Abstract
Vascular malformations, affecting ~1%-1.5% of the population, comprise a spectrum of developmental patterning defects of capillaries, arteries, veins, and/or lymphatics. The majority of vascular malformations occur sporadically; however, inherited malformations exist as a part of complex congenital diseases. The malformations, ranging from birthmarks to life-threatening conditions, are present at birth, but may reveal signs and symptoms – including pain, bleeding, disfigurement, and functional defects of vital organs – in infancy, childhood, or adulthood. Vascular malformations often exhibit recurrent patterns at affected sites due to the lack of curative treatments. This review series provides a state-of-the-art assessment of vascular malformation research at basic, clinical, genetic, and translational levels.
Keywords: Vascular malformation, lymphatic malformation, Cerebral cavernous malformation, Hepatic cavernous malformation, Hemangiomas
Subject Terms: Vascular Disease
In 1863, Rudolf Virchow classified vascular anomalies into three categories based on the microscopic vascular architecture: angioma simplex, angioma cavernosum, and angioma racemosum1. Over the next 150 years, these categories became synonymous with superficial hemangioma, venous malformation or deep hemangioma, and arteriovenous malformation due to innate differences in anatomy, physiology, morphology and clinical presentation2. Based on endothelial cell proliferation rate, Mulliken and Glowacki characterized vascular anomalies into binary categories: 1) hemangiomas – benign vascular neoplasms with pathologic proliferation of endothelium, and 2) vascular malformations – defective vascular morphogenesis with normal rate of endothelial proliferation3. According to blood flow characteristics and the type of vessels involved, vascular malformations have been classified into low flow: lymphatic, venous, capillary, or combination; and high flow: arterial or arteriovenous2. Among vascular malformations, venous malformations are the most common (~50%), followed by arterial and arterio-venous malformations (~35%), lymphatic malformations (~10%), and mixed combined malformations (~5%)4.
After working with Rudolf Virchow, Wegner classified lymphatic defects into lymphangioma simplex, cavernosum, and cystoides5. Based on the size of localized dilation of lymphatic vessels, lymphatic malformations are characterized as macrocystic (> 2 cm), microcystic (< 2 cm), or mixed6. Macrocystic lymphatic malformations are composed of varying size cysts filled with eosinophilic and protein-rich fluid appearing as smooth and translucent masses. Aberrant expansion of lymphatic macrocysts impairs organ function and the anatomy of soft tissues, often leading to bleeding and cellulitis. Microcystic lymphatic malformation, mostly affecting skin and mucosal surfaces, present as small, translucent or hemorrhagic vesicles, resembling brawny edema7. Lymphatic malformations often disrupt interstitial fluid drainage promoting primary lymphedema, defective immune function, and malnutrition. Patients with chromosomal anomalies – including Turner’s, Noonan’s, and Down syndrome – also present with lymphatic malformations. Although the majority of lymphatic malformation cases are sporadic, inherited anomalies, like distichiasis or myelodysplasia, are also frequently associated with lymphatic malformations8. During the past two decades, studies of human genetics and vertebrate systems have identified somatic mutations of transcription factors, cofactors, kinases, receptors and ligands in lymphatic anomalies often leading to lymphedema9, 10. Emerging studies also highlight critical relationship between genetic mutations and epigenetic factors in lymphatic anomalies. For instance, human genetic mutations within evolutionarily conserved GATA2 intronic super-enhancer in patients with Emberger syndrome, alter recruitment of HDAC3, thus disrupting formation of enhanceosome complex, which in turn reduces GATA2 expression and cause lymphedema9.
In this review series, Mäkinen T et al11, provide an insightful update on somatic mutation-driven pathophysiology of sporadic lymphatic malformations. The authors also highlight the intricate relationship between disease-driving mutations and multifocal complex lymphatic anomalies affecting central collecting lymphatic vessels. Novel therapeutic approaches, like the repurposing of targeted cancer therapeutics, for both lymphatic malformations and complex lymphatic anomalies, have been discussed.
Expanding on the concept of novel therapeutic approaches, Queisser A et al12 provides an in-depth review of theranostics, a combination of diagnosis and therapeutics, for the personalized treatment of vascular anomalies, including benign vascular tumors and malformations. Conventional treatment approaches – like sclerotherapy, embolization, surgery, or laser resection – often cause severe chronic pain, yet rarely cure vascular anomalies. Lessons from animal models and human patients have helped to identify existing Food and Drug Administration-approved antineoplastics for the treatment of vascular malformations13. Consistent with this, the authors provide a brief overview of the ongoing clinical trials which have been designed to evaluate efficacy of rapamycin and trametinib for slow-flow and fast-flow vascular malformations, respectively.
Critical relationship between the immune system and the endothelium in the pathophysiology of vascular diseases has long been established. Rustenhoven J et al14 introduce a notion that aberrant innate immune system activation coupled with disrupted Cerebrospinal fluid flow predominantly drive the formation of cerebrovascular malformations, mainly arteriovenous malformations and dural arteriovenous fistula. In this in-depth review, authors discuss genetic, environmental, aging, and immune factors contributing to the formation of cerebrovascular anomalies. In addition to underlying mechanisms, they also discuss adverse consequences of cerebrovascular anomalies. Finally, the authors discuss homeostasis of the Cerebrospinal fluid clearance by highlighting a functional relationship between the glymphatic system, a perivascular channel system promoting drainage of soluble proteins and metabolites, and the lymphatic system.
Snellings DA et al15 present the innovative concept of how combinatorial mutations in Cerebral cavernous malformations genes and PIK3CA or MAP3K3 genes regulate progression of Cerebral cavernous malformations, a simple venous malformation broadly classified as either sporadic or familial. The authors provide in-depth analysis of the major developments over the past few decades in the field of Cerebral cavernous malformations. Notably, they highlight the causal relationship between the microbiome / gut barrier and Cerebral cavernous malformations. Based on studies characterizing human and murine Cerebral cavernous malformation lesions, novel candidate diagnostic markers and compelling therapeutic approaches, including repurposing Food and Drug Administration-approved antineoplastics, have been discussed. Encouraging results from pre-clinical trials and multisite therapeutic initiatives show great promise for the future treatment of patients with Cerebral cavernous malformations lesions.
Among provisionally unclassified vascular malformations by the International Society for the Study of Vascular Anomalies16, Hepatic cavernomas or ‘hepatic hemangiomas’ or sinusoidal hemangioma, is the most common sporadic benign venous malformation that present histologically as multiple contiguous dilated vascular spaces lined by a single layer of non-proliferating endothelial cells. Often diagnosed in asymptomatic individuals undergoing abdominal ultrasound, computed tomography, or magnetic resonance imaging, hepatic cavernomas can manifest clinically with abdominal pain or, more rarely, with spontaneous rupture resulting in abdominal hemorrhage and hemodynamic collapse17. The liver is embedded with a vascular network to aid its critical bodily metabolic functions. Blood supply to the liver involves two major inflow conduits, the portal vein draining nutrient rich blood from the intestine and the hepatic artery delivering blood rich in oxygen. Both vessels branch and merge into an intricately patterned sinusoidal system of vessels that drain into systemic hepatic veins18. Vascular malformations affecting the liver can occur as isolated focal, multifocal or diffuse lesions; or as part of a wider spectrum of vascular disease involving multiple organs. Detected incidentally during imaging for other conditions, hepatic vascular lesions are often asymptomatic and can manifest at any age17.
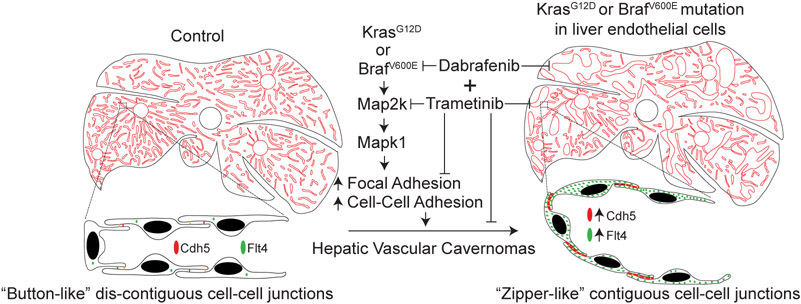
Despite its diagnostic commonplace, the molecular mechanisms of hepatic cavernomas have been largely unknown. However, recent studies revealed the first and unexpected causal link between KRAS or BRAF mutations and hepatic vascular cavernomas (Figure)13. In a cohort of 39 patients, targeted sequencing of genes in the RAS/MAPK pathway revealed a subset of patients with low frequency gain-of-function mutations in two components, the upstream GTPase KRAS and downstream kinase BRAF13. Mice expressing human gain-of-function KRASG12D or BRAFV600E mutations in sinusoidal endothelium recapitutaled the human hepatic cavernous hemangioma phenotype of dilated sinuosoidal capillaries with defective branching pattern13. Consistent with Mulliken and Glowacki’s binary characterization of vascular malformation described in 19823, study demonstrates that KRASG12D or BRAFV600E mutations cause hepatic vascular cavernomas without modulating endothelial cell proliferation13. Mechanistically, these findings support a model in which discontiguous adhesion drives intercalation, and thus sinusoidal capillary branching, whereas contiguous adhesion disrupts intercalation, and thereby expansion of sinusoidal capillary diameter. Repurposing Food and Drug Administration-approved dabrafenib and trametinib, two kinases within the RAS-RAF-MAP2K-MAPK1 signaling pathway, inhibited the growth of BRAFV600E-dependent hepatic sinusoidal cavernomas13. In addition, genetic deletion of Mapk1 also rescued this phenotype in mice, suggesting Mapk1 as a novel therapeutic target. Animal models mimicking human vascular malformations provide an invaluable platform for pre-clinical studies13. A recent study also revealed recurrent missense mutations in GJA4 (p.Gly41Cys) in a majority of patients diagnosed with hepatic hemangiomas19.
Figure.
Hepatic endothelial gain-of-function KRASG12D or BRAFV600E mutations cause cavernous vascular malformations via activation of KRAS-BRAF-MAP2K-MAPK1 signaling pathway. Contiguous expression of adherens junctional proteins in KRAS or BRAF mutant hepatic endothelium promote cavernous sinusoidal expansion instead of branching. Concurrent administration of Food and Drug Administration-approved dabrafenib and trametinib inhibits growth of BRAFV600E-driven cavernous sinusoidal malformations. Adapted from Janardhan HP et al.13
Hereditary Hemorrhagic Telangiectasia (HHT) is an inherited arterio-venous malformation affecting multiple organs including the liver, spleen, pancreas, and stomach20. Similar to hepatic cavernomas, the majority of patients with hepatic arterio-venous malformations are asymptomatic. Clinical signs and symptoms when present are, however, dictated by the nature of the vascular shunt. Arterio-systemic shunts, where the hepatic artery connects directly with the central vein, results in high output heart failure due to increased cardiac preload. In contrast, arterio-portal shunts, where a direct connection is established between the hepatic artery and the portal vein; results in portal hypertension, ascites, splenomegaly and varices21. Initial linkage studies in families with HHT identified chromosomal locations of candidate genes. Additional molecular genetic analysis of these individuals revealed one of the mutated genes to be ENG coding for Endoglin, a co-receptor for BMP/TGFβ ligands22. Subsequent studies revealed additional genes of the BMP/TGFβ signaling pathway being mutated including ACVRL1 and SMAD423, 24. Since the identification of mutations families, several murine models of HHT have been described and used to elucidate the molecular pathogenesis. For example, mice with ALK1 haploinsufficiency demonstrate hepatic Arterio-venous malformations similar to that seen in humans. Murine models have revealed that endothelial cells are the primary cell type responsible for the development of HHT25. Because several different types of mutations have been found to cause HHT, future studies using mice engineered with specific mutations should help further our understanding of this common vascular disorder.
HHT lesions are observed in the gastrointestinal tract, mostly in patients with recurrent nose bleeds (epistaxis), chronic gastrointestinal bleeding and anemia. Diagnosis and management of vascular lesions due to HHT in the gastrointestinal tract remain challenging. Histologically, lesions exhibit clusters of abnormally dilated thin-walled vessels26. In addition to controlling the source of bleeding, treatment is focused on resolving anemia and iron deficiency in these patients. Specific treatments like oral estrogen-progesterone or tranexamic acid, anti-angiogenic drug bevacizumab have been reported to be very effective. Argon plasma coagulation is an effective treatment for gastrointestinal bleeding, particularly in the proximal small intestine, the major site of localization of Arterio-venous malformations in HHT patients.
Pancreatic arterio-venous malformations are the second most common type of abdominal vascular abnormality, some adopting an appearance similar to hypervascular tumors27, 28. While the majority of cases are asymptomatic and, hence, under-reported, symptomatic patients present with gastrointestinal bleeding, portal hypertension, pain and/or pancreatitis29. Diagnosis is confirmed via angiogram and computed tomography often revealing tortuous feeding arteries, a racemose vascular network, and early draining veins29. Approximately 90% of cases are congenital, with the remainder caused by trauma, inflammation, or other processes29, 30. Congenital pancreatic arterio-venous malformations has been attributed to aberrant differentiation of blood vessels and hypothesized to originate from fetal pancreatic vasculature31. Although genetic mutations in ENG, ALK1 and SMAD4 have been associated with vascular malformations in pancreas32; comprehensive analyses will be required to identify additional mutations. Arterio-venous malformations affecting the spleen have also been reported as isolated lesions, associated with splenic aneurysms, or in the context of underlying systemic HHT33, 34. However, genetic drivers of isolated lesions remain undefined.
Together this collection of state-of-the-art reviews11, 12, 14, 15 provide the comprehensive genetic, developmental, pathophysiologic, clinical, and translational basis of vascular and lymphatic malformations affecting multi-organ systems. These articles will provide not only recent developments in the field of vascular malformations at an in-depth level, but also highlight critical gaps in knowledge. While multiple laboratories have made seminal contributions to advance the field of vascular malformations, we have a task to harness the cutting-edge technologies to elucidate novel cellular, molecular, and developmental mechanisms. We hope these discoveries along with the necessary clinical trials will advance our abilities to provide personalized treatments for the patients suffering from vascular and lymphatic malformations.
Acknowledgments:
We thank Timothy J. Cashman for critical reading of the manuscript.
Sources of funding
C.M.T is supported by National Institutes of Health grant HL118100 and HL141377.
Nonstandard Abbreviations and Acronyms:
- PIK3CA
Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha
- MAP3K3
Mitogen-Activated Protein Kinase Kinase Kinase 3
- KRAS
KRAS Proto-Oncogene, GTPase
- BRAF
B-Raf Proto-Oncogene, Serine/Threonine Kinase
- MAPK1
Mitogen-Activated Protein Kinase 1
- RAF
Raf-1 Proto-Oncogene, Serine/Threonine Kinase
- MAP2K
Mitogen-Activated Protein Kinase Kinase
- GJA4
Gap Junction Protein Alpha 4
- HHT
Hereditary Hemorrhagic Telangiectasia
- ENG
Endoglin
- BMP
Bone Morphogenetic Protein
- TGFβ
Transforming Growth Factor Beta
- ACVRL1
Activin A Receptor Like Type 1
- SMAD4
SMAD Family Member 4
- ALK1
ALK Receptor Tyrosine Kinase 1
Footnotes
Disclosures:
The authors have declared that no conflict of interest exists.
References
- 1.Virchow R. Angioma in die krankhaften geschwülste. Berlin, Germany: Hirschwald. 1863 [Google Scholar]
- 2.Steiner J, Drolet B. Classification of vascular anomalies: An update. Seminars in Interventional Radiology. 2017;34:225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: A classification based on endothelial characteristics. Plastic and Reconstructive Surgery. 1982;69:412–420 [DOI] [PubMed] [Google Scholar]
- 4.Rendón-Elías FG, Hernández-Sánchez M, Albores-Figueroa R, Montes-Tapia FF, Gómez-Danés LH. Congenital vascular malformations update. Medicina Universitaria. 2014;16:184–198 [Google Scholar]
- 5.Wegner G. Ueber lymphangiome. Arch. Klin. Chir 1877;20:641 [Google Scholar]
- 6.De Serres LM, Sie KCY, Richardson MA. Lymphatic malformations of the head and neck: A proposal for staging. Archives of Otolaryngology - Head and Neck Surgery. 1995;121:577–582 [DOI] [PubMed] [Google Scholar]
- 7.Elluru RG, Balakrishnan K, Padua HM. Lymphatic malformations: Diagnosis and management. Seminars in Pediatric Surgery. 2014;23:178–185 [DOI] [PubMed] [Google Scholar]
- 8.Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, Burrows P, Frieden IJ, Garzon MC, Lopez-Gutierrez JC, et al. Vascular anomalies classification: Recommendations from the international society for the study of vascular anomalies. PEDIATRICS. 2015;136:e203–e214 [DOI] [PubMed] [Google Scholar]
- 9.Janardhan HP, Milstone ZJ, Shin M, Lawson ND, Keaney JF Jr., Trivedi CM. Hdac3 regulates lymphovenous and lymphatic valve formation. J Clin Invest. 2017;127:4193–4206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janardhan HP, Trivedi CM. Establishment and maintenance of blood-lymph separation. Cell Mol Life Sci. 2019;76:1865–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mäkinen T, Boon LM, Vikkula M, Alitalo K. Lymphatic malformations: Genetics, mechanisms and therapeutic strategies. Circulation Research. 2021;129:XX-XXX [DOI] [PubMed] [Google Scholar]
- 12.Queisser A, Seront E, Boon LM, Vikkula M. Genetic basis and therapies for vascular anomalies. Circulation Research. 2021;129:xx-xxx [DOI] [PubMed] [Google Scholar]
- 13.Janardhan HP, Meng X, Dresser K, Hutchinson L, Trivedi CM. Kras or braf mutations cause hepatic vascular cavernomas treatable with map2k-mapk1 inhibition. J Exp Med. 2020;217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rustenhoven J, Tanumihardja C, Kipnis J. Cerebrovascular anomalies: Perspectives from immunology and cerebrospinal fluid flow. Circulation Research. 2021;129:xx-xxx [DOI] [PubMed] [Google Scholar]
- 15.Snellings DA, Hong CC, Ren AA, Lopez-Ramirez MA, Girard R, Srinath A, March DA, Ginsberg MH, Award IA, Kahn ML. Cerebral cavernous malformation: From mechanism to therapy. Circulation Research. 2021;129:xx-xxx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anomalies ICoV. International Society for the Study of Vascular Anomalies. revised in May 2018;Available at: https://www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf
- 17.Evans J, Willyard CE, Sabih DE. Cavernous hepatic hemangiomas. Statpearls. Treasure Island (FL): StatPearls Publishing; Copyright © 2021, StatPearls Publishing LLC.; 2021. [Google Scholar]
- 18.Greenway CV, Stark RD. Hepatic vascular bed. Physiol Rev. 1971;51:23–65 [DOI] [PubMed] [Google Scholar]
- 19.Ugwu N, Atzmony L, Ellis KT, Panse G, Jain D, Ko CJ, Nassiri N, Choate KA. Cutaneous and hepatic vascular lesions due to a recurrent somatic gja4 mutation reveal a pathway for vascular malformation. HGG Adv. 2021;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kühnel T, Wirsching K, Wohlgemuth W, Chavan A, Evert K, Vielsmeier V. Hereditary hemorrhagic telangiectasia. Otolaryngol Clin North Am. 2018;51:237–254 [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Tsao G. Liver involvement in hereditary hemorrhagic telangiectasia (hht). J Hepatol. 2007;46:499–507 [DOI] [PubMed] [Google Scholar]
- 22.McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, et al. Endoglin, a tgf-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351 [DOI] [PubMed] [Google Scholar]
- 23.Gallione CJ, Repetto GM, Legius E, Rustgi AK, Schelley SL, Tejpar S, Mitchell G, Drouin E, Westermann CJ, Marchuk DA. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in madh4 (smad4). Lancet. 2004;363:852–859 [DOI] [PubMed] [Google Scholar]
- 24.Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13:189–195 [DOI] [PubMed] [Google Scholar]
- 25.Tual-Chalot S, Oh SP, Arthur HM. Mouse models of hereditary hemorrhagic telangiectasia: Recent advances and future challenges. Front Genet. 2015;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tortora A, Riccioni ME, Gaetani E, Ojetti V, Holleran G, Gasbarrini A. Rendu-osler-weber disease: A gastroenterologist’s perspective. Orphanet Journal of Rare Diseases. 2019;14:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welle CL, Welch BT, Brinjikji W, Ehman EC, Venkatesh SK, Johnson MP, Iyer VN, Leise MD, Wood CP. Abdominal manifestations of hereditary hemorrhagic telangiectasia: A series of 333 patients over 15 years. Abdom Radiol (NY). 2019;44:2384–2391 [DOI] [PubMed] [Google Scholar]
- 28.Ishigami K, Sakuma T, Saito M, Kawakami Y, Masaki Y, Murota A, Motoya M, Kimura Y, Nakase H. Arteriovenous malformation in pancreas mimicking hypervascular tumor. JGH Open. 2020;4:773–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishiyama R, Kawanishi Y, Mitsuhashi H, Kanai T, Ohba K, Mori T, Hamabe N, Watahiki Y, Nakamura S. Management of pancreatic arteriovenous malformation. J Hepatobiliary Pancreat Surg. 2000;7:438–442 [DOI] [PubMed] [Google Scholar]
- 30.Nikolaidou O, Xinou E, Papakotoulas P, Philippides A, Panagiotopoulou-Boukla D. Pancreatic arteriovenous malformation mimicking pancreatic neoplasm: A systematic multimodality diagnostic approach and treatment. Radiol Case Rep. 2018;13:305–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shearer DD, Demos TC, Sichlau MJ. Pancreatic arteriovenous malformation: A case report and literature review. J Radiol Case Rep. 2011;5:8–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacout A, Pelage JP, Lesur G, Chinet T, Beauchet A, Roume J, Lacombe P. Pancreatic involvement in hereditary hemorrhagic telangiectasia: Assessment with multidetector helical ct. Radiology. 2010;254:479–484 [DOI] [PubMed] [Google Scholar]
- 33.Agrawal A, Whitehouse R, Johnson RW, Augustine T. Giant splenic artery aneurysm associated with arteriovenous malformation. J Vasc Surg. 2006;44:1345–1349 [DOI] [PubMed] [Google Scholar]
- 34.Ono S, Obara H, Shimoda M, Kitagawa Y. Idiopathic splenic arteriovenous fistula without splenic artery aneurysm. BMJ Case Rep. 2015;2015 [DOI] [PMC free article] [PubMed] [Google Scholar]