Abstract
The aim of this study was to identify viral exposure (VE) measures and their relationship to mortality risk among persons with HIV.
Prospective multicenter observational study to compare VE formulae.
Eligible participants initiated first combination antiretroviral therapy (cART) between March 1, 1995 and June 30, 2015. We included 1645 participants followed for ≥6 months after starting first cART, with cART prescribed ≥75% of time, who underwent ≥2 plasma viral load (VL) and ≥1 CD4+ T-lymphocyte cell (CD4) measurement during observation. We evaluated all-cause mortality from 6 months after cART initiation until June 30, 2016. VE was quantified using 2 time-updated variables: viremia copy-years and percent of person-years (%PY) spent >200 or 50 copies/mL. Cox models were fit to estimate associations between VE and mortality.
Participants contributed 10,453 person years [py], with median 14 VLs per patient. Median %PY >200 or >50 were 10% (interquartile range: 1%–47%) and 26% (interquartile range: 6%–72%), respectively. There were 115 deaths, for an overall mortality rate of 1.19 per 100 person years. In univariate models, each measure of VE was significantly associated with mortality risk, as were older age, public insurance, injection drug use HIV risk history, and lower pre-cART CD4. Based on model fit, most recent viral load and %PY >200 copies/mL provided the best combination of VE factors to predict mortality, although all VE combinations evaluated performed well.
The combination of most recent VL and %PY >200 copies/mL best predicted mortality, although all evaluated VE measures performed well.
Keywords: cumulative HIV viral exposure, HIV viremia, mortality, viremia copy-years
1. Introduction
The use of plasma HIV RNA levels (viral load [VL]) to assess response to combination antiretroviral treatment (cART) and HIV disease progression risk is well established and a standard of care for persons living with HIV (PWH).[1–7] Achievement and maintenance of VL suppression on cART have been associated with reduction in mortality risk, other clinical benefits, and with marked reduction in risk for sexual transmission of HIV.[8–15] Since virologic nonsuppression occurs, at least intermittently, in many PWH receiving long-term cART, considerable[16–18] interest has emerged in recent years to develop longer-term within-person HIV virologic exposure (VE) measures that correlate better with important clinical endpoints than peripheral blood VL assessments taken at a single time point.[18] Although “most recent” measures of VL levels have been used in epidemiologic research and clinically to generate estimates of overall viral suppression and ART efficacy across populations[19] as well as to generate estimates of long-term risk for death, such measures are cross-sectional and do not account for multiple VL measures obtained as part of clinical care for PWH over time periods spanning years.
Principal among the longitudinal measures of VE that have been studied to assess so-called “cumulative viremia” are: viremia copy-years[20–22]; and estimated percentage of person-time under observation spent with a VL above a threshold value.[23] To date, robust associations between various longer-term VE measures and important clinical endpoints such as death,[20,24–27] cardiovascular disease (CVD),[28] and cancer[29] have been reported from several cohorts of PWH, and VE measures have also informed estimation of risk of HIV transmission by PWH in care.[30,31]
In the present report we sought to evaluate several different individual measures of longitudinal VE and multiple combinations of measures of VE to assess comparatively their ability to predict mortality. Participants were from the large, multicenter, prospectively followed, diverse group of cART-treated adults with HIV who participate in the Centers for Disease Control and Prevention-sponsored HIV Outpatient Study (HOPS).
2. Methods
2.1. HIV outpatient study
We conducted an observational prospective cohort study utilizing data from an ongoing longitudinal cohort of >10,000 adults with HIV (aged 18 years and older), seen in HIV specialty clinics since 1993.[32] The reporting guidelines for STROBE were used in the design and implementation of our research (https://www.strobe-statement.org). Patient data were collected from electronic medical records. This study was conducted at 12 clinics located in 9 US cities: Tampa, FL; Washington, DC; Denver, CO (3 sites); Chicago, IL (2 sites); Stony Brook, NY; Oakland, CA; Walnut Creek, CA; Portland, OR; and Philadelphia, PA.
2.2. Study procedures
We analyzed medical records data of ART-naive HOPS participants seen at 12 US HIV clinics who initiated first cART between March 1, 1995 and June 30, 2015. Abstracted information included: demographic characteristics, risk factors for HIV infection, diagnoses, prescribed medications, laboratory values (including CD4+ T-lymphocyte cell counts/mm3 [CD4] and plasma HIV RNA copies/mL3), mortality, and hospitalization records (primarily from discharge summaries). All HOPS clinicians have extensive experience treating HIV-infected patients. Information was abstracted from outpatient charts and/or electronic medical records at each visit, entered electronically by trained staff, compiled centrally, reviewed, and cleaned before being analyzed.
2.3. Study population
For this analysis, we selected 1645 HOPS participants who had complete antiretroviral history, and were followed for ≥6 months after starting first cART, had cART prescribed ≥75% of time, and underwent ≥2 plasma VL and ≥1 CD4 measurements during observation. Observation began at baseline, continued until death (if documented within 183 days of last patient contact), and was censored (patient assumed alive) at last patient contact plus 183 days if the patient was not known to be deceased by then, or at 31 December 2016, whichever occurred first. We used a HOPS dataset available as of June 30, 2017.
2.4. Outcome and predictor variables
We evaluated all-cause mortality from 6 months after cART initiation until June 30, 2016. During the period of this analysis, the HOPS sites routinely used the US Social Security Death Index to ascertain which patients were deceased. Cumulative viremia measures accrued after 6 months of cART (ie, the baseline date in the analysis). VE was quantified using 2 time-updated variables: viremia copy-years (VCY) and percent of person-years (%PY) spent >200 or 50 copies/mL. For VLs with lower limit of detection >50 copies/mL, we imputed VL values, separately for persons alive and deceased at observation end.
HIV transmission risk group was defined using the following hierarchy: persons who inject drugs; then gay, bisexual, and other men who have sex with men (MSM); and persons whose only self-reported risk was heterosexual contact. Health insurance payor was characterized as private, public, or none/unknown/other. CD4 values analyzed were those closest to ART initiation, within a window of six months prior to three months after.
2.5. Ethics statement
The HOPS protocol has been approved and renewed annually by each participating institution's ethical review board and by the Centers for Disease Control and Prevention Institutional Review Board. All study participants have provided written, informed consent.
2.6. Statistical analyses
In descriptive analyses, Wilcoxon rank-sum tests were used to compare distributions for continuous variables, Yates-corrected χ2 test for categorical variables. We fit 9 Cox models to estimate associations between VE and mortality. For both unadjusted and adjusted Cox regression models, results are reported using hazard ratios and 95% confidence intervals. Akaike information criterion scores were used to compare models (lower score = better fit). The 9 models were created using the 4 measures of viremia: most recent log10 viral load, VCY, %PY >50 and >200 copies/mL. Four models had one of these measures, and 5 models had 2 of the measures. For the 2-measure models, only 1 of the 2%PY measures was selected for each 2-measure model. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Patient selection and characteristics
Table 1 profiles demographics of the analysis cohort. Of 10,818 participants in the HOPS as of June 30, 2017, there were 4989 whose first ART regimen was considered to be cART, 3568 with complete ART information recorded, 2122 started first cART regimen while under observation in the HOPS, 1976 had at least 6 months of observation while taking their first cART regimen, and 1645 with a CD4 cell count within 90 days of observation start as well as at least 2 viral load tests recorded during observation (Fig. 1).
Table 1.
Baseline cohort characteristics, overall and by vital status at the end of observation, the HIV Outpatient Study, 1995–2016 (N = 1645).
| Characteristics∗ median (IQR) or N (column %) | Overall (n = 1645) | Deceased (n = 115) | Not deceased (n = 1530) | P† |
| Baseline age, y | 38 (31–45) | 44 (38–52) | 37 (31–45) | <.001 |
| Sex | .89 | |||
| Male | 1289 (78.4) | 89 (77.4) | 1,200 (78.4) | |
| Female | 356 (21.6) | 26 (22.6) | 330 (21.6) | |
| Race/ethnicity | .008 | |||
| Non-Hispanic/Latino white | 743 (45.2) | 38 (33.0) | 705 (46.1) | |
| Non-Hispanic/Latino black | 606 (36.8) | 55 (47.8) | 551 (36.0) | |
| Hispanic/Latino ethnicity | 224 (13.6) | 20 (17.4) | 204 (13.3) | |
| Other race/ethnicity | 72 (4.4) | 2 (1.7) | 70 (4.6) | |
| HIV risk activity | <.001 | |||
| MSM | 929 (56.5) | 34 (29.6) | 895 (58.5) | |
| PWID | 90 (5.5) | 24 (20.9) | 66 (4.3) | |
| Heterosexual | 525 (31.9) | 49 (42.6) | 476 (31.1) | |
| Unknown/other risk | 101 (6.1) | 8 (7.0) | 93 (6.1) | |
| Payer | <.001 | |||
| Private | 882 (53.6) | 45 (39.1) | 837 (54.7) | |
| Public | 506 (30.8) | 57 (49.6) | 449 (29.4) | |
| None/unknown/other | 257 (15.6) | 13 (11.3) | 244 (15.9) | |
| CD4+ cell count (cells/mm3), pre-cART | 293 (140–460) | 144 (40–274) | 308 (154–473) | <.001 |
| Observation duration, median y | 5.2 (2.5–9.1) | 4.3 (2.0–8.6) | 5.2 (2.5–9.2) | .07 |
cART = combination antiretroviral therapy, IQR = interquartile range, MSM = men who have sex with men, PWID = persons who inject drugs.
Characteristics are summarized at baseline (defined as the date 6 months after cART initiation), unless otherwise indicated.
Wilcoxon rank-sum tests for continuous variables, Yates-corrected chi-square test for categorical variables comparing characteristics of deceased and nondeceased participants.
Figure 1.
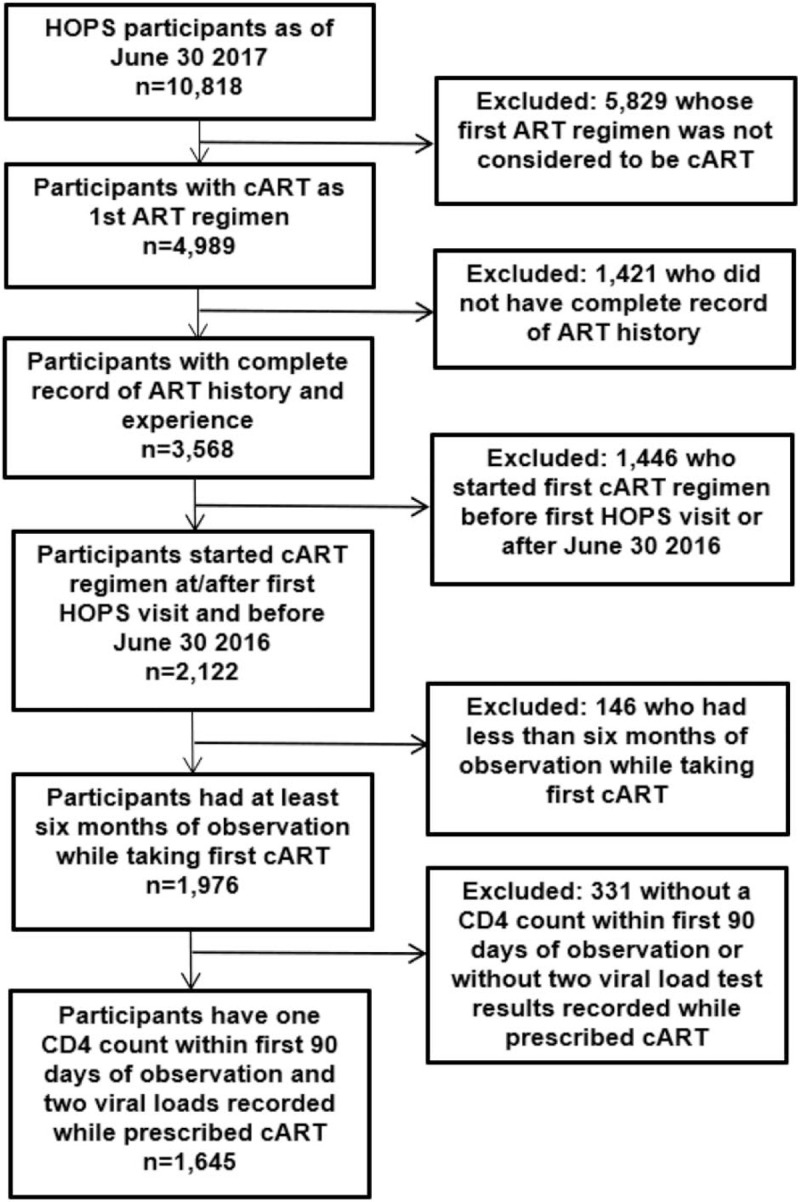
Analysis selection steps, the HIV Outpatient Study, 1995–2016.
There were 115 deaths recorded, for an overall mortality rate of 1.19 per 100 person years. Compared to nondecedents, deceased persons were more often older at baseline, non-Hispanic/Latino black (compared to non-Hispanic/Latino white), had history injection drug use (IDU) or heterosexual (vs MSM) risk for HIV acquisition, had public (vs private) sources of health care payment at baseline, and had lower pre-cART CD4 counts (Table 1).
3.2. Viral load measures
The 1645 participants contributed 10,453 person years, with a median of 14 VL measurements (interquartile range: 7–24) per patient. For the 27,763 VL measurements recorded during observation, if the lower limit of detection was >50 copies/mL and the test result was undetectable, VL values were imputed; this was the case for 1232 (4.4%) measurements. Overall, the median VCY for all participants was 3.0 (interquartile range: 2.3–4.2) log10 copy-years during the observation. Related to %PY above a given VL threshold, participants spent a median of 9.7% of person-years with VL >200 copies/mL and a median of 25.5% of person-years with VL >50 copies/mL during 1995 to 2017, with substantial variability across patient subgroups (Table 2). By all 3 evaluated measures of VE, women had greater VE than men, non-Hispanic blacks greater than non-Hispanic whites, persons with IDU risk and heterosexuals more than MSM, and decedents much greater than nondecedents. Persons with public insurance at baseline versus privately insured persons had significantly greater VE when measuring %PY >200 copies/mL and %PY >50 copies/mL (P < .001) but not VCY (P = .42) (Table 2).
Table 2.
VCY and %PY with viral load >200 or >50 copies/mL by participant characteristics, the HIV Outpatient Study, 1995–2016 (N = 1645).
| Characteristics∗ median (IQR), or N (column %) | Overall (n = 1645) | VCY, log10 copy-years | P | %PY with viral load >200 copies/mL | P | %PY with viral load > 50 copies/mL | P† |
| All participants | 1645 (100.0) | 3.0 (2.3–4.2) | 9.7 (0.9–46.7) | 25.5 (6.0–72.0) | |||
| Age, y | <.001 | <.001 | <.001 | ||||
| <35 | 630 (38.3) | 3.0 (2.2–4.3) | 12.4 (0.8–58.8) | 29.0 (7.2–80.1) | |||
| 35–45 | 557 (33.9) | 3.2 (2.4–4.4) | 12.2 (1.8–45.8) | 27.7 (8.4–76.8) | |||
| >45 | 458 (27.8) | 2.7 (2.1–3.7) | 6.1 (0.0–28.7) | 19.1 (3.8–52.2) | |||
| Sex | .019 | <.001 | <.001 | ||||
| Male | 1289 (78.4) | 3.0 (2.2–4.1) | 8.0 (0.6–41.3) | 23.6 (5.5–67.7) | |||
| Female | 356 (21.6) | 3.1 (2.4–4.5) | 18.0 (2.4–63.1) | 35.9 (9.1–86.7) | |||
| Race/ethnicity | <.001 | <.001 | <.001 | ||||
| Non-Hispanic/Latino white | 743 (45.2) | 3.0 (2.2–4.1) | 6.2 (0.7–30.6) | 20.1 (4.4–58.0) | |||
| Non-Hispanic/Latino black | 606 (36.8) | 3.1 (2.4–4.5) | 18.0 (2.3–66.4) | 35.9 (12.6–86.8) | |||
| Hispanic/Latino ethnicity | 224 (13.6) | 2.9 (2.2–4.2) | 9.0 (0.3–41.8) | 24.1 (4.7–66.4) | |||
| Other race/ethnicity | 72 (4.4) | 2.5 (2.1–3.5) | 5.3 (0.0–21.1) | 17.4 (3.6–40.9) | |||
| HIV risk activity | <.001 | <.001 | <.001 | ||||
| MSM | 929 (56.5) | 2.9 (2.2–4.0) | 6.0 (0.5–30.7) | 19.1 (4.4–56.0) | |||
| PWID | 90 (5.5) | 3.1 (2.4–4.6) | 23.9 (3.7–74.1) | 46.7 (15.8–92.2) | |||
| Heterosexual | 525 (31.9) | 3.1 (2.4–4.4) | 19.3 (2.9–64.4) | 36.3 (12.4–85.6) | |||
| Unknown/other risk | 101 (6.1) | 3.3 (2.3–4.5) | 12.1 (0.0–57.5) | 26.8 (4.2–82.5) | |||
| Payer | .42 | <.001 | <.001 | ||||
| Private | 882 (53.6) | 2.9 (2.2–4.1) | 6.5 (0.5–33.0) | 20.2 (4.5–60.1) | |||
| Public | 506 (30.8) | 3.0 (2.2–4.4) | 18.6 (1.5–62.4) | 35.4 (10.8–86.1) | |||
| None/unknown/other | 257 (15.6) | 3.1 (2.4–4.1) | 12.5 (2.0–48.4) | 28.6 (10.3–75.0) | |||
| Died | <.001 | <.001 | <.001 | ||||
| Yes | 115 (7.0) | 3.7 (2.7–4.9) | 44.1 (11.8–91.6) | 81.9 (26.2–100.0) | |||
| No | 1530 (93.0) | 2.9 (2.2–4.2) | 8.5 (0.6–41.9) | 23.8 (5.5–66.7) |
%PY = percent person-years, IQR = interquartile range, MSM = men who have sex with men, PWID = persons who inject drugs, VCY = viremia copy-years.
Characteristics are summarized at baseline (defined as the date 6 months after cART initiation), unless otherwise indicated.
Wilcoxon rank-sum tests for continuous variables, Yates-corrected chi-square test for categorical variables.
3.3. Associations of viral exposure with mortality
In univariate Cox regression models, each measure of individual VE evaluated was associated with mortality risk, as were known risks for death in this cohort such as older age, other demographics, public insurance, IDU HIV risk activity, or lower pre-cART CD4,[33–37] (Table 3). In multivariable Cox proportional hazard analyses, in all 9 evaluated models which included various measures and combinations of measures of VE, including adjustment for known correlates for mortality in the HOPS listed above, we found that all evaluated combinations of VE were highly predictive of mortality. Based on Akaike information criterion (model fit index, lower being better), the model that included most recent viral load and %PY >200 copies/mL comprised the best combination of VE factors that predicted mortality (Table 4).
Table 3.
Participant characteristics associated with mortality, the HIV Outpatient Study, 1995–2016 (N = 1645).
| Participant characteristics | Univariate Cox proportional hazards analyses HR (95% CI) | P |
| Baseline age, per 10 y | 1.83 (1.54–2.17) | <.001 |
| Public insurance at baseline | 2.72 (1.89–3.93) | <.001 |
| PWID HIV risk activity | 6.13 (3.71–10.13) | <.001 |
| Heterosexual HIV risk activity | 2.46 (1.63–3.71) | <.001 |
| CD4+ cell count, pre cART, per 100 cells/mm3 | 0.80 (0.73–0.89) | <.001 |
| Most recent log10 viral load, per 1 log copies/mL∗ | 1.63 (1.43–1.85) | <.001 |
| Viremia log10 copy-years, copies/mL times years∗ | 1.69 (1.46–1.97) | <.001 |
| %PY with viral load >200 copies/mL, per 10% increment∗ | 1.22 (1.16–1.28) | <.001 |
| %PY with viral load >50 copies/mL, per 10% increment∗ | 1.20 (1.14–1.27) | <.001 |
Characteristics are summarized at baseline (defined as the date 6 months after cART initiation), unless otherwise indicated. %PY = percent person-years, cART = combination antiretroviral therapy, CI = confidence interval, HR = hazard ratio, PWID = persons who inject drugs.
Time-updated variables.
Table 4.
Multivariable Cox proportional hazards analyses of time-updated VE measures associated with mortality and sorted by lowest to highest AIC score, the HIV Outpatient Study, 1995–2016 (N = 1645).
| Most recent log10 viral load | Viremia log10 copy-years | Proportion attributable time spend at viral load: >50 copies/mL | Proportion attributable time spent at viral load: >200 copies/mL | Model fit index: AIC score (lower = better)∗ | |
| aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | ||
| Model 1 | 1.30 (1.07–1.58) | 1.15 (1.07–1.23) | 1350 | ||
| Model 2 | 1.21 (0.96–1.52) | 1.17 (1.08–1.26) | 1355 | ||
| Model 3 | 1.22 (1.15–1.28) | 1355 | |||
| Model 4 | 1.40 (1.20–1.64) | 1.43 (1.20–1.72) | 1357 | ||
| Model 5 | 1.44 (1.21–1.72) | 1.10 (1.03–1.18) | 1357 | ||
| Model 6 | 1.39 (1.13–1.71) | 1.11 (1.03–1.19) | 1363 | ||
| Model 7 | 1.19 (1.12–1.25) | 1370 | |||
| Model 8 | 1.67 (1.46–1.90) | 1370 | |||
| Model 9 | 1.70 (1.45–1.99) | 1371 |
Each model included factors listed and adjusted for baseline factors: age, public insurance, HIV risk activity, and pre-cART CD4+ cell count.
aHR = adjusted hazard ratio, AIC = Akaike's information criterion, CI = confidence interval, HR = hazard ratio, PWID = persons who inject drugs, VE = viral exposure.
4. Discussion
We found that each measure of long-term VE evaluated was independently associated with mortality. Most recent viral load and %PY >200 copies/mL was the best combination of VE factors that predicted mortality although essentially all VE measures, individually and in combination, performed well. Although %PY >50 copies/mL and VCY were independently associated with mortality, in multivariable analyses %PY >200 copies/mL remained associated with mortality but VCY did not. This suggests that if a higher VL threshold is used (eg, 200 copies/mL), that %PY above this threshold acts as an adequate summary of VE for purposes of mortality risk assessment.
To our knowledge, this is the first study to comparatively evaluate the mortality predictive performance of combinations of specific VE measures that have, in the past, been analyzed individually for their clinical prognostic utility. Although published data from other cohorts have evaluated and identified associations between individual types of VE measures with death,[20,24,25,26,27] risk for HIV transmission[23,30] and risk for AIDS opportunistic diseases, cancer, or myocardial infarction,[25,27,28] ours is the first to systematically compare and combine VE measures to evaluate their predictive value regarding a specific clinical event (death).
We consider our findings compelling in that they confirm the independent mortality predictive value of various longitudinal and cross-sectional measures of viremia over time, both individually and in pairs. These findings not only attest to the potential clinical value of these measures, but also provide comparative estimates of the different longitudinal and point-in-time measures of viral exposure considered. Although most recent viral load and %PY >200 copies/mL were the optimal combination of VE measures to predict mortality, all individual and combinations of VE measures evaluated performed quite well, with the range of mortality predictive values (model “fitness” estimates) across models being very narrow, indicating similar high precision of prognostic performance for each VE measure and combination of measures evaluated.
Ours was a large, well-characterized prospectively followed, diverse group of ART-treated adults with HIV who were observed from 6 months after cART initiation during 1995 to 2016 and who were mostly virally suppressed. There were multiple measures of VE available for the participants, most of whom were followed for extended periods of time (4- to 5-year median for both decedents and nondecedents), and we observed a sufficient number of deaths to be able to investigate potential differences in the strength of the association between various VE measures and all-cause mortality.
Limitations to our study also existed. First, routinely collected medical abstraction data involves variability in the timing of participant health care contact screenings, including viral loads, and thus not all patients have equally detailed clinical history. Also, the overall inferences regarding the combination of VE measures that best predict mortality were generated from observational data that spanned a prolonged calendar time period in this analysis (21 years), during which cART use patterns evolved. Further, our findings may not necessarily reflect outcomes that would be expected had we restricted our analysis to persons treated exclusively with newer cART regimens; this latter group would be challenging to study because of the decreasing overall number of deaths observed over calendar time. Furthermore, the variability across participants as to the length of calendar time over which VL measurements were available and the number of VL values available per participant may have been associated with biases that we could not ascertain. However, such limitations are germane to studies that rely on data from routine HIV care, and emblematic of the challenges faced in clinical practice in which clinicians often have limited patient historical information upon which to base treatment decisions.
In conclusion, although we determined that most recent viral load and %PY >200 copies/mL was the best pair of VE factors for mortality prediction, all VE measures evaluated, individually and in combination, performed quite well. These findings imply that clinicians can use diverse measures of VE that can reliably predict the risk of mortality among PWH, and that this ability may not be hampered by more limited VE measures in situations where complete VE histories are not available. Topic areas requiring further careful elucidation include identifying associations between various measures of VE and selected clinical endpoints; such information could potentially have substantial value to inform more nuanced medical management and monitoring of ART-treated patients. Hence, future work should focus on ascertaining whether and the extent to which various VE measures, used individually and in combination, increase the ability to discriminate and to predict with more precision the risk for specific clinical events other than death.
Author contributions
Conceptualization: Frank J Palella Jr, Ellen Tedaldi, Richard Novak, Linda Battalora, Jun Li.
Data curation: Carl Armon, Rachel Hart, Stephen R. Cole.
Investigation: Frank J Palella Jr.
Methodology: Stephen R. Cole.
Project administration: Rachel Hart, Stacey Purinton, Jun Li, Kate Buchacz.
Supervision: Jun Li, Kate Buchacz.
Validation: Carl Armon, Stephen R. Cole.
Visualization: Carl Armon.
Writing – original draft: Frank J Palella Jr.
Writing – review & editing: Frank J Palella Jr, Carl Armon, Ellen Tedaldi, Richard Novak, Linda Battalora, Stacey Purinton, Jun Li, Kate Buchacz, Stephen R. Cole.
Supplementary Material
Footnotes
Abbreviations: %PY = Percent person-years, cART = combination antiretroviral therapy, HOPS = HIV Outpatient Study, IDU = injection drug use, MSM = men who have sex with men, PWH = persons living with HIV, VCY = Viremia copy-years, VE = viral exposure, VL = viral load.
How to cite this article: Palella FJ, Armon C, Cole SR, Hart R, Tedaldi E, Novak R, Battalora L, Purinton S, Li J, Buchacz K. HIV viral exposure and mortality in a multicenter ambulatory HIV adult cohort, United States, 1995–2016. Medicine. 2021;100:25(e26285).
The HIV Outpatient Study (HOPS) investigators are listed in the Appendix.
Funding: This work was supported by the Centers for Disease Control and Prevention (contract nos. 200-2001-00133, 200-2006-18797, 200-2011-41872, 200-2015-63931).
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflicts of interest: FJP has been a consultant and/or on the Speakers’ Bureau for Gilead Sciences, Janssen Pharmaceuticals, Merck and Co. and ViiV.
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
References
- [1].Human immunodeficiency virus type 1 RNA level and CD4 count as prognostic markers and surrogate end points: a, meta-analysis. HIV Surrogate Marker Collaborative Group. AIDS Res Hum Retroviruses 2000;16:1123–33. [DOI] [PubMed] [Google Scholar]
- [2].Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort, studies. Lancet (London, England) 2008;372:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chene G, Sterne JA, May M, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet (London, England) 2003;362:679–86. [DOI] [PubMed] [Google Scholar]
- [4].Marschner IC, Collier AC, Coombs RW, et al. Use of changes in plasma levels of human immunodeficiency virus type 1 RNA to assess the clinical benefit of antiretroviral therapy. J Infect Dis 1998;177:40–7. [DOI] [PubMed] [Google Scholar]
- [5].Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 1997;126:946–54. [DOI] [PubMed] [Google Scholar]
- [6].Murray JS, Elashoff MR, Iacono-Connors LC, Cvetkovich TA, Struble KA. The use of plasma HIV RNA as a study endpoint in efficacy trials of antiretroviral drugs. AIDS (London, England) 1999;13:797–804. [DOI] [PubMed] [Google Scholar]
- [7].Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 2008;105:3879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016;375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Doshi RK, Milberg J, Isenberg D, et al. High rates of retention and viral suppression in the US HIV safety net system: HIV care continuum in the Ryan White HIV/AIDS Program, 2011. Clin Infect Dis 2015;60:117–25. [DOI] [PubMed] [Google Scholar]
- [10].Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet (London, England) 2002;360:119–29. [DOI] [PubMed] [Google Scholar]
- [11].Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Justice AC, Modur SP, Tate JP, et al. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr 2013;62:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kowalkowski MA, Day RS, Du XL, Chan W, Chiao EY. Cumulative HIV viremia and non-AIDS-defining malignancies among a sample of HIV-infected male veterans. J Acquir Immune Defic Syndr 2014;67:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Laut KG, Shepherd LC, Pedersen C, et al. Associations between HIV-RNA-based indicators and virological and clinical outcomes. AIDS (London, England) 2016;30:1961–72. [DOI] [PubMed] [Google Scholar]
- [15].Rodger AJ, Cambiano V, Bruun T, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet (London, England) 2019;393:2428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Althoff KN, Buchacz K, Hall HI, et al. U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med 2012;157:325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Laprise C, de Pokomandy A, Baril JG, Dufresne S, Trottier H. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis 2013;57:1489–96. [DOI] [PubMed] [Google Scholar]
- [18].Sklar PA, Ward DJ, Baker RK, et al. Prevalence and clinical correlates of HIV viremia (‘blips’) in patients with previous suppression below the limits of quantification. AIDS (London, England) 2002;16:2035–41. [DOI] [PubMed] [Google Scholar]
- [19].Marks G, Patel U, Stirratt MJ, et al. Single viral load measurements overestimate stable viral suppression among HIV patients in care: clinical and public health implications. J Acquir Immune Defic Syndr 2016;73:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chirouze C, Journot V, Le Moing V, et al. Viremia copy-years as a predictive marker of all-cause mortality in HIV-1-infected patients initiating a protease inhibitor-containing antiretroviral treatment. J Acquir Immune Defic Syndr 2015;68:204–8. [DOI] [PubMed] [Google Scholar]
- [21].Cole SR, Napravnik S, Mugavero MJ, Lau B, Eron JJ, Jr, Saag MS. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol 2010;171:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wright ST, Hoy J, Mulhall B, et al. Determinants of viremia copy-years in people with HIV/AIDS after initiation of antiretroviral therapy. J Acquir Immune Defic Syndr 2014;66:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Marks G, Gardner LI, Rose CE, et al. Time above 1500 copies: a viral load measure for assessing transmission risk of HIV-positive patients in care. AIDS (London, England) 2015;29:947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mugavero MJ, Napravnik S, Cole SR, et al. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis 2011;53:927–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sempa JB, Dushoff J, Daniels MJ, et al. Reevaluating cumulative HIV-1 viral load as a prognostic predictor: predicting opportunistic infection incidence and mortality in a Ugandan cohort. Am J Epidemiol 2016;184:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang R, Haberlen SA, Palella FJ, Jr, et al. Viremia copy-years and mortality among combination antiretroviral therapy-initiating HIV-positive individuals: how much viral load history is enough? AIDS (London, England) 2018;32:2547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Quiros-Roldan E, Raffetti E, Castelli F, et al. Low-level viraemia, measured as viraemia copy-years, as a prognostic factor for medium-long-term all-cause mortality: a MASTER cohort study. J Antimicrob Chemother 2016;71:3519–27. [DOI] [PubMed] [Google Scholar]
- [28].Salinas JL, Rentsch C, Marconi VC, et al. Baseline, time-updated, and cumulative HIV care metrics for predicting acute myocardial infarction and all-cause mortality. Clin Infect Dis 2016;63:1423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zoufaly A, Stellbrink HJ, Heiden MA, et al. Cumulative HIV viremia during highly active antiretroviral therapy is a strong predictor of AIDS-related lymphoma. J Infect Dis 2009;200:79–87. [DOI] [PubMed] [Google Scholar]
- [30].Crepaz N, Tang T, Marks G, Mugavero MJ, Espinoza L, Hall HI. Durable viral suppression and transmission risk potential among persons with diagnosed HIV infection: United States, 2012-2013. Clin Infect Dis 2016;63:976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mendoza MCB, Gardner L, Armon C, et al. Time spent with HIV viral load above 1500 copies/ml among patients in HIV care. 2000-2014 AIDS (London, England) 2018;32:2033–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Moorman AC, Holmberg SD, Marlowe SI, et al. Changing conditions and treatments in a dynamic cohort of ambulatory HIV patients: the HIV outpatient study (HOPS). Ann Epidemiol 1999;9:349–57. [DOI] [PubMed] [Google Scholar]
- [33].Holmberg SD, Hamburger ME, Moorman AC, Wood KC, Palella FJ, Jr. Factors associated with maintenance of long-term plasma human immunodeficiency virus RNA suppression. Clin Infect Dis 2003;37:702–7. [DOI] [PubMed] [Google Scholar]
- [34].Palella FJ, Jr, Armon C, Buchacz K, et al. The association of HIV susceptibility testing with survival among HIV-infected patients receiving antiretroviral therapy: a cohort study. Ann Intern Med 2009;151:73–84. [DOI] [PubMed] [Google Scholar]
- [35].Palella FJ, Jr, Baker RK, Buchacz K, et al. Increased mortality among publicly insured participants in the HIV Outpatient Study despite HAART treatment. AIDS (London, England) 2011;25:1865–76. [DOI] [PubMed] [Google Scholar]
- [36].Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006;43:27–34. [DOI] [PubMed] [Google Scholar]
- [37].Palella FJ, Jr, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med 2003;138:620–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


