Abstract
The acute ischemic stroke (AIS) is a devastating disease and remains the leading cause of death and disability. This study aims to evaluate the role of hematological inflammatory markers (neutrophil-to-lymphocyte ratio [NLR], platelet-to-lymphocyte ratio [PLR], and systemic immune inflammation index [SII]) in predicting the neurological recovery in acute cerebrovascular events over 1-year follow-up.
Adult patients diagnosed with AIS within 3 hours from January 2016 to December 2018 were recruited retrospectively. The modified Rankin Scale (mRS) was recorded upon admission to the emergency department (ED) and 1, 3, 6, and 12 months after a stroke. The primary outcome measure was the neurological recovery. The neurological recovery was defined as an improvement in mRS score ≥ 1 compared with that at the ED admission baseline.
A total of 277 consecutive adult patients with AIS within 3 hours were enrolled. The initial average of the National Institute of Health Stroke Scale was 9.2 ± 7.8, and 90.3% of patients had an mRS ≥ 2 at ED admission baseline. The overall neurological recovery rates of 48.7%, 53.7%, 59.2%, and 55.9% were observed at 1, 3, 6, and 12 months follow-up, respectively. The multivariate analysis revealed that the baseline NLR value was a significant predictor of neurological recovery at 3 months after a stroke (adjusted odds ratio = 0.89, 95% confidence interval = 0.80–0.99, P = .035).
A low NLR at ED admission could be useful marker for predicting neurological recovery at 3 months after stroke.
Keywords: acute ischemic stroke, neurological recovery, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, Sankey diagram, systemic immune inflammation index
1. Introduction
The stroke is defined as a sudden impairment in the brain function of an area of the injured brain.[1] The acute ischemic stroke (AIS) is a devastating disease and remains the leading cause of death and disability worldwide.[1] The modulation of the inflammation cell function plays a role in postischemic brain injury and repair. Evidence supports that systemic inflammatory response makers may have a role in the prognosis of patients with AIS.[2,3] In response to the inflammatory immune system, white blood cells (WBCs) are important parts of our body's immune system.[3] The acute inflammation is the body's immediate response to infection or injury, and the inflammation has a key role in the initiation and the progression of atherosclerosis.[4] The activation of the inflammatory process usually involves leukocyte infiltration into the ischemic tissue.[1]
In Taiwan, around 30,000 patients are hospitalized due to acute stroke annually.[5] The immediate treatment for stroke may help minimize the brain cell damage and death. Current reperfusion treatment strategies for AIS include intravenous recombinant tissue plasminogen activators (IV-rtPA) thrombolytic therapy or intra-arterial (IA) endovascular management. However, these treatments are limited to a narrow therapeutic window.[6] An accurate prediction or measurement tool to assess neurological recovery after AIS is important for patients and physicians in the emergency department (ED). This tool would help guide acute treatment decisions; help physicians, patients, and families to develop realistic prognostic expectations in the future; and plan resource use and long-term living settings.[7] Thus, a related prognostic factor or model needs to be easily applicable in the clinical setting.
Nowadays, state-of-the-art hematology analyzers are fully automated systems. These analyzers provide a complete blood count (CBC) panel, which includes 5-part differential WBCs and platelet within a short time. The hematological markers of inflammation in the CBC panel are potentially useful in determining the prognosis of the diseases. The acute stress can increase the number of neutrophils but reduce the number of lymphocytes. Previous studies have reported that higher neutrophil counts before thrombolysis for cerebral ischemia can result in poor outcome and degree of stroke severity.[8,9] The platelet, another well-known parameter, reflects platelet consumption, thrombopoiesis, and senescence for a constant balance of platelets.[3,9] Recently, several new WBC-based inflammatory indices namely, neutrophil-to-lymphocyte ratio (NLR),[9,10] platelet-to-lymphocyte ratio (PLR),[11,12] and systemic immune inflammation index (SII),[13] have been introduced as prognostic markers.
NLR and PLR represent the peripheral neutrophil and lymphocyte counts and the peripheral platelet and lymphocyte counts; respectively, and SII integrates the lymphocyte, neutrophil, and platelet counts into an index. This study aims to evaluate the prognostic values of NLR, PLR, and SII upon ED admission to predict neurological recovery in acute cerebrovascular events over 1-year follow-up. The significance of these parameters has not been studied much in relation to the functional outcome especially in patients with AIS within 3 hours.
2. Material and methods
2.1. Study population
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The institutional review board (IRB) of Taipei Veterans General Hospital (VGH) approved the study (IRB: 2019–04–009BC) and waived the requirement for informed consent because it was a retrospective, registry-based study. Adult patients presented to the ED diagnosed with AIS within 3 hours from January 2016 to December 2018 in the Taipei VGH, Taiwan was recruited retrospectively by trained nurses. The following items were recorded for each patient: patient demographic data, medical history, vital signs, initial National Institutes of Health Stroke Scale (NIHSS), laboratory values, radiographic images, and treatment provided. The follow-up information after hospital discharge was collected through phone interviews with patients or caregivers by a trained stroke case manager. This study did not include healthy controls.
2.2. Functional outcome
The modified Rankin Scale (mRS) was used to assess the degree of disability or dependence upon ED admission and 1, 3, 6, and 12 months follow-up after a stroke. The score ranged from 0 (no disability) to 6 (death), and each subsequent level indicated progressive functional impairment.[14] The primary outcome measure was the neurological recovery, which was defined as an improvement of mRS ≥ 1 score compared with ED admission baseline over a 12 months follow-up.
2.3. Laboratory measurement
Blood samples for routine blood tests were drawn prior to any treatment upon ED arrival. The CBC, including WBCs, neutrophil counts, lymphocyte counts, and platelet counts, was determined using the Beckman Counter UniCel DxH800 Automated Hematology Analyzer (Beckman Coulter, Inc, Miami, FL). The hemoglobin A1c and the lipid profile (i.e., total cholesterol, triglyceride, LDL cholesterol, and HDL cholesterol) were obtained using the Cobas 8000 modular analyzer (Roche Diagnostics, Penzberg, Germany), which used the enzymatic colorimetric analysis.
The NLR, the PLR, and the SII were determined and calculated as follows: NLR = neutrophil counts/lymphocyte counts, PLR = platelet counts/lymphocyte counts, and SII = platelet counts × neutrophil counts/lymphocyte counts.
2.4. Sankey diagram illustration
The transition of patient functional neurological outcomes (i.e., improved, remained, and worse) based on its mRS score change over 12 months postdischarge compared with the ED admission baseline was illustrated using the Sankey diagram (http://sankeymatic.com/).[15,16] Each node in the graph presented the mRS score. The links represented the transitions of each mRS score in relation to the clinical reality. The height of nodes and links in the Sankey diagram represented the relative number of patients that shared the mRS scores and transition, respectively. Hovering the time over a specific node or a link showed the exact mRS score of observations in that flow. The widths of the bands were linearly proportional to the neurological recovery. The color was mapped to the relative proportion of patients that presented with an outcome of mRS. Yellow, green, and red colors were used to distinguish the patient's neurological recovery and represented improved, remained, and worse, respectively. Our overall visual design followed the principle of the Sankey diagram, which visualized the transfers between mRS scores with lines and quantiles by line width.
2.5. Statistical analysis
Descriptive statistics were reported as mean ± standard deviation for continuous variables. The groups were compared using the Mann–Whitney U test for numerical data and the Pearson χ2 test for categorical data. Logistic regression models were used to explore independent predictors of neurological recovery. Univariate analyses were performed separately for each factor to ascertain the odds ratio (OR) and 95% confidence interval (CI). All biologically plausible variables with P < .20 in the univariate analysis were included in the logistic regression model using a backward selection process during the multivariate analysis. Regression testing was performed on neurological recovery vs NLR, PLR, and SII values independently. The receiver operating characteristic (ROC) curve was applied to determine the capacity to predict functional neurological outcome improvement for different variables. All statistical analyses were completed using the R (version 3.5.1) software, and a 2-tailed P < .05 was considered significant.
3. Results
3.1. Patient characteristics
A total of 277 consecutive adult patients with AIS within 3 hours from onset of symptoms admitted to our ED were included in this study. The patients had a mean age of 73.2 ± 13.4 years, and 56.6% of the patients were males. The initial average of NIHSS was 9.2 ± 7.8, and 90.3% (250/277) of the patients had mRS ≥ 2 at the ED baseline. IV-rtPA, IA thrombolysis, and IV-rtPA combined with IA endovascular management were administered to 103 (37.2%), 55 (19.9%), and 35 (12.6%) patients, respectively. Moreover, 30.3% (84/277) of the patients had neither thrombolysis nor thrombectomy treatment. The demographic and the clinical characteristics of patients with AIS within 3 hours are summarized in Table 1.
Table 1.
Clinical and biological data at admission in acute ischemic stroke patients within 3 hours.
| All patients (n = 277) | |
| Age, y | 73.2 ± 13.4 |
| Gender | |
| Male, N (%) | 157 (56.6%) |
| Female, N (%) | 120 (43.4%) |
| Clinical data | |
| NIHSS | 9.2 ± 7.8 |
| mRS ≥ 2, N (%) | 250 (90.3%) |
| Current cigarette smoking, N (%) | 76 (27.4%) |
| Current alcohol drinking, N (%) | 46 (16.6%) |
| Systolic blood pressure, mm Hg | 159.9 ± 30.9 |
| Diastolic blood pressure, mm Hg | 88.2 ± 19.7 |
| Diabetes mellitus, N (%) | 79 (28.5%) |
| Heart disease, N (%) | 98 (35.4%) |
| Dyslipidemia, N (%) | 137 (49.5%) |
| Initial laboratories | |
| Triglyceride, mg/dL | 123.0 ± 95.3 |
| Total cholesterol, mg/dL | 173.3 ± 79.1 |
| LDL-cholesterol, mg/dL | 104.3 ± 38.4 |
| HDL-cholesterol, mg/dL | 44.4 ± 14.6 |
| HbA1C, % | 6.3 ± 1.3 |
| Prothrombin time, INR | 1.1 ± 0.2 |
| White blood cell count, ×103/μL | 8.1 ± 2.9 |
| Platelete count, ×103/μL | 214.1 ± 74.9 |
| Neutrophil count, ×103/μL | 5.3 ± 2.8 |
| Lymphocyte count, ×103/μL | 2.0 ± 11.0 |
| Monocyte count, ×103/μL | 0.6 ± 0.2 |
| Reperfusion therapy | |
| IV-rtPA, N (%) | 103 (37.2%) |
| IA thrombolysis, N (%) | 55 (19.9%) |
| IV-rtPA + IA endovascular management, N (%) | 35 (12.6%) |
NIHH = National Institute of Health Stroke Scale, mRS = modified Rankin Scale, HBA1C = hemoglobin A1c, INR = international normalized ratio, IV-rtPA = intra-venous recombinant tissue plasminogen activators, IA = intra-arterial.
3.2. Changes in the functional scales 1 year after acute cerebrovascular events
Figure 1 shows the changes in the functional scales 1 year after acute cerebrovascular events. The overall neurological recovery rates of 48.7%, 53.7%, 59.2%, and 55.9% were observed at 1, 3, 6, and 12 months follow-up, respectively. In the subgroup analysis, at 1, 3, 6, and 12 months follow-up, the neurological recovery rates were 60.1%, 60.1%, 65.0%, and 64.0%, respectively, for patients who received the IV-rtPA treatment; 32.7%, 41.8%, 49.1%, and 43.6%, respectively, for patients who received the IA thrombolysis; and 42.8%, 42.8%, 51.4%, and 51.4%, respectively, for patients who received the IV-rtPA combined with IA endovascular management.
Figure 1.
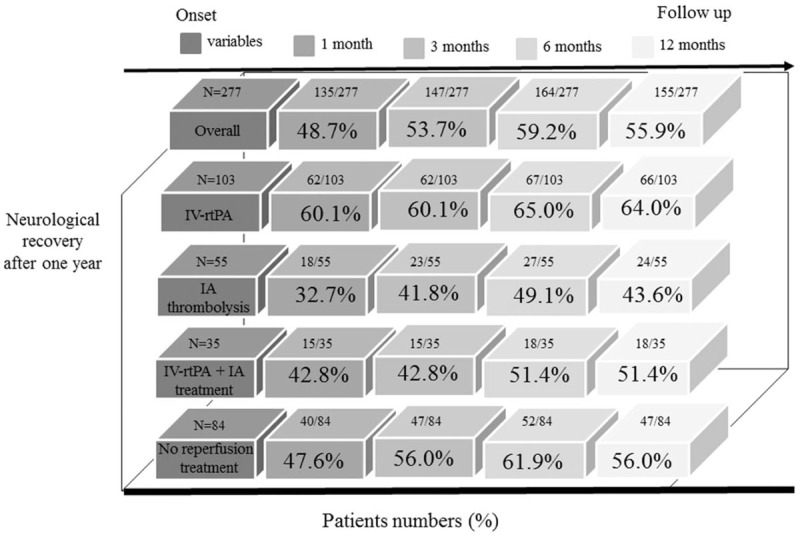
The numbers and percentages of neurological recovery in AIS patients 1 year after a stroke. Neurological recovery was defined as an improvement of mRS ≥ 1 score compared to ED admission baseline.
The Sankey configurations presented that many patients with AIS within 3 hours had high mRS scores in the beginning of stroke onset. Two trends in this map were observed after the postischemic stroke. Patients with AIS had more chances of neurological recovery within 3 months. Minimal improvement in mRS scores were observed at 6 and 12 months poststroke (Fig. 2).
Figure 2.
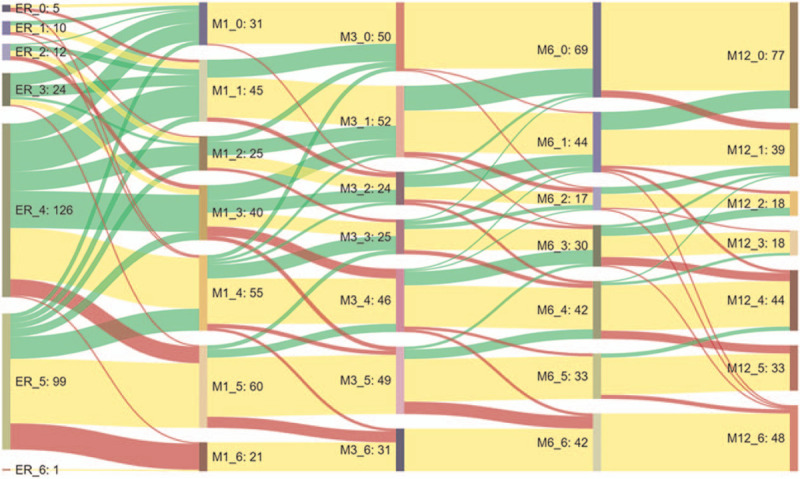
Sankey diagram for the visualization of temporal patterns for AIS patient and its mRS change over time. Yellow, green, and red colors were used to distinguish patient's neurological recovery: improved, remained, and worse, respectively.
3.3. Baseline NLR, PLR, and SII values and neurological recovery in patients with AIS over 1-year poststroke follow-up
Baseline NLR values (2.9 ± 1.9 vs 4.2 ± 4.0, P = .004) were significantly lower in patients with AIS and neurological recovery at 3 months poststroke only. However, no difference was observed in PLR and SII values upon admission between patients with AIS and neurological recovery and patients with no improvement at 1, 3, 6, and 12 months poststroke. The multivariate analysis revealed that baseline NLR values were significant predictors of neurological recovery at 3 months after stroke (adjusted OR = 0.89, 95% CI = 0.80–0.99, P = .035; Table 2). Initial NIHSS, age, gender, and IV-rtPA treatment were also significantly associated with neurological recovery at 3 months after stroke. The capacity of baseline NLR values to predict the presence from the absence of neurological recovery at 3 months poststroke was assessed using the ROC curve analysis. The area under the ROC curve (AUC) for NLR was 0.57 (95% CI = 0.54–0.60).
Table 2.
Logistic regression analysis of predictors of neurological recovery at 3 month poststroke follow-up in this cohort.
| Univariable analysis | Multivariable analysis | |||
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| NIHSS | 0.932 (0.903–0.962) | <.001 | 0.932 (0.894–0.971) | .001∗ |
| Age | 0.928 (0.907–0.950) | <.001 | 0.938 (0.916–0.961) | <.001∗ |
| Sex, male | 2.390 (1.469–3.887) | <.001 | 1.863 (1.057–3.283) | .031∗ |
| NLR | 0.876 (0.800–0.959) | .004 | 0.893 (0.804–0.992) | .035∗ |
| PLR | 0.997 (0.994–1.000) | .063 | ||
| SII | 1.000 (0.999–1.000) | .071 | ||
| Systolic blood pressure | 0.987 (0.987–1.001) | .110 | ||
| Diastolic blood pressure | 1.003 (0.992–1.015) | .562 | ||
| Diabetes mellitus | 0.701 (0.416–1.181) | .182 | ||
| Heart disease | 0.773 (0.472–1.268) | .308 | ||
| High-density lipoprotein | 0.986 (0.963–1.010) | .258 | ||
| Low-density lipoprotein | 1.007 (1.000–1.015) | .046 | ||
| IV-rtPA | 1.512 (0.923–2.478) | .101 | 2.073 (1.078–3.986) | .029∗ |
| IA thrombolysis | 0.548 (0.301–0.996) | .048 | ||
| IV-rtPA + IA endovascular management | 0.604 (0.295–1.237) | .168 | ||
NIHSS = National Institutes of Health Stroke Scale, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio, SII = systemic immune inflammation index, IV-rtPA = intra-venous recombinant tissue plasminogen activators, IA = intra-arterial.
P < .05.
4. Discussion
This study has investigated the effect of baseline NLR, PLR, and SII values in predicting the neurological recovery of patients with AIS within 3 hours over 1-year follow-up. The main findings are as follows:
-
1.
The represented long-term follow-up confirms the importance of the first 90 days for functional outcome improvement in patients with AIS. Improvement is observed within the first 3 months and can take up to a year or even longer.
-
2.
The baseline NLR value is an independent predictor of neurological recovery at 3 months after a stroke.
-
3.
The Sankey configurations are helpful in improving the long-term functional outcome change clarity.
A previous study shows that the survival time of 30 to 60 minutes of occlusion results in a steady increase in the temporal infiltration of neutrophils.[1] Blood samples are drawn from our ED prior to any treatment, which means that the study is conducted at ideal conditions to evaluate the influence of neutrophils, lymphocytes, and platelets at baseline in response to acute cerebrovascular events. The difference in hematological parameters may fluctuate due to the timing and frequency of data collection, thereby influencing the accuracy of prediction.
After the AIS, the computerized tomography (CT) scan and magnetic resonance imaging may play as predictors of functional outcomes. Johnston et al have shown that the CT scan can determine the 3-months excellent outcome in patients with AIS with AUC of 0.70.[17] The results of their study rely on the CT imaging evaluation, which may be influenced by different radiographic interpreters. However, the CBC parameters can easily predict functional outcome changes after a stroke. In the present study, these 3 hematological inflammatory biomarkers with the advantage of free available in the routine blood test are evaluated.[13,18] Previous literature has demonstrated that these inflammatory markers may be correlated with the initial stage of AIS and may predict the severity of stroke.[9,19–21] Our results show that the NLR values at admission are significantly negatively correlated with clinical outcome improvement in patients with AIS at the first 90 days poststroke. These results agree with those of previous studies.[9,19–21] Nevertheless, the AUC ranges from 0.5 to 0.6, indicating that these hematological prognostic biomarkers are not good.
The reperfusion therapy with IV-rtPA or IA endovascular management improves functional outcomes after AIS, but the benefits of these treatment modalities are highly dependent to time.[6,22,23] The IV-rtPA can destroy blood clots. However, the narrow therapeutic window (3 hours from symptom onset) and the strict patient eligibility criteria all limit its use. The IA thrombectomy can afford good results in the first 6 to 12 hours from the symptom onset.[22] Our patients with AIS within 3 hours eligible for IV-rtPA treatment have more favorable neurological recovery over 1-year follow-up. Furthermore, patients with large vessel occlusion receiving IV-rtPA thrombolysis combined with IA thrombectomy has more than 50% chance of 6 and 12 months functional outcome improvement.
In this study, an innovative approach for the visualization of temporal patterns for AIS and the change in functional outcomes over time is demonstrated. Our results show that the Sankey configuration is helpful in improving the long-term functional outcome change clarity. The map visualizes the data by classifying into mRS scores to represent the neurological recovery over time. Patients with AIS have more chances of neurological recovery within 3 months after a stroke. Minimal improvement in mRS scores is observed at 6 to 12 months poststroke. Therefore, with the help of the Sankey diagrams, a unique visualization technique can provide authentic and interesting disease spectra over time.
After a stroke, patients may develop immune responses against their own brain tissue. When ischemia happens, which leads to brain cell death, damage-associated molecular patterns (DAMPs) are released. These DAMPs trigger inflammatory response, along with the subsequent increase of inflammatory biomarkers, such as NLR.[9,10] The NLR levels of nonimprovement AIS in our data are higher than that in patients with neurological recovery. The activation of inflammation may be necessary to repair the cerebral injuries after the stroke onset. However, the overactivation of inflammation can cause harm to brain tissues, resulting in neurological deterioration, reduced circulating lymphocytes, and increased risk of infection.[24] The entry of inflammatory cells into the bloodstream triggers platelet-mediated recognition, which is amplified by cell surface receptors and immune cells. The inflammation markers NLR and PLR may reflect the imbalance of overactive inflammation and the protective regulation rather than using indices alone. The association between SII values and patients with AIS is further explored. Our study is the first to highlight the role of NLR, PLR, and SII at admission to predict the change in functional outcomes over 1-year poststroke.
It has been known that infarct volume may be closely related with initial neurological deficits and serve as a predictor of functional outcome in patients with AIS.[25] The Alberta Stroke Program Early CT Score (ASPECT) is a 10-point quantitative tool used the summed score for functional outcome estimation, but does not account for the stroke location.[26,27] Phan et al had proved the concept of relating infarct location to stroke disability using the method of partial least squares with penalized logistic regression.[27] However, in patients with AIS, the relationship between stroke volume, location, and functional recovery remains controversial and challenging to compare across studies. In addition, low serum 25-Hydroxyvitamin D is a common condition in patients with AIS, and several studies suggested that it may be associated with a poor prognosis.[28,29] Vitamin D deficiency-associated larger infarct volume, cognitive decline, activation of inflammatory pathways, leukoaraiosis, and derangement of bone metabolism were plausible mechanisms of poor outcome in patients with AIS.[29]
Several limitations of this study need to be addressed. First, this study was conducted using registry data and subject to the limitations of any retrospective analysis. Second, we only gathered NLR, PLR, and SII data at ED admission. Studies showed that these biomarkers could fluctuate in postischemic brain injury and repair. Serial changes in these data may give more information and increased accuracy. Third, this study focused on patients with AIS within 3 hours. Our results could not be applicable in other types of stroke. Fourth, the parameters related to etiology, severity, and treatment might have more effect on poststroke outcome than inflammatory indices. Limitations remain present in our study even though we attempted to compensate for all the possible biases by adjusting for all known confounders that may have influenced our results in a multivariate regression model. Finally, the neurological recovery largely depended on poststroke care standards and rehabilitation adherence. The patient compliance may influence the prognostic values of the various parameters for long-term outcomes in ischemic stroke.
5. Conclusion
Our cohort study showed that a low NLR at ED admission could be useful marker for predicting neurological recovery at 3 months after stroke. The Sankey configurations were helpful in improving the long-term functional outcome change clarity.
Acknowledgments
The authors thank stroke case managers of Taipei Veterans General Hospital, Taiwan, for their assistance with the data collection.
Author contributions
Conceptualization: Li-Hua Li, Chung-Ting Chen, Chorng-Kuang How.
Data curation: Li-Hua Li, Chung-Ting Chen, Yun-Chin Chang, Ying-Ju Chen.
Formal analysis: Li-Hua Li, Chung-Ting Chen, I-Hui Lee, Chorng-Kuang How.
Investigation: Li-Hua Li, Yun-Chin Chang, Ying-Ju Chen.
Methodology: Li-Hua Li, Chung-Ting Chen.
Supervision: I-Hui Lee, Chorng-Kuang How.
Writing – original draft: Li-Hua Li, Chung-Ting Chen, Chorng-Kuang How.
Writing – review & editing: Yun-Chin Chang, Ying-Ju Chen, I-Hui Lee.
Footnotes
Abbreviations: AIS = acute ischemic stroke, AUC = area under the ROC curve, CBC = complete blood count, CT = computerized tomography, CI = confidence interval, ED = emergency department, IV-rtPA = intravenous recombinant tissue plasminogen activators, IA = intra-arterial, mRS = modified Rankin Scale, NIHSS = National Institutes of Health Stroke Scale, NLR = neutrophil-to-lymphocyte ratio, OR = odds ratio, PLR = platelet-to-lymphocyte ratio, ROC = receiver operating characteristic, SII = systemic immune inflammation index, WBCs = white blood cells.
How to cite this article: Li LH, Chen CT, Chang YC, Chen YJ, Lee IH, How CK. Prognostic role of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune inflammation index in acute ischemic stroke: a STROBE-compliant retrospective study. Medicine. 2021;100:25(e26354).
The data that support the findings of this study are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Grønberg NV, Johansen FF, Kristiansen U, et al. Leukocyte infiltration in experimental stroke. J Neuroinflamm 2013;10:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jiang X, Andjelkovic AV, Zhu L, et al. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol 2018;163-164:144–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jayaraj RL, Azimullah S, Beiram R, et al. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflamm 2019;16:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105:1135–43. [DOI] [PubMed] [Google Scholar]
- [5].Hsieh CY, Tsao WC, Lin RT, et al. Three years of the nationwide post-acute stroke care program in Taiwan. J Chin Med Assoc 2018;81:87–8. [DOI] [PubMed] [Google Scholar]
- [6].Li We, Yin Q, Xu G, et al. Treatment strategies for acute ischemic stroke caused by carotid artery occlusion. Interv Neurol 2016;5:148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol 2006;5:603–12. [DOI] [PubMed] [Google Scholar]
- [8].Maestrini I, Strbian D, Gautier S, et al. Higher neutrophil counts before thrombolysis for cerebral ischemia predict worse outcomes. Neurology 2015;85:1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim J, Song TJ, Park JH, et al. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis 2012;222:464–7. [DOI] [PubMed] [Google Scholar]
- [10].Wang L, Song Q, Wang C, et al. Neutrophil to lymphocyte ratio predicts poor outcomes after acute ischemic stroke: a cohort study and systematic review. J Neurol Sci 2019;406:116445. [DOI] [PubMed] [Google Scholar]
- [11].Akkaya E, Gul M, Ugur M. Platelet to lymphocyte ratio: a simple and valuable prognostic marker for acute coronary syndrome. Int J Cardiol 2014;177:597–8. [DOI] [PubMed] [Google Scholar]
- [12].Altintas O, Altintas MO, Tasal A, et al. The relationship of platelet-to-lymphocyte ratio with clinical outcome and final infarct core in acute ischemic stroke patients who have undergone endovascular therapy. Neurol Res 2016;38:759–65. [DOI] [PubMed] [Google Scholar]
- [13].Fest J, Ruiter R, Ikram MA, et al. Reference values for white blood-cell-based inflammatory markers in the Rotterdam study: a population-based prospective cohort study. Sci Rep 2018;8:10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wilson JT, Hareendran A, Grant M, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke 2002;33:2243–6. [DOI] [PubMed] [Google Scholar]
- [15].Mica L, Niggli C, Bak P, et al. Development of a visual analytics tool for polytrauma patients: proof of concept for a new assessment tool using a multiple layer Sankey diagram in a single-center database. World J Surg 2020;44:764–72. [DOI] [PubMed] [Google Scholar]
- [16].Schmidt M. The Sankey diagram in energy and material flow management. J Ind Ecol 2008;12:82–94. [Google Scholar]
- [17].Johnston KC, Wagner DP, Haley EC, Jr, et al. Combined clinical and imaging information as an early stroke outcome measure. Stroke 2002;33:466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vis JY, Huisman A. Verification and quality control of routine hematology analyzers. Int J Lab Hematol 2016;38:100–9. [DOI] [PubMed] [Google Scholar]
- [19].Buck BH, Liebeskind DS, Saver JL, et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke 2008;39:355–60. [DOI] [PubMed] [Google Scholar]
- [20].Akil E, Akil MA, Varol S, et al. Echocardiographic epicardial fat thickness and neutrophil to lymphocyte ratio are novel inflammatory predictors of cerebral ischemic stroke. J Stroke Cerebrovasc Dis 2014;23:2328–34. [DOI] [PubMed] [Google Scholar]
- [21].Lim HH, Jeong IH, An GD, et al. Early prediction of severity in acute ischemic stroke and transient ischemic attack using platelet parameters and neutrophil-to-lymphocyte ratio. J Clin Lab Anal 2019;33:e22714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jovin TG, Chamorro E, Cobo E, et al. REVASCAT trial investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–306. [DOI] [PubMed] [Google Scholar]
- [23].National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–7. [DOI] [PubMed] [Google Scholar]
- [24].Liesz A, Suri-Payer E, Veltkamp C, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 2009;15:192–9. [DOI] [PubMed] [Google Scholar]
- [25].Schiemanck SK, Kwakkel G, Post MW, et al. Predictive value of ischemic lesion volume assessed with magnetic resonance imaging for neurological deficits and functional outcome poststroke: a critical review of the literature. Neurorehabil Neural Repair 2006;20:492–502. [DOI] [PubMed] [Google Scholar]
- [26].Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000;355:1670–4. [DOI] [PubMed] [Google Scholar]
- [27].Phan TG, Demchuk A, Srikanth V, et al. Proof of concept study: relating infarct location to stroke disability in the NINDS rt-PA trial. Cerebrovasc Dis 2013;35:560–5. [DOI] [PubMed] [Google Scholar]
- [28].Tu WJ, Zhao SJ, Xu DJ, et al. Serum 25-hydroxyvitamin D predicts the short-term outcomes of Chinese patients with acute ischaemic stroke. Clin Sci (Lond) 2014;126:339–46. [DOI] [PubMed] [Google Scholar]
- [29].Park KY, Chung PW, Kim YB, et al. Serum vitamin D status as a predictor of prognosis in patients with acute ischemic stroke. Cerebrovasc Dis 2015;40:73–80. [DOI] [PubMed] [Google Scholar]


