and in Alzheimer’s disease (38). We examined the robustness of the cf-mRNA platform in liver disease specifically, by evaluating the technical reproducibility of libraries generated from cf-RNA in all the serum samples in this study. For most samples (239/247, 97%) multiple serum aliquots were available and technical replicates were analyzed. Whole transcriptome comparison [transcripts per million (TPM) ≥ 10] of technical replicates showed high correlation indicative of robust technical reproducibility (Pearson’s correlation ≥ 0.94 for 95% of NAFLD samples) (Fig. 2A). In addition, the high correlation between observed versus expected number of extracellular RNA consensus consortium (ERCC) molecules demonstrated high quantification accuracy (Fig. 2B and Supplemental Fig. S1A), 95% of samples with Pearson’s correlation r ≥ 0.93) (39). A median of 7,120 transcripts with ≥ 10 TPM were identified per sample (Fig. 2C), highlighting the diversity of the circulating mRNA transcripts quantified by the assay. To further validate the robustness of the cf-mRNA RNA-Seq assay, we compared the levels of circulating transcripts assessed by cf-mRNA RNA-Seq with qPCR. We used multiplex qPCR (Fluidigm BioMark) to assess the expression of 96 gene targets known to be expressed in healthy and NAFLD liver tissue at different levels (40, 41) (Fig. 2D, Supplemental Table 3). Twelve plasma samples from the discovery cohort, separate aliquots to those used for RNA-Seq, were used to extract RNA and subsequently used for multiplex qPCR profiling of 96 genes. RNA-Seq TPM values inversely correlated with the qPCR cycle threshold (CT) value (Pearson’s correlation, r = −0.86), indicating a high degree of concordance between cf-mRNA-seq and qPCR platforms (Fig. 2D). Collectively, these data highlight the technical robustness of the cf-mRNA RNA-Seq assay in individuals with NAFLD.
Figure 2.
Cell-free (cf)-mRNA-based high-throughput sequencing is an accurate, robust, and reproducible approach for characterizing serum transcriptome. A: histogram representing correlation between the cf-mRNA transcriptomes of technical replicates using Pearson’s correlation analysis (n = 239). B: histogram compiling r values of expected extracellular RNA consensus consortium (ERCC) transcripts vs. observed level of ERCC from each sample (n = 239). C: histogram of number of transcripts detected (transcripts per million, TPM ≥10) per sample (n = 239). D: schematics for comparison between next-generation sequencing assay and qPCR (top). Correlation between sequencing TPM against qPCR Ct value (96 genes in total, n = 12) (bottom).
Identification of Transcriptomic Signatures in Circulation Associated with NAFLD Compared with Healthy Controls
To evaluate dysregulated cf-mRNA transcripts that are distinct to patients with NAFLD, we conducted differential gene expression analysis between NAFLD (discovery cohort) and healthy controls. Applying the DEseq algorithm (using a negative binomial model) to raw read counts (36), and adjusting for sex, we identified 2,498 differentially expressed genes [false discovery rate (FDR) < 0.05, Fig. 3A and Supplemental File S1]. Of these genes, 1,527 genes were upregulated and 971 genes were downregulated. Subsequently, using these dysregulated genes, we applied Gene Ontology and Reactome pathway analyses to identify pathways that are dysregulated in the cf-mRNA transcriptomes of patients with NAFLD. Gene Ontology analysis revealed dysregulation of immune system process and metabolic process as well as change in cellular component organization or biogenesis; all common processes that are associated with fibrosis (33) (Fig. 3B, Supplemental Fig. S2A, and Supplemental File 1). Interestingly, we identified “localization” as the most dysregulated pathway for both upregulated and downregulated genes. Previous studies have indicated that liver fibrosis dysregulates genes that are associated with cellular localization (42, 43). Considering that dysregulation of genes that are associated with “localization” can be overexpressed or inhibited in the liver, we speculate that this GO term was identified in both groups. Similarly, Reactome pathway analysis identified dysregulation of fibrosis-associated pathways, including the adaptive immune system, Rho GTPase signaling, and angiogenesis (Supplemental Fig. 2B and Supplemental File 1).
Figure 3.

Cell-free (cf)-mRNA profile shows distinct profile for patients with nonalcoholic fatty liver disease (NAFLD) compared with control subjects. A: volcano plot depicting the cf-mRNA genes that are differentially expressed between patients with NAFLD and healthy controls (n = 176 and 23, respectively, n = number of subjects). Significantly dysregulated genes are denoted in red, false discovery rate (FDR) < 0.05 was used as the cutoff criterion. B: most significant biological pathways identified using upregulated (top) and downregulated genes (bottom) identified in A as inputs (Gene Ontology). C: heat map showing nonnegative matrix factorization (NMF) gene clusters within patients with NAFLD. D: top Gene Ontology biological process categories associated with each cluster.
Next, to examine the overall biology of the cf-mRNA transcriptome in patients with NAFLD, we applied unsupervised nonnegative matrix factorization (NMF) (27) decomposition of cf-mRNA transcriptomes using NAFLD samples from the discovery cohort. Subsequently, we identified 12 distinct gene clusters (Fig. 3C and Supplemental File S2). Of 12 clusters, we focused specifically on six clusters where the number of genes in the cluster was greater than 200 (Supplemental Fig. 2C). We then used Gene Ontology to identify key biological processes and pathways that are associated with individual clusters (Fig. 3D and Supplemental Fig. S2C). Functional analyses revealed identification of the following clusters: cellular component organization, immune systems 1 and 2, metabolic process, hemostasis, and cellular localization (Supplemental Fig. S2C). Interestingly, several clusters such as metabolic process, cellular component organization, and immune systems are well-recognized biological processes that are associated with NAFLD, indicating that molecular alterations in the liver might be reflected in the circulation. Collectively, our data indicate that the cf-mRNA profile can be used to noninvasively identify molecular alterations that are specific to patients with NAFLD, and the gene-expression profile of patients with NAFLD may reflect the molecular characteristics of the disease.
Identification of Transcriptomic Signatures in Circulation Associated with Fibrosis Stage within the NAFLD Cohort
NAFLD-related transcriptomic changes in serum cf-mRNA were evaluated in the discovery NAFLD cohort samples to identify markers of fibrosis. Applying the DESeq algorithm to raw read counts and treating fibrosis as a continuous variable, we applied a less stringent threshold of FDR < 0.1 due to the more challenging problem of identifying differentially expressed genes within the NAFLD patient cohort. We identified 134 differentially expressed genes (FDR < 0.1, Fig. 4A, Supplemental File S2). The majority (79, 59%) of genes were upregulated, whereas 55 (41%) genes were downregulated (Fig. 4A). The terms “upregulated” and “downregulated” are used to describe changes in the number of RNA molecules in the circulation of higher fibrosis patients. Next, we used Gene Ontology and Reactome pathway analysis algorithms to evaluate the functional roles and biological processes reflected by these differentially expressed genes (Fig. 4B and Supplemental Fig. S3, A and B). Using Gene Ontology enrichment analysis, cf-mRNA genes that are associated with fibrosis stages were associated with biological processes of NAFLD, including metabolic processes and immune system processes, both well-known processes that are commonly dysregulated in patients with NAFLD (Fig. 4B). In addition, we used Reactome pathway analysis to examine biological pathways that are linked to cf-mRNA fibrosis-associated genes (Supplemental Fig. 3B). The Reactome pathway analysis identified pathways that are known to be associated with fibrosis and NAFLD, including notch pathways and lipid metabolism (44, 45). We then used publicly available liver tissue RNA sequencing data that were generated from patients with NAFLD and liver fibrosis (34) and compared genes that are associated with fibrosis between hepatic tissue and cf-mRNA. The tissue RNA-Seq data were generated from liver biopsy samples from 72 patients with NAFLD, and ordinal regression was applied to identify genes that are associated with fibrosis stages (34). A total of 4,010 genes were identified using FDR < 0.05 as the cutoff criterion. The comparison between differentially expressed genes between tissues and cf-mRNA resulted in identification of 43 common genes (32% of fibrosis associated cf-mRNA genes) (Fig. 4C). Furthermore, we evaluated the common pathways that are dysregulated in fibrosis among both tissue and cf-mRNA (Fig. 4C). We identified that of the 21 tissue and 17 cf-mRNA Gene Ontology biological pathways identified, 15 pathways were common between liver tissues and cf-mRNA (Fig. 4C). Collectively, our data suggest that dysregulated genes in the cf-mRNA reflect those of tissues and frequently display common dysregulated pathways.
Figure 4.
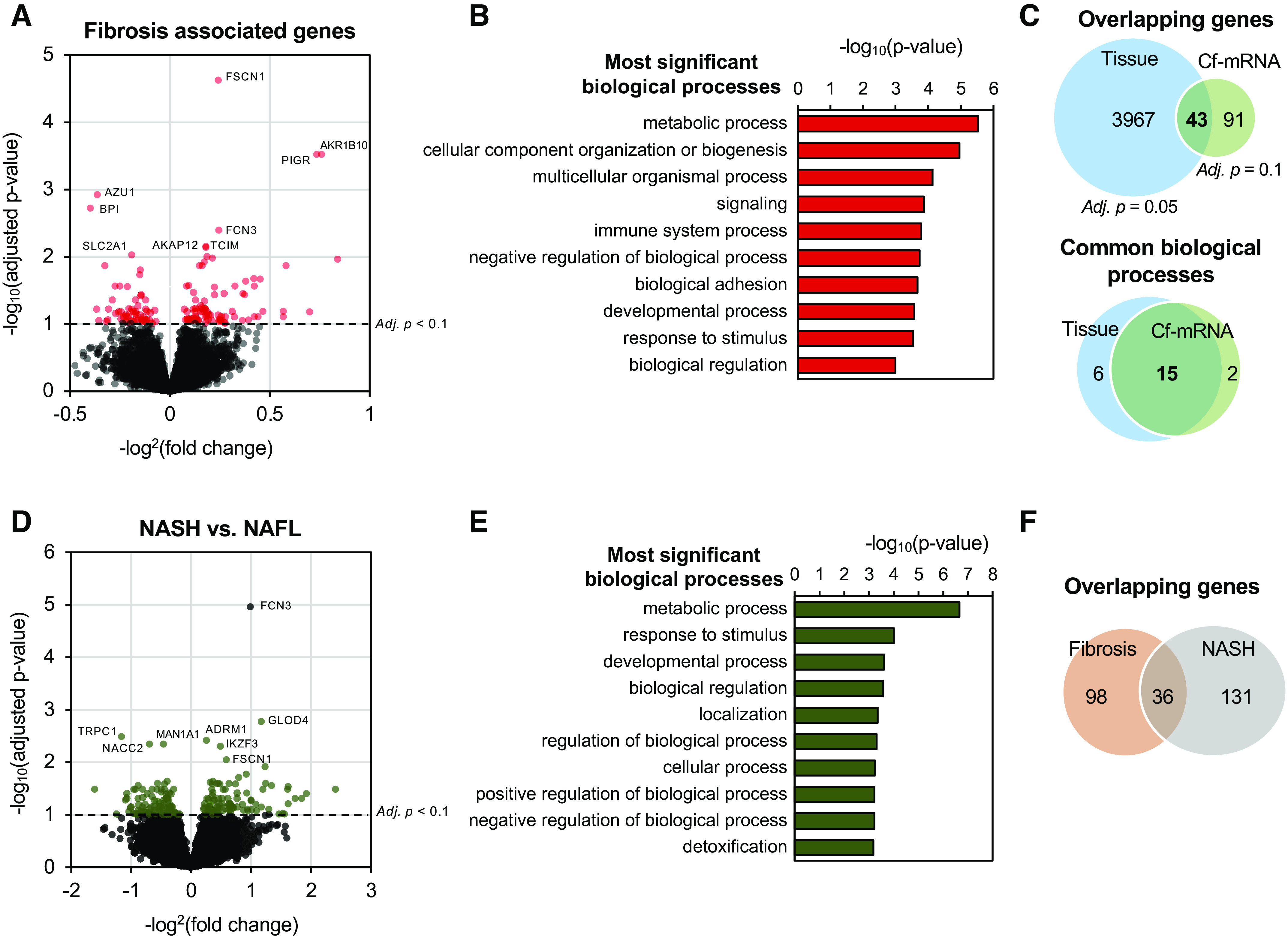
Cell-free (cf)-mRNA profiling reveals key biological pathways and processes associated with advanced fibrosis. A: volcano plot depicting the differential expression analysis associated with fibrosis in cf-mRNA (n = 176). Significantly dysregulated genes are denoted in red, false discovery rate (FDR) < 0.10 was used as the cutoff criterion. B: most significantly enriched pathways identified using genes significantly dysregulated with fibrosis stages (Gene Ontology). C: overlap of genes (top) and pathways (bottom) between tissue and cf-mRNA fibrosis stage-associated genes [tissue data obtained from Hoang et al. (34)]. Pathway analysis was conducted using Gene Ontology (biological processes). D: volcano plot depicting the differential expression analysis between nonalcoholic steatohepatitis (NASH) and NAFL in cf-mRNA (132 NASH vs. 44 NAFL). Significantly dysregulated genes are denoted in green, FDR < 0.10 was used as the cutoff criterion. E: most significantly enriched pathways identified using genes significantly dysregulated between NASH and NAFLD (Gene Ontology). F: overlap of differentially dysregulated genes between fibrosis and NASH vs. NAFLD comparison.
Although many patients with NASH have advanced liver fibrosis, NASH is a distinct histological entity that is diagnosed based on pattern recognition of steatosis, inflammation, and ballooning. We, therefore, examined cf-mRNA genes that are dysregulated in patients with NASH compared with in those with NAFL (Fig. 4D). We identified 167 differentially expressed genes between NASH and NAFL (FDR < 0.10, Fig. 4D, Supplemental File 3). Whereas 88 (53%) genes were upregulated, 79 (47%) genes were downregulated (Fig. 4D). Pathway analyses of these dysregulated genes revealed that metabolic pathways were prominent (Fig. 4E and Supplemental Fig. 3C) for both Gene Ontology and Reactome (Fig. 4E and Supplemental Fig. 3, C and D). In addition, we compared dysregulated genes between fibrosis and NASH versus NAFL comparison. Interestingly, only 36 genes that were dysregulated in both analyses overlapped, indicating that the molecular profile of NASH substantially differs from that of advanced fibrosis and would require a separate set of genes to effectively identify patients with NASH (Fig. 4F).
Stratification of NAFLD Fibrosis Stages
Since biopsy-measured fibrosis is an imperfect gold standard, we developed a classifier of fibrosis stage that yields the patient’s estimated probability of having F2–F4 fibrosis. To assess the feasibility of distinguishing fibrosis stages for detection, disease management, and therapeutic trials, we developed this classifier based on the discovery cohort. We modeled fibrosis as an ordinal variable (F0/F1, F2, F3/F4) and applied an elastic net penalty. The resulting biomarker score was applied to the independent prospective NAFLD cohort. Rather than reporting AUC, sensitivity, and specificity, which assume a clinically actionable risk threshold and are dependent on the cohort in which these parameters are estimated, we display the predicted probability of having clinically significant fibrosis (F2 to F4) on the y-axis as a function of the percentile of the biomarker score on the x-axis (Fig. 5A). This predictiveness curve shows the range and distribution of estimated risk levels associated with the model when it is applied to the population from which the cohort was drawn (46). Although the predictiveness curve relates to classification performance, the curve also displays essential information about risk that is not displayed by the receiver operating characteristic curve and highlights the ability of the biomarker score to tease apart risk from the average risk in the cohort (40% in this cohort). Therefore, the predictiveness curve reflects both risk modeling and population performance approach, providing a more complete and comprehensive analysis than an evaluation of classifier performance. The predictiveness curve depicted in Fig. 5A shows 25% of patients, the majority of whom are labeled F0/1, with less than 25% probability of having F2–F4 disease. The figure also shows 40% of patients, the majority of whom are labeled F2–F4, with higher than 90% probability of having F2–F4 disease. Similarly, we developed a classifier for advanced fibrosis (F3/F4). Thirty-four percent of patients were classified with less than 25% probability of having advanced fibrosis, a majority of whom were F0/1/2; 36% of patients had higher than 90% probability of having advanced fibrosis, a majority of whom were F3–F4 (Fig. 5B). Collectively, these data show that cf-mRNA transcriptome can be used to develop effective classifiers for liver fibrosis.
Figure 5.

Performance of a cell-free (cf)-mRNA fibrosis classifier. A: predicted probability of clinically significant fibrosis (F2, F3, and F4) in the validation set. B: predicted probability of advanced fibrosis (F3 and F4) in the validation set.
DISCUSSION
NAFLD is a disorder that affects a quarter of the worldwide population, resulting in a considerable global health and economic burden (47). Although liver biopsy is currently the reference method for NAFLD characterization, this invasive approach has several key limitations. The biopsy samples are equivalent to only one fifty-thousandth of the liver volume and may not reflect the pathological status of the entire liver. Pathological grading of the biopsy samples is subject to inter- and intraobserver variation (48, 49). Furthermore, liver biopsies are expensive, are invasive, and carry the risk, though infrequent, of serious complications, thereby making them unsuitable for disease monitoring. For these reasons, there is an urgent need for noninvasive tools to diagnose and monitor the disease status of NAFLD, as well as to identify novel therapeutic targets. Blood-based noninvasive assays may potentially overcome many of the limitations intrinsic to liver biopsy and enable better disease screening, diagnosis, and monitoring (50). In the present study, we utilized cohorts with biopsy-confirmed fibrosis covering a broad spectrum of disease. Subsequently, we identified cf-mRNA markers of NAFLD and NAFLD severity that are common to those observed in liver tissue of patients with NAFLD (34). We used two cohorts to demonstrate that molecular alterations associated with liver fibrosis are reflected in the circulation, and these transcriptional changes may be used to develop classifiers to assess the severity and perhaps ongoing diverse pathological changes with intervention of liver fibrosis. We acknowledge that the present study is a proof-of-concept study and has limitations in its design that may have introduced selection bias as well as unmeasured confounders. Further analytical and clinical development and validation studies in the intended-use population are needed as a next step to developing a robust clinical-grade NASH fibrosis diagnostic.
Over the past decade, blood-based molecular biomarkers have been identified as key candidates for the development of clinically relevant noninvasive disease biomarkers. Accordingly, several circulating DNA-based biomarkers have been developed for prenatal diagnostics, transplant rejection, and cancer monitoring (51–56). Recent gene-expression profiling of liver tissues obtained from patients with NAFLD/NASH revealed unique gene-expression alterations for patients with NAFLD compared with those of healthy individuals (26). For this reason, we believe that the signals observed from liver reflect the expression of active processes and are reflective of disease. In the present study, we demonstrated the utility of circulating transcriptomic cf-mRNA profiling to evaluate molecular alterations associated with patients with NAFLD. Although RNA-Seq-based cf-mRNA profiling has been used previously in other diseases (27–29), this is the first study, to our knowledge, to utilize the cf-mRNA RNA-Seq technology platform to comprehensively examine the transcriptional alterations in patients with NAFLD and to evaluate the association of cf-mRNA profiles with severity of liver diseases. We showed that cf-mRNA profiling reflected that of previously performed liver tissue sequencing data and identified several pathways that were associated with key biological processes that are linked to NAFLD, including lipid metabolism and extracellular matrix dysregulation. Our results demonstrate that cf-mRNA profiling can potentially be used beyond simple disease diagnosis and fibrosis assessment in liver disease by utilizing the genes and pathways that are dysregulated in liver for therapeutic target identification, patient selection, and the monitoring of efficacy of pharmacotherapy, lifestyle intervention, and bariatric surgery.
With numerous drugs currently being developed for NAFLD and a need to inform the encouragement of lifestyle intervention or bariatric surgery, it is critical to identify the stage of disease (57, 58). Although most current clinical trials utilize a liver biopsy to identify a suitable patient population, the invasive nature of biopsy limits its utility as a broader patient-screening tool for clinical trials (50), patient monitoring during trials, or for monitoring intervention efficacy. The fibrosis-4 index (FIB4), NAFLD fibrosis score (NFS), NIS4, and vibration-controlled transient elastography (VCTE) are currently available noninvasive tests to predict the presence and severity of hepatic fibrosis in NAFLD (16, 17, 19, 50, 59). However, these tests are based on simple variables such as age, liver enzymes, platelets, and liver physical stiffness, which unlike our cf-mRNA classifier are unlikely to reflect the real-time dynamics of NAFLD pathophysiology or hepatic transcriptome changes in response to therapeutic interventions for NAFLD. We utilized whole transcriptome cf-mRNA profiling to develop a classifier for fibrosis. The classifier was able to identify a significant proportion of patients at either very high or low probability of having clinically significant fibrosis (F2–F4) in the validation cohort. Since the classifier includes multiple gene transcripts, it is more likely to reflect the multiple underlying pathologies known to be involved in this chronic complex disease. Because an expression-based assay could be used to evaluate multiple aspects of the disease and of patient response over time, our study demonstrates the potential utility of cf-mRNA as a promising alternative to liver biopsies, imaging, and conventional blood tests.
DATA AVAILABILITY
Sequencing data generated in this study was deposited in Sequence Read Archive (SRA) under accession number PRJNA701722 (https://www.ncbi.nlm.nih.gov/sra/PRJNA701722). All data needed to evaluate the conclusions in the paper are present in the paper and/or the supplementary materials.
SUPPLEMENTAL DATA
Supplemental Figures S1–3 and Tables S1–3: https://doi.org/10.5061/dryad.1jwstqjt1)
DISCLOSURES
S.T., J.J.S., R.P.R., J.V.B., J.Z., M.N., and T.M. are current or past employees at Molecular Stethoscope, Inc. S.T., J.Z., and M.N. are named as inventors in patent applications related to the technologies used in this manuscript. S.T., J.Z., and M.N. are named as inventors in pending patent applications related to the technologies used in this manuscript filed by Molecular Stethoscope Inc. [WO2020092646A1 filed on October 30, 2019 (J.Z., M.N.), WO2020087037A2 filed on October 25, 2019 (S.T., J.Z., M.N.), and WO2019060369A1 filed on September 18, 2018 (M.N.)]. S.R.Q. is a founder of Molecular Stethoscope, Inc., and a member of its scientific advisory board. N.C. has ongoing consulting agreements with Abbvie, Madrigal, Foresite, Zydus, ObsEva, and Galectin and research support from DSM, Exact Sciences, Zydus, and Intercept. At the time of this work, J.V.B. had ongoing consulting agreements from Exact Sciences, CareDX, and Grail. These outside interests are not directly or significantly related to this paper. At the time of this work, T.M. had ongoing consulting agreements and/or other compensation from CareDX, Delfi, Grail, and Lexent Bio. These outside interests are not directly or significantly related to this paper. S.G. consults for TransMedics and Pfizer and receives research grant support from Zydus, Galmed and Viking. None of these interests is related to this work. These outside interests are not directly or significantly related to this paper. All authors declare that they have no additional competing interests. No external funding was used for this study.
AUTHOR CONTRIBUTIONS
N.C., S.T., M.N., and T.M. conceived and designed research; N.C., S.T., J.J.S., R.P.R., J.V.B., J.Z., and T.M. analyzed data; N.C., S.T., J.J.S., R.P.R., J.V.B., S.G., J.Z., M.N., S.R.Q. and T.M. interpreted results of experiments; S.T., J.V.B., and J.Z. prepared figures; N.C., S.T., J.Z., and T.M. drafted manuscript; N.C., S.T., J.J.S., R.P.R., J.V.B., S.G., J.Z., M.N., S.R.Q., and T.M. edited and revised manuscript; N.C., S.T., J.J.S., R.P.R., J.V.B., S.G., J.Z., M.N., S.R.Q., and T.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Teresa Wright and Guillermo Elias for conceptual discussions and critical reading of the manuscript; Neeraj Salathia, Arkaitz Ibarra, John Aballi, Alex Acosta, Lucy Guo, Vera Huang, Amy P Karns, Julianna R Parks, and Yue Zhao for technical input and assistance; Kayla Gelow and Emily R Smith for sample collection and data management; Brooke McMillen Patz and the Clinical and Translational Sciences Institute (CTSI) Biorepository group at Indiana University, and Erin Kelly and the CTSI Biorepository group at University of Florida, for sourcing patient samples; and Ruben Rodrigues and Brian Read at the Development Research Services for assistance with sourcing samples from the San Diego Blood Bank.
REFERENCES
- 1.Danford CJ, Sanchez JE, Corey KE. Managing the burden of non-NASH NAFLD. Curr Hepatol Rep 16: 326–334, 2017. doi: 10.1007/s11901-017-0371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: mayo clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 55: 434–438, 1980. [PubMed] [Google Scholar]
- 3.Brunt EM. Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 7: 195–203, 2010. doi: 10.1038/nrgastro.2010.21. [DOI] [PubMed] [Google Scholar]
- 4.Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, Couzigou P, de Lédinghen V. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 51: 828–835, 2010. doi: 10.1002/hep.23425. [DOI] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67: 328–357, 2018. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 6.Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, Francque S, Farrell G, Kowdley KV, Craxi A, Simon K, Fischer L, Melchor-Khan L, Vest J, Wiens BL, Vig P, Seyedkazemi S, Goodman Z, Wong VW, Loomba R, Tacke F, Sanyal A, Lefebvre E. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 67: 1754–1767, 2018. doi: 10.1002/hep.29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E; NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385: 956–965, 2015. [Erratum in Lancet 385: 946, 2015]. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, Drenth J, Anstee QM, Hum D, Hanf R, Roudot A, Megnien S, Staels B, Sanyal A; GOLDEN-505 Investigator Study Group. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 150: 1147–1159, 2016. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 9.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 362: 1675–1685, 2010. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee Y, Doumouras AG, Yu J, Brar K, Banfield L, Gmora S, Anvari M, Hong D. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 17: 1040–1060, 2019. doi: 10.1016/j.cgh.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 67: 829–846, 2017. doi: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149: 367–378, 2015. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Vilar-Gomez E, Yasells-Garcia A, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Villa-Jimenez O, Friedman SL, Diago M, Romero-Gomez M. Development and validation of a noninvasive prediction model for nonalcoholic steatohepatitis resolution after lifestyle intervention. Hepatology 63: 1875–1887, 2016. doi: 10.1002/hep.28484. [DOI] [PubMed] [Google Scholar]
- 14.Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 156: 1264–1281, 2019. e1264 doi: 10.1053/j.gastro.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nobili V, Parkes J, Bottazzo G, Marcellini M, Cross R, Newman D, Vizzutti F, Pinzani M, Rosenberg WM. Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology 136: 160–167, 2009. doi: 10.1053/j.gastro.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Siddiqui MS, Vuppalanchi R, Van Natta ML, Hallinan E, Kowdley KV, Abdelmalek M, Neuschwander-Tetri BA, Loomba R, Dasarathy S, Brandman D, Doo E, Tonascia JA, Kleiner DE, Chalasani N, Sanyal AJ; NASH Clinical Research Network. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 17: 156–163.e2, 2019. doi: 10.1016/j.cgh.2018.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tapper EB, Castera L, Afdhal NH. FibroScan (vibration-controlled transient elastography): where does it stand in the United States practice. Clin Gastroenterol Hepatol 13: 27–36, 2015. doi: 10.1016/j.cgh.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 18.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 7: 1104–1112, 2009. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison SA, Ratziu V, Boursier J, Francque S, Bedossa P, Majd Z, Cordonnier G, Sudrik Fb Darteil R, Liebe R, Magnanensi J, Hajji Y, Brozek J, Roudot A, Staels B, Hum DW, Megnien SJ, Hosmane S, Dam N, Chaumat P, Hanf R, Anstee QM, Sanyal AJ. A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 5: 970–985, 2020. doi: 10.1016/S2468-1253(20)30252-1. [DOI] [PubMed] [Google Scholar]
- 20.Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: clinical prediction rules and blood-based biomarkers. J Hepatol 68: 305–315, 2018. doi: 10.1016/j.jhep.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 21.De Rubis G, Krishnan SR, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci 40: 172–186, 2019. doi: 10.1016/j.tips.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Xiao Y, Wu X, Jiang L, Yang S, Ding Z, Fang Z, Hua H, Kirby MS, Shou J. A circulating microRNA signature as noninvasive diagnostic and prognostic biomarkers for nonalcoholic steatohepatitis. BMC Genomics 19: 188, 2018. doi: 10.1186/s12864-018-4575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK, Jones MB, Sirlin CB, Schnabl B, Brinkac L, Schork N, Chen CH, Brenner DA, Biggs W, Yooseph S, Venter JC, Nelson KE. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab 25: 1054–1062, 2017. doi: 10.1016/j.cmet.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Keuren-Jensen KR, Malenica I, Courtright AL, Ghaffari LT, Starr AP, Metpally RP, Beecroft TA, Carlson EW, Kiefer JA, Pockros PJ, Rakela J. microRNA changes in liver tissue associated with fibrosis progression in patients with hepatitis C. Liver Int 36: 334–343, 2016. doi: 10.1111/liv.12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeybel M, Hardy T, Robinson SM, Fox C, Anstee QM, Ness T, Masson S, Mathers JC, French J, White S, Mann J. Differential DNA methylation of genes involved in fibrosis progression in non-alcoholic fatty liver disease and alcoholic liver disease. Clin Epigenetics 7: 25, 2015. doi: 10.1186/s13148-015-0056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryaboshapkina M, Hammar M. Human hepatic gene expression signature of non-alcoholic fatty liver disease progression, a meta-analysis. Sci Rep 7: 12361, 2017. doi: 10.1038/s41598-017-10930-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koh W, Pan W, Gawad C, Fan HC, Kerchner GA, Wyss-Coray T, Blumenfeld YJ, El-Sayed YY, Quake SR. Noninvasive in vivo monitoring of tissue-specific global gene expression in humans. Proc Natl Acad Sci USA 111: 7361–7366, 2014. [Erratum in Proc Natl Acad Sci USA 111: 11223, 2014]. doi: 10.1073/pnas.1405528111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munchel S, Rohrback S, Randise-Hinchliff C, Kinnings S, Deshmukh S, Alla N, Tan C, Kia A, Greene G, Leety L, Rhoa M, Yeats S, Saul M, Chou J, Bianco K, O'Shea K, Bujold R, Norwitz E, Wapner E, Saade G, Kaper F. Circulating transcripts in maternal blood reflect a molecular signature of early-onset preeclampsia. Sci Transl Med 12: eaaz0131, 2020. doi: 10.1126/scitranslmed.aaz0131. [DOI] [PubMed] [Google Scholar]
- 29.Ngo TTM, Moufarrej MN, Rasmussen MH, Camunas-Soler J, Pan W, Okamoto J, Neff NF, Liu K, Wong RJ, Downes K, Tibshirani R, Shaw GM, Skotte L, Stevenson DK, Biggio JR, Elovitz MA, Melbye M, Quake SR. Noninvasive blood tests for fetal development predict gestational age and preterm delivery. Science 360: 1133–1136, 2018. doi: 10.1126/science.aar3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibarra A, Zhuang J, Zhao Y, Salathia NS, Huang V, Acosta AD, Aballi J, Toden S, Karns AP, Purnajo I, Parks JR, Guo L, Mason J, Sigal D, Nova TS, Quake SR, Nerenberg M. Non-invasive characterization of human bone marrow stimulation and reconstitution by cell-free messenger RNA sequencing. Nat Commun 11: 400, 2020. doi: 10.1038/s41467-019-14253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simons M, Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol 21: 575–581, 2009. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 32.van den Boorn JG, Dassler J, Coch C, Schlee M, Hartmann G. Exosomes as nucleic acid nanocarriers. Adv Drug Deliv Rev 65: 331–335, 2013. doi: 10.1016/j.addr.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Chang M-L, Yang S-S. Metabolic signature of hepatic fibrosis: from individual pathways to systems biology. Cells 8: 1423, 2019. doi: 10.3390/cells8111423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoang SA, Oseini A, Feaver RE, Cole BK, Asgharpour A, Vincent R, Siddiqui M, Lawson MJ, Day NC, Taylor JM, Wamhoff BR, Mirshahi F, Contos MJ, Idowu M, Sanyal AJ. Gene expression predicts histological severity and reveals distinct molecular profiles of nonalcoholic fatty liver disease. Sci Rep 9: 12541, 2019. doi: 10.1038/s41598-019-48746-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321, 2005. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 36.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, Chanda SK. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun 10: 1523, 2019. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toden S, Zhuang J, Acosta AD, Karns AP, Salathia NS, Brewer JB, Wilcock DM, Aballi J, Nerenberg M, Quake SR, Ibarra A. Non-invasive characterization of Alzheimer’s disease by circulating, cell-free messenger RNA next generation sequencing. Sci Adv 6: eabb1654, 2020. doi: 10.1126/sciadv.abb1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker SC, Bauer SR, Beyer RP, Brenton JD, Bromley B, Burrill J; External RNA Controls Consortium, , et al. The external RNA controls consortium: a progress report. Nat Methods 2: 731–734, 2005. doi: 10.1038/nmeth1005-731. [DOI] [PubMed] [Google Scholar]
- 40.GTEx Consortium. The genotype-tissue expression (GTEx) project. Nat Genet 45: 580–585, 2013. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moylan CA, Pang H, Dellinger A, Suzuki A, Garrett ME, Guy CD, Murphy SK, Ashley-Koch AE, Choi SS, Michelotti GA, Hampton DD, Chen Y, Tillmann HL, Hauser MA, Abdelmalek MF, Diehl AM. Hepatic gene expression profiles differentiate presymptomatic patients with mild versus severe nonalcoholic fatty liver disease. Hepatology 59: 471–482, 2014. doi: 10.1002/hep.26661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karsdal MA, Detlefsen S, Daniels SJ, Nielsen MJ, Krag A, Schuppan D. Is the total amount as important as localization and type of collagen in liver fibrosis attributable to steatohepatitis? Hepatology 71: 346–351, 2020. doi: 10.1002/hep.30969. [DOI] [PubMed] [Google Scholar]
- 43.Zhong Y, Qin Y, Dang L, Jia L, Zhang Z, Wu H, Cui J, Bian H, Li Z. Alteration and localization of glycan-binding proteins in human hepatic stellate cells during liver fibrosis. Proteomics 15: 3283–3295, 2015. doi: 10.1002/pmic.201500030. [DOI] [PubMed] [Google Scholar]
- 44.Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol 290: G852–G858, 2006. doi: 10.1152/ajpgi.00521.2005. [DOI] [PubMed] [Google Scholar]
- 45.Zhu C, Kim K, Wang X, Bartolome A, Salomao M, Dongiovanni P, Meroni M, Graham MJ, Yates KP, Diehl AM, Schwabe RF, Tabas I, Valenti L, Lavine JE, Pajvani UB. Hepatocyte notch activation induces liver fibrosis in nonalcoholic steatohepatitis. Sci Transl Med 10: eaat0344, 2018. doi: 10.1126/scitranslmed.aat0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pepe MS, Feng Z, Huang Y, Longton G, Prentice R, Thompson IM, Zheng Y. Integrating the predictiveness of a marker with its performance as a classifier. Am J Epidemiol 167: 362–368, 2008. doi: 10.1093/aje/kwm305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 64: 1577–1586, 2016. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 48.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 344: 495–500, 2001. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 49.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T; LIDO Study Group. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 128: 1898–1906, 2005. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 50.Wong VW, Adams LA, de Lédinghen V, Wong GL, Sookoian S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol 15: 461–478, 2018. doi: 10.1038/s41575-018-0014-9. [DOI] [PubMed] [Google Scholar]
- 51.Bianchi DW, Chiu RWK. Sequencing of circulating cell-free DNA during pregnancy. N Engl J Med 379: 464–473, 2018. doi: 10.1056/NEJMra1705345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Vlaminck I, Valantine HA, Snyder TM, Strehl C, Cohen G, Luikart H, Neff NF, Okamoto J, Bernstein D, Weisshaar D, Quake SR, Khush KK. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med 6: 241ra77, 2014. doi: 10.1126/scitranslmed.3007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan HC, Blumenfeld YJ, Chitkara U, Hudgins L, Quake SR. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proc Natl Acad Sci USA 105: 16266–16271, 2008. doi: 10.1073/pnas.0808319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan HC, Gu W, Wang J, Blumenfeld YJ, El-Sayed YY, Quake SR. Non-invasive prenatal measurement of the fetal genome. Nature 487: 320–324, 2012. [Erratum in Nature 489: 326, 2012]. doi: 10.1038/nature11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grskovic M, Hiller DJ, Eubank LA, Sninsky JJ, Christopherson C, Collins JP, Thompson K, Song M, Wang YS, Ross D, Nelles MJ, Yee JP, Wilber JC, Crespo-Leiro MG, Scott SL, Woodward RN. Validation of a clinical-grade assay to measure donor-derived cell-free DNA in solid organ transplant recipients. J Mol Diagn 18: 890–902, 2016. doi: 10.1016/j.jmoldx.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Snyder TM, Khush KK, Valantine HA, Quake SR. Universal noninvasive detection of solid organ transplant rejection. Proc Natl Acad Sci USA 108: 6229–6234, 2011. doi: 10.1073/pnas.1013924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol 53: 362–376, 2018. doi: 10.1007/s00535-017-1415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong VW, Chitturi S, Wong GL, Yu J, Chan HL, Farrell GC. Pathogenesis and novel treatment options for non-alcoholic steatohepatitis. Lancet Gastroenterol Hepatol 1: 56–67, 2016. doi: 10.1016/S2468-1253(16)30011-5. [DOI] [PubMed] [Google Scholar]
- 59.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology 66: 1486–1501, 2017. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequencing data generated in this study was deposited in Sequence Read Archive (SRA) under accession number PRJNA701722 (https://www.ncbi.nlm.nih.gov/sra/PRJNA701722). All data needed to evaluate the conclusions in the paper are present in the paper and/or the supplementary materials.