Abstract
To investigate the importance of pulmonary vascular measurements on computed tomography (CT) in predicting pulmonary hypertension (PH) and worse outcomes in diffuse cystic lung diseases (DCLDs).
We conducted a cross-sectional study of patients with DCLDs. Patients underwent pulmonary function tests, a six-minute walk test (6MWT), chest CT, transthoracic echocardiography, and right heart catheterization. Pulmonary artery (PA) diameter and PA-ascending aorta ratio (PA-Ao ratio) were obtained from CT. Mean pulmonary artery pressure (mPAP) from right heart catheterization was correlated with tomographic, functional, and echocardiographic variables. The association between the PA-Ao ratio with outcomes was determined by Kaplan–Meier curves.
Thirty-four patients were included (18 with pulmonary Langerhans cell histiocytosis and 16 with lymphangioleiomyomatosis, mean age 46 ± 9 years). Forced expiratory volume in the first second and lung diffusing capacity for carbon monoxide were 47 ± 20% and 38 ± 21% predicted, respectively. PA diameter and PA-Ao ratio were 29 ± 6 mm and 0.95 ± 0.24, respectively. PA-Ao ratio > 1 occurred in 38.2% of patients. PA-Ao ratio was a good predictor of PH. mPAP correlated best with PA-Ao ratio, PA diameter, oxygen desaturation during six-minute walk test, and echocardiographic variables. Patients with PA-Ao ratio > 1 had greater mPAP, and a higher risk of death or lung transplantation (log-rank, P < .001) than those with PA-Ao ratio ≤ 1.
The PA-Ao ratio measured on CT scan has a potential role as a non-invasive tool to predict the presence of PH and as a prognostic parameter in patients with DCLDs.
Keywords: catheterization, lung cysts, lung function testing, pulmonary hypertension, tomography
1. Introduction
Diffuse cystic lung diseases (DCLDs) are characterized by the presence of thin-walled and air-filled spaces (cysts) in more than one lobe of the lungs, usually bilateral. The most common etiologies of DCLDs include lymphangioleiomyomatosis (LAM), pulmonary Langerhans cell histiocytosis (PLCH), lymphoid interstitial pneumonia, and Birt-Hogg-Dubé syndrome.[1,2] LAM is a rare and low-grade neoplasm that occurs mostly in women at their reproductive age. It occurs in a sporadic form or associated with tuberous sclerosis complex.[3–5] PLCH is an inflammatory myeloid neoplasm that is often associated with smoking and is characterized by the infiltration of organs with dendritic and other inflammatory cells.[6,7]
Pulmonary hypertension (PH) may occur in LAM and PLCH. According to current PH classification, LAM falls within group three (lung diseases and/or hypoxia), whereas PLCH is included in group five (unclear and/or multifactorial mechanisms).[8] The prevalence of PH is approximately 8% in LAM, usually mild and suggestive to be associated more with pulmonary parenchymal involvement.[9–11] However, the prevalence of PH is higher in PLCH, varying from 41% in patients with different severities, to 100% in those with end-stage disease, and is usually more severe than LAM.[12–14] Furthermore, PH is multifactorial and associated with arterial, venular, and/or parenchymal involvement in PLCH.[12–15]
The gold standard for the confirmation of PH is the right heart catheterization (RHC), which is an invasive and more expensive procedure. Therefore, the identification of non-invasive tools to act as surrogates for the presence of PH is needed in patients with chronic lung diseases. The most used non-invasive screening tool for PH is the echocardiogram. However, the accuracy of transthoracic echocardiography (TTE) is controversial in the prediction of the occurrence of PH in DCLDs, due to a potential limitation of the acoustic chest window. Although lung diffusing capacity for carbon monoxide (DLCO) is considered a functional parameter that can be used to predict the presence of PH in DCLDs, it has limited sensitivity when isolated.[9,14]
In this context, variables assessed in computed tomography (CT) scan, including pulmonary artery-ascending aorta (PA-Ao) ratio, may be used as predictors of PH, which has already been described in several respiratory diseases, such as chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), chronic hypersensitivity pneumonitis, cystic fibrosis, and chronic thromboembolic PH, with a reasonable correlation with mean pulmonary artery pressure (mPAP) from RHC and survival.[16–20,22] PA-Ao ratio was also associated with an increased risk of acute exacerbations in patients with COPD.[19,21] Several studies in various lung diseases showed that a PA-Ao ratio > 1 is associated with a greater specificity to detect PH and with worse outcomes.[16,19,20,22,23] Although a previous investigation evaluated the PA-Ao ratio as a surrogate marker of PH in LAM, the analysis of such tomographic variable with parameters obtained in the RHC was not performed.[24]
To the best of our knowledge, there is no previous study that assessed the importance of the PA-Ao ratio or the PA diameter, measured by CT, as markers of resting PH in DCLDs. Therefore, the aims of this study were:
-
1)
investigate whether PA-Ao ratio and the PA diameter could be used as predictors of PH in patients with DCLDs;
-
2)
evaluate whether such tomographic parameters have a greater correlation with mPAP measured by RHC than functional and echocardiographic variables, and their association with worse outcomes.
2. Materials and methods
2.1. Study design and subjects
This was a cross-sectional and single-center study. Data of patients with PLCH and LAM who underwent RHC between 2014 and 2018 were analyzed. The diagnoses of LAM and PLCH were made according to the current recommendations.[3,5–7]
Subjects of this investigation were participants in two previous studies that assessed the prevalence of PH in LAM and in PLCH at our center,[9,14] which were approved by the local ethics committee (2015/06604-0 and 759.676). All patients signed the informed consent form.
2.2. Measurements
All patients underwent pulmonary function tests (PFTs), six-minute walk test (6MWT), CT scan, TTE, and RHC.
2.2.1. Clinical and demographic features
The following demographic and clinical variables were obtained from medical records: age, sex, smoking history, time from diagnosis, method of diagnosis, the presence of dyspnea, history of pneumothorax, and use of supplemental oxygen at rest. Dyspnea was evaluated using the modified Medical Research Council dyspnea scale.
2.2.2. Pulmonary function tests and 6MWT
Pulmonary function tests were performed according to recommended standards.[25–27] Forced expiratory volume in the first second (FEV1), forced vital capacity, FEV1/forced vital capacity ratio, residual volume, total lung capacity, residual volume/total lung capacity ratio, and DLCO were measured. Spirometry was performed using a calibrated pneumotachograph (Medical Graphics Corporation, St Paul, MN), and lung volumes and DLCO measurements were obtained with a body plethysmograph (Elite Dx, Elite Series; Medical Graphics Corporation). Predicted values were obtained from the Brazilian population.[28–30]
A 6MWT was performed according to recommended standards in a 30-m long sides corridor.[31] Oxygen saturation and Borg scales for dyspnea and fatigue were obtained at rest and at the end of the exercise.[32] The distance walked was obtained in meters and as predicted values for the Brazilian population.[33]
2.2.3. Chest computed tomography
Multidetector CT scanners were used (MX8000 IDT10 or IDT16, Philips Medical Systems, Cleveland, OH). All CTs were performed in supine position, with breath-holding at full inspiration, without the administration of intravenous contrast. Chest CT was performed within 3 months of the RHC. The diameters of the main PA and the Ao were measured at the level of the PA bifurcation from axial CT images on inspiration and at the mediastinal window, as previously described.[19] The PA bifurcation was defined as the level where the right and left PAs showed equal size. The ratio of the two measurements (PA-Ao ratio) was obtained.
An investigator blinded to the clinical information and the results of the procedures evaluated the tomographic parameters. A second blinded reviewer also measured the PA and Ao diameters to obtain the interobserver agreement.
2.2.4. Transthoracic echocardiography
A two-dimensional TTE (IE 33 equipment, Philips Medical Systems, Bothell) was performed to evaluate the tricuspid regurgitation velocity (TRV) and the estimated systolic pulmonary artery pressure (sPAP).
2.2.5. Right heart catheterization
The RHC was performed at rest and with a pulmonary artery catheter 7F inserted through the jugular vein, as previously described.[34] The following variables were obtained: cardiac output, mPAP, pulmonary vascular resistance (PVR), and wedge pressure. The diagnosis of PH was based on the following findings: pre-capillary PH, mPAP > 20 mm Hg, wedge pressure ≤ 15 mm Hg, and PVR ≥ 2.2 Wood units; post-capillary PH, mPAP > 20 mm Hg, wedge pressure > 15 mm Hg, and PVR < 2.2 Wood units; and pre- and post-capillary PH, mPAP > 20 mm Hg, wedge pressure > 15 mm Hg and PVR ≥ 2.2 Wood units.[8,35]
2.3. Statistical analysis
Data are reported as the mean ± standard deviation for variables with normal distribution, as the median (25th–75th percentiles) for variables with non-normal distribution, or as numbers (percentiles). Unpaired t tests or the Mann–Whitney U test was used to compare continuous variables, whereas Fisher exact test or the Chi-square test was used to compare categorical variables. Interobserver reproducibility of the measurements of the PA-Ao ratio and the diameter of PA for two experienced readers were determined by the intraclass correlation coefficient. The prevalence of patients with PA-Ao ratio > 1 was reported as the percentage with a 95% confidence interval (95% CI). The sensitivity, specificity, positive-predictive value (PPV), and negative-predictive value (NPV) for the PA-Ao ratio > 1 to diagnose PH were calculated. The receiver operating characteristic curve was used to evaluate the optimal cut-off PA-Ao ratio with the best accuracy to predict the presence of PH in RHC. Spearman's correlation analysis was used to determine the association between mPAP and other variables. Time-to-event (death or lung transplantation, whichever event occurred first) analysis from the date of the chest CT scan was performed with Kaplan–Meier curves and differences between groups were evaluated with Log-Rank tests. P-values less than .05 were considered statistically significant. Data were analyzed using SigmaStat version 3.5 (Systat Software, Inc., San Jose, CA).
3. Results
Thirty-four patients with diffuse cystic lung disease (DCLD) were included in the study (18 with PLCH and 16 with LAM). Patients had a mean age of 46 ± 9 years, 82.4% were women, and 52.9% had a history of smoking. FEV1 and DLCO were 47 ± 20% and 38 ± 21% of predicted, respectively. The distance walked and the minimum oxygen saturation in the 6MWT were 392 ± 116 m (96 ± 54% of predicted) and 79 ± 9%, respectively. Table 1 presents demographic, clinical, and functional features.
Table 1.
Demographic, clinical, and functional characteristics of patients with diffuse cystic lung diseases (n = 34).
| Demographic and clinical data | ||
| Age (yrs) | 46 ± 9 | |
| Female (n, %) | 28 (82.4%) | |
| Smoking (n, %) | 18 (52.9%) | |
| Pack-years | 34 ± 22 | |
| Time from diagnosis (yrs) | 2.5 (1–13) | |
| Diagnosis | ||
| Clinical-tomographic (n, %) | 15 (44.1%) | |
| Lung biopsy (n, %) | 18 (52.9%) | |
| Renal biopsy (n, %) | 1 (3%) | |
| Dyspnea (n, %) | 30 (88.2%) | |
| mMRC | 2 (1–2) | |
| History of pneumothorax (n, %) | 12 (35.3%) | |
| SpO2 at rest (%) | 93 (89–95) | |
| Use of supplemental oxygen at rest (n, %) | 12 (35.3%) | |
| Pulmonary function tests | ||
| FEV1 (L) | 1.32 ± 61 | |
| FEV1 (% predicted) | 47 ± 20 | |
| FVC (L) | 2.47 ± 0.82 | |
| FVC (% predicted) | 71 ± 20 | |
| FEV1/FVC | 0.53 ± 0.15 | |
| TLC (L) | 5.13 ± 1.1 | |
| TLC (% predicted) | 104 ± 17 | |
| RV (L) | 2.60 ± 0.91 | |
| RV (% predicted) | 177 ± 62 | |
| RV/TLC | 0.51 ± 0.12 | |
| DLCO (mL/min/mm Hg) | 10.08 ± 5.84 | |
| DLCO (% predicted) | 38 ± 21 | |
| Six-minute walk test | ||
| Distance (m) | 392 ± 116 | |
| Distance (% predicted) | 96 ± 54 | |
| Minimum SpO2 (%) | 79 ± 9 | |
| Oxygen desaturation (%) | −12 ± 7 | |
| Borg dyspnea score | 7 (3–8) | |
| Borg leg discomfort score | 4 (0–7) | |
Data are presented as mean ± SD, median (25th–75th percentile), or numbers (percentiles). DLCO = lung diffusing capacity for carbon monoxide, FEV1 = forced expiratory volume in the first second, FVC = forced vital capacity, mMRC = modified Medical Research Council Dyspnea scale, RV = residual volume, SpO2 = oxygen saturation, TLC = total lung capacity.
Table 2 demonstrates tomographic, echocardiographic, and hemodynamic data. The Ao and PA diameters and PA-Ao ratio were 31 ± 4, 29 ± 6 mm, and 0.95 ± 0.24, respectively. The intraclass correlation coefficients for the PA-Ao ratio and PA diameter were 0.949 (CI 95%: 0.898–0.975), P < .001, and 0.945 (CI 95% 0.945: 0.890–0.973), P < .001, respectively. Figure 1 demonstrates vascular tomographic measurements in patients with PLCH and LAM. PA-Ao ratio > 1 occurred in 38.2% (CI 95%: 23.9–55%) of all patients and 48.1% (CI 95%: 30.7–66%) of those with confirmed PH on RHC. All patients with PA-Ao ratio > 1 had mPAP > 20 mm Hg on RHC. The mPAP was 28.6 ± 10.1 mm Hg. Twenty-seven patients had the diagnosis of PH confirmed, 11 with LAM, and 16 with PLCH. Thus, the prevalence of PH in this sample was 79.4% (CI 95%: 63.2–89.5%), 81.5% was pre-capillary, 7.4% was post-capillary, and 11.1% was pre- and post-capillary. The prevalence of patients with PA-Ao ratio > 1 was 50% in PLCH and 25% in LAM.
Table 2.
Tomographic, echocardiographic, and hemodynamic data in patients with diffuse cystic lung diseases (n = 34).
| Computed tomography | |
| Ao diameter (mm) | 31 ± 4 |
| PA diameter (mm) | 29 ± 6 |
| PA-Ao ratio | 0.95 ± 0.24 |
| Transthoracic echocardiogram | |
| sPAP (mm Hg)∗ | 43 (33–46) |
| TRV (m/s)† | 3.08 ± 0.68 |
| Right heart catheterization | |
| mPAP (mm Hg) | 28.6 ± 10.1 |
| PVR (wood units) | 3.2 (2.4–4.1) |
| CO (L/min) | 4.7 ± 1.0 |
| Wedge pressure (mm Hg) | 11.6 ± 4.9 |
Data are presented as mean ± SD or median (25th–75th percentile). Ao = ascending aorta, CO = cardiac output, mPAP = mean pulmonary artery pressure, PA = pulmonary artery, PVR = pulmonary vascular resistance, sPAP = systolic pulmonary artery pressure, TRV = tricuspid regurgitation velocity.
n = 24.
n = 22.
Figure 1.
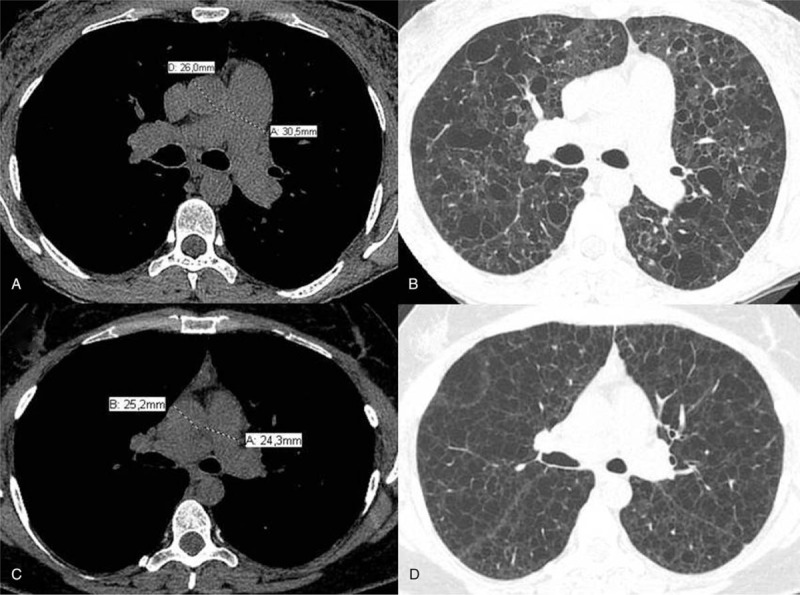
(A) Axial computed tomography slice at the level of bifurcation of the main pulmonary artery demonstrates measurements of the diameters of the main pulmonary artery (30.5 mm) and ascending aorta (26 mm) in a patient with pulmonary Langerhans cell histiocytosis. PA/Ao ratio in this patient was 1.17. (B) Axial computed tomography image shows irregular pulmonary cysts in the patient presented in (A). (C) Axial computed tomography slice at the level of bifurcation of the main pulmonary artery demonstrates measurements of the diameters of the main pulmonary artery (24.3 mm) and ascending aorta (25.2 mm) in a patient with lymphangioleiomyomatosis. PA/Ao ratio in this patient was 0.96. (D) Axial computed tomography image shows extensive and diffuse pulmonary cysts with regular walls in the patient presented in (C). Ao = ascending aorta, PA = pulmonary artery.
Patients with PA-Ao ratio > 1 had higher use of supplemental oxygen, and greater sPAP and TRV on echocardiogram, and mPAP and PVR on RHC, than those with PA-Ao ratio ≤ 1 (Table 3). The sensitivity, specificity, PPV, and NPV for the PA-Ao ratio > 1 to identify PH were 48%, 100%, 100%, and 33%, respectively.
Table 3.
Comparison of clinical, functional, tomographic, echocardiographic, and hemodynamic data between patients with diffuse cystic lung diseases with PA-Ao ratio > 1 and those with PA-Ao ratio ≤ 1.
| PA-Ao ratio > 1 (n = 13) | PA-Ao ratio ≤ 1 (n = 21) | P | |
| Age (yrs) | 43 ± 8 | 48 ± 9 | .11 |
| Smoking (n, %) | 8 (62%) | 10 (48%) | .66 |
| Time from diagnosis (yrs) | 2 (1–3.5) | 5 (2–13.5) | .04 |
| Dyspnea (n, %) | 12 (92%) | 18 (86%) | 1.00 |
| mMRC | 2 (1–3) | 2 (1–2) | .31 |
| SpO2 at rest (%) | 92 (86–95) | 93 (90–95) | .32 |
| Supplemental oxygen at rest (n, %) | 8 (62%) | 4 (19%) | .02 |
| FEV1 (L) | 1.37 ± 0.53 | 1.29 ± 0.66 | .72 |
| FEV1 (% predicted) | 45 ± 17 | 47 ± 23 | .75 |
| FEV1/FVC | 0.55 ± 0.16 | 0.52 ± 0.15 | .54 |
| RV (L) | 2.58 ± 0.67 | 2.61 ± 1.05 | .91 |
| RV (% predicted) | 167 ± 37 | 183 ± 73 | .47 |
| RV/TLC | 0.50 ± 0.08 | 0.51 ± 0.14 | .82 |
| DLCO (mL/min/mm Hg) | 8.94 (6.57–13.33) | 8.70 (6.34–14.17) | .86 |
| DLCO (% predicted) | 35 ± 11 | 40 ± 25 | .54 |
| Distance in the 6MWT (m) | 403 ± 91 | 386 ± 130 | .68 |
| Distance in the 6MWT (% predicted) | 106 (85–114) | 75 (61–114) | .18 |
| Minimum SpO2 (%) | 77 ± 10 | 81 ± 9 | .32 |
| Oxygen desaturation (%) | −13 ± 5 | −12 ± 7 | .82 |
| Borg dyspnea score | 5 (1–9) | 7 (5–8) | .28 |
| Ao diameter (mm) | 29 ± 4 | 32 ± 5 | .04 |
| PA diameter (mm) | 33 ± 7 | 26 ± 4 | <.001 |
| PA-Ao ratio | 1.16 ± 0.26 | 0.83 ± 0.10 | <.001 |
| sPAP on echocardiogram (mm Hg) | 58 ± 29∗ | 38 ± 9† | .02 |
| TRV on echocardiogram (m/s) | 3.40 ± 0.81∗ | 2.81 ± 0.39‡ | .04 |
| mPAP on RHC (mm Hg) | 37 ± 10 | 23 ± 6 | <.001 |
| PVR on RHC (Wood units) | 4.8 (3.4–6.9) | 2.6 (1.9–3.2) | <.001 |
| Wedge pressure (mm Hg) | 11.8 ± 4.3 | 11.5 ± 5.4 | .84 |
Data are presented as mean ± SD, median (25th–75th percentile), or numbers (percentiles). 6MWT = six-minute walk test, Ao = ascending aorta, DLCO = lung diffusing capacity for carbon monoxide, FEV1 = forced expiratory volume in the first second, FVC = forced vital capacity, mMRC = modified Medical Research Council Dyspnea scale, mPAP = mean pulmonary artery pressure, PA = pulmonary artery, PVR = pulmonary vascular resistance, RHC = right heart catheterization, RV = residual volume, sPAP = systolic pulmonary artery pressure, SpO2 = oxygen saturation, TLC = total lung capacity, TRV = tricuspid regurgitation velocity.
n = 10.
n = 14.
n = 12.
The receiver operating characteristic curve analysis (Fig. 2) demonstrated that the PA-Ao ratio with the best accuracy to determine the presence of PH on RHC was 0.86 (area under the curve = 0.735, 95% CI = 0.573–0.898, P = .05). PA-Ao ratio > 0.86 occurred in 64.7% (CI 95%: 47.9–78.5%) of all patients. The sensitivity, specificity, PPV, and NPV for the PA-Ao ratio > 0.86 to identify PH were 74%, 71%, 91%, and 42%, respectively. Patients with PA-Ao ratio > 0.86 had higher mPAP and PVR on RHC than those with PA-Ao ratio ≤ 0.86 (Table 1 on Supplementary Material).
Figure 2.
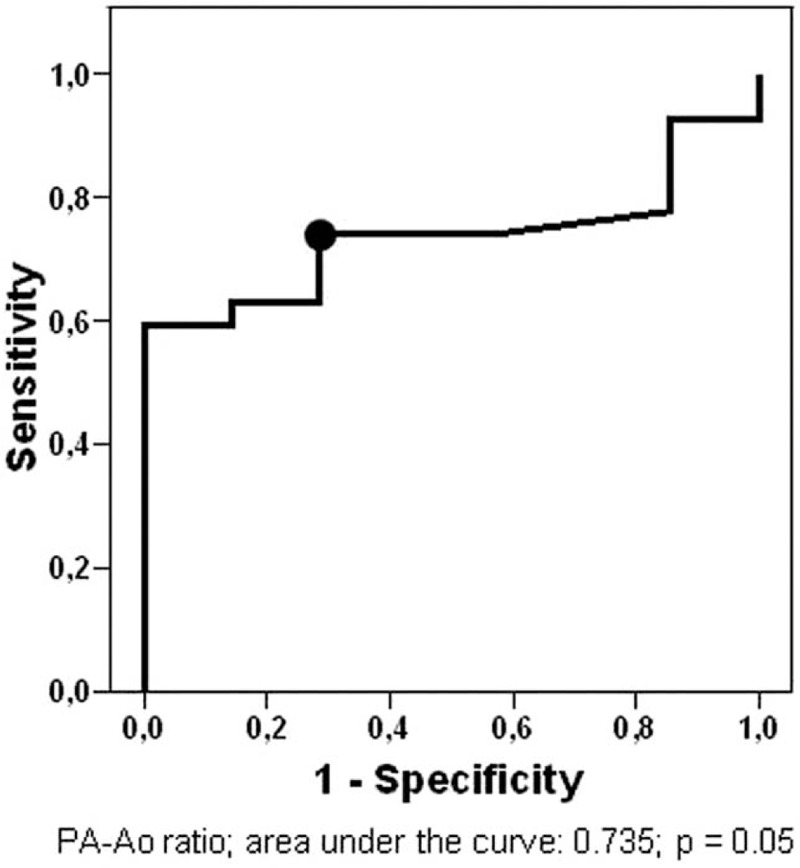
ROC curve for PA-Ao ratio for predicting the presence of PH on RHC in LAM and PLCH patients. Black dot mark cutoff value for the PA-Ao ratio (0.86) with the highest accuracy. Ao = ascending aorta, LAM = lymphangioleiomyomatosis, PA = pulmonary artery, PH = pulmonary hypertension, PLCH = pulmonary Langerhans cell histiocytosis, RHC = right heart catheterization, ROC = receiver operating characteristic.
Mean pulmonary artery pressure (PAP) correlated significantly with PA-Ao ratio (r = 0.788, P < .0001), PA diameter (mm) (r = 0.666, P < .0001), oxygen desaturation during the 6MWT (r = 0.453, P = .009), sPAP on TTE (r = 0.675, P < .0001), and TRV (m/s) (r = 0.459, P = .03), which are described in Table 4 and in Figure 3. Mean PAP correlated with PA-Ao ratio in PLCH (r = 0.802, P < .0001) and in LAM (r = 0.704, P = .002).
Table 4.
Correlations between mPAP obtained in the right heart catheterization with tomographic, functional, and echocardiographic variables.
| Variable | r | P |
| PA-Ao ratio | 0.788 | <.0001 |
| PA (mm) | 0.666 | <.0001 |
| DLCO (% predicted) | −0.023 | .89 |
| FEV1 (% predicted) | −0.203 | .25 |
| FEV1/FVC | 0.122 | .49 |
| RV/TLC | 0.084 | .63 |
| SpO2 at rest (%) | −0.248 | .16 |
| Distance in the 6MWT (m) | 0.075 | .68 |
| Distance in the 6MWT (% predicted) | 0.254 | .16 |
| Oxygen desaturation during 6MWT (%) | 0.453 | .009 |
| sPAP (mm Hg)∗ | 0.675 | <.0001 |
| TRV (m/s)† | 0.459 | .03 |
6MWT = six-minute walk test, Ao = ascending aorta, DLCO = lung diffusing capacity for carbon monoxide, FEV1 = forced expiratory volume in the first second, FVC = forced vital capacity, mPAP = mean pulmonary arterial pressure, PA = pulmonary artery, RV = residual volume, sPAP = systolic pulmonary artery pressure, SpO2 = oxygen saturation, TLC = total lung capacity, TRV = tricuspid regurgitation velocity.
n = 24.
n = 22.
Figure 3.
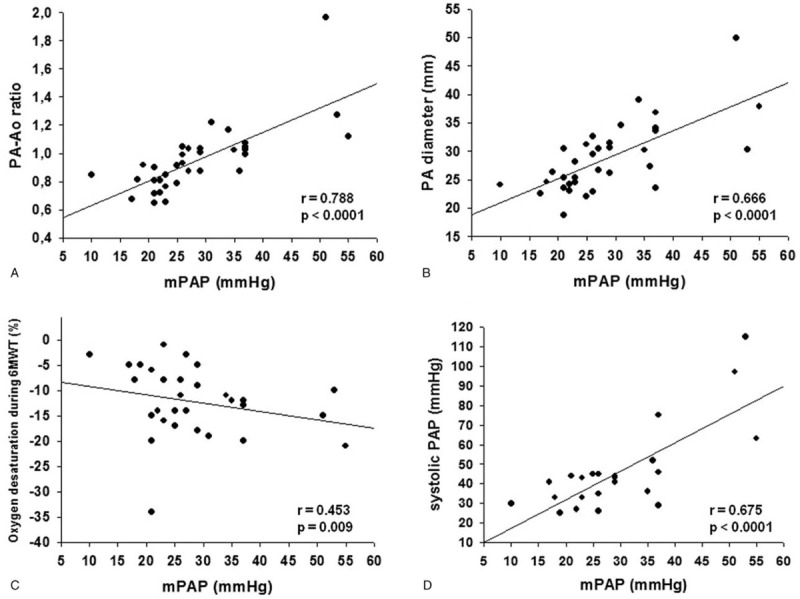
The best correlations of mPAP (mm Hg) obtained in RHC with tomographic, functional, and echocardiographic were as follows: (A) PA-Ao ratio; (B) PA diameter (mm); (C) oxygen desaturation during 6MWT (%); (D) systolic PAP (mm Hg). 6MWT = six-minute walk test, Ao = ascending aorta, mPAP = mean pulmonary artery pressure, PA = pulmonary artery, PAP = pulmonary artery pressure, r = Spearman correlation coefficient, RHC = right heart catheterization.
Kaplan–Meier curve of time to death or lung transplant demonstrated that patients with Pa-Ao ratio > 1 had a worse prognosis than those with PA-Ao ratio ≤ 1 (Fig. 4A, P < .001). In patients with PA-Ao ratio > 1, six (46%) patients died or underwent lung transplantation, whereas, in those with PA-Ao ratio ≤ 1, only one (5%) patient had such outcomes. The mean survival or transplant-free times were 35 and 76 months, respectively. The median time from diagnosis to the time of the CT scan were 2 (1–3.5) and 5 (2–13.5) years in patients with PA-A ratio > 1 and in those with PA-Ao ratio ≤ 1, respectively (P = .045). However, there was no difference in the prognosis when patients with PA-Ao ratio > 0.86 were compared with those with PA-Ao ratio ≤ 0.86 (Fig. 4B, P = .171).
Figure 4.
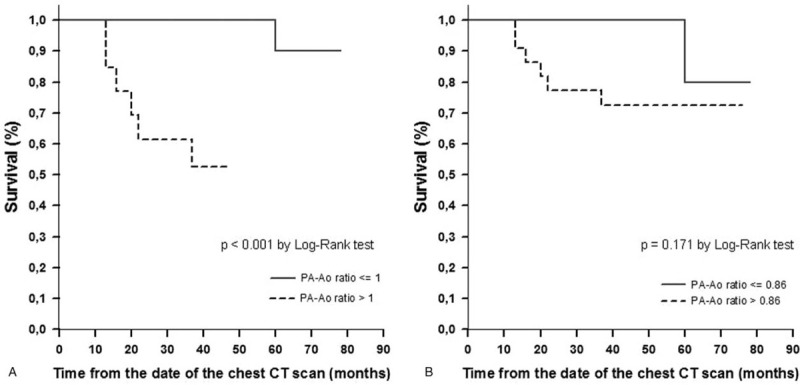
Kaplan–Meier curves for freedom for death or lung transplantation according to: (A) PA-Ao ratio > 1 vs PA-Ao ratio ≤ 1; (B) PA-Ao ratio > 0.86 vs PA-Ao ratio ≤ 0.86. Ao = ascending aorta, PA = pulmonary artery.
4. Discussion
To the best of our knowledge, this is the first study that assessed the measurements of vascular tomographic parameters, such as PA-Ao ratio and PA diameter, and their association with mPAP and outcomes in patients with PLCH and LAM that underwent RHC. The main findings of our study are as follows:
-
1)
PA-Ao ratio and PA diameter have a good correlation with mPAP on RHC and, therefore, are potential surrogate markers of PH;
-
2)
the measurement of pulmonary vascular diameter on CT is reliable when evaluated by different readers;
-
3)
PA-Ao ratio > 1 is frequent in patients with DCLDs with PH and is associated with higher use of supplemental oxygen, and with echocardiographic parameters;
-
4)
PA-Ao ratio > 1 has high specificity and PPV to identify PH and is associated with worse outcomes.
Although RHC is still the gold standard for confirming the diagnosis of PH, it is a more invasive tool with potential complications. Thus, it is desirable to identify non-invasive measures that could predict the presence of PH in DCLDs. There are several advantages in assessing the vascular measurements on CT scans, including its availability, simplicity, non-invasiveness, and no use of contrast. Despite being the main tool for PH screening in pulmonary chronic diseases, the echocardiogram has several limitations, particularly in this population. Hyperinflation is a common feature in DCLD and might impose technical complexity for the echocardiogram to access a proper evaluation window which tends to be very narrow in hyperinflated patients.[9,10,14] This limitation is not associated with the vascular assessment on CT scan. Other studies identified high values for interobserver agreement for vascular tomographic measurements in parenchymal lung diseases, which was confirmed by our findings.[16,19,20,22,23] Therefore, the use of tomographic findings to further enhance the sensitivity of conventional PH screening in DCLD is potentially useful and may help to anticipate PH diagnosis in this population.
Previous investigations in other chronic pulmonary diseases, such as COPD, IPF, chronic thromboembolic PH, chronic hypersensitivity pneumonitis, and cystic fibrosis demonstrated the association between PA and PA-Ao ratio with mPAP obtained in the RHC and suggested that PA-Ao ratio is as an index of PH, supporting our findings.[16–20,22] Our results also reinforced the fact that the PA-Ao ratio has good specificity and PPV for the presence of PH. Generally, a higher probability of PH was associated with PA-Ao ratio > 1.[19,20,22] Additionally, several studies demonstrated that PA-Ao ratio > 1 was associated with a higher risk of death and lung transplantation in different pulmonary diseases, such as IPF, COPD, and cystic fibrosis, which was also observed in our study.[16,20,22,23] Thus, our results reinforce the association between PA-Ao ratio > 1 and the prognosis. Another investigation showed that an increase in the PA-Ao ratio during acute exacerbation was associated with worse clinical outcomes in COPD.[21]
Only one study evaluated the PA-Ao ratio in LAM and showed that 22% out of 123 patients had an index > 1. However, this study did not assess the correlation between the PA-Ao ratio with mPAP obtained in the RHC.[24] Then, to our knowledge, this is the first study that assessed the PA-Ao ratio obtained by CT scan and correlated with the mPAP obtained in the RHC and with outcomes in patients with DCLDs. Our findings might help to identify PH in such a population. It is possible that the identification of an increased PA-Ao ratio in a patient with DCLD may abbreviate the length of time required to confirm PH because the indication of RHC is mainly based on echocardiographic findings, which may not be accurate in patients with hyperinflation, such as those with DCLDs. Our results did not change even when we assessed the correlation between PA-Ao ratio and mPAP in LAM and PLCH isolatedly (data not shown). We found that 0.86 was the cut-off with the highest accuracy for predicting PH, which was quite similar to that obtained in investigations with patients with IPF and COPD.[36,37] The lack of difference in pulmonary function parameters between patients with PA-Ao ratio > 1 and those with PA-Ao ratio ≤ 1 suggests that other mechanisms in addition to parenchymal involvement and hypoxic vasoconstriction, such as pulmonary vascular involvement and cardiac dysfunction, may have contributed to the enlargement of PA-Ao ratio in our study.
If we used the previous definition of PH (mPAP ≥ 25 mm Hg at rest), the prevalence of PH in our study would be 61.8% (CI 95%: 45–76.1%) and PA-Ao ratio > 1 would occur in 61.9% (CI 95%: 40.9–79.2%) in those with confirmed PH on RHC.[38] However, the correlations found and the comparisons between patients with PA-Ao ratio > 1 and ≤1 would not modify with the use of the previous definition of PH.[38]
Our study has limitations that need to be addressed. Our sample size was small, and this was a single-center analysis, which included only patients with LAM and PLCH, with no other DCLD, that underwent RHC in previous studies. We included only patients that underwent RHC, who probably had more advanced disease due to the fact that the screening method for RHC were DLCO < 40%, systolic PAP ≥ 35 mm Hg, TRV ≥ 2.5 m/s, and/or indirect signs of PH at TTE. However, even with these limitations a robust association was found between vascular parameters on CT scan and hemodynamic data on RHC. A multivariate analysis was not performed to avoid the phenomenon of over-fitting of the model.
5. Conclusions
In conclusion, our study demonstrates a potential role of PA-Ao ratio as a non-invasive, reproducible, and available surrogate marker to predict the presence of PH and as a prognostic parameter in patients with DCLD, therefore adding value to the CT scan evaluation in such population. Future and multi-center studies with a higher number of patients with different severities are necessary to further determine the role of serial vascular measurements assessed on CT scan as complementary markers of prognosis and treatment response.
Author contributions
Bruno Guedes Baldi contributed to the study design, data collection, data analysis, writing and manuscript review, and is the guarantor of the paper.
Caio Júlio César dos Santos Fernandes contributed to the study design, data collection, data analysis, and manuscript review.
Gláucia Itamaro Heiden contributed to the study design, data collection, data analysis, writing, and manuscript review.
Carolina Salim Gonçalves Freitas contributed to the data collection and manuscript review.
Juliana Barbosa Sobral contributed to the data collection and manuscript review.
Ronaldo Adib Kairalla contributed to the study design, writing, and manuscript review.
Carlos Roberto Ribeiro Carvalho contributed to the study design, writing, and manuscript review.
Rogério Souza contributed to the study design, data analysis, writing, and manuscript review.
Conceptualization: Bruno Guedes Baldi, Caio Júlio César dos Santos Fernandes, Carlos Roberto Ribeiro Carvalho, Rogério Souza.
Data curation: Bruno Guedes Baldi.
Formal analysis: Bruno Guedes Baldi, Caio Júlio César dos Santos Fernandes, Gláucia Itamaro Heiden, Carolina Salim Gonçalves Freitas, Juliana Barbosa Sobral, Ronaldo Adib Kairalla, Rogério Souza.
Investigation: Bruno Guedes Baldi, Gláucia Itamaro Heiden, Carolina Salim Gonçalves Freitas, Juliana Barbosa Sobral, Ronaldo Adib Kairalla, Carlos Roberto Ribeiro Carvalho, Rogério Souza.
Methodology: Bruno Guedes Baldi, Caio Júlio César dos Santos Fernandes, Gláucia Itamaro Heiden, Carolina Salim Gonçalves Freitas, Juliana Barbosa Sobral, Ronaldo Adib Kairalla, Carlos Roberto Ribeiro Carvalho, Rogério Souza.
Project administration: Bruno Guedes Baldi, Carolina Salim Gonçalves Freitas.
Supervision: Bruno Guedes Baldi, Ronaldo Adib Kairalla, Carlos Roberto Ribeiro Carvalho, Rogério Souza.
Writing – original draft: Bruno Guedes Baldi, Caio Júlio César dos Santos Fernandes, Rogério Souza.
Writing – review & editing: Caio Júlio César dos Santos Fernandes, Gláucia Itamaro Heiden, Carolina Salim Gonçalves Freitas, Juliana Barbosa Sobral, Ronaldo Adib Kairalla, Carlos Roberto Ribeiro Carvalho, Rogério Souza.
Supplementary Material
Footnotes
Abbreviations: 6MWT = six-minute walk test, Ao = ascending aorta, CI = confidence interval, CT = computed tomography, DCLD = diffuse cystic lung disease, DLCO = lung diffusing capacity for carbon monoxide, FEV1 = forced expiratory volume in the first second, IPF = idiopathic pulmonary fibrosis, LAM = lymphangioleiomyomatosis, mPAP = mean pulmonary artery pressure, NPV = negative-predictive value, PA = pulmonary artery, PAP = pulmonary artery pressure, PH = pulmonary hypertension, PLCH = pulmonary Langerhans cell histiocytosis, PPV = positive-predictive value, PVR = pulmonary vascular resistance, RHC = right heart catheterization, RV = residual volume, sPAP = systolic pulmonary artery pressure, SpO2 = oxygen saturation, TLC = total lung capacity, TRV = tricuspid regurgitation velocity, TTE = transthoracic echocardiography.
How to cite this article: Baldi BG, Fernandes CJ, Heiden GI, Freitas CS, Sobral JB, Kairalla RA, Carvalho CR, Souza R. Association between pulmonary artery to aorta diameter ratio with pulmonary hypertension and outcomes in diffuse cystic lung diseases. Medicine. 2021;100:25(e26483).
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
References
- [1].Obaidat B, Yasdani D, Wikenheiser-Brokamp KA, Gupta N. Diffuse cystic lung diseases. Respir Care 2020;65:111–26. DOI 10.4187/respcare.07117. [DOI] [PubMed] [Google Scholar]
- [2].Baldi BG, Carvalho CRR, Dias OM, Marchiori E, Hochhegger B. Diffuse cystic lung diseases. J Bras Pneumol 2017;43:140–9. DOI 10.1590/S1806-37562016000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Johnson SR, Cordier JF, Lazor R, et al. Review Panel of the ERS LAM task Force. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J 2010;35:14–26. DOI 10.1183/09031936.00076209. [DOI] [PubMed] [Google Scholar]
- [4].McCormack FX, Travis WD, Colby TV, Henske EP, Moss J. Lymphangioleiomyomatosis: calling it what it is: a low-grade, destructive, metastasizing neoplasm. Am J Respir Crit Care Med 2012;186:1210–2. DOI 10.1164/rccm.201205-0848OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McCormack FX, Gupta N, Finlay GR, et al. ATS/JRS Committee on Lymphangioleiomyomatosis. Official American Thoracic Society/Japanese Respiratory Society Clinical Practice Guidelines: Lymphangioleiomyomatosis Diagnosis and Management. Am J Respir Crit Care Med 2016;194:748–61. DOI 10.1164/rccm.201607-1384ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vassallo R, Harari S, Tazi A. Current understanding and management of pulmonary Langerhans cell histiocytosis. Thorax 2017;72:937–45. DOI 10.1136/thoraxjnl-2017-210125. [DOI] [PubMed] [Google Scholar]
- [7].Shaw B, Borchers M, Zander D, Gupta N. Pulmonary Langerhans cell histiocytosis. Semin Respir Crit Care Med 2020;41:269–79. DOI 10.1055/s-0039-1700996. [DOI] [PubMed] [Google Scholar]
- [8].Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53: DOI 10.1183/13993003.01913-2018. pii: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Freitas CSG, Baldi BG, Jardim C, et al. Pulmonary hypertension in lymphangioleiomyomatosis: prevalence, severity and the role of carbon monoxide diffusion capacity as a screening method. Orphanet J Rare Dis 2017;12:74.DOI 10.1186/s13023-017-0626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Taveira-DaSilva AM, Hathaway OM, Sachdev V, Shizukuda V, Birdsall CW, Moss J. Pulmonary artery pressure in lymphangioleiomyomatosis: an echocardiographic study. Chest 2007;132:1573–8. DOI 10.1378/chest.07-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cottin V, Harari S, Humbert M, et al. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM“O”P). Pulmonary hypertension in lymphangioleiomyomatosis: characteristics in 20 patients. Eur Respir J 2012;40:630–40. DOI 10.1183/09031936.00093111. [DOI] [PubMed] [Google Scholar]
- [12].Fartoukh M, Humbert M, Capron F, et al. Severe pulmonary hypertension in histiocytosis X. Am J Respir Crit Care Med 2000;161:216–23. DOI 10.1164/ajrccm.161.1.9807024. [DOI] [PubMed] [Google Scholar]
- [13].Dauriat G, Mal H, Thabut G, et al. Lung transplantation for pulmonary langerhans’ cell histiocytosis: a multicenter analysis. Transplant 2006;81:746–50. DOI 10.1097/01.tp.0000200304.64613.af. [DOI] [PubMed] [Google Scholar]
- [14].Heiden GI, Sobral JB, Freitas CSG, et al. Mechanisms of exercise limitation and prevalence of pulmonary hypertension in pulmonary Langerhans cell histiocytosis. Chest 2020;158:2440–8. DOI 10.1016/j.chest.2020.05.609. [DOI] [PubMed] [Google Scholar]
- [15].Le Pavec J, Lorillon G, Jais X, et al. Pulmonary Langerhans cell histiocytosis-associated pulmonary hypertension: clinical characteristics and impact of pulmonary arterial hypertension therapies. Chest 2012;142:1150–7. DOI 10.1378/chest.11-2490. [DOI] [PubMed] [Google Scholar]
- [16].Choi JS, Lee SH, Leem Y, et al. Prognostic impact of the ratio of the main pulmonary artery to that of the aorta on chest computed tomography in patients with idiopathic pulmonary fibrosis. BMC Pulm Med 2019;19:81.DOI 10.1186/s12890-019-0843-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chung JH, Montner SM, Adegunsoye A, et al. CT findings associated with survival in chronic hypersensitivity pneumonitis. Eur Radiol 2017;27:5127–35. DOI 10.1007/s00330-017-4936-3. [DOI] [PubMed] [Google Scholar]
- [18].Grosse A, Grosse C, Lang I. Evaluation of the CT imaging findings in patients newly diagnosed with chronic thromboembolic pulmonary hypertension. PLoS One 2018;13:e0201468.DOI 10.1371/journal.pone.0201468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wells JM, Washko GR, Han MK, et al. ECLIPSE Study Investigators. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 2012;367:913–21. DOI 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shin S, King CS, Brown AW, et al. Pulmonary artery size as a predictor of pulmonary hypertension and outcomes in patients with chronic obstrutctive pulmonary disease. Respir Med 2014;108:1626–32. DOI 10.1016/j.rmed.2014.08.009. [DOI] [PubMed] [Google Scholar]
- [21].Wells JM, Morrison JB, Bhatt SP, Nath H, Dransfield MT. Pulmonary artery enlargement is associated with cardiac injury during severe exacerbations of COPD. Chest 2016;149:1197–204. DOI 10.1378/chest.15-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zouk AN, Gulati S, Xing D, Wille KM, Rowe SM, Wells JM. Pulmonary artery enlargement is associated with pulmonary hypertension and decreased survival in severe cystic fibrosis: a cohort study. PLoS One 2020;15:e0229173.DOI 10.1371/journal.pone.0229173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shin S, King CS, Puri N, et al. Pulmonary artery size as a predictor of outcomes in idiopathic pulmonary fibrosis. Eur Respir J 2016;47:1445–51. DOI 10.1183/13993003.01532-2015. [DOI] [PubMed] [Google Scholar]
- [24].Courtwright AM, Baldi BG, Kidambi P, et al. Characterization of lymphangioleiomyomatosis patients with discordance between spirometric and diffusion measurements of pulmonary function. Sarcoidosis Vasc Diffuse Lung Dis 2018;35:206–12. DOI 10.36141/svdld.v35i3.6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 2005;26:319–38. DOI 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- [26].Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005;26:511–22. DOI 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- [27].Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720–35. DOI 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- [28].Pereira CA, Sato T, Rodrigues SC. New reference values for forced spirometry in white adults in Brazil. J Bras Pneumol 2007;33:397–406. DOI 10.1590/s1806-37132007000400008. [DOI] [PubMed] [Google Scholar]
- [29].Neder JA, Andreoni S, Castelo-Filho A, Nery LE. Reference values for lung function tests. I. Static volumes. Braz J Med Biol Res 1999;32:703–17. [DOI] [PubMed] [Google Scholar]
- [30].Neder JA, Andreoni S, Peres C, Nery LE. Reference values for lung function tests. III. Carbon monoxide diffusing capacity (transfer factor). Braz J Med Biol Res 1999;32:729–37. DOI 10.1590/s0100-879x1999000600008. [DOI] [PubMed] [Google Scholar]
- [31].ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. DOI 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- [32].Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–81. [PubMed] [Google Scholar]
- [33].Soaresa MR, Pereira CA. Six-minute walk test: reference values for healthy adults in Brazil. J Bras Pneumol 2011;37:576–83. DOI 10.1590/s1806-37132011000500003. [DOI] [PubMed] [Google Scholar]
- [34].Hoette S, Jardim C, Souza R. Diagnosis and treatment of pulmonary hypertension: an update. J Bras Pneumol 2010;36:795–811. DOI 10.1590/s1806-37132010000600018. [DOI] [PubMed] [Google Scholar]
- [35].Maron BA, Brittan EL, Hess E, et al. Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: a retrospective cohort study. Lancet Respir Med 2020;8:873–84. DOI 10.1016/S2213-2600(20)30317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yagi M, Taniguchi H, Kondoh Y, et al. CT-determined pulmonary artery to aorta ratio as a predictor of elevator pulmonary artery pressure and survival in idiopathic pulmonary fibrosis. Respirology 2017;22:1393–9. DOI 10.1111/resp.13066. [DOI] [PubMed] [Google Scholar]
- [37].Ratanawatkul P, Oh A, Richards JC, Swigris JJ, et al. Performance of pulmonary artery dimensions measured on high-resolution computed tomography scan for identifying pulmonary hypertension. ERJ Open Res 2020;6:00232–2019. DOI 10.1183/23120541.00232-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62: Suppl: D34–41. DOI 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


